Abstract
A range of chemical reactions occurring on the surfaces of metal nanoparticles exhibit enhanced rates under plasmonic excitation. It is not straightforward to distinguish between photochemical and photothermal effect using Arrhenius fitting of the reaction rates alone.
In the recently published article: “Thermal effects – an alternative mechanism for plasmon-assisted photocatalysis”, Dubi et al.1 argue that the results of multiple works on plasmon-excited-induced bond dissociation reactions can be explained by a purely photothermal enhancement of the reaction rates and that no non-thermal effects are required to explain the enhanced rates resulting from plasmonic excitation. Their argument rests on a reproduction of the reaction rate data by an Arrhenius expression with a light-intensity-dependent local temperature at the surface of the nanoparticles.
Dubi et al.‘s straightforward analysis may have general appeal for explaining rate enhancements in bond dissociation reactions observed under plasmonic excitation of metal nanostructures without invoking hot electron contributions. But there is one caveat that deserves recognition when undertaking such an analysis. As shown below, under certain common scenarios, it is practically impossible to distinguish between a photochemical (non-thermal) effect of light excitation and a purely photothermal one using a phenomenological Arrhenius fitting of the data alone.
As per the Arrhenius equation, the rate of a reaction depends on the set temperature Ts as:
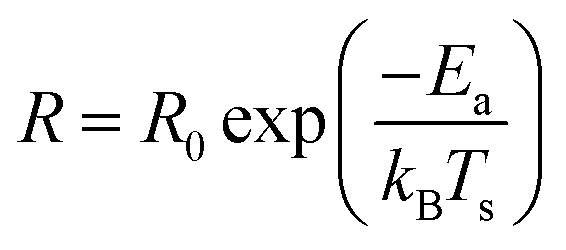 |
1 |
where R0 is a constant for a given reaction and reaction conditions and Ea is the apparent activation energy barrier for the reaction. As an aside, one should note that unlike the Eyring equation, which is preferred for non-gas-phase reaction kinetics involving a vibrational reaction co-ordinate, the pre-exponential factor in the Arrhenius equation is assumed to have a negligible temperature dependence.
A photochemical explanation of plasmon-enhanced catalysis is that the apparent activation energy Ea is lower under plasmonic excitation as compared to its value, Edarka, in the dark. Thus, as per eqn (1), at a fixed temperature Ts, R will be higher under light excitation. In fact, the measured apparent activation barrier has been found to be dependent on the light intensity I. For the sake of the following argument, let us assume that the decrease in Ea is linearly dependent on the light intensity:
| Ea = Edarka − BI | 2 |
where B is a proportionality constant with units of eV cm2 W−1 when Ea is expressed in units of eV and I in units of W cm−2. Note that B is expected to be wavelength-dependent. Eqn (2) can be written alternatively as:
| Ea = Edarka(1 − bI) | 3 |
where b is simply B/Edarka and has units of cm2 W−1. From eqn (1) and (3):
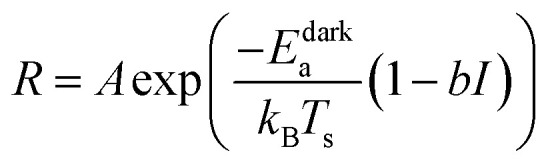 |
4 |
Using a Taylor's expansion around I = 0 (dark condition),
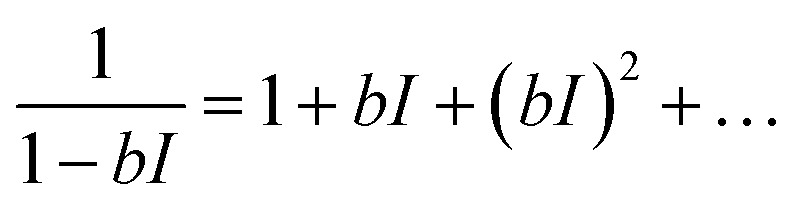 |
5 |
For the light-intensity regime (I ≪ 1/b), the higher order terms can be neglected, so one gets from eqn (4) and (5):
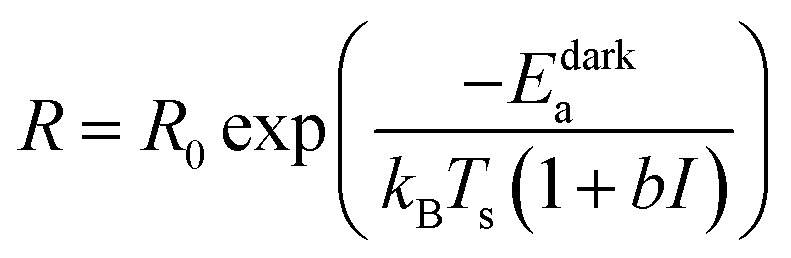 |
6 |
Thus, if one simply uses an Arrhenius analysis of the reaction rate, the reaction appears to be carried out at a hypothetical temperature that is higher than the actual temperature Ts by an amount proportional to the light intensity I:
| Tdummy = Ts(1 + bI) | 7 |
where this hypothetical temperature is referred to as Tdummy. Eqn (7) is equivalently expressed as:
| Tdummy = Ts + aI | 8 |
where a = bTs is the photothermal conversion coefficient with units of K cm2 W−1. Eqn (8) is identical to the expression used by Dubi et al. in their argument in favor of a purely photothermal effect. In other words, it would appear as if plasmonic excitation led to an increase in the temperature, but led to no change in the apparent activation barrier. Effectively, in a phenomenological Arrhenius analysis, the photochemical (non-thermal) effect of plasmonic excitation on the reaction is simply masked as a temperature increase.
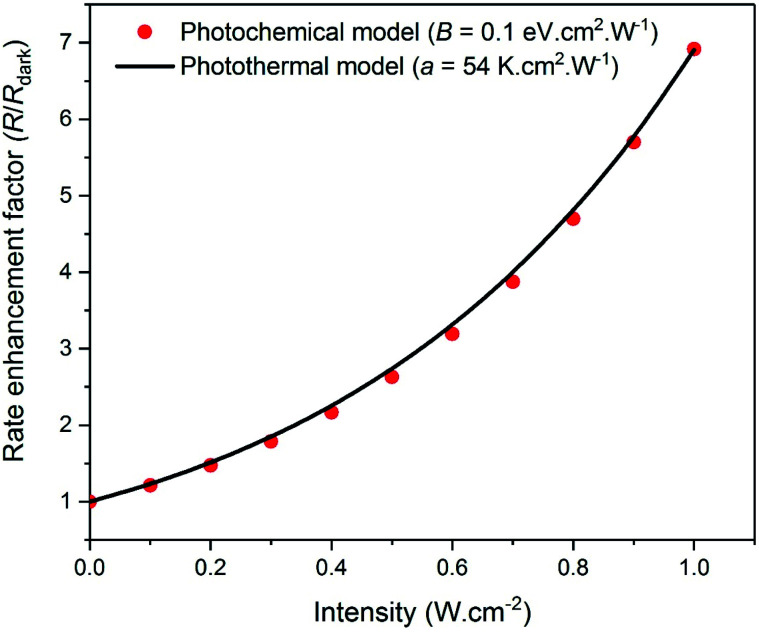
Thus, as shown in Fig. 1, an Arrhenius analysis with a as an adjustable fit parameter may be futile for practically distinguishing the photochemical action of plasmonic excitation, (i.e., a rate enhancement caused by a decrease in the activation barrier) from a purely photothermal effect (i.e., a rate enhancement caused by an increase in the surface temperature). Under such a scenario, for distinguishing these effects, it is necessary to have precise knowledge and/or control over the temperature at the surface of the nanoparticles, as correctly argued by Dubi et al.,1 but also acknowledged by practitioners2–4 in the field. It is well appreciated that the localized inhomogeneous nature of photothermal heating results in a temperature gradient extending out from the surface of the nanoparticles to the bulk of the medium. These gradients are small in magnitude under conditions where the heat dissipation rate can keep up with the energy deposition rate. However, in systems where heat transfer rates are limiting, significant non-uniformities in temperature and thermal bottlenecks can arise. Such cases necessitate spatially precise temperature-probing localized to the nanoparticle surface.
Fig. 1. The reaction rate under plasmonic excitation, R, relative to that in the dark, Rdark, is plotted as a function of light intensity for (i) the photochemical case (red dots), where the activation barrier is decreased by plasmonic excitation (eqn (1) and (2) with B = 0.1 eV cm2 W−1) while the temperature is kept fixed and (ii) the purely photothermal model (black line), where the temperature is increased by plasmonic excitation (eqn (1) and (8)) with a = 54 K cm2 W−1) but the activation barrier remains unchanged. In both cases, Edarka = 1.21 eV and Ts = 600 K. The two models yield trends that are practically indistinguishable.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This material is based upon work supported by the National Science Foundation under Grant (NSF CHE-1455011). The author thanks Varun Mohan for proof-reading. The author has no competing interests.
References
- Dubi Y. Un I. W. Sivan Y. Thermal effects – an alternative mechanism for plasmon-assisted photocatalysis. Chem. Sci. 2020;11:5017–5027. doi: 10.1039/C9SC06480J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L. Swearer D. F. Zhang C. Robatjazi H. Zhao H. Henderson L. Dong L. Christopher P. Carter E. A. Nordlander P. Halas N. J. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science. 2018;362:69–72. doi: 10.1126/science.aat6967. [DOI] [PubMed] [Google Scholar]
- Jain P. K. Taking the heat off of plasmonic chemistry. J. Phys. Chem. C. 2019;123:24347–24351. doi: 10.1021/acs.jpcc.9b08143. [DOI] [Google Scholar]
- Li X. Everitt H. O. Liu J. Confirming nonthermal plasmonic effects enhance CO2 methanation on Rh/TiO2 catalysts. Nano Res. 2019;12:1906–1911. doi: 10.1007/s12274-019-2457-x. [DOI] [Google Scholar]