Abstract
Cancer is a clonal disease, i.e., all tumor cells within a malignant lesion trace their lineage back to a precursor somatic cell that acquired oncogenic mutations during development and aging. And yet, those tumor cells tend to have genetic and nongenetic variations among themselves—which is denoted as intratumor heterogeneity. Although some of these variations are inconsequential, others tend to contribute to cell state transition and phenotypic heterogeneity, providing a substrate for somatic evolution. Tumor cell phenotypes can dynamically change under the influence of genetic mutations, epigenetic modifications, and microenvironmental contexts. Although epigenetic and microenvironmental changes are adaptive, genetic mutations are usually considered permanent. Emerging reports suggest that certain classes of genetic alterations show extensive reversibility in tumors in clinically relevant timescales, contributing as major drivers of dynamic intratumor heterogeneity and phenotypic plasticity. Dynamic heterogeneity and phenotypic plasticity can confer resistance to treatment, promote metastasis, and enhance evolvability in cancer. Here, we first highlight recent efforts to characterize intratumor heterogeneity at genetic, epigenetic, and microenvironmental levels. We then discuss phenotypic plasticity and cell state transition by tumor cells, under the influence of genetic and nongenetic determinants and their clinical significance in classification of tumors and therapeutic decision-making.
Keywords: cancer evolution, cell state transition, drug resistance, intratumor heterogeneity, phenotypic plasticity
INTRODUCTION
“All happy families are alike; each unhappy family is unhappy in its own way” wrote Leo Tolstoy in Anna Karenina. Just like the unhappy families of Tolstoy, no two tumors are exactly alike, both at the clinical and molecular levels. Even within a cancerous lesion, tumor cells, which trace their lineage back to a single, renegade somatic cell, tend to have genetic and nongenetic differences among themselves—which is referred to as intratumor heterogeneity. Genetic and nongenetic variations between tumor cells introduce phenotypic variations in a heterogeneous tumor, providing substrates for somatic evolution, and also contributing to tumorigenesis, emergence of drug resistance, dormancy, and metastasis (1–4).
Heterogeneity in cancer has been recognized since early days in biomedical research. In the 1800s, pathologist Rudolf Virchow discussed morphological diversity of cells within individual tumors (5). Cytogenetic studies in the 1960s and 70s have identified genetic and phenotypic variations in tumor cell populations. Mitelman (6) used cytogenetic approach to study chromosomal defects in primary Rous rat sarcomas, and found evidence for clonal evolution, and heterogeneity at the levels of chromosome numbers and karyotypes in these tumors. In 1984, Heppner (7) published an elegant review summarizing contemporary studies that identified multiple subpopulations within heterogeneous tumors derived from both cancer patients and model systems in laboratories. Subsequently, investigation of tumor heterogeneity using morphological studies was followed by advances in subtyping methods using histopathological markers, karyotyping genetic and cytogenetic abnormalities, treatment sensitivity, and in recent times next generation sequencing platforms (3, 4, 8). Recent efforts have characterized intratumor heterogeneity in most major cancer types, and also within individual patients during the course of tumor progression (9–11).
Intratumor heterogeneity is frequently discussed in the context of 1) clonal evolution of tumor cells marked by progressive accumulation of somatic mutations, such that the tumor cells within a lesion have different mutational makeups; a subset of those mutations offers selective advantage to the tumor cells as they compete for resources promoting somatic evolution during tumor progression (4), and 2) developmental hierarchy of tumor cells starting from tumor stem cells (12, 13). Epigenetic and non-cell-autonomous changes in the tumor microenvironment (TME) can alter cellular characteristics of tumor cells reversibly, contributing to phenotypic plasticity both at the levels of individual cells and tumor microenvironment (14–16). It is important to note that tumors typically have heterogeneity concurrently at genetic and nongenetic levels (Fig. 1), and that heterogeneity at multiple levels might be related or independent. Although genetic mutations are usually considered irreversible, emerging reports suggest that certain classes of genetic alterations can show reversion during tumor progression, and those appear to contribute as major drivers of dynamic intratumor heterogeneity and phenotypic plasticity in tumors. In this article, we first highlight recent efforts to characterize intratumor heterogeneity at genetic, epigenetic, and microenvironment levels. We will then discuss phenotypic plasticity and phenotypic switching by tumor cells, under the influence of genetic and nongenetic determinants and their clinical significance in classification of cancer and therapeutic decision-making.
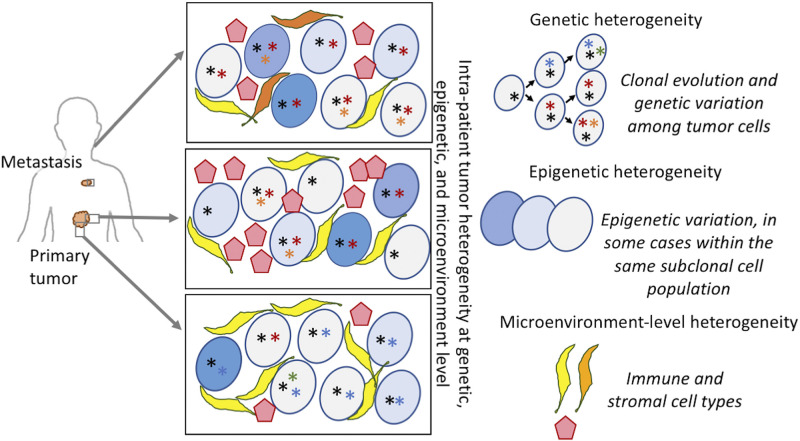
Figure 1.
Intrapatient tumor heterogeneity at multiple levels. Tumors represent complex dynamic systems comprising different subclones, heterogeneous in both genotype and phenotype, and regulated by cell-intrinsic and -extrinsic factors. The clonal evolution model (top right) depicts the accumulation of mutations and clonal evolutionary dynamics that follows. Genetic heterogeneity arises due to somatic mutations occurring in different tumor cell lineages within the same malignant growth. Epigenetic heterogeneity (middle right) develops due to changes in epigenetic states in different tumor cells, sometimes within the same tumor subclones, and such changes can be potentially reversible. Genetic and nongenetic variations collectively result in phenotypic diversity of the tumor cells. Phenotypically diverse tumor cells interact with immune and stromal cells in varied microenvironmental contexts (bottom right) to promote tumor heterogeneity. *Genetic alterations, while the shades of blue represent epigenetic and/or phenotypic heterogeneity in tumor cells. Stromal and immune cells are shown in yellow, orange, and red, respectively.
INTRATUMOR GENETIC HETEROGENEITY
Traditional histopathological examination was one of the earliest methods used to assess intratumor heterogeneity. Apart from scoring tumors they also provide information regarding tumor cellularity, nuclear characteristics, and immune cell infiltration. Staining for cancer-related biomarkers enable simultaneous interrogation of genetic and phenotypic characteristics of tumors and associated intratumor heterogeneity at single-cell resolution. In addition, cytogenetic analyses can identify chromosomal abnormalities including ploidy changes, large-scale rearrangements, and micronuclei in cancer cells. Pathological and cytogenetic analysis established that inter- and intratumor heterogeneity are hallmarks of all cancers, and that evolutionary trajectory of cancer can be predicted from heterogeneity patterns (3, 8). For instance, using histopathological analysis of breast cancer samples and mathematical modeling, Martins et al. (17) identified that BRCA1 loss promotes intratumor heterogeneity, and that although there was no obligatory order of events, loss of PTEN was identified as the most common early event in basal-like subtypes, whereas TP53 mutation was considered as the first oncogenic event in a majority of luminal tumors. But pathological and cytogenetic studies are usually low throughput and labor-intensive, such that large-scale investigation was challenging. Nonetheless, aforementioned studies demonstrated that combining genomic and phenotypic analysis can help better characterize tumor heterogeneity. Recently, expert-curated histopathological and cytogenetic analyses have paved the way for development of automated image analysis and digital techniques with machine learning algorithms complementing traditional pathological characterization to analyze large number of tumor and stromal cells at single-cell level with their spatial context to guide clinical diagnostics (18). For instance, Yuan et al. (19) integrated large-scale quantitative image analysis to complement genomic profiling to infer multilevel heterogeneity in patients with ER-negative breast cancer. Through this approach they derived tumor cellular composition, which showed the importance of spatial relationships between cells in the tumor tissue and predicted that spatial distribution of stromal cells was an independent prognostic factor for survival. Convergence of high-throughput spatial and single-cell genomics has the potential to transform our understanding of spatiotemporal heterogeneity in cancer at remarkable resolution, as discussed in future directions.
In the past decade, advances in next generation sequencing approaches have enabled large-scale characterization of intratumor heterogeneity in patient-derived samples at a genome-wide scale. A pioneering study of whole genome sequencing of paired primary tumor and paired metastatic lesion from a patient with breast cancer identified clonal and subclonal mutations, and provided early insights into clonal dynamics during metastatic progression; of the 32 somatic nonsynonymous coding mutations present in the metastasis, only 5 were prevalent in the primary tumor, 6 were present at low frequencies, suggesting significant evolution during metastatic progression (20). Subsequently, a number of studies, including the Cancer Genome Atlas Project have examined patterns of genetic heterogeneity in all major cancer types (4, 10, 11). Owing to high cost and technical challenges, initial efforts involved sequencing the whole tumor tissue samples. But tumor tissues are spatially heterogeneous, such that bulk tumor sequencing provides only limited insights into the extent of intratumor spatial heterogeneity. Furthermore, some subclones might be dominant locally but undetectable elsewhere in a spatially heterogeneous tumor, such that caution is recommended while interpreting observed heterogeneity patterns from bulk sequencing data.
In a classic paper, using genomic profiling of multiple geographic regions from a primary renal tumor and metastatic lesions, Gerlinger et al. (21) showed that only ∼30% mutations were clonal, i.e., common to all tumor cells across all regions, and only a locally restricted, minor subpopulation of tumor cells was associated with metastatic dissemination—highlighting extensive genetic and spatial heterogeneity. Subsequently, investigator-initiated and multi-institutional initiatives such as the TracerX project have characterized intratumor spatial and genetic heterogeneity using multiregion sequencing of many cancer types (10, 11, 22–24). Although cancer is usually a clonal disease, pathological characterization and genetic assessments have established that tumors with mixed histology and/or mixed grades are prevalent in many cancer types, and genetic changes explain subtype classification to some extent, whereas some other tumors might be admixture of concurrent lesions. Occasionally, patients are presented with multiple synchronous tumors at various distal regions in the same organ [e.g., in lung (25, 26)], but those appear to be of independent origins. Sequencing of primary and multiple metastatic lesions from the same patients provided evidence for polyclonal seeding and secondary metastatic dissemination highlighting complex patterns of tumor progression (27). These and other investigations indicate that certain mutagenic processes are common early during development and aging, whereas a few others are dominantly late during tumor progression, driving intratumor genetic heterogeneity (11, 28–31).
More recently, single-cell genomics has enabled characterization of intratumor heterogeneity in patient-derived tumor samples at the resolution of individual cells in most major cancer types, and identified genetic and nongenetic determinants of tumor evolution, as discussed in detail elsewhere (32). For instance, using integrative omics-analysis of subclonal cell populations in non-small cell lung cancer, Sharma et al. (22) reported that the extent of nongenetic intratumor heterogeneity was not sufficiently evident from the patterns of intratumor genetic variations; furthermore nongenetic heterogeneity impacted molecular pathways and proliferative potential of tumor cell populations, which in turn influenced clonal dynamics in vivo. Alongside, mathematical modeling of subclonal mutation burden and their cell fractions in tumors inferred from bulk and single-cell data have helped generate provocative hypotheses about the mode of evolutionary dynamics in individual tumors (33–35). For example, using genomic data-driven mathematical modeling approaches, Gao et al. (36) established that a subset of triple negative breast tumors undergo punctuated evolution such that a substantial fraction of copy number alterations arises early, in short punctuated bursts followed by period of relatively stable clonal growth, which challenges the paradigm of gradual somatic evolution.
Early cancer progression is often asymptomatic, and even during treatment and follow-up period it remains challenging to track tumor evolution using invasive procedures. But tumors shed intact tumor cells and tumor-derived DNA in blood and other body fluids, such that sensitive sequencing of cell-free DNA in blood can noninvasively identify cancer-associated mutations with clinically acceptable specificity and sensitivity. It has been possible to identify minimal residual disease, and also infer approximate clonal architecture and clonal dynamics from noninvasive profiling of serum-derived cell-free DNA from cancer patients and at-risk population (9, 37, 38). For example, using whole exome followed by deep amplicon sequencing, Murtaza et al. (37) quantified and compared mutation levels in biopsy and plasma samples over a clinical course and showed that a minor subclone in the lymph node gave rise to distant metastases, suggesting multifocal clonal evolution.
INTRATUMOR NONGENETIC AND CELL STATE HETEROGENEITY
Unlike genetic makeup, the epigenomic, transcriptomic, and proteomic profiles of somatic cells are more adaptive, and heterogeneity therein can ultimately influence tumor cell morphologies, developmental hierarchies, and evolutionary trajectories (14, 16, 32). Intratumor epigenetic heterogeneity, at least partly could contribute to cancer stem cell phenotypes, dormancy, and metastasis (1, 12). Immunostaining, fluorescence-activated cell sorting, single-molecule RNA FISH, and more recently single-cell genomics approaches have enabled profiling of thousands of transcriptome and/or epigenome of tumor cells in a single assay. Massively parallel single cell RNA sequencing has enabled deep characterization of intratumor transcriptomic heterogeneity in tumor, immune, and stromal cell populations in most major cancer types (32, 39). Alongside, a number of single-cell genomic assays have emerged, which include scATAC-Seq for chromatin accessibility (40), scHi-C for chromosomal topological alteration (41), and cNOMe-seq for scChIC-seq for histones and transcription factors profiling (42). Innovative usage of omics assays and computational genomic resources have enabled multi-omics profiling of tissue samples at single-cell level (43, 44), presenting opportunities to identify the key drivers of phenotypic heterogeneity in tumors (32). In a pioneering work, La Manno et al. (45) estimated the rate of change in transcript abundance, termed as “RNA velocity” and predicted cell states by measuring the ratio of unspliced to spliced mRNA in scRNA-seq transcripts, which allowed deriving pseudotime measurements from single cells and decoupling cell state dynamics of both normal and cancerous tissues.
Cell-to-cell epigenetic and transcriptomic heterogeneity in tumor cells tend to be much higher than that among normal cells of the same cell type within somatic tissues. Although a proportion of cell-to-cell variation in nongenetic attributes may be noise attributed to deregulation of gene regulatory programs in cancer cells, some variations may reflect systematic modulation of cellular processes, promoting cell state transition in tumor cell populations. The cancer stem cell model (12) argues that a subpopulation of tumor cells might have biomarkers and/or characteristics of stem or progenitor cells, which maintain higher seeding and regeneration potential within a tumor. Stem-like cells have been observed in multiple cancers including hematopoietic cancers (46). Cancer stem cells may directly descend from the cell-of-origin of the tumor in tissue stem cells or arise due to cell state transition during tumorigenesis. During metastasis, disseminated tumor cells from solid tumors tend to undergo epithelial-mesenchymal transition, and show dormant and stem cell-like phenotype (1), whereas tumor cells with such characteristics are minorities in the bulk primary tumor and also macro-metastases.
Tumors usually show heterogeneity concurrently at genetic, transcriptomic, and other levels, but it remains challenging to compare heterogeneity at genetic and nongenetic levels directly. Sharma et al. (22, 47) used multiregion sequencing and properties of distance-based trees to compare patterns of regional heterogeneity at genetic, transcriptomic, immune, and phenotypic level in lung tumors, and inferred that nongenetic intratumor heterogeneity might be higher than that observed at the genetic level and impact the regional variations in tumor phenotype and histological subtyping differences. It is also to be noted that genetic and epigenetic heterogeneities need not be entirely independent of each other, rather certain epigenetic states may either promote genomic instability or genetic changes may drive specific epigenetic changes (48–50). Tumor microenvironment, which is naturally heterogeneous, also plays an active role in this, as discussed later. Taken together, a complex interplay of heterogeneity across multiple levels shapes up phenotypic heterogeneity in tumors.
DYNAMIC ASPECTS OF TUMOR HETEROGENEITY
Collectively, genetic and nongenetic heterogeneities lead to phenotypic variation among tumor cells, which promotes natural selection and somatic evolution during tumor progression and clinical management of patients with cancer. Although multiple tumor cell populations coexist during neutral evolution, clonal sweep can enable one tumor subclone to dominate, transiently limiting clonal diversity, before secondary subclones arise within the dominant clonal population with time (51). Solid tumors can have spatially segregated microenvironmental contexts within a lesion, and also between the primary tumor and metastases—providing opportunities for parallel evolutionary processes with independent outcomes. During the course of tumor progression, the phenotypic states of tumor cells, as well as the patterns and extent of intratumor heterogeneity tend to change as well, and can have a multitude of implications in cancer contexts.
Cell state transition within the developmental hierarchy is an important component of the dynamic aspects of intratumor heterogeneity (52). Tumor cells hijack many normal cellular processes, and many embryogenesis-related programs are activated during cancer progression (53). Unlimited proliferation and migratory potential of cancer cells mimic the mechanisms of normal embryonic stem cells, often expressing similar repertoire of markers genes like Oct-4, hCG, and exhibiting dependence on similar signaling pathways including Notch, Wnt, etc. that are associated with cancer stemness. It is debated whether a tumor stem cell state is a persistor phenotype, or whether at least in some cases tumor cells can transition in and out of stem-like cell states (12, 54). Sharma et al. (22) reported that tumor cells belonging to the same subclonal cell population show differences in transcriptomic makeup, proliferative potential, and tumor hallmarks depending on their localization in the tumor margin or resource-limited interior. Cell-to-cell variation in protein abundance can influence key cellular phenotypes, as has been shown for proteotoxic stress response network (55). Data-driven modeling indicated that dynamic cell state transition and epithelial-mesenchymal heterogeneity can emerge from unequal segregation of RNAs and proteins among daughter cells during cell division in tumor cell populations (56). Metastasis involves reversible epithelial-mesenchymal cell state transition during dissemination, seeding, dormancy, and reawakening cycle of tumor cells, and there is evidence that primary metastatic sites could seed secondary metastases in some cases (1, 27, 57). Newly developed mouse models of tumor metastasis underscore reversible phenotypic switching associated with metastatic dissemination, dormancy, and reawakening at distal metastatic sites (58, 59). In many instances, these transitions do not involve discrete cell states, but rather a quasi-continuous spectrum of transient cell states depending on physiological status of the tissue and microenvironmental cues.
Epigenetic alterations are usually considered as predominant drivers of the cell state dynamics (50, 60). Epigenetic alterations can affect multiple loci simultaneously, changing regulatory programs and ultimately cell states very quickly. Both DNA methylation and histone modifications, the two primary epigenetic codes, are reversible processes with high-error rate in replicating DNA and therefore potentially less faithfully propagated through cell division than genetic information indicating possibility of more epigenetic variability as tumors evolve (61, 62). Acquired epigenetic alterations and cell states tend to be heritable, maintained over multiple generations before being reversed spontaneously or under local cues. Cell tracking and laboratory fluctuation experiments indicate that, in many cases, tumor cells show that coordinated transcriptional bursting in gene modules can guide cell state transition of tumor cells, and such events persisting for several generations in rare cells can confer resistance to anticancer drugs (63, 64).
GENETIC DRIVERS OF DYNAMIC HETEROGENEITY AND PHENOTYPIC PLASTICITY
Genetic changes during clonal evolution are usually considered permanent, but in some cases genetic changes can dynamically modulate cellular properties leading to phenotypic plasticity (Fig. 2). These changes can be broadly grouped into two categories. First, accumulation of secondary mutations can negate oncogenic genetic alterations on the gene product or compensate them in the same pathway, erasing their effects. The examples include reversal mutations in BRCA1 and DYNLL1 loss that rescue BRCA deficiency as bona fide mechanisms of resistance to PARP inhibitors and chemotherapy (65, 66). In other cases, genetic alterations may impact the cellular phenotype via a dose-dependent mechanism. For example, within a clonal lineage of tumor cells, variation in the number of copies of the genetic element may contribute to phenotypic heterogeneity. These changes can occur in the chromosomal DNA, or extrachromosomal DNA (ecDNA) and mitochondria.
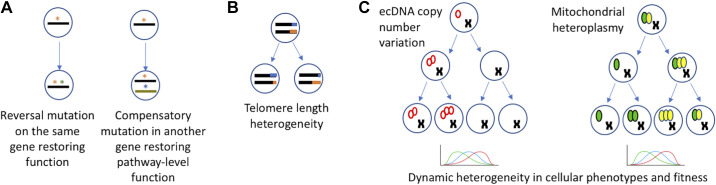
Figure 2.
Genetic drivers of dynamic heterogeneity in tumors. A: reversal mutations during tumor progression restore cancer gene function or compensate for gain- or loss-of-function at the pathway-level. *Genetic alterations on the same gene or on different genes. B: dynamic changes in telomerase expression and telomere length heterogeneity with implications for genomic instability during cancer progression. Different colors (blue and orange) display telomeres of different lengths. C: examples of genetic drivers of dynamic heterogeneity in cancer by copy number variation. Red empty ovals represent extrachromosomal circular DNA (left), and green and yellow filled ovals represent varied proportion of mutated and wild-type mitochondrial DNA (right). Replication and asymmetric distributions can lead to skewed distributions, and dynamic heterogeneity in tumors.
Extrachromosomal DNA elements tend to have sizes ranging from a few bases to Mb of sequences; a subset of those could be circularized, which increase their stability, and have their own origins of replication and chromatin states, allowing for replicative copy number increase (67). During cell division, unequal distribution of these ecDNA copies between the two daughter cells can lead to copy number differences, such that within a few generations the progenitor cell populations can have a wide variation in copy number, contributing to genetic heterogeneity. While also detected in normal cells, ecDNA appears to be very common in most major cancer types, and focal high-copy number gains of oncogenes are frequently attributed to ecDNAs (68–70). It appears that in the nucleus rather than individual ecDNAs, clusters of ∼10–100 ecDNAs, dubbed as ecDNA hubs drive amplified oncogene expression, as observed for MYC, epidermal growth factor receptor (EGFR), FGFR2, etc (71). ecDNA heterogeneity can confer adaptive characteristics at the tumor cell population level. Although cells with high copy numbers of oncogenic loci may have proliferative advantage during tumor progression, the cells with lower copy numbers might be transiently less sensitive to treatment or stress, and can attain high-copy number states within a few cell divisions under favorable conditions. For example, EGFR mutant glioblastoma cells propagate copies of mutant EGFR as extrachromosomal DNA whose abundance can change rapidly in the face of treatment (72). However, when therapy-related selection pressure is removed, its progenitors can regain high copy number via asymmetric segregation of ecDNAs within a relatively short time frame. It is noted that selection induced by tumor microenvironment contexts, e.g., between hypoxic core versus invasive margin can also modulate oncogenic expression, often via epigenetic regulation (73), but further studies are needed to establish whether those also involve dynamic ecDNA copy number variation within tumor cell lineages.
Similar adaptive response is also possible via dynamic changes in mitochondrial copy numbers in tumors. Normal cells typically contain thousands of mitochondria, which can replicate independent of chromosomal DNA, and can segregate between daughter cells asymmetrically. In cancer, changes in mitochondrial copy numbers and heteroplasmy have been well-documented (74–76). There is evidence for change in allele frequency of mtDNA between different geographic regions within the same tumor suggesting that some mtDNA mutations may undergo positive selection during the clonal expansion and promote heteroplasmy (77).
Telomere regions of chromosomes in cancer genomes are eroded, and can affect cellular response to replication and affect potential for break-bridge-fusion-mediated chromosomal instability, replicative stress, and cell death. Telomere length is regulated by telomerase-mediated and alternative pathways, which may be context-dependent, such that intratumor telomere length variation occurs, even among the chromosomes in the same subclone. Multiple studies have reported intratumor heterogeneity in telomere length and telomerase expression in tumor cell populations depending on tumor tissue contexts (22, 78, 79). Although eroded telomere can increase genomic instability and break-bridge-fusion-mediated rearrangements, telomere restoration can suppress such events—modulating the tempo of genome evolution during cancer progression.
Mathematical models provide a framework for analyzing the timescale of fluctuation of genetic drivers, e.g., copy numbers of extrachromosomal DNA, and their effects on cellular phenotypes under neutral evolution and selection (80–82). Both ecDNA and mitochondria can replicate independent of chromosomal DNA, which coupled with unequal segregation provide opportunities for rapid changes in copy numbers within a few generations. Although the rate of copy number fluctuation seems much higher than the somatic mutation rate, it remains to be established if the timescale of fluctuation in these genetic drivers is comparable with that of nongenetic sources in vivo.
MICROENVIRONMENTAL DRIVERS OF INTRATUMOR HETEROGENEITY DYNAMICS
Tumor microenvironment represents a complex ecosystem comprising of tumor, stromal, and immune cells. Parallel to the tumor cells, the tumor microenvironment also experiences changes throughout the course of tumor evolution. This dynamic TME provides tumor plasticity for adaptation, which has a significant impact on tumor progression and clinical outcome. Recently, Lambrechts et al. (83) identified 52 stromal cell subtypes in TME of human lung tumors demonstrating underlying functional heterogeneity at single-cell level. Adding to this complexity is the functionally diverse subpopulations of cancer-associated fibroblasts and their variable potential of tumor-promoting activities (84). Oncogenic signaling in the TME, e.g., via tumor-derived exosomes can prime cancer-associated fibroblasts in the stroma (85), and in turn cancer-associated fibroblast heterogeneity in the TME can promote cell migration and initiate an epithelial-to-mesenchymal transition through CXCL12 and TGFβ pathways, driving metastases (86). Using single-cell RNA sequencing in a murine melanoma model, it was shown that a dynamic stromal niche comprising of temporally distinct stromal populations with unique functional signatures modulates immune landscapes from early disease and supports tumor growth (87). Similar to MSC to stromal differentiation and stromal to stromal trans-differentiation events (88), tumor cells are in fact reported to be capable of transdifferentiation from tumor cell to stromal-like cell, to facilitate tumor development (89) contributing to dynamically heterogeneous stromal cell population with distinct subtypes.
Tumor immune microenvironment is inherently heterogeneous, due to intratumor vasculature, cell migration, and at least partly by immunoediting (90). Immune system can both contain and support tumor development by immunoediting, which typically proceeds through three stages—elimination, equilibrium and escape phases, modulating immunogenicity of a tumor along the way. During tumor progression, a subset of somatic mutations in tumor cells generates neoepitopes, which elicit immune response. As a result, the subclonal populations of tumor cells that harbor the strong neoepitopes are purged and less immunogenic subclones grow, maintaining a dynamic equilibrium between tumor and immune cells; in some cases, additional immunosuppressive mechanisms are acquired later which facilitate immune escape and unrestrained growth. Tumor cells might deploy multiple mechanisms for immune escape. Tumors evade immune surveillance by reduced antigen presentation, triggered by loss of heterozygosity at the HLA locus and epigenetic repression of neoantigen expression (91), continued antigen exposure resulting in immune exhaustion (92, 93) and immuno-suppressive microenvironment (94). Kalvala et al. (95) showed that exosomal mutant KRAS induced the conversion of naive CD4+ T cells into Treg‐like cells, possessing immune-suppressive function. Recently, Jiménez-Sánchez et al. (96) proposed coexistence of immune-cell-excluded and inflammatory microenvironments within the same individuals and within the same tumor sites, by demonstrating marked variation in T-cell infiltration across individuals and sites. In multiple cancer types, regional mutational diversity appeared to drive intratumor immune heterogeneity, moderating immune surveillance leading to weaker antitumor activity (97, 98), such that intratumor mutational heterogeneity can predict efficacy of immunotherapy even in immunologically hot tumors. Using multiregion whole genome sequencing, immunohistochemistry, histologic image analysis, gene expression profiling, and T- and B-cell receptor sequencing of patients with ovarian cancer, Zhang et al. (99) suggested that within-patient spatial immune microenvironment variation shapes nongenetic heterogeneity and ultimately intraperitoneal malignant spread.
Tumor microenvironment also evolves with time. An orthotopic, immunocompetent mouse model of lung cancer suggested that different immune cell types populate tumor microenvironment at temporal order during tumor growth, indicating an interplay between tumor preventing (M1 macrophages) and tumor promoting phenotypes (M2 macrophages) (100). Deligne et al. (101) showed that tumor cells synthesized extracellular matrix protein tenascin-C that promoted a switch of macrophages from proinflammatory tumor preventing phenotypic to immuno-suppressive tumor supportive phenotype, evading immune elimination. It is likely that the temporal patterns of immune and stromal cell-type heterogeneity might be part of a complex system dynamics. Single-cell sequencing in a melanoma model identified temporally distinct, evolutionarily conserved stromal cell populations with immune, desmoplastic, and contractile signatures with tumor progression; stromal cells with “immune”-signature were observed in early tumors whereas cells with contractile signatures were more common at later time points (87).
The concept of heterogeneity in evolutionary dynamics in tumors can be extended beyond the differences observed within a single lesion. Genomic profiling of multiple samples from primary and metastatic sites from the same patients revealed different trajectories of tumor progression—while evidence for parallel evolution was observed in breast and pancreatic cancer patients, those observed in a lung tumor model followed linear evolution (20, 102, 103). Although these specific examples may not necessarily be representative of the preferred modes of evolution in these cancer types, they underscore substantial heterogeneity in evolutionary dynamics across cancer types.
CELL STATE PLASTICITY AND DRUG RESISTANCE
Even when tumor cell populations do not harbor pre-existing resistant mutations, variation in treatment sensitivity due to nonheritable modifications and stochastic fluctuations in gene expression confers apparent treatment insensitivity to a subset of tumor cells (104, 105). For instance, a transiently acquired reversible drug-tolerant state was detected in cancer cell lines treated with a range of different agents which appeared to be mediated via IGF-1 receptor signaling and was a consequence of stochastic phenotypic switch (105). Memory-seq based fluctuation experiments suggest that at least in some cases the transiently resistant phenotype may persist for several generations that may represent clinically significant timescales (64). Random segregation of ecDNAs to daughter cells during each division may result in one of the daughter cells randomly inheriting a higher copy number of ecDNAs with a driver oncogene, which can be advantageous during tumor growth, but the other daughter cell with fewer oncogenic ecDNA may be relatively resistant to targeted therapy. For example in glioblastomas, variable EGFR copy number confers resistance to epidermal growth factor receptor inhibitor treatment, by reversible loss of mutant EGFR from ecDNA, which re-emerges upon drug withdrawal, contributing to eventual relapse of tumor (70).
Mathematical models (106) suggests that even short-term reversible (epi)mutations and fluctuations in gene expression can confer transient drug resistance due to spontaneous phenotypic switching, ultimately promoting long-term drug resistance in the absence of any bona fide resistance mechanisms. Although these transiently resistant phenotypes are eventually erased from the memory of individual cells, at the cell population-level this phenomenon can rescue the tumor from extinction during treatment and enable the emergence of more permanent resistance mechanisms. In some cases, drug-sensitive and transiently resistant cell populations may coexist within tumors, such that the latter may persist during treatment and maintain a minimal residual disease that increases the risk of relapse.
FUTURE DIRECTIONS
Although intratumor heterogeneity has been recognized since early days in cancer research, clinically relevant model systems remain under-represented. Recent studies have generated a deluge of data to characterize intratumor heterogeneity at multiple levels in all major cancer types, which underscore the multilevel genetic and nongenetic heterogeneity within complex ecosystems in tumors. Convergence of spatial and single-cell genomics (39, 107) presents exciting opportunities to better characterize complex interactions within tumor microenvironment at single-molecule resolution. But omics profiling of resected tumors provides only a snapshot of tumor progression, which does not fully reveal the past or future trajectory of clonal dynamics of that tumor. Innovative barcoding and lineage tracing (108–110) have potentials to allow tracking developmental history of somatic cells before and after tumor initiation and study the dynamics of cellular phenotypes in model systems. Patient-derived xenografts and organoids also present platforms for systematic assessment of phenotypic plasticity of tumor cells in response to treatment in controlled conditions (111). Noninvasive profiling of circulating tumor cells has the potential to track both genomic and phenotypic characteristics of circulating tumor cells during treatment in real time (112, 113). Systematic characterization of dynamic properties of intratumor heterogeneity and phenotypic plasticity can guide treatment strategies and improve clinical outcome for cancer patients.
GRANTS
The authors acknowledge financial support from NIH (R01GM129066 and R21CA248122) to S. De.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B. and S.D. prepared figures; A.B. and S.D. drafted manuscript; A.B. and S.D. edited and revised manuscript; A.B. and S.D. approved final version of manuscript.
REFERENCES
- 1.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7: 834–846, 2007. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maley CC, Aktipis A, Graham TA, Sottoriva A, Boddy AM, Janiszewska M, Silva AS, Gerlinger M, Yuan Y, Pienta KJ, Anderson KS, Gatenby R, Swanton C, Posada D, Wu CI, Schiffman JD, Hwang ES, Polyak K, Anderson ARA, Brown JS, Greaves M, Shibata D. Classifying the evolutionary and ecological features of neoplasms. Nat Rev Cancer 17: 605–619, 2017. doi: 10.1038/nrc.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 12: 323–334, 2012. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 4.McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168: 613–628, 2017. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 5.David H. Rudolf Virchow and modern aspects of tumor pathology. Pathol Res Pract 183: 356–364, 1988. doi: 10.1016/S0344-0338(88)80138-9. [DOI] [PubMed] [Google Scholar]
- 6.Mitelman F. The chromosomes of fifty primary Rous rat sarcomas. Hereditas 69: 155–186, 1971. doi: 10.1111/j.1601-5223.1971.tb02431.x. [DOI] [PubMed] [Google Scholar]
- 7.Heppner GH. Tumor heterogeneity. Cancer Res 44: 2259–2265, 1984. [PubMed] [Google Scholar]
- 8.Heppner GH, Miller BE. Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metastasis Rev 2: 5–23, 1983. doi: 10.1007/BF00046903. [DOI] [PubMed] [Google Scholar]
- 9.Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R , et al.; TRACERx consortium C, PEACE consortium. Phylogenetic ctDNA analysis depicts early stage lung cancer evolution. Nature 545: 446–451. 2017. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andor N, Graham TA, Jansen M, Xia LC, Aktipis CA, Petritsch C, Ji HP, Maley CC. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med 22: 105–113, 2016. doi: 10.1038/nm.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S , et al.; TRACERx Consortium. Tracking the evolution of non–small-cell lung cancer. N Engl J Med 376: 2109–2121, 2017. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 12.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med 23: 1124–1134, 2017. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 13.Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—a clinical update. Nat Rev Clin Oncol 17: 204–232, 2020. doi: 10.1038/s41571-019-0293-2. [DOI] [PubMed] [Google Scholar]
- 14.Brock A, Chang H, Huang S. Non-genetic heterogeneity—a mutation-independent driving force for the somatic evolution of tumours. Nat Rev Genet 10: 336–342, 2009. doi: 10.1038/nrg2556. [DOI] [PubMed] [Google Scholar]
- 15.Dirkse A, Golebiewska A, Buder T, Nazarov PV., Muller A, Poovathingal S, Brons NHC, Leite S, Sauvageot N, Sarkisjan D, Seyfrid M, Fritah S, Stieber D, Michelucci A, Hertel F, Herold-Mende C, Azuaje F, Skupin A, Bjerkvig R, Deutsch A, Voss-Böhme A, Niclou SP. Stem cell-associated heterogeneity in glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat Commun 10: 1787, 2019. doi: 10.1038/s41467-019-09853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinohara K, Polyak K. Intratumoral heterogeneity: more than just mutations. Trends Cell Biol 29: 569–579, 2019. doi: 10.1016/j.tcb.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins FC, De S, Almendro V, Gönen M, Park SY, Blum JL, Herlihy W, Ethington G, Schnitt SJ, Tung N, Garber JE, Fetten K, Michor F, Polyak K. Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov 2: 503–511, 2012. doi: 10.1158/2159-8290.CD-11-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heindl A, Nawaz S, Yuan Y. Mapping spatial heterogeneity in the tumor microenvironment: a new era for digital pathology. Lab Invest 95: 377–384, 2015. doi: 10.1038/labinvest.2014.155. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, Failmezger H, Rueda OM, Raza AH, Gräf S, Chin SF, Schwarz RF, Curtis C, Dunning MJ, Bardwell H, Johnson N, Doyle S, Turashvili G, Provenzano E, Aparicio S, Caldas C, Markowetz F. Quantitative image analysis of cellular heterogeneity in breast tumors complements genomic profiling. Sci Transl Med 4: 157ra143, 2012. doi: 10.1126/scitranslmed.3004330. [DOI] [PubMed] [Google Scholar]
- 20.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, Delaney A, Gelmon K, Guliany R, Senz J, Steidl C, Holt RA, Jones S, Sun M, Leung G, Moore R, Severson T, Taylor GA, Teschendorff AE, Tse K, Turashvili G, Varhol R, Warren RL, Watson P, Zhao Y, Caldas C, Huntsman D, Hirst M, Marra MA, Aparicio S. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461: 809–813, 2009. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 21.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D , et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366: 883–892, 2012. [Erratum in N Engl J Med 367: 976, 2012]. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A, Merritt E, Hu X, Cruz A, Jiang C, Sarkodie H, Zhou Z, Malhotra J, Riedlinger GM, De S. Non-genetic intra-tumor heterogeneity is a major predictor of phenotypic heterogeneity and ongoing evolutionary dynamics in lung tumors. Cell Rep 29: 2164–2174.e5, 2019. doi: 10.1016/j.celrep.2019.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI , et al.; TRACERx Renal Consortium. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173: 581–594.e12, 2018. doi: 10.1016/j.cell.2018.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Fujimoto J, Zhang J, Wedge DC, Song X, Zhang J, Seth S, Chow C-W, Cao Y, Gumbs C, Gold KA, Kalhor N, Little L, Mahadeshwar H, Moran C, Protopopov A, Sun H, Tang J, Wu X, Ye Y, William WN, Lee JJ, Heymach JV, Hong WK, Swisher S, Wistuba II, Futreal PA. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 346: 256–259, 2014. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Zhang J, Li L, Yin G, Zhang J, Zheng S , et al. Genomic heterogeneity of multiple synchronous lung cancer. Nat Commun 7: 13200, 2016. doi: 10.1038/ncomms13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma P, Fu Y, Cai MC, Yan Y, Jing Y, Zhang S, Chen M, Wu J, Shen Y, Zhu L, Chen HZ, Gao WQ, Wang M, Gu Z, Bivona TG, Zhao X, Zhuang G. Simultaneous evolutionary expansion and constraint of genomic heterogeneity in multifocal lung cancer. Nat Commun 8: 823, 2017. doi: 10.1038/s41467-017-00963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Kebir M, Satas G, Raphael BJ. Inferring parsimonious migration histories for metastatic cancers. Nat Genet 50: 718–726, 2018. doi: 10.1038/s41588-018-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandrov LB, Jones PH, Wedge DC, Sale JE, Campbell PJ, Nik-Zainal S, Stratton MR. Clock-like mutational processes in human somatic cells. Nat Genet 47: 1402–1407, 2015. doi: 10.1038/ng.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D , et al.; PCAWG Consortium . The evolutionary history of 2,658 cancers. Nature 578: 122–128, 2020. doi: 10.1038/s41586-019-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X, Xu Z, De S. Characteristics of mutational signatures of unknown etiology. NAR Cancer 2: zcaa026, 2020. doi: 10.1093/narcan/zcaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh VK, Rastogi A, Hu X, Wang Y, De S. Mutational signature SBS8 predominantly arises due to late replication errors in cancer. Commun Biol 3: 421, 2020. doi: 10.1038/s42003-020-01119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam AS, Chaligne R, Landau DA. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat Rev Genet 22: 3–18, 2020. doi: 10.1038/s41576-020-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altrock PM, Liu LL, Michor F. The mathematics of cancer: integrating quantitative models. Nat Rev Cancer 15: 730–745, 2015. doi: 10.1038/nrc4029. [DOI] [PubMed] [Google Scholar]
- 34.Bozic I, Wu CJ. Delineating the evolutionary dynamics of cancer from theory to reality. Nat Cancer 1: 580–588, 2020. doi: 10.1038/s43018-020-0079-6. [DOI] [PubMed] [Google Scholar]
- 35.Durrett R, Foo J, Leder K, Mayberry J, Michor F. Intratumor heterogeneity in evolutionary models of tumor progression. Genetics 188: 461–477, 2011. doi: 10.1534/genetics.110.125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao R, Davis A, McDonald TO, Sei E, Shi X, Wang Y, Tsai P-C, Casasent A, Waters J, Zhang H, Meric-Bernstam F, Michor F, Navin NE. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat Genet 48: 1119–1130, 2016. doi: 10.1038/ng.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murtaza M, Dawson S-J, Pogrebniak K, Rueda OM, Provenzano E, Grant J, Chin S-F, Tsui DWY, Marass F, Gale D, Ali HR, Shah P, Contente-Cuomo T, Farahani H, Shumansky K, Kingsbury Z, Humphray S, Bentley D, Shah SP, Wallis M, Rosenfeld N, Caldas C. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat Commun 6: 8760, 2015. doi: 10.1038/ncomms9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 11: 426–437, 2011. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 39.Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol 33: 495–502, 2015. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cusanovich DA, Daza R, Adey A, Pliner HA, Christiansen L, Gunderson KL, Steemers FJ, Trapnell C, Shendure J. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348: 910–914, 2015. doi: 10.1126/science.aab1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagano T, Lubling Y, Várnai C, Dudley C, Leung W, Baran Y, Mendelson Cohen N, Wingett S, Fraser P, Tanay A. Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547: 61–67, 2017. doi: 10.1038/nature23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ku WL, Nakamura K, Gao W, Cui K, Hu G, Tang Q, Ni B, Zhao K. Single-cell chromatin immunocleavage sequencing (scChIC-seq) to profile histone modification. Nat Methods 16: 323–325, 2019. doi: 10.1038/s41592-019-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chappell L, Russell AJC, Voet T. Single-cell (multi)omics technologies. Annu Rev Genomics Hum Genet 19: 15–41, 2018. doi: 10.1146/annurev-genom-091416-035324. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Guo F, Gao Y, Ren Y, Yuan P, Yan L, Li R, Lian Y, Li J, Hu B, Gao J, Wen L, Tang F, Qiao J. Single-cell multi-omics sequencing of human early embryos. Nat Cell Biol 20: 847–858, 2018. doi: 10.1038/s41556-018-0123-2. [DOI] [PubMed] [Google Scholar]
- 45.La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, Lidschreiber K, Kastriti ME, Lönnerberg P, Furlan A, Fan J, Borm LE, Liu Z, van Bruggen D, Guo J, He X, Barker R, Sundström E, Castelo-Branco G, Cramer P, Adameyko I, Linnarsson S, Kharchenko PV. RNA velocity of single cells. Nature 560: 494–498, 2018. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vetrie D, Helgason GV, Copland M. The leukaemia stem cell: similarities, differences and clinical prospects in CML and AML. Nat Rev Cancer 20: 158–173, 2020. doi: 10.1038/s41568-019-0230-9. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A, Jiang C, De S. Dissecting the sources of gene expression variation in a pan-cancer analysis identifies novel regulatory mutations. Nucleic Acids Res 46: 4370–4381, 2018. doi: 10.1093/nar/gky271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De S, Michor F. DNA secondary structures and epigenetic determinants of cancer genome evolution. Nat Struct Mol Biol 18: 950–955, 2011. doi: 10.1038/nsmb.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De S, Shaknovich R, Riester M, Elemento O, Geng H, Kormaksson M, Jiang Y, Woolcock B, Johnson N, Polo JM, Cerchietti L, Gascoyne RD, Melnick A, Michor F. Aberration in DNA methylation in B-cell lymphomas has a complex origin and increases with disease severity. PLoS Genet 9: e1003137, 2013. doi: 10.1371/journal.pgen.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feinberg AP, Koldobskiy MA, Göndör A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet 17: 284–299, 2016. doi: 10.1038/nrg.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De S, Ganesan S. Looking beyond drivers and passengers in cancer genome sequencing data. Ann Oncol 28: 938–945, 2017. doi: 10.1093/annonc/mdw677. [DOI] [PubMed] [Google Scholar]
- 52.Tripathi S, Levine H, Jolly MK. The physics of cellular decision making during epithelial-mesenchymal transition. Annu Rev Biophys 49: 1–18, 2020. doi: 10.1146/annurev-biophys-121219-081557. [DOI] [PubMed] [Google Scholar]
- 53.Ratajczak MZ, Bujko K, Mack A, Kucia M, Ratajczak J. Cancer from the perspective of stem cells and misappropriated tissue regeneration mechanisms. Leukemia 32: 2519–2526, 2018. doi: 10.1038/s41375-018-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carvalho J. Cell reversal from a differentiated to a stem-like state at cancer initiation. Front Oncol 10: 541, 2020. doi: 10.3389/fonc.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guilbert M, Anquez F, Pruvost A, Thommen Q, Courtade E. Protein level variability determines phenotypic heterogeneity in proteotoxic stress response. FEBS J 287: 5345–5361, 2020. doi: 10.1111/febs.15297. [DOI] [PubMed] [Google Scholar]
- 56.Tripathi S, Chakraborty P, Levine H, Jolly MK. A mechanism for epithelial-mesenchymal heterogeneity in a population of cancer cells. PLoS Comput Biol 16: e1007619, 2020. doi: 10.1371/journal.pcbi.1007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell 168: 670–691, 2017. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis RJ, Farias EF, Condeelis J, Klein CA, Aguirre-Ghiso JA. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540: 588–592, 2016. doi: 10.1038/nature20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rambow F, Marine JC, Goding CR. Melanoma plasticity and phenotypic diversity: therapeutic barriers and opportunities. Genes Dev 33: 1295–1318, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones PA, Issa JPJ, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet 17: 630–641, 2016. doi: 10.1038/nrg.2016.93. [DOI] [PubMed] [Google Scholar]
- 61.Gaiti F, Chaligne R, Gu H, Brand RM, Kothen-Hill S, Schulman RC, Grigorev K, Risso D, Kim KT, Pastore A, Huang KY, Alonso A, Sheridan C, Omans ND, Biederstedt E, Clement K, Wang L, Felsenfeld JA, Bhavsar EB, Aryee MJ, Allan JN, Furman R, Gnirke A, Wu CJ, Meissner A, Landau DA. Epigenetic evolution and lineage histories of chronic lymphocytic leukaemia. Nature 569: 576–580, 2019. doi: 10.1038/s41586-019-1198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pastore A, Gaiti F, Lu SX, Brand RM, Kulm S, Chaligne R, Gu H, Huang KY, Stamenova EK, Béguelin W, Jiang Y, Schulman RC, Kim KT, Alonso A, Allan JN, Furman RR, Gnirke A, Wu CJ, Melnick AM, Meissner A, Bernstein BE, Abdel-Wahab O, Landau DA. Corrupted coordination of epigenetic modifications leads to diverging chromatin states and transcriptional heterogeneity in CLL. Nat Commun 10: 1874, 2019. doi: 10.1038/s41467-019-09645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schuh L, Saint-Antoine M, Sanford EM, Emert BL, Singh A, Marr C, Raj A, Goyal Y. Gene networks with transcriptional bursting recapitulate rare transient coordinated high expression states in cancer. Cell Syst 10: 363–378.e12, 2020. doi: 10.1016/j.cels.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaffer SM, Emert BL, Reyes Hueros RA, Cote C, Harmange G, Schaff DL, Sizemore AE, Gupte R, Torre E, Singh A, Bassett DS, Raj A. Memory sequencing reveals heritable single-cell gene expression programs associated with distinct cellular behaviors. Cell 182: 947–959.e17, 2020. doi: 10.1016/j.cell.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He YJ, Meghani K, Caron M-C, Yang C, Ronato DA, Bian J, Sharma A, Moore J, Niraj J, Detappe A, Doench JG, Legube G, Root DE, D’Andrea AD, Drané P, De S, Konstantinopoulos PA, Masson J-Y, Chowdhury D. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature 563: 522–526, 2018. doi: 10.1038/s41586-018-0670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tobalina L, Armenia J, Irving E, O’Connor MJ, Forment JV. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol 32: 103–112, 2021. doi: 10.1016/j.annonc.2020.10.470. [DOI] [PubMed] [Google Scholar]
- 67.Verhaak RGW, Bafna V, Mischel PS. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution. Nat Rev Cancer 19: 283–288, 2019. doi: 10.1038/s41568-019-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H, Nguyen NP, Turner K, Wu S, Gujar AD, Luebeck J, Liu J, Deshpande V, Rajkumar U, Namburi S, Amin SB, Yi E, Menghi F, Schulte JH, Henssen AG, Chang HY, Beck CR, Mischel PS, Bafna V, Verhaak RGW. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat Genet 52: 891–897, 2020. doi: 10.1038/s41588-020-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumar P, Kiran S, Saha S, Su Z, Paulsen T, Chatrath A, Shibata Y, Shibata E, Dutta A. ATAC-seq identifies thousands of extrachromosomal circular DNA in cancer and cell lines. Sci Adv 6: eaba2489, 2020. doi: 10.1126/sciadv.aba1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, Li B, Arden K, Ren B, Nathanson DA, Kornblum HI, Taylor MD, Kaushal S, Cavenee WK, Wechsler-Reya R, Furnari FB, Vandenberg SR, Rao PN, Wahl GM, Bafna V, Mischel PS. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 543: 122–125, 2017. doi: 10.1038/nature21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hung KL, Yost KE, Xie L, Wu S, Lange JT, Duffy CV, Kraft K, Tang J, Shi Q, Rose JC, Corces MR, Granja JM, Li R, Rajkumar U, Tjian R, Bafna V, Mischel PS, Liu Z, Chang HY. EcDNA hubs drive cooperative intermolecular oncogene expression. bioRxiv, 2020. doi: 10.1101/2020.11.19.390278. [DOI] [PMC free article] [PubMed]
- 72.Nathanson DA, Gini B, Mottahedeh J, Visnyei K, Koga T, Gomez G, Eskin A, Hwang K, Wang J, Masui K, Paucar A, Yang H, Ohashi M, Zhu S, Wykosky J, Reed R, Nelson SF, Cloughesy TF, James CD, Rao PN, Kornblum HI, Heath JR, Cavenee WK, Furnari FB, Mischel PS. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 343: 72–76, 2014. doi: 10.1126/science.1241328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke TL, Tang R, Chakraborty D, Van Rechem C, Ji F, Mishra S, Ma A, Kaniskan HÜ, Jin J, Lawrence MS, Sadreyev RI, Whetstine JR. Histone lysine methylation dynamics control EGFR DNA copy-number amplification. Cancer Discov 10: 306–325, 2020. doi: 10.1158/2159-8290.CD-19-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He Y, Wu J, Dressman DC, Iacobuzio-Donahue C, Markowitz SD, Velculescu VE, Diaz LA, Kinzler KW, Vogelstein B, Papadopoulos N. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature 464: 610–614, 2010. doi: 10.1038/nature08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK, Al-Ahmadie HA, Lee W, Seshan VE, Hakimi AA, Sander C. Mitochondrial DNA copy number variation across human cancers. eLife 5: e10769, 2016. doi: 10.7554/eLife.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zong WX, Rabinowitz JD, White E. Mitochondria and cancer. Mol Cell 61: 667–676, 2016. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Guo X, Li D, Du X, Yin C, Chen C, Fang W, Bian Z, Zhang J, Li B, Yang H, Xing J. Multi-regional sequencing reveals intratumor heterogeneity and positive selection of somatic mtDNA mutations in hepatocellular carcinoma and colorectal cancer. Int J Cancer 143: 1143–1152, 2018. doi: 10.1002/ijc.31395. [DOI] [PubMed] [Google Scholar]
- 78.Pezzolo A, Pistorio A, Gambini C, Haupt R, Ferraro M, Erminio G, De Bernardi B, Garaventa A, Pistoia V. Intratumoral diversity of telomere length in individual neuroblastoma tumors. Oncotarget 6: 7493–7503, 2015. doi: 10.18632/oncotarget.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rowland TJ, Dumbović G, Hass EP, Rinn JL, Cech TR. Single-cell imaging reveals unexpected heterogeneity of telomerase reverse transcriptase expression across human cancer cell lines. Proc Natl Acad Sci USA 116: 18488–18497, 2019. doi: 10.1073/pnas.1908275116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chinnery PF, Samuels DC, Elson J, Turnbull DM. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: is there a common mechanism? Lancet 360: 1323–1325, 2002. doi: 10.1016/S0140-6736(02)11310-9. [DOI] [PubMed] [Google Scholar]
- 81.Pichugin Y, Huang W, Werner B. Stochastic dynamics of extra-chromosomal DNA. bioRxiv 2019. doi: 10.1101/2019.12.15.876714. [DOI]
- 82.van Gisbergen MW, Voets AM, Starmans MHW, de Coo IFM, Yadak R, Hoffmann RF, Boutros PC, Smeets HJM, Dubois L, Lambin P. How do changes in the mtDNA and mitochondrial dysfunction influence cancer and cancer therapy? Challenges, opportunities and models. Mutat Res Rev 764: 16–30, 2015. doi: 10.1016/j.mrrev.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 83.Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, Bassez A, Decaluwé H, Pircher A, Van den Eynde K, Weynand B, Verbeken E, De Leyn P, Liston A, Vansteenkiste J, Carmeliet P, Aerts S, Thienpont B. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 24: 1277–1289, 2018. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 84.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, Bonneau C, Kondratova M, Kuperstein I, Zinovyev A, Givel AM, Parrini MC, Soumelis V, Vincent-Salomon A, Mechta-Grigoriou F. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33: 463–479.e10, 2018. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 85.Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, Cao D, Tang L, Tang S, Wu M, Yang W, Wang H. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun 9: 191, 2018. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pelon F, Bourachot B, Kieffer Y, Magagna I, Mermet-Meillon F, Bonnet I, Costa A, Givel AM, Attieh Y, Barbazan J, Bonneau C, Fuhrmann L, Descroix S, Vignjevic D, Silberzan P, Parrini MC, Vincent-Salomon A, Mechta-Grigoriou F. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat Commun 11: 404, 2020. doi: 10.1038/s41467-019-14134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davidson S, Efremova M, Riedel A, Mahata B, Pramanik J, Huuhtanen J, Kar G, Vento-Tormo R, Hagai T, Chen X, Haniffa MA, Shields JD, Teichmann SA. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep 31: 107628, 2020. doi: 10.1016/j.celrep.2020.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Midgley AC, Rogers M, Hallett MB, Clayton A, Bowen T, Phillips AO, Steadman R. Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J Biol Chem 288: 14824–14838, 2013. doi: 10.1074/jbc.M113.451336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scully S, Francescone R, Faibish M, Bentley B, Taylor SL, Oh D, Schapiro R, Moral L, Yan W, Shao R. Transdifferentiation of glioblastoma stem-like cells into mural cells drives vasculogenic mimicry in glioblastomas. J Neurosci 32: 12950–12960, 2012. doi: 10.1523/JNEUROSCI.2017-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 16: 151–167, 2019. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 91.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, Greenbaum B, Carroll J, Garon E, Hyman DM, Zehir A, Solit D, Berger M, Zhou R, Rizvi NA, Chan TA. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359: 582–587, 2018. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, Crowley D, Chen J, Jacks T. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 19: 72–85, 2011. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 37: 457–495, 2019. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 94.Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, Makarev E, Artemov AV, Wysocki PT, Mehra R, Nimmagadda S, Marchionni L, Sidransky D, Borrello IM, Izumchenko E, Bedi A. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun 9: 741, 2018. doi: 10.1038/s41467-017-02696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalvala A, Wallet P, Yang L, Wang C, Li H, Nam A, Nathan A, Mambetsariev I, Poroyko V, Gao H, Chu P, Sattler M, Bild A, Manuel ER, Lee PP, Jolly MK, Kulkarni P, Salgia R. Phenotypic switching of naïve T cells to immune-suppressive treg-like cells by mutant KRAS. J Clin Med 8: 1726, 2019. doi: 10.3390/jcm8101726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiménez-Sánchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, Ricca J, Zamarin D, Walther T, Aghajanian C, Wolchok JD, Sala E, Merghoub T, Snyder A, Miller ML. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 170: 927–938.e20, 2017. doi: 10.1016/j.cell.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jia Q, Wu W, Wang Y, Alexander PB, Sun C, Gong Z, Cheng J-N, Sun H, Guan Y, Xia X, Yang L, Yi X, Wan YY, Wang H, He J, Futreal PA, Li Q-J, Zhu B. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun 9: 5361, 2018. doi: 10.1038/s41467-018-07767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolf Y, Bartok O, Patkar S, Eli GB, Cohen S, Litchfield K, Levy R, Jiménez-Sánchez A, Trabish S, Lee JS, Karathia H, Barnea E, Day CP, Cinnamon E, Stein I, Solomon A, Bitton L, Pérez-Guijarro E, Dubovik T, Shen-Orr SS, Miller ML, Merlino G, Levin Y, Pikarsky E, Eisenbach L, Admon A, Swanton C, Ruppin E, Samuels Y. UVB-induced tumor heterogeneity diminishes immune response in melanoma. Cell 179: 219–235.e21, 2019. doi: 10.1016/j.cell.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang AW, McPherson A, Milne K, Kroeger DR, Hamilton PT, Miranda A , et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell 173: 1755–1769.e22, 2018. doi: 10.1016/j.cell.2018.03.073. [DOI] [PubMed] [Google Scholar]
- 100.Poczobutt JM, De S, Yadav VK, Nguyen TT, Li H, Sippel TR, Weiser-Evans MCM, Nemenoff RA. Expression profiling of macrophages reveals multiple populations with distinct biological roles in an immunocompetent orthotopic model of lung cancer. J Immunol 196: 2847–2859, 2016. doi: 10.4049/jimmunol.1502364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deligne C, Murdamoothoo D, Gammage AN, Gschwandtner M, Erne W, Loustau T, Marzeda AM, Carapito R, Paul N, Velazquez-Quesada I, Mazzier I, Sun Z, Orend G, Midwood KS. Matrix-targeting immunotherapy controls tumor growth and spread by switching macrophage phenotype. Cancer Immunol Res 8: 368–382, 2020. doi: 10.1158/2326-6066.CIR-19-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467: 1109–1113, 2010. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caswell DR, Chuang CH, Yang D, Chiou SH, Cheemalavagu S, Kim-Kiselak C, Connolly A, Winslow MM. Obligate progression precedes lung adenocarcinoma dissemination. Cancer Discov 4: 781–789, 2014. doi: 10.1158/2159-8290.CD-13-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hinohara K, Wu HJ, Vigneau S, McDonald TO, Igarashi KJ, Yamamoto KN, Madsen T, Fassl A, Egri SB, Papanastasiou M, Ding L, Peluffo G, Cohen O, Kales SC, Lal-Nag M, Rai G, Maloney DJ, Jadhav A, Simeonov A, Wagle N, Brown M, Meissner A, Sicinski P, Jaffe JD, Jeselsohn R, Gimelbrant AA, Michor F, Polyak K. KDM5 histone demethylase activity links cellular transcriptomic heterogeneity to therapeutic resistance. Cancer Cell 34: 939–953.e9, 2018. doi: 10.1016/j.ccell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma SV., Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141: 69–80, 2010. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gunnarsson EB, De S, Leder K, Foo J. Understanding the role of phenotypic switching in cancer drug resistance. J Theor Biol 490: 110162, 2020. doi: 10.1016/j.jtbi.2020.110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burgess DJ. Spatial transcriptomics coming of age. Nat Rev Genet 20: 317, 2019. doi: 10.1038/s41576-019-0129-z. [DOI] [PubMed] [Google Scholar]
- 108.Guo C, Kong W, Kamimoto K, Rivera-Gonzalez GC, Yang X, Kirita Y, Morris SA. CellTag indexing: genetic barcode-based sample multiplexing for single-cell genomics. Genome Biol 20: 90, 2019. doi: 10.1186/s13059-019-1699-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalhor R, Kalhor K, Mejia L, Leeper K, Graveline A, Mali P, Church GM. Developmental barcoding of whole mouse via homing CRISPR. Science 361: eaat9804, 2018. doi: 10.1126/science.aat9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, Shendure J. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353: aaf7907, 2016. doi: 10.1126/science.aaf7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roerink SF, Sasaki N, Lee-Six H, Young MD, Alexandrov LB, Behjati S, Mitchell TJ, Grossmann S, Lightfoot H, Egan DA, Pronk A, Smakman N, van Gorp J, Anderson E, Gamble SJ, Alder C, van de Wetering M, Campbell PJ, Stratton MR, Clevers H. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556: 457–462, 2018. doi: 10.1038/s41586-018-0024-3. [DOI] [PubMed] [Google Scholar]
- 112.Jiang P, Lo YMD. The long and short of circulating cell-free DNA and the ins and outs of molecular diagnostics. Trends Genet 32: 360–371, 2016. doi: 10.1016/j.tig.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 113.Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 17: 223–238, 2017. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]