Abstract
Lipid oxidation products, including lysophosphatidylcholine (lysoPC) inhibit endothelial cell (EC) migration in vitro and impair EC healing of arterial injuries in vivo, in part by activating phosphatidylinositol 3-kinase (PI3K), which increases the externalization of canonical transient receptor potential 6 (TRPC6) channels and the subsequent increase in intracellular calcium. Inhibition of PI3K is a potential method to decrease TRPC6 activation and restore migration, but PI3K is involved in multiple intracellular signaling pathways and has multiple downstream effectors. The goal of this study is to identify the specific p110 catalytic subunit isoforms responsible for lysoPC-induced TRPC6 externalization to identify a target for intervention while minimizing impact on alternative signaling pathways. Down-regulation of the p110α and p110δ isoforms, but not the p110β or p110γ isoforms, with small interfering RNA significantly decreased phosphatidylinositol (3,4,5)-trisphosphate production and TRPC6 externalization, and significantly improved EC migration in the presence of lysoPC. These results identify an additional role of p110α in EC and reveal for the first time a specific role of p110δ in EC, providing a foundation for subsequent in vivo studies to investigate the impact of p110 isoform inhibition on arterial healing after injury.
Keywords: endothelial cell migration, PI3-kinase, p110α, p110δ, TRPC6
INTRODUCTION
Endothelial cell (EC) migration is essential for healing intimal injuries, such as those that occur with angioplasty, but hypercholesterolemia reduces endothelial healing (1, 2). Lipid oxidation products, including lysophosphatidylcholine (lysoPC), accumulate in atherosclerotic arteries (3) and at regions of injury. LysoPC causes cellular dysfunction and plays an important role in atherogenesis and other inflammatory diseases (4). LysoPC also inhibits EC migration in vitro (5, 6), and increased lysoPC is associated with decreased endothelial healing in vivo (1, 2, 7). Limited re-endothelialization contributes to thrombogenicity, smooth muscle cell proliferation, and restenosis after arterial injury.
Oxidized low-density lipoprotein (oxLDL) and lysoPC cause an inappropriate increase in intracellular free calcium ion concentration ([Ca2+]i). Importantly, the Ca2+ influx is not through classical voltage-gated channels, but through canonical transient receptor potential (TRPC) channels, specifically TRPC6 (8). Activation of TRPC6 by oxLDL and lysoPC causes an increase in [Ca2+]i that results in activation of TRPC5 and a prolonged rise in [Ca2+]i (8). The increased [Ca2+]i activates calpains that break down cytoskeletal proteins, inhibiting EC migration (9). Our studies in TRPC6−/− mice provide compelling evidence of the importance of this cascade in vivo. Re-endothelialization of injured carotid arteries is dramatically reduced in wild-type mice on a high fat diet compared with chow-fed mice, but in TRPC6−/− mice, hypercholesterolemia does not inhibit re-endothelialization of the injury (10).
Considerable effort has been directed at identifying a TRPC6 inhibitor with limited success. We have discovered that lipid oxidation products induce TRPC6 externalization by activating phosphatidylinositol 3-kinase (PI3K), which generates phosphatidylinositol (3,4,5)-trisphosphate (PIP3) (12). PIP3 in the cell membrane promotes TRPC6 translocation to the cell membrane, anchoring it there, and promoting increased [Ca2+]i (13).
PI3 kinases are grouped into three classes: class I, II, and III. All three classes can synthesize phosphatidylinositol 3-phosphate (PIP), class I and II can synthesize phosphatidylinositol (3,4)-bisphosphate (PIP2), but only class I can produce phosphatidylinositol (3,4,5)-trisphosphate (PIP3) (14) in the inner leaflet of the plasma membrane. In vascular biology, class I isoforms are the most well characterized (15). Class I PI3K is composed of a regulatory subunit (p85α, p85β, p50α, p55α or p55γ) and a catalytic subunit (p110α, p110β, p110δ or p110γ). PI3Ks regulate a wide range of signaling pathways, trafficking to the cell membrane, cell growth, cell proliferation, cell survival, metabolic processes, and autophagy (14, 16). In vascular cells, class I isoforms participate in a variety of intracellular signaling processes, including TRPC6 activation (12), nitric oxide synthesis, endothelial cell-leukocyte interaction, angiogenesis, and endothelial progenitor cell biology (15).
We have previously shown that lysoPC inhibits EC migration in part by activating PI3K (12). The resultant increase in PIP3 production leads to TRPC6 externalization followed by a rise in [Ca2+]i and alteration of the cytoskeleton. Alteration of the cytoskeleton inhibits EC migration across an area of injury. The specific isoform(s) of the PI3K p110 catalytic subunit that plays a role in TRPC6 externalization is unknown. The goal of this study is to identify the isoform(s) of PI3 kinase activated by lysoPC and determine if down-regulation of the specific isoform(s) could preserve EC migration in the presence of lipid oxidation products.
MATERIALS AND METHODS
EC Harvest and Culture
Bovine aortic ECs (BAECs) (unknown sex) were isolated from fresh bovine aortas (source: abattoir) by scraping after collagenase treatment and cultured as previously described (8). ECs from passage 4 to 10 were used for experiments. EA.hy926 cells (unknown sex), an immortalized human umbilical vein EC line, were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT) with 1 μg/mL gentamicin.
Immunoprecipitation and Immunoblot Analysis of Proteins
Confluent ECs were made quiescent in serum-free DMEM for 12-13 h before treatment was initiated. Target proteins were immunoprecipitated as previously described (17). Cell lysates were stored at −20° C until analyzed.
Lysate proteins (50 μg/lane of total protein) were separated by 4–20% gradient SDS-PAGE as previously described (17). Target proteins were detected by antibodies specific for p110α subunit of PI3K (1:500; Cell Signaling Technology, Danvers, MA; Cat. No. 4255S), p110β (1:500; Novus Biologicals, Centennial, CO; Cat. No. NBP1 33116), p110δ (1:500; Cell Signaling Technology; Cat. No. 34050S), p110γ (1:500; Cell Signaling Technology; Cat. No. 5405S), or TRPC6 (1:500; Cell Signaling Technology; Cat. No. 16716). Blots were incubated with a horseradish peroxidase-conjugated secondary antibody (1:1,000; Cell Signaling Technology; Cat. No. 7074) and the signal was developed using a chemiluminescent reagent (Invitrogen, Brookfield, WI). To verify equal loading, the blots were stripped and reprobed using an anti-actin antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA; Cat. No. MA1-744). The images were acquired on HyBlot CL autoradiography film (Denville Scientific, Metuchen, NJ) and then scanned with an Epson Perfection V600 Photo using EPSON scan Software. Protein band density was quantitated using ImageJ software (NIH, Bethesda, MD).
Biotinylation of Proteins on EC Cell Surface to Detect TRPC6 Externalization
Externalization of TRPC6 channels was determined by biotinylation assay as previously described (8). Briefly, ECs were treated with Sulfo-NHS-Biotin (2 mg/mL; Calbiochem, La Jolla, CA) to biotinylate externalized proteins. ECs were lysed and lysates were incubated with streptavidin-agarose beads. After collecting the beads, the biotinylated proteins were released from the beads, separated and resolved by SDS-PAGE, and detected by immunoblot analysis. To determine total TRCP6 protein in the lysates, immunoblot analysis was performed on an aliquot of cell lysate removed prior to incubation with the streptavidin-agarose beads.
Target Protein Down-Regulation Using Small Interfering RNA (siRNA)
ECs at 70–80% confluence were transiently transfected with the siRNA duplex specific to p110α (10–20 nmol/L, Dharmacon, Lafayette, CO), p110β (20–30 nmol/L, Dharmacon), p110δ (20–30 nmol/L, Dharmacon), p110γ (20–30 nmol/L, Dharmacon), or p110α (20 nmol/L) + p110δ (20 nmol/L) using a transfection kit (RNAiFect; Qiagen, Germantown, MD) following the manufacturer’s protocol. Down-regulation of the endogenous target protein level was verified after 48 h by immunoblot analysis. NsiRNA (20–40 nmol/L; Ambion, Austin, TX) with no known homology to any gene sequence was used as a negative control.
Measurement of [Ca2+]i
ECs at 80–90% confluence were loaded with the FITC-conjugated fluorophore Calbryte 520 AM dye (AAT Bioquest, Sunnyvale CA; Cat. No. 36310) following the manufacturer’s protocol. After 35 min, the EC were suspended and loaded into the sort chamber of a BD FACSMelody cell sorter (BD Biosciences, San Jose, CA) maintained at 37°C. After adjusting the baseline, lysoPC (12.5 μmol/L; 1-palmitol-2-hydroxy-sn-glycero-3-phosphocholine; Avanti Polar Lipids, Alabaster, AL) was added and relative changes in [Ca2+]i were read using the kinetic reading mode at Ex/Em 490/525 nm. Kinetics data was analyzed using the FlowJo v10 software (BD Biosciences).
EC migration Assay
Confluent ECs were made quiescent in serum-free DMEM for 16–18 h prior to the migration assay. The ECs were incubated with specified agents, and migration was assessed by the razor scrape assay, as previously described (9). After 24 h, migrating ECs were quantitated by an observer blinded to the experimental condition using NIH ImageJ (NIH, Bethesda, MD).
PIP3 Production by ELISA
Confluent ECs were made quiescent in serum-free medium for 12–13 h and agents added as indicated. PIP3 was extracted using a PIP3 Mass ELISA kit (Echelon Biosciences, Salt Lake City, UT), following the manufacturer’s protocol. PIP3 levels were measured at 450 nm using a Spectramax 190 microplate reader (Molecular Devices, San Jose, CA).
Proliferation Assay
EC were cultured to 70–80% confluence in DMEM containing 10% fetal calf serum. The medium was removed, ECs washed twice with PBS, and DMEM without FBS was applied. Cell proliferation was assessed using the Quick Cell Proliferation Kit II (Abcam, Cambridge, MA; Cat. No. ab65475), following the manufacturer’s protocol. Absorbance was measured at 450 nm using a Spectramax 190 microplate reader (Molecular Devices). Fibroblast growth factor (FGF; 20 nM) and camptothecin (8 µM) were used as positive and negative controls, respectively.
Statistical Methods
Experimental results are represented as mean (SD). Unless otherwise specified, experiments were performed in triplicate with cells cultured from three different animals or independent biological samples. All Western blots and EC migration images are representative images of at least three separate experiments. GraphPad Prism 8.2 (GraphPad Software, Inc., San Diego, CA) was used to perform the statistical analysis. Analysis of variance (ANOVA) with Tukey’s multiple comparison’s test or Dunnett’s multiple comparison test was performed. Differences were considered statistically significant at P < 0.05.
RESULTS
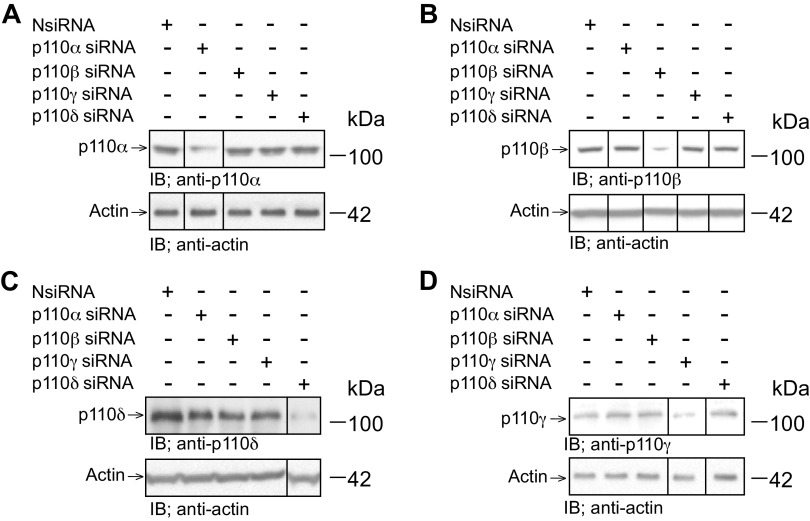
LysoPC-induced activation of the p110α and the p110δ catalytic subunit isoforms of PI3K. In ECs incubated with lysoPC, PI3K is activated, leading to increased TRPC6 externalization, increased [Ca2+]i and inhibition of migration (12). The specific catalytic subunit isoform(s) responsible for TRPC6 externalization has not been identified. To identify the isoform(s) involved, each of the four PI3K p110 isoforms (p110α, p110β, and p110δ, and p110γ) was down-regulated with isoform specific siRNA. Transient transfection of BAECs with p110α siRNA decreased p110α protein levels to 27% (SD 2) of basal level (n = 3, P < 0.02 compared with NsiRNA) but had no effect on the level of p110β, p110δ, or p110γ protein (Fig. 1, A–D). Similarly, transient transfection of BAECs with p110β siRNA decreased p110β protein levels to 23% (SD 2) of basal level (n = 3, P < 0.01 compared with NsiRNA) but had no effect on the level of p110α, p110δ, or p110γ protein (Fig. 1, A–D). P110δ siRNA decreased p110δ protein levels in transfected BAECs to 12% (SD 2) of basal level (n = 3, P <0.02 compared with NsiRNA) but had no effect on the levels of p110α, p110β, or p110γ protein (Fig. 1, A–D). Transfection of BAECs with p110 γ siRNA decreased p110 γ protein levels to 17% (SD 1) of basal level (n = 3, P < 0.01 compared with NsiRNA) but had no effect on the levels of p110α, p110β, or p110δ protein (Fig. 1, A–D). These studies confirmed the effectiveness and specificity of the siRNAs for down-regulation of p110 isoforms.
Figure 1.
siRNA for the p110 catalytic subunit isoforms selectively down-regulates the corresponding p110 isoform of PI3K. Bovine aortic ECs were transiently transfected with control siRNA (NsiRNA) (40 nmol/L), p110α siRNA (20 nmol/L), p110β siRNA (30 nmol/L), p110δ siRNA (20 nmol/L), or p110γ siRNA (30 nmol/L) for 24 hours. The siRNA was then removed. At 48 h after siRNA removal, the cells were lysed and p110α (A), p110β (B), p110δ (C), or p110γ (D) was identified by immunoblot analysis. Actin served as the loading control (n = 3 independent biological samples). Black lines indicate lanes rearranged from the same gel. All bands are from the same gel.
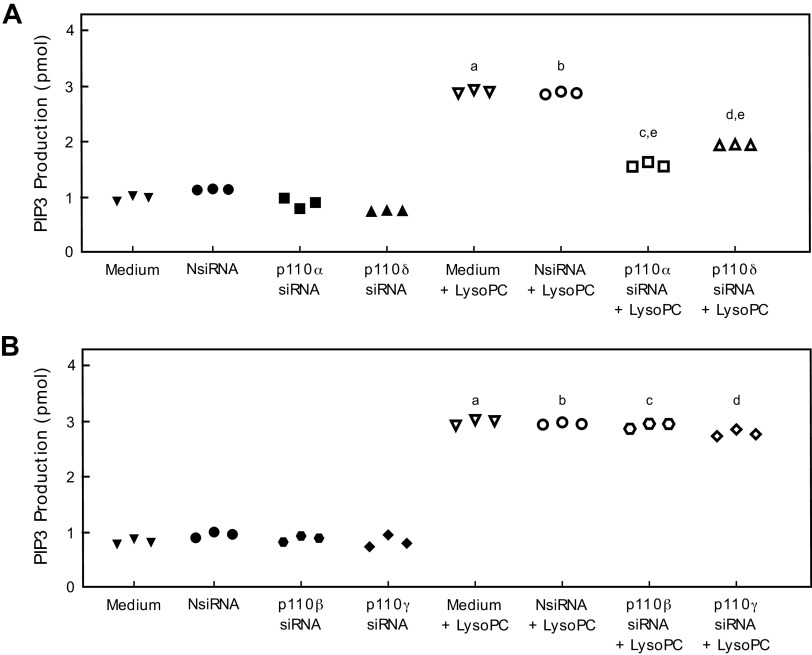
PIP3 production was measured to determine the effect of down-regulation of the p110 isoforms on lysoPC-induced PI3K activation. Basal PIP3 production was similar in control BAECs and BAECs transfected with NsiRNA, p110α siRNA, p110β siRNA, p110δ, or p110γ siRNA (Fig. 2, A, B). LysoPC significantly increased PIP3 production in control BAECs (n = 3; P < 0.001 compared with no lysoPC; Fig. 2, A, B) and in cells transfected with NsiRNA (n = 3; P<0.001 compared with NsiRNA with no lysoPC; Fig. 2, A, B). The increase in PIP3 production by lysoPC was significantly less when p110α or p110δ protein was down-regulated with the respective siRNA. Down-regulation of p110α reduced lysoPC-induced PIP3 production by 46% (n = 3; P < 0.001 compared with NsiRNA + lysoPC; Fig. 2A) and down-regulation of p110δ siRNA decreased lysoPC-induced PIP3 production by 32% (n = 3; P < 0.001 compared with NsiRNA + lysoPC; Fig. 2A). Down-regulation of p110β or p110γ did not alter the increased PIP3 production in response to lysoPC (n = 3; Fig. 2B).
Figure 2.
Down-regulation of p110α or p110δ reduces lysoPC-induced PIP3 production. A: Bovine aortic ECs were transiently transfected with NsiRNA (40 nmol/L), p110α siRNA (20 nmol/L), or p110δ siRNA (20 nmol/L). PIP3 production in the presence or absence of lysoPC (12.5 μmol/L) was determined by ELISA and represented by scatter plot (n = 3 independent biological samples, a. P < 0.001 compared with medium, b. P < 0.001 compared with NsiRNA, c. P < 0.001 compared with p110α siRNA, d. P < 0.001 compared with p110δ siRNA, e. P<0.001 compared with NsiRNA + lysoPC). B: Bovine aortic ECs were transiently transfected with NsiRNA (40 nmol/L), p110β siRNA (30 nmol/L), or p110γ siRNA (30 nmol/L). PIP3 production in the presence or absence of lysoPC (12.5 μmol/L) was determined by ELISA and represented by scatter plot (n = 3 independent biological samples, a. P < 0.001 compared with medium, b. P < 0.001 compared with NsiRNA, c. P < 0.001 compared with p110β siRNA, d. P < 0.001 compared with p110γ siRNA). Statistical analysis for A, B performed using One-Way ANOVA with Tukey’s Multiple Comparisons Test.
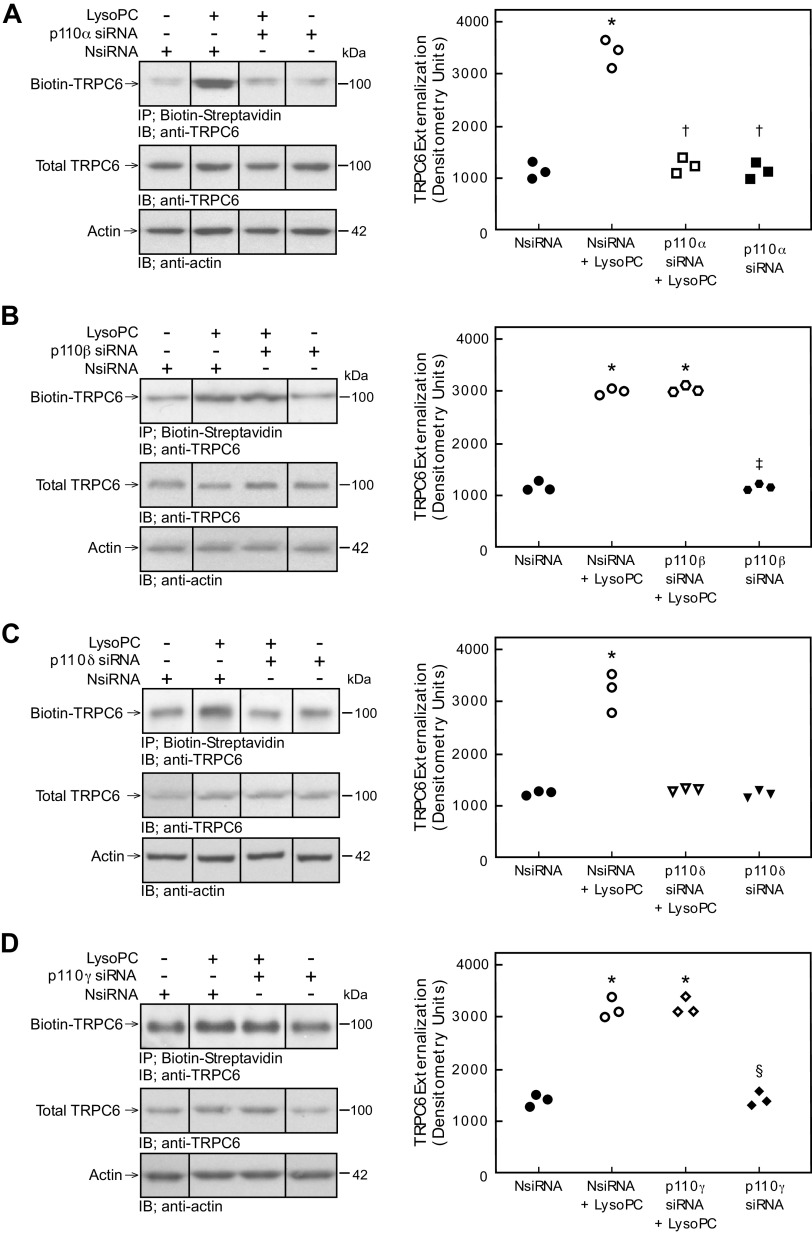
The effect of PI3K isoform down-regulation on lysoPC-induced TRPC6 externalization was evaluated by biotinylation assay. Compared with NsiRNA transfected cells, down-regulation of p110α significantly decreased TRPC6 externalization in response to lysoPC (n = 3; Fig. 3A). Similarly, down-regulation of p110δ significantly decreased TRPC6 externalization in response to lysoPC compared with NsiRNA transfected cells (n = 3; Fig. 3C). In contrast, down-regulation of p110β or p110γ did not affect lysoPC-induced TRPC6 externalization compared with ECs transfected with NsiRNA (n = 3; Fig. 3, B and D). These results suggested the p110α and p110δ catalytic subunit isoforms of PI3K are involved in lysoPC-induced TRPC6 externalization, but the p110β and p110γ isoforms are not involved.
Figure 3.
Down-regulation of p110α and p110δ inhibits lysoPC-induced TRPC6 externalization. Bovine aortic ECs were transiently transfected with control siRNA (NsiRNA) (40 nmol/L), p110α siRNA (20 nmol/L), p110β siRNA (30 nmol/L), p110δ siRNA (20 nmol/L), or p110γ siRNA (30 nmol/L) for 24 h before incubation with lysoPC (12.5 μmol/L) for 15 min. Externalization of TRPC6 after down-regulation of p110α (A: left panel), p110β (B: left panel), p110δ (C: left panel), or p110γ (D: left panel) was detected by biotinylation assay and total TRPC6 by immunoblot analysis. Actin served as loading control (n = 3 independent biological samples). Black lines indicate lanes rearranged from the same gel. All bands are from the same gel. Densitometry measurements of Biotin-TRPC6 are represented in scatter plots after down-regulation of p110α (A: right panel), p110β (B: right panel), p110δ (C: right panel), or p110γ (D: right panel) (n = 3 independent biological samples, *P < 0.001 compared with NsiRNA, †P < 0.001 compared with NsiRNA + LysoPC, ‡P < 0.001 compared with p110β + LysoPC, and §P < 0.001 compared with p110γ + LysoPC). Statistical analysis performed using One-Way ANOVA with Tukey’s Multiple Comparisons Test.
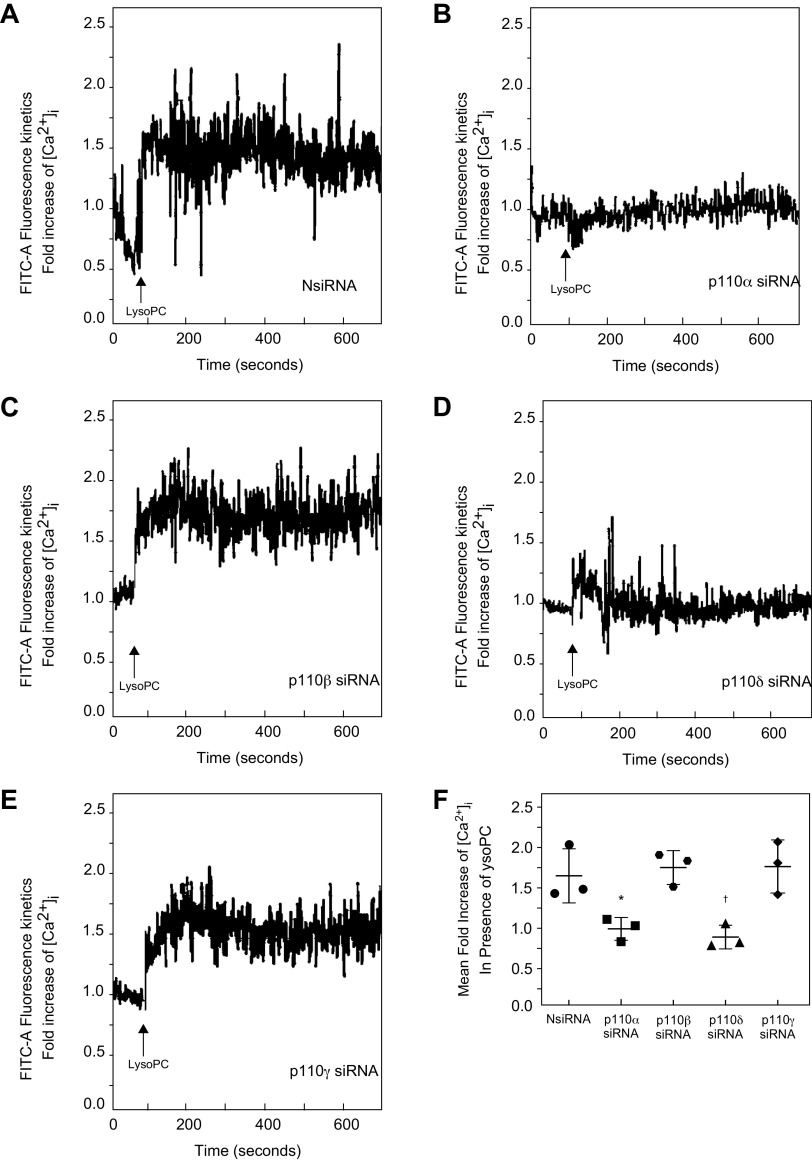
The effect of PI3K isoform down-regulation on [Ca2+]i in the presence of lysoPC was evaluated. LysoPC significantly increased [Ca2+]i in NsiRNA transfected cells (Fig. 4A). Down-regulation of p110α inhibited the rise in [Ca2+]i in response to lysoPC compared with NsiRNA transfected cells (n = 3; Fig. 4, B and F). Similarly, down-regulation of p110δ blocked the rise in [Ca2+]i after the addition of lysoPC compared with NsiRNA transfected cells (n = 3; Fig. 4, D and F). In contrast, down-regulation of p110β or p110γ did not alter the lysoPC-induced rise in [Ca2+]i compared with ECs transfected with NsiRNA (n = 3; Fig. 4, C, E, and F). These results support the important role of the p110α and p110δ catalytic subunit isoforms of PI3K in lysoPC-induced TRPC6 activation.
Figure 4.
Down-regulation of p110α and p110δ inhibits lysoPC-induced increase in [Ca2+]i. Bovine aortic ECs were transiently transfected with control siRNA (NsiRNA) (40 nmol/L), p110α siRNA (20 nmol/L), p110β siRNA (30 nmol/L), p110δ siRNA (20 nmol/L), or p110γ siRNA (30 nmol/L) for 24 h. ECs were loaded with the FITC-conjugated fluorophore Calbryte 520 AM dye. The EC were suspended and loaded into the sort chamber of a BD FACSMelody cell sorter maintained at 37°C. After adjusting the baseline, lysoPC (12.5 μmol/L) was added. A–D: Using the kinetic reading mode at Ex/Em 490/525 nm, relative changes in [Ca2+]i after transfection with NsiRNA (A), p110α (B), p110β (C), p110δ (D), or p110γ (E) were determined (Representative images of n = 3 independent biologic samples). F: Fold increase of [Ca2+]i measured by difference in mean [Ca2+]i at baseline and after addition of lysoPC (12.5 μmol/L) presented as a dot whisker plot (n = 3 independent biological samples; *P = 0.02 compared with NsiRNA, †P = 0.01 compared with NsiRNA). Statistical analysis performed using One-Way ANOVA with Dunnett’s Multiple Comparison Test against NsiRNA.
Down-regulation of the p110α and p110δ Catalytic Subunit Isoforms of PI3K Preserved EC Migration in Presence of LysoPC
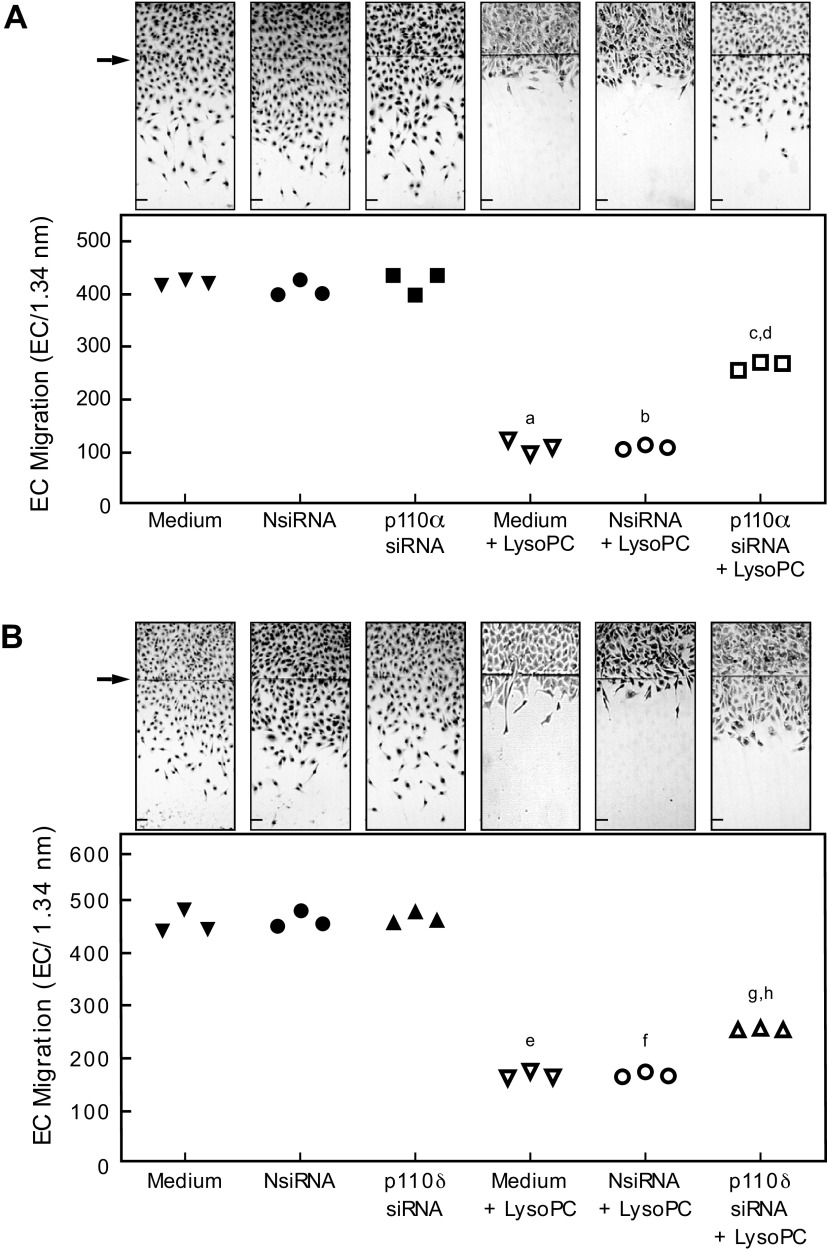
Based on the above results indicating the role of the p110α and p110δ catalytic subunit isoforms of PI3K in lysoPC-induced TRPC6 externalization, the effect of p110α and p110δ down-regulation on EC migration in the presence of lysoPC was evaluated. Migration of control BAECs and BAECs transfected with NsiRNA, p110α, or p110δ siRNA was similar (Fig. 5, A and B). In the presence of lysoPC, migration in control BAECs decreased by 74% (n = 3; P < 0.001 compared with no lysoPC; Fig. 5A) and by 73% in BAECs transfected with NsiRNA (n = 3; P < 0.001 compared with NsiRNA with no lysoPC; Fig. 5A). In BAECs transfected with p110α siRNA, however, lysoPC inhibited migration by only 38% (n = 3; P < 0.001 compared with p110α siRNA and no lysoPC; Fig. 5A). Similarly, in BAECs transfected with p110δ siRNA, lysoPC inhibited migration by only 38% (n = 3; P < 0.001 compared with p110δ siRNA with no lysoPC; Fig. 5B). The partial preservation in BAEC migration indicated the p110α and p110δ isoforms are involved in the lysoPC-induced inhibition of BAEC migration.
Figure 5.
Down-regulation of p110α and p110δ partially preserves EC migration in presence of lysoPC. Bovine aortic ECs were transiently transfected for 24 h with NsiRNA (40 nmol/L), p110α siRNA (20 nmol/L), or p110δ siRNA (20 nmol/L) and then made quiescent for 12 h. The migration assay was initiated and migration in the presence or absence of lysoPC (12.5 μmol/L) was assessed after 24 h. (Upper) Representative images are shown at 40x magnification (Scale Bar, 200 µm). Arrow indicates the starting line of EC migration. (Lower) EC migration represented in scatter plots for (A) p110α down-regulation (n = 3 independent biological samples, a. P < 0.001 compared with medium, b. P < 0.001 compared with NsiRNA, c. P < 0.001 compared with p110α siRNA, and d. P < 0.001 compared with p110α siRNA + lysoPC) and (B) p110δ down-regulation (n = 3 independent biological samples, e. P < 0.001 compared with medium, f. P < 0.001 compared with NsiRNA, g. P < 0.001 compared with p110δ siRNA, and h. P < 0.001 compared with p110δ siRNA + lysoPC). Statistical analysis performed using One-Way ANOVA with Tukey’s Multiple Comparisons Test.
To verify the changes in BAEC migration were not related to changes in BAEC proliferation, proliferation assays were performed. Transfection with NsiRNA, p110α siRNA, or p110δ siRNA had no effect on BAEC proliferation in the presence or absence of lysoPC (Supplemental Fig. S1, all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.12881900).
Down-regulation of p110α and p110δ Simultaneously Has Similar Effect on TRPC6 Externalization and BAEC Migration in the Presence of lysoPC as Down-regulation of Each Isoform Separately Despite a More Significant Decrease in PIP3 Production
Based on the involvement of p110α and p110δ in PIP3 production, TRPC6 externalization and EC migration, the effect of simultaneous down-regulation of both isoforms was assessed. Down-regulation of both p110α and p110δ significantly reduced lysoPC-induced PIP3 production by 67% (n = 3; P < 0.001 compared with NsiRNA + LysoPC; Fig. 6A). In fact, lysoPC-induced PIP3 production was significantly less when both isoforms were down-regulated simultaneously compared with down-regulation of either isoform alone. Despite a more significant decrease in PIP3 production, the impact of lysoPC on TRPC6 externalization and EC migration in BAECs transfected with both p110α and p110δ siRNA was not significantly different than when each isoform was down-regulated separately (Fig. 6 B and C). Down-regulation of both p110α and p110δ had no effect on BAEC proliferation in the presence or absence of lysoPC (Supplemental Fig. S1), indicating the changes in EC migration were not related to changes in EC proliferation.
Figure 6.
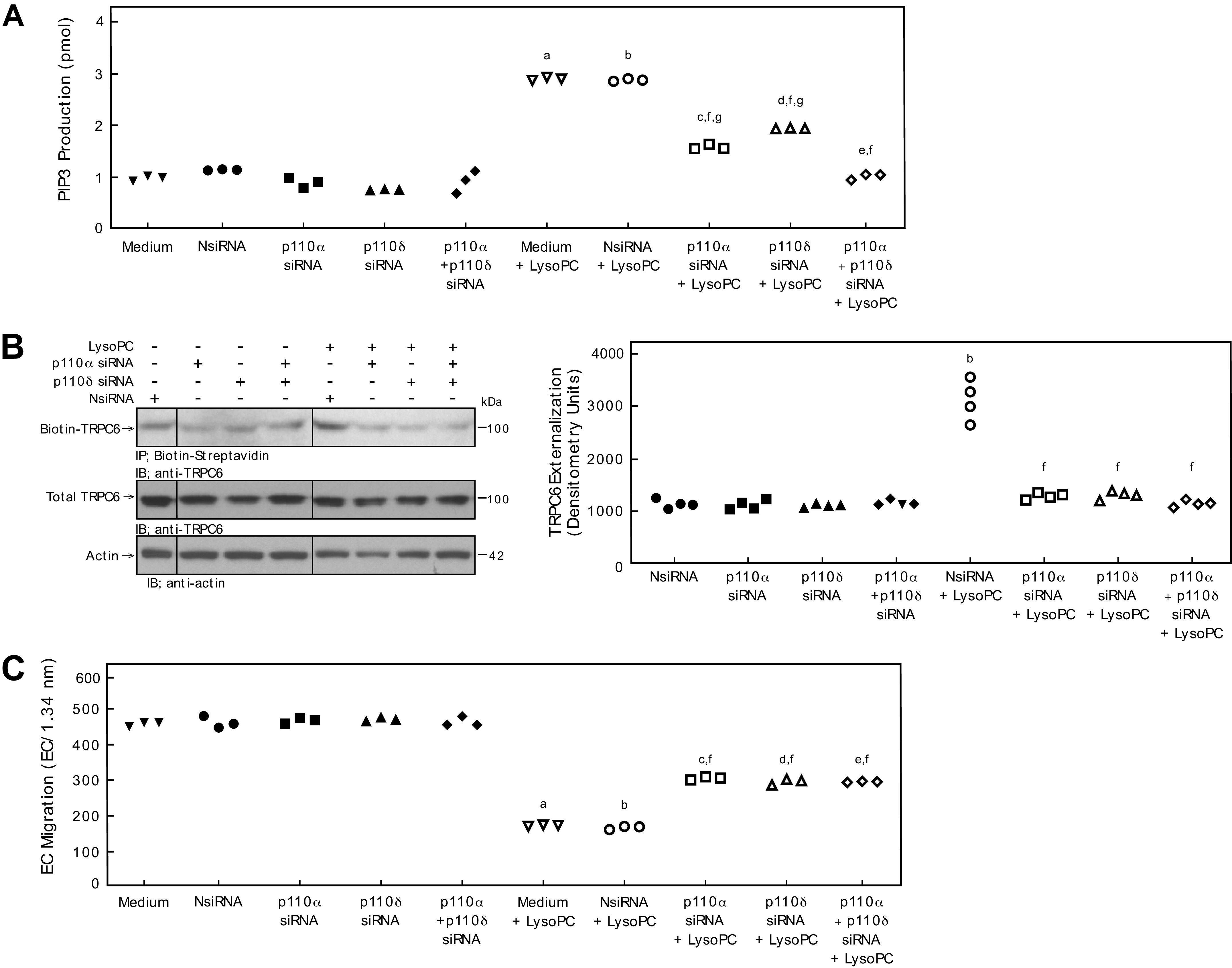
Combined down-regulation of p110α and p110δ significantly decreases PIP3 production, but does not significantly decrease TRPC externalization or improve EC migration compared with p110α or p110δ alone. Bovine aortic ECs were transiently transfected with control siRNA (NsiRNA) (40 nmol/L), p110α siRNA (20 nmol/L), p110β siRNA (30 nmol/L), p110δ siRNA (20 nmol/L), p110γ siRNA (30 nmol/L), or p110α siRNA (20 nmol/L) + p110δ siRNA (20 nmol/L) for 24 h. A: PIP3 production in the presence or absence of lysoPC (12.5 μmol/L) was determined by ELISA and represented by scatter plot (n = 3 independent biological samples). B: Externalization of TRPC6 after down-regulation of p110α, p110δ, or p110α + p110δ (left panel) was detected by biotinylation assay and total TRPC6 by immunoblot analysis. Actin served as loading control (n = 4 independent biological samples). Black lines indicate lanes rearranged from the same gel. All bands are from the same gel. Densitometry measurements of Biotin-TRPC6 are represented in a scatter plot after down-regulation of p110α, p110δ, or p110α + p110δ (right panel) (n = 4 independent biological samples). C: ECs were made quiescent for 12 h. The migration assay was initiated, and the migration in the presence or absence of lysoPC (12.5 μmol/L) was assessed after 24 h. Results are presented as scatter plots (n = 3 independent biological samples). Statistical analysis for A–C performed using One-Way ANOVA with Tukey’s Multiple Comparisons Test (a. P < 0.001 compared with medium, b. P < 0.001 compared with NsiRNA, c. P < 0.001 compared with p110α siRNA, d. P < 0.001 compared with p110δ, e. P < 0.001 compared with p110α siRNA + p110δ siRNA, f. P < 0.001 compared with NsiRNA + LysoPC, g. P < 0.001 compared with p110α siRNA + p110δ siRNA + LysoPC).
Effects of Down-regulation of p110α, p110δ, or Both Isoforms Simultaneously on PIP3 Production, TRPC6 Externalization, [Ca2+]i, and EC Migration in Human ECs Are Consistent with Effects Seen in BAECs
To ensure the effects of down-regulation of p110α, p110δ, or p110α + p110δ on lysoPC-induced PIP3 production, TRPC6 externalization, [Ca2+]i, and EC migration are not species-specific to bovine ECs, the results were verified in human EA.hy926 ECs. Transient transfection of EA.hy926 ECs with p110α siRNA or p110δ siRNA significantly decreased p110α or p110δ (Fig. 7A) protein levels, respectively.
Figure 7.
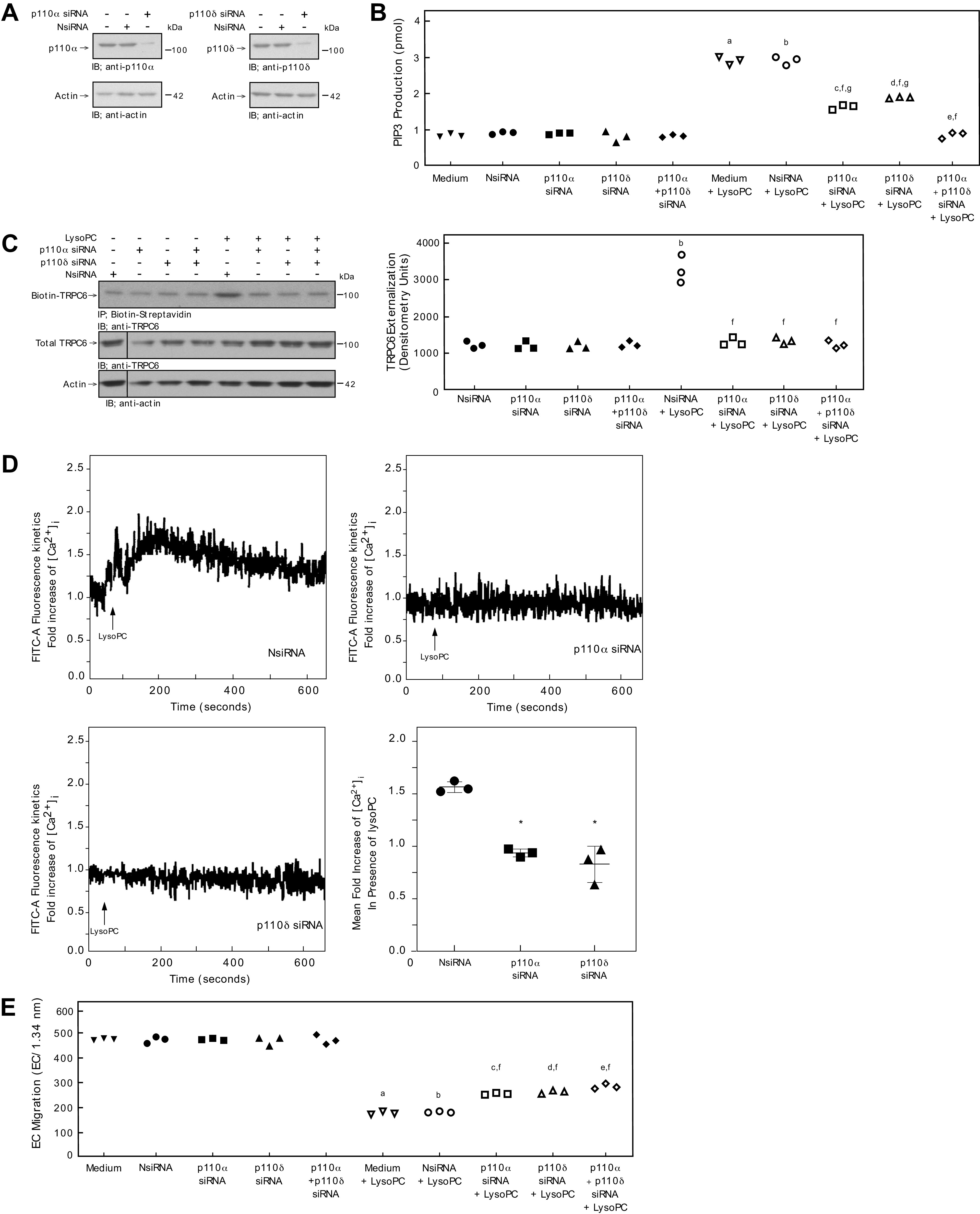
Down-regulation of p110α or p110δ inhibits lysoPC-induced PIP3 production, blocks TRPC6 externalization, and partially restores EC migration in the presence of lysoPC in human EC. EAhy926 ECs were transiently transfected with NsiRNA (40 nmol/L), p110α siRNA (20 nmol/L), p110δ siRNA (20 nmol/L), or p110α siRNA (20 nmol/L) + p110δ siRNA (20 nmol/L) for 24 h. A: The siRNA was then removed. At 48 h after siRNA removal, the cells were lysed and p110α (left panel) or p110δ (right panel) was identified by immunoblot analysis. Actin served as the loading control (n = 3 independent biological samples). B: PIP3 production in the presence or absence of lysoPC (12.5 μmol/L) was determined by ELISA and represented by scatter plot (n = 3 independent biological samples). C: ECs were incubated with lysoPC (12.5 μmol/L) for 15 minutes. Externalization of TRPC6 after down-regulation of p110α, p110δ, orp110α + p110δ (left panel) was detected by biotinylation assay and total TRPC6 by immunoblot analysis. Actin served as loading control (n = 3 independent biological samples). Black lines indicate lanes rearranged from the same gel. All bands are from the same gel. Densitometry measurements of Biotin-TRPC6 are represented in a scatter plot after down-regulation of p110α, p110δ, or p110α + p110δ (right panel) (n = 3 independent biological samples). D: ECs were loaded with the FITC-conjugated fluorophore Calbryte 520 AM dye. The EC were suspended and loaded into the sort chamber of a BD FACSMelody cell sorter maintained at 37°C. After adjusting the baseline, lysoPC (12.5 μmol/L) was added. Using the kinetic reading mode at Ex/Em 490/525 nm, relative changes in [Ca2+]i after transfection with NsiRNA (top left panel), p110α (top right panel), p110δ (bottom left panel) were read (Representative images of n = 3 independent biologic samples). Fold increase of [Ca2+]i measured by difference in mean [Ca2+]i at baseline and after addition of lysoPC (12.5 μmol/L) presented as a dot whisker (bottom right panel) (n = 3 independent biological samples). E. ECs were made quiescent for 12 h. The migration assay was initiated, and the migration in the presence or absence of lysoPC (12.5 μmol/L) was assessed after 24 h. Results are presented as scatter plots (n = 3 independent biological samples). Statistical analysis for B, C, and E performed using One-Way ANOVA with Tukey’s Multiple Comparisons Test.(a. P < 0.001 compared with medium, b. P < 0.001 compared with NsiRNA, c. P < 0.001 compared with p110α siRNA, d. P < 0.001 compared with p110δ, e. P < 0.001 compared p110α siRNA + p110δ siRNA, f. P < 0.001 compared with NsiRNA + LysoPC, g. P < 0.001 compared with p110α siRNA + p110δ siRNA + LysoPC). Statistical analysis for D performed using One-Way ANOVA with Dunnett’s Multiple Comparison Test against NsiRNA (*P < 0.001 compared with NsiRNA).
In EA.hy926 ECs, baseline PIP3 levels of control ECs and ECs transfected with NsiRNA, p110α siRNA, p110δ siRNA, or p110α siRNA + p110δ siRNA were similar (Fig. 7B). Exposure to lysoPC significantly increased PIP3 production in control (n = 3; P<0.001 compared with medium with no lysoPC; Fig. 7B) and NsiRNA transfected cells (n = 3; P<0.001 compared with NsiRNA with no lysoPC; Fig. 7B). The increase in PIP3 production by lysoPC was significantly less when p110α, p110δ, or both p110α and p110δ protein was down-regulated with the respective siRNA. Down-regulation of p110α reduced lysoPC-induced PIP3 production by 45% (n = 3; P < 0.001 compared with NsiRNA + lysoPC; Fig. 7B) and down-regulation of p110δ decreased lysoPC-induced PIP3 production by 35% (n = 3; P < 0.001 compared with NsiRNA + lysoPC; Fig. 7B). Down-regulation of both p110α siRNA and p110δ simultaneously decreased lysoPC-induced PIP3 production by 65% (n = 3; P < 0.001 compared with NsiRNA + lysoPC, p110α siRNA + lysoPC, and p110δ siRNA + lysoPC).
To verify that the decrease in PIP3 production was accompanied by a decrease in TRPC6 externalization, biotinylation assays were performed. As in BAECs, down-regulation of the p110α subunit, the p110δ subunit, or both the p110α and p110δ subunits simultaneously with the respective siRNA in EA.hy926 cells significantly decreased lysoPC-induced TRPC6 externalization (Fig. 7C). TRPC6 externalization in EA.hy926 cells transfected with both p110α and p110δ siRNA was not significantly different than when each isoform was down-regulated separately (Fig. 7C).
To determine the functional impact of p110α and p110δ downregulation, [Ca2+]i was evaluated in EA.hy926 cells. As in BAEC, LysoPC caused a significant increase in [Ca2+]i in NsiRNA transfected cells (Fig. 7D). Similarly, down-regulation of the p110α subunit or the p110δ subunit in EA.hy926 cells inhibited the lysoPC-induced rise in [Ca2+]i (Fig. 7D).
Basal migration of control (medium) EA.hy926 ECs and EA.hy926 ECs transfected with NsiRNA, p110α siRNA, or p110δ siRNA was similar (Fig. 7E). In the presence of lysoPC, migration in control EA.hy926 ECs decreased by 64% (n = 3; P < 0.001 compared with no lysoPC; Fig. 7E) and by 62% in EA.hy926 ECs transfected with NsiRNA (n = 3; P < 0.001 compared with NsiRNA with no lysoPC; Fig. 7E). However, in EA.hy926 ECs transfected with p110α siRNA or p110δ siRNA, lysoPC inhibited migration by only 48% (n = 3; P < 0.001 compared with p110α siRNA with no lysoPC; Fig. 7E) and 47% (n = 3; P < 0.001 compared with p110δ siRNA with no lysoPC; Fig. 7E), respectively. With simultaneous down-regulation of both p110α and p110δ, lysoPC inhibited migration by only 37% (n = 3; P < 0.001 compared with p110α siRNA + p110δ siRNA with no lysoPC; Fig. 7E). EC migration in EA.hy926 cells transfected with both p110α and p110δ siRNA was not significantly different than when each isoform was down-regulated separately (Fig. 7C).
These results indicated that the changes in PIP3 production, TRPC6 externalization, and EC migration in response to lysoPC are not species specific and strengthen case that p110α and p110δ are the PI3K catalytic subunit isoforms involved in externalization of TRPC6 channels in the presence of lysoPC.
DISCUSSION
Lipid oxidation products increase TRPC6 externalization and activation, at least in part by activating PI3K, increasing PIP3 levels, and increasing the TRPC6 co-localized with PIP3 in the cell membrane (12). PIP3 associates with C-terminal of TRPC6 to anchor TRPC6-containing vesicles in the plasma membrane (13). Anchoring of TRPC6 in the membrane increases its activation and leads to increased [Ca2+]i (13, 18, 19). Increased [Ca2+]i activates calpain causing cytoskeletal protein alterations and resulting in the inhibition of EC migration (9).
The role of PI3K in TRPC6 externalization makes it a candidate for therapeutic intervention to improve EC migration and arterial healing after injury. PI3K is estimated to have 50-100 down-stream effectors and is involved in multiple intracellular signaling pathways (16). To minimize the off-target effect of PI3K inhibition to improve arterial healing, the goal of the current study is to identify the specific PI3K p110 catalytic subunit isoforms responsible for the lysoPC-induced increase in TRPC6 externalization and decreased EC migration. The results of this study show that the p110α and p110δ isoforms, but not the p110β or p110γ isoforms, are responsible for the increased PIP3 production, increased TRPC6 externalization, and decreased migration of EC in the presence of lysoPC.
P110α is ubiquitous and abundant (15) in EC and primarily plays a role in angiogenesis and vascular development (20). In contrast to our results indicating that inhibition of p110α improves EC migration in the presence of lysoPC, inhibition of p110α during angiogenesis impairs cell junction stabilization, decreases actomyosin contractility, and ultimately leads to decreased cell migration and vessel growth (20, 21). To our knowledge, this study is the first to report the specific role of p110α in EC migration in response to lipid oxidation products.
The role p110δ in inflammation and immune functions is well described (11, 22), but no previous reports suggest a role in EC migration, despite p110δ being present in EC. Bilancio et al. show that p110δ promotes neointimal thickening through smooth muscle cell proliferation and the inflammatory processes associated with vascular stenosis and that inhibition of p110δ leads to significant decrease in restenosis after a carotid crush injury in a mouse model (23). The improved EC migration after down-regulation of p110δ in our studies may help explain the findings of decreased restenosis shown by Bilancio et al. If an EC surface is re-established early after arterial injury, intimal hyperplasia is minimized (24).
Identifying the specific PI3K p110 catalytic subunits involved in lysoPC-induced TRPC6 externalization provides the opportunity to use pharmacologic inhibitors of the specific p110 isoforms to improve EC migration in vivo. Pan-class I PI3K inhibitors and isoform-specific inhibitors to p110α and p110δ are FDA approved or currently in late-stage clinical trials for various solid tumor cancers, lymphoma, and leukemia (25). Repurposing existing isoform-specific PI3K inhibitors may provide a novel approach to promoting EC healing after vascular intervention such as angioplasty or implantation of vascular bypass grafts.
GRANTS
This project was supported by Career Development Award #IK2BX003628 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service and Grant Number HL064357 from the National Institutes of Health (NIH/NHLBI).
DISCLAIMERS
The content is solely the responsibility of the authors and does not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health, the National Heart, Lung, and Blood Institute, or the United States Government.
DISCLOSURES
The authors have no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
P.C., L.M.G., and M.A.R. conceived and designed research; P.C. and M.A.R. performed experiments; P.C., L.M.G., and M.A.R. analyzed data; P.C., A.H.S., P.P., L.M.G., and M.A.R. interpreted results of experiments; P.C. and M.A.R. prepared figures; P.C., L.M.G., and M.A.R. drafted manuscript; P.C., A.H.S., P.P., L.M.G., and M.A.R. edited and revised manuscript; P.C., A.H.S., P.P., L.M.G., and M.A.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Kewal Asosingh, Director of the Cleveland Clinic Lerner Research Institute Flow Cytometry Core facility, for his invaluable assistance in analysis of the calcium data. The authors would also like to thank Amy Graham, technologist in the Flow Cytometry Core facility, for her technical assistance with calcium studies.
References
- 1.Rosenbaum MA, Miyazaki K, Colles SM, Graham LM. Anti-oxidant therapy reverses impaired graft healing in hypercholesterolemic rabbits. J Vasc Surg 51: 184–193, 2010. doi: 10.1016/j.jvs.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum MA, Miyazaki K, Graham LM. Hypercholesterolemia and oxidative stress inhibit endothelial cell healing after arterial injury. J Vasc Surg 55: 489–496, 2012. doi: 10.1016/j.jvs.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 73: 321–354, 2004. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem 14: 3209–3220, 2007. doi: 10.2174/092986707782793899. [DOI] [PubMed] [Google Scholar]
- 5.Murugesan G, Fox PL. Role of lysophosphatidylcholine in the inhibition of endothelial cell motility by oxidized low density lipoprotein. J Clin Invest 97: 2736–2744, 1996. doi: 10.1172/JCI118728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Aalst JA, Burmeister W, Fox PL, Graham LM. a-Tocopherol preserves endothelial cell migration in the presence of cell-oxidized low density lipids by inhibiting changes in cell membrane fluidity. J Vasc Surg 39: 229–237, 2004. doi: 10.1016/S0741-5214(03)01038-3. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki K, Colles SM, Graham LM. Impaired graft healing due to hypercholesterolemia is prevented by dietary suplementation with a-tocopherol. JVascSurg 48: 986–993, 2008. doi: 10.1016/j.jvs.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri P, Colles SM, Bhat M, Van Wagoner DR, Birnbaumer L, Graham LM. Elucidation of a TRPC6-TRPC5 channel cascade that restricts endothelial cell movement. Mol Biol Cell 19: 3203–3211, 2008. doi: 10.1091/mbc.e07-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhuri P, Colles SM, Damron DS, Graham LM. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arterioscler Thromb Vasc Biol 23: 218–223, 2003. doi: 10.1161/01.atv.0000052673.77316.01. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum MA, Chaudhuri P, Graham LM. Hypercholesterolemia inhibits re-endothelialization of arterial injuries by TRPC channel activation. J Vasc Surg 62: 1040–1047, 2015. doi: 10.1016/j.jvs.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantry D VA, Holtzman DA, Wood C, Gray PW, Cooper JA, Hoekstra MF. p110delta, a novel phophatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem 272: 19236–19241, 1997. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri P, Rosenbaum MA, Sinharoy P, Damron DS, Birnbaumer L, Graham LM. Membrane translocation of TRPC6 channels and endothelial migration are regulated by calmodulin and PI3 kinase activation. Proc Natl Acad Sci U S A 113: 2110–2115, 2016. doi: 10.1073/pnas.1600371113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell 25: 491–503, 2007. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jean S, Kiger AA. Classes of phosphoinositide 3-kinases at a glance. J Cell Sci 127: 923–928, 2014. doi: 10.1242/jcs.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res 82: 261–271, 2009. doi: 10.1093/cvr/cvn325. [DOI] [PubMed] [Google Scholar]
- 16.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13: 195–203, 2012. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhuri P, Colles SM, Fox PL, Graham LM. Protein kinase Cδ-dependent phosphorylation of syndecan-4 regulates cell migration. Circ Res 97: 674–681, 2005. doi: 10.1161/01.RES.0000184667.82354.b1. [DOI] [PubMed] [Google Scholar]
- 18.Monet M, Francoeur N, Boulay G. Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of TRPC6 protein in vascular smooth muscle cells. J Biol Chem 287: 17672–17681, 2012. doi: 10.1074/jbc.M112.341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng PH, Lin HP, Hu H, Wang C, Zhu MX, Chen CS. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry 43: 11701–11708, 2004. doi: 10.1021/bi049349f. [DOI] [PubMed] [Google Scholar]
- 20.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 453: 662–666, 2008. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 21.Angulo-Urarte A, Casado P, Castillo SD, Kobialka P, Kotini MP, Figueiredo AM, Castel P, Rajeeve V, Milá-Guasch M, Millan J, Wiesner C, Serra H, Muixi L, Casanovas O, Viñals F, Affolter M, Gerhardt H, Huveneers S, Belting HG, Cutillas PR, Graupera M. Endothelial cell rearrangements during vascular patterning require PI3-kinase-mediated inhibition of actomyosin contractility. Nat Commun 9: 4826, 2018. doi: 10.1038/s41467-018-07172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri KD, Doggett TA, Douangpanya J, Hou Y, Tino WT, Wilson T, Graf T, Clayton E, Turner M, Hayflick JS, Diacovo TG. Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood 103: 3448–3456, 2004. doi: 10.1182/blood-2003-05-1667. [DOI] [PubMed] [Google Scholar]
- 23.Bilancio A, Rinaldi B, Oliviero MA, Donniacuo M, Monti MG, Boscaino A, Marino I, Friedman L, Rossi F, Vanhaesebroeck B, Migliaccio A. Inhibition of p110delta PI3K prevents inflammatory response and restenosis after artery injury. Biosci Rep 37: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest 41: 407–418, 1979. [PubMed] [Google Scholar]
- 25.Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 15: 273–291, 2018. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]