Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease of unknown etiology with limited treatment options. It is characterized by repetitive injury to alveolar epithelial cells and aberrant activation of numerous signaling pathways. Recent evidence suggests that metabolic reprogramming, metabolic dysregulation, and mitochondria dysfunction are distinctive features of the IPF lungs. Through numerous mechanisms, metabolomic abnormalities in alveolar epithelial cells, myofibroblast, macrophages, and fibroblasts contribute to the abnormal collagen synthesis and dysregulated airway remodeling described in lung fibrosis. This review summarizes the metabolomic changes in amino acids, lipids, glucose, and heme seen in IPF lungs. Simultaneously, we provide new insights into potential therapeutic strategies by targeting a variety of metabolites.
Keywords: IPF, metabolomics, senescence
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic and irreversible disease characterized by progressive scarring of the lung parenchyma, leading to respiratory failure and, ultimately, death. It is a heterogeneous disease with an unknown etiology, poor prognosis, and a median survival of 3–5 yr after diagnosis. In the United States, ∼100,000 people are affected by IPF, and 30,000–40,000 new cases are diagnosed every year. IPF has been considered as an age-associated disease, it typically affects adults over the age of 50 yr, and its incidence increases with age (1). Little is known about the pathophysiology of IPF, thus presenting a challenge in treatment development. Despite multiple clinical trials, only two antifibrotic agents have been approved as therapy in IPF (2). Thus, to pursue more effective pharmacological approaches, we must first deeply understand the pathophysiology of this disease. Specifically, the metabolomics of IPF is of interest, as evidence suggests that modulating specific metabolites can provide clues for new therapeutic avenues. This review provides an overview of selected metabolic molecules described in IPF and explores potential strategies targeting these pathways.
AMINO ACIDS
Amino acids are organic molecules required for numerous physiologic functions, including synthesis of polypeptides and proteins, cell signaling, nucleotide and lipid biosynthesis, redox homeostasis, epigenetic regulation, protein phosphorylation cascade, and gene expression (3). Recent evidence has suggested that alterations in the metabolism of the nonessential amino acids, namely, glycine, glutamine, and arginine drive the profibrotic cellular phenotype seen in IPF (4). Understanding the specific role of these amino acids in this disease pathogenesis might allow the development of potential therapeutic approaches targeting these molecules.
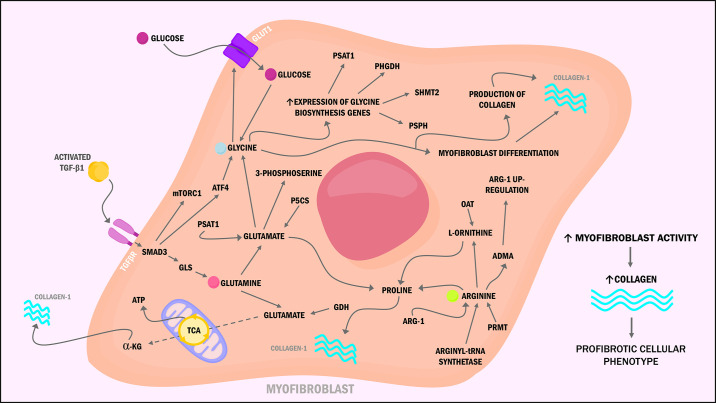
Glutamine (Gln) is the most abundant amino acid in the bloodstream. It is an essential nutrient for energy production through the tricarboxylic acid (TCA) cycle, lymphocyte proliferation, cytokine production, macrophage function, and synthesis of proline, a key component of collagen and the extracellular matrix (ECM) (5). Glutamine undergoes a process termed glutaminolysis, in which glutamine is converted to glutamate by the enzyme glutaminase (GLS) and then to α-ketoglutarate (α-KG) by glutamate dehydrogenase (GDH) to enter the tricarboxylic acid (TCA) cycle. In lung fibroblast, transforming growth factor β1 (TGF-β1) stimulates glutaminolysis by upregulating the expression of GLS through activation of Smad3 and p38-MAPK-dependent signaling (6). Conversion of Gln to α-KG allows the metabolic reprogramming needed for myofibroblast differentiation by increasing TCA cycle metabolites. Thus, providing reducing equivalents to the mitochondrial oxidative phosphorylation (OXPHOS) to produce adenosine triphosphate (ATP), sustain cell growth, and proliferation (7). Moreover, Hamanaka et al. (8) reported that lung fibroblasts required Gln and GLS for the TGF-β1-induced production of collagen. Simultaneously, the metabolism of glutamate by the phosphoserine aminotransferase (PSAT1) produces 3-phosphoserine, an intermediate of the de novo serine and glycine synthesis, both amino acids needed for collagen production. Similarly, glutamate can be metabolized by the enzyme pyrroline-5-carboxylate synthase (P5CS) to generate proline and contribute to collagen production. These findings add to the growing evidence that pharmacological targeting of glutamine metabolism could inhibit the collagen production seen in pulmonary fibrosis.
Moreover, glycine (Gly) represents the highest prevalent amino acid in collagen and is a major precursor of numerous proteins (9). In lung fibroblast, TGF-β1 binds to its receptor, leading to Smad3-dependent activating transcription factor 4 (ATF4) and mechanistic target of rapamycin complex 1/eukaryotic initiation factor 4E-binding protein 1 (mTORC1/4E-BP1) axis activation. ATF4 activation increases the expression of glycine biosynthesis enzymes, phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), phosphoserine phosphatase (PSPH), and serine hydroxymethyltransferase 2 (SHMT2). Simultaneously, ATF4 stimulates the production of the glucose transporter GLUT1, allowing glycolysis for the synthesis of glucose-derived glycine biosynthesis, and subsequently, collagen production in activated myofibroblast (10, 11). These metabolic changes suggest that myofibroblast differentiation requires not only modifications in the metabolism of amino acids but also of glucose and, most importantly, identifies the TGF-β1–mTORC1–ATF4 axis as a novel therapeutic approach to diminish the aberrant synthesis and deposition of collagen.
Next, arginine (Arg), a semiessential amino acid in adults and rodents, participates in multiple cellular functions, including cell division, ammonia removal, nitric oxide (NO) synthesis, glycogenesis, protein synthesis (through mTORC induction), and synthesis of collagen (12). Arginine is used as the substrate for five different enzymes: 1) arginyl-tRNA synthetase required for protein synthesis and, subsequently, the production of asymmetric dimethyl-l-arginine (ADMA), symmetric dimethyl-l-arginine (SDMA), and NG-monomethyl-l-arginine (L-NMMA) after posttranslational methylation by the protein arginine methyltransferase (PRMT); 2) NO synthases (NOS) for NO generation; 3) arginases (Arg-1 or Arg-2) for the production of urea, ornithine, proline, glutamate, and polyamines; 4) arginine-glycine amidinotransferase to generate creatine; and 5) arginine decarboxylase, which catalyzes the conversion of l-arginine to agmatine and carbon oxide (13). The precise role of Arg in IPF remains controversial; evidence indicates that its metabolism is altered in lung fibrosis, predisposing patients to abnormal collagen synthesis and dysregulated airway remodeling (14, 15).
For instance, l-proline, a precursor of collagen, is synthesized from l-ornithine and l-arginine by the actions of ornithine aminotransferase (Oat) and arginases (16). Arginase-1 (Arg-1) is highly expressed in mice models of bleomycin-induced lung fibrosis and alveolar macrophages of patients with IPF (17). Interestingly, increased expression of arginases in macrophages is associated with TGF-β1 stimulation. Thus, suggesting that macrophages contribute to the supply of l-proline needed by fibroblasts to synthesize collagen (18). Kitowska et al. (19) tested the effects of Arg-1 activity inhibition in lung fibroblast, showing diminished collagen deposition but without decreased mRNA expression or TGF-β1-dependent Smad-signaling. This study suggests that chronic inhibition of Arg-1 may represent a potential therapeutic approach; however, studies in other cell lines have shown poor outcomes, including worsened fibrosis.
The arginine by-product ADMA has been linked to fibrosis promotion by upregulating Arg-1 and collagen synthesis; metabolism and inactivation of this by-product are carried by the enzyme dimethylarginine dimethylaminohydrolase (DDAH). Pullamsetti et al. (20) found increased activity of DDAH in alveolar epithelial type II (AE2) cells from the lungs of bleomycin-injured mice fibrosis and patients with IPF, as a result of increased TGF-β1 and IL-6 production. The same study reported that inhibition of DDAHs promoted apoptosis in an ADMA-dependent manner (by the ADMA-p53-p21 signaling) while reducing collagen deposition by fibroblasts in an ADMA-independent manner. Moreover, overexpression of other enzymes involved in the metabolism of arginine by-products has been described in IPF fibroblasts, including peptidylarginine deiminase-4 (PAD4), which converts arginine residues to citrulline and the enzyme PRMT that catalyzes the methylation of arginine residues, thereby generating ADMA, L-NMMA, or SDMA. Moreover, IPF lungs have shown to have increased levels of arginine metabolites (creatine, putrescine, spermidine, 4-hydroxyproline, and proline-hydroxyproline dipeptide) that are crucial for ATP-production, ECM collagen formation, and cell proliferation (14, 21). Further studies are needed to fully elucidate the role of arginine in the pathogenesis of IPF. Nonetheless, the evidence points out that modulating this amino acid metabolism or its by-products may present a new therapeutic approach (Fig. 1).
Figure 1.
Schematic representation of altered amino acids metabolism in myofibroblast of idiopathic pulmonary fibrosis (IPF). Glutamine is converted to glutamate and then to α-ketoglutarate (α-KG) to enter the tricarboxylic acid (TCA) cycle, thus providing enough adenosine triphosphate (ATP) to sustain cell growth, proliferation, and collagen production. Transforming growth factor β1 (TGF-β1) activates the activating transcription factor 4-mechanistic target of rapamycin complex 1 (ATF4-mTORC1) axis, increasing glycine biosynthesis, stimulating glucose transporter 1 (GLUT1) production, and allowing subsequent glycolysis needed for collagen production. Upregulation of arginine by-products and arginase-1 (Arg-1) has been linked to collagen synthesis through increased proline production.
GLUCOSE
Glucose is composed of 12-carbon, 12-hydrogens, and 6-oxygen atoms; it can exist in a six-sided ring structure or open-chain structure. Glucose itself plays an integral role in cellular functioning by generating energy. It is the essential substrate that all eukaryotic cells break down during a process known as glycolysis to produce energy in the form of ATP (22). Glycolysis is a sequence of 10 enzyme-catalyzed reactions that metabolize glucose into pyruvate and it simultaneously generates two ATP molecules and transforms two molecules of oxidized nicotinamide adenine dinucleotide (NAD+) to its reduced form NADH. Traditionally, it was believed that oxygen presence dictates the fate of pyruvate. Under aerobic conditions, pyruvate undergoes oxidative decarboxylation to form acetyl coenzyme A (Acetyl-CoA), which enters the mitochondria for breakdown via the TCA cycle, resulting in ∼32 molecules of ATP per molecule of glucose via the electron transport chain (ETC) in the mitochondria. In contrast, during anaerobic conditions, only two molecules of ATP can be produced, where pyruvate is fermented to lactate by the enzyme lactate dehydrogenase (LDH) remaining in the cytoplasm (23). This is not the case for all cells. Malignant cells prefer to metabolize glucose to lactate even in the presence of oxygen, a phenomenon termed “the Warburg effect,” described as a physiological response by tumor cells to balance energy production and facilitate the uptake of nutrients necessary to proliferate, including amino acids, lipids, and nucleotides (24). Evidence suggests that glucose metabolism becomes dysregulated as cells age, therefore likely that glycolysis and glucose metabolism is altered in IPF and contribute to disease progression.
Mass spectroscopy and microarrays of total IPF lungs demonstrated to have decreased levels of the later-stage glycolysis metabolites fructose 1,6-bisphosphate (fructose 1,6-BP) and phosphoenolpyruvate (PEP), possibly due to decreased expression of phosphofructokinase (PFK). PFK is one of the rate-limiting enzymes in glycolysis that converts fructose 6-P to fructose 1,6-BP. This enzyme is activated by fructose 2,6 bisphosphate (fructose 2,6-BP), and the enzyme that generates fructose 2,6-BP [6-phosphofructo-2-kinase/fructose-2,6-biosphosphatase 3 (PFKFB3)] is also decreased (21). All in all, the decreased activity of PFK in IPF lungs contributes to diminished levels of late products of glycolysis. Though the production of late-glycolysis metabolites might be diminished in IPF lungs, early-stage glycolysis metabolites levels were observed to be unchanged.
Simultaneously, elevated lactic acid levels have also been observed in IPF lungs, signifying that early glycolysis products may be diverted to lactic acid production instead of the citric acid cycle as part of the Warburg effect in AE2 (25, 26). The Warburg effect is well known as a factor causing tumor progression in malignancy but has recently been shown to be partly responsible for many phenotypes of chronic diseases related to aging. In IPF, the Warburg effect induces myofibroblast differentiation via TGF-β and contributes to fibrosis. Though evidence suggests that AE2 cells might have decreased levels of late glycolysis metabolites, myofibroblasts in IPF have shown the opposite. TGF-β induces the expression of PFKFB3, the enzyme that contributes to the formation of 2,6-BP, was found to be upregulated in IPF myofibroblast (21, 27). Furthermore, Xie et al. (28) reported that augmented glycolysis stabilizes the hypoxia-inducible factor 1a (HIF-1α), an element required for the myofibroblast differentiation, adding to the previous reports that have identified HIF-1a as a crucial key in the pathogenesis of lung fibrosis (Fig. 2A). Altogether, these findings heighten the role of glycolytic reprogramming as a novel therapeutic avenue for IPF.
Figure 2.
A: glucose metabolism is altered in idiopathic pulmonary fibrosis (IPF). In alveolar epithelial cells, early glycolysis products are likely shifted to lactic acid production through the Warburg effect, inducing myofibroblast differentiation via transforming growth factor β (TGF-β) and further fibrosis. B: schematic diagram of dysregulated fatty acid (FA) metabolism in alveolar epithelial cells and macrophages contributing to lung fibrosis. Decreased carnitine synthesis leads to downregulation of β-oxidation and subsequent accumulation of lipids in the cytoplasm.
LIPIDS
Lipids are the general group of molecules that contain hydrophobic or amphipathic properties. The varying structures of lipids, from fatty acids to triglycerides to sphingolipids, lend themselves to specific functions within the eukaryotic cell.
Fatty acids (FAs) are a large group of molecules with a basic structure that contains a carboxylic acid group attached to a chain of carbon and hydrogen atoms; they can range from under five carbons to over twenty-two. FA can be either saturated or unsaturated: structurally, saturated-FAs have hydrocarbon chains connected by single bonds between carbons, whereas unsaturated-FAs have double bonds between carbons, giving the chain a bent shape. FAs have numerous functions throughout the eukaryotic cell as part of larger molecules from the phospholipid bilayer that constitutes most organelle and cell membranes to intracellular messengers (29). From a metabolic standpoint, these molecules are crucial as they can be broken down to generate ATP via β-oxidation. In β-oxidation, FAs attach to coenzyme A and are transported into mitochondria via the carnitine shuttle. Inside the inner mitochondrial matrix, intermediates are oxidized to produce NADH and FADH2, which generate ATP in the electron transport chain. Like glucose metabolism and other energy-generating processes affected by aging, β-oxidation is likely altered in IPF lungs.
Increased levels of long-chain and medium-chain FAs (caproate, caprylate, myristate, and palmitoleate) as well as significantly decreased levels of carnitine and medium-chain acyl-carnitines (hexanoyl-carnitine, octanoyl-carnitine, palmitoylcarnitine, and succinyl-carnitine) have been found in IPF lungs (21). As the successful transport of FAs into the inner mitochondrial matrix requires the action of carnitine (via the carnitine shuttle) to allow β-oxidation, this finding proposes a mechanism for the lipid accumulation and downregulated β-oxidation reported in IPF lungs. Though the transport of FAs into mitochondria of alveolar epithelial cells is impaired, it appears to be increased in lung macrophages of IPF lungs.
Lung macrophages of patients with IPF and bleomycin-injured mice have increased expression of mitochondrial calcium uniporter (MCU). This transporter allows the passage of Ca+ into the mitochondrial matrix and activates a key regulator of lipid metabolism in the mitochondria, the peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC-1α) (30). Moreover, IPF lung macrophages have shown increased β-oxidation, indicating that FA metabolism is shifted toward the cells that contribute to the fibrotic response by increasing Ca+ transport through the MCU and activation of PGC-1α (31, 32). Together, these findings suggest that lung macrophages undergo metabolic reprogramming to support tissue remodeling through FA oxidation as an energy source.
Although FAs allow for the formation of lipid bilayers and energy generation through oxidation, sphingolipids’ structure gives these lipids varying roles in the cell. Sphingolipids are a broad class of compounds derived from an 18 carbon amino alcohol, known as a sphingoid base and synthesized in the endoplasmic reticulum (ER). Modification of this base allows sphingolipids to participate in many pathophysiological processes within the cell, such as cellular structure, storage, and signaling (33). Current evidence suggests that these molecules are critical mediators of immune reactions, and abnormalities in sphingolipid metabolism are now associated with numerous respiratory diseases, including IPF. Sphingolipids have emerged as potential regulators of tissue fibrosis, given their ability to regulate cell migration, gene expression, and cell-to-cell interactions (34). Particularly, sphingosine 1-phosphate (S1P) and lysophosphatidic acid (LPA) play an essential role in the pathogenesis of IPF (35).
Serum and bronchoalveolar lavage (BAL) of patients with IPF have shown an increase in S1P concentration. Furthermore, inhibition of their synthesis by blocking the actions of sphingosine kinase-1 (SphK-1) using SKI-II reduced the levels of lung fibrosis in mice after bleomycin challenge (36, 37). Similarly, delayed onset of radiation-induced pulmonary fibrosis was seen by inhibition of sphingolipid de novo synthesis with myriocin, a serine palmitoyltransferase blocker, by decreasing the levels of S1P in mouse lung (38). Conversely, metabolomic analysis of IPF lungs revealed the downregulation of sphingomyelinases SMPD1 and SMPD4 with associated decreased sphinganine and sphingosine levels. This analysis showed that samples taken from these patients had downregulation of SphK-1 and S1P receptors (S1PR1 and S1PR4), suggesting that S1P production and signaling are diminished, contrary to the BAL findings of prior studies (39). These findings suggest that sphingolipids’ role in the development of lung fibrosis has not yet been fully elucidated, and further evidence is required as their metabolism may represent a new therapeutic approach for this disease (Fig. 2B).
HEME
Heme is an essential molecule to living aerobic organisms and plays an essential role in various biological reactions, such as oxygen transport, respiration, drug detoxification, and signal transduction. It interacts with various inactive apo-proteins giving rise to functional hemeproteins, and its function is ultimately determined by the properties of the polypeptide bound to it. Each heme group can bind up to one molecule of oxygen and is the essential component that gives hemoglobin and myoglobin their oxygen-carrying capacity. Heme is incorporated into cytochromes, catalases, and other biologically critical enzymes (40). In mammals, heme is catabolized inside splenic macrophages to form the compound bilirubin in two major steps. The first reaction involves cleavage of the heme ring by heme oxygenase-1 (HO-1) to form the intermediate biliverdin. In the second step, biliverdin reductase reduces the central methene bridge of biliverdin, producing bilirubin. Bilirubin is then conjugated with glucuronic acid to become more water-soluble for excretion (41). Evidence suggests that heme metabolism is altered in IPF, and it contributes to elevated reactive oxygen species (ROS) production seen in this disease.
Multiple forms of heme are required for the proper functioning of mitochondrial OXPHOS complexes. Heme serves as the prosthetic group for many mitochondrial respiratory complexes; heme a/a3 acts as a prosthetic group for complex IV, heme b for complex II, heme b11-562 heme c/c1 for complex III. Hence, serving a pivotal role in OXPHOS and energy production. IPF lungs have shown to have decreased levels of heme, a finding that coupled with the many other factors involved in mitochondrial dysfunction could explain the metabolic reprogramming from oxidative phosphorylation to glycolysis seen in AE2 (25). Reduced biliverdin levels but increased levels of bilirubin ZZ and bilirubin EE were also described in a cohort of patients with IPF. Moreover, increased gene expression of 5′-diphospho-glucuronosyltransferase (UGT1A1), which converts bilirubin ZZ to bilirubin glucuronide for excretion, was also found in IPF lungs (21). These findings may indicate that the heme degradation pathway in IPF lungs is increased, thus depleting heme while simultaneously accumulating bilirubin.
Furthermore, iron-derived ROS are implicated in the pathogenesis of numerous disorders, and one abundant source of redox-active iron is heme released from intracellular heme proteins. Free heme damages lipids, proteins, and DNA through the generation of ROS and has been implicated in various toxic effects through oxidation of lipids (42). Heme oxygenase plays a significant role in protecting cells from heme-induced oxidative stress by breaking down heme into biliverdin, free iron (Fe2+), and carbon monoxide (CO). Biliverdin is further metabolized to bilirubin [by biliverdin reductase (BVR)], which efficiently scavenges peroxyl radicals, thereby inhibits lipid peroxidation (43). Furthermore, CO inhibits the expression of proinflammatory cytokines TNFα, IL-1β, and macrophage inflammatory protein-1β (MIP-1β) through MAPK signaling activation (44). Moreover, Ito et al. (45) looked at stress-induced upregulation of heme oxygenase (HO-1) in alveolar macrophages of aged-mice and found that after intratracheal administration of lipopolysaccharide (LPS), the HO-1 expression was significantly reduced when compared with young mice. In summary, the breakdown of heme may induce an anti-inflammatory and protective response in alveolar macrophages against tissue damage. The upregulation of HO-1 is essential during elevated oxidative stress. However, the expression of this enzyme has shown to be limited in aging, suggesting that this protective mechanism might be impaired in age-related lung fibrosis and exacerbates the chronic epithelial injury seen in this condition (Fig. 3).
Figure 3.
Schematic representation of altered heme metabolism in macrophages of idiopathic pulmonary fibrosis (IPF). These pulmonary macrophages might have lower heme and biliverdin levels, leading to increased oxidative stress and expression of proinflammatory cytokines that can exacerbate fibrosis. BVR, biliverdin reductase; CO, carbon monoxide; Fe2, free iron; HO-1, heme oxygenase-1; ROS, reactive oxygen species.
CONCLUDING REMARKS
Presently, the therapeutic options for fibrotic lung disease remain limited. Evidence suggests that the dysregulated metabolism of alveolar epithelial cells, macrophages, fibroblast, and myofibroblast contribute to the pathogenesis and further progression of lung fibrosis. Although further studies are needed, we are convinced that linking aberrant tissue repair with impaired metabolomics will serve as the key to further understanding IPF and lay a foundation for more effective treatments.
GRANTS
This work was supported by NIH Grant R01HL136833.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.R. prepared figures; W.R. and F.R. drafted manuscript; W.R. and F.R. edited and revised manuscript; W.R. and F.R. approved final version of manuscript.
REFERENCES
- 1.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174: 810–816, 2006. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 2.Margaritopoulos GA, Trachalaki A, Wells AU, Vasarmidi E, Bibaki E, Papastratigakis G, Detorakis S, Tzanakis N, Antoniou KM. Pirfenidone improves survival in IPF: results from a real-life study. BMC Pulm Med 18: 177, 2018. doi: 10.1186/s12890-018-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruhat A, Cherasse Y, Chaveroux C, Maurin A-C, Jousse C, Fafournoux P. Amino acids as regulators of gene expression in mammals: molecular mechanisms. Biofactors 35: 249–257, 2009. doi: 10.1002/biof.40. [DOI] [PubMed] [Google Scholar]
- 4.Gaugg MT, Engler A, Bregy L, Nussbaumer-Ochsner Y, Eiffert L, Bruderer T, Zenobi R, Sinues P, Kohler M. Molecular breath analysis supports altered amino acid metabolism in idiopathic pulmonary fibrosis. Respirology 24: 437–444, 2019. doi: 10.1111/resp.13465. [DOI] [PubMed] [Google Scholar]
- 5.Cruzat V, Macedo RM, Noel KK, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients 10: 1564, 2018. doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard K, Logsdon NJ, Benavides GA, Sanders Y, Zhang J, Darley-Usmar VM, Thannickal VJ. Glutaminolysis is required for transforming growth factor-β1-induced myofibroblast differentiation and activation. J Biol Chem 293: 1218–1228, 2018. doi: 10.1074/jbc.RA117.000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge J, Cui H, Xie N, Banerjee S, Guo S, Dubey S, Barnes S, Liu G. Glutaminolysis promotes collagen translation and stability via alpha-ketoglutarate-mediated mTOR activation and proline hydroxylation. Am J Respir Cell Mol Biol 58: 378–390, 2018. doi: 10.1165/rcmb.2017-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamanaka RB, O'Leary EM, Witt LJ, Tian Y, Gokalp GA, Meliton AY, Dulin NO, Mutlu GM. Glutamine metabolism is required for collagen protein synthesis in lung fibroblasts. Am J Respir Cell Mol Biol 61: 597–606, 2019. doi: 10.1165/rcmb.2019-0008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhagavan NV, Ha CE. Protein and amino acid metabolism. In: Essentials of Medical Biochemistry (2nd ed.). Cambridge, MA: Academic Press, 2015, p. 227–268. [Google Scholar]
- 10.Park Y, Reyna-Neyra A, Philippe L, Thoreen CC. mTORC1 balances cellular amino acid supply with demand for protein synthesis through post-transcriptional control of ATF4. Cell Rep 19: 1083–1090, 2017. doi: 10.1016/j.celrep.2017.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvarajah B, Azuelos I, Plate M, Guillotin D, Forty EJ, Contento G, Woodcock HV, Redding M, Taylor A, Brunori G, Durrenberger PF, Ronzoni R, Blanchard AD, Mercer PF, Anastasiou D, Chambers RC. mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-β1-induced collagen biosynthesis. Sci Signal 12: eaav3048, 2019. doi: 10.1126/scisignal.aav3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2: 387–417, 2014. doi: 10.1146/annurev-animal-022513-114113. [DOI] [PubMed] [Google Scholar]
- 13.Fulton MD, Brown T, Zheng YG. The biological axis of protein arginine methylation and asymmetric dimethylarginine. Int J Mol Sci 20: 3322, 2019. doi: 10.3390/ijms20133322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endo M, Oyadomari S, Terasaki Y, Takeya M, Suga M, Mori M, Gotoh T. Induction of arginase I and II in bleomycin-induced fibrosis of mouse lung. Am J Physiol Lung Cell Mol Physiol 285: L313–L321, 2003. doi: 10.1152/ajplung.00434.2002. [DOI] [PubMed] [Google Scholar]
- 15.Mora AL, Torres-González E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 35: 466–473, 2006. doi: 10.1165/rcmb.2006-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-beta(1) stimulates L-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation 103: 1121–1127, 2001. doi: 10.1161/01.cir.103.8.1121. [DOI] [PubMed] [Google Scholar]
- 17.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol 167: 6533–6544, 2001. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 18.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5: e1000371, 2009. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitowska K, Zakrzewicz D, Konigshoff M, Chrobak I, Grimminger F, Seeger W, Bulau P, Eickelberg O. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L34–L45, 2008. doi: 10.1152/ajplung.00007.2007. [DOI] [PubMed] [Google Scholar]
- 20.Pullamsetti SS, Savai R, Dumitrascu R, Dahal BK, Wilhelm J, Konigshoff M, Zakrzewicz D, Ghofrani HA, Weissmann N, Eickelberg O, Guenther A, Leiper J, Seeger W, Grimminger F, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase in idiopathic pulmonary fibrosis. Sci Transl Med 3: 87ra53, 2011. doi: 10.1126/scitranslmed.3001725. [DOI] [PubMed] [Google Scholar]
- 21.Zhao YD, Yin L, Archer S, Lu C, Zhao G, Yao Y, Wu L, Hsin M, Waddell TK, Keshavjee S, Granton J, de Perrot M. Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir Res 4: e000183, 2017. doi: 10.1136/bmjresp-2017-000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummings JH, Stephen AM. Carbohydrate terminology and classification. Eur J Clin Nutr 61 Suppl 1: S5–S18, 2007. doi: 10.1038/sj.ejcn.1602936. [DOI] [PubMed] [Google Scholar]
- 23.Bar-Even A, Flamholz A, Noor E, Milo R. Rethinking glycolysis: on the biochemical logic of metabolic pathways. Nat Chem Biol 8: 509–517, 2012. doi: 10.1038/nchembio.971. [DOI] [PubMed] [Google Scholar]
- 24.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41: 211–218, 2016. [Erratum in Trends Biochem Sci 41: 287, 2016]. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alysandratos K-D, Russo SJ, Petcherski A, Taddeo EP, Acín-Pérez R, Villacorta-Martin C, Jean JC, Mulugeta S, Blum BC, Hekman RM, Vedaie M, Kook S, Wambach JA, Cole FS, Hamvas A, Emili A, Guttentag SH, Shirihai OS, Beers MF, Kotton DN. Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease (Preprint). bioRxiv 201: A2512, 2020. doi: 10.1101/2020.11.13.382390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Para R, Romero F, George G, Summer R. Metabolic reprogramming as a driver of fibroblast activation in pulmonary fibrosis. Am J Med Sci 357: 394–398, 2019. doi: 10.1016/j.amjms.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Kwan JYY, Yip K, Liu PP, Liu F-F. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov 19: 57–75, 2020. doi: 10.1038/s41573-019-0040-5. [DOI] [PubMed] [Google Scholar]
- 28.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu R-M, Bernard K, Thannickal VJ, Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 192: 1462–1474, 2015. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratnayake WM, Galli C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: a background review paper. Ann Nutr Metab 55: 8–43, 2009. doi: 10.1159/000228994. [DOI] [PubMed] [Google Scholar]
- 30.Gu L, Larson-Casey JL, Carter AB. Macrophages utilize the mitochondrial calcium uniporter for profibrotic polarization. FASEB J 31: 3072–3083, 2017. doi: 10.1096/fj.201601371R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu L, Larson Casey JL, Andrabi SA, Lee JH, Meza-Perez S, Randall TD, Carter AB. Mitochondrial calcium uniporter regulates PGC-1α expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol 26: 101307, 2019. doi: 10.1016/j.redox.2019.101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tedesco S, Scattolini V, Albiero M, Bortolozzi M, Avogaro A, Cignarella A, Fadini GP. Mitochondrial calcium uptake is instrumental to alternative macrophage polarization and phagocytic activity. Int J Mol Sci 20: 4966, 2019. doi: 10.3390/ijms20194966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. In: Sphingolipids as Signaling and Regulatory Molecules, edited by Chalfant C, Poeta MD.. New York, NY: Springer New York, 2010, p. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyne NJ, Dubois G, Pyne S. Role of sphingosine 1-phosphate and lysophosphatidic acid in fibrosis. Biochim Biophys Acta 1831: 228–238, 2013. doi: 10.1016/j.bbalip.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Shea BS, Tager AM. Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc 9: 102–110, 2012. doi: 10.1513/pats.201201-005AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, Ma W, Noth I, Ma S-F, Pendyala S, Reddy SP, Zhou T, Zhang W, Garzon SA, Garcia JG, Natarajan V. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J 27: 1749–1760, 2013. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Cai Y, Zhang W, Chen X. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem Cell Biol 96: 742–751, 2018. doi: 10.1139/bcb-2017-0302. [DOI] [PubMed] [Google Scholar]
- 38.Milara J, Navarro R, Juan G, Peiro T, Serrano A, Ramon M, Morcillo E, Cortijo J. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax 67: 147–156, 2012. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- 39.Rindlisbacher B, Schmid C, Geiser T, Bovet C, Funke-Chambour M. Serum metabolic profiling identified a distinct metabolic signature in patients with idiopathic pulmonary fibrosis - a potential biomarker role for LysoPC. Respir Res 19: 7, 2018. doi: 10.1186/s12931-018-0714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiabrando D, Vinchi F, Fiorito V, Mercurio S, Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol 5: 61, 2014. doi: 10.3389/fphar.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser ST, Midwinter RG, Berger BS, Stocker R. Heme oxygenase-1: a critical link between iron metabolism, erythropoiesis, and development. Adv Hematol 2011: 473709, 2011. doi: 10.1155/2011/473709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res 66: 121–127, 2009. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- 43.Kim SY, Park SC. Physiological antioxidative network of the bilirubin system in aging and age-related diseases. Front Pharmacol 3: 45, 2012. doi: 10.3389/fphar.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim MX, Png CW, Tay CY, Teo JDW, Jiao H, Lehming N, Tan KSW, Zhang Y. Differential regulation of proinflammatory cytokine expression by mitogen-activated protein kinases in macrophages in response to intestinal parasite infection. Infect Immun 82: 4789–4801, 2014. doi: 10.1128/IAI.02279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito Y, Betsuyaku T, Moriyama C, Nasuhara Y, Nishimura M. Aging affects lipopolysaccharide-induced upregulation of heme oxygenase-1 in the lungs and alveolar macrophages. Biogerontology 10: 173–180, 2009. doi: 10.1007/s10522-008-9164-4. [DOI] [PubMed] [Google Scholar]