Abstract
Adenosine receptors (ADORs) are G protein-coupled purinoceptors that have several functions including regulation of chloride secretion via cystic fibrosis transmembrane conductance regulator (CFTR) in human airway and kidney. We cloned an ADOR from Squalus acanthias (shark) that likely regulates CFTR in the rectal gland. Phylogenic and expression analyses indicate that elasmobranch ADORs are nonolfactory and appear to represent extant predecessors of mammalian ADORs. We therefore designate the shark ADOR as the A0 receptor. We coexpressed A0 with CFTR in Xenopus laevis oocytes and characterized the coupling of A0 to the chloride channel. Two-electrode voltage clamping was performed, and current-voltage (I-V) responses were recorded to monitor CFTR status. Only in A0- and CFTR-coinjected oocytes did adenosine analogs produce a significant concentration-dependent activation of CFTR consistent with its electrophysiological signature. A pharmacological profile for A0 was obtained for ADOR agonists and antagonists that differed markedly from all mammalian ADOR subtypes [agonists: R-phenyl-isopropyl adenosine (R-PIA) > S-phenyl-isopropyl adenosine (S-PIA) > CGS21680 > N6-cyclopentyladenosine (CPA) > 2-chloroadenosine (2ClAdo) > CV1808 = N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine (DPMA) > N-ethyl-carboxyl adenosine (NECA); and antagonists: 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) > PD115199 > 1,3-dimethyl-8-phenylxanthine (8PT) > CGS15943]. Structures of human ADORs permitted a high-confidence homology model of the shark A0 core that revealed unique structural features of ancestral receptors. We conclude that 1) A0 is a novel and unique adenosine receptor ancestor by functional and structural criteria; 2) A0 likely activates CFTR in vivo, and this receptor activates CFTR in oocytes, indicating an evolutionary coupling between ADORs and chloride secretion; and 3) A0 appears to be a nonolfactory evolutionary ancestor of all four mammalian ADOR subtypes.
Keywords: adenosine receptor, CFTR, chloride secretion, molecular evolution, shark, structural biology
INTRODUCTION
Two broad classes of purinoceptors, P1 and P2, recognize and bind adenosine-based nucleosides and nucleotides and can be distinguished by two antiparallel pharmacological profiles (P1: adenosine > AMP > ADP > ATP and P2: ATP > ADP > AMP > adenosine) (1). Receptors selective for adenosine (ADORs) are divided into four nonolfactory subtypes including A1, A2a, A2b, and A3, which couple to Go,i(1–3), Gs, Gs, and Gi(2,3),q-like G proteins, respectively (2–7). Typically, all ADORs modulate the activity of at least one of a number of different isoforms of adenylyl cyclase through the coupling to a G protein. The A1 ADOR can also modulate other effector systems, namely potassium channels, calcium channels, and phospholipase A and C (2).
One major unifying theme in the function of ADORs in mammals appears to be a feedback mechanism to either curb metabolic activity of the cell or oppositely vasodilate to increase oxygen supply and meet higher metabolic demand (8). Relatedly, in sharks, which evolved before any land-based vertebrate, low concentrations of adenosine released from metabolic activity by the highly active shark rectal gland of Squalus acanthias serve as an autacoid feedback inhibitor to slow ion transport (9). Curiously, high concentrations of adenosine (∼10 µM) increased ion transport activity at both the apical and basolateral membranes and stimulated chloride secretion in rectal gland via the shark ortholog of the cystic fibrosis transmembrane conductance regulator (CFTR) (10, 11). Shark CFTR is an impressive 70% identical to human CFTR at the amino acid residue sequence level (12), and an ancient evolutionary link in ADOR signaling of CFTR activity in both fish and mammals is now well established.
The four main ADOR subtypes (A1, A2a, A2b, and A3), first identified in many different mammals and now observed in the genomes of birds, reptiles, amphibians, and fish, can be distinguished at phylogenetic and pharmacological levels. For the A1 receptor, the adenosine analogs N6-cyclopentyladenosine (CPA) and R-phenyl-isopropyl adenosine (R-PIA) are the most potent whereas CV1808 and CGS21680 are weaker agonists. For A2a, N-ethyl-carboxyl adenosine (NECA), CV1808, and CGS21680 are the most potent agonists, and CPA is less potent. The A2b receptor subtype responds with high potency to NECA but exhibits little or no response to CV1808 and CGS21680. A fifth ADOR subtype, designated A2c, was more recently identified and was shown to be an olfactory ADOR that allows fish to sense adenosine released into the water by potential prey (13).
We sought to examine ADORs in S. acanthias, and we identified a single receptor with highest expression in the rectal gland and stomach but very low levels in brain and other tissues. Phylogenetic analysis shows that the S. acanthias ADOR as well as putative ADORs identified by genome sequencing from other sharks, amphibians, and reptiles form a separate clade that is distinct from the other five ADOR subtypes. We used the Xenopus laevis oocyte system to coexpress the S. acanthias ADOR with CFTR to demonstrate the coupling of receptor to chloride channel. Coinjected oocytes revealed a pharmacological profile of S. acanthias ADOR that is unique from all four major subtypes of ADORs. We argue that the S. acanthias ADOR is not olfactory and that the clade containing the elasmobranch ADORs is ancestral to the four major subtypes. We therefore designated the shark ADOR as A0-ADOR and characterize this ancestral receptor here, including novel structural features revealed by three-dimensional atomic homology modeling.
MATERIALS AND METHODS
Molecular Biology
Tissues were obtained from a male dogfish shark, S. acanthias, weighing 2–4 kg, that was caught by gill nets in Frenchman’s Bay, ME, and kept in marine live cars until use, usually within 3 days of capture. One male dogfish shark was killed by pithing the spinal cord in accordance with protocols approved by the Mount Desert Island Biological Laboratory. Details describing the molecular biology [preparation of total RNA from shark tissue, degenerate PCR, library screening, rapid amplification of cDNA ends-PCR (RACE-PCR), Northern blot, quantitative RT-PCR (qRT-PCR), and electrophysiology in oocytes] are presented in the Supplemental Material [see Supplemental Figs. S1 (https://doi.org/10.6084/m9.figshare.14248805.v1), S2 (https://doi.org/10.6084/m9.figshare.14248820.v1), S3 (https://doi.org/10.6084/m9.figshare.14248817.v1), S4 (https://doi.org/10.6084/m9.figshare.14248811.v1), and S5 (https://doi.org/10.6084/m9.figshare.14248799.v1)]. Human CFTR was obtained from Dr. Carol Semrad in the pKS-βglobin-CFTR-polyAC plasmid, and human adenosine receptors were provided by Dr. Joel Linden. Capped messenger RNA for all receptors was synthesized from linearized plasmid templates with the Ambion mMessage mMachine T7 in vitro transcription kit according to the manufacturer’s protocol.
To retrieve putative adenosine receptor coding sequences with good representation from mammals, birds, reptiles, amphibians, and fish, multiple database searches were carried out with the NCBI BlastP server against the full nonredundant data set. To find potential ancestral receptor sequences, additional searches using shark A0 sequence were performed, excluding the Mammalia taxid (40674). Global alignments were performed with the Clustal method (14) within the Lasergene software package (DNASTAR, Inc), and similar results were obtained with the implementation of Clustal Omega (15) in the EMBOSS package (16) and standard parameters (17). Alignments for phylogenetic analyses including the production of the phylogenetic tree in Fig. 1 were conducted with MEGA7 (18).
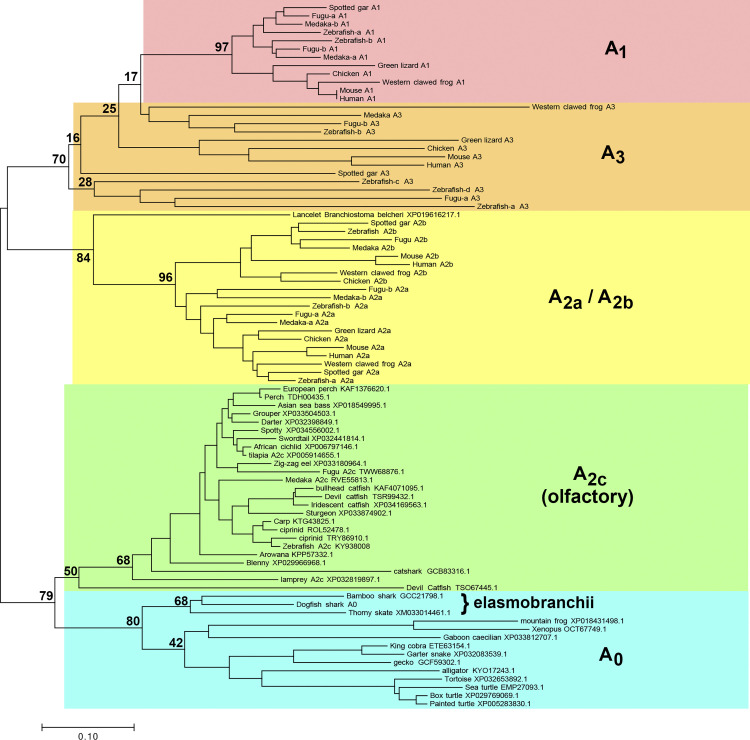
Figure 1.
Phylogenetic relationships of adenosine receptor (ADOR) taxa. Evolutionary history is inferred with the neighbor-joining method (14). The optimal tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the tree. Distances were calculated with the Poisson correction method (36) and are in the units of the number of amino acids substitutions per site. A1, A2a, A2b, A3, and olfactory (A2c) ADORs shown in Wakisaka et al. (13) are shown, using the same names as in that paper. The first characterized A2c (zebrafish, KY938008) by Wakisaka et al. (13) is also shown. Additional putative ADORs found in this work are shown by species and accession. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test using 500 replicates (40) are shown at selected branchpoints. Analyses were conducted with MEGA7 (18), using only full-length sequences for all receptors.
Xenopus laevis Expression System
Adult female X. laevis were obtained from Nasco and Xenopus 1 and housed in the animal care facilities at Yale University. Surgeries to harvest oocytes were performed 4–7 days before experiments according to a protocol approved by the Yale Animal Care Committee. Injection pipettes pulled on a Sachs-Flaming micropipette puller model PC-84, using Drummond 210/310/510 glass pipettes, were beveled to a tip diameter of 10–15 µm. The pipettes were back-filled with mineral oil and attached to a Drummond microinjector, in preparation for cRNA aspiration and oocyte injection. Oocytes were injected with 50 nL (∼15 ng) of cRNA encoding shark A0 or human A1, A2a, A2b, or A3 receptors and 5–10 ng of human CFTR and subsequently incubated for 3–5 days at 18–20°C before experiments.
Oocytes were prepared for two-electrode voltage clamping by insertion of two 0.6- to 0.9-MΩ, 3 M KCl-filled Ag electrodes, pulled on a Sachs-Flaming micropipette puller model PC-84, using borosilicated glass obtained from Sutter Instrument (BF120-69-10). Oocytes were perifused with frog Ringer solution (FR; in mM: 98 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES, adjusted to pH 7.4), which was also used in the preparation of solutions for the pharmacological agents tested. Oocytes were voltage clamped with the Dagan TEV-200 amplifier along with the Dagan LM-12 Lab Interface on a PC computer running pCLAMP version 5.5 software. The Soltec 1242 plotter was used to acquire the data. Oocytes were clamped at −60 mV, and the current was measured as a function of time. Moreover, frequent voltage pulses (−120 mV to +60 mV) were performed to determine the current-voltage (I-V) relationship at any given time point. Data were corrected for any capacitive current incurred because of the voltage pulses. Inconclusive data were obtained with the human A2a and A3 receptors, which presumably do not couple efficiently to frog G proteins or the proper adenylyl cyclase(s) for CFTR activation, and were omitted from the analysis.
Data analysis was performed with Microsoft Excel. Some of the data were analyzed with an Excel macro provided by the laboratory of Dr. David Dawson, which allowed the subtraction of the capacitive current from the current-voltage relationship incurred during the voltage pulses. Statistical analysis was limited to standard deviation performed on the concentration response of the shark A0 receptor.
Homology Model of the Shark A0
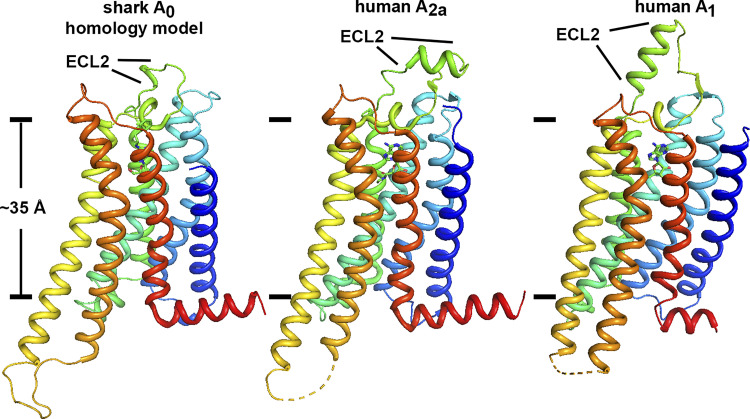
At the time of this writing there were 48 high-resolution structures of adenosine receptors in the Protein Data Bank (PDB) as determined by either X-ray crystallography or cryo-electron microscopy. All structures are human in origin, and only two of the four known subtypes were represented, A2a (n = 45) and A1 (n = 3). We chose a small subset for consideration as templates for homology modeling of the shark A0 receptor: A2a with adenosine bound (PDB code 2ydv) (19), A1 with adenosine (6d9h) (20), A2a with NECA (6gdg) (21), and A2a with CGS21680 (4ug2) (22). Primary amino acid sequence alignments between shark A0 sequence and the selected subset were performed with NCBI Cobalt (23). Amino acid sequences present in the human receptors but not present in shark A0 were removed [see full alignment between shark A0 and human A2a in Supplemental Fig. S6 (https://doi.org/10.6084/m9.figshare.14248823.v1)]. The human A2a receptor appeared to be a sufficiently good template for A0 modeling, as it shares 45% identical amino acid sequence with A0 and exhibited an NCBI Cobalt 3-bit amino acid chemical similarity of 81% after removal of disordered sequences from the A2a structure and after removal of sequences present in either receptor but not the other (see Supplemental Fig. S7; https://doi.org/10.6084/m9.figshare.14248814.v1). With the A2a X-ray crystal structure template (PDB code 2ydv), A2a insertion sequences were removed and mutagenesis to A0 was performed with scripts written in Perl and PyMOL version 2.0.6 (Schrödinger, LLC). Two short amino acid sequences of shark A0 that are not present in the human receptors (i.e., A0-Arg95 and A0-Asn228-Ser230) were inserted into the three-dimensional model with Coot version 0.8.9 (24) and model minimization/regularization. Refinements using phenix (25) of A0 against the A2a data set (PDB code 2ydv) were performed by replacing the human receptor with the shark starting model. Three rounds of rigid body, XYZ-reciprocal space, and B-factor refinement were performed, including secondary-structure restrains and Ramachandran/rotamer optimization, all with resolution cutoff set to 4.0-Å resolution. Parameterization of the R-PIA ligand was accomplished with phenix (25) starting with the Simplified Molecular-Input Line-Entry System (SMILES) string for the ligand (26): C[C@H](Cc1ccccc1)Nc2ncnc3n(cnc23)[C@@H]4O[C@H](CO)[C@@H](O)[C@H]4O. The position of the lipid bilayer was predicted based on the location of conserved aromatic amino acids known to interact with lipid head groups spaced ∼35 Å apart (27).
RESULTS
Identification of Shark A0 ADOR
Since the S. acanthias genome is not yet available and there is some uncertainty associated with the search for bona fide protein-coding sequences from genomic data, we sought to clone shark adenosine receptors with reverse transcription procedures from mRNA/cDNA prepared from shark tissues. PCR of shark rectal gland cDNA using degenerate primers to conserved regions of transmembrane domains 5 and 7 of previously cloned ADORs yielded a single 352-bp product (see Supplemental Fig. S1). The translated amino acid sequence of this fragment (P-SH12) had highest homology to ADORs that was only 39–45% identical to the human receptor subtypes (A1, A2a, A2b, and A3). Northern blot and qRT-PCR revealed strongest expression of the putative shark ADOR in the rectal gland and stomach, with weak levels in other tissues including the brain [see Supplemental Figs. S2 and S3 and Supplemental Tables S1 (https://doi.org/10.6084/m9.figshare.14248880) and S2 (https://doi.org/10.6084/m9.figshare.14248883)]. To control for the quality of cDNA template used in the qRT-PCR experiments, shark CFTR was amplified from the same cDNA templates prepared from the various shark tissues. Results showed abundant CFTR expression in rectal gland, intestine, brain, and testis (see Supplemental Fig. S4).
Screening of a shark rectal gland cDNA library using 32P-labeled P-SH12 yielded more nucleotide sequence from a single putative ADOR and allowed the design of primers for RACE-PCR. Extensive rounds of both cDNA library screening and RACE-PCR using total RNA from shark rectal gland revealed only a single putative ADOR entity, which we designate here as the shark A0 receptor (for details see Supplemental Material).
Hydropathy analysis of the putative shark A0 ADOR amino acid sequence revealed seven predicted transmembrane regions and a predicted extracellular orientation of the NH2 terminus that was consistent with all known G protein-coupled receptors (GPCRs) (see Supplemental Fig. S5). Phylogenetic analysis of known and putative ADORs revealed an ancestral branch that included dogfish shark A0 and other putative ADORs from nonmammalian species that are distinct from the recently discovered olfactory ADORs (13) (Fig. 1). Ancestral olfactory and nonolfactory receptors could be distinguished from the four specialized mammalian subtypes (i.e., A1, A2a, A2b, and A3) as having an abbreviated second extracellular loop (ECL2) at 9 amino acid (aa) residues (see Supplemental Fig. S8; https://doi.org/10.6084/m9.figshare.14248802.v1). ECL2 of A1 is elongated by 4–17 aa (n = 42 receptor species examined), ECL2 of A2a is elongated by 8–14 aa (n = 22), ECL2 of A2b is elongated by 10–19 aa (n = 28), and ECL2 of A3 is elongated by 1–7 aa (n = 18; see Supplemental Fig. S9; https://doi.org/10.6084/m9.figshare.14248796.v1). Assuming that mutagenesis rates of all ADORs are similar among species, we interpret the phylogram to indicate that the elasmobranch A0 ADOR belongs to an evolutionary ancestral branch of the specialized ADOR subtypes. We next surmised that shark A0 is responsive to adenosine analogs with potentially unique properties compared with known receptor subtypes, and we sought to test this hypothesis with functional and pharmacological characterization in X. laevis oocytes.
CFTR Expression
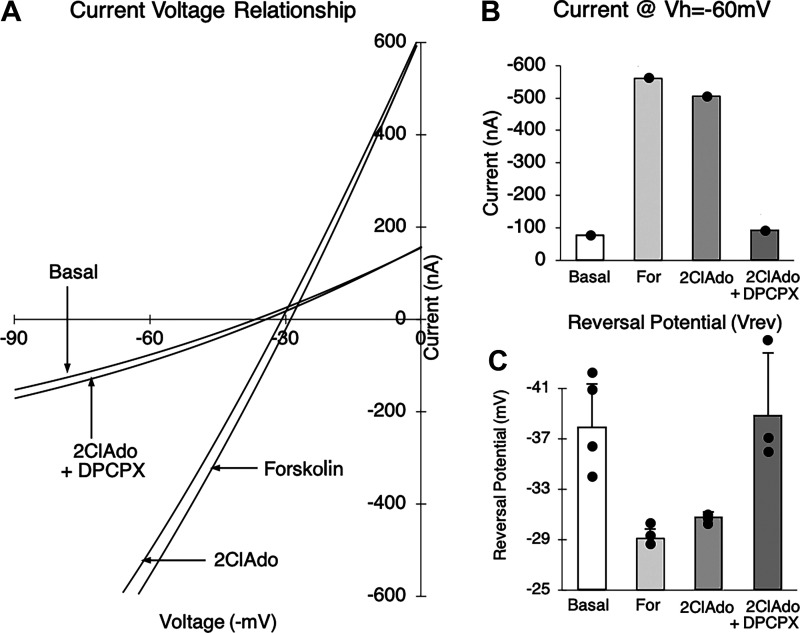
Expression studies were conducted in oocytes coinjected with human CFTR and shark A0 cRNA, and current-voltage (I-V) relationships were determined by imposing voltage pulses (−120 mV to +60 mV) and measuring the clamping current. Expression of CFTR was determined by the use of forskolin, a well-known activator of adenylyl cyclase that increases intracellular cAMP levels and activates CFTR by phosphorylation of the R domain by a cAMP-dependent protein kinase A (PKA) (28). Figure 2 illustrates the I-V relationships of a single representative experiment on an oocyte 3 days after coinjection. Addition of 5 µM forskolin produced an increase in conductance (increase in the slope of the I-V relationship) from the basal level, an increase in the magnitude of the clamping current at −60 mV from −76 nA to −563 nA, and a rightward shift in the reversal potential to −29.4 ± 0.75 mV (n = 3 I-V ramps ± SD) from a basal value of −38.0 ± 3.8 mV (n = 5 ramps ± SD). This negative, nonrectifying current with a reversal potential close to that of Cl− ( = approximately −30 mV) has the electrophysiological characteristics of a CFTR Cl− current. Furthermore, this response was sensitive to 300 µM glibenclamide, an inhibitor of the CFTR Cl− channel (29) (n = 1; data not shown). The response to forskolin was also inhibited by the PKA antagonist H-89 (25 µM, n = 3) in oocytes injected with human CFTR cRNA (data not shown), suggesting that CFTR is activated by a cAMP-dependent protein kinase (PKA) in this system. To confirm, we measured total cAMP in oocytes expressing the shark A0 receptor and observed an increase in total cAMP that paralleled the order of agonist potency we observed in electrophysiology experiments (see Supplemental Fig. S10; https://doi.org/10.6084/m9.figshare.14248787.v1). The negative, nonrectifying CFTR current was absent in uninjected and water-injected oocytes (n = 4) under identical conditions and was reproducible both in the same oocyte and in all oocytes injected with human CFTR cRNA. These data established expression of CFTR in our system.
Figure 2.
Current-voltage (I-V) relationship illustrating the effects of forskolin (For), 2-chloroadenosine (2ClAdo; a deaminase-resistant form of adenosine), and 2ClAdo + 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; a known adenosine receptor antagonist) in a representative oocyte coexpressing the shark A0 receptor and human CFTR Cl− channel. A: selected single I-V plots for basal, For, 2ClAdo, and 2ClAdo+DPCPX. B: corresponding total oocyte current at −60 mV clamping voltage (Vh) for the plots in A. C: reversal potentials (means ± SD) were obtained for basal (−38.0 ± 3.8 mV, n = 5), For (−29.5 ± 0.74 mV, n = 3), 2ClAdo (−30.5 ± 0.28 mV, n = 4), and 2ClAdo+DPCPX (−39.4 ± 4.8 mV, n = 3).
Addition of 10 µM 2-chloroadenosine (2ClAdo) in the representative oocyte (Fig. 2) also produced an increase in total current by a similar nonrectifying negative current of the magnitude approximately −500 nA and a reversal potential of approximately −30.5 ± 0.28 mV (n = 3 ramps ± SD). This response was reversibly inhibited by 10 µM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), an adenosine receptor antagonist, where the clamping current at −60 mV was approximately −90 nA with a reversal potential of −39.4 ± 4.8 mV (n = 3 ramps ± SD), both of which are reminiscent of the basal state (Fig. 2). This response suggested that the expression and stimulation of the shark A0 receptor are associated with an increase in Cl− current that has characteristics consistent with CFTR C1− currents. Our results also established a coupling of the shark A0 adenosine receptor and human CFTR in the Xenopus oocyte expression system.
Pharmacological Characterization of Shark A0
Determination of the pharmacological profiles of adenosine receptor agonists and antagonists in the oocyte necessitated the development of protocols in the X. laevis oocyte expression system. We first compared three agonists in a series of identical experiments to provide quantitative data for statistical analysis. We then developed a second protocol to determine a relative response to a larger number of agonists and antagonists to provide a more comprehensive profile for comparison with other receptor profiles in the literature.
Quantitative concentration response to agonists.
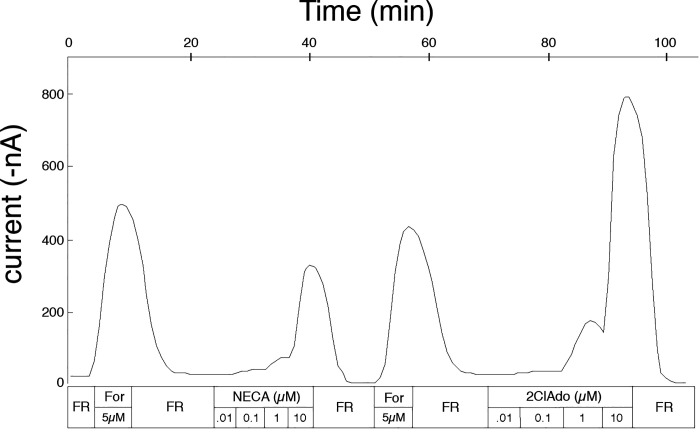
Based on preliminary data, NECA, 2-chloroadenosine (2ClAdo), and R-PIA were selected to determine potency order in a quantitative manner. The concentration response to each agonist was measured as in the representative experiment shown in Fig. 3. Oocytes were first stimulated with 5 µM forskolin in the absence of IBMX, since IBMX is thought to antagonize adenosine receptors (30), and subsequently brought to a basal state with frog Ringer solution (FR). Five micromolar forskolin alone appears to be submaximal for stimulating CFTR in oocytes (31, 32) and avoids desensitization of the adenylyl cyclase/CFTR pathway, since each oocyte was stimulated multiple times during experiments. Next, the oocytes were exposed to increasing concentrations of NECA (0.01 µM, 0.1 µM, 1 µM, and 10 µM); each incremental concentration of the agonist was added once the response to the prior concentration had peaked (n = 3). This sequence was followed by an identical protocol for 2ClAdo (n = 3) and R-PIA (n = 6). Variation in the sequence of the agonists tested gave similar results.
Figure 3.
Representative tracing for the determination of the concentration response profile for the shark A0 adenosine receptor. A total of 4 oocytes were used for the determination of the concentration response reported in the main text. Three oocytes, including results from 1 representative shown in the figure, were exposed to multiple sequences of incremental concentrations of N-ethyl-carboxyl adenosine (NECA), 2-chloroadenosine (2ClAdo), and R-phenyl-isopropyl adenosine (R-PIA; not shown), while the fourth was used for the study of R-PIA exclusively. After any stimulation of the oocytes [with forskolin (For) or adenosine analogs], oocytes were returned to basal levels by perifusion with frog Ringer solution (FR).
The initial 5 µM forskolin response was used as an internal control (comparison of the agonist response to forskolin) in these studies because of the variability of the expression of CFTR secondary to imprecision in injection of RNA, interoocyte variability in CFTR expression, and interfrog and intrafrog variability in the quality of the oocytes due to environmental factors. Data were normalized to the response to 5 µM forskolin preceding each concentration response.
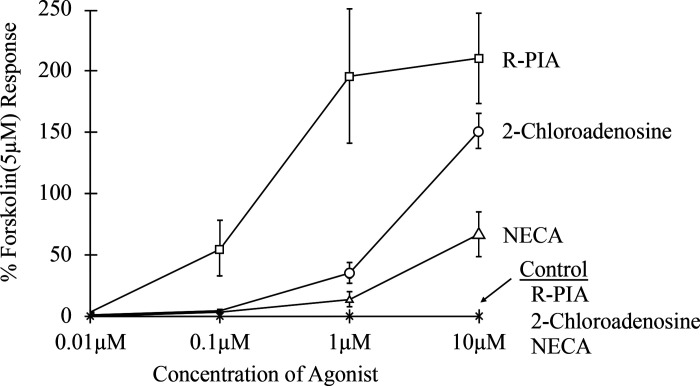
Results from the concentration-response experiments are summarized in Fig. 4. The peak normalized stimulation of the control oocytes (injected with human CFTR cRNA only) at 10 µM of NECA, 2-chloroadenosine, and R-PIA are −0.01%, 0.04%, and 0.03%, respectively (normalized to 5 µM forskolin responses, n = 6, with mean ± SD of 239 ± 66 nA), showing very poor coupling of any endogenous oocyte adenosine receptor to human CFTR. The responses of oocytes injected with human CFTR and shark A0 cRNA, at concentrations of 0.01 µM NECA, 2-chloroadenosine, and R-PIA and 0.1 µM NECA and 2-chloroadenosine were not significantly different from uninjected controls (P > 0.05). The response to R-PIA at 0.1 µM (55.6%) was greater than NECA and 2-chloroadenosine (2.7% and 4.2%, respectively). At an agonist concentration of 1 µM the differences between the three agonists, NECA, 2-chloroadenosine, and R-PIA (13.0%, 35.1%, and 196%, respectively) were appreciable and significant (P < 0.05). The responses at 10 µM (66.5%, 151%, and 210%, respectively) were markedly different (P < 0.05). The probability values for determining a significant main effect for each of these observations were obtained with an ANOVA. These data established the potency order of R-PIA > 2-chloroadenosine > NECA for the shark A0 adenosine receptor in X. laevis oocytes.
Figure 4.
Concentration response of the shark A0 adenosine receptor in both A0- and CFTR-coinjected oocytes as well as CFTR only-injected oocytes (Control). The controls were conducted in 3 oocytes, with each agonist tested in each of the 3 oocytes (see asterisks on the x-axis). The experimental group was tested in 4 oocytes [N-ethyl-carboxyl adenosine (NECA), n = 3; 2-chloroadenosine, n = 3; R-phenyl-isopropyl adenosine (R-PIA), n = 6). The y-axis is expressed in terms of % response to 5 μM forskolin in total oocyte current (nA).
Qualitative profile for agonists and antagonists.
With the above profile established for the three agonists, a more extensive profile was ascertained for eight agonists [NECA, N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl) ethyl]adenosine (DPMA), CV1808, 2-chloroadenosine, CPA, S-phenyl-isopropyl adenosine (S-PIA), CGS21680, R-PIA] and five antagonists [8-(3-chlorostyryl)caffeine (CSC), 1,3-dimethyl-8-phenylxanthine (8PT), DPCPX, CGS15943, PD115199] to allow comparison with profiles established for the presently cloned adenosine receptors. The analysis was carried out at an agonist concentration of 1 µM and an antagonist concentration of 10 µM to optimize the analysis.
The protocol required oocyte perifusion with one agonist at 1 µM and substitution with a second after stabilization of the signal. The responses were judged as an increase, a decrease, or equivocal. The data were compiled into the agonist profile for the shark A0 receptor of R-PIA > S-PIA > CGS21680>CPA > 2-chloroadenosine > CV1808 = DPMA > NECA (n = 7). Varying the order of agonists produced highly consistent results, in that the profiles conducted in order of increasing agonist potency were usually no different from profiles conducted in order of decreasing agonist potency.
The antagonist order of potency was carried out with a similar protocol. Oocytes were perifused with 1 µM R-PIA to generate a stable stimulated state, followed by perifusion with 1 µM R-PIA and the various antagonists at 10 µM. The antagonist profile obtained for the shark A0 receptor was DPCPX > PD115199 > 8PT > CSC > CGS15943 (n = 5). The relative potency orders of agonists and antagonists were determined qualitatively in 10 different oocytes, including 186 pairwise comparisons (see Supplemental Table S3; https://doi.org/10.6084/m9.figshare.14248886.v1), and 91% (n = 169) of all pairwise observations agree with our final conclusion of potency order.
Protocol Validation Using Human ADORs
The human A1, A2a, A2b, and A3 receptors, obtained from the Linden laboratory at the University of Virginia, were also examined in our oocyte system using the qualitative protocol to provide validation for this form of analysis. Although four human ADORs were expressed in the oocyte, reproducible profiles were obtained for two receptors, the human A1 (agonist profile, n = 3) and human A2b (agonist profile, n = 4, antagonist profile, n = 3) (Table 1). The human A1 and A2b receptor agonist profiles determined in oocytes are in excellent agreement with binding studies and functional studies (see Supplemental Table S4; https://doi.org/10.6084/m9.figshare.14248877). However, the A2b antagonist profile shows an apparent discrepancy in the potency order of CGS15943 and 8PT. This difference may be attributable to a cross-species comparison (rat vs. human). Taken together, these data provide strong support for the validity of our protocols used in pharmacological characterization of human and shark ADORs.
Table 1.
Pharmacological profiles of human adenosine receptors in Xenopus oocytes obtained from this study
| A1 agonist | CPA > R-PIA = NECA > 2ClAdo > S-PIA>DPMA > CV1808 > CGS21680 |
| A2b agonist | NECA > R-PIA > 2ClAdo > CPA > DPMA > S-PIA > CV1808 > CGS21680 |
| A2b antagonist | 8PT > PD115199 > DPCPX > CGS15943 > CSC |
2ClAdo, 2-chloroadenosine; CPA, N6-cyclopentyladenosine; CSC, 8-(3-chlorostyryl)caffeine; DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; DPMA, N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine; NECA, N-ethyl-carboxyl adenosine; R-PIA, R-phenyl-isopropyl adenosine; S-PIA, S-phenyl-isopropyl adenosine; 8PT, 1,3-dimethyl-8-phenylxanthine.
Homology Model of Shark A0
Given the reasonable sequence homology of shark A0 between ADORs that have been well characterized and for which structures are known (see Supplemental Fig. S11; https://doi.org/10.6084/m9.figshare.14248808.v1), we conducted exploratory refinements of the A0 starting homology model against the deposited structure factors of the human A2a X-ray crystal structure (PDB 2ydv), replacing the coordinates of A2a with A0. Refinement was restricted to low resolution (4.0 Å) to effectively downweight the contribution of mismatched side chain sequences between the two species while providing a data-driven constraint for backbone atoms and secondary structure. The resulting model exhibited good protein Ramachandran and side chain rotameric geometry (see Supplemental Table S5; https://doi.org/10.6084/m9.figshare.14248865.v1) and had convincing secondary structure for the transmembrane helices and extramembrane segments (Fig. 5). Both sequence alignments and the shark A0 homology model revealed an abbreviated extracellular loop 2 (ECL2) in ancestral receptors compared with the more specialized subtypes. Although it is not yet known what, if any, functional consequence arises from different lengths of the ECL2 specifically, >30 ancestral receptors in the database have a minimized ECL2 (see Supplemental Fig. S8). Thus, it would seem that ECL2 of ancestral receptors is at the minimum length necessary to maintain the structure and/or function of the adenosine receptor architecture.
Figure 5.
Overall view of the shark A0 homology model compared with human A2a and A1 receptors (PDB codes 2ydv and 6d9h). The approximate position of the hydrophobic stretch of the lipid bilayer is depicted with lines separated by ∼35 Å using conserved aromatic side chains as fiducials (17). A striking feature of shark A0 compared with the more specialized adenosine receptors (ADORs) is an abbreviated extracellular loop 2 (ECL2, labeled) and the relatively low profile that the A0 receptor presents to the extracellular milieu. Multiple sequence alignment of all known and putative receptor orthologs (see Supplemental Figs. S9 and S10) reveals that an abbreviated ECL2 appears to be a unique feature of ancestral ADORs.
Conserved Ligand-Receptor Interactions
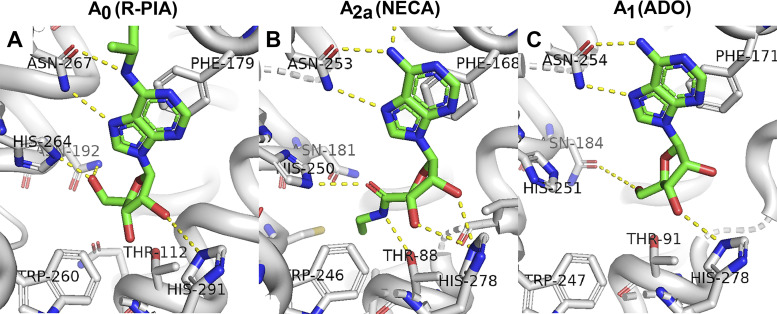
To explore the extent to which our homology model recapitulates known fundamental interactions between ligand and receptor, we separately modeled adenosine (ADO), 2-chloroadenosine, and the most potent ligand in this work, R-phenyl-isopropyl adenosine (R-PIA), into the A0 binding pocket, conducted refinements, and inspected the receptor-ligand interactions. Although we recognize that there will be error in the process of homology modeling A0 in the absence of an actual structure, potential fundamental interactions between A0 and ligands are well conserved in the refined A0 model compared with human ADOR structures (Fig. 6; see Supplemental Table S6; https://doi.org/10.6084/m9.figshare.14248874.v1).
Figure 6.
Binding pockets for adenosine and adenosine agonists. A: a potential binding mode of R-phenyl-isopropyl adenosine (R-PIA) as it appears in our refined homology model of the shark A0 receptor. The purpose is not to highlight precise interactions, bonds, or geometry but to indicate that the major known adenosine-binding ligands are conserved in the shark A0 ancestor receptor and are well positioned to potentially interact with ligands. For comparison, the location of N-ethyl-carboxyl adenosine (NECA) binding in the 2.6-Å X-ray structure of human A2a receptor (PDB code 2ydv) is shown in B. C: the location of adenosine (ADO) binding in the 3.6-Å cryo-electron microscopy (cryo-EM) structure of the human A1 receptor (PDB code 6d9h).
Some of the most important known interactions between receptor and ligand occur between ADOR and the purine pharmacophore of adenosine and adenosine analogs. The structures of human A2a and A1 reveal that an aromatic residue (F168 and F171, respectively) protrudes down into the ligand binding pocket and contributes an intimate π-π stacking interaction with the purine ring of adenosine (33). Our refined homology model of shark preserves this interaction convincingly well, with the corresponding A0 aromatic residue being F179 (Fig. 6). A second critical interaction revealed by the structures of A2a and A1 is a strong polar interaction, most likely hydrogen bonding, between an asparagine residue (N253 and N254, respectively) and the N7 nitrogen and the amino group of C6 of the purine ring. The corresponding A0 asparagine is N267 (Fig. 6). These two interactions form the fundamental basis for recognizing the adenosine pharmacophore and are invariant in all ADORs we have examined [see multiple sequence alignments in Supplemental Figs. S12 (https://doi.org/10.6084/m9.figshare.14248790.v1) and S13 (https://doi.org/10.6084/m9.figshare.14248793.v1]. In totality, nine amino acid residues in close proximity to the adenosine analog in our shark A0 homology model are highly conserved in all known and putative ADORs, including the ancestral branch (see also Supplemental Table S6).
DISCUSSION
Coexpression of Shark A0 and Human CFTR
We successfully coexpressed the shark A0 adenosine receptor with human CFTR in X. laevis oocytes, which, to our knowledge, represents the first heterologous coexpression of any adenosine receptor with CFTR in the literature. Oocytes appear to be an ideal system to characterize shark A0, since neither frog ADORs nor frog CFTR appears to be active or coupled. We coupled an adenosine receptor from an elasmobranch shark, a subclass of Chondrichthyes fish with a temporal range dating back to at least the Devonian period (∼419–360 million years ago), to human CFTR, using the endogenous G proteins and cellular enzymes of the oocyte. Other investigators have in the past exploited CFTR for coexpression studies, specifically with α2-adrenergic, β-adrenergic, δ-opioid, and 5-HT1A receptors (34, 35); however, those receptors were mammalian in origin, which would not be viewed nearly as long-lived as receptors identified in the shark. A0 function in oocytes suggests that the interaction between receptors and frog G proteins is maintained despite the ancestral properties and structural differences A0 has with respect to more specialized ADORs. This highlights a likely conservation of the interface between G proteins and receptors with which they interact, across species and through evolution. Our results also emphasize the versatility and value of the X. laevis expression system for studies of GPCR molecular evolution.
A0 Exhibits Unique ADOR Pharmacology
We characterized the shark A0 receptor in Xenopus oocytes using a novel protocol, in contrast to previous studies using binding assays, in vivo functional studies, and cAMP measurements. To this end it was necessary to couple the shark A0 with a reporter target protein, in this case human CFTR, to permit measurement of expression by electrophysiology. The data from these studies revealed a unique profile for the shark A0 receptor. Table 2 summarizes the comparison of the A0 receptor to other adenosine receptors with respect to agonist potency.
Table 2.
Summary of agonist potency order of shark A0 determined from this study compared with potencies for A1, A2a, A2b, and A3 receptors from binding/functional studies in the literature
| Receptor Subtype | Potency Order |
|---|---|
| Shark A0 | R-PIA > 2ClAdo > NECA |
| A1 | R-PIA > NECA > 2ClAdo |
| A2a | NECA > 2ClAdo > R-PIA |
| A2b | NECA > 2ClAdo > R-PIA |
| A3 | R-PIA ≈ NECA |
2ClAdo, 2-chloroadenosine; NECA, N-ethyl-carboxyl adenosine; R-PIA, R-phenyl-isopropyl adenosine. See expanded Supplemental Tables S7 and S8, including references.
The shark A0 differs 1) from A1 in its potency order of 2ClAdo and NECA, 2) from A2a and A2b, which have the reverse potency order, and 3) from A3, in which the least potent shark A0 agonist, NECA, and the most potent shark A0 agonist, R-PIA, have equal potencies. Therefore, the shark A0 appears to have a unique agonist profile, judging from the potency order of R-PIA > 2ClAdo > NECA.
Subsequent qualitative studies further established the unique nature of the shark A0. Table 3 compares the pharmacological profile for the shark A0 to mammalian A1, A2a, A2b, and A3 adenosine receptor subtypes. Analysis reveals that the A1-selective agonist R-PIA is the most potent in the shark; however, the A1-selective agonist CPA is of intermediate potency, and S-PIA and CGS21680 are more potent in the shark A0 than in the A1 receptors. All of the above are inconsistent with an A1-like receptor profile for A0.
Table 3.
Comparison of data from qualitative studies of shark A0 in Xenopus oocytes and functional studies of adenosine A1, A2a, A2b, and A3 receptor subtypes from the literature
| Subtype | Ag/Antag | Functional Profiles |
|---|---|---|
| A0 | Ag | R-PIA > S-PIA>CGS21680 > CPA > 2ClAdo > CV1808 ≥ DPMA > NECA |
| Antag | DPCPX > PD115199 > 8PT > CSC > CGS15943 | |
| A1 | Ag | CPA > R-PIA = CHA ≥ NECA > 2ClAdo > S-PIA > CV1808 ≥ CGS21680 |
| Antag | DPCPX > PD115199 > 8PT | |
| A2a | Ag | CGS21680 = NECA > CV1808 ≥ 2ClAdo>R-PIA = CHA = CPA > S-PIA |
| Antag | PD115199 > DPCPX = 8PT | |
| A2b | Ag | NECA > 2ClAdo > R-PIA = CHA > S-PIA ≥ CV1808 ≥ CGS21680 |
| Antag | DPCPX = 8PT ≥ PD115199 | |
| A3 | Ag | APNEA > R-PIA = NECA > CGS21680 |
| Antag | None identified in this review |
Ag, agonist; Antag, antagonist; 2ClAdo, 2-chloroadenosine; CPA, N6-cyclopentyladenosine; CSC, 8-(3-chlorostyryl)caffeine; DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; DPMA, N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]adenosine; NECA, N-ethyl-carboxyl adenosine; R-PIA, R-phenyl-isopropyl adenosine; S-PIA, S-phenyl-isopropyl adenosine; 8PT, 1,3-dimethyl-8-phenylxanthine. See expanded Supplemental Tables S7 and S8, including references. Data adapted from Collis and Hourani (36).
The shark A0 receptor is dissimilar to A2a or A2b with respect to its low potency to the A2-preferring agonist NECA. Furthermore, response of A0 to S-PIA was unexpectedly high considering that S-PIA is not relatively potent at A1, A2a, or A2b receptors. Interestingly, the A2a-preferring agonist CGS21680 has relatively high potency in the shark A0 profile, whereas CV1808, also A2a preferring, has a much lower potency in the profile. The shark A0 profile bears little resemblance to that of A2b receptors, especially with respect to the position of CGS21680, NECA, R-PIA, and S-PIA. Therefore, the shark A0 resembles neither the A2a nor the A2b adenosine receptors (Table 3). Similarly, it is quite different from the known A3 adenosine receptor profiles (Table 3), with low potency of NECA (intermediate potency in A3), relatively high potency of CGS21680 and S-PIA (lower in the profile for A3), and the large difference in potency of R-PIA and NECA, which are of equal potency in the A3 adenosine receptor profiles.
A comparison of the antagonist profiles yields similar results. Although the shark A0 antagonist profile is reminiscent of the A1 antagonist functional profile (Table 3), compared with binding studies for the rat A1 (see Supplemental Table S7; https://doi.org/10.6084/m9.figshare.14248862.v1), the low potency of CGS15943 in the shark A0 profile is striking. The relative potency of CGS15943 is also incongruent with the rat A2a and rat A2b profiles (see Supplemental Table S8; https://doi.org/10.6084/m9.figshare.14248871.v1). Moreover, the potency order of DPCPX > PDl15199 in the shark A0 is reversed for A2a receptors and A2b receptors. It is clear that shark A0 also possess a unique antagonist profile.
In summary, the agonist and antagonist profiles do not allow the classification of this receptor into any known receptor subtype, providing functional evidence that A0 is a unique ADOR.
A0 Is an Extant ADOR Ancestor
Since the initial characterization of specialized adenosine receptor (ADOR) subtypes ∼40 yr ago, hundreds of ADORs have been identified. Many examples arise from genomic sequencing but have not been characterized structurally or functionally. Phylogenetic analysis shows that examples of specialized ADORs (i.e., A1, A2a, A2b, and A3) are apparent throughout the animal kingdom, including fish, amphibians, reptiles, birds, and mammals. However, a separate phylogenetic branch emerges from the analysis that contains ADORs in reptiles, amphibians, and fish, including the shark A0 ADOR we have characterized here. Shark A0 and the related members on the branch appear to be ancestral in nature. We draw this conclusion based on the following: 1) amino acid sequence similarity of A0 is roughly equally different from any specific specialized subtype (A1, A2a, A2b, and A3; see Supplemental Table S9; https://doi.org/10.6084/m9.figshare.14248868.v1); 2) the pharmacological profile of A0 is significantly different from the profiles of any subtype; 3) A0 belongs to a phylogenetic branch that is distinct from the branches formed by the other specialized subtypes; and 4) ADOR members of the ancestor branch contain structural features unique only to themselves, such as a minimal ECL2.
A1, A2a, A2b, and A3 appear to be specialized for several reasons: 1) they have distinct and consistent pharmacological profiles among their respective subbranches; 2) they prefer to couple to one G protein α-subunit (e.g., inhibitory Giα or stimulatory Gsα on adenylyl cyclase activity); and 3) they are abundant in birds and mammals, which are thought to have arrived later in evolution. The latter point highlights an interesting observation: specialized ADORs are prevalent in mammals, which arrived more recently in evolution timescales, but the ancestral receptors, including olfactory and nonolfactory ADORs, appear to have been lost in mammals.
Conclusions
We have cloned, expressed, and characterized an adenosine receptor (A0) from the dogfish shark (S. acanthias) that exhibits novel pharmacology compared to all known subtypes (A1, A2a, A2b, and A3). A0 and its closest relatives form a distinct ancestral phylogenetic branch from the known subtypes and do not appear to have representation in birds or mammals. All ancestral ADORs appear to have distinguishing structural elements, such as a minimally short extracellular loop 2. Shark A0 expression is strong in rectal gland, where it likely regulates chloride secretion, but its expression is weak in brain, allowing us to conclude that it is not olfactory in primary function. A nonolfactory ancestral branch of ADORs including A0 from elasmobranchs, amphibians, and reptiles is now apparent from the wealth of genomic sequencing data.
In contrast to the recently identified olfactory ADOR (A2c) found exclusively in fish (13), A0 was cloned from the shark rectal gland, a nonolfactory epithelial organ known for diverse GPCR-mediated regulation of chloride secretion via the CFTR chloride channel (37). Shark CFTR is >70% identical to human CFTR, and ADOR regulation of CFTR in sharks and mammals is well described (38, 39). Taken together, a long-standing evolutionary functional coupling between ADORs and CFTR-mediated chloride secretion in epithelial tissues is evident, and the elasmobranch A0 receptor emerges as a nonolfactory extant ancestor of specialized ADOR subtypes that most likely regulates CFTR in the shark.
GRANTS
This work was supported by a Howard Hughes Medical Institute grant to S.B. and internal University of Alabama funding to S.G.A. and by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK034208 and National Institute of Environmental Health Sciences Grant P30 ES003828 and National Science Foundation Grant DBI-0453391 to J.N.F.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B., G.H., and J.N.F. conceived and designed research; S.B. and G.H. performed experiments; S.B., G.H., C.L.M., S.G.A., and J.N.F. analyzed data; S.B., G.H., S.G.A., and J.N.F. interpreted results of experiments; S.B., G.H., and S.G.A. prepared figures; S.B., S.G.A., and J.N.F. drafted manuscript; S.G.A., and J.N.F. edited and revised manuscript; S.B., G.H., C.L.M., S.G.A., and J.N.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Stephanie Halene and Kristina Lübbe for important contributions toward the manuscript.
REFERENCES
- 1.Burnstock G. A Basis for Distinguishing Two Types of Purinergic Receptor. New York: Raven Press, 1978. [Google Scholar]
- 2.Fredholm B, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev 46: 143–156, 1994. [PMC free article] [PubMed] [Google Scholar]
- 3.Jockers R, Linder ME, Hohenegger M, Nanoff C, Bertin B, Strosberg AD, Marullo S, Freissmuth M. Species difference in the G protein selectivity of the human and bovine A1-adenosine receptor. J Biol Chem 269: 32077–32084, 1994. doi: 10.1016/S0021-9258(18)31603-X. [DOI] [PubMed] [Google Scholar]
- 4.Marala RB, Mustafa SJ. Direct evidence for the coupling of A2-adenosine receptor to stimulatory guanine nucleotide-binding-protein in bovine brain striatum. J Pharmacol Exp Ther 266: 294–300, 1993. [PubMed] [Google Scholar]
- 5.Olah ME. Identification of A2a adenosine receptor domains involved in selective coupling to Gs. Analysis of chimeric A1/A2a adenosine receptors. J Biol Chem 272: 337–344, 1997. doi: 10.1074/jbc.272.1.337. [DOI] [PubMed] [Google Scholar]
- 6.Palmer TM, Stiles GL. Adenosine receptors. Neuropharmacology 34: 683–694, 1995. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- 7.Sweeney MI, Dolphin AC. Adenosine A1 agonists and the Ca2+ channel agonist bay K 8644 produce a synergistic stimulation of the GTPase activity of Go in rat frontal cortical membranes. J Neurochem 64: 2034–2042, 1995. doi: 10.1046/j.1471-4159.1995.64052034.x. [DOI] [PubMed] [Google Scholar]
- 8.Burns RF, Lu GH, Pugsley TA. Adenosine receptor subtypes: binding studies. In: Topics and Perspectives in Adenosine Research, edited by Gerlach E, Becker BF.. Berlin: Springer-Verlag, 1987, p. 59–73. [Google Scholar]
- 9.Kelley G, Aassar O, and Forrest JJ. Endogenous adenosine is an autacoid feedback inhibitor f chloride transport in the shark rectal gland. J Clin Invest 88: 1933–1939, 1991. doi: 10.1172/JCI115517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest JN Jr, Aller SG, Wood SJ, Ratner MA, Forrest JK, Kelley GG. Cadmium disrupts the signal transduction pathway of both inhibitory and stimulatory receptors regulating chloride secretion in the shark rectal gland. J Exp Zool 279: 530–536, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Forrest JN Jr. Cellular and molecular biology of chloride secretion in the shark rectal gland: regulation by adenosine receptors. Kidney Int 49: 1557–1562, 1996. doi: 10.1038/ki.1996.224. [DOI] [PubMed] [Google Scholar]
- 12.Marshall J, Martin K, Picciotto M, Hockfield S, Nairn A, Kaczmarek L. Identification and localization of a dogfish homolog of human cystic fibrosis transmembrane conductance regulator. J Biol Chem 266: 22749–22754, 1991. doi: 10.1016/S0021-9258(18)54631-7. [DOI] [PubMed] [Google Scholar]
- 13.Wakisaka N, Miyasaka N, Koide T, Masuda M, Hiraki-Kajiyama T, Yoshihara Y. An adenosine receptor for olfaction in fish. Curr Biol 27: 1437–1447.e4, 2017. doi: 10.1016/j.cub.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680, 1994. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey AR, Potter SC, Finn RD, Lopez R. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47: W636–W641, 2019. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16: 276–277, 2000. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 17.Nordström KJ, Sällman Almén M, Edstam MM, Fredriksson R, Schiöth HB. Independent HHsearch, Needleman-Wunsch-based, and motif analyses reveal the overall hierarchy for most of the G protein-coupled receptor families. Mol Biol Evol 28: 2471–2480, 2011. doi: 10.1093/molbev/msr061. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874, 2016. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, and Tate CG. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474: 521–525, 2011. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Draper-Joyce CJ, Khoshouei M, Thal DM, Liang YL, Nguyen AT, Furness SG, Venugopal H, Baltos JA, Plitzko JM, Danev R, Baumeister W, May LT, Wootten D, Sexton PM, Glukhova A, Christopoulos A. Structure of the adenosine-bound human adenosine A1 receptor-Gi complex. Nature 558: 559–563, 2018. doi: 10.1038/s41586-018-0236-6. [DOI] [PubMed] [Google Scholar]
- 21.García-Nafría J, Lee Y, Bai X, Carpenter B, Tate CG. Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. Elife 7: e35946, 2018. doi: 10.7554/eLife.35946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebon G, Edwards PC, Leslie AG, and Tate CG. Molecular determinants of CGS21680 binding to the human adenosine A2A receptor. Mol Pharmacol 87: 907–915, 2015. doi: 10.1124/mol.114.097360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 45: D12–D17, 2017. doi: 10.1093/nar/gkw1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501, 2010. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221, 2010. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weininger D. SMILES, a chemical language and information system. J Chem Inf Comput Sci 28: 31–36, 1988. doi: 10.1021/ci00057a005. [DOI] [Google Scholar]
- 27.de Planque MR, Kruijtzer JA, Liskamp RM, Marsh D, Greathouse DV, Koeppe RE 2nd, de Kruijff B, Killian JA. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane alpha-helical peptides. J Biol Chem 274: 20839–20846, 1999. doi: 10.1074/jbc.274.30.20839. [DOI] [PubMed] [Google Scholar]
- 28.Dulhanty AM, Riordan JR. Phosphorylation by cAMP-dependent protein kinase causes a conformational change in the R domain of the cystic fibrosis transmembrane conductance regulator. Biochemistry 33: 4072–4079, 1994. doi: 10.1021/bi00179a036. [DOI] [PubMed] [Google Scholar]
- 29.Devor DC, Forrest JN , Jr, Suggs WK, Frizzell RA. cAMP-activated Cl- channels in primary cultures of spiny dogfish (Squalus acanthias) rectal gland. Am J Physiol Cell Physiol 268: C70–C79, 1995. doi: 10.1152/ajpcell.1995.268.1.C70. [DOI] [PubMed] [Google Scholar]
- 30.Daly JW, Jacobson KA, Ukena D. Adenosine receptors: development of selective agonists and antagonists. Prog Clin Biol Res 230: 41–63, 1987. [PubMed] [Google Scholar]
- 31.Artigas P, Al’aref SJ, Hobart EA, Díaz LF, Sakaguchi M, Straw S, Andersen OS. 2,3-Butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: the role of bilayer material properties. Mol Pharmacol 70: 2015–2026, 2006. doi: 10.1124/mol.106.026070. [DOI] [PubMed] [Google Scholar]
- 32.Stauffer BB, Cui G, Cottrill KA, Infield DT, McCarty NA. Bacterial sphingomyelinase is a state-dependent inhibitor of the cystic fibrosis transmembrane conductance regulator (CFTR). Sci Rep 7: 2931, 2017. doi: 10.1038/s41598-017-03103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science 332: 322–327, 2011. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birnbaum AK, Wotta DR, Law PY, Wilcox GL. Functional expression of adrenergic and opioid receptors in Xenopus oocytes: interaction between alpha 2- and beta 2-adrenergic receptors. Brain Res Mol Brain Res 28: 72–80, 1995. doi: 10.1016/0169-328X(94)00185-H. [DOI] [PubMed] [Google Scholar]
- 35.Uezono Y, Bradley J, Min C, McCarty NA, Quick M, Riordan JR, Chavkin C, Zinn K, Lester HA, Davidson N. Receptors that couple to 2 classes of G proteins increase cAMP and activate CFTR expressed in Xenopus oocytes. Recept Channels 1: 233–241, 1993. [PubMed] [Google Scholar]
- 36.Collis MG, Hourani SM. Adenosine receptor subtypes. Trends Pharmacol Sci 14: 360–366, 1993. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- 37.Forrest JN Jr. The shark rectal gland model: a champion of receptor mediated chloride secretion through Cftr. Trans Am Clin Climatol Assoc 127: 162–175, 2016. [PMC free article] [PubMed] [Google Scholar]
- 38.Clancy JP, Ruiz FE, Sorscher EJ. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am J Physiol Cell Physiol 276: C361–C369, 1999. doi: 10.1152/ajpcell.1999.276.2.C361. [DOI] [PubMed] [Google Scholar]
- 39.Kelley GG, Poeschla EM, Barron HV, and Forrest JN Jr.. A1 adenosine receptors inhibit chloride transport in the shark rectal gland. Dissociation of inhibition and cyclic AMP. J Clin Invest 85: 1629–1636, 1990. doi: 10.1172/JCI114614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791, 1985. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]