Abstract
During the recent years, immune checkpoint-based therapy has proven highly effective in microsatellite instable (MSI) solid tumors irrespective of organ site. MSI tumors are associated with a defective mismatch repair (MMR) system and a highly immune-infiltrative tumor microenvironment—both characteristics of Lynch syndrome. Lynch syndrome is a multi-tumor syndrome that not only confers a high risk of colorectal and endometrial cancer but also cancer in, eg the upper urinary tract, ovaries, and small bowel. Since the genetic predisposition for Lynch syndrome are pathogenic variants in one of the four MMR genes, MLH1, MSH2, MSH6 or PMS2, most of the Lynch syndrome cancers show MMR deficiency, MSI, and activation of the immune response system. Hence, Lynch syndrome cancer patients may be optimal candidates for immune checkpoint-based therapies. However, molecular differences have been described between sporadic MSI tumors (developed due to MLH1 promoter hypermethylation) and Lynch syndrome tumors, which may result in different treatment responses. Furthermore, the response profile of the rare Lynch syndrome cases may be masked by the more frequent cases of sporadic MSI tumors in large clinical trials. With this review, we systematically collected response data on Lynch syndrome patients treated with FDA- and EMA-approved immune checkpoint-based drugs (pembrolizumab, atezolizumab, durvalumab, avelumab, ipilimumab, and nivolumab) to elucidate the objective response rate and progression-free survival of cancer in Lynch syndrome patients. Herein, we report Lynch syndrome-related objective response rates between 46 and 71% for colorectal cancer and 14–100% for noncolorectal cancer in unselected cohorts as well as an overview of the Lynch syndrome case reports. To date, no difference in the response rates has been reported between Lynch syndrome and sporadic MSI cancer patients.
Keywords: hereditary colorectal cancer, HNPCC, Lynch syndrome, endometrial cancer, germline mismatch repair defect
Introduction
Lynch syndrome, caused by germline pathogenic variants in the mismatch repair (MMR) genes, MLH1, PMS2, MSH2, and MSH6, is the most common type of hereditary colorectal cancer. The syndrome is, however, also associated with a series of other cancer types, including endometrial cancer, ovarian cancer, urothelial tract cancer, small bowel cancer, gastric cancer, brain tumor, and sebaceous skin tumor.1–3 Lynch syndrome-associated tumors develop through inactivation of the second MMR allele leading to biallelic loss of MMR protein expression and hence deficient MMR (dMMR). Tumors with d-MMR have lost the ability to repair DNA errors introduced during replication and these tumors often present with high levels of mutation, reflected as microsatellite instability (MSI).4 The increased number of mutations are often presented as neoantigens that recruit and activate the host immune cells.5 Further tumor progression can be facilitated through immunoediting like T cell exhaustion, eg, by targeting immune checkpoints like the programmed death 1 (PD-1) or cytotoxic T lymphocyte antigen 4 (CTLA-4) receptors.6 Inhibiting these checkpoint blockades may reactivate the anti-tumorigenic T cells.
In 2017, MSI or dMMR were approved as pan-cancer biomarkers for the anti-PD-1 checkpoint therapy pembrolizumab by the American Food and Drug Administration (FDA).7 The approval was based on multicenter, multicohort, single-arm trials, some of which included Lynch syndrome data.4,8 Shortly after, nivolumab was approved by the FDA in 2017 for MSI colorectal cancer and in 2018 in combination with ipilimumab based on the CheckMate-142 study.7,8 Pembrolizumab has not received a tissue-agnostic indication by the European Medicines Agency (EMA) and despite comparable mechanism of action, the other immune checkpoint inhibitors, like atezolizumab, durvalumab, and avelumab, have neither been approved by the FDA nor the EMA in an MSI/dMMR pan-cancer setting.
Based on the expected tumor-agnostic effects, many Lynch syndrome MSI tumors may have been enrolled in clinical trials using these drugs. However, Lynch syndrome may only be the causative reason for tumor development in a smaller subset of all MSI tumors (3–5%),9–12 hence, their specific response rate may be masked by the responses of sporadic MSI cancers, that may differ molecularly from Lynch syndrome tumors.13–16 Here, we review large clinical trials that have presented data separately for Lynch syndrome and sporadic MSI cancer patients to elucidate the clinical benefit from immune checkpoint-based therapy in Lynch syndrome. Furthermore, we summarize current data on Lynch syndrome case reports, although these may be publication biased.
Materials and Methods
Systematic Literature Search
A systematic literature search was performed to identify studies with Lynch syndrome specific treatment data to evaluate the clinical benefit of immune checkpoint-based therapies in this cohort. All published studies including patients with Lynch syndrome-associated cancer, who had been treated with one or more of the FDA- and EMA-approved checkpoint-based immunotherapies targeting CTLA-4 (ipilimumab), PD-1 (pembrolizumab and nivolumab), or PD-L1 (atezolizumab, avelumab, and durvalumab) and where data was available on clinical outcomes, were considered eligible for this review.
The search strategy was developed in collaboration with a research librarian at the Medical Library, Aalborg University Hospital, Denmark. The search string was assembled from MeSH and non-MeSH terms included in the two categories: a cancer subtype specific domain [“Lynch syndrome” OR “hereditary MSI” OR “hereditary MMR-deficiency” OR “colorectal neoplasm, hereditary nonpolyposis”] and a treatment domain [“Ipilimumab” OR “Nivolumab” OR “Atezolizumab” OR “Durvalumab” OR “Pembrolizumab” OR “Avelumab”] combined with a Boolean logical “AND”. In PubMed, all search terms were coined as MESH terms, as SUPPLEMENTARY CONCEPT, or as TEXT WORD (combined with “OR”) securing capture of yet unindexed articles. In Embase, the search terms were used as EMTREE terms and TEXT WORDS. In Web of Science, the search terms were used as TOPIC. In the Cochrane Library, the search terms were used as MeSH terms and as Title, abstract, and keywords terms. No constraints related to language or publication type were applied—except for exclusion of conference abstracts on Embase. Detailed search terms are available from the authors upon request.
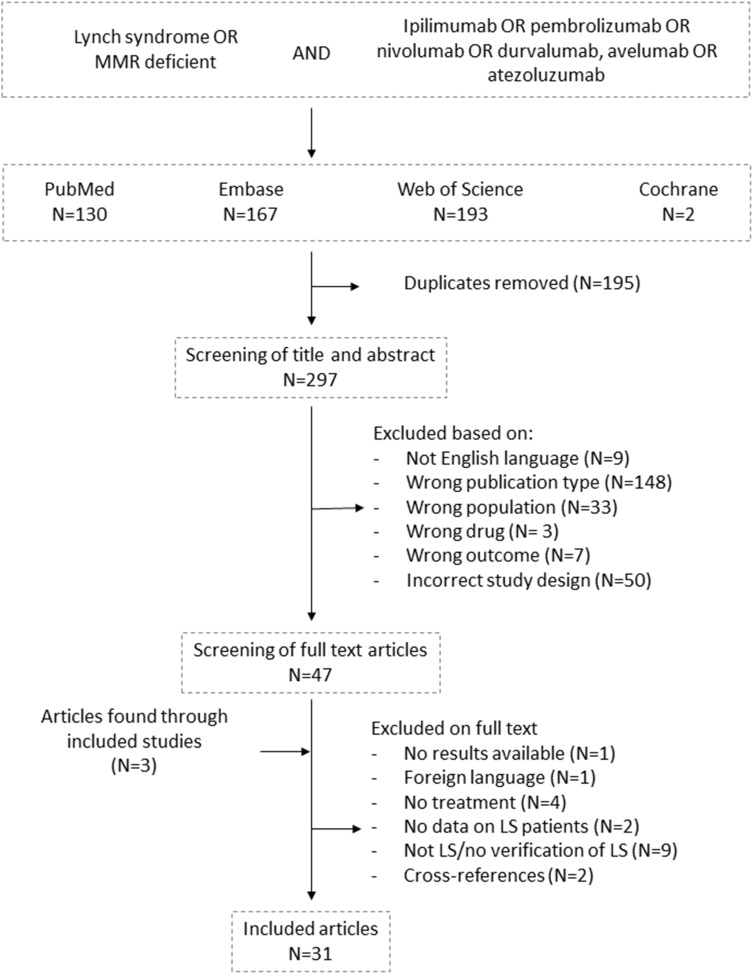
The final search in the four databases was conducted on November 3, 2020. The combined results of the analog searches in PubMed, Embase, Web of Science, and Cochrane Library were imported into the Rayyan QCRI application (Qatar Computing Research Institute, https://libraryguides.mcgill.ca/rayyan, last updated on October 7, 2020).17 Herein, all the items identified from the four databases were imported and the software identified 196 duplicates, which were manually checked before removal (N=195). Studies identified through reference lists from the included studies (N=3)8,18,19 were included if they were scored as relevant (Figure 1).
Figure 1.
Flowchart showing the systematic literature search and screening procedure following the preferred reporting items for systematic reviews and meta-analyses (PRISMA). Data extraction was performed using modified criteria based on the guidelines given by the Cochrane Collaboration for the 31 studies included.
Data Extraction
All studies identified were independently reviewed by four authors (IB, LHJ, MR, or CT) with at least two reviewers per item analyzing inclusion/exclusion criteria defining the study population. Whenever discrepancy was met, consensus was reached involving a third reviewer.
Study eligibility was performed following the preferred reporting items for systematic reviews and meta-analyses (PRISMA), while data extraction was performed using modified criteria based on the guidelines given by the Cochrane Collaboration.20 Data were extracted regarding study population (eg, age, sex, race, and MMR germline mutation), tumor type (eg, location, organ, dMMR/MSI status, and stage), treatment regimen (eg, pharmaceutical drug used, line of treatment, combinational treatment, and period), and outcome (eg, objective response rate, overall survival, progression-free survival, and alternative endpoints).
Since this was a scoping review, quality assessment was not conducted. Publication bias was considered, as case reports may only be reported when interesting results are available. Likewise, funding sources were extracted to assess any conflicts of interest. All studies were requested to be on Homo sapiens and written in English and published as original articles unless sufficient data could be extracted from an English abstract (N=1).21 Redundant data, which was published in overlapping studies, motivated exclusion (N=2) or merging of the studies (N=4) (Figure 1).18,22–24 When information on overlapping data was missing, data from the two studies was presented separately according to the outcome in focus.19,25 One study included two cases with two different tumor types and responses and was for clarity depicted as two separate case reports in Table 2.26
Table 2.
Study Characteristics and Response Data from Included Case Reports
| Study | Country | Cancer Center | Study Design | Cancer Type | Treatment | Line of Therapy | Response | Criteria | Progression-free survival | MMR Germline Mutation | Details/Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan et al. 202037 | USA | Saint Michael’s Medical Center | Case report (N=1) | Colon cancer | Pembrolizumab | 3. line | PD (N=1) | RECIST | PFS: 1 month | MSH6 mutation carrier | 1 pt treated with PD after 1 month (1 cycle) |
| Salman et al, 201836 | Chile | Arturo Lopez Pérez | Case report (N=1) | Colorectal cancer | Pembrolizumab | 2. line | SD (N=1) | RECIST | PFS: 7 months | MSH2/EPCAM mutation carrier | 1 pt treated with SD at 2.8 months (mean of 2 metastases) and PD at 7 months (mean of 2 metastases) |
| Keating et al, 201935 | USA | Roger Williams Medical Center | Case report (N=1) | Metastatic colorectal cancer | Pembrolizumab | 4. line | SD (N=1) | Clinical benefit | PFS: 42 months | MSH2 mutation carrier | 1 pt treated with SD at 42 months |
| Demisse et al, 202028 | USA | UC Davis Comprehensive Cancer Center | Case report (N=1) | Advanced rectal cancer | Pembrolizumab | 1. line | PR (N=1) | RECIST | PFS: 10 months | Unspecified clinical history, no MMR gene test available | 1 pt treated with pathologic CR after surgery at 10 months |
| Zhang et al, 201929 | China | The Sixth Affiliated Hospital, Sun Yat-sen University | Case report (N=2) | Locally advanced rectal cancer | Nivolumab | 1. line (pt #1) and 2. line (pts #2) | CR (N=2) | Pathologic complete response | PFS: Mean 12 months | Amsterdam I, no MMR gene test available | 2 pts treated with pathological and clinical CR at 1 year |
| Patel et al, 201826 | USA | Stanford Cancer University | Case report (N=1) | Rectal cancer | Pembrolizumab | 2. line | PD (N=1) | Tumor increase | PFS: 1.4 months | Carrier of two MSH6 VUS | 1 pt treated tumor progression after 1.4 month |
| Feng et al, 201938 | China | Bejing National Cancer Center | Case report (N=1) |
Ureter + colorectal cancer | Pembrolizumab | 4. line | PR (N=1) PD (N=1) |
RECIST | PFS: 7 months | MSH2 mutation carrier | 1 pt treated: PR in ureter at 7 months and PD in CRC |
| Ghatalia et al, 201718 and Winer et al, 201924 |
USA | Brown University | Case report (N=1) | Metastatic colorectal cancer + bilateral ureter cancer + bladder cancer | Pembrolizumab Atezolizumab Pembrolizumab Nivolumab + Ipilimumab Nivolumab | 3. line for CRC and 1 line for urothelial cancers | SD (CRC) SD (N=3 urothelial cancers) | Tumor decrease | PFS: 26 months | MSH2 mutation carrier | 1 pt with 4 cancers (3 urothelial tumors and liver metastases from a previous CRC) treated with different regimens due to progression three times—tumor decrease of urothelial tumors and disease control for CRC metastasis at 26 months |
| Musher and Rahal, 201934 | USA | Baylor College of Medicine | Case report (N=1) | Colorectal cancer (N=1) and intrahepatic cholangiocarcinoma (N=1) | Pembrolizumab | 1. line | PR (N=2) | RECIST | PFS: 18 months | MLH1 mutation carrier | 1 pt with 2 primary tumors—both responsive at 16 months |
| Yang et al, 201948 | USA | Virginia Commonwealth University | Case report (N=1) | Glioblastoma multiforme | Pembrolizumab | 2. line | SD (N=1) | RECIST | PFS: 12 months | MSH6 mutation carrier | 1 patient treated with SD at 12 months |
| Bouffet et al, 201623 and Larouche et al, 201822 | Canada | The hospital for sick children and Montreal Children’s hospital | Case report (N=2) | Glioblastoma multiforme | Nivolumab Nivolumab Nivolumab + ipilimumab Nivolumab | 2. line(pt #1) and 1. line (pt #2) | PR (N=1) (pt #1) CR (N=1) (pt #2) | RECIST | PFS: 11 months (pt #1) and 30 months (pt #2) | Homozygous biallelic PMS2 mutations | Siblings with biallelic MMR mutations—one had PR at 1,4 months and PD at 11 months and the other had PR at 9 months and CR at 30 months |
| Kawashima et al, 201939 | Japan | Japanese Foundation for Cancer research | Case report (N=1) | Lung cancer | Nivolumab | 2. line | SD (N=1) | Tumor decrease | PFS: 2 months | MSH2 mutation carrier | 1 pt treated with tumor decrease at 2 months |
| Masuzawa et al, 202040 | Japan | Keio University School of Medicine | Case report (N=1) | metastatic non-small-cell lung cancer | Nivolumab | 3. line | PR (N=1) | RECIST | PFS: 27 months | MLH1 mutation carrier | 1 pt treated with response at 7 months |
| Nevgi et al, 202041 | Australia | University of Melbourne | Case report (N=1) | Adrenocortical carcinoma | Nivolumab + ipilimumab | 2. line | PR (N=1) | Tumor decrease | PFS: 23 months | MSH2 mutation carrier | 1 pt treated with PR of liver metastases at 23 months |
| Casey et al, 201842 | UK | Cambridge University | Case report (N=1) | Adrenocortical carcinoma | Pembrolizumab | 2. line | PD (N=1) | RECIST | PFS: 2.8 months | MSH2 mutation carrier | 1 pt with recurrence and liver metastases treated with progression at 2.8 months |
| Mancuso et al, 202043 | Canada | Cancer Prevention Centre | Case report (N=1) | Muscle invasive bladder cancer | Pembrolizumab | 2. line | CR (N=1) | RECIST | PFS: 22 months | MSH2 mutation carrier | 1 pt treated with CR at 22 months |
| Graham et al, 202046 | USA | University of Washington and University of Michigan | Case report (N=2) | Metastatic prostate cancer | Pembrolizumab | 2. line | SD (N=2) | PSA decrease | PFS: Mean 12,5 months | MSH2 mutation carriers | 2 pts treated with decreasing PSA and clinical response at 10 and 15 months |
| Patil and Khan, 202047 | USA | Henry Ford Health System | Case report (N=1) | Pancreatic cancer | Pembrolizumab | 3. line | PR (N=1) | Tumor decrease | PFS: 11 months | MLH1 mutation carrier | 1 pt treated with PR in liver metastases at 11 months |
| Hu et al, 201849 | USA | Memorial Sloan Kettering Cancer Center | Case report (N=1) | Pancreatic cancer | Anti-PD-L1-therapy | 2. line | PD (N=1) | Clinical benefit | PFS: 22 months | MLH1 mutation carrier | 1 pt treated with PD at 22 months. Immunotherapy continued despite gynecological metastases |
| Ngo et al, 202044 | USA | University of Louisville School of Medicine | Case report (N=1) | Pancreatic cancer | Pembrolizumab | 3. line | PR (N=1) | Tumor decrease | PFS: 35 months | MSH2 mutation carrier | 1 pt treated with two liver metastases—1 responded all 35 months and the other progressed after 8 months but remained decreased after radiation |
| Patel et al, 201826 | USA | Stanford Cancer Institute | Case report (N=1) | Pancreatic cancer | Pembrolizumab | 2. line | SD (N=1) | Tumor decrease | PFS: 11.9 months | MLH1 VUS carrier | 1 pt treated with pancreatic cancer and 3 vetebral metastases—tumor decrease after 11.9 months |
| Tlemsani et al, 202045 | France | Hôpital Cochin | Case report (N=1) | Rhabdomyosarcoma | Nivolumab | 2. line | CR (N=1) | RECIST | PFS: 12 months | MLH1 mutation carrier | 1 pt treated with lung metastases completely removed at 12 months |
| Carvalho et al, 202027 | Brazil | University of Sao Paulo | Case report (N=1) | Endometrial cancer | Pembrolizumab | 1. line | PR (N=1) | RECIST | PFS: 24 months | MLH1 VUS carrier | 1 pt treated after hysterectomy with lymph node metastases—PR at 2.1 month and sustained for 24 months |
Abbreviations: ORR, objective response rate; PFS, progression-free survival; CRC, colorectal cancer; MSI, microsatellite instable; MSS, microsatellite stable; LS, Lynch syndrome; VUS, variant of unknown significance; NA, not available; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PSA, prostate specific antigen; pt/pts, patient/patients.
As this review included all types of MSI or dMMR Lynch syndrome-associated cancers (irrespective of organ or tumor stage) with all lines of therapy, and primarily case reports, meta-analysis was not considered appropriate. Instead, data was grouped by study design with clinical trials in unselected MSI/dMMR cancer cohorts scored as a high level of evidence, while case reports were considered to have a lower level of evidence and a higher level of bias.
Definitions
Lynch syndrome diagnostics were based on individuals with pathogenic or likely pathogenic variants in one of the four MMR genes: MLH1, PMS2, MSH2/EPCAM, and MSH6 found by germline DNA sequencing (18 case studies and three cohort studies). Since some of the cohort studies included few variants of unknown significance (VUS) as causative for Lynch syndrome25 or included Lynch syndrome families based on individuals with dMMR/MSI tumors in families with a cancer history,8,21 we chose to include two case studies with VUS26,27 and two case studies with clinical Lynch syndrome diagnostics, but with unknown germline MMR gene variant.28,29 Lynch syndrome individuals with biallelic MMR variants (also referred to as constitutional MMR deficiency (CMMR-D) syndrome) were also included in this study, since these tumors show the same molecular phenotype as monoallelic Lynch syndrome tumors (N=1). There were no selection criteria regarding tumor type and no restrictions in period.
Outcome Data
The primary endpoints were objective response rate (ORR), progression-free survival (PFS), and overall survival (OS). ORR was measured as the best response during treatment or alternating immune checkpoint-based therapies using the response evaluation criteria in solid tumors (RECIST) guidelines, with partial response (PR) and complete response (CR) categorized as response. In cases where the RECIST criteria were not used, pathological complete response was classified as CR, while nonqualified tumor decrease was categorized as stable disease (SD). Disease control rates were calculated as all CR, PR and SD divided by the total number of treated and evaluable patients. PFS was measured in months from first dose of immune checkpoint-based therapy to tumor progression or end of follow-up. In case of alternating immunotherapeutic regimens, PFS were defined as the time from first dose of immunotherapy to end of the last regimen of immunotherapy caused by disease progression. OS was measured in months from the first dose of immune checkpoint-based therapy to death or end of follow-up. Inclusion of alternative endpoints was motivated when ORR data was not available, as these may translate into a clinically meaningful benefit in PFS and OS. Decreasing prostate specific antigen (PSA) level was categorized as disease control for one case according to the prostate cancer clinical trial working group (PCWG3) guideline.30 Whenever possible, the analyses were based on original raw data and Lynch syndrome cases were sought and extracted from larger studies with separate endpoint data from the sporadic cancers.
Results
Literature Review
In total, 492 studies were identified (two from Cochrane Library, 167 from Embase, 130 from PubMed, and 193 from Web of Science) (Figure 1). After removal of 195 duplicates, 297 studies were reviewed on title and abstract level following the PRISMA guidelines. Hereafter, 47 studies were screened on full text level and three additional studies were added to the search based on the references of the reviewed publications.8,18,19 In total, 31 articles were included for this review, six of which included overlapping cases: two cases were described in two case reports18,22–24 and one clinical trial was updated with different data presented in two papers.19,25
Cohort Studies
Colorectal Cancer
Large cohort studies of consecutive unselected patients were considered to be less biased by publication and were initially investigated for specific data on Lynch syndrome cases. Seven studies were identified and presented in Table 1. Three studies investigated the response rates in colorectal cancer and found that Lynch syndrome patients had an ORR between 46 and 71% after immune checkpoint-based therapy.8,19 Two of the articles presented data from the MK-3475 study covering three to six centers in the US (NCT01876511), in which Lynch syndrome-associated ORR were calculated in the study from Le et al,25 while individual Lynch syndrome cases could be extracted in the study from Le et al.19,25 In Le et al,19 eight Lynch syndrome patients with colorectal cancer were treated with pembrolizumab, of which two showed PR giving an ORR of 25% and six showed SD reaching disease control in 100% of the patients. In the updated paper from 2017, the Lynch syndrome-associated ORR had increased to 46%.25 The percentage of Lynch syndrome cases presenting with disease control was not specified in here, but 23% of the entire cohort (covering 86 patients) showed SD reaching disease control in 77% of the unselected MSI/dMMR cohort. Furthermore, mean time to response was 21 weeks and mean time for complete response was 42 weeks.25 Corresponding data from the sporadic MSI/dMMR cohort were only specified in the paper from 2015 with two colorectal cancer patients both showing PR (ORR=100%).19 Though similar data was missing in the updated paper from 201725 statistical analyses did not identify significant difference in ORR between Lynch syndrome and sporadic MSI/dMMR colorectal cancers (ORR of the entire study cohort was 52%). The CheckMate-142 study presented by Overman et al, which is a multicenter study covering 28 centers in eight countries, included 35 Lynch syndrome patients of which 25 patients showed objective response (ORR=71%).8 In comparison, sporadic (non-Lynch syndrome) MSI/dMMR colorectal cancers showed an ORR of 48%. No comparison was made between the two groups and lack of individual data restrained further analyses. For both clinical trials, sufficient follow-up to calculate PFS and OS was not reached, and not specified for Lynch syndrome and sporadic MSI cancers individually.
Table 1.
Study Characteristics and Response Data from Included Cohort Studies
| Study | Country | Cancer Center | Study Design | LS Cases | Total Cohort | Cancer Type | Treatment | ORR (LS) | PFS (LS) | ORR (Spor. MSI) | MMR Germline Mutation | Details/Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Le et al, 201519 | USA | Johns Hopkins University | Prospective, multicenter study (3 centers included) | N=13 | N=41 (MSS and MSI tumors) | Colorectal cancer and noncolorectal cancer | Pembrolizumab | CRC: 25% NonCRC: 33% Total: 27% | PFS: none | CRC: 100% NonCRC: 100% | CRC: MLH1 (N=3), MSH2 (N=3), unknown (N=2), non-RC: MSH2 (N=3) | Phase II trial incl. 13 LS pts. Response evaluable for 11 LS pts and 4 sporadic MSI cases. LS CRC: 2 PR, 5 SD, 1 PD; LS nonCRC: 1 PR, 2 PD; sporadic CRC: 2 PR; sporadic nonCRC: 2 PR |
| Le et al, 201725 | USA | Johns Hopkins University | Prospective, multicenter study (6 centers included) | N=39 | N=86 (only MSI tumors) | Colorectal cancer and noncolorectal cancer | Pembrolizumab | CRC: 46% NonCRC: 59% | Not specified for LS | Not specified | 3 unknowns, CRC: MLH1 (N=6), PMS2 (N=2), MSH2 (N=9), MSH6 (N=1), VUS (N=2); nonCRC: MLH1 (N=3), MSH2 (N=7), MSH6 (N=3), VUS (N=3) | Phase II trials incl. 39 LS pts. ORR are calculated for all 39 LS, though germline mutation was only reported for 36 cases including 5 VUS cases. Average time to response for the entire study cohort was 21 weeks. No data available for sporadic MSI alone and no specific PFS or responses for LS alone. ORR for the total cohorts: CRC: 52%; nonCRC: 54% |
| Overman et al, 20188 | USA | MD Andersen Cancer Center | Prospective, multicenter study (28 centers) | N=35 | N=119 (MSI or dMMR tumors) | Metastatic colorectal cancer | Nivolumab + ipilimumab | 71% | Not specified for LS | 48% | 35 LS cases (clinical history, germline testing not mandatory) | Phase II trial incl. 35 LS pts (25 responders), 31 pts with sporadic MSI tumors (15 responders), and 53 pts with unknown LS status (25 responders). |
| Hu et al, 201833 | USA | Memorial Sloan Kettering Cancer Center | Retrospective cohort study | N=7 | N=833 (MSS and MSI tumors) | Pancreatic ductal adenocarcinoma | Anti-PD-1/anti-PD-L1 | 60% | PFS: mean 12,5 months (of 4 pts) | NA | MLH1 (N=1), PMS2 (N=1), MSH2 (N=2), MSH6 (N=1) | 833 consecutively collected pancreatic cancers investigated for dMMR/MSI—7 cases with MSI (all LS), of which 5 were treated with evaluable response: 1 CR, 2 PR, 1 SD and 1 PD |
| Raj et al, 201932 | USA | Memorial Sloan Kettering Cancer Center | Prospective, single center study | N=2 | N=39 (MSS and MSI tumors) | Adrenocortical carcinoma | Pembrolizumab | 100% | PFS: mean 27.5 months | 0% | MSH2 (N=1) MSH6 (N=1) | Phase II trial with 39 pts not selected for MSI/dMMR. 6 tumors showed MSI/dMMR, incl. 2 LS pts with PR response. 4 pts with sporadic MSI tumors: 2 SD, 2 PD |
| Abida et al, 201931 | USA | Memorial Sloan Kettering Cancer Center | Prospective, single center study | N=2 | N=1033 (MSS and MSI tumors) | Prostate cancer | Anti-PD1/PDL1 therapy | 50% | Mean PFS: 5.6 months | 50% | MSH2 (N=1) MSH6 (N=1) | 1033 consecutively collected prostate cancer patients of which 11 pts were treated, 8 pts completed/evaluated. Sporadic MSI: 3 PR + 1 SD + 2 PD; LS: 1 PR + 1 PD. Only MSI/dMMR tumors were evaluated for response to therapy. One MSS tumor in a patient with an MSH6 germline variant was not treated. |
| Bari et al, 202021 | USA | H. Lee Moffitt Cancer Center | Retrospective, single center study | N=22 | N=194 (only LS tumors—MSS and MSI) | Different cancer types | Immune checkpoint inhibitors (80% pembrolizumab) | 14% | NA | NA | 262 LS cases (mutated genes not reported) | Retrospectively collected cohort focused on LS cases identified 22 pts treated, 1 PD after 1 month, 21 pts evaluated: 2 CR, 1 PR, 13 SD and 5 PD |
Abbreviations: ORR, objective response rate; PFS, progression-free survival; CRC, colorectal cancer; MSI, microsatellite instable; MSS, microsatellite stable; LS, Lynch syndrome; NA, not available; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; pts, patients.
Noncolorectal Cancer
Investigating noncolorectal cancers, we found four studies with specified Lynch syndrome response data, three of which published from the Memorial Sloan Kettering Cancer Center, New York, USA.21,31–33 Hu et al identified five Lynch syndrome patients in a retrospective cohort of 833 consecutively collected patients with pancreatic ductal carcinomas, of which three responded (ORR=60%) to anti-PD1/anti-PD-L1 drugs—one with a mixed response involving a complete response followed by metastasis eight months later.33 The mean PFS of four cases with available data was 12.5 months, although the responses appeared after 22 and 24 months. No sporadic cases with an MSI phenotype were identified nor treated with immunotherapy. Abida et al, 2019 identified two Lynch syndrome patients among a prospectively collected cohort of 1033 patients with prostate cancer, of which one showed PR and the other had PD after anti-PD-1/anti-PD-L1 therapy.31 Likewise, Raj et al found two Lynch syndrome patients in a prospective phase II study of 39 patients with adrenocortical carcinomas; both showed PR and a mean PFS of 27.5 months when treated with pembrolizumab.32 In 2015, the MK-3475 study from Johns Hopkins University presented three Lynch syndrome-associated noncolorectal cancer patients with an ORR of 33%.19 In the updated cohort from 2017, this increased to 59%.25
At the American Society of Clinical Oncology (ASCO) in May 2020, Bari et al, presented a large retrospective study design, in which they aimed to describe the response rates in a Lynch syndrome cohort irrespective of tumor type.21 They identified 194 Lynch syndrome patients with different types of solid tumors, of which 22 had received treatment with immune checkpoint-based therapies. Of the 22 patients, two showed CR, one had PR, 13 had SD, and six showed PD giving an ORR of 14% and a disease control rate of 73%. In contrast to the other studies, treatment responses were measured irrespective of MSI status and showed continued response after nine months of treatment in one (out of three) microsatellite stable (MSS) Lynch syndrome tumors.21 Detailed PFS and OS were not calculated but 15 out of 22 patients showed continuous disease control or complete remission at 48 months of follow-up.
In summary, these studies identified 107 Lynch syndrome cancer patients and individual response data could be collected from 77 cases (excluding Le et al).25 Thirty-six of these cases responded to treatment, giving a summarized ORR of 47% (63% for CRC and 29% for noncolorectal cancer). In comparison, summarized ORR for sporadic MSI colorectal cancer patients were 55% (17 out of 33) and 42% for sporadic MSI noncolorectal cancer patients (five out of 12). PFS was only reported in three studies (all regarding noncolorectal cancers) and summarized to 15.2 months for Lynch syndrome cancer patients.
Case Studies
Study Eligibility/Data Quality
Next, we reviewed the Lynch syndrome cancer case reports. Twenty-four case reports covering 26 patients (three with multiple cancers) presented treatment response. Two cases were presented in two papers each, but since data was overlapping the studies were merged to two case reports.18,22–24 In contrast, one study presented to cases with two different types of MSI cancers and was for simplicity presented as two separate cases (Table 2).26 Cancer center, country, MMR gene affected, immune checkpoint-based treatment, therapeutic setting, and outcome results for the 26 unique cases are presented in Table 2. Two of the studies did not present data on the MMR gene test analyses nor the MMR variant identified in the patients, and it remains uncertain how Lynch syndrome was diagnosed in these individuals.28,29 The cases were identified in USA (N=13), Canada (N=3), China (N=3), Japan (N=2), France (N=1), Chile (N=1), UK (N=1), Brazil (N=1), and Australia (N=1). The responses were evaluated using the RECIST criteria (N=13), pathologic complete response with no viable tumor cells after surgery (N=2), tumor decrease with unknown percentage of decrease (N=7), clinical response with proceeded treatment due to no or little tumor progression (N=2), and as a decrease in prostate specific antigen (PSA) (N=2).
The case reports either presented cases with clinical responses/disease control (N=21), or disease progression (N=4), or cases with two primary tumors, of which one progressed and the other responded (N=1). The majority of studies presenting positive treatment responses may indicate publication bias as these cases are more likely to be published.
Colorectal Cancer
In summary, 10 cases were included with a colorectal cancer (three with multiple cancers in other organs as well) and one patient with liver metastases from a previously removed colorectal cancer.18,24,26,28,29,34–38 Two cases obtained CR, two showed PR, three had SD, and three showed PD giving an ORR of 40% and disease control rate of 70%. All the patients, who showed positive response, were given immunotherapy as first (N=3) or second (N=1) line treatment. The mean PFS was 14.9 months with a mean time to response of 13.3 months. As only one patient died one month after treatment end,18,24 PFS was equal to OS in these cases.
Noncolorectal Cancer
Investigating noncolorectal cancers, 21 MSI/dMMR Lynch syndrome-associated solid tumors developed (three glioblastoma multiforme, four pancreatic cancers, three ureteral cancers, two lung cancers, two prostate cancers, two adrenocortical carcinomas, two bladder cancers, one endometrial cancer, one rhabdomyosarcoma, and one intrahepatic cholangiocarcinoma).18,22–24,26,34,38–49 Although it was not stated, it was considered highly likely that the pancreatic cancer showing PD at 22 months by Hu et al,49 was included in the large pancreatic cancer cohort study.33,49 However, in order to reduce publication bias, this case was included in the following summary. Among the 21 noncolorectal cancers, three showed CR, eight showed PR, eight had SD (two of which had decreased PSA levels as the only response data), and two experienced PD resulting in a summarized ORR of 53% and disease control in 90%. Mean PFS was 14.1 months with a mean time to positive response of 18.3 months. Again, OS was not reported for the majority of the studies as follow-up was ended at tumor progression or with PFS.
Multiple Lines of Immunotherapy
Irrespective of the cancer type, treatment lines of which the immune checkpoint-based therapy was introduced varied across the included studies with seven tumors receiving immunotherapy in first line, 14 in second line, seven in third line, and three in fourth line. Two cases received different regimens of immunotherapy due to local progression or adverse events with the selected treatment. A male 64-year-old Lynch syndrome carrier presenting with three urothelial cancers (bladder and bilateral ureter) and a liver metastasis 10 years after previously removed colorectal cancer was treated with pembrolizumab at the indication of dMMR in one of the urothelial tumors. At nine months, the patient was treated with atezolizumab based on progression of the urothelial cancers. The patient obtained eight months of disease control before progression of the liver metastasis. Pembrolizumab was reintroduced for three months until progression and the patient switched to combination therapy with nivolumab and ipilimumab resulting in tumor decrease after two months. The patient continued therapy with nivolumab alone with disease control for seven months. At this time, his bilirubin levels increased probably attributed to immunotherapy-related adverse events and the patient declined continued therapy and passed away one month later.18,24
The second case report presented a boy at 3.5 years with homozygous biallelic PMS2 pathogenic variants with a glioblastoma multiforme tumor in the frontal cortex that was surgically removed. Ten months later multinodular recurrence was observed, and he was treated with nivolumab with initial response. Six months later, a new nodal glioblastoma reoccurred at the primary surgical site and ipilimumab was added to the nivolumab treatment for four doses. Significant response was observed after three months (nine months from first immunotherapy dose) and complete response was reached after one year. The patient continued on nivolumab for maintenance and magnetic resonance imaging confirmed CR 30 months from first glioblastoma recurrence and first immunotherapeutic dose.
Discussion
Herein, we summarized the clinical responses among Lynch syndrome cancer patients treated with FDA- and EMA-approved checkpoint-based immune therapies. We identified 31 studies including 133 unique Lynch syndrome cancer patients. For Lynch syndrome, the large cohort studies showed ORRs between 46–71% for MSI/dMMR colorectal cancers and 14–100% for noncolorectal MSI/dMMR cancers. The corresponding ORRs for sporadic MSI/dMMR cancers were 48–100% and 50–100%, respectively. Summarizing the Lynch syndrome case reports, the ORRs were 40% and 53%, respectively. In addition, the only study investigating a systematic difference between Lynch syndrome and sporadic MSI cancers did not reach any significance. Together the data indicates that Lynch syndrome cancer patients may benefit from immune checkpoint-based therapy to the same extend as sporadic MSI/dMMR tumors, though the sample size is limited and confidence intervals large.
Since the approval of pembrolizumab as a tissue-agnostic drug against MSI/dMMR solid tumors, more than 100 clinical trials have been registered at ClinicalTrial.gov and are still ongoing, testing immunotherapy in a wide range of solid tumors. Many of these studies select tumors based on MSI or dMMR status, but only a few studies choose to investigate hereditary germline MMR variants and even fewer to report the outcome data according to germline MMR status. One of the pioneering studies within this field is Le et al, who showed that Lynch syndrome cancer patients had an ORR of 27% compared to an ORR of 100% for sporadic MSI tumors.19 The reduced response rate observed in these preliminary results is hypothetically supported by molecular differences between the sporadic and hereditary MSI tumors, including different immune evasion mechanism affecting, eg, the antigen processing and presentation pathway.14–16 However, the updated study from Le et al,25 reported no significant difference in response rates between the two subsets though separate ORRs for sporadic or Lynch syndrome MSI/dMMR tumors are not reported. The PFS and OS measures are still not complete and separate data for these groups is awaited.
Although many of the immune-checkpoint-based drugs are not approved for MSI/dMMR noncolorectal solid tumors, the case reports and cohort studies presented in here show that Lynch syndrome cancer patients may be potential candidates for such treatments. Complete responses (five out of 26 cases) were reported in advanced rectal cancer, glioblastoma, muscle invasive bladder cancer, and cases with lung metastases, albeit these stories may be more likely to get published than negative findings.22,43,45 It remains very important to publish Lynch syndrome cancer cases with resistance or tumor progression in response to immune checkpoint-based therapy as these tumors may evade the immune system through alternative routes. One such case was presented by Hu et al,49 in which mutation analyses was conducted on the primary pancreatic cancer and the ovarian metastasis. IImmunoediting was suspected to be the cause of acquired resistance, but no mutations were found in the antigen processing and presentation genes, eg the HLA genes, B2M, JAK1, JAK2, PTEN, or TAP1.49 Future molecular studies revealing the genetic makeup of resistant tumors are needed to elucidate why some Lynch syndrome tumors may not respond to immunotherapy.
The majority of the cases presented here were offered immunotherapy due to an MSI phenotype. It is important to note that while dMMR is largely associated with MSI in Lynch syndrome colorectal (98%)50 and endometrial (94%)51 cancers, the concordance is much lower for other Lynch syndrome cancer types such as urothelial cancer (23%)52 and brain tumors (0%).53 In accordance with this, an MSS phenotype has been found in 36% of Lynch syndrome tumors, with associations to noncolorectal, nonendometrial tumors and MSH6 and PMS2 gene variants.54 Abida et al, presented one case with prostate cancer and an MSH6 germline pathogenic variant, but due to an MSS phenotype this patient was not treated.31 In contrast, Bari et al, reported one MSS Lynch syndrome tumor that had continued response at nine months, but immunohistochemical dMMR was not reported in this abstract.21 Though data is still scarce, it is possible that thorough molecular diagnostics, eg, both dMMR and MSI in addition to deeper analyses such as tumor mutation burden, may help guide treatment decision.21,25,31
Tumor mutation burden may add valuable knowledge in the selection of Lynch syndrome patients to immunotherapy, although this has only been investigated and associated with positive responses in some of the included case reports.29,31,32,49 Two cases with locally advanced rectal cancer showed high tumor mutation burden and experienced complete pathological response,29 while two of the cohort studies only associated high tumor mutation burden with MSI status and did not use it as an independent indicator for treatment response.29,31,32 In contrast, a recent case study reported discrepancies between MSI and tumor mutation burden.55 Two Lynch syndrome cancer patients: one with a dMMR and MSI hepatic cholangiocarcinoma and one with a dMMR but MSS neuroendocrine carcinoma were treated with nivolumab in combination with ipilimumab and pembrolizumab, respectively, and both progressed after three cycles of treatment. The authors suspected that the resistance was caused by lack of neoantigen presentation as these tumors showed low tumor mutation burden.55 Yet, the nonresponsive pancreatic cancer reported by Hu et al, presented with a high tumor mutation burden with massive tumor-infiltrating lymphocytes.49 These data emphasize the tumor heterogeneity and lack of solid molecular markers to select responsive cases. To this end, we may add that it is possible that MSS tumors appearing in Lynch syndrome cancer patients may simply not arise from the pathogenic germline MMR variants, and that the specific MMR gene mutated as well as the mutation type may affect cancer risk and survival.56,57 It is possible that the type of mutation may affect the tumor mutation burden and the number of neoantigens presented, hypothetically triggering the immune system differently and affect responses to treatment with immune checkpoint inhibitors.
Owing to scarce Lynch syndrome specific response data, we included case reports in our review. The majority of these cases presented with positive treatment responses indicating publication bias. Hence, these results should be interpreted with caution. However, the summarized ORR from all the cases was 40% for colorectal cancer and 53% for noncolorectal cancers, which is in alignment with the Lynch syndrome-specific ORR presented in the larger cohort studies (ORRs between 46 and 71% for colorectal cancers and between 14 and 100% noncolorectal cancers). Another limitation to this study is the inclusion of eight individuals with VUS alterations. These individuals have previously been categorized as Lynch-like or unexplained MMR deficiency, as they may be caused by biallelic somatic MMR mutations within the tumor.58,59 Though these tumors may mimic Lynch syndrome tumors with an MSI phenotype, the colorectal cancer risk is somewhat lower compared to Lynch syndrome carriers of pathogenic variants.59 Specific response data were not reported according to MMR variants in the unselected cohort described by Le et al,25 but focusing on the case reports with VUS individuals, we observed ORRs of 0% and 50% for colorectal and noncolorectal cancer, respectively. The corresponding numbers were 17% and 53% for verified pathogenic MMR gene variant carriers.
Conclusion
Despite the fact that data is still scarce, we have found that Lynch syndrome cancer patients may benefit from immune checkpoint-based therapies and that no difference in the response rates has been reported to date between Lynch syndrome and sporadic MSI cancer patients. It remains unknown, however, why some Lynch syndrome cancer patients do not respond to immune checkpoint-based therapies and thorough molecular profiling including dMMR, MSI, tumor mutation burden, and immunoediting driver mutations may aid in the selection of patients, who are more likely to respond. Until the routes of resistance are clarified, it is encouraged to report Lynch syndrome-specific outcome data from the large clinical trials.
Acknowledgments
The authors would like to acknowledge the medical librarian Jette Frost, Aalborg University Hospital, for assisting in the different database searches.
Disclosure
Dr Lars Henrik Jensen reports he is an investigator for clinical trials run by MSD, BMS and INCYTE. All payments are to institution for the work performed, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Therkildsen C, Ladelund S, Smith-Hansen L, et al. Towards gene- and gender-based risk estimates in Lynch syndrome; age-specific incidences for 13 extra-colorectal cancer types. Br J Cancer. 2017;117:1702–1710. doi: 10.1038/bjc.2017.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67:1306–1316. doi: 10.1136/gutjnl-2017-314057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow P, Khan M, Lalloo F, et al. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg. 2013;100:1719–1731. doi: 10.1002/bjs.9316 [DOI] [PubMed] [Google Scholar]
- 4.Lynch HT, Snyder CL, Shaw TG, et al. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer. 2015;15:181–194. doi: 10.1038/nrc3878 [DOI] [PubMed] [Google Scholar]
- 5.Kloor M, Becker C, Benner A, et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–6424. doi: 10.1158/0008-5472.CAN-05-0044 [DOI] [PubMed] [Google Scholar]
- 6.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan L, Zhang W. Precision medicine becomes reality-tumor type-agnostic therapy. Cancer Commun Lond Engl. 2018;38:6. doi: 10.1186/s40880-018-0274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol off J Am Soc Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 9.Bhalla A, Zulfiqar M, Weindel M, et al. Molecular diagnostics in colorectal carcinoma. Clin Lab Med. 2013;33:835–859. doi: 10.1016/j.cll.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 10.Ryan NAJ, Glaire MA, Blake D, et al. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreira L, Balaguer F, Lindor N, et al. Identification of lynch syndrome among patients with colorectal cancer. JAMA J Am Med Assoc. 2012;308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe MJ, Petros FG, Rao P, et al. Universal point of care testing for lynch syndrome in patients with upper tract urothelial carcinoma. J Urol. 2018;199:60–65. doi: 10.1016/j.juro.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 13.Bohaumilitzky L, von Knebel Doeberitz M, Kloor M, et al. Implications of hereditary origin on the immune phenotype of mismatch repair-deficient cancers: systematic literature review. J Clin Med. 2020;9(6):9. doi: 10.3390/jcm9061741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada R, Yamaguchi T, Lijima T, et al. Differences in histological features and PD-L1 expression between sporadic microsatellite instability and Lynch-syndrome-associated disease in Japanese patients with colorectal cancer. Int J Clin Oncol. 2018;23:504–513. doi: 10.1007/s10147-018-1238-y [DOI] [PubMed] [Google Scholar]
- 15.Kloor M, Michel S, Buckowitz B, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121(2):454–458. doi: 10.1002/ijc.22691 [DOI] [PubMed] [Google Scholar]
- 16.Dierssen JWF, de Miranda NFCC, Ferrone S, et al. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer. 2007;7:33. doi: 10.1186/1471-2407-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):5. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghatalia P, Nagarathinam R, Cooper H, et al. Mismatch repair deficient metastatic colon cancer and urothelial cancer: a case report of sequential immune checkpoint therapy. Cancer Biol Ther. 2017;18:651–654. doi: 10.1080/15384047.2017.1356506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020. www.training.cochrane.org/handbook. [Google Scholar]
- 21.Bari S, Kim RD, Wang X, et al. Outcomes of Lynch syndrome (LS) patients treated with immune checkpoint inhibitors (ICI). J Clin Oncol. 2020;38:1548. doi: 10.1200/JCO.2020.38.15_suppl.1548 [DOI] [Google Scholar]
- 22.Larouche V, Atkinson J, Albrecht S, et al. Sustained complete response of recurrent glioblastoma to combined checkpoint inhibition in a young patient with constitutional mismatch repair deficiency. Pediatr Blood Cancer. 2018;65:e27389. doi: 10.1002/pbc.27389 [DOI] [PubMed] [Google Scholar]
- 23.Bouffet E, Larouche V, Campel BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol off J Am Soc Clin Oncol. 2016;34:2206–2211. doi: 10.1200/JCO.2016.66.6552 [DOI] [PubMed] [Google Scholar]
- 24.Winer A, Ghatalia P, Bubes N, et al. Dual checkpoint inhibition with ipilimumab plus nivolumab after progression on sequential PD-1/PDL-1 inhibitors pembrolizumab and atezolizumab in a patient with lynch syndrome, metastatic colon, and localized urothelial cancer. The Oncologist. 2019;24:1416–1419. doi: 10.1634/theoncologist.2018-0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SA, Longacre TA, Ladabaum U, et al. Tumor Molecular Testing Guides Anti-PD-1 therapy and provides evidence for pathogenicity of mismatch repair variants. The Oncologist. 2018;23:1395–1400. doi: 10.1634/theoncologist.2018-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvalho JP, Del Giglio A, Achatz MI, et al. Complete clinical response in stage IVB endometrioid endometrial carcinoma after first-line pembrolizumab therapy: report of a case with isolated loss of PMS2 protein. Case Rep Oncol. 2020;13:1067–1074. doi: 10.1159/000510000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demisse R, Damle N, Kim E, et al. Neoadjuvant immunotherapy-based systemic treatment in MMR-Deficient or MSI-high rectal cancer: case series. J Natl Compr Cancer Netw JNCCN. 2020;18:798–804. doi: 10.6004/jnccn.2020.7558 [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Cai J, Deng Y, et al. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology. 2019;8(12):e1663108. doi: 10.1080/2162402X.2019.1663108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol off J Am Soc Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abida W, ML ChengArmenia J et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–478. doi: 10.1001/jamaoncol.2018.5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raj N, Zheng Y, Kelly V, et al. PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol off J Am Soc Clin Oncol. 2020;38:71–80. doi: 10.1200/JCO.19.01586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res off J Am Assoc Cancer Res. 2018;24:1326–1336. doi: 10.1158/1078-0432.CCR-17-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musher B, Rahal A. Single-agent immunotherapy for two types of cancer in one patient. Ann Intern Med. 2019;170:210–211. doi: 10.7326/L18-0360 [DOI] [PubMed] [Google Scholar]
- 35.Keating M, Giscombe L, Tannous T, et al. Prolonged treatment response to pembrolizumab in a patient with pretreated metastatic colon cancer and lynch syndrome. Case Rep Oncol Med. 2019;2019:3847672. doi: 10.1155/2019/3847672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salman P, Panay S, Fernández R, et al. Evidence of response to pembrolizumab in a patient with Lynch syndrome-related metastatic colon cancer. OncoTargets Ther. 2018;11:7295–7300. doi: 10.2147/OTT.S167645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan KH, Lakkasani S, Ramahi A, et al. Hyperprogressive Disease in an Advanced Stage Colon Cancer Patient on Pembrolizumab: a Case Report. Cureus. 2020;12:e7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Y, Cao Y, Yuan M, et al. Different responses to anti-programmed cell death protein 1 (PD-1) immunotherapy in a patient with Lynch syndrome and metachronous urothelial and colon cancer: a case report. Oncol Lett. 2019;18:5085–5090. doi: 10.3892/ol.2019.10909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawashima Y, Nishikawa S, Ninomiya H, et al. Lung adenocarcinoma with lynch syndrome and the response to nivolumab. Intern Med Tokyo Jpn. 2019;58:1479–1484. doi: 10.2169/internalmedicine.1673-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masuzawa K, Asakura T, Ikemura S. et al. Long-lasting response to nivolumab for a patient with lynch syndrome-associated lung adenocarcinoma. JCO Precis Oncol;2020. 4. doi: 10.1200/PO.19.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nevgi A, Klein O, Cheung AS. Sustained remission of Lynch syndrome-associated metastatic adrenocortical carcinoma following checkpoint inhibitor therapy-associated multiorgan autoimmunity. Clin Endocrinol (Oxf). 2020;93:214–216. doi: 10.1111/cen.14258 [DOI] [PubMed] [Google Scholar]
- 42.Casey RT, Giger O, Seetho I, et al. Rapid disease progression in a patient with mismatch repair-deficient and cortisol secreting adrenocortical carcinoma treated with pembrolizumab. Semin Oncol. 2018;45:151–155. doi: 10.1053/j.seminoncol.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancuso JG, Foulkes WD, Pollak MN. Cancer immunoprevention: a case report raising the possibility of ‘immuno-interception’. Cancer Prev Res Phila Pa. 2020;13:351–356. doi: 10.1158/1940-6207.CAPR-19-0528 [DOI] [PubMed] [Google Scholar]
- 44.Ngo P, Shanshal M, Rojan A. Immunotherapy in pancreatic cancer and the importance of tumour testing. BMJ Case Rep. 2020;13. doi: 10.1136/bcr-2020-235774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tlemsani C, Leroy K, Gimenez-Roqueplo A-P. et al. Chemoresistant pleomorphic rhabdomyosarcoma: whole exome sequencing reveals underlying cancer predisposition and therapeutic options. J Med Genet. 2020;57:104–108. doi: 10.1136/jmedgenet-2018-105594 [DOI] [PubMed] [Google Scholar]
- 46.Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: response to PD-1 blockade and standard therapies. PLoS One. 2020;15:e0233260. doi: 10.1371/journal.pone.0233260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patil NR, Khan GN. Exceptional response to a single cycle of immunotherapy in a lynch syndrome patient with metastatic pancreatic adenocarcinoma. Am J Case Rep. 2020;21:e923803. doi: 10.12659/AJCR.923803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang C, Austin F, Richard H, et al. Lynch syndrome-associated ultra-hypermutated pediatric glioblastoma mimicking a constitutional mismatch repair deficiency syndrome. Cold Spring Harb Mol Case Stud. 2019;5(5):5. doi: 10.1101/mcs.a003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu ZI, Hellmann MD, Wolchok JD, et al. Acquired resistance to immunotherapy in MMR-D pancreatic cancer. J Immunother Cancer. 2018;6:127. doi: 10.1186/s40425-018-0448-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loughrey MB, McGrath J, Coleman HG, et al. Identifying mismatch repair-deficient colon cancer: near-perfect concordance between immunohistochemistry and microsatellite instability testing in a large, population-based series. Histopathology. 2020;3:401–413. [DOI] [PubMed] [Google Scholar]
- 51.Stelloo E, Jansen AML, Osse EM, et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol. 2017;28:96–102. doi: 10.1093/annonc/mdw542 [DOI] [PubMed] [Google Scholar]
- 52.Joost P, Therkildsen C, Dominguez-Valentin M, et al. Urinary Tract Cancer in Lynch Syndrome; Increased Risk in Carriers of MSH2 Mutations. Urology. 2015;86:1212–1217. doi: 10.1016/j.urology.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 53.Gylling AHS, Nieminen TT, Abdel-Rahman WM, et al. Differential cancer predisposition in Lynch syndrome: insights from molecular analysis of brain and urinary tract tumors. Carcinogenesis. 2008;29:1351–1359. doi: 10.1093/carcin/bgn133 [DOI] [PubMed] [Google Scholar]
- 54.Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J Clin Oncol. 2019;37:286–295. doi: 10.1200/JCO.18.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bielska AA, Chatila WK, Walch H, et al. Tumor mutational burden and mismatch repair deficiency discordance as a mechanism of immunotherapy resistance. J Natl Compr Cancer Netw JNCCN. 2021;19:130–133. doi: 10.6004/jnccn.2020.7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dominguez-Valentin M, Sampson JR, Seppälä TT, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med off J Am Coll Med Genet. 2020;22:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maccaroni E, Bracci R, Giampieri R, et al. Prognostic impact of mismatch repair genes germline defects in colorectal cancer patients: are all mutations equal? Oncotarget. 2015;6:38737–38748. doi: 10.18632/oncotarget.5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omark J, Vilar E, You YN, et al. Patients with unexplained mismatch repair deficiency are interested in updated genetic testing. Hered Cancer Clin Pract. 2020;18:19. doi: 10.1186/s13053-020-00150-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodríguez-Soler M, Pérez-Carbonell L, Guarinos C, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144:926–932. doi: 10.1053/j.gastro.2013.01.044 [DOI] [PubMed] [Google Scholar]