Abstract
Muscle fatigue induced by voluntary exercise, which requires central motor drive, causes central fatigue that impairs endurance performance of a different, nonfatigued muscle. This study investigated the impact of quadriceps fatigue induced by electrically induced (no central motor drive) contractions on single-leg knee-extension (KE) performance of the subsequently exercising ipsilateral quadriceps. On two separate occasions, eight males completed constant-load (85% of maximal power-output) KE exercise to exhaustion. In a counterbalanced manner, subjects performed the KE exercise with no pre-existing quadriceps fatigue in the contralateral leg on one day (No-PreF), whereas on the other day, the same KE exercise was repeated following electrically induced quadriceps fatigue in the contralateral leg (PreF). Quadriceps fatigue was assessed by evaluating pre- to postexercise changes in potentiated twitch force (ΔQtw,pot; peripheral fatigue), and voluntary muscle activation (ΔVA; central fatigue). As reflected by the 57 ± 11% reduction in electrically evoked pulse force, the electrically induced fatigue protocol caused significant knee-extensors fatigue. KE endurance time to exhaustion was shorter during PreF compared with No-PreF (4.6 ± 1.2 vs 7.7 ± 2.4 min; P < 0.01). Although ΔQtw,pot was significantly larger in No-PreF compared with PreF (−60% vs −52%, P < 0.05), ΔVA was greater in PreF (−14% vs −10%, P < 0.05). Taken together, electrically induced quadriceps fatigue in the contralateral leg limits KE endurance performance and the development of peripheral fatigue in the ipsilateral leg. These findings support the hypothesis that the crossover effect of central fatigue is mainly mediated by group III/IV muscle afferent feedback and suggest that impairments associated with central motor drive may only play a minor role in this phenomenon.
Keywords: central command, electrical stimulation, group III and IV afferents, muscle fatigue
INTRODUCTION
The progressive reduction in the ability to exert muscle force or power during exercise is defined as muscle fatigue (1) and has a central and a peripheral component. Central fatigue describes the impairment of the neural drive to a muscle or muscle group, whereas peripheral fatigue entails biochemical changes occurring at or distally to the neuromuscular junction (2). Previous studies demonstrated that peripheral fatigue in one limb may induce changes in the central nervous system (CNS), potentially affecting exercise performance of a remote muscle group (3–8). This “crossover” effect of fatigue is usually secondary to a compromised activation of the remote muscle group by the CNS. Although several factors have been identified as possible contributors (9), the mechanistic basis for this phenomenon is still unclear.
In previous investigations, preinduced fatigue in one limb has been shown to directly affect neuromuscular function and exercise performance during the successive exercise bout carried out with a previously rested muscle (3, 10, 11). For example, voluntary knee-extension (KE) exercise-induced fatigue in the contralateral leg impaired spinal motoneuronal output and endurance performance of the ipsilateral quadriceps (3). A reduction in endurance performance and a faster decline in voluntary muscle activation (VA) were also found when arm-cranking exercise was performed before leg cycling exercise (4). Since exercising with prior fatigue entails increased afferent feedback signaling arising from both the active and recovering muscles, strong evidence suggests that group III/IV afferent-mediated inhibition of central motor drive may play a pivotal role in the crossover effect of central fatigue and the impairment of performance (3, 8). Importantly, when leg cycling exercise is carried out with pharmacologically blocked group III/IV afferent feedback from the lower limbs, the decrease in VA in a nonexercising, remote muscle (i.e., elbow flexors) is prevented, emphasizing the importance of these sensory neurons in mediating the crossover effect of fatigue (8). However, controversy exists on the role of group III/IV on the crossover of central fatigue. For example, Kennedy and colleagues (12) did not find any decrease in VA or maximal voluntary force when ischemia was maintained on the contralateral limb after a 2-min isometric fatiguing task. Also, it is important to note that all the above-mentioned studies utilized voluntary exercise to induce prior fatigue.
When voluntary exercise is used to prefatigue a limb, the effects associated with central motor drive may, by itself, impair performance during subsequent tasks. In fact, parallel to the activation of spinal motor neurons directly involved in the exercise, a “copy” of the neural signal (i.e., corollary discharge) is sent from the premotor areas to the sensory areas of the brain, ultimately facilitating effort perception and potentially impairing exercise performance (13, 14). Moreover, several studies using different neurophysiological approaches have shown that the activity in different brain areas, such as the prefrontal cortex (15), the thalamus, and the insula (16) progressively increases during fatiguing voluntary exercise, and possibly contributes to the development of fatigue and, ultimately, exercise cessation. Furthermore, repetitive central motor pathway activation associated with voluntary fatiguing exercise may, by itself, reduce motoneuron excitability (17–20). On the other hand, the preinduction of fatigue through electrical stimulation of the limb that is successively exercised compromises exercise performance by causing peripheral fatigue that diminishes the ability of the muscle to respond to a given motor input. Therefore, this approach may confound the potentially performance limiting effects of increased group III/IV afferents discharge (21).
To circumvent these issues, we used electrical muscle stimulation to induce quadriceps fatigue in the contralateral leg immediately before the start of voluntary KE exercise with the ipsilateral leg. This strategy elevated, compared with the control trial performed without pre-existing fatigue in the contralateral leg, ensemble afferent feedback to the CNS during KE exercise. We hypothesized that 1) electrically induced peripheral fatigue in the contralateral leg would compromise exercise performance of the ipsilateral leg, and 2) end-exercise peripheral fatigue would be lower when exercise is executed with pre-existing fatigue in the contralateral leg.
METHODS
Subjects and Ethical Approval
Eight active healthy young males (age: 26 ± 1 yr; height: 179 ± 2 cm; body mass: 76 ± 2 kg) were recruited for the purpose of this study. All subjects were right-leg dominant. The study was approved by the local ethical committee of the University of Verona (No. 30444) and was carried out following the Declaration of Helsinki. All subjects read and signed an informed consent before enrolling in the study. All the experiments were performed following the safety procedure for exercise testing in the scenario of COVID-19 (22).
Study Design
The subjects reported to the laboratory on four occasions, interspersed by at least 48 h between sessions. Also, the two finals visits were separated by a minimum of 72 h. Following preliminary familiarization trials conducted to practice exhaustive KE exercise, on the first experimental day, anthropometric data were collected, and the subjects were familiarized with the study instrumentation and procedures including exhaustive voluntary KE exercise and electrically evoked muscle contractions to the limit of tolerance. On a separate occasion (day 2), a single-leg KE incremental test to exhaustion (15 W + 5 W min−1) was administered on the dominant leg to determine the individual peak power output (PPO). On the two following visits, the subjects carried out a constant-load exercise to exhaustion on the single-leg KE ergometer at 60 RPM and 85% PPO in a nonprefatigued condition (No-PreF) or with preinduced fatigue in the contralateral leg (PreF). The induction of fatigue (detailed in a following section: see “Electrically Induced Fatiguing Protocol”) was achieved by electrical stimulation on the nondominant, contralateral limb. The order of the two experimental sessions was counterbalanced. In both sessions, participants were evaluated for neuromuscular function before and right after the end of the single-leg KE exercise. The constant-load test was terminated when the subjects were unable to maintain 50 RPM for more than 10 s. At the beginning of each constant-load exercise test, the ergometer was manually accelerated by a member of the research team, to avoid initial peak force outputs.
Exercise Responses
Pulmonary gas exchange (V̇o2 and V̇co2) and ventilatory (V̇e) responses were measured breath-by-breath with a metabolic cart (Quark b2, Cosmed, Italy). Before each session, after an appropriate warm-up, the gas analyzer and the turbine flowmeter were calibrated according to the instructions of the manufacturer. The data were averaged over the last 30 s of each minute. Rate of perceived exertion (RPE) was obtained at rest and after every minute of exercise using the Borg’s modified Category Ratio 10 scale (23).
During the voluntary KE exercise, a Doppler ultrasound (Logic 7 Doppler system; General Electric Medical Systems, Milwaukee, WI) equipped with a 12–14 MHz linear array transducer was utilized to assess femoral blood flow and vascular conductance, which may have an influence on the development of neuromuscular fatigue (24). Femoral artery blood velocity (Vmean) and arterial diameter were measured distally from the inguinal ligament and proximal to the bifurcation of the superficial and deep femoral artery. Vmean was measured using the same probe utilizing a frequency of 5 MHz with the probe positioned and maintained at an insonation angle of 60° or less, and the sample volume was centered and maximized according to vessel size. Then, femoral blood flow (FBF) was calculated as Vmean·π·(arterial diameter· 2−1)2·60.
A noninvasive thoracic impedance cardiograph (Physio Flow, Manatec, Strasbourg, France) was utilized to measure heart rate (HR) and estimate stroke volume. Cardiac output (CO) was calculated as stroke volume·HR. The validity and reliability of this method has previously been established (25). The data of the last 30 s of each minute were averaged. Systolic and diastolic blood pressures were measured manually, by means of a sphygmomanometer and an adult size cuff at each minute of exercise. Mean arterial pressure (MAP) was calculated as: diastolic blood pressure + 1/3·(systolic blood pressure − diastolic blood pressure). Leg vascular conductance (LVC) was calculated as FBF·MAP−1.
Neuromuscular Function Assessment of the Ipsilateral Limb
Surface electromyography.
Vastus lateralis electromyography (EMG) was continuously recorded with a wireless system (ZeroWire, Aurion, Italy). Two surface Ag/AgCl electrodes (Blue sensor, Ambu, Ballerup, Denmark) were attached to the skin with a 2-cm interelectrode distance. The electrodes were placed longitudinally, in line with the underlying muscle fibers arrangement, at two-thirds of the distance between the anterior iliac spine and the lateral part of the patella (26). Before electrodes application, the skin was shaved and cleaned with alcohol to minimize impedance. The placement of the electrodes was marked to allow a consistent repositioning of the electrodes between the two experimental sessions. The EMG transmitter connected to the electrodes was secured with adhesive tape to avoid movement-induced artefacts. The raw EMG signal was amplified, band-pass filtered (10–1,000 Hz), and digitized online at a 5-kHz sampling frequency. Acquisition of the EMG data was done using a computer-based data acquisition and analysis system (hardware: PowerLab 16/30; ML880, ADInstruments, Colorado Springs, CO; and software: LabChart 6, ADInstruments, Colorado Springs, CO). Burst onset was established when the EMG signal rose >2.5 SD above baseline noise. On the contrary, burst offset was determined by the signal declining below the same threshold. Integrated electromyography (iEMG) for each muscle contraction was calculated and averaged over the last 30 s of each minute and normalized to the EMG signal obtained during an maximal voluntary contraction (MVC) performed before the exercise.
Femoral nerve stimulation.
The motor nerve was stimulated with the anode placed between the greater trochanter and the iliac crest and the cathode placed over the femoral nerve in the femoral triangle via a constant current electrical stimulator (Digitimer DS7AH, Welwyn Garden City, UK). Electrical stimuli were delivered through rectangular (50 × 90 mm) self-adhesive electrodes (Myotrode Plus, Globus G0465). The evoked twitch force was measured by a force transducer (model UU2; DaCell, Korea) previously calibrated, connected to a custom-made chair through a noncompliant strap placed around the subject ankle. The subjects were seated with a 90° knee flexion. The output from the force transducer was amplified (INT2-L, London Electronics Limited, Sandy Bedfordshire, UK) and recorded at a sampling rate of 5 kHz. Once the electrodes were in place, stimulation intensity was increased by 25-mA increments until the size of the evoked twitch and compound muscle action potential (M-wave) demonstrated no further increase (i.e., amplitude of maximal M-wave; Mmax). Stimulation intensity was set at 125% of this value (range utilized: 343–469 mA) and was kept constant throughout the experimental session.
For the evaluation of quadriceps function, we measured potentiated twitch force (Qtw,pot) 2 s after a 5-s MVC of the knee extensors and repeated this procedure six times (27). These MVCs were interspersed by at least 60 s of rest. VA was assessed using the interpolated twitch technique by comparing the force produced during a superimposed single twitch on the MVC with the potentiated single twitch delivered 2 s afterward (27). VA was then calculated as [1 − (superimposed twitch force/Qtw,pot)·100]. Peak force, maximal rate of force development (MRFD), and maximal rate of relaxation (MRR) were analyzed for all Qtw,pot. Peak force was calculated as the highest value reached for every Qtw,pot. MRFD and MRR represent the maximal force increase and decrease, respectively, occurring between 10% and 90% of the peak force of Qtw,pot.
Electrically Induced Fatiguing Protocol
In PreF, neuromuscular electrical stimulation was used to fatigue the contralateral quadriceps before the voluntary KE exercise to exhaustion of the ipsilateral leg. After completion of the neuromuscular assessment of the right leg, subjects were accommodated on the single-leg KE ergometer. After adequate preparation of the skin, two 50- × 90-mm self-adhesive electrodes (Myotrode Plus, Globus G0465) were positioned on the left quadriceps. The left ankle was then connected to the force transducer via a noncompliant velcro strap to measure the force evoked by the train pulses. The anode was placed on the proximal part of the rectus femoris while the cathode was placed ∼3 cm above the patella. To elicit the train pulses and to administer the stimulation, we used a custom-made system previously used in our laboratory (28). Briefly, the same constant current electrical stimulator previously mentioned was interfaced with an Arduino microcontroller (Arduino Uno, Arduino, Italy) to generate the train pulses. The stimulation intensity was progressively increased to find the maximal tolerable intensity. The subjects were given instructions to indicate their discomfort levels on a visual analogue scale (VAS) from 0 to 10, where 0 was no discomfort and 10 maximal discomfort that they considered tolerable for at least 5 min. When the maximal tolerable intensity was reached, the ramp protocol was interrupted, and the electrically induced fatiguing protocol began. Stimulation intensity was kept constant throughout the trial. Every train of stimulation consisted of twenty 1-ms square wave pulses, duty cycle 20% (0.2 s on/0.8s off) delivered at 100 Hz to evoke a tetanic contraction. The decrease in the force evoked by the train pulses was expressed as the difference between the average of the peak force reached in the first five and last five contractions (Fig. 1A). Once the subjects reached their subjective point of intolerable discomfort, the stimulation was terminated, and the performance test of the ipsilateral leg started within 10 s.
Figure 1.

A: representative quadriceps force output obtained from a subject during the prefatigue protocol using electrically evoked knee-extensors contractions. B: individual (triangles) and mean (-) changes in the evoked force during the electrical stimulation protocol. Δ: average of the evoked peak force reached in the first 5 contractions, ▴: average of the evoked peak force achieved during the last 5 contractions; *Significantly different than Pre (paired samples t test, P < 0.05). Number of participants (n) = 8.
Statistical Analysis
For the neuromuscular assessments, where a pre–post design was applied, a two-way ANOVA with repeated measures (conditions: No-PreF and PreF, time: pre and post exercise) was performed to evaluate differences between means. For the in-exercise data, also a two-way repeated measures ANOVA was carried out in which the last time point was the subjective time to exhaustion. If the sphericity assumption was violated, the Greenhouse–Geisser correction coefficient was reported. When a significant condition × time interaction was found, pairwise differences were identified using Bonferroni post hoc test correction for multiple comparisons. A two-tailed paired samples t test was used to assess the differences in time to exhaustion between conditions, to compare baseline data for the neuromuscular assessment, and to assess the difference between the force of the first five versus last five evoked contractions. Significance level was set at α ≤ 0.05. Data are expressed as means ± SD unless otherwise stated. Statistical analysis was performed using IBM SPSS Statistics 24 (IBM Corp, 2016). Graphs and figures were made with GraphPad Prism 6.0 (GraphPad Software, Inc., 2012).
RESULTS
Electrically Induced Fatiguing Protocol
Electrically evoked quadriceps contractions significantly decreased the evoked force response to the train pulses by 57 ± 11% (from 318 ± 124 N to 136 ± 54 N, t7 = 6.16, P < 0.01, Fig. 1B). Furthermore, the point of intolerable discomfort was reached after 6.2 ± 1.5 min.
Exercise Performance and Neuromuscular Function
Time to exhaustion was 38 ± 13% shorter in PreF compared with No-PreF (4.6 ± 1.2 vs. 7.7 ± 2.4 min, t7 = 5.01, P < 0.01). The changes in neuromuscular function for the two trials are presented in Fig. 2. At baseline, quadriceps MVC was similar between conditions (625 ± 111 N vs. 618 ± 101 N, t7 = 0.46, P = 0.66). Immediately after the two protocols, quadriceps MVC was reduced both in No-PreF (from 625 ± 111 N to 391 ± 76 N) and in PreF (from 618 ± 101 N to 430 ± 51 N) with a significant difference between the two conditions (F1,7 = 9.3, η2p = 0.57, P = 0.02). Pre-exercise Qtw,pot was not different between experimental days (191 ± 47 N vs. 189 ± 43 N, t7 = 1.22, P = 0.26). Also, Qtw,pot was reduced after both trials with a higher exercise-induced decline (F1,7 = 24.7, η2p = 0.78, P < 0.01) after No-PreF (191 ± 47 N to 75 ± 20 N) compared with PreF (189 ± 43 N to 87 ± 21 N). Furthermore, although not being different at baseline (No-PreF: 92 ± 3% vs. PreF: 91 ± 4%, t7 = 1.29, P = 0.24), the decrease in VA was more accentuated (F1,7 = 8.3, η2p = 0.54, P = 0.02) in PreF (from 91 ± 4% to 78 ± 7%) compared with No-PreF (from 92 ± 3% to 83 ± 5%). Finally, within-twitch variables showed a significant greater reduction in MRFD (F1,7 = 5.4, η2p = 0.44, P = 0.05) after No-PreF (5.24 ± 1.44 N/ms to 1.57 ± 0.56 N/ms) compared with PreF (5.10 ± 1.44 N/ms to 2.10 ± 0.81 N/ms) and also in MRR (F1,7 = 17.6, η2p = 0.72, P < 0.01) between No-PreF (1.05 ± 0.38 N/ms to 0.32 ± 0.13 N/ms) and PreF (1.00 ± 0.28 N/ms to 0.40 ± 0.18 N/ms).
Figure 2.
Individual (circles) and group mean (−) data showing the changes in (A) time to exhaustion, (B) maximal voluntary contraction (MVC), (C) potentiated twitch force (Qtw,pot), and (D) voluntary muscle activation (VA) under control condition (No-PreF, ○) and under experimental condition (PreF, ●, i.e., exercise performed with prior fatigue induced by electrical stimulation in the contralateral leg). *Significantly different from No-PreF (ANOVA, condition × time interaction, P < 0.05); Number of participants (n) = 8. No-PreF, nonprefatigued condition; PreF, prefatigued condition.
Ventilatory and Hemodynamic Variables
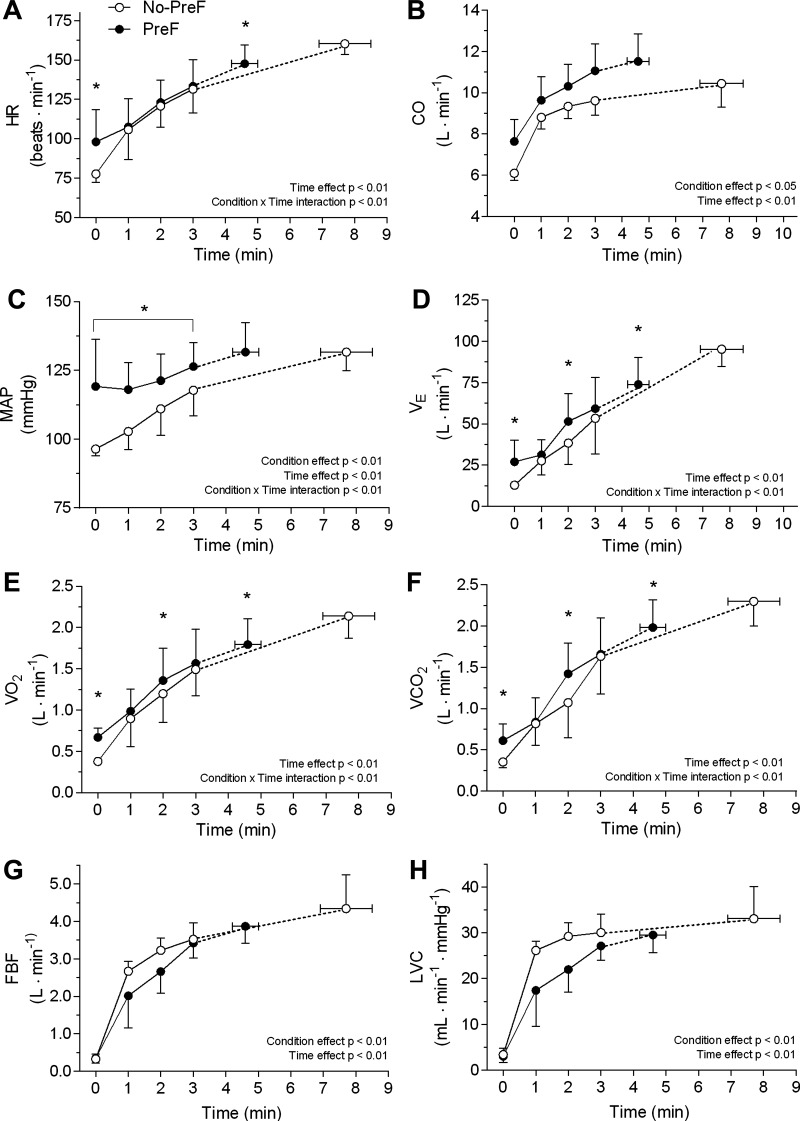
The ventilatory and hemodynamic responses collected during both performance trials are reported in Fig. 3. A condition × time interaction was found for HR (F4,28 = 7.3, η2p = 0.51, P < 0.01), MAP (F4,28 = 9.0, η2p = 0.56, P < 0.01), V̇o2 (F1.7,11.8 = 9.6, η2p = 0.58, P < 0.01), V̇co2 (F4,28 = 5.3, η2p = 0.43, P < 0.01), and V̇e (F4,28 = 16.1, η2p = 0.70, P < 0.01) during the successive knee-extensors performance test with all variables being heightened at min 0. Also, the two-way ANOVA showed a main effect of time (F4,28 = 196.9, η2p = 0.97, P < 0.01) and condition (F1,7 = 8.2, η2p = 0.54, P < 0.02) for CO. FBF also presented a significant main effect of time (F4,28 = 155.4, η2p = 0.96, P < 0.01) and condition (F1,7 = 19.1, η2p = 0.73, P < 0.01). Finally, a significant effect of time (F4,28 = 143.8, η2p = 0.96, P < 0.01) and condition (F1,7 = 22.4, η2p = 0.76, P < 0.01) was also found for LVC.
Figure 3.
Pulmonary and hemodynamic responses to constant-load single leg knee-extensor exercise at 85% of the individual peak power output without (No-PreF, ○) and with preinduced fatigue in the contralateral leg (PreF, ●). A: HR, heart rate; B: CO, cardiac output; C: MAP, mean arterial pressure; D: V̇e, minute ventilation; E: V̇o2, oxygen consumption; F: V̇co2, carbon dioxide production; G: FBF, femoral blood flow; H: LVC, leg vascular conductance. *Significantly higher than No-PreF following Bonferroni post hoc correction for multiple comparisons (P < 0.05). The exhaustion point is presented as mean ± SE (horizontal bars); number of participants (n) = 8. No-PreF, nonprefatigued condition; PreF, prefatigued condition.
M-Waves Characteristics, Integrated Electromyography, and Rate of Perceived Exertion
Peak-to-peak M-wave amplitude was similar between experimental days (No-PreF: 10.02 ± 1.37 mV vs. PreF: 9.78 ± 0.35 mV, t7 = 0.4, P = 0.69). Sarcolemmal excitability was maintained from pre- to postexercise in all trials as indicated by unchanged M-wave characteristics (peak-to-peak amplitude: No-PreF: from 10.02 ± 1.37 mV to 9.97 ± 1.41 mV; PreF: from 9.78 ± 0.35 mV to 9.74 ± 0.47 mV, F1,7 = 0.46, η2p = 0.01, P = 0.84).
The iEMG data (Fig. 4A) showed a main effect of time (F3,21 = 35.7, η2p = 0.84, P < 0.01) and condition (F1,7 = 8.0, η2p = 0.54, P = 0.03). Finally, apart from the exhaustion time point, RPE was different throughout exercise (F3,21 = 12.9, η2p = 0.65, P < 0.01, Fig. 4B). RPE was higher in PreF at min 1 and continued to increase at the same rate as in No-PreF until reaching the maximal value at the point of exhaustion.
Figure 4.
Integrated electromyography (iEMG; A), and rate of perceived exertion (RPE; B) during constant-load single leg knee-extensor exercise at 85% of the individual peak power output without (No-PreF, ○) and with preinduced fatigue in the contralateral leg (PreF, ●). Mean values for iEMG during each concentric knee extension were averaged during the last 30 s of every minute and normalized to the EMG activity during an MVC performed before exercise. *Significantly higher than No-PreF following Bonferroni post hoc correction for multiple comparisons (P < 0.05). The exhaustion point is presented as mean ± SEM (horizontal bars); n = 8. MVC, maximal voluntary contraction; No-PreF, nonprefatigued condition; PreF, prefatigued condition.
DISCUSSION
The purpose of this study was to investigate whether electrically induced quadriceps fatigue in the contralateral leg would affect endurance performance and fatigue development of the subsequently exercising ipsilateral leg. In agreement with our hypothesis, we found that endurance time to exhaustion was 38% lower, and end-exercise quadriceps fatigue (ΔQtw,pot) 8% less when the exercise was performed with pre-existing fatigue. Since the magnitude of the effects associated with contralateral fatigue induced by voluntary exercise (3) is only slightly larger compared to the one observed in the current study, together, these results suggest that central motor drive and associated CNS effects might only play a minor role in the crossover effect of central fatigue and further support the hypothesis that group III/IV-mediated afferent feedback is the main determinant of this phenomenon.
Evidence That Crossover of Central Fatigue Is Mediated by Group III/IV Muscle Afferent Feedback
Previous studies have shown that group III/IV muscle afferent feedback-mediated inhibition affects exercise performance and VA in a remote, nonexercising muscle (3, 8). However, the involvement of central command in the induction of fatigue may have been a confounding factor in these studies. We circumvented this caveat in the current study by employing electrically evoked KE exercise to induce fatigue in the contralateral quadriceps. This paradigm allowed us to study the group III/IV-mediated crossover of central fatigue in a condition in which confounding influences associated with central motor drive and volitional effort were eliminated during the process of prefatiguing a remote muscle.
Since group III/IV muscle afferents play an important role in regulating the cardiovascular and ventilatory response to exercise independently from central command (29, 30), the augmented cardiorespiratory response found in PreF (Fig. 3) represents indirect evidence that electrically induced fatigue in the contralateral leg successfully raised group III/IV afferents firing during the successive performance test. It is, however, important to recognize that pain associated with the electrical stimulation likely also contributed to the increase in the cardiorespiratory response at the beginning of the fatiguing task in PreF (10, 31).
An experimental setup and conceptual framework similar to the current study was previously used to raise ensemble afferent feedback and evaluate its effect on exercise performance and neuromuscular function in a remote muscle (3). In that study, voluntary KE exercise to exhaustion in the contralateral leg was employed to increase group III/IV afferents firing immediately before the start of constant-load KE exercise to exhaustion in the ipsilateral leg. Although the magnitude of end-exercise quadriceps fatigue was similar in the current (Fig. 2) and the previous (3) study, the impact on endurance performance was slightly less pronounced when prefatigue was induced via electrically evoked (i.e., current study) compared with voluntary (3) KE exercise [−38% (range: 20%–58%) vs. −49% (range: 33%–75%)]. Although speculative, the larger impact on performance in the previous study might, at least in part, be due to the involvement of central command in the prefatigue process. Specifically, the impact on performance might not only have resulted from the group III/IV-mediated inhibition (as was the case in the current study), but additionally from CNS-related implications associated with central motor drive.
Studies utilizing postexercise circulatory occlusion (PECO) following fatiguing voluntary isometric muscle contractions document that metabo-nociceptive muscle afferent feedback only impairs neuromuscular function in the rested antagonist (13) or proximal (32) ipsilateral muscle, but not in the contralateral homologous (13). Based on these observations, the authors concluded that nociceptive group III/IV muscle afferents, a subset of group III/IV muscle afferents not active during freely perfused conventional exercise (33), are not responsible for the crossover effect of fatigue to the contralateral limb but may reduce central motor drive to an ipsilateral muscle (13). The current study employed a different exercise paradigm during the prefatigue task (i.e., rhythmic contractions following electrical muscle stimulation) and might therefore have engaged a different subset of group III/IV muscle afferents. This could, at least in part, account for the discrepancy between the current study and these earlier observations. Importantly, however, the recruitment pattern during electric muscle stimulation (i.e., earlier recruitment of high threshold, fatigue-prone motor units) does not follow the Henneman size principle (34), and the exercise-induced intramuscular changes might therefore not only have differed from that during voluntary exercise (35) but also from that during PECO. Taken together, these observations suggest that the role of group III/IV-mediated muscle afferent feedback in the crossover of fatigue is highly dependent on the exercise modality utilized for the prefatigue task (and consequently the subtype of muscle afferents activated), and the outcome measures (e.g., VA/MVC vs. time to task failure). Also, our findings and those of others (9) suggest that the impact of the crossover of central fatigue may be more relevant in the context of submaximal, rhythmic contractions compared to changes in the neuromuscular function obtained during brief, maximal, isometric contractions.
The mechanism by which group III/IV muscle afferent feedback modulates the crossover in central fatigue is not completely clear. Interestingly, however, electrically induced muscle fatigue has been shown to affect supraspinal areas of the CNS (36, 37). A study based on near-infrared spectroscopy showed that when electrical stimulation was used to induce plantar flexor fatigue, the decline in VA, measured in the stimulated limb, was mirrored by a decline in both somatosensory and motor cortex activity (36). Since indices of spinal excitability, quantified by H-reflex, were unchanged, and cortical activity (changes in oxy- and deoxy-hemoglobin) decreased, these findings suggest that afferent feedback may also act at a higher level than the motor cortex, compromising the synaptic input into it. However, to the best of our knowledge, no study has measured whether central impairments are also detected in a rested muscle when electrically induced contractions are performed on the contralateral side. Therefore, even though Alexandre et al. (36) evaluated neuromuscular function on the same limb on which fatigue was electrically induced, we speculate that this effect may carry over to the contralateral muscles. In fact, various crossover effects between the two sides have been identified and are possibly mediated by shared neural networks between the two brain hemispheres (e.g., transcallosal connections) (38) and between upper and lower limbs (39).
It is recognized that electrical stimulation involving high frequency and wide pulses may depolarize sensory neurons and therefore recruit descending spinal motor neurons by an evoked afferent volley (40, 41). Even though we cannot exclude that central fatigue in the current study was also influenced by spinal factors, previous studies suggest that electrically induced muscle contractions do not cause spinal fatigue (17, 36, 42). Specifically, fatigue induced by electrical stimulation of the plantar flexors (42) or the adductor pollicis (17) does not change spinal/motoneuronal excitability. Therefore, supraspinal mechanisms are likely to be the main determinants of central fatigue evoked by electrically induced muscle contractions.
Evidence That Central Fatigue Restricts the Development of Peripheral Fatigue and Impairs Exercise Performance of a Remote Muscle
Central fatigue develops during constant-load, dynamic endurance exercise until a subject reaches his/her sensory tolerance limit, a point where the CNS fails to sufficiently activate the exercising muscle and task failure occurs (2, 13). Although numerous factors contribute to the development of central fatigue and the attainment of the sensory tolerance limit during exercise, group III/IV muscle afferent feedback, triggered by the intramuscular metabolic perturbations associated with peripheral fatigue (43), has been identified as a key determinant (2, 3, 13, 44).
In the current study, task failure occurred sooner, and the sensory tolerance limit was reached with less peripheral fatigue in the ipsilateral leg, during PreF compared with No-PreF. We hypothesize that, compared with No-PreF, the additional source of afferent feedback during PreF facilitated the development of central fatigue and accelerated the attainment of the sensory tolerance limit which reduced exercise time to task failure and, as a consequence, end-exercise peripheral fatigue. Specifically, since the discharge frequency of group IV muscle afferents remains elevated for up to 15 min following electric muscle stimulation (32), our results suggest that during PreF, inhibitory afferent feedback was arising from both the electrically fatigued and the voluntarily exercising quadriceps.
Given the differences in V̇e between the two trials, it is reasonable to assume that the work carried out by the respiratory muscles was higher during PreF. Thus, a potentially stronger respiratory metaboreflex during PreF cannot be excluded as a factor contributing to the exacerbated development of fatigue and the compromised performance during this trial. However, although respiratory muscle work can negatively affect endurance performance and fatigue development during heavy whole body exercise (45, 46), single-leg exercise is characterized by a significant cardiac reserve allowing to satisfy the metabolic demand of all working muscles, even at maximal intensities. We therefore consider it unlikely that the relatively small difference in V̇e between PreF and No-PreF had a significant impact on performance or fatigue.
Considerations on the Use of Voluntary Activation as an Index of Central Fatigue Crossover
The crossover effect of central fatigue is often illustrated by the decrease in VA in the nonexercised muscle (7, 47, 48). The design utilized in the present study prevents the use of changes in VA as an indication of fatigue crossover. Specifically, in order to use differences in the exercise-induced fall in VA between conditions as an indicator of fatigue crossover, the assessment should be carried out in the fresh, rested muscle immediately after the prefatiguing bout is performed. If the assessment is done at the end of the fatiguing task, as in the present study, VA data may be confounding, since they would result from the sum of the prefatiguing and the voluntary exercise tasks. Instead, relevant information could be extrapolated by the rate of development of central fatigue (ΔVA/exercise time). For instance, in two recent studies (4, 10) the exercise-induced fall in VA was the same across conditions, but the prefatigued groups reached it in a significantly shorter time. This finding, in line with the outcome of the current study, agrees with the concept of a crossover of central fatigue, suggesting that more central motor drive was needed to overcome inhibitory feedback, ultimately leading to a premature termination of exercise.
Perspectives and Significance
The current study provides further evidence that group III/IV muscle afferent feedback plays a crucial role in the development of central fatigue and therefore in limiting endurance exercise performance of a remote muscle. Since the augmented firing of these afferent fibers was obtained bypassing central command, it is suggested that impairments associated with repetitive activation of the central motor pathway may only play a minor role in this phenomenon.
GRANTS
The work was partially supported by the Italian Ministry of Research and University (MIUR—Rome, Italy) 5-yr special funding (https://www.miur.gov.it/dipartimenti-di-eccellenza). M. Amann is supported by the US National Heart, Lung, and Blood Institute (HL-116579, HL-139451) and the U.S. Department of Veterans Affairs (E3343-R).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.G.L., M.V., G.G., C.B., F.R., and A.P. conceived and designed research; F.G.L., M.V., E.K., G.G., F.R., A.P., C.M., and C.T. performed experiments; F.G.L., G.G., C.M., and C.T. analyzed data; F.G.L., M.V., and M.A. interpreted results of experiments; F.G.L. and M.V. prepared figures; F.G.L., M.V., M.A., C.B., and A.P. drafted manuscript; F.G.L., F.S., M.V., M.A., E.K., G.G., C.B., F.R., A.P., and C.T. edited and revised manuscript; F.G.L., F.S., M.V., M.A., E.K., G.G., C.B., F.R., A.P., C.M., and C.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the participants for their time and effort.
REFERENCES
- 1.Bigland-Ritchie B, Woods JJ. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7: 691–699, 1984. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- 2.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, Richardson RS. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol (1985) 115: 355–364, 2013. doi: 10.1152/japplphysiol.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson MA, Sharpe GR, Williams NC, Hannah R. Locomotor muscle fatigue is not critically regulated after prior upper body exercise. J Appl Physiol (1985) 119: 840–850, 2015. doi: 10.1152/japplphysiol.00072.2015. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL. Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol (1985) 116: 385–394, 2014. doi: 10.1152/japplphysiol.01166.2013. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL. Firing of antagonist small-diameter muscle afferents reduces voluntary activation and torque of elbow flexors. J Physiol 591: 3591–3604, 2013. doi: 10.1113/jphysiol.2012.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rattey J, Martin PG, Kay D, Cannon J, Marino FE. Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Pflugers Arch 452: 199–207, 2006. doi: 10.1007/s00424-005-0027-4. [DOI] [PubMed] [Google Scholar]
- 8.Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M. Spinal mu-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592: 5011–5024, 2014. doi: 10.1113/jphysiol.2014.275438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halperin I, Chapman DW, Behm DG. Non-local muscle fatigue: effects and possible mechanisms. Eur J Appl Physiol 115: 2031–2048, 2015. doi: 10.1007/s00421-015-3249-y. [DOI] [PubMed] [Google Scholar]
- 10.Aboodarda SJ, Iannetta D, Emami N, Varesco G, Murias JM, Millet GY. Effects of pre-induced fatigue vs. concurrent pain on exercise tolerance, neuromuscular performance and corticospinal responses of locomotor muscles. J Physiol 598: 285–302, 2020. doi: 10.1113/JP278943. [DOI] [PubMed] [Google Scholar]
- 11.Finn HT, Kennedy DS, Green S, Taylor JL. Fatigue-related feedback from calf muscles impairs knee extensor voluntary activation. Med Sci Sports Exerc 52: 2136–2144, 2020. doi: 10.1249/MSS.0000000000002362. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy DS, Fitzpatrick SC, Gandevia SC, Taylor JL. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J Appl Physiol (1985) 118: 408–418, 2015. doi: 10.1152/japplphysiol.00375.2014. [DOI] [PubMed] [Google Scholar]
- 13.Hureau TJ, Romer LM, Amann M. The 'sensory tolerance limit': a hypothetical construct determining exercise performance? Eur J Sport Sci 18: 13–24, 2018. doi: 10.1080/17461391.2016.1252428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulet JF, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci 30: 14–21, 2007. doi: 10.1016/j.tins.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Berchicci M, Menotti F, Macaluso A, Di Russo F. The neurophysiology of central and peripheral fatigue during sub-maximal lower limb isometric contractions. Front Hum Neurosci 7: 135, 2013. doi: 10.3389/fnhum.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilty L, Jancke L, Luechinger R, Boutellier U, Lutz K. Limitation of physical performance in a muscle fatiguing handgrip exercise is mediated by thalamo-insular activity. Hum Brain Mapp 32: 2151–2160, 2011. doi: 10.1002/hbm.21177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Amico JM, Rouffet DM, Gandevia SC, Taylor JL. Unlike voluntary contractions, stimulated contractions of a hand muscle do not reduce voluntary activation or motoneuronal excitability. J Appl Physiol (1985) 128: 1412–1422, 2020. doi: 10.1152/japplphysiol.00553.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finn HT, Rouffet DM, Kennedy DS, Green S, Taylor JL. Motoneuron excitability of the quadriceps decreases during a fatiguing submaximal isometric contraction. J Appl Physiol (1985) 124: 970–979, 2018. doi: 10.1152/japplphysiol.00739.2017. [DOI] [PubMed] [Google Scholar]
- 19.Weavil JC, Amann M. Corticospinal excitability during fatiguing whole body exercise. Prog Brain Res 240: 219–246, 2018. doi: 10.1016/bs.pbr.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Fatigue diminishes motoneuronal excitability during cycling exercise. J Neurophysiol 116: 1743–1751, 2016. doi: 10.1152/jn.00300.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S, Maltais F. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol (1985) 107: 832–840, 2009. doi: 10.1152/japplphysiol.91546.2008. [DOI] [PubMed] [Google Scholar]
- 22.Venturelli M, Cè E, Paneroni M, Guazzi M, Lippi G, Paoli A, Baldari C, Schena F, Esposito F. Safety procedures for exercise testing in the scenario of COVID-19: a position statement of the Società Italiana Scienze Motorie e Sportive. Sport Sci Health 16: 601–607, 2020. doi: 10.1007/s11332-020-00694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics, 1998. [Google Scholar]
- 24.Dempsey JA, Amann M, Romer LM, Miller JD. Respiratory system determinants of peripheral fatigue and endurance performance. Med Sci Sports Exerc 40: 457–461, 2008. doi: 10.1249/MSS.0b013e31815f8957. [DOI] [PubMed] [Google Scholar]
- 25.Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M, Lampert E, Mettauer B, Geny B, Lonsdorfer J. Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol 85: 202–207, 2001. doi: 10.1007/s004210100458. [DOI] [PubMed] [Google Scholar]
- 26.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 27.Venturelli M, Villa F, Ruzzante F, Tarperi C, Rudi D, Milanese C, Cavedon V, Fonte C, Picelli A, Smania N, Calabria E, Skafidas S, Layec G, Schena F. Neuromuscular and muscle metabolic functions in MELAS before and after resistance training: a case study. Front Physiol 10: 503, 2019. doi: 10.3389/fphys.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giuriato G, Ives SJ, Tarperi C, Bortolan L, Ruzzante F, Pedrinolla A, Martignon C, Laginestra FG, Cevese A, Schena F, Venturelli M. Timed synchronization of muscle contraction to heartbeat enhances muscle hyperemia. J Appl Physiol (1985) 128: 805–812, 2020. doi: 10.1152/japplphysiol.00898.2019. [DOI] [PubMed] [Google Scholar]
- 29.Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199: 367–383, 2010. doi: 10.1111/j.1748-1716.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 31.Smith SA, Micklewright D, Winter SL, Mauger AR. Muscle pain induced by hypertonic saline in the knee extensors decreases single-limb isometric time to task failure. Eur J Appl Physiol 120: 2047–2058, 2020. doi: 10.1007/s00421-020-04425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darques JL, Decherchi P, Jammes Y. Mechanisms of fatigue-induced activation of group IV muscle afferents: the roles played by lactic acid and inflammatory mediators. Neurosci Lett 257: 109–112, 1998. doi: 10.1016/s0304-3940(98)00816-7. [DOI] [PubMed] [Google Scholar]
- 33.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Falces J, Place N. Recruitment order of quadriceps motor units: femoral nerve vs. direct quadriceps stimulation. Eur J Appl Physiol 113: 3069–3077, 2013. doi: 10.1007/s00421-013-2736-2. [DOI] [PubMed] [Google Scholar]
- 35.Vanderthommen M, Duteil S, Wary C, Raynaud JS, Leroy-Willig A, Crielaard JM, Carlier PG. A comparison of voluntary and electrically induced contractions by interleaved 1H- and 31P-NMRS in humans. J Appl Physiol (1985) 94: 1012–1024, 2003. doi: 10.1152/japplphysiol.00887.2001. [DOI] [PubMed] [Google Scholar]
- 36.Alexandre F, Derosiere G, Papaiordanidou M, Billot M, Varray A. Cortical motor output decreases after neuromuscular fatigue induced by electrical stimulation of the plantar flexor muscles. Acta Physiol 214: 124–134, 2015. doi: 10.1111/apha.12478. [DOI] [PubMed] [Google Scholar]
- 37.Papaiordanidou M, Guiraud D, Varray A. Kinetics of neuromuscular changes during low-frequency electrical stimulation. Muscle Nerve 41: 54–62, 2010. doi: 10.1002/mus.21427. [DOI] [PubMed] [Google Scholar]
- 38.Baumer T, Munchau A, Weiller C, Liepert J. Fatigue suppresses ipsilateral intracortical facilitation. Exp Brain Res 146: 467–473, 2002. doi: 10.1007/s00221-002-1202-x. [DOI] [PubMed] [Google Scholar]
- 39.Huang HJ, Ferris DP. Neural coupling between upper and lower limbs during recumbent stepping. J Appl Physiol (1985) 97: 1299–1308, 2004. doi: 10.1152/japplphysiol.01350.2003. [DOI] [PubMed] [Google Scholar]
- 40.Barss TS, Ainsley EN, Claveria-Gonzalez FC, Luu MJ, Miller DJ, Wiest MJ, Collins DF. Utilizing physiological principles of motor unit recruitment to reduce fatigability of electrically-evoked contractions: a narrative review. Arch Phys Med Rehabil 99: 779–791, 2018. doi: 10.1016/j.apmr.2017.08.478. [DOI] [PubMed] [Google Scholar]
- 41.Collins DF. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc Sport Sci Rev 35: 102–109, 2007. doi: 10.1097/jes.0b013e3180a0321b. [DOI] [PubMed] [Google Scholar]
- 42.Boerio D, Jubeau M, Zory R, Maffiuletti NA. Central and peripheral fatigue after electrostimulation-induced resistance exercise. Med Sci Sports Exerc 37: 973–978, 2005. [PubMed] [Google Scholar]
- 43.Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315, 2016. doi: 10.1113/JP272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matkowski B, Place N, Martin A, Lepers R. Neuromuscular fatigue differs following unilateral vs bilateral sustained submaximal contractions. Scand J Med Sci Sports 21: 268–276, 2011. doi: 10.1111/j.1600-0838.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- 45.Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol 293: R2036–R2045, 2007. doi: 10.1152/ajpregu.00442.2007. [DOI] [PubMed] [Google Scholar]
- 46.Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol 571: 425–439, 2006. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin PG, Rattey J. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch Eur J Physiol 454: 957–969, 2007. doi: 10.1007/s00424-007-0243-1. [DOI] [PubMed] [Google Scholar]
- 48.Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res 150: 308–313, 2003. doi: 10.1007/s00221-003-1379-7. [DOI] [PubMed] [Google Scholar]