Abstract
Exposure to early life stress (ELS) is associated with a greater risk of chronic disease development including depression and cardiovascular disease. Altered gut microbiota has been linked to both depression and cardiovascular disease in mice and humans. Rodent models of early life neglect are used to characterize the mechanistic links between early life stress (ELS) and the risk of disease later in life. However, little is understood about ELS exposure and the gut microbiota in the young mice and the influence of the maternal inheritance of the gut microbiota. We used a mouse model of ELS, maternal separation with early weaning (MSEW), and normally reared mice to determine whether the neonate microbiota is altered, and if so, are the differences attributable to changes in dam microbiota that are then transmitted to their offspring. Individual amplicon sequence variants (ASVs) displayed differential abundance in the microbiota of MSEW compared with normally reared pups at postnatal day (PD) 28. Additionally, ELS exposure reduced the alpha diversity and altered microbial community composition at PD28. The composition, levels of alpha diversity, and abundance of individual ASVs in the microbiota of dams were similar from MSEW or normally reared cohorts. Thus, the observed shifts in the abundance of individual bacterial ASVs in the neonates and young pups are likely driven by endogenous effects of MSEW in the offspring host and are not due to inherited differences from the dam. This knowledge suggests that exposure to ELS has a direct effect on microbial factors on the risk of chronic disease development.
Keywords: early life stress, gut microbiota, maternal inheritance, microbiome
INTRODUCTION
The long-term health effects of early life stress (ELS) are well documented; abuse, neglect, and household dysfunction during childhood increase the risk of chronic diseases such as depression and anxiety, cardiovascular disease, and autoimmune diseases later in life (1–4). However, little is known about the impact of ELS on the individual before adulthood, including how exposure to ELS may affect the trajectory of microbial colonization of the gastrointestinal (GI) tract. A number of studies suggest that experiencing chronic stress as an adult can alter microbial composition, and that gut microbiota can in turn shape the host’s stress responsiveness (5–7). The gut is the most heavily colonized area of the human body with concentrations ranging from 1011 to 1012 bacteria per gram in the colon (8). The gut microbiota plays a key role in host health by protecting against pathogen colonization, metabolizing nutrients indigestible by the host, influencing nutrient absorption, and producing hormones, neurotransmitters, and other signaling molecules (7, 9, 10). Therefore, the previously characterized effects of ELS and chronic disease development in adults may have their origins in adversity-dependent effects on composition and functions of the gut microbiota.
The human neonatal intestinal tract is gradually populated with maternally and environmentally derived microbes, which combine to form a stable community within 2–3 years (11, 12). In mouse models, cross-fostering experiments have shown the importance of maternal transfer of microbiota in the first hours following birth (13). Similar to humans, the gut microbiota of mice undergoes a period of maturation over the first 12 wk of life before a stable adult-like community is reached (14). In humans and rodents, colonization and maturation of the gut microbiota coincides with critical development windows for the immune and central nervous systems (CNS) (9, 15, 16). Perturbations in the trajectory of gut microbiota colonization can lead to immune and GI diseases, metabolic disorders, and neurodevelopmental impairments (15–17). Rodent models of ELS involving maternal separation have shown that exposure to stress as neonates leads to altered microbial composition in adult animals (18–20). However, since these analyses were conducted at least 7–20 wk following termination of the stressor, it is still not known when the effects of stress on the microbiota actually begin to manifest. Second, it is unknown if the dams also experience stress-dependent alterations in their microbiota due to separation from their pups. Considering that the neonate is initially colonized by maternal microbiota, it is reasonable that maternal separation-induced microbial differences in the dams would be inherited by their progeny. Therefore, we hypothesized that exposure to ELS directly alters the diversity, abundance, and composition of microbial taxa in young mice. To test this hypothesis, we pursued two major objectives. First, we examined whether ELS-induced changes in the gut microbiota begin to manifest in neonate and adolescent mice. Second, we sought to determine if any potential effect(s) of ELS on the microbiota may be attributed to stress-induced differences in the maternal microbiota or whether they are due to direct effects of ELS on the pup.
We utilized a mouse model of ELS, maternal separation with early weaning (MSEW), which has been used to characterize the mechanistic links between ELS and depression, anxiety, and cardiovascular disease risk in adult life (4, 21–23). We show that MSEW induces shifts in the abundances of specific bacterial taxa, a reduction of microbial diversity, and altered community composition at PD28, with a reduction in potentially beneficial taxa and an expansion of opportunistic taxa. No appreciable effects of ELS on the dam microbiota were found, indicating that MSEW-induced microbial differences in the pups were not inherited from their mothers. Collectively, our study establishes that ELS has a direct impact on the neonate gut microbiota and are likely due to endogenous mechanisms within the offspring.
MATERIALS AND METHODS
Animals
C57BL/6J mice were purchased from Jackson Labs (Bar Harbor, ME) and bred after 2 wk of acclimation to the animal housing facility. Mice were maintained under a 12-h/12-h light-dark cycle in specific pathogen-free conditions with sterilized bedding and free access to standard chow (NIH-31; NSN 8710-01-005-8438) and sterile water. Male-female breeding pairs were cohoused for 17 days after which the males were removed, and females were observed daily to document the exact day of birth termed postnatal day zero (PD0). All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Maternal Separation with Early Weaning
The Maternal Separation with Early Weaning (MSEW) protocol was performed as previously described (24). Briefly, from PD2 to PD5 MSEW litters were separated from dams for 4 h each day (0900 h to 1300 h). From PD6 to PD16, pups were separated for 8 h each day (0900 h to 1700 h), and the MSEW group were weaned at PD17 with free access to food and water. During the separation period, pups were housed in an incubator [Animal Intensive Care Unit (AICU) by Lyon Technologies, Catalog No. 912-062], which was maintained at 37.5°C and 60% humidity. Normally reared, control litters remained undisturbed with dams until weaning on PD21. All mice were maintained on standard chow (NIH-31; NSN 8710-01-005-8438) following weaning. Two rounds of breeding were conducted in which the MSEW treatment or normal rearing assigned to each dam was switched in a crossover design, i.e., dams with normally reared control litters in the first breeding round had litters assigned to the MSEW treatment in the second breeding round or dams with MSEW treatment in the first breeding round had litters assigned to the normally reared control litters in the second breeding round. After their litters were weaned, females were allowed to rest for a minimum of 7 days before being mated again. Microbial samples were sequenced from a total of 11 litters produced by six dams in this study, totaling 68 pups (PD2: n = 9 normally reared and 9 MSEW; PD10: n = 9 normally reared and 16 MSEW; PD28: n = 13 normally reared and 12 MSEW). We have previously shown that the MSEW protocol does not affect the trajectory of body weight gain of pups throughout the separation periods as well as up to 12 wk of age (24). To determine whether the MSEW protocol affected weight gain in the present study, body weights were determined for MSEW (n = 14) and control pups (n = 17) at PD2, 5, 11, and 15 from a separate breeding round that was conducted in the same manner and the same facility over the same timeframe as the two breeding rounds from which microbiota samples were sequenced. Generalized linear mixed models (GLMMs) were used to compare the body weights for MSEW and control pups at each PD timepoint using lme4 v1.1.21 (71) and adjusted for dam identity as a random factor using the form: body weight ∼ Treatment + (1 | Dam Identity). F tests, denominator degrees of freedom, and P values were approximated with Satterthwaite’s degrees of freedom method using lmerTest v3.1.1 (72).
Sample Collection
On PD2, two pups from each litter were terminated using sterile instruments, and the colon was removed and butterflied open to collect the entire luminal contents into a sterile microfuge tube. On PD10, half of the remaining pups from each litter were anesthetized with 2.5% isoflurane, and colonic luminal contents were collected in the same manner as PD2 pups. It was necessary to collect the entire luminal content of PD2 and PD10 pups to assure sufficient DNA yields for sequencing. The other half of the remaining pups in each litter were aged to PD28, anesthetized (isoflurane), and the most distal luminal pellet was collected into a sterile microfuge tube. When possible, a sample was collected from at least 1 male and 1 female pup per litter at each timepoint. One pellet was collected at weaning from each dam for both of her breeding cycles. To collect pellets, dams were removed from their cage into sterile containers for a period of 2–5 min and immediately returned to their cage once a pellet was produced. All samples were immediately flash frozen in liquid nitrogen before being transferred to −80°C. Metadata associated with each microbial sample sequenced in this study is included in Supplemental Table S1 (all Supplemental material is available at http://doi.org/10.5281/zenodo.4485113).
DNA Extraction and Sequencing
DNA from the luminal contents of PD2 and PD10 pups was extracted using the Zymo Quick-DN Fecal/Soil Microbe Microprep Kit (Zymo Research Corp., Irvine, CA, D6012). PD28 luminal pellets and fecal pellets collected from dams were extracted using the Zymo Quick-DN Fecal/Soil Microbe Miniprep Kit (D6010). All samples were mechanically disrupted in a high-speed bead beater (Biospec Products, Bartlesville, OK) for 45 s and then extracted following the manufacturer’s protocol. PD2 and PD10 samples were eluted in 20 µL and PD28 and dam samples were eluted in 100 µL of Zymo DNA Elution Buffer. Reagent-only controls were processed through each of the extraction kits, and no DNA was recovered from these extraction controls. All samples were quantified using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA) and the Qubit dsDNA BR Assay kit. Each sample was normalized to 15 ng/µL and sent to the Georgia Genomics and Bioinformatics Core at the University of Georgia (Athens, GA) for paired-end sequencing on a Illumina MiSeq flow cell v2 with a 500 cycle (PE250) reagent kit. The V4 region of the 16S rRNA gene was amplified using the primers 515FB18 (5′-GTGYCAGCMGCCGCGGTAA) and 806RB19 (5′-GGACTACNVGGGTWTCTAAT) modified from Caporaso and colleagues (27).
Sequence Quality Control and Initial Processing
Raw sequences were trimmed of Illumina adapters using trimmomatic v0.39 (28). Then, primers were removed using cutadapt v2.10 in the linked primer mode (29). The quality of trimmed reads was verified by Fastqc v0.11.9 (30) and MultiQC v1.9 (31). Further processing of amplicon libraries was completed in R v4.0.3 (32). Exact amplicon sequence variants (ASVs) were inferred from raw reads using DADA2 v1.16.0 (33) following the bioinformatic workflow of Callahan et al. (34). DADA2 was used for quality filtering with the options maxEE = c(3,3) and truncLen = c(220,150), dereplication, error estimation, inference of ASVs with the pool = "pseudo" option, and merging of paired reads. Merged reads were required to pass filtering within a 252–254-bp window, the expected size range of the amplified 16S rRNA gene V4 hypervariable region. Taxonomy was assigned to ASVs with DADA2 using the SILVA small subunit ribosomal RNA database v138 (35). Then, a second step was implemented with the DADA2 addSpecies command to make species-level assignments based on exact matching (or 100% identity) between ASVs and sequenced reference strains. A multiple alignment of ASVs was performed with DECIPHER v2.16.1 (36) and used to build a phylogenetic tree de novo (reference-free) with phangorn v2.5.5 (37).
The resulting ASV and taxonomy tables, phylogenetic tree, and metadata were then imported R (32) using phyloseq v1.32.0 (38) for visualizations and statistical analyses. Sequences that could not be assigned as bacteria or archaea or that were identified as mitochondria were removed from further analysis. ASVs that had a mean read count across all samples of less than 5 were filtered from the data set to reduce artifacts in the data due to PCR and sequencing error. One PD2 control sample with less than 3,000 reads was removed from the data set.
Differential Abundance Testing of Microbial Taxa
Analysis of composition of microbiomes (ANCOM) was used to test for differential abundance of individual ASVs between MSEW and normally reared groups for PD2, PD10, and PD28 samples (39). The raw count ASV table was filtered to remove ASVs that were not present in at least 30% of the samples. The ANCOM script was downloaded from https://github.com/sidhujyatha/ANCOM and run on centered log-ratio transformed (CLR) count data using a Benjamini–Hochberg-corrected significance level of 0.05 and adjusting for Dam Identity as a covariant. ANCOM calculates a W test statistic that reflects the number of times that the log ratio of a given taxa compared with each of the other taxa present in the microbial community is detected to be significantly different across the treatment groups. The ANCOM detection limit was set to the default value of 0.7 so that only ASVs differentially abundant in at least 70% of samples are reported. Paired ANCOM was conducted for dam samples to test whether ASVs of an individual dam changed in abundance with treatment assignment. This was done by setting Dam Identity as the repeat.var option with all other parameters being equal to those utilized for pup data. ANCOM was repeated on ASV CLR-transformed count data that was summarized at the Genus and Family level with phyloseq for PD2, PD10, PD28, and dam samples. Differential abundance of ASVs was visualized with heat maps that were generated by the R package pheatmap v1.0.12 (26) based on CLR-transformed count data.
Alpha Diversity
Two measurements of alpha diversity (within sample diversity), Chao1 species richness and Faith’s phylogenetic diversity, were estimated based on data rarefied to 3,200 sequences per sample using the vegan v2.4.2 and btools v0.0.1 packages (25, 40). Alpha diversity metrics were checked for normality with histograms and normal probability plots and Shapiro–Wilk normality tests. Log transformation was applied to data that failed the normality checks and normality improvement was verified in the same manner. The crossover design, in which dams that were assigned to the control treatment in the first breeding round were then assigned to the MSEW treatment in the second breeding, and vice versa, allowed for paired t tests to be conducted on the alpha diversity metrics of individual dams.
Generalized linear mixed models (GLMMs) were used to compare the alpha diversity for MSEW and control pups at each PD timepoint using lme4 v1.1.21 (71) and adjusted for dam identity as a random factor using the form: alpha diversity metric ∼ Treatment + (1 | Dam Identity). F tests, denominator degrees of freedom, and P values were approximated with Satterthwaite’s degrees of freedom method using lmerTest v3.1.1 (72).
Community Composition (Beta Diversity)
Compositional differences (beta diversity) were calculated based on the Aitchison distance, which is calculated as the Euclidean distance of centered log-ratio transformed (CLR) count data (41). Before the CLR transformation with the CoDaSeq package v0.99.6 (42) was performed, zero counts were imputed using the Bayesian-multiplicative replacement function cmultRepl in the zCompositions package v1.3.4 (43). Principal component analysis of the Aitchison distance was performed with prcomp in the R base stats package and plotted with ggplot2 (44). Significance of compositional differences was evaluated with permutational multivariate analysis of variance (PERMANOVA) using the vegan function “adonis” with 9,999 permutations and the linear model: Aitchison distance ∼ Treatment, strata = Dam Identity. The strata function allowed permutations in a paired manner based on dam identity. A linear model was used because the adonis package does not support PERMANOVA of GLMMs. An Aitchison distance matrix was also calculated for dam samples to examine potential shifts in community composition when individual dams were assigned to control and MSEW treatments. Next, permutation tests for homogeneity in multivariate dispersion (PERMDISP) were conducted using the vegan function “betadisper.” This function quantifies the distances from each sample to its group’s centroid and was used to compare the mean dispersion level for control and MSEW groups. Together PERMANOVA and PERMDISP provide a comprehensive analysis of changes in microbial composition by evaluating between-group and within-group differences, respectively.
Similarity of Pup and Dam Microbiota
To address whether the MSEW protocol, and reduced maternal contact, affected the level of similarity between the microbiota of pups and their mothers, the number of ASVs each pup shared in common with its mother (referred to as shared ASVs) was calculated. Additionally, the proportion of the gut microbial community composed of shared ASVs was analyzed based on relative abundance. Finally, the mean Aitchison distance between each pup and its respective mother was compared for MSEW and normally reared control groups. These two measures of similarity were conducted for both PD10 and PD28 pups, and significance was assessed adjusting for dam identity as a random factor using the GLMM: similarity metric ∼ Treatment + (1 | dam identity), and F tests, denominator degrees of freedom, and P values were approximated with Satterthwaite’s degrees of freedom method using lmerTest v3.1.1 (72).
RESULTS
Weight Gain
No significant difference in body weight between mice normally reared or exposed to MSEW was observed during the separation protocol (Supplemental Fig. S1 and Supplemental Table S2).
Differentially Abundant Amplicon Sequence Variants
A total of ∼10.23 million sequences passed initial quality filtering of amplicon sequence variants (ASVs), and filtering rare amplicon sequence variants (ASVs) from the data set resulted in ∼10.20 million sequences with an average of 127,450 sequences per sample. Analysis of composition of microbiomes (ANCOM) was utilized to examine ASVs that had statistically different abundance between treatment groups at PD2, PD10, and PD28 and adjusted for Dam Identity/Litter. ANCOM analysis did not identify any differentially abundant ASVs between treatments in baseline samples collected at PD2. ANCOM identified one ASV classified as Enterococcus (family Enterococcaceae) that was significantly lower in PD10 MSEW samples relative to normally reared control samples (Fig. 1A, Supplemental Table S3). However, PD10 samples did cluster based only on treatment or litter. Twenty-three ASVs were enriched in PD28 control samples, 11 of which were classified as members of the Lactobacillaceae family, five were classified as members of the Clostridiaceae family, three were classified as members of the Oscillospiraceae family, and the remaining two were classified as members of the Ruminococcaceae family (Fig. 1B, Supplemental Table S4). Nine ASVs were more abundant in PD28 MSEW samples relative to controls, of which two were classified as Clostridium senso stricto 1 and three were classified as Lachnoclostridium. PD28 pup samples clustered into two distinct groups that reflected treatment based on significant differential abundance of 32 ASVs and minor subgroupings reflecting litter.
Figure 1.
ELS exposure significantly altered the abundance of amplicon sequence variants (ASVs) at PD10 and PD28. Differentially abundant ASVs identified by analysis of composition of microbiomes (ANCOM) analysis in MSEW relative to normally reared control pups at PD10 (n = 9 control animals and 16 MSEW animals) (A) and PD28 (n = 13 control animals and 12 MSEW animals) (B). ANCOM analysis utilized the centered log-ratio (CLR) transformed ASV count table and adjusted for dam identity. The threshold for significance was set to a Benjamini–Hochberg false discovery rate-corrected P value < 0.05. Abundance of significant ASVs is shown based on CLR transformation of the ASV count table. ASV color corresponds to the SILVA138 SSU database annotation. ELS, early life stress; MSEW, maternal separation with early weaning; PD, postnatal day.
Differentially Abundant Genera and Families at PD28
Related taxa, with similar niches or functional traits, may respond in a similar manner to drivers of microbial abundance (45). Thus, differential abundance patterns at the ASV level may be reflected at higher taxonomic levels. Alternatively, the effects of the driver may be diffuse with no single ASV responding in a consistent and significant manner across a host population, whereas the summarized change of all ASVs belonging to a particular taxonomic group, with similar ecological niches within the gut environment, is statistically significant. ANCOM revealed three bacterial genera, Tyzzerella, Ruminococcus, and Oscillospiraceae uncultured genus-level group (UCG)-005, were significantly depleted in MSEW mice compared with normally reared control mice at PD28 (Fig. 2A, Supplemental Table S7). The relative abundance of these genera and the ASVs that comprise them is shown in Supplemental Fig. S2. ANCOM at the family level detected one bacterial family, Peptostreptococcaceae, that was enriched in MSEW mice compared with normally reared control mice at PD28 (Fig. 2B, Supplemental Table S8) though no ASVs belonging to this family were significantly different alone. The relative abundance of Peptostreptococcaceae at the family and ASV levels at PD28 is shown in Supplemental Fig. S3. At PD10, ANCOM detected that the Enterococcus genus and the Enterococcaceae family were enriched in normally reared control animals compared with MSEW animals (Supplemental Tables S5 and S6). Upon further investigation, it was found that these taxonomic groups contained only a single ASV (ASV22). Thus, ANCOM results at these higher taxonomic levels are reflective of ANCOM results at the ASV level for PD10 samples. No bacterial genera or families were differentially abundant between normally reared control and MSEW mice at PD2.
Figure 2.
ELS exposure altered microbial composition and diversity at PD28. Analysis of composition of microbiomes (ANCOM) differential abundance volcano plots at the bacterial genus (A) and family (B) levels. ANCOM analysis utilized the centered log-ratio (CLR) transformed ASV count table and adjusted for Dam Identity. In A and B, the W value represents the number of times the ratio between the given taxon and each of the other taxa in the community was significantly different between the two treatment groups. W = 30 means the ratio of the given taxon and 30 other taxa were detected to be significantly different between the two treatment groups. The threshold for significance was set to a Benjamini–Hochberg false discovery rate corrected P value < 0.05. The x-axis value represents the CLR transformed mean difference in abundance of a given taxon between the MSEW and normally reared control groups. A positive x-axis means a taxon is enriched in MSEW pups (colored red) and a negative x-axis value means a taxon is depleted in MSEW pups (colored black). All nonsignificant taxa are shown in gray. Only significant taxa are labeled. Alpha diversity metrics were reduced in PD28 samples: mean (± SE) Chao1-estimated richness (C) and mean (± SE) Faith’s phylogenetic diversity (D). In C and D, P values denote significance of Treatment from the Satterthwaite’s approximation of F tests for generalized linear mixed effects models (GLMMs) while adjusting for Dam Identity. ELS exposure significantly altered microbial composition as visualized by principal component analysis (PCA) of Aitchison distances of CLR-transformed counts (E). In E, each solid point corresponds to an individual sample and open circles represent the estimated mean of each treatment. The P value indicates significance of grouping by treatment with permutational analysis of variance (PERMANOVA), and dashed ovals represent 95% confidence intervals of groupings. For all panels, n = 13 normally reared control animals and n = 12 MSEW animals. ASV, amplicon sequence variant; ELS, early life stress; MSEW, maternal separation with early weaning; PD, postnatal day.
ELS Exposure Significantly Reduced Microbial Alpha Diversity at PD28
The alpha diversity metrics Chao1-estimated ASV richness and Faith’s phylogenetic diversity were compared for normally reared control and MSEW samples at each PD timepoint using generalized linear mixed models (GLMMs) with Treatment and the random factor Dam Identity as predictors. Alpha diversity values and results from GLMMs are provided in Supplemental Tables S9 and S10. Log transformation was applied to PD10 Chao1 data and PD2 phylogenetic diversity data before statistical analysis. Improved normality of transformed data was verified with histograms and normal probability plots and Shapiro–Wilk normality tests. All other alpha diversity data were normally distributed and did not require transformation. There were no statistically significant differences in alpha diversity levels between MSEW and normally reared control microbiota for Chao1 ASV richness at PD2 (Treatment: F(1,14.2) = 1.04, P = 0.3) and PD10 (Treatment: F(1,23.0) = 0.70, P = 0.4). There was also no difference in Faith’s phylogenetic diversity between treatment groups at PD2 (Treatment: F(1,13.8) = 0.59, P = 0.5) and PD10 (Treatment: F(1,23.0) = 0.25, P = 0.6). However, at PD28 MSEW microbiota had lower Chao1 ASV richness (Fig. 2C, 175 ± 8 compared with 198 ± 8 in control samples; Treatment: F(1,20.4) = 5.0, P = 0.04) and lower phylogenetically diversity (Fig. 2D, 18.7 ± 0.3 compared with 20.3 ± 0.3 in control samples; Treatment: F(1,19.0) = 11.95, P = 0.003).
ELS Exposure Significantly Altered Microbial Composition at PD28
Microbial composition was compared for normally reared control and MSEW samples at each PD timepoint for Aitchison distance matrices of centered log-ratio transformed (CLR) counts using permutational analysis of variance (PERMANOVA) of linear models with Treatment and Dam Identity as predictors (Supplemental Table S11). No compositional differences were detected between pups assigned to normally reared control and MSEW at PD2 before the separation protocol began (Treatment: F(1,18) = 1.03, P = 0.3). There were no statistically significant compositional differences at PD10, after 8 days of separation (Treatment: F(1,24) = 1.52, P = 0.06). However, MSEW significantly altered microbial composition by PD28 (Fig. 2E, Treatment: F(1,24) = 2.29, P = 0.002). There was no difference in group dispersions of PD28 normally reared control samples (44.7 ± 1.0) and PD28 MSEW samples (43.1 ± 2.4; PERMDISP, F(1,24) = 0.44, P = 0.5). This indicates that compositional differences detected by PERMANOVA analyses were due to PD28 normally reared control and MSEW samples occupying a different location in ordination space (i.e., a shift in community composition), and not an artifact of variances in group dispersion.
Effect of ELS Exposure on the Level of Similarity between Pup and Dam Microbiota
There was no difference in the number of ASVs that a pup shared with its mother between treatment groups at PD10 (Fig. 3A, Supplemental Table S12; Treatment: F(1,23.0) = 0.46, P = 0.5). However, at PD28, MSEW mice shared fewer ASVs with their mothers compared with normally reared control animals (Fig. 3B, Treatment: F(1,20.3) = 27.58, P < 0.001). The total relative abundance of all ASVs that a pup shared with its mother was no different for normally reared control and MSEW pups at PD10 (Fig. 3C, Treatment: F(1,21.6) = 0.45, P = 0.51). PD28 MSEW and control mice had similar total relative abundance of ASVs (Fig. 3D, Treatment: F(1,18.5) = 2.5, P = 0.1). The mean Aitchison distance between the microbial community composition of pups and their respective mothers was higher for the MSEW group compared with the normally reared control group at PD10 (Fig. 3E, Treatment: F(1,19.9) = 12.71, P = 0.002). At PD28 this trend was reversed, and the Aitchison distance between MSEW pups and their respective mothers was greater than that between normally reared control pups and their respective mothers (Fig. 3F, Treatment: F(1,15.0) = 4.99, P = 0.04).
Figure 3.
Similarity between pups and their respective mothers. The number of amplicon sequence variants (ASVs) that a pup shared in common with its mother at PD10 (A) and PD28 (B). Total relative abundance of ASVs that a pup shared with its mother for normally reared control and MSEW groups at PD10 (C) and PD28 (D). The level of similarity between each pup’s microbial community composition and that of its mother for normally reared control and MSEW groups at PD10 (E) and PD28 (F). For all panels, error bars depict mean ± SE and P values denote significance from the Satterthwaite’s approximation of F tests for generalized linear mixed effects models (GLMMs). For A, C, and E, n = 9 control and n = 16 MSEW PD10 animals and for B, D, and F, n = 13 control and n = 12 MSEW PD28 animals. MSEW, maternal separation with early weaning; PD, postnatal day.
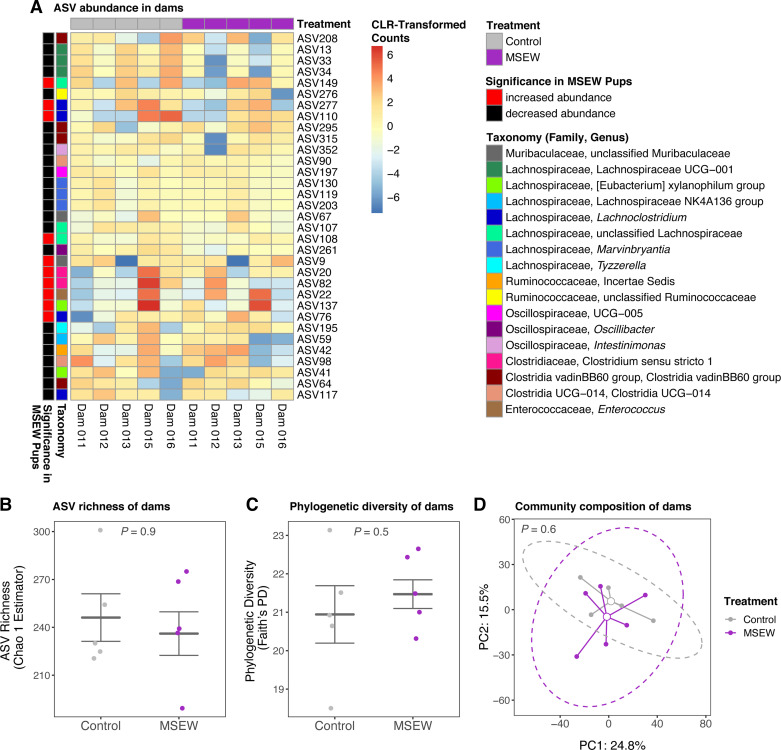
Separation and Early Weaning Did Not Affect the ASV Abundance, Diversity, or Composition of Dam Microbiota
No ASVs changed significantly in abundance within the microbiota of dams when they were assigned to normally reared control versus MSEW treatments based on paired ANCOM. All of the ASVs that were differentially abundant at PD10 and PD28 were present in dam samples, and the abundance pattern of these 33 ASVs across dam samples was random with respect to treatment (Fig. 4A). Likewise, no taxa were significantly different in dams between treatment assignments based on paired ANCOM at the Genus and Family levels. The alpha diversity of dam microbiota was not affected by treatment assignment. One dam did not have a litter in the second breeding round and was not included in paired t tests of alpha diversity metrics. Excluding this individual sample did not significantly change group means of alpha diversity metrics for dams (Supplemental Table S9). Chao1-estimated ASV richness did not differ when dams were assigned to MSEW (242 ± 15) or normally reared control treatments (Fig. 4B, 246 ± 15; n = 5; t(4) = 0.16, P = 0.9). Faith’s phylogenetic also did not change when dams were assigned to MSEW (21.6 ± 0.4) or normally reared control treatments (Fig. 4C, 20.9 ± 0.7; n = 5; t(4) = −0.66, P = 0.5). The microbial community composition quantified by Aitchison distance did not shift in dams with treatment assignment based on paired PERMANOVA analysis that stratified permutations within the factor Dam Identity in a paired test (Fig. 4D, Supplemental Table S11; n = 5 control and n = 6 MSEW; Treatment: F(1,10) = 0.80, P = 0.6).
Figure 4.
No effect of maternal separation on dam microbiota. Analysis of composition of microbiomes (ANCOM) analysis did not identify any amplicon sequence variants (ASVs) as significantly different when dams (n = 5 animals) were assigned to MSEW or normally reared control treatments. ANCOM analysis utilized the centered log-ratio (CLR) transformed ASV count table and was conducted in a paired manner based on Dam Identity. For visualization, the CLR transformation of raw count abundance of the ASVs that were identified as significantly different in PD10 and PD28 pups is shown (A). There was no difference in the alpha diversity metrics mean (± SE) estimated Chao1 richness (B) and mean (± SE) Faith’s phylogenetic diversity (C). In B and C, P-values denote significance from the Satterthwaite’s approximation of F tests for generalized linear mixed effects models (GLMMs). Community composition of dams did not shift with treatment assignment as visualized by principal component analysis (PCA) of Aitchison distances of CLR-transformed counts. In D, each solid point corresponds to an individual sample and open circles represent the estimated mean of each treatment. The P value indicates significance of grouping by Treatment with paired permutational analysis of variance (PERMANOVA) that stratified permutations by Dam Identity and dashed ovals represent 95% confidence intervals of grouping. MSEW, maternal separation with early weaning; PD, postnatal day.
DISCUSSION
Adverse childhood experiences (ACEs) or early life stress (ELS), such as abuse, neglect, and household dysfunction during childhood, are well-recognized to be associated with negative outcomes in long-term adult health especially in the development of depression, anxiety, hypertension, and cardiovascular disease (2–4). Rodent models of early life neglect have been utilized to determine the mechanistic links between ELS exposure and risk of developing depression and anxiety (23) as well as hypertension and cardiovascular disease later in life (4). Rodent models of ELS have only recently been applied to understanding how stress exposure in early life may affect the gut microbiota (18–20). We designed studies in mice to fill key knowledge gaps in the literature with respect to the impact of ELS on the gut microbiota during the neonatal to adolescent timeframe as well as on the microbiota of dams. Specifically, the objectives of this study were to determine when the effects of maternal separation with early weaning (MSEW) on the gut microbiota of mice begin to manifest and whether any of these effects may be attributed to heritable impacts of stress on the maternal microbiota that is subsequently transferred to the offspring.
The main findings of this study documented the following differences with exposure to ELS and normally reared mice: 1) shifts in the abundance of individual bacterial amplicon sequence variants (ASVs) and overall compositional differences in offspring were found as early as postnatal day (PD) 28, and, 2) no detectable effects of ELS exposure on the dam microbiota were found. Overall, we conclude that exposure to ELS impacts microbial colonization of the gut independent of maternal inheritance of microbiota. The distinct microbial colonization occurs during the critical developmental window (adolescence) for the central nervous system, immune system, and metabolic programming (15, 16, 46).
Maternally derived microbiota are the first to colonize the infant intestinal tract (12). It is possible that in rodent models involving maternal separation, dams also experience cumulative stress with possible consequences for their microbiota. However, previous studies utilizing maternal separation rodent models of ELS have not examined potential changes in dam microbiota (18–20). This current study found no differences in the diversity, composition, and differential abundance of dam gut microbiota when dams were assigned to MSEW or normally reared protocols. Dams may have experienced psychological stress due to separation from their pups, but this did not result in detectable changes in their gut microbiota abundance and composition. We also found no difference in body weights between MSEW and normally reared pups in agreement with previous studies utilizing this mouse model (21, 24). Thus, differences in the microbiota of MSEW pups documented in this study were likely not inherited from their mothers or due to a lower volume of milk intake. It is possible that disruption of dam/pup contact in ELS rodent models involving maternal separation reduces the chance for vertical transmission of microbiota from dams to their offspring. However, we found no difference in the number of shared ASVs between MSEW and control pups and their respective mothers at PD10. There was a significant reduction in the number of shared ASVs between MSEW pups and their respective mothers compared with the control group at PD28. PD28 MSEW mice were also compositionally less similar to their respective mothers, evident by larger Aitchison distance values, compared with normally reared control mice. In the MSEW protocol, separation does not begin until PD2 and cross-fostering studies have shown that maternal transmission of microbiota likely occurs within the first 48 h of birth (13). Thus, this initial period of uninterrupted contact immediately after birth in the MSEW protocol may be sufficient for acquisition of maternal microbiota and may account for why there was no difference in the number of shared ASVs between control and MSEW pups and their respective mothers at PD10. One limitation of this study is that sequencing data from fresh fecal pellets of dams were compared with sequencing data of the most distal luminal pellet of PD28 mice (47). This may account for why some maternal ASVs were not detected in the pup samples. However, this cannot account for the lesser amount of compositional similarity seen between MSEW pups and their respective mothers compared with that between control pups and their respective mothers. The reduction of shared taxa and greater dissimilarity of community composition between MSEW pups and their respective mothers at PD28 is likely driven by stress-dependent host factors affecting the abundance and ability to maintain acquired microbiota. Maternal separation of mice and rats has been shown to promote intestinal hyperpermeability (20, 48, 49), visceral hypersensitivity (19, 20, 50), bile acid malabsorption, and low-grade inflammation (19, 20, 48–50), potentially representing a different ecological niche for colonizing microbiota. Future interventional studies will be helpful in linking ELS-dependent effects on the host to changes in the gut microbial community development.
The microbiota of mice exposed to ELS was enriched with ASVs belonging to the bacterial genera Clostridium senso stricto 1 and Lachnoclostridium, which may affect the inflammatory state of the host. The three clostridia ASVs that were elevated with ELS exposure fall within the clostridia cluster I (Clostridium sensu stricto), which contains many toxin-producing strains including enterotoxigenic C. perfringes that has been recently implicated in pediatric inflammatory bowel disease (IBD) (51), and neurotoxin-producing C. tenani and C. botulinum (52). Clostridia cluster I is only distantly related to enterotoxigenic C. difficile, which is associated with ulcerative colitis and Crohn’s disease (51, 53), the two major forms of IBD, and clostridia clusters XIVa and IV that contain commensals important for maintaining gut homeostasis and fermenting short-chain fatty acids (SCFAs) (54). The metabolic potential and toxin production of the highly diverse Clostridium genus has yet to be fully described (52), and it is unknown whether the clostridia ASVs associated with ELS in this study have pathogenic potential or act more as beneficial commensals. Likewise, it is difficult to predict the functional role of the differentially abundant Lachnoclostridium ASVs in this study based on current evidence. This newly described genus within the Lachnospiraceae family (55) is strictly anaerobic, spore-forming, and capable of producing short-chain fatty acids (SCFAs) including acetate and butyrate, which may promote intestinal homeostasis (56). Several novel Lachnoclostridium species have been recently isolated from healthy individuals (57, 58), whereas other Lachnoclostridium have been identified as potential biomarkers for chronic kidney disease (59) and colorectal cancer (60). Thus, it is likely that some species within the Lachnoclostridium genus provide more of a protective effect, whereas others have a detrimental health impact.
MSEW mice also had a greater abundance of Peptostreptococcacea, which is a family of strict anaerobes that is associated with colorectal cancer (61, 62). In a mouse model of dextran sulfate sodium (DSS)-induced colitis, chronic stress caused a proliferation of Peptostreptococcacea and degradation of the colonic mucus barrier (7). An enrichment of Peptostreptococcacea in MSEW mice may be an indication of a proinflammatory environment and warrants further investigation.
A reduction of microbial diversity has been associated with “loss of function dysbiosis,” which is defined by shifts in the microbiota that lead to the loss of bacteria that normally provide beneficial functions protective of host health (63). This study found lower alpha diversity estimates for the microbiota of MSEW mice compared with normally reared control mice at PD28. Low microbial diversity is frequently observed in patients with IBD (64, 65), liver disease (66), and some forms of cancer (67) and is often marked by a reduction of Lachnospiraceae and Ruminococcaceae species, which are important anaerobic fermenters of butyrate and other SCFAs (68). In this study, 57% (13 of 23) of the significant ASVs that decreased in abundance at PD28 following ELS exposure were classified as Lachnospiracea (other than Lachnoclostrium spp.) or Ruminococcaceae. In fact, there was a greater than twofold reduction in the genus Ruminococcus in ELS-exposed mice compared with normally reared mice. This suggests that ELS may alter the SCFA-producing component of the developing gut microbiota and affect the balance of SCFAs in early life. SCFAs, mainly acetate, propionate, and butyrate, are known to modulate gut barrier function, induce anti-inflammatory signaling pathways such as activating G protein-coupled receptors (GPCRs), and inhibit histone deacetylase (HDAC), and stimulate histone acetyl transferase (69, 70). Thus, future studies are warranted to determine the effect of ELS on SCFA fermenters and whether a change in SCFA production plays a mechanistic role in the development of ELS-specific phenotypes.
In summary, our study provides new insights into changes in the gut microbiota in early life in a widely used murine model of ELS. We have demonstrated that differentially abundant taxa were observed in neonate mice at PD28, and these changes are likely due to strong endogenous effects rather than inherited differences from the dams. This knowledge promotes the usefulness of the MSEW ELS mouse model to examine host and microbial interactions that may influence the early life stress phenotype in adulthood.
DATA AVAILABILITY STATEMENT
All R scripts necessary to reproduce the statistical analyses and visualizations in this manuscript and the supplemental materials are available at the Github link: https://github.com/KMKemp/ELS. The Github repository and supplemental materials have been archived at the DOI link: http://doi.org/10.5281/zenodo.4485113.
The raw fastq files generated for this study are available under the NCBI BioProject number PRJNA605008.
GRANTS
The authors gratefully acknowledge funding from the Environmental Triggers Research Initiatives Award 558420 from the Crohn’s and Colitis Foundation (to C. L. Maynard and J. S. Pollock) and a National Institute of General Medical Sciences Award K12GM088010 (to K. M. Kemp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.K., R.G.L., C.L.M., and J.S.P. conceived and designed research; K.M.K. and J.C. performed experiments; K.M.K. analyzed data; K.M.K., C.L.M., and J.S.P. interpreted results of experiments; K.M.K. prepared figures; K.M.K. drafted manuscript; K.M.K., R.G.L., C.L.M., and J.S.P. edited and revised manuscript; K.M.K., J.C., R.G.L., C.L.M., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Barbara Klocke for her outstanding technical support throughout this study.
REFERENCES
- 1.Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med 71: 243–250, 2009. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Reprint of: Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 56: 774–786, 2019. doi: 10.1016/j.amepre.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 67: 113–123, 2010. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obi IE, McPherson KC, Pollock JS. Childhood adversity and mechanistic links to hypertension risk in adulthood. Br J Pharmacol 176: 1932–6019, 2019. doi: 10.1111/bph.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25: 397–407, 2011. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural and functional consequences of chronic psychosocial stress on the microbiome and host. Psychoneuroendocrinology 63: 217–227, 2016. doi: 10.1016/j.psyneuen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Cao Q, Cheng Y, Zhao D, Wang Z, Yang H, Wu Q, You L, Wang Y, Lin Y, Li X, Wang Y, Bian J-S, Sun D, Kong L, Birnbaumer L, Yang Y. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci USA 115: E2960–E2969, 2018. [Erratum in Proc Natl Acad Sci USA 115: E4542, 2018]. doi: 10.1073/pnas.1720696115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14: 20–32, 2016. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 13: 260–270, 2012. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab 26: 110–130, 2017. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J, Jun W. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17: 690–703, 2015. [Erratum in Cell Host Microbe 17: 852, 2015]. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA 108: 4578–4585, 2011. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daft JG, Ptacek T, Kumar R, Morrow C, Lorenz RG. Cross-fostering immediately after birth induces a permanent microbiota shift that is shaped by the nursing mother. Microbiome 3: 17, 2015. doi: 10.1186/s40168-015-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schloss PD, Schubert AM, Zackular JP, Iverson KD, Young VB, Petrosino JF. Stabilization of the murine gut microbiome following weaning. Gut Microbes 3: 383–393, 2012. . doi: 10.4161/gmic.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20: 509–518, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science 352: 539–544, 2016. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Mahony SM, Clarke G, Dinan TG, Cryan JF. Early-life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience 342: 37–54, 2017. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 18.De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, Denou E, Silva MA, Santacruz A, Sanz Y, Surette MG, Verdu EF, Collins SM, Bercik P. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun 6: 7735, 2015. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- 19.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65: 263–267, 2009. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Riba A, Olier M, Lacroix-Lamandé S, Lencina C, Bacquié V, Harkat C, Van Langendonck N, Gillet M, Cartier C, Baron M, Sommer C, Mallet V, Zill M, Robert H, Laurent F, Ellero-Simatos S, Théodorou V, Ménard S. Early life stress in mice is a suitable model for irritable bowel syndrome but does not predispose to colitis nor increase susceptibility to enteric infections. Brain Behav Immun 73: 403–415, 2018. doi: 10.1016/j.bbi.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 21.George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci 11: 123–114, 2010. doi: 10.1186/1471-2202-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pryce CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev 29: 649–674, 2005. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep 65: 1451–1461, 2013. doi: 10.1016/s1734-1140(13)71505-6. [DOI] [PubMed] [Google Scholar]
- 24.Ho DH, Burch ML, Musall B, Musall JB, Hyndman KA, Pollock JS. Early life stress in male mice induces superoxide production and endothelial dysfunction in adulthood. Am J Physiol Heart Circ Physiol 310: H1267–H1274, 2016. doi: 10.1152/ajpheart.00016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battaglia T. btools: A suite of R function for all types of microbial diversity analyses. R package version 0.0.1. 2020.
- 26.Kolde R. pheatmap: Pretty Heatmaps. R package version 1.0.12. https://CRAN.R-project.org/package=pheatmap. 2019.
- 27.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108: 4516–4522, 2011. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120, 2014. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17: 10, 2011. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 30.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Cambridge, UK: Babraham Bioinformatics, Babraham Institute, 2010. [Google Scholar]
- 31.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32: 3047–3048, 2016. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. 2020.
- 33.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callahan B, Sankaran K, Fukuyama J, McMurdie P, Holmes S. Bioconductor workflow for microbiome data analysis: from raw reads to community analyses [version 2; peer review: 3 approved]. F1000Res 5: 1492, 2016. doi: 10.12688/f1000research.8986.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaren MR. Silva SSU Taxonomic Training Data Formatted for DADA2 (Silva version 138) (Version 2) [Data set]. Zenodo. http://doi.org/105281/zenodo3986799 2020.
- 36.Wright ES. Using DECIPHER v2.0 to analyze big biological sequence data in R. R J 8: 352, 2016. doi: 10.32614/RJ-2016-025. [DOI] [Google Scholar]
- 37.Schliep KP. phangorn: phylogenetic analysis in R. Bioinformatics 27: 592–593, 2015. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8: e61217–61211, 2013. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis 26: 27663, 2015. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, Ohara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. vegan: Community Ecology Package. R package version 2.4-2. https://CRAN.R-project.org/package=vegan. 2017.
- 41.Kaul A, Mandal S, Davidov O, Peddada SD. Analysis of microbiome data in the presence of excess zeros. Front Microbiol 8: 2114–2114, 2017. doi: 10.3389/fmicb.2017.02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gloor GB, Reid G. Compositional analysis: a valid approach to analyze microbiome high-throughput sequencing data. Can J Microbiol 62: 692–703, 2015. doi: 10.1139/cjm-2015-0821. [DOI] [PubMed] [Google Scholar]
- 43.Palarea-Albaladejo J, Martín-Fernández JA. zCompositions — R package for multivariate imputation of left-censored data under a compositional approach. Chemometr Intell Lab Syst 143: 85–96, 2015. doi: 10.1016/j.chemolab.2015.02.019. [DOI] [Google Scholar]
- 44.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2016. [Google Scholar]
- 45.Philippot L, Andersson SGE, Battin TJ, Prosser JI, Schimel JP, Whitman WB, Hallin S. The ecological coherence of high bacterial taxonomic ranks. Nat Rev Microbiol 8: 523–529, 2010. doi: 10.1038/nrmicro2367. [DOI] [PubMed] [Google Scholar]
- 46.Fall CHD, Kumaran K. Metabolic programming in early life in humans. Philos Trans R Soc Lond B Biol Sci 374: 20180123, 2019. doi: 10.1098/rstb.2018.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozik AJ, Nakatsu CH, Chun H, Jones-Hall YL. Comparison of the fecal, cecal, and mucus microbiome in male and female mice after TNBS-induced colitis. PLoS One 14: e0225079, 2019. doi: 10.1371/journal.pone.0225079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut 53: 501–506, 2004. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varghese AK, Verdu EF, Bercik P, Khan WI, Blennerhassett PA, Szechtman H, Collins SM. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology 130: 1743–1753, 2006. doi: 10.1053/j.gastro.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology 127: 524–534, 2004. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 51.Banaszkiewicz A, Kądzielska J, Gawrońska A, Pituch H, Obuch-Woszczatyński P, Albrecht P, Młynarczyk G, Radzikowski A. Enterotoxigenic Clostridium perfringens infection and pediatric patients with inflammatory bowel disease. J Crohns Colitis 8: 276–281, 2014. doi: 10.1016/j.crohns.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Cruz-Morales P, Orellana CA, Moutafis G, Moonen G, Rincon G, Nielsen LK, Marcellin E. Revisiting the evolution and taxonomy of clostridia, a phylogenomic update. Genome Biol Evol 11: 2035–2044, 2019. doi: 10.1093/gbe/evz096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Therap Adv Gastroenterol 6: 53–68, 2013. doi: 10.1177/1756283X12454590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog 5: 23, 2013. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yutin N, Galperin MY. A genomic update on clostridial phylogeny: gram-negative spore formers and other misplaced clostridia. Environ Microbiol 15: 2631–2641, 2013. doi: 10.1111/1462-2920.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gutiérrez N, Garrido D. Species deletions from microbiome consortia reveal key metabolic interactions between gut microbes. mSystems 4: e00185–e00219, 2019. doi: 10.1128/mSystems.00185-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tidjani Alou M, Lagier JC, La Scola B, Cassir N. ‘Lachnoclostridium massiliosenegalense’, a new bacterial species isolated from the human gut microbiota. New Microbes New Infect 14: 4–5, 2016. doi: 10.1016/j.nmni.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Traore SI, Azhar EI, Yasir M, Bibi F, Fournier PE, Jiman-Fatani AA, Delerce J, Cadoret F, Lagier JC, Raoult D. Description of ‘Blautia phocaeensis’ sp. nov. and ‘Lachnoclostridium edouardi’ sp. nov., isolated from healthy fresh stools of Saudi Arabia Bedouins by culturomics. New Microbes New Infect 19: 129–131, 2017. doi: 10.1016/j.nmni.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Su X, Zhang L, Liu Y, Shi M, Lv C, Gao Y, Xu D, Wang Z. Dysbiosis of the gut microbiome is associated with CKD5 and correlated with clinical indices of the disease: a case–controlled study. J Transl Med 17: 228, 2019. doi: 10.1186/s12967-019-1969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang JQ, Li T, Nakatsu G, Chen YX, Yau TO, Chu E, Wong S, Szeto CH, Ng SC, Chan FKL, Fang JY, Sung JJY, Yu J. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 69: 1248–1257, 2020. doi: 10.1136/gutjnl-2019-318532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst 105: 1907–1911, 2013. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsoi H, Chu ESH, Zhang X, Sheng J, Nakatsu G, Ng SC, Chan AWH, Chan FKL, Sung JJY, Yu J. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology 152: 1419–1433.e5, 2017. doi: 10.1053/j.gastro.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Miller AW, Orr T, Dearing D, Monga M. Loss of function dysbiosis associated with antibiotics and high fat, high sugar diet. ISME J 13: 1379–1390, 2019. doi: 10.1038/s41396-019-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alam MT, Amos GCA, Murphy ARJ, Murch S, Wellington EMH, Arasaradnam RP. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog 12: 1, 2020. doi: 10.1186/s13099-019-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat Commun 8: 1784, 2017. doi: 10.1038/s41467-017-01973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Chen Y, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature 513: 59–64, 2014. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 67.Liu C, Frank DN, Horch M, Chau S, Ir D, Horch EA, Tretina K, van Besien K, Lozupone CA, Nguyen VH. Associations between acute gastrointestinal GvHD and the baseline gut microbiota of allogeneic hematopoietic stem cell transplant recipients and donors. Bone Marrow Transplant 52: 1643–1650, 2017. doi: 10.1038/bmt.2017.200. [DOI] [PubMed] [Google Scholar]
- 68.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol 44: 34–40, 2018. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. Chapter three - the role of short-chain fatty acids in health and disease. In: Adv Immunol, edited by Alt FW. Cambridge, MA: Academic Press, 2014, p. 91–119. [DOI] [PubMed] [Google Scholar]
- 71.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Software 67: 1–48, 2015. doi: 10.18637/jss.v067.i01. 30036705 [DOI] [Google Scholar]
- 72.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: Tests in linear mixed effects models. J Stat Software 82: 1–26, 2017. doi: 10.18637/jss.v082.i13. 30036705 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All R scripts necessary to reproduce the statistical analyses and visualizations in this manuscript and the supplemental materials are available at the Github link: https://github.com/KMKemp/ELS. The Github repository and supplemental materials have been archived at the DOI link: http://doi.org/10.5281/zenodo.4485113.
The raw fastq files generated for this study are available under the NCBI BioProject number PRJNA605008.