Abstract
Light therapy for older persons with dementia is often administered with light boxes, even though indoor ambient light may more comfortably support the diverse lighting needs of this population. Our objective is to investigate the influence of indoor daylight and lighting on the health of older adults with dementia living in long-term care facilities. A systematic literature search was performed within PubMed, CINAHL, PsycINFO, Web of Science and Scopus databases. The included articles (n=37) were published from 1991 to 2020. These articles researched the influence of existing and changed indoor light conditions on health and resulted in seven categories of health outcomes. Although no conclusive evidence was found to support the ability of indoor light to decrease challenging behaviors or improve circadian rhythms, findings of two studies indicate that exposure to (very) cool light of moderate intensity diminished agitation. Promising effects of indoor light were to reduce depressive symptoms and facilitate spatial orientation. Furthermore, there were indications that indoor light improved one’s quality of life. Despite interventions with dynamic lighting having yielded little evidence of its efficacy, its potential has been insufficiently researched among this study population. This review provides a clear and comprehensive description of the impact of diverse indoor light conditions on the health of older adults with dementia living in long-term care facilities. Variation was seen in terms of research methods, (the description of) light conditions, and participants’ characteristics (types and severity of dementia), thus confounding the reliability of the findings. The authors recommend further research to corroborate the beneficial effects of indoor light on depression and to clarify its role in supporting everyday activities of this population. An implication for practice in long-term care facilities is raising the awareness of the increased lighting needs of aged residents.
Keywords: lighting, Alzheimer’s disease, assisted living, nursing homes, indoor daylight, light therapy
Introduction
Although Western governmental policies encourage aging-in-place, the number of persons with dementia living in long-term care facilities is still growing. According to the World Health Organization,1 dementia is one of the major causes of disability and dependency among older people. Therefore, we would expect long-term care facilities to offer an optimal physical environment that would support older persons with dementia and accommodate for their losses. However, the physical environment, including indoor light, is often an undervalued and even ignored resource in dementia care.2–4 Indoor daylight and lighting are essential elements of an optimal environment to compensate for age- and dementia-related sensory changes.5,6
Due to the aging of the eyes, older people have an increasing demand for higher light levels that support good vision and help synchronize their biological clock.7,8 Sufficient light for visual needs helps older people to (independently) execute activities of daily living, hobbies and social activities. The light aids them in not only moving safely but to also feeling safe.9 In addition, high-intensity light during daytime is needed, because it is the strongest cue for synchronizing the biological clock with the 24-hour rhythm of the earth.10,11 In turn, the biological clock plays an important role in the timing and coordination of physiological and psychological processes with a circadian (24 h) rhythm, including hormone levels, body temperature, alertness, urine production and composition, sleep–wake rhythm, mood and performance.12–15 Despite the lighting needs of older persons, the literature shows that the light conditions in long-term care facilities are poor, both for visual needs as well as for entraining the biological clock.16–19
Ensuring good quantity and quality of indoor light is even more important for older persons with dementia. For instance, in winter, this group is more sensitive to circadian disruption than healthy older adults.20 Increasing dementia severity can lead to increasing sleep–wake rhythm disturbances, which in extreme cases may lead to complete day and night sleep pattern reversals.21,22 In addition, specific dementia-related changes in the brain result in difficulties in finding objects, reading, depth perception, perceiving structure from motion, color recognition and impairment in spatial contrast sensitivity.23 It is to be expected that sufficient light that supports good vision can help compensate for these changes as well as improve orientation of older adults with dementia.
Light therapy, which focuses on changes in the circadian pacemaker in the brain, is an emerging therapy within the domain of dementia care.24 While light therapy can be administered in a number of ways, the use of a light box standing on a table in front of the person with dementia is to date the most frequently applied and researched method.25 However, using light boxes for persons with dementia presents some disadvantages. To remain sitting in front of a light box for a minimum of 30 minutes and up to 2 hours per day may be difficult for persons with dementia,26 even if the intervention takes place while performing other activities, such as having meals or watching television. The exposure may require supervision,26,27 which in turn puts a strain on the participants as well as on the supervisor. In contrast, indoor environmental light may be a preferable source because it allows for free movement.28 In recent years, the use of dynamic lighting for this population has become increasingly popular, not in the least due to the often unproven claims of their suppliers. Dynamic lighting changes during the day in illuminance or spectral composition, or both, in such a way that the variation in light can be perceived by people.29 Long-term care facilities have purchased dynamic lighting with the intention of improving the well-being and day–night rhythm of persons with dementia.30
The foregoing paragraphs highlight the importance of indoor daylight and lighting and raises questions about the influence thereof. To date, there is no systematic review published that exclusively documents the influence of indoor environmental light conditions (daylight and lighting) on the health of older persons with dementia living in a long-term care facility. A Cochrane review on the effects of light therapy on persons with dementia included only one study with indoor environmental light.31 Their conclusions were based on studies where the light sources (light boxes, light visors, light fixtures) differed to such an extent that the results might not be comparable. Other reviews did not fully combine all three distinctive elements that this research is interested in: older adults with dementia, indoor environmental light and long-term care facilities. These reviews, for instance, were concerned with light therapy and dementia,32 daylight and health in general33 or a therapeutic lighting design and older adults.34 Therefore, the research question for this systematic literature review is the following: What does scientific literature tell us about the influence of indoor environmental light conditions (daylight and lighting) on the health of older adults with dementia living in a long-term care facility? Health is thereby defined as the ability to adapt and self-manage in the face of social, physical and emotional challenges.35 Compared to the WHO-definition of health,36 this definition of Huber et al better reflects the everyday reality of people with chronic diseases, including people with dementia.
Methods
Search Strategy
To identify relevant empirical studies, we conducted a systematic literature search in five scientific databases. We focused on databases related to health, medicine, nursing, behavioral and social sciences, as well as the built environment. Therefore, the databases PubMed, CINAHL, PsycINFO, Web of Science and Scopus were selected. Published studies were next identified using a search strategy based on the three facets of the research question: persons with dementia, light and long-term care facilities (Supplementary Table). The search string encompassed specific indexing terms and subject headings from the different databases as well as keywords, synonyms and some additional words that best represented the facet. The search included articles published up to May 2020 and resulted in 810 unique articles.
Article Selection
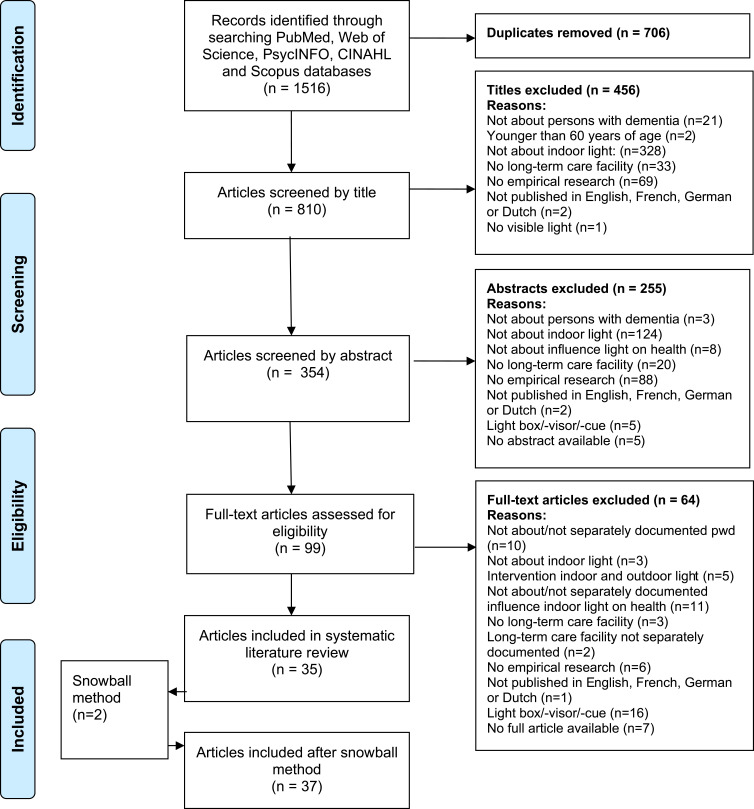
Figure 1 shows the selection process of the articles. In the first selection phase, all duplicates were removed, and the remaining titles (n=810) were screened for inclusion by the first author (IG). When all selection criteria (Table 1) were met or in any cases of doubt, articles proceeded for further screening. In this second phase, two reviewers (IG and MV) independently assessed the abstracts of the remaining articles (n=354) and discussed the eligibility until they reached consensus. If consensus was not reached, the full research team was consulted. In the third phase, an identical procedure was followed for assessing the remaining full-text articles (n=99). Finally, the first author (IG) screened all references of the 35 included articles for any additional potentially relevant articles (snowball method). Eligibility of these articles was again checked by two reviewers independently (IG and LVB) and yielded two additional articles, which resulted in a total of 37 articles in this review.
Figure 1.
PRISMA flowchart of the literature search on the influence of indoor environmental light on the health of older persons with dementia in long-term care facilities. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6(7):e1000097.37
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Research investigating the influence of indoor daylight and/or lighting on health | Research on interventions with (supplementary) light boxes, light visors, light cues or light tables |
| Research in which participants are 60 years and older and have dementia | Research on immune suppression by light |
| Research in which participants live in a long-term care facility | Research aimed at extra visual effects of light on health (eg, through the skin instead of the eye) and/or on effects of non-visible light |
| Empirical research with a qualitative, quantitative or mixed methods design | Research conducted exclusively in (geriatric) hospitals, long-term care facilities for persons with psychiatric problems or for persons with intellectual disabilities who also have dementia |
| Original and peer-reviewed articles written in English, French, German or Dutch |
Data Processing
Data from the 37 included articles were extracted independently by the first author and one of the other reviewers, and subsequently discussed in the same pairs (IG and LVB/MV/JVH). All reviewers used the same data extraction form consisting of the following categories:
Study, sample and setting characteristics,
Specifics on the light condition, lighting systems, light measurements and light-measuring equipment (based on recommendations by Aarts, Aries, Diakoumis, van Hoof),38
Environmental properties (eg, location, date, weather conditions, daylight openings),
Health outcomes and assessment tools used,
Specifics on data collection methods and (statistical) analyses,
Appraisal of the methodological quality.
Because we included empirical studies regardless of their research design, we used the Mixed Methods Appraisal Tool (MMAT) for appraising a study’s methodological quality.39 The MMAT was comprised of two general screening questions and five specific methodological criteria for each type of research design. The maximum score of each type of design was five.
Results
Characteristics of Included Articles
The majority of the studies were conducted in North America (51.4%), followed by Europe (32.4%), Asia (10.8%), Australasia (2.7%) and in both Asia and Australasia (2.7%). For more detailed information about the characteristics and the results of the included articles see . The articles were published from 1991 to 2020 and written in English. Most studies took place in (dementia-specific care units of) nursing homes, assisted living facilities or both. The research designs included the following categories: 12 quantitative descriptive studies, 19 quantitative non-randomized studies, 2 quantitative randomized controlled trials, 3 qualitative studies and 1 mixed methods study. Although the methodological quality of the studies varied, most studies reached MMAT quality scores of three or more out of five. Less attention was given to the results of two studies that received lower MMAT scores.30,40 The light conditions were often insufficiently described; eg, a number of studies lacked relevant data, such as characteristics of the light sources, or used subjective light measurements.
Table 2.
Characteristics of the Studies
| Author (Year) | Methods, MMAT Type (Quality Score) | Participants | Setting (Country) | Light Conditions (Light Assessment) | Duration Light Intervention (Study Duration) | Health Categories and Health Outcomes | Health Assessment | Results |
|---|---|---|---|---|---|---|---|---|
| Aarts et al (2015)30 | Survey; QDS (2/5) | n=17 care professionals | 4 Psychogeriatric care facilities (The Netherlands) | Change in light conditions: 4 conditions 3 facilities: DLS and 1 facility: SLS. Average: activating: vertical 370 lx; 4130 K. rest light condition: vertical 217 lx; 3259 K (Light meter) |
≈ 9–17 am (Not reported) | Be: behavior OH: use of sleep medication OH: visual performance DF: number of falls ReAc: activity nighttime, sleep, activity daytime |
All: specifically designed questionnaires: effects on clients | No significant influences on all health outcomes. |
| Algase et al (2010)53 | Descriptive, cross-sectional, correlational design; QDS (4/5) | n=122 individuals with dementia diagnosis and wandering behavior; M MMSE = 7.4 (n=114; SD=7.2) F 77%; M age 83.7 (SD = 6.48) y |
22 Nursing homes + 6 assisted living facilities with dementia specific units (USA) | Existing light conditions: M ambient light level = 151.46 lx (SD = 298.70) (Light meter) |
NA (2 nonconsecutive days) | Be: wandering behavior | Be: videotapes of 10–12 20-minute observations: rate and duration | -Light was significantly positively associated with wandering. -Ambient light level significantly predicted wandering. |
| Barrick et al (2010)27 | Cluster-unit crossover design; QNRS (3/5) | n=20 older persons with moderate-very severe dementia (Oregon); F 95%; age 65–79 y (n=4) and ≥80 y (n=16) | 1 Dementia-specific residential care facility (USA) | Change in light conditions: 4 conditions SLS in activity and dining areas. Bright (very high-intensity) light M 2638 lx: am (7–11), pm (4–8), all day (7 am–8 pm) and ‘standard’ light M 591 lx. Mean exposure was 2.64, 2.87, and 8.40 h daily during am, pm, and all day, resp. (Not reported) |
8 Intervention periods of 3 weeks for all light conditions | Be: agitation | Be: hourly direct researchers’ observation (6 am–8 pm; total 48h) Be: CMAI: reported observations care professionals |
-CMAI was significantly higher in all day bright light than in am bright light. -No significant differences in CMAI for am, pm and all day bright light vs standard light. -Observed agitation: participants with moderate dementia were significantly more agitated under all three bright light conditions. than in standard conditions. -No significant differences between CMAI and day length (h) nor with the rate of change in day length over the 3-week intervention period (min/day). |
| Bicket et al (2010)48 | Analytical cross-sectional study; QNRS (3/5) | n=194 residents with dementia; F 77.8%; M age 86.1 (± 8.1) y | 21 Assisted Living Facilities (USA) | Existing light conditions: No specific information reported about the light conditions in the ALFs Light intensity; light glare and light evenness (TESS-NH/RC) |
NA (3 weeks) TESS 1.5–5.5 years after baseline assessment |
Be: neuropsychiatric symptoms OH: risk of falls QoL: quality of life |
Be: NPI BF: falls in the last month as recalled by the resident and chart review QoL: ADRQL |
-No significant relationship of light intensity, - glare and - evenness with NPI total score and with ADRQL. -Light glare was significantly negatively correlated with fall risk (n=187). |
| Bliwise et al (1993)60 | Behavioral observation study; QDS (4/5) | n=9 older adults with moderate-severe dementia; F 77.8%; age 82–92 y | Skilled Nursing Facility (USA) | Existing light conditions Averaged highest recorded illumination exposure for subjects: Autumn: 647 lx (SD = 372). Winter: 277 lx (SD = 164) (Light meter) |
NA (From 1 pm to 1 am Autumn: 9 days in 3 weeks; Winter: 6 days in 3 weeks) | Be: agitation ReAc:sleep |
Be: ABRS; ReAc: observations of being awake or asleep |
-Agitation: autumn/winter: no significant effect for time of day. -Agitation: autumn: no significant effect for day of the week. Not measured in winter. -Agitation/sleep: No seasonal differences in the proportion of observations spent asleep/agitated before or after sunset. -Sleep: autumn: no significant effect for time of day. -Sleep: winter: significantly less sleep during the sunset period. |
| Bromundt et al (2019)47 | A balanced crossover, within-subject study; QNRS (4/5) | n=20 participants with (suspected) dementia: n=10 AD, n=5 VD, n=3 MD, n=1 FTD and LBD F 85% Age 85.6 ± 5.8 y |
Nursing Home (Switzerland) | Change in light conditions: 2 conditions An 8-week individually timed dawn–dusk simulation (DDS) in which polychromatic white LED lighting gradually changed light level with constant CCT: (4000 K), followed by 8 weeks without the DDS. Order semi-randomized between two groups (n=10) (Photometer) |
8 weeks (17 weeks in fall and winter) | Be: agitation; social behavior+; verbal interaction* DF: independence in daily life activities; ADL+ MF: alertness*; memory+ Mo: cheerfulness*; mood both * and + QoL: quality of life; well-being* ReAc: Rest–activity rhythms and sleep (many parameters) |
5 Visual analogue scales* NOSGER+ Be: CMAI DF: CADS QoL: QUALID ReAc: wrist-actimetry |
- Significantly better mood (VAS) and greater cheerfulness upon awakening during the second 4 weeks with DDS (DDS2) compared to no DDS2. -The younger subgroup (<86, n=10) had better mood in DDS1 and DDS 2 (compared to no DDS2). -No statistically significant impact of DDS on NOSGER; CADS; CMAI; QUALID. -Neither circadian nor sleep parameters were significantly influenced by DDS. |
| Brush et al (2002)63 | Pretest–posttest design; QNRS (3/5) | n=11 residents NH and n=14 ALF residents all diagnosed with dementia; F 88%; age > 70 y | 1 Nursing Home and 1 Assisted Living Facility (USA) | Change in light conditions: 1 condition Improved lighting and table setting contrast during breakfast, lunch and dinner in NH from 266 to 377 lx and in ALF from 95 to 247 lx. (Light meter) |
4 weeks (4 weeks) | DF: caloric intake DF: functional abilities |
DF: nutritional analysis for food and beverage; COMFI; MAST | −23 of 25 residents had an increased caloric intake (ALF significant; NH not). - total COMFI scores increased (NH significant; ALF not). -MAST NH: consistent; ALF: non-significant decrease. |
| Chang et al (2017)62 | Cross-sectional study design; QDS (3/5) | n=213 residents with dementia; M MMSE = 8.9 (SD=8.2); F 57.3%; M age 82.6 (SD=6.7) y | 8 Nursing Homes (Taiwan) | Existing light conditions M 474.0 ± 417.3 lx; M-Lunch: 550.1 ± 646.7 lx; M-Dinner: 398.0 ± 290.2 lx (Light meter) |
NA (not reported) | DF: food intake difficulties | DF: EdFED; Ch-FDI | -Ch-FDI dinner was significantly negatively correlated with the illuminance level; Ch-FDI lunch was not. -Illuminance level was a significant negative predictor of Ch-FDI dinner. |
| Cohen-Mansfield et al (2010)65 | Randomized, controlled, observational cross-sectional study; QNRS (3/5) | n=193 residents with mild-severe dementia M MMSE = 7.2 (SD=6.3); F 78%; M age 86 y | 7 Nursing Homes (USA) | Existing light conditions (Environmental portion ot the ABMI) | NA (3 weeks) | MF: avoiding dark areas MF: quality of engagement to a stimulus (attention; attitude) MF:duration of engagement to a stimulus |
MF: ABMI; OME | -A dark setting was associated with few people in the room. -Attention and engagement duration were significantly higher in normal light than in a dark room. -Attention and attitude were significantly less positive with bright than normal lighting. |
| Cohen-Mansfield et al (2012)49 | Randomized, controlled, observational cross-sectional study; QNRS (3/5) | n=193 residents with mild-severe dementia; F 78%; M age 86 y | 7 Nursing Homes (USA) | Existing light conditions (Environmental portion ot the ABMI) | NA (3 weeks) | Be: agitation | Be: researcher obersvations with the ABMI | -Light level was not associated with significant changes in agitation levels (total agitation, verbal agitation and physical agitation). |
| Cohen-Mansfield et al (1991)54 | Observational study (Study 2); QDS (5/5) | n=6 severely cognitively impaired residents with a high level of pacing; F 83.3%; Age 62–93 y | 1 Nursing Home (USA) | Existing light conditions (Bright, normal and dark light with ABMI) | NA (3 months) | Be: pacing | Be: researcher obersvations with the ABMI | -No significant differences in pacing for different days of the week. -Pacing occurred significantly less often when it was dark. -Significant less pacing during mealtimes than in other periods. |
| Coulson & White (1997)61 | Triangulation study; QS (5/5) | n=64 residents with dementia; 34 professional caregivers; 4 managers/directors of nursing | 2 Dementia units of a Nursing Home (Australia) | Existing light conditions Light levels of different spaces of 1 unit (lights on) at 10:30 am and 8 pm: 0–700 lx. Mostly fluorescent lighting and some incandescent lighting fixtures. (electronic lux meter; Physical Environment Rating Scale) |
NA 3–4 days of observations(not reported) |
Be: resident’s behaviors MF: avoiding dark areas |
Be and MF: nurse manager interviews; researcher observations; caregiver feedback sessions | - Professional caregivers stated resident’s behaviors to be more difficult to manage in the evening. -Poorly lighted areas were avoided, creating overcrowding in other areas. - In one unit: when toilets were not occupied, the lights turned off and the door closed they were not used by residents. Male residents urinated in the ‘slop hopper’ in the ‘pan room’ since it was easily visible (lights were on) and resembles a toilet. |
| Elmståhl et al (1997)50 | Part of a prospective follow-up study; QNRS (4/5) | n=105 older adults with dementia: AD (39), VD (61), MD (5). M MMSE among units 11.3–15.7 (SD=0.9–2.7); F 88.6%; M age 83.0±5.8 y | 18 Group Living Units for demented elderly (Sweden) | Existing light conditions Lighting in hallways, activity areas and residents’ rooms (TESS-2) |
NA (14 months for each individual) | Be: psychiatric symptoms | Be: two subscales of the OBS | The observed psychiatric symptoms did not differ in units with ample lighting of the hallways compared to the other hallways. |
| Figueiro et al (2014)45 | Pretest-posttest design; QNRS (3/5) | n=14 residents with dementia (severity unclear: mild-moderate in inclusion criteria and moderate-severe in text); sleep and agitation problems; tending to stay in their rooms; no severe visual problems; F 64.3%; M age 86.9 ± 4.4 y | x Skilled nursing homes (USA) | Change in light conditions: 1 condition A tailored lighting intervention of ambient lighting with four floor lamps in the bedroom designed to deliver high circadian stimulation at moderate light levels (M 324 ±190 lx) from a high-CCT white light source (9325 K) (Light meter) |
4-week lighting intervention; 8–10h per day; (8 weeks) Daysimeter data: December- March and April-September |
Be: agitation DF: activities of daily living Mo: depression, ReAc: sleep quality; total sleep time; sleep efficiency; sleep-onset latency; phasor magnitude; interdaily stability; intradaily variabilty |
Questionnaires: Be: CMAI DF: MDS-ADL Mo: CSDD ReA with PSQI ReAc with Daysimeter: total sleep time; sleep efficiency; sleep-onset latency; phasor magnitude; interdaily stability; intradaily variablility |
Between baseline and during intervention -PSQI; total sleep time; sleep efficiency; phasor magnitude: significantly higher. CMAI; CSDD: significantly lower. -MDS-ADL; sleep-onset latency; interdaily stability; Intradaily variability: not significant. Between baseline and post-intervention -CMAI: significantly lower. |
| Garre-Olmo et al (2012)68 | Analytical cross-sectional study; QNRS (4/5) | n=160 residents with severe dementia F 76.9% M age 82.6 (SD=11.60) y |
8 Nursing Homes (Spain) | Existing light conditions M light level all rooms was 362.8 ± 240.5 lx. Median (range) in lx per room: Bedroom Morning 134.6 (6–1140.2) Bedroom Afternoon 85.2 (0.5–1025.2) Dining room Morning 452.0 (31.0–1342.0) Dining room Afternoon 364.7 (22.0–1195.0) Living room Morning 493.0 (20.0–1342.0) Living room Afternoon 250.5 (14.7–1195.1). (Environment meter) |
NA (April 21–July 4, 2008) | QoL quality of life: (incl. behavioral signs of discomfort; behavioral signs of social interaction; signs of negative affective mood) | QoL: QUALID | -Total QUALID score significantly correlated with light level of the dining room. -Low light levels in the bedroom for participants who spent many hours there were significantly associated with more signs of a negative affective mood. |
| Ho et al (2013)70 | Descriptive cross-sectional study; QDS (3/5) | n=77 residents with dementia (in Sydney H 40, in Sydney QT 24, in Macao 13) F Macao 38.5% M age total group 82.2 ± 8.4 y |
3 Nursing Facilities (2 in Australia; 1 in Macao SAR China) | Existing light conditions Light exposure (daytime: 6 am-8 pm): SH: M = 102.8 ± 112.8 lx; Median = 45.9 lx SQT M = 16.1 ± 19.3 lx; Median = 7.5 lx Ma M = 29.9 ± 32.3 lx; Median = 28.2 lx Light exposure (nighttime): SH M = 0.7 ± 1.1 lx; Median = 0.3 lx SQT M = 1.1 ± 1.4 lx; Median = 0.8 lx Ma M = 0.5 ± 0.7 lx; Median = 0.3 lx (Actiwatch) |
NA (6 days: 24-h a day) | ReAc:sleep–wake patterns: total sleeptime (24h, daytime, nighttime), sleep efficiency, sleep onset latency, wake after sleep onset, the number of awakenings ReAc: (physical) activity level: total activity count per min; activity count daytime and activity count nighttime |
ReAc: actiwatch | -The activity counts per minute were positively correlated with light daytime exposure. -Activity (daytime) was positively correlated with light daytime exposure. -No significant outcomes for sleep–wake patterns. |
| Jao et al (2015)55 | Exploratory study with a descriptive and repeated observation design; QDS (4/5) | n=40 participants: (in NH 26, in ALF 14) with mild-very severe dementia (DSM-IV) F 76.3% M age 82.7 (SD=6.3) y |
In parent study: 22 Nursing homes and 6 Assisted Living Facilities (USA) | Existing light conditions Light level was categorized into three groups: low light level ≤ 74 lx moderate light level 75 to ≤ 170 lx high light level > 170 lx (Light meter) |
NA [parent study of Algase et al (2010) recruited participants from 2000–2004] | Be: Apathy level | Be: 360 video segments were coded to measure apathy level with the PEAR – Apathy subscale | -Light did not show significant effects on apathy. |
| Konis et al (2018)44 | Non-randomized clustered trial, using a two-arm parallel intervention study design;QNRS (4/5) | n=77 (NPI-NH); n=64 (CSDD) residents with different types of dementia;MMSE ≥10 (mild-moderate dementia) F 72.7% M age 85.3 (SD=7.0) y |
8 Senior living dementia care communities (USA) | Change in light conditions: 2 conditions Increasing daylight exposure of participants of 4 communities by taking them each day to the perimeter zone of a daylit room from 8 am to 10 am for socialization (mlx avg 159.3). Participants of the other 4 went to a similar sized non-daylit room with typical electrical lighting conditions (mlx avg 42.3). (Spectrometer) |
12 weeks (NR) |
Be: neuropsychiatric symptoms Mo: depression |
Be: NPI-NH Mo: CSDD |
-The group differences in outcome changes achieved statistical significance for CSDD, but not for NPI-NH. -Significant group differences in the CSDD change among nine participants with baseline CSDD > 10 (major depression) in which the intervention subgroup (n=5) had a significant reduction in symptoms. |
| Leung et al (2020)64 | Empirical study; QDS (3/5) | n=40 residents with dementia (observation) n=96 residents with mild (44.8%)- moderate (55.2%) dementia (survey) F 79.2% (survey) Age all > 65 y; 78.1% over 81 y (survey) |
8 Care and Attention Homes (Hong Kong: SAR China) | Existing light conditions Satisfaction level with indoor light was measured (survey). Remark about glare due to poorly shielded lighting lamps (Survey) |
NA 10–12 am observation |
DF: limited mobility Mo: negative emotion; positive emotion; loneliness ReAc:: sleep disturbance |
DF, Mo, ReAc: DEMQOL and Dementia Care Mapping | Pearsons correlations: -Negative emotion and loneliness had significant negative relationships with indoor light. -Positive emotion was positively correlated with indoor light. -Sleep disturbance had significant negative interactions with indoor light. Multiple regression analysis: -Positive emotion was positively predicted by indoor light. -Sleep disturbance was negatively associated with indoor light. -Loneliness was negatively predicted by indoor light. |
| Martin et al (2000)51 | Descriptive cross-sectional study; QDS (3/5) | n=85 residents with mild-severe AD; F 68.5%; Women significantly older than men M age 82.5 (SD = 7.6) y. |
5 Nursing Homes (USA) | Existing light conditions M level of light exposure daytime: 476 lux (SD = 1551, range = 18–12,900 lux, median = 131 lux). M number of minutes of exposure >1000 lux was 19.6 minutes per day (SD = 37.2, range = 0–201 minutes, median = 3.6 minutes). (Actillume) |
NA (3 days per person over a 4-year period) | Be: circadian agitation rhythms | Be: ABRS; Actillume | -Higher levels of illumination exposure during the night was significantly associated with agitation througout the day and night. -Exposure to more minutes of light over 1000 lx during the night was significantly associated with later agitation rhythm peaks. -There was no significant relationship between amount of light during the day and agitation. |
| Mobley et al (2017)52 | Instrumental case study; MMS (4/5); QS (5/5); QDS (0/5) | n=9 residents with moderate-severe dementia and n=6 certified nursing assistants (observations), n=3 CNAs (e-survey); F residents NR; age: 84–100 y | 1 Dementia Special Care Unit of a Nursing Home (USA) | Existing light conditions Artificial lighting was exclusively provided by ceiling-mounted fluorescent fixtures, which caused flooring glare. There appeared o opportunity to adjust lighting levels within the unit to reduce glare or help balance residents’ circadian rhythms and sleep patterns. (Spatial inventory with photo documentation) |
NA (10 weeks; January–March: 6 am–9 pm) | Be: environmental adaptation-coping behaviors Be: environmental maladjustment-stress behaviors |
Be: quantitative and qualitative data were collected through a spatial inventory, staff e-survey, and behavioral observations in the unit’s public spaces | - Daylighting (including views provided by windows) were found to foster adapation-coping behaviors. -Certified nursing assistants described problems with sun-setting behaviors, although none were observed during researcher observations. |
| Münch et al (2017)42 | Between-subjects study; QNRS (5/5) | n=89 residents with severe dementia: AD (50), MD (20), VD (11), FTD (5), PD (2), KS (1); F 65.2%; M age = 78.4 ± 9.0 y |
1 Nursing Home (Switzerland) | Change in light conditions: 2 conditions At first a DLS and SLS group. DLS: max. ≈ Ev=1000 lx from 10.30 am–2.30 pm at eye level sitting; combining 2700 and 6500 K during the day. Producing max. 6000 K. SLS: ≈ 500 lux; 2700 K. Because there was no statistical difference in individual light exposures (in lx) of the activity-light watches between the participants with the dynamic or conventional lighting during daytime, ultimately a high light exposure group (HLG average > 417 lx) and a low light exposure group (average < 417.24 lx); 8 am–6 pm were researched. (Spectroradiometer; activity-light watches) |
8 weeks; October-December 2012 | Be: agitation DF: independence of daily life activities Mo: subjective emotions QoL: quality of life ReAc: sleep; activity |
Be: CMAI DF: CADS Mo: OERS QoL: QUALID ReAc:Daily 24-h measurements with activity-light watches |
DLS vs SLS: -No significant differences in CMAI; CADS; OERS. HLG vs LLG - No significant differences in CMAI; CADS. -Significantly more pleasure and higher general alertness. -Pleasure significantly higher in the morning and anger in the evening. -QUALID significantly higher. -No significant difference for sleep latency; sleep end; sleep fragmentation. -Significantly later bedtimes, less time in bed; later sleep time. Gender-specifiic HLG vs LLG: -Men in HLG had significantly higher activity than men in LLG and women in HLG. -Higher daytime light exposure significantly predicted increase in amplitude in men. |
| Netten (1989)66 | Observational, exploratory study; QNRS (4/5) | n=104 older residents with moderate-severe dementia; Group Homes: n=50, Communal homes: n=52 in the analysis. F and age not reported |
6 Group Homes; 7 Communal Homes (United Kingdom) | Existing light conditions Ratings of light levels not reported. The light level tended to be lower in the group-designed homes. (Moos’ Rating Scale) |
NA (not reported) | MF: The residents’ capacity to find their way around the house | MF: FIND | Light level significantly predicted residents’ capacity to find their way in smaller scale group homes, where the light level tended to be lower than in traditional nursing homes. |
| Rheaume (1998)40 | 3 Case reports; QS (1/5) | 3 Cases: n=1; probable AD; F 0% Age resp 73; 64; 75 y |
1 Special Care Dementia Unit (USA) | Change in light conditions: 3×1 condition A light treatment room (approximately 2.5 to 5 m), furnished as a living room, with a ceiling with high-intensity fluorescent lights that gradually raise in 30-second intervals to 10,000 lux. The level of light at eye level is approximately 2.500 lx (Not reported) |
Case 1: usually 3 am–6/7 am Case 2: 10–12 pm Case 3: 2 hours in the late evening (Case 1: ≥ 1 month) |
Be: disturbed behaviors (eg agitated behavior) ReAc: insomnia/sleep |
Be/ReAc: Predominantly clinical observation and in case 1: also heart rate; motor activity. | These case reports suggest that an exposure to bright light, can improve, and in some cases even eliminate, insomnia and disturbed behaviors of residents with AD which are resistant to other therapeutic strategies. |
| Riemersma-van der Lek et al (2008)43 | Long-term, double-blind, placebo- controlled, 2×2 factorial randomized trial; QRCT (4/5) | n=94 residents light only: 49; placebo:45. n=59 probable AD; n=35 other types (incl. unknown) M MMSE Light only: 14.5 (SD=6.2). Placebo: 14.3 (SD=7.0) F light only 91.8%; placebo 88.9% M age light only 85 (SD=6) y; placebo 85 (SD=5) y |
12 Assisted Care Facilities (The Netherlands) | Change in light conditions: 2 conditions SLS. 6 Ceiling mounted lighting in the common living rooms of 6 facilities reaching Ev = ±1000 lx, CCT= 4000K from ±10am – 5pm and in 6 control facilities Ev= ± 300 lx (Light meter) |
Range: 0–3.5 y. Mean duration 15 months (SD=12) (3.5 y) | Be: agitation; psychopathological behaviors; withdrawn behavior DF: functional limitations MF: cognition Mo: depression; negative mood; self-esteem ReAc: sleep efficiency; sleep onset latency; total sleep duration; nocturnal restlessness; duration of nocturnal awakenings; duration of uninterrupted sleep epochs OH: adverse effects; prescription use of psychotropic medication |
Be: CMAI; NPI-Q; subscale of MOSES DF: NI-ADL MF: MMSE Mo: CSDD; PGCARS; PGCMS ReAc: Actiwatch BF: list adverse effects; medical record |
All outcomes concern light only compared to placebo Be: -No significant treatment effect for agitation; NPI-Q severity; withdrawn behavior. DF: -Light significantly attenuated the gradual increase in functional limitations after 6 weeks and 2 years. MF:-Light significantly ameliorated cognitive deterioration. Mo:-No significant treatment effect for PGCARS positive; idem negative; PGCMS. -Light ameliorated depressive symptoms. ReAc:- No significant treatment effect on duration of awakenings; uninterrupted sleep epochs; nocturnal restlessness; sleep efficiency; sleep onset latency. -An increase in efficacy over time was found for sleep duration BF: No effects on prescription use of psychotropic medication. - Light significantly lowered irritability; dizziness, headache, constipation; inability to sleep. |
| Schnelle et al (1999)72 | A randomized control group design with a delayed intervention for the control group; QNRS (5/5) | n=184 incontinent residents: n=90 immediate intervention; n=94 delayed intervention. M MMSE (SD) resp 11.1 (9.4) and 10.7 (9.1) F resp 85%; 79% M age (SD) resp 82.6 (7.4); 85.3 (11.9) |
8 Nursing Homes (USA) | Change in light conditions: 2 conditions Light is part of individualized incontinence care at night (1 of 4 components of the study). Efforts were made to reduce noise and light levels whenever a resident was changed. (Bedside monitor) |
5 nights (4 y) | ReAc: number of awakenings | ReAc 1-minute observations of sleep status upon entering and leaving the room in on average 10 rounds per night; wrist activity monitor when in bed | Significant decreases in wakes associated with light only and with noise plus light. |
| Shochat et al (2000)71 | Analytical cross-sectional study; QNRS (5/5) | n=66 institutionalized older individuals: mild-severe dementia (n=63) no dementia (n=3) F 75.3% (n=77) M age 85.76 (SD 7.3) y (n=77) |
1 Nursing Home (USA) | Existing light conditions M daytime light exposure = 485 lx (SD = 761); range = 43–3565. Median time > 1000 lx = 10.5 min (mean = 34 min, SD = 63, range. 0–314). Median time > 2000 lx = 4 min (mean = 19 min, SD = 39, range = 0–242). 17% was never exposed to light > 1000 lx, and 26% not to light > 2000 lx (Actillume) |
3 days (NR) | ReAc:Sleep–wake behavior and 24h-rhythms of activity | ReAc: Actillume | -Residents exposed to higher light levels had significant fewer awakenings at night. -Residents whose peak of light exposure occurred early in the day also had an early peak in activity; for most residents the peak of light provoked the peak of activity. -Residents with number of minutes >2000 lx had significantly later activity peaks. |
| Sloane et al (1998)58 | Cross-sectional study; QDS (4/5) | x Residents with on average severe dementia; M MDS-COGS (SD)= 4.94 (1.31); F NR; Age NR |
53 Alzheimer’s Disease Special Care Units in Nursing Homes (USA) | Existing light conditions No information reported about the light levels in the 53 Alzheimer’s Disease Special Care Units(SCUEQS; a subscale of TESS-2+) |
1x morning and 1x afternoon (1 full day data collection site visit for each AD SPCU) | Be: agitation | Be: RSOC | -Significant negative correlation between light level/intensity index and overall and weighted agitation level. -No significant correlation between resident room and actitvity room light level and agitation outcomes. |
| Sloane et al (2005)76 | Clinical trial; QNRS (4/5) | n=38 residents with dementia (Oregon NH) F NR; Age NR | 1 Dementia-specific Residential Care Facility (USA) | Change in light conditions: 2 Ambient lighting and skylights together producing > 2000 lx and a control conditiion of 500–600 lx daytime (Light meter) |
3-week periods (NR) |
OH; side effects reported by staff and staff perception about resident reactions on high-intensity light | OH: Questionnaire Postintervention survey |
-No significant difference in side effects during high intensity compared to control lighting. -Staff felt residents were somewhat or much better. |
| Sloane et al (2007)28 | Cluster-unit crossover intervention trial; QNRS (4/5) | n=20 older adults with dementia (Oregon NH): type of dementia and severity NR; F NR; M Age NR | 1 Dementia-specific residential care facility (USA) | Change in light conditions: 4 conditions SLS in activity and dining areas. Bright (very high intensity) light M Eh 2641 lx (SD=259): am (7–11), pm (4–8), all day (7 am–8 pm) and ‘standard’ light Eh M 606 lx (SD=179). Mean exposure was 2.57 ± 0.98 h;, 2.70 ± 1.28 h, and 8.44 ± 2.73 h daily during am, pm, and all day, resp. (Light meter) |
8×3-week periods (5.5 months: August 17, 2004–January 31, 2005) | ReAc: nighttime sleep (h) and nighttime bouts; daytime activity; circadian rhythms: intradaily variability, interdaily stability, mesor, amplitude and acrophase. | ReAc: Wrist actigraphy; daytime observations | -In persons with severe or very severe dementia (Oregon nursing home), evening light was associated with a significant increase in daytime sleepiness. |
| Song et al (2009)69 | Pilot study; QDS (4/5) | n=11 participants with dementia: AD(4), VD (1), DNFS (6); M MMSE-K (SD) = 13.1 (4.0); F 100%; M Age 85.6 (7.2) |
1 Assisted Living Facility and 1 Nursing Home (South Korea) | Existing light conditions Light level at eye level measured every day during the study period. Average afternoon light level: -common areas: 2038 lx (SD = 288) Institution A: 2378 lx, SD = 41 Institution B: 1832 lx, SD = 51; -bedrooms: 591 lx (SD = 498) Institution A: 1048 lx, SD = 601 Institution B: 330 lx, SD = 121 (Light meter) |
NA (April–May 2007) | ReAc: Rest–activity rhythm: intradaily variability; interdaily stability; (onset of) L5; (onset of) M10; (relative) amplitude. Sleep time and wake time: -nighttime (6 pm–8 am): total sleep time; wake time; mean duration/number of wake episodes -daytime (8 am–6 pm): napping behavior; sleep time; number of sleep events |
ReAc: Actiwatch | -No significant differences in sleep parameters for light levels -No significant relationships of rest–activity characteristics and light levels. |
| Van Hoof, Aarts et al (2009)56 | Intervention study; QNRS (4/5) | n=26 residents with probable AD (16), VD (6); MD (4) F 73.1% M Age 85.6 (SD=6.9) y |
1 Psychogeriatric Ward of a Nursing Home (The Netherlands) | Change in light conditions: 3 Ceiling mounted illumination (SLS). Intervention group: alternating interventions of very high-intensity light Ev > 1000 lx, with a high CCT (6500 K) in intervention I and a low CCT in intervention II (2700 K). Both from ± 8 am to 6 pm. Control group: M Ev < 200 lx with a low CCT (2700 K) (Light meter) |
High and low CCT intervention both 3 weeks (May–August 2006) | Be: apathic behavior, restless behavior MF: disturbances of consciousness ReAc:circadian rhythmicity |
Be and MF: GIP ReAc: tympanic temperature |
- No significant improvements in apathic or restless behavior nor in disturbances of consciousness after the high CCT intervention compared to the control group. - Tympanic temperature increased after the high CCT and decreased after the low CCT intervention, both significantly compared to the control group. |
| Van Hoof, Schoutens et al (2009)57 | Randomized cluster-unit cross-over intervention trial; QRCT (3/5) | n=22 residents with dementia (VD, AD, MD and LBD) F 77.3% M Age 88.2 (SD 5.5) y |
1Psychogeriatric day care ward of a Care Home (The Netherlands) | Change in light conditions: 2 Ceiling mounted illumination (SLS). Comparing 2 intervention groups with prolonged exposure to standard intensity white light (Eh=500 lx at eye level) with a low CCT (2700 K) alternating with the same intensity and a very high CCT (17,000 K). Both from 8 amto 6 pm. (Light meter) |
Each intervention’s duration is approx 4–5 days (May 9–June 24, 2008) | Be: anxious, apathic and restless behavior MF: disturbances of consciousness Mo: depressive/sad behavior ReAc: circadian rhythmicity |
Be, MF and Mo: GIP ReAc: tympanic temperature |
-No significant differences in anxious, apathic, restless and depressive behavior between groups. -Significant more disturbances of consciousness in the 17,000 K than in the 2700 K scenario, but within-group comparisons showed this difference was not significant. -No circadian effects of the 17,000 K scenario. |
| Van Someren et al (1997)74 | Repeated measurement study; NRS (4/5) | n=22 residents with severe dementia: probable AD (16), VD (3), KS (2), and 1 normal pressure hydrocephalus; M GDS = 6.3± 0.13 (range 5–7) F 68.2% M age 79 ± 2 |
1 Psychogeriatric Ward of a Nursing Home (The Netherlands) | Change in light conditions: 1 condition Ceiling-mounted illumination with high-intensity white fluorescent tubes in five living rooms during the daytime. Average light level=1136±89 lx, range 790–2190 lx (Luxmeter) |
4 weeks of bright light therapy daytime (November–April) | ReAc: intradaily variability; interdaily stability; circadian amplitude | ReAc: Wrist-worn actigraph | After excluding 5 persons with severe visual deficiency: -IS during light treatment was significantly higher than the pooled baseline level. - IV during light treatment was significantly lower than the pooled baseline level. - AMP during light treatment did not differ significantly from the pooled baseline level. |
| Wahnschaffe, Nowozin, Haedel et al (2017)46 | Pretest–posttest design; QNRS (5/5) | n=12 residents with dementia: AD (3), VD (3), other types of dementia (6) M MMSE (SD): 12.1 (9.2) F 58.3% M Age (SD) 79.1 (11) y |
1 Nursing home for residents with dementia (Germany) | Change in light conditions:1 condition Ceiling mounted DLS consisting of two lamps (6500 K and 3000 K) that were dimmed in and out vice versa during changing times from 5 am to 10 am and 3 pm to 8 pm. Peak illuminance from 10 am to 3 pm. M Ev=389.1 lx (SD=23.2) and M CCT 4440 K (SD=517) at 10.30 am; M Ev=33.8 lx (SD=23.2) and M CCT 1747 K (SD=60) at 10.30 pm (Spectroradiometer) |
4 months: December 20, 2012–April 20, 2013 (7 months) | Be: agitation ReAc: interdaily stability; intradaily variability; relative amplitude |
Be: CMAI ReAc: Actiwatch |
-Significantly lower amount of agitated behavior during intervention than before. -The subscore “physically nonaggressive behaviors” was significantly lower during intervention than before. -No significant differences of RA, IS or IV between different periods before and during intervention. |
| Wahnschaffe, Nowozin, Rath et al (2017)73 | Longitudinal, retrospective, explorative analysis of data set; QDS (4/5) | n=20 residents with dementia AD (9),VD (2), FTD (1), KS (1), DNFS (7) F 95% M Age (SD) 83.8 (8.8) |
1 Nursing Home for people with dementia (Germany) | Existing light conditions: 1 condition Exposure of natural daylight (day length and cloud amount) in an old villa with large windows allowing a high amount of natural light exposure on residents who spent the majority of their waking time in the common living room or, if the weather allowed it in the garden. Electrical lighting E 70–170 lx at eye level; CCT: 2700 K (Season and weather data) |
NA (3 years: July 21, 2009- June 17, 2012.) |
ReAc:circadian rest–activity cycles (IS; IV; RA; (onset of) L5; (onset of) M10) | ReAc: Actiwatch | -Night-time activity (L5) was significantly higher during cloudy short days when compared to clear short days and cloudy long days. |
| Wong et al (2014)59 | Qualitative study; QS (5/5) | n=36 participants: n=27 care professionals and n=6 RCH staff involved in care for older adults with dementia; n=3 architects F 91.7% |
4 Residential Care Homes for Dementia (Hong Kong SAR China) | Existing light conditions Poor lighting as part of the indoor environment triggering behavioural and psychological symptoms of dementia. (Experiences of participants) |
6 Focus groups each lasting 1.5–2 h (Not reported) | Be: indoor environment-related behavioral and psychological symptoms of dementia | Be: Critical Incident Technique | -Glare, eg light reflection from glass can lead to hallucination and emotional disorders. -BPSD eg dysphoria, wandering, emotional disorders when light was dim or at dusk. -During late afternoon many experience being nervous and anxious about ‘going home’ or ‘looking for relatives’ (sun downing). |
Abbreviations: Quality assessment: MMAT, Mixed Methods Appraisal Tool; MMS, mixed methods studies; QDS, quantitative descriptive studies; QNRS, quantitative non-randomized studies; QRCT, quantitative randomized controlled trials; QS, qualitative studies. Type of dementia: AD, Alzheimer’s disease; DNFS, Dementia Not Further Specified; FTD, frontotemporal dementia; KS, Korsakoff Syndrome; LBD, Lewy-Body Dementia; MD, mixed dementia; PD, Parkinson dementia; VD, vascular dementia. Environmental assessment: ABMI, (environmental portion of the) Agitation Behavior Mapping Inventory; SCUEQS, Special Care Unit Environmental Quality Scale; TESS-2, Therapeutic Environment Screening Survey version 2; TESS 2+, TESS version 2+; TESS NH-RC, TESS for Nursing Homes and Residential Care. Health outcome categories: Be, behavior; DF, daily functioning; MF, mental functions; Mo, mood and emotions; OH, other health outcomes; QoL, quality of life; ReAc, rest–activity. Health assessment: ABMI, Agitation Behavior Mapping Inventory; ABRS, Agitated Behavior Rating Scale (Bliwise and Lee, 1993); ADRQL, Alzheimer Disease Related Quality of Life; BPSD, Behavioral and Psychological Symptoms of Dementia; CADS, Changes in Advanced Dementia Scale; Ch-FDI, Chinese Feeding Difficulty Index; CMAI, Cohen–Mansfield Agitation Inventory; COMFI, Communication Outcome Measure of Functional Independence; CSDD, Cornell Scale for Depression in Dementia; DEMQOL, Dementia Quality of Life; EdFED, Edinburgh Feeding Evaluation in Dementia; GIP, Dutch Behavior Observation Scale for Intramural Psychogeriatrics; MAST, Meal Assistance Screening Tool; MDS-ADL, Minimum Data Set Activities of Daily Living Scale; MDS-COGS, Minimum Data Set Cognition Scale; MMSE, Mini-Mental State Examination; MMSE-K idem Korean version; MOSES, Multi Observational Scale for Elderly Subjects; NI-ADL, nurse informant activities of daily living scale; NOSGER, Nurses’ Observation Scale for Geriatric Patients; NPI, Neuropsychiatric Inventory; NPI-NH, Neuropsychiatric Inventory – Nursing Home version; NPI-Q, questionnaire format of the Neuropsychiatric Inventory; OBS, Organic Brain Syndrome; OERS, Observed Emotion Rating Scale; OME, Observational Measurement of Engagement; PEAR, Person-Environment Rating (Jao et al, 2013); PGCARS, Philadelphia Geriatric Centre Affect Rating Scale; PGCMS, Philadelphia Geriatric Centre Morale Scale; PSQI, Pittsburgh Sleep Quality Index; QUALID, Quality of Life Scale for Severe Dementia; RSOC, Resident and Staff Observation Checklist. Rest–activity variables: A, amplitude; AMP, circadian amplitude; IS, interdaily stability; IV, intradaily variability; (onset of) L5, (onset of) activity during 5 least active hours; (onset of) M10, (onset of) activity during 10 most active hours; RA, relative amplitude. Other abbreviations: Avg, average; CCT, correlated color temperature; DLS, dynamic lighting system; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; Eh, light intensity horizontal; Ev, light intensity vertical; F, female; GDS, Global Deterioration Scale; h, hour; lx, lux; M, mean; mlx, melanopic lux; K, kelvin; NA, not applicable; NH, nursing home; NR, not reported; Resp, respectively; SAR, special administrative region; SLS, static lighting system; vs, versus; y, year(s).
Almost 60% (n=21) of the studies investigated the influence of existing indoor light conditions on health. In 16 studies (43.2%), the indoor light conditions were purposely changed before or during the study. These light interventions during the day consisted of an increase in the light intensity, color temperature or both. This was done either by adding luminaires or replacing light bulbs, adding static or dynamic lighting systems, increasing the use of incident daylight through newly built skylights or by taking residents to a daylit room near the windows for socialization. Two interventions took place around night-time, namely reduction of the frequency of light changes during incontinence care and a naturalistic simulation of dawn–dusk signals in the bedrooms of persons with dementia. Most of the light interventions took place in communal living rooms, dining rooms, or both (n=12) and the rest occurred in bedrooms (n=3) or a small light therapy living room (n=1).
Influence of Light on Health
By examining and reporting the influence of light on health, two specific characteristics of light were classified: light level and color temperature. Light can differ in intensity, the light level, and its spectrum can, for example, be experienced as warm white or yellowish, cool white or even bluish, often referred to as the color temperature. The correct technical term is correlated color temperature (CCT) and depends on the type of light source. In this review, these two characteristics are used separately or in different combinations to indicate the light level and (correlated) color temperature of light (Table 3).
Table 3.
Characteristics of Light
| Light Level (Illuminance) in lux (lx) | CCT in kelvin (K) |
|---|---|
| Very low intensity <50 | Warm (white) light 2700−3200 |
| Low intensity 50-200 | Neutral (white) light 3200−4200 |
| Moderate intensity 200−400 | Cool (white) light 4200−6200 |
| Standard intensity 400−600 | Very cool (blue) light 6200−9500 |
| High intensity 600−1000 | Extremely cool (blue) light >9500 |
| Very high intensity >1000 |
Notes: These light levels can be measured horizontally or vertically.
Abbreviation: CCT, correlated color temperature.
By categorizing all health outcomes in the literature into thematic groups, we identified seven categories of health outcomes (number of articles; percentage). We will discuss the outcomes in the following order:
Behavior, such as agitation, (neuro)psychiatric symptoms and apathy (n=22; 59.5%)
Daily functioning, such as activities of daily living, falls and food intake (n=9; 24.3%)
Mental functions, such as orientation, cognition and disturbances of consciousness (n=7; 18.9%)
Mood and emotions, such as depression and subjective emotions (n=8; 21.6%)
Quality of life (n=4; 10.8%)
(24h) Rest–activity rhythms or certain aspects thereof, such as daytime activity, rest–activity cycles, nocturnal restlessness and sleep (n=18; 48.6%)
Bodily functions: adverse effects of light, use of medication and visual performance (n=3; 8.1%).
Behavior
Behavior was the most investigated health outcome (n=22) in the literature, and most articles reported on sets of challenging behaviors (n=18; 81.8%), like agitation. The Cohen–Mansfield Agitation Inventory (CMAI), which consists of 29 distinctive behaviors, was often used in the research.41 Almost 30% (n=6) of the articles (also) researched one or more separate challenging behaviors, such as pacing or apathy. Only two studies also involved positive behaviors. In this review two types of influences of light on behavior were distinguished. First, articles were identified that investigated a direct influence of the quantity, quality or color of light on behavior, which we will discuss first (n=21). Second, a more indirect influence of light on behavior was found, namely temporal aspects of light, like day length or time of day (n=7).
Direct Influence of Light on Behavior
Six methodologically sound light intervention studies investigated the influence of cooler light (neutral to very cool) during the day on sets of challenging behaviors (MMAT score ≥3/5). The majority of these light interventions (n=4; 66.7%) did not yield any significant influences on sets of challenging behaviors in the intervention group compared to a control condition.27,42–44 These ineffective interventions concerned light with a (very) high light intensity (Table 3) which was produced by lighting installations or was a result of incident daylight through a window. In two other studies, the lighting interventions resulted in a daytime light exposure of moderate intensity of (very) cool light.45,46 In these before–after studies (n=14;45 n=1246), agitation decreased significantly compared to the baseline condition. One of these studies found that the decrease in agitation was attributable to physically nonaggressive behaviors.46 In addition to agitation assessed by staff caregivers with the CMAI, the study of Barrick et al27 also used direct observations of research personnel to evaluate agitation. Analyzing these observations, nursing home participants with moderate dementia were significantly more agitated under all three bright light conditions (7−11 am, 4−8 pm and all day 7 am−8 pm) than in standard light conditions. Therefore, Barrick et al27 noted that very high-intensity cool light may even exacerbate agitation. An individually timed light intervention simulating a natural pattern of dusk and dawn did not have an effect on agitation.47
Most studies regarding existing light conditions (n=5; 71.4%) did not find a relationship between light during the day and sets of challenging behaviors.48–52 However, one of these studies investigating agitation rhythms found a relationship of agitation and very high-intensity light during the night.51 Participants with more minutes of exposure to very high-intensity light during the night displayed their agitation later in the day. They also found that exposure to higher light levels during the night resulted in experiencing some agitation throughout the day and night, with no consistent periods without agitation. Possible sources of the nocturnal light exposure were lights being left on in the bedrooms or participants wandering over to a brightly lit nurses’ station.51
Six articles reported on one or more distinct challenging behaviors, namely anxious behaviors, apathy, pacing, restless behavior, wandering behavior and withdrawn behavior.43,53–57 Only two of these studies (33.3%) found the behaviors to have a significant relationship with indoor light. These quantitative descriptive studies conducted in existing light conditions investigated physically active challenging behaviors. The concerned studies reported significantly less wandering53 or pacing,54 when light levels were low, which is contrary to findings in similar light conditions on sets of challenging behaviors.58,59
Two studies investigated a direct influence of light on positive behaviors, namely adaptation-coping and social behavior. A mixed methods study found that indoor daylight improved adaptation-coping behaviors. Residents seemed to orient strolling behaviors toward areas with increased daylight and frequently paused to look through the glass in two exit doors.52 Using a crossover design, a dawn–dusk simulation did not produce significant effects on social behavior.47
Indirect Influence of Light on Behavior
Six studies reported on the relationship of indirect aspects of light exposure and sets of challenging behaviors. Most studies (n=4; 66.7%) found no significant temporal aspects regarding sets of challenging behaviors. They did not find any significant effects in these behaviors before, during or after sunset, nor for any parts of the day, different days of the week, daylengths, months or seasons.27,51,52,60
With regard to sundowning behavior, the results depended on the perspective the observations were made from. Whereas care professionals mentioned that persons with dementia showed (more) challenging behaviors around sunset or in the evening,52,59,61 none of these behaviors were observed during researchers’ observations.52 Furthermore, two quantitative descriptive studies that specifically focused on the timing of these challenging behaviors and used researcher assessments, found no significant proof of these behaviors during or after sunset either.51,60 Bliwise et al60 stated, therefore, that these disruptive behaviors might occur with identical frequency throughout the day, but might have more impact on the nursing staff later in the day. The study focusing on agitation rhythms showed a substantial amount of variation in these rhythms.51 This study identified only two persons with dementia (2.4%) having an agitation peak at sundown.
Daily Functioning
Studies investigating the impact of indoor light on daily functioning of persons with dementia focused on independence in activities of daily living (ADL), aspects of food intake and, mobility or falls. A randomized controlled trial (RCT) examining the effectiveness of a long-term intervention (up to 3.5 years) of high-intensity neutral light showed that the ADL in the intervention group decreased significantly less compared to the control group.43 Two studies using different, but short-term (4 and 8 weeks) light interventions during the day did not produce significant effects on ADL compared to baseline45 or the control group.42 In the study of Münch et al,42 the actual 24-h light exposure of persons with dementia was measured using activity watches with a light sensor. This study, comparing the influence of a dynamic lighting system to standard lighting, did not find any significant differences in the average daily light exposure between the groups. After dividing the sample into a high light and a low light group, based on their actual light exposure, the researchers did not find any significant differences in ADL either. A short-term dawn–dusk light intervention (8 weeks) also did not have a significant impact on ADL.47
Regarding food intake, beneficial effects of light have been found. A cross-sectional study, examining the influence of existing light conditions, showed a significant negative association of indoor light with food intake difficulties during dinnertime, but not during lunchtime.62 An intervention with improved lighting and table setting contrast during breakfast, lunch and dinner increased 3-day caloric intake of almost all participants.63 This before–after study also showed higher functioning during meals.
Investigating the relationship of light and falls, a cross-sectional study using an environmental assessment tool (Table 2) found that light glare was significantly correlated with a higher risk of falls, while light intensity and light evenness were not.48 A quantitative descriptive study found that satisfaction with indoor light had no significant correlation with limited mobility.64
Mental Functions
Seven articles reported on different kinds of mental functions, including disturbances of consciousness, spatial orientation, cognition, memory and engagement. No significant effects were found of the two very different light interventions on disturbances of consciousness compared to the control condition.56,57 Studies in existing light conditions demonstrated indoor light being an important aid for spatial orientation of persons with dementia.52,61,65,66 Observations showed that poorly lighted areas were avoided by people with dementia, thus creating overcrowding in other areas.61,65 In a qualitative study, men were observed to urinate in the “slop hopper” because, unlike the toilet areas, this area was easily visible with the lights on and it looked like a toilet.61 As mentioned in the behavior section, persons with dementia also seemed to orient strolling behaviors toward areas with increased daylight.52 An observational study showed that light level significantly predicted the capacity of residents with dementia to find their way in small-scale group homes, where the light level tended to be lower than in more traditional nursing homes.66 These group homes had a larger number of corridors that relied heavily on artificial lighting, Staff knowing their way around and unaware of the importance of light for residents in finding their way around, forgot to switch on the lights and the defunct bulbs made these corridors, even in the middle of the day, very dark.
A long-term intervention with high-intensity neutral light during the day resulted in a significant decrease of cognitive deterioration in the intervention group compared to the control group.43 Concerning memory, no significant impact was found of a dawn–dusk simulation.47
When investigating engagement while offering stimuli, researchers hypothesized that because of common vision problems persons with dementia would benefit from bright light, and their engagement would be adversely affected by dark surroundings.65 Data, however, showed the contrary, quality of engagement (attention and attitude) was significantly more positive in normal light than in bright light. Nonetheless, duration of engagement was significantly more positive when light was normal than in a dark room.
Mood and Emotions
In all three light intervention studies, depressive symptoms, measured by the Cornell Scale for Depression in Dementia67 were significantly improved.43–45 However, these light interventions differed considerably in source, color, intensity as well as in duration. A long-term lighting intervention showed a significant decrease in depressive symptoms in the intervention group compared to the control group.43 In a before–after study, a 4-week light intervention significantly decreased depressive symptoms during the intervention period compared to baseline, but the difference lost significance 4 weeks post-intervention.45 In the third study, the daylight exposure of the intervention group was increased over a period of 12 weeks.44 Every morning the participants of the intervention group were taken to a communal room within 3 meters of a daylit window. The control group was taken to a similar sized area indoors under typical electrical lighting conditions without daylight. This resulted in the daylight intervention group showing a significant decrease in depressive symptoms compared to an increase in the control group. Significant group differences were also found for participants with a probable baseline major depression (CSDD > 10; n=9). The intervention subgroup (n=5) had a significant reduction in symptoms, comparable to an effective pharmacological treatment.
Five articles reported on the relationship between light and negative affective mood. Two very different light interventions (neutral high-intensity light and extremely cool standard light) yielded no beneficial effects.43,57 In a cross-sectional study (n=160) with existing light conditions, persons with dementia who spent many hours in low light levels in their bedrooms showed significant more signs of a negative affective mood, yet no associations were found with light in the living and dining room, where light levels were considerably higher.68 As far as negative subjective emotions (anger, sadness and fear) were concerned, no significant differences were found comparing a high light and a low light group.42 In a quantitative descriptive study, loneliness and negative emotion had significant negative relationships with satisfaction with indoor light, which in turn was a significant predictor of loneliness.64
Positive affective mood was investigated in four very different light conditions and mostly yielded positive results.42,47,64 Persons with dementia in a high light group showed significantly more pleasure and higher general alertness than those in a low light group.42 However, an intervention with high-intensity neutral light during the day did not produce significant effects on positive affective mood nor on self-esteem in comparison with a control group.43 An individually timed dawn–dusk simulation exposure (DDS) for older adults with dementia significantly produced better mood and greater cheerfulness upon awakening in the second 4-week period of the DDS compared to the second 4-week period without DDS.47 Only the relatively younger subgroup (<86 years; n=10) expressed a better mood earlier, namely in the first 4 weeks of the DDS compared to the first 4 weeks without DDS, suggesting that the response may need more time to manifest with higher age. In a quantitative descriptive study, positive emotion, including cheerfulness, was positively predicted by satisfaction with indoor light of persons with dementia.64
Quality of Life
We found results suggesting that daytime light affects the quality of life of persons with dementia in long-term care facilities. Two studies in nursing homes reported a significant positive relationship between light and quality of life of persons with dementia.42,68 Measuring the light levels in different rooms (bed room, dining room and living room), the quality of life was positively correlated with the light level of the dining room.68 This large cross-sectional study (n=160) found that persons with dementia spending many hours in low light levels in their bedrooms showed more signs of a negative affective mood, which is an element of quality of life. In a between-subjects study (n=89), quality of life was also higher in the high light than in the low light group, but not in the dynamic lighting group when compared to the conventional lighting group.42 As mentioned before, this study found no statistical differences in actual light exposure between the dynamic and conventional lighting group. A large cross-sectional study (n=194) in assisted living facilities using an environmental assessment tool found no significant correlation between quality of life and light intensity, light glare and light evenness.48 Finally, DDS had no effect on quality of life either.47
Rest–Activity
The following results concern studies examining the influence of light on rest–activity during the day (n=8), on rest–activity at night (n=12) and on circadian rest–activity rhythms (n=11).
Rest–Activity Daytime
In general, the light interventions had no effect on daytime rest–activity, apart from the results for some specific subgroups. However, studies in existing light conditions predominantly found a positive correlation between light and activity. Three studies using light interventions of (very) cool light with different light levels during the day showed no significant influence of light exposure on daytime rest–activity of persons with dementia.28,42,45 However, two of these studies did find significant relationships for specific characteristics, namely severity of dementia and gender. First, in persons with (very) severe dementia, exposure to high-intensity light between 4 and 8 pm was associated with a significant increase in daytime sleepiness.28 Second, men in the high light group had significantly higher activity (from 10 am to 8 pm) than men in the low light group and women in both groups.42 A quantitative descriptive study (n=12) in existing light conditions found no relationship between light level and activity during the day.69 However, this sample may have been too small to reach significance. In addition, the sample consisted only of women. Two larger cross-sectional studies (n=77;70 n=6671) in existing light conditions found a positive relationship between (the peak of) light exposure and (the peak of) activity during the day.70,71 One of these studies found, “persons with dementia whose peak of light exposure occurred early in the day also had an early peak in activity.”71 Thus, for most of these individuals, the peak of light exposure preceded the peak of activity. This study also showed that persons with dementia who spent more time in very high light levels (>2000 lx) had significantly later activity peaks.
Rest–Activity Nighttime
Most studies (n=7; 70%; MMAT-score ≥3) that examined nighttime rest–activity variables found significant influences of light.42,43,45,64,71–73 However, these results were difficult to compare, because of the variety in light conditions and the use of many different − not always clearly defined − sleep variables.
With regard to nocturnal restlessness, no significant influences were found from different light conditions.43,47,69 However, a 3-year retrospective longitudinal study did find a specific combination of day length and cloud amount to have an influence on nocturnal restlessness of persons with dementia in nursing homes.73 In this study, nighttime activity (indicating nocturnal restlessness) of persons with dementia was significantly higher on cloudy short days than on cloudy long days or on clear short days.
Most studies, including three intervention studies, investigating awakenings at night found no significant influence of light exposure.42,43,47,69,70 In contrast, a high quality cross-sectional study in existing light conditions found persons with dementia who had been exposed to higher light levels during the day had significantly fewer awakenings during the night.71 Two other studies researched indoor light in a different way. Making efforts to reduce noise and light levels during incontinence care at night, led to a significant decrease in the number of awakenings (related to “light only” and “light and sound”) in the intervention group compared to the control group.72 Satisfaction with indoor light was another factor associated with less sleep disturbance.64
There was no light condition that significantly influenced sleep onset latency.42,43,45,70 In a between-subjects study, the high light group showed significant later bedtimes and spent less time in bed than the low light group.42 Most of the studies investigating sleep efficiency (including a long-term RCT)43,47,70 did not yield any significant results. Only a before–after study showed a significant increase in sleep efficiency as well as in sleep quality during an intervention with very cool light of moderate intensity.45 Two light intervention studies, the aforementioned study and an RCT, yielded significant positive results on total sleep time during the night. In this RCT, an intervention with high-intensity neutral light increased total sleep duration by 10 minutes per year for up to 3.5 years.43 Studies in existing light conditions and a DDS had no influence on total sleep time during the night.47,69,70
24-Hour Rest–Activity Rhythms
Using the following circadian rest–activity parameters, only a few studies found an influence of light on circadian rest–activity rhythms, such as interdaily stability, intradaily variability, circadian amplitude, 24h-sleep and 24h-activity time. Interdaily stability is used to compare the day-by-day regularity of the 24-hour sleep–wake pattern.45 Intradaily variability represents the frequency and extent of transitions between periods of rest and activity during the day.45 One repeated measurement study found favorable results for interdaily stability (an increase) and intradaily variability (a decrease) during a light intervention with very high-intensity light, but only when removing persons with severe visual deficiencies from the sample.74 Five later published studies exploring different light conditions (not including high-intensity light), yielded no significant results for these two variables,42,45–47,69 not even when excluding persons with severe visual deficiencies.42,45 One of these studies originally used high-intensity dynamic lighting, but compared circadian rhythms only between the high light and low light group (not being equal to the dynamic and conventional lighting group).42
The definitions used for circadian amplitude, amplitude or relative amplitude for the rest–activity cycles are largely, but not entirely, similar. They all represent a difference between the means of the most active 10-hour period (“day”) and the least active 5-hour period (“night”) in the average 24-hour pattern.75 No significant relationships were found between light exposure and amplitude,42,46,47,69,74 not even if the studies excluded people with severe eye diseases.42,74 The between-subjects study of Münch et al,42 however, has found a gender-specific susceptibility, noting that “higher daily light exposures significantly predicted an increase in relative amplitude only for men.” As far as total activity is concerned, this study found no overall greater activity in the high light than in the low light group. In a cross-sectional study in existing light conditions, daytime light exposure was positively correlated with 24-h activity, but not with 24h-sleep time.70
When other parameters were used to investigate 24-hour rest–activity rhythms (phasor magnitude; tympanic temperature), two intervention studies both using very cool light, but of different intensity, found significant evidence for improved circadian rhythms.45,56 Nonetheless, an intervention of extremely cool high-intensity light, using tympanic temperature, did not find any notable circadian effects.57
Bodily Functions
Both studies (an RCT43 and a clinical trial76) showed no increase of adverse effects of the intervention group compared to the control group. Instead, the RCT, a long-term intervention study using high-intensity neutral light significantly lowered the ratings on five items: irritability, dizziness, headache, constipation and inability to sleep.43 No significant effects were found on the prescription use of psychotropic medication.43 Visual performance was only touched upon in one article. In this study, six caregivers (35.3%) indicated by questionnaire that the visual performance of their clients improved after installing (dynamic) lighting systems.30
Discussion
This systematic literature review demonstrated that researchers have investigated many different health outcomes in relation to the potential effects of indoor light on older persons with dementia living in long-term care facilities. Behavior was the most researched health outcome, and within this area researchers focused almost exclusively on challenging behaviors. Only two before–after studies in which (very) cool light of moderate intensity was provided showed a significant decrease in agitation.45,46 We found no conclusive evidence that periods with less light due to the sunset or to dark seasons caused sundowning behavior. Further, there was no convincing evidence that high-intensity light positively influenced the circadian day–night rhythm, despite a much-cited study from the 1990s showing promising results.74 Although many studies showed a beneficial influence of daytime light on nighttime sleep and activity, for each of the variables, it often concerned a single study. In contrast, a 3-year longitudinal study found nocturnal restlessness to be related to a specific combination of day length and cloud amount, namely cloudy short days,73 while other light conditions showed no influence. We found that dynamic lighting did not improve day–night rhythm, nor did it improve quality of life.30,42,46 However, in one before–after study dynamic lighting decreased agitation.46 There were some indications that indoor light exposure during the day positively affected the quality of life of older persons with dementia in long-term care facilities.42,68 There are quite strong indications that indoor light can help reduce depressive symptoms and that incident daylight may play an important role in this effect. None of the included studies focused on light for good vision, although light showed to be an important aid for spatial orientation in older persons with dementia.52,61,65,66
Non-Seasonal Depression
Non-seasonal depression is common in older adults with different types of dementia, causing personal (and professional) caregiver distress.77–80 Nevertheless, only three studies researched the influence of indoor daylight and lighting on depression in long-term care facilities. These light intervention studies showed promising beneficial effects on depression, sometimes even comparable to an effective pharmacological treatment.43–45 An equally small number of research evaluated the treatment of depression in this population using light boxes.81–84 The intervention study with the longest treatment period (8 weeks) had significant treatment effects on depression, regardless of the severity of dementia.83,84 A recent study using aggregated data of three light intervention methods (floor luminaires, light boxes, and light tables) showed a beneficial influence on depressive symptoms as well.85 Despite the limited body of research, these combined results confirm the applicability of light to treat and possibly prevent non-seasonal depression among older adults with dementia in long-term care facilities. Probably, indoor daylight and lighting can play an important role in ameliorating depressive symptoms in a more comfortable way than light boxes and with fewer side effects than pharmacological treatment.
Dynamic Lighting
Imitating natural light indoors was found in the use of dynamic lighting systems. Following up on claims from suppliers, long-term care facilities are installing dynamic lighting systems to improve the well-being and circadian rhythms of older adults with dementia.30 Contrary to the industry’s compelling claims, research on this topic is still in its early stages and far from conclusive.10 The term dynamic lighting itself does not provide much clarity. Dynamic lighting scenarios can differ in terms of light intensity, CCT and the timing and duration of both elements. The two dynamic lighting interventions in our review differed in light intensity and CCT, but were similar in duration of light exposure.42,46 One of these studies measured the actual daytime light exposure with individual light sensors, and showed that the average daily light exposure did not differ significantly between the group with dynamic lighting and the group with standard lighting.42 Despite the fact that both studies were executed in darker seasons, persons in the standard lighting group could go outside or sit in the vicinity of daylit windows, so their light exposure was effectively dynamic too. A review on the rationale for dynamic lighting found several reasons for applying dynamic lighting scenarios, which call for different requirements of the dynamic lighting system.10 Using dynamic lighting to entrain the biological clock, for instance, might require another lighting scenario than the ones needed for executing different tasks or activities. Developing effective dynamic lighting scenarios for the different needs of older adults with dementia in long-term care facilities still requires further new high-quality research. For now, the results for this particular study population are inconclusive.
Limitations and Strengths
This review focused on a clearly defined target group, which enabled us to unravel specific influences of indoor light on older persons with dementia living in long-term care facilities. In addition, we focused on indoor daylight and lighting and excluded studies that (also) focused on other methods of light administration, such as light boxes and light visors, which have different (dis)advantages and characteristics. By combining the results of quantitative, qualitative and mixed-method studies we were able to include a wide range of research focusing on a broad spectrum of health outcomes. Because of this heterogeneity, however, it was not feasible to undertake a meta-analysis. This heterogeneity also made it difficult to find strong evidence and draw unambiguous conclusions. Additionally, several included studies lacked relevant light data, which hindered comparing and interpreting the results. Finally, though we focused on studies of older adults with dementia, persons with different types of dementia can possibly react differently to light.
Recommendations for Future Research and Practice
Although ensuring good quality and quantity of light may support older adults with dementia to (more) independently perform everyday activities, only a few of the included studies have focused on this topic. A review on the use of the physical environment to support everyday activities for people with dementia showed overall ADL scores, orientation in space and eating and drinking to be the most frequently researched everyday activities.86 The studies included in our review focused precisely on these everyday activities. We recommend further research on the influence and requirements of light to support persons with dementia in performing these and other important everyday activities, such as carrying out hobbies, communicating and getting dressed.
Second, we recommend future research to include accurate descriptions of the light conditions, since almost every study was missing essential elements. To compare and interpret the results, it is imperative that researchers give a clear description of the lighting equipment, the light measurements, the building or room and the exposure to the different light sources, including daylight.10,38 Therefore, it is necessary to develop and use consolidated strategies for the description and assessment of both static and dynamic lighting.10
Third, future randomized studies are needed to obtain stronger evidence of (very) cool light of moderate intensity to reduce agitation. In addition, further research is needed to clarify the most effective indoor light conditions to prevent or alleviate depressive symptoms. In doing so, we strongly suggest to research the influence of indoor daylight exposure for more than 2 hours per day. We also recommend to clarify which light conditions significantly influence quality of life.
Finally, we recommend both management and care professionals of long-term care facilities to increase their awareness of the potential impact and risks of inadequate light in the daily life of older adults with dementia. Many included articles reported insufficient light conditions in long-term care facilities. A higher quality and quantity of light can support residents to perform everyday activities, to feel better and to prevent unpleasant situations, such as falls and feeling unsafe in dark places. Moreover, this greater quality and quantity of light is also needed for the task performance of (aging) care professionals, for example, to reduce errors in administering medication.87
Conclusions and Future Directions
Overall, we found moderate evidence for the influence of indoor light on the health of older persons with dementia living in long-term care facilities. The most promising results of indoor light concerned the reduction of depressive symptoms and the facilitation of spatial orientation. With regard to challenging behaviors, we only found indications for a very specific light intervention to decrease agitation. Further research is needed to corroborate these results in which it is important to describe the characteristics of the light conditions more clearly. No conclusive evidence was found for the influence of indoor light on sundowning behavior, nor on circadian rest–activity rhythms. To date, the research on dynamic lighting has yielded little evidence of its efficacy and has not been sufficiently researched among this study population. Based on our findings, we recommend long-term care facilities to raise awareness of the increased lighting needs of older adults with dementia and the potential beneficial effects of indoor light on health.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. The Global Dementia Observatory Reference Guide. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 2.Sloane PD, Mitchell CM, Weisman G, et al. The Therapeutic Environment Screening Survey for Nursing Homes (TESS-NH): an observational instrument for assessing the physical environment of institutional settings for persons with dementia. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):S69–S78. doi: 10.1093/geronb/57.2.S69 [DOI] [PubMed] [Google Scholar]
- 3.Nolan L. Caring for people with dementia in the acute setting: a study of nurses’ views. Br J Nurs. 2007;16(7):419–422. doi: 10.12968/bjon.2007.16.7.23245 [DOI] [PubMed] [Google Scholar]
- 4.Day K, Carreon D, Stump C. The therapeutic design of environments for people with dementia: a review of the empirical research. Gerontologist. 2000;40(4):397–416. doi: 10.1093/geront/40.4.397 [DOI] [PubMed] [Google Scholar]
- 5.Van Hoof J, Kort HSM. Supportive living environments: a first concept of a dwelling designed for older adults with dementia. Dementia. 2009;8(2):293–316. doi: 10.1177/1471301209103276 [DOI] [Google Scholar]
- 6.Van Hoof J, Wouters EJM, Schräder B, et al. Intelligent light therapy for older adults: ambient assisted living. In: Agah A, editor. Medical Applications of Artificial Intelligence. Boca Raton: CRC Press, Taylor & Francis Group; 2013:343–353. [Google Scholar]
- 7.Bouma H, Weale RA, McCreadie C. Technological environments for visual independence in later years. Gerontechnology. 2006;5(4):187–194. https://journal.gerontechnology.org/archives/641-643-1-PB.pdf [Google Scholar]
- 8.Van Someren EJW, Hagebeuk EEO, Lijzenga C, et al. Circadian rest—activity rhythm disturbances in alzheimer’s disease. Biol Psychiatry. 1996;40(4):259–270. doi: 10.1016/0006-3223(95)00370-3 [DOI] [PubMed] [Google Scholar]
- 9.Aries MBC, Vlies RD, Westerlaken AC. Inventarisatie en vastlegging van de State-of-Art kennis over licht en ouderen. Utrecht: TNO; 2010. [Google Scholar]
- 10.Kompier ME, Smolders KCHJ, de Kort YAW. A systematic literature review on the rationale for and effects of dynamic light scenarios. Build Environ. 2020;186:1–12. doi: 10.1016/j.buildenv.2020.107326 [DOI] [Google Scholar]
- 11.Wever RA, Polášek J, Wildgruber CM. Bright light affects human circadian rhythms. Pflugers Arch. 1983;396(1):85–87. doi: 10.1007/BF00584704 [DOI] [PubMed] [Google Scholar]
- 12.Campbell SS, Dijk D-J, Boulos Z, Eastman CI, Lewy AJ, Terman M. Light treatment for sleep disorders: consensus report: III. Alerting and activating effects. J Biol Rhythms. 1995;10(2):129–132. doi: 10.1177/074873049501000205 [DOI] [PubMed] [Google Scholar]
- 13.Lockley SW. Circadian rhythms: influence of light in humans. In: Squire LR, editor. Encyclopedia of Neuroscience. 5th ed. Oxford, England: Academic Press; 2009:971–986. [Google Scholar]
- 14.Turner PL, Van Someren EJW, Mainster MA. The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep Med Rev. 2010;14(4):269–280. doi: 10.1016/j.smrv.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 15.Van Someren EJW, Riemersma-van Der Lek RF. Live to the rhythm, slave to the rhythm. Sleep Med Rev. 2007;11(6):465–484. doi: 10.1016/j.smrv.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 16.De Lepeleire J, Bouwen A, De Coninck L, Buntinx F. Insufficient lighting in nursing homes. J Am Med Dir Assoc. 2007;8(5):314–317. doi: 10.1016/j.jamda.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 17.Sinoo MM, Van Hoof J, Kort HSM. Light conditions for older adults in the nursing home: assessment of environmental illuminances and colour temperature. Build Environ. 2011;46(10):1917–1927. doi: 10.1016/j.buildenv.2011.03.013 [DOI] [Google Scholar]
- 18.Sloane PD, Mitchell CM, Calkins M, Zimmerman SI. Light and Noise Levels in Alzheimer’s Disease Special Care Units. Vol. 4. New York: Springer; 2000. [Google Scholar]
- 19.Moore KJ, Hill KD, Robinson AL, Haines TP, Haralambous B, Nitz JC. The state of physical environments in Australian residential aged care facilities. Aust Health Rev. 2011;35(4):412–417. doi: 10.1071/AH10932 [DOI] [PubMed] [Google Scholar]
- 20.Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis. 2012;31(4):711–715. doi: 10.3233/JAD-2012-120484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev. 2000;4(6):603–628. doi: 10.1053/smrv.2000.0127 [DOI] [PubMed] [Google Scholar]
- 22.Roccaro I, Smirni D. Fiat lux: the light became therapy. An overview on the bright light therapy in Alzheimer’s disease sleep disorders. J Alzheimers Dis. 2020;77(1):113–125. doi: 10.3233/JAD-200478 [DOI] [PubMed] [Google Scholar]
- 23.Guo L, Duggan J, Cordeiro MF. Alzheimer’s disease and retinal neurodegeneration. Curr Alzheimer Res. 2010;7(1):3–14. doi: 10.2174/156720510790274491 [DOI] [PubMed] [Google Scholar]
- 24.Van Hoof J, Aarts MPJ, Westerlaken AC, et al. Light therapy in smart healthcare facilities for older adults: an overview. In: Curran K, editor. Recent Advances in Ambient Intelligence and Context-Aware Computing. Hershey, PA, USA: IGI Global; 2015:303–310. [Google Scholar]
- 25.Konis K. Field evaluation of the circadian stimulus potential of daylit and non-daylit spaces in dementia care facilities. Build Environ. 2018;135:112–123. doi: 10.1016/j.buildenv.2018.03.007 [DOI] [Google Scholar]
- 26.Colenda CC, Cohen W, McCall WV, Rosenquist PB. Phototherapy for patients with Alzheimer disease with disturbed sleep patterns: results of a community-based pilot study. Alzheimer Dis Assoc Disord. 1997;11(3):175–178. doi: 10.1097/00002093-199709000-00011 [DOI] [PubMed] [Google Scholar]
- 27.Barrick AL, Sloane PD, Williams CS, et al. Impact of ambient bright light on agitation in dementia. Int J Geriatr Psychiatry. 2010;25(10):1013–1021. doi: 10.1002/gps.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloane PD, Williams CS, Mitchell CM, et al. High-intensity environmental light in dementia: effect on sleep and activity. J Am Geriatr Soc. 2007;55(10):1524–1533. doi: 10.1111/j.1532-5415.2007.01358.x [DOI] [PubMed] [Google Scholar]
- 29.Izsó L, Laufer L, Suplicz S. Effects of dynamic lighting on the visual performance of older adults. Light Res Technol. 2009;41:361–370. doi: 10.1177/1477153509336802 [DOI] [Google Scholar]
- 30.Aarts MPJ, Aries MBC, Straathof J, Van Hoof J. Dynamic lighting systems in psychogeriatric care facilities in the Netherlands: a quantitative and qualitative analysis of stakeholders’ responses and applied technology. Indoor Built Environ. 2015;24(5):617–630. doi: 10.1177/1420326X14532387 [DOI] [Google Scholar]
- 31.Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2014;(2). doi: 10.1002/14651858.CD003946.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Missotten P, Farag L, Delye S, Muller A, Grotz C, Adam S. Role of “light therapy” among older adults with dementia: an overview and future perspectives. Geriatr Psychol Neuropsychiatr Vieil. 2019;17(1):83–91. doi: 10.1684/pnv.2019.0786 [DOI] [PubMed] [Google Scholar]
- 33.Aries MBC, Aarts MPJ, Van Hoof J. Daylight and health: a review of the evidence and consequences for the built environment. Light Res Technol. 2015;47(1):6–27. doi: 10.1177/1477153513509258 [DOI] [Google Scholar]
- 34.Shikder S, Mourshed M, Price A. Therapeutic lighting design for the elderly: a review. Perspect Public Health. 2012;132(6):282–291. doi: 10.1177/1757913911422288 [DOI] [PubMed] [Google Scholar]
- 35.Huber M, Knottnerus JA, Green L, et al. How should we define health? Br Med J. 2011;343:d4163–d4163. doi: 10.1136/bmj.d4163 [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Constitution of the World Health Organzation; 2006. Available from: www.who.int/governance/eb/. Accessed April21, 2021.
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aarts MPJ, Aries MBC, Diakoumis A, Van Hoof J. Shedding a light on phototherapy studies with people having dementia: a critical review of the methodology from a light perspective. Am J Alzheimers Dis Other Demen. 2016;31(7):551–563. doi: 10.1177/1533317515628046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong QN, Pluye P, Fàbregues S, et al. Mixed methods appraisal tool (MMAT), version 2018. Registr Copyright. 2018;1148552. [Google Scholar]
- 40.Rheaume YL, Manning BC, Harper DG, Volicer L. Effect of light therapy upon disturbed behaviors in Alzheimer patients. Am J Alzheimers Dis Other Demen. 1998;13(6):291–295. [Google Scholar]
- 41.Cohen-Mansfield J, Marx MS, Rosentahl AS. A description of agitation in a nursing home. J Gerontol. 1989;44(3):M77–M84. doi: 10.1093/geronj/44.3.M77 [DOI] [PubMed] [Google Scholar]
- 42.Münch M, Schmieder M, Bieler K, et al. Bright light delights: effects of daily light exposure on emotions, rest-activity cycles, sleep and melatonin secretion in severely demented patients. Curr Alzheimer Res. 2017;14(10):1063–1075. doi: 10.2174/1567205014666170523092858 [DOI] [PubMed] [Google Scholar]
- 43.Riemersma-van der Lek RF, Swaab DF, Twisk J, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299(22):2642–2655. doi: 10.1001/jama.299.22.2642 [DOI] [PubMed] [Google Scholar]
- 44.Konis K, Mack WJ, Schneider EL. Pilot study to examine the effects of indoor daylight exposure on depression and other neuropsychiatric symptoms in people living with dementia in long-term care communities. Clin Interv Aging. 2018;13:1071–1077. doi: 10.2147/CIA.S165224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahnschaffe A, Nowozin C, Haedel S, et al. Implementation of dynamic lighting in a nursing home: impact on agitation but not on rest-activity patterns. Curr Alzheimer Res. 2017;14(10):1076–1083. doi: 10.2174/1567205014666170608092411 [DOI] [PubMed] [Google Scholar]
- 47.Bromundt V, Wirz-Justice A, Boutellier M, et al. Effects of a dawn-dusk simulation on circadian rest-activity cycles, sleep, mood and well-being in dementia patients. Exp Gerontol. 2019;24(110641):1–8. doi: 10.1016/j.exger.2019.110641 [DOI] [PubMed] [Google Scholar]
- 48.Bicket MC, Samus QM, McNabney M, et al. The physical environment influences neuropsychiatric symptoms and other outcomes in assisted living residents. Int J Geriatr Psychiatry. 2010;25(10):1044–1054. doi: 10.1002/gps.2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen-Mansfield J, Thein K, Marx MS, Dakheel-Ali M, Murad H, Freedman LS. The relationships of environment and personal characteristics to agitated behaviors in nursing home residents with dementia. J Clin Psychiatry. 2012;73(3):392–399. doi: 10.4088/JCP.10m06605 [DOI] [PubMed] [Google Scholar]
- 50.Elmståhl S, Annerstedt L, Ahlund O. How should a group living unit for demented elderly be designed to decrease psychiatric symptoms? Alzheimer Dis Assoc Disord. 1997;11(1):47–52. doi: 10.1097/00002093-199703000-00008 [DOI] [PubMed] [Google Scholar]
- 51.Martin J, Marler M, Shochat T, Ancoli-Israel S. Circadian rhythms of agitation in institutionalized patients with Alzheimer’s disease. Chronobiol Int. 2000;17(3):405–418. doi: 10.1081/CBI-100101054 [DOI] [PubMed] [Google Scholar]
- 52.Mobley C, Leigh K, Malinin L. Examining relationships between physical environments and behaviors of residents with dementia in a retrofit special care unit. J Inter Des. 2017;42(2):49–69. doi: 10.1111/joid.12094 [DOI] [Google Scholar]
- 53.Algase DL, Beattie ERA, Antonakos C, Beel-Bates CA, Lan Y. Wandering and the physical environment. Am J Alzheimers Dis Other Demen. 2010;25(4):340–346. doi: 10.1177/1533317510365342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen-Mansfield J, Werner P, Marx MS, Freedman L. 2 studies of pacing in the nursing home. J Gerontol. 1991;46(3):M77–M83. doi: 10.1093/geronj/46.3.M77 [DOI] [PubMed] [Google Scholar]
- 55.Jao YL, Algase DL, Specht JK, Williams K. The association between characteristics of care environments and apathy in residents with dementia in long-term care facilities. Gerontologist. 2015;55(Suppl 1):S27–39. doi: 10.1093/geront/gnu166 [DOI] [PubMed] [Google Scholar]
- 56.Van Hoof J, Aarts MPJ, Rense CG, Schoutens AMC. Ambient bright light in dementia: effects on behaviour and circadian rhythmicity. Build Environ. 2009;44(1):146–155. doi: 10.1016/j.buildenv.2008.02.005 [DOI] [Google Scholar]
- 57.Van Hoof J, Schoutens AMC, Aarts MPJ. High colour temperature lighting for institutionalised older people with dementia. Build Environ. 2009;44(9):1959–1969. doi: 10.1016/j.buildenv.2009.01.009 [DOI] [Google Scholar]
- 58.Sloane PD, Mitchell CM, Preisser JS, Phillips C, Commander C, Burker E. Environmental correlates of resident agitation in Alzheimer’s disease special care units. J A Geriatr Soc. 1998;46(7):862–869. doi: 10.1111/j.1532-5415.1998.tb02720.x [DOI] [PubMed] [Google Scholar]
- 59.Wong JKW, Skitmore M, Buys L, Wang K. The effects of the indoor environment of residential care homes on dementia suffers in Hong Kong: a critical incident technique approach. Build Environ. 2014;73:32–39. doi: 10.1016/j.buildenv.2013.12.001 [DOI] [Google Scholar]
- 60.Bliwise DL, Carroll JS, Lee KA, Nekich JC, Dement WC. Sleep and “sundowning” in nursing home patients with dementia. Psychiatry Res. 1993;48(3):277–292. doi: 10.1016/0165-1781(93)90078-U [DOI] [PubMed] [Google Scholar]
- 61.Coulson I, White J. A total environment quality of care approach to evaluation of management and care at two dementia care units in Tasmania. Am J Alzheimers Dis Other Demen. 1997;12(3):128–137. doi: 10.1177/153331759701200306 [DOI] [Google Scholar]
- 62.Chang CC, Lin YF, Chiu CH, et al. Prevalence and factors associated with food intake difficulties among residents with dementia. PLoS One. 2017;12(2):e0171770. doi: 10.1371/journal.pone.0171770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brush JA, Meehan RA, Calkins MP. Using the environment to improve intake for people with dementia. Alzheimers Care Q. 2002;3(4):330–338. [Google Scholar]
- 64.Leung MY, Wang C, Wei X. Structural model for the relationships between indoor built environment and behaviors of residents with dementia in care and attention homes. Build Environ. 2020;169:106532. doi: 10.1016/j.buildenv.2019.106532 [DOI] [Google Scholar]
- 65.Cohen-Mansfield J, Thein K, Dakheel-Ali M, Marx MS. Engaging nursing home residents with dementia in activities: the effects of modeling, presentation order, time of day, and setting characteristics. Aging Ment Health. 2010;14(4):471–480. doi: 10.1080/13607860903586102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Netten A. The effect of design of residential homes in creating dependency among confused elderly residents: a study of elderly demented residents and their ability to find their way around homes for the elderly. Int J Geriatr Psychiatry. 1989;4(3):143–153. doi: 10.1002/gps.930040305 [DOI] [Google Scholar]
- 67.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23(3):271–284. doi: 10.1016/0006-3223(88)90038-8 [DOI] [PubMed] [Google Scholar]
- 68.Garre-Olmo J, López-Pousa S, Turon-Estrada A, Juvinyà D, Ballester D, Vilalta-Franch J. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230–1236. doi: 10.1111/j.1532-5415.2012.04040.x [DOI] [PubMed] [Google Scholar]
- 69.Song Y, Dowling GA, Wallhagen MI, Lee KA, Strawbridge WJ, Hubbard EM. Rest-activity patterns in institutionalized Korean older adults with dementia: a pilot study. J Gerontol Nurs. 2009;35(12):20–30. doi: 10.3928/00989134-20091109-99 [DOI] [PubMed] [Google Scholar]
- 70.Ho J, Mathews RM, Heard R, Chin Moi C. Differences in sleep of dementia residents between Macao (China) and Sydney (Australia). Macau J Nurs. 2013;12(2):52. [Google Scholar]
- 71.Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res. 2000;9(4):373–379. doi: 10.1046/j.1365-2869.2000.00221.x [DOI] [PubMed] [Google Scholar]
- 72.Schnelle JF, Alessi CA, Al-Samarrai NR, Fricker RD, Ouslander JG. The nursing home at night: effects of an intervention on noise, light, and sleep. J Am Geriatr Soc. 1999;47(4):430–438. doi: 10.1111/j.1532-5415.1999.tb07235.x [DOI] [PubMed] [Google Scholar]
- 73.Wahnschaffe A, Nowozin C, Rath A, et al. Night-time activity forecast by season and weather in a longitudinal design – natural light effects on three years’ rest-activity cycles in nursing home residents with dementia. Int Psychogeriatr. 2017;1–10. doi: 10.1017/S1041610217001235 [DOI] [PubMed] [Google Scholar]
- 74.Van Someren EJW, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41(9):955–963. doi: 10.1016/S0006-3223(97)89928-3 [DOI] [PubMed] [Google Scholar]
- 75.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27:536–572. doi: 10.1016/0006-3223(90)90523-5 [DOI] [PubMed] [Google Scholar]
- 76.Sloane PD, Noell-Waggoner E, Hickman S, et al. Implementing a lighting intervention in public areas of long-term care facilities: lessons learned. Alzheimers Care Q. 2005;6(4):280–293. [Google Scholar]
- 77.Andreasen P, Lönnroos E, von Euler-chelpin MC. Prevalence of depression among older adults with dementia living in low-and middle-income countries: a cross-sectional study. Eur J Public Health. 2014;24(1):40–44. doi: 10.1093/eurpub/ckt014 [DOI] [PubMed] [Google Scholar]
- 78.Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry. 2011;24(6):461–472. doi: 10.1097/YCO.0b013e32834bb9d4 [DOI] [PubMed] [Google Scholar]
- 79.Steinberg M, Shao H, Zandi P, et al. Point and 5‐year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. doi: 10.1002/gps.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan LL, Wong HB, Allen H. The impact of neuropsychiatric symptoms of dementia on distress in family and professional caregivers in Singapore. Int Psychogeriatr. 2005;17(2):253. doi: 10.1017/S1041610205001523 [DOI] [PubMed] [Google Scholar]
- 81.Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: a randomized controlled trial. Int Psychogeriatr. 2009;21(4):711. doi: 10.1017/S1041610209008886 [DOI] [PubMed] [Google Scholar]
- 82.Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long‐term care. Int J Geriatr Psychiatry. 1999;14(7):520–525. doi: [DOI] [PubMed] [Google Scholar]
- 83.Onega LL, Pierce TW, Epperly L. Effect of bright light exposure on depression and agitation in older adults with dementia. Issues Ment Health Nurs. 2016;37(9):660–667. doi: 10.1080/01612840.2016.1183736 [DOI] [PubMed] [Google Scholar]
- 84.Onega LL, Pierce TW, Epperly L. Bright light therapy to treat depression in individuals with mild/moderate or severe dementia. Issues Ment Health Nurs. 2018;39(5):370–373. doi: 10.1080/01612840.2018.1437648 [DOI] [PubMed] [Google Scholar]
- 85.Figueiro MG, Plitnick B, Roohan C, Sahin L, Kalsher M, Rea MS. Effects of a tailored lighting intervention on sleep quality, rest–activity, mood, and behavior in older adults with Alzheimer disease and related dementias: a randomized clinical trial. J Clin Sleep Med. 2019;15(12):1757–1767. doi: 10.5664/jcsm.8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woodbridge R, Sullivan MP, Harding E, et al. Use of the physical environment to support everyday activities for people with dementia: a systematic review. Dementia (London). 2018;17(5):533–572. doi: 10.1177/1471301216648670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aarts MPJ, Craenmehr G, Rosemann ALP, Van Loenen EJ, Kort HSM. Light for patient safety: impact of light on reading errors of medication labels. Int J Ind Ergon. 2019;71:145–154. doi: 10.1016/j.ergon.2019.03.004 [DOI] [Google Scholar]