Abstract
Cardiac myosin binding protein-C (cMyBP-C) is a thick filament protein that influences sarcomere stiffness and modulates cardiac contraction-relaxation through its phosphorylation. Phosphorylation of cMyBP-C and ablation of cMyBP-C have been shown to increase the rate of MgADP release in the acto-myosin cross-bridge cycle in the intact sarcomere. The influence of cMyBP-C on Pi-dependent myosin kinetics has not yet been examined. We investigated the effect of cMyBP-C, and its phosphorylation, on myosin kinetics in demembranated papillary muscle strips bearing the β-cardiac myosin isoform from nontransgenic and homozygous transgenic mice lacking cMyBP-C. We used quick stretch and stochastic length-perturbation analysis to characterize rates of myosin detachment and force development over 0–12 mM Pi and at maximal (pCa 4.8) and near-half maximal (pCa 5.75) Ca2+ activation. Protein kinase A (PKA) treatment was applied to half the strips to probe the effect of cMyBP-C phosphorylation on Pi sensitivity of myosin kinetics. Increasing Pi increased myosin cross-bridge detachment rate similarly for muscles with and without cMyBP-C, although these rates were higher in muscle without cMyBP-C. Treating myocardial strips with PKA accelerated detachment rate when cMyBP-C was present over all Pi, but not when cMyBP-C was absent. The rate of force development increased with Pi in all muscles. However, Pi sensitivity of the rate force development was reduced when cMyBP-C was present versus absent, suggesting that cMyBP-C inhibits Pi-dependent reversal of the power stroke or stabilizes cross-bridge attachment to enhance the probability of completing the power stroke. These results support a functional role for cMyBP-C in slowing myosin detachment rate, possibly through a direct interaction with myosin or by altering strain-dependent myosin detachment via cMyBP-C-dependent stiffness of the thick filament and myofilament lattice. PKA treatment reduces the role for cMyBP-C to slow myosin detachment and thus effectively accelerates β-myosin detachment in the intact myofilament lattice.
NEW & NOTEWORTHY Length perturbation analysis was used to demonstrate that β-cardiac myosin characteristic rates of detachment and recruitment in the intact myofilament lattice are accelerated by Pi, phosphorylation of cMyBP-C, and the absence of cMyBP-C. The results suggest that cMyBP-C normally slows myosin detachment, including Pi-dependent detachment, and that this inhibition is released with phosphorylation or absence of cMyBP-C.
Keywords: cardiac, myofilament, myosin binding protein-C, step response, stretch activation
INTRODUCTION
The NH2-terminus of cardiac myosin binding protein-C (cMyBP-C) binds to several functionally relevant sarcomeric proteins: actin (1–3), myosin S2 (1, 4), and myosin regulatory light chain (5). The NH2-terminus also bears a phosphorylation motif that affects its preferred binding partner and thereby can modify sarcomeric mechanics. Protein kinase A (PKA) phosphorylation of the NH2-terminus of cMyBP-C diminishes its binding affinity to actin (6–8) and myosin S2 (4), which in turn leads to a faster actin-filament sliding velocity in vitro (6, 8) and a faster rate of force development after a quick stretch in skinned myocardial strips (9, 10). Thus, cMyBP-C phosphorylation has been identified as a significant molecular regulator of myosin function that can enhance cardiac contractility in response to β-adrenergic stimulation in part by enhancing myosin cross-bridge characteristic rates of detachment and recruitment. Functional mechanics of skinned mouse myocardium lacking cMyBP-C (11–13) also show faster rates of force development (14–16), higher frequencies of oscillatory work production (17), and faster loaded shortening velocities (14). These similarities in the functional consequences of phosphorylated cMyBP-C and the loss of cMyBP-C on myosin-dependent rates suggest that cMyBP-C phosphorylation effectively eliminates interactions between the NH2-terminus of cMyBP-C and other myofilament proteins within the sarcomere, as the binding data suggest (4, 7, 8). This concept is perhaps illustrated best in Stelzer et al. (9) where the two conditions, phosphorylation and absence of cMyBP-C, result in force responses after a quick stretch that are nearly superimposable. Nevertheless, it must be noted that chronic phosphorylation of cMyBP-C, as mimicked in the transgenic AllP+ and t3SD mice (18, 19), does not lead to a cardiomyopathy like that observed in mice lacking cMyBP-C and could be cardioprotective in vivo.
Although myosin detachment rate is determined principally by MgADP release rate, inorganic phosphate (Pi) can influence detachment by reversing the myosin power stroke and detaching the force-producing myosin cross-bridge (20–24). We previously reported increased Pi sensitivity of the frequency of oscillatory work production in mouse myocardium lacking cMyBP-C and expressing predominantly β-cardiac myosin (17). That observation suggested that cMyBP-C slows Pi-dependent myosin cross-bridge detachment at least in the β-cardiac myosin background. In light of the similar functional consequences between phosphorylation and removal of cMyBP-C previously reported, we hypothesized that PKA-phosphorylated cMyBP-C would effectively remove its influence on Pi-dependent detachment in β-cardiac myosin, thereby resulting in greater sensitivity of cross-bridge detachment rate to Pi.
Herein, we present characteristics of Pi-dependent β-cardiac myosin cross-bridge detachment due to cMyBP-C phosphorylation in skinned mouse myocardial strips and compare these characteristics with those when cMyBP-C is absent. The rates of cross-bridge detachment rate and force development were measured using a step length change and stochastic length perturbation analysis techniques, thus allowing a direct comparison of the PKA effects on these rate parameters with previous studies (9, 10, 15, 17, 25). These measurements were also performed at maximal and submaximal Ca2+ activation to compare with previous observations. With our study design, a significant statistical interaction detected by repeated measures ANOVA involving the absence of cMyBP-C would indicate that myosin-sensitive mechanics are differentially affected by the presence or absence of cMyBP-C.
METHODS
Animal Models
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of the University of Vermont College of Medicine and the University of Cincinnati Children’s Hospital and complied with the Guide for the Use and Care of Laboratory Animals, published by the National Institutes of Health. Adult male mice were acquired from the University of Cincinnati and were either nontransgenic (NTG) or homozygous transgenic lacking cMyBP-C (t/t) due to truncated mutation of the MYBPC3 gene (13). To mitigate the added difficulty of inferring cross-bridge detachment rates due to the absence of cMyBP-C or due to a mixture of α- and β-cardiac myosin (25), all mice were fed an iodine-deficient 0.15% propylthiouracil diet for at least 12 wk (Harlan Teklad, Indianapolis, IN), resulting in hypothyroidism and a complete shift to β-cardiac myosin expressed in the LV (17, 26, 27). Mouse populations are referred to as NTGβ (n = 5) and t/tβ (n = 6) to denote the β-cardiac myosin background. The two populations were of comparable age (NTGβ 33.2 ± 2.0 wk vs. t/tβ 34.5 ± 1.3 wk, P = 0.589 by two-tailed t test). The t/tβ mice were smaller by body mass (NTGβ 33.1 ± 2.0 g vs. t/tβ 27.5 ± 1.2 g, P = 0.033) while also having larger LVs. (NTGβ 75 ± 5 mg vs. t/tβ 89 ± 4 mg, P = 0.039).
Solutions for Skinned Myocardial Strips
Chemicals and reagents were obtained from Sigma Corp. (St. Louis, MO) unless otherwise noted. Solution concentrations (mmol/L) were formulated by solving equations describing ionic equilibria according to Godt and Lindley (28). The types of solutions are as follows: relaxing solution: pCa 8.0 (pCa = −log10[Ca2+]), 5 EGTA, 5 MgATP, 1 Mg2+, 0 Pi, 35 phosphocreatine, 300 U/mL creatine kinase (CK), 200 ionic strength, pH 7.0; maximal activation solution: same as relaxing with pCa 4.8 and 0 or 16 Pi; half-maximal activation solution: same as activating at pCa 5.75; skinning solution: same as relaxing without CK, with 1% Triton X-100 wt/vol and 50% glycerol wt/vol; storage solution: same as skinning without Triton, with 10 µg/mL leupeptin; alkaline phosphatase (AP) solution: same as relaxing solution with 6 U/mL recombinant AP from Escherichia coli (P-4252, Sigma); cAMP-dependent PKA solution: same as relaxing solution with 1 U/µL recombinant PKA from E. coli (V516A, Promega, Madison, WI).
cMyBP-C Phosphorylation and Mass Spectrometry
Phosphorylated amino acids were identified at four specific sites within cMyBP-C isolated from NTGβ expressing wild-type MyBP-C, and the degree of phosphorylation was quantified by label-free liquid chromatography-mass spectrometry (LC-MS). An explanation of these methods is provided elsewhere (29).
Skinned Myocardial Strips
Skinned myocardial strips were studied as previously described (25, 27). Papillary muscles from the LV were dissected to yield at least two thin strips (∼140 µm diameter) with longitudinally oriented parallel fibers, skinned for 2 h at room temperature, and stored at –20°C. Aluminum T-clips were attached to the ends of a strip ∼300 µm apart. Immediately before mechanical analysis, all strips were incubated for 10 min in AP solution at room temperature. Some strips received a second incubation in PKA solution for 1 h at room temperature. Strips were mounted between a piezoelectric motor and a strain gauge, lowered into a 30 µL droplet of relaxing solution maintained at 17°C, chosen to ease future use of Q10 to infer rates at body temperature, and stretched to 2.2 µm sarcomere length measured by digital Fourier transform. Strips were mounted at pCa 8.0 and then activated at pCa 4.8. Activating solution containing 16 mM Pi was then exchanged to raise Pi concentration from 0 to 12 mM. Strips were then activated at pCa 5.75, and a second Pi titration performed. Calcium activation at pCa 5.75 was chosen to provide a submaximal calcium activation near or below 50% activation. Our previously measured tension-pCa curves between two similar genotypes also fed 6-propyl-2-thiouracil (PTU) (17) and both to have similar pCa50 ∼5.65. PKA would be expected to lower the calcium sensitivity of the thin filament; therefore, pCa 5.75 is expected to be near or below 50% activation. Recorded forces were normalized to cross-sectional area to calculate isometric tension (T), with individual recordings of developed tension (Tdev) calculated by subtracting the relaxed tension (Tmin) value: Tdev = T – Tmin.
Mechanics Analysis
Step length changes of amplitude 1% strip length were applied under increasing Pi conditions. Estimates of rates of force release (kf,rel) and subsequent force development (kf,dev) were calculated from the half-time of the decaying (t1,2, phase 3) and rising (t2,3, phase 3) force transients following step length change (15, 30):
| (1) |
The force release rate, kf,rel, reflects the rate of myosin cross-bridge detachment apparent in phase 2 (31–33). The force development rate, kf,dev, reflects the rate of myosin cross-bridge recruitment in phase 3 (31).
Stochastic length perturbations were also applied at each Pi condition for a period of 40 s as previously described (34), using an amplitude distribution with a standard deviation of 0.1% strip length that covered the frequency range 0.25–250 Hz. Elastic and viscous moduli as a function of angular frequency (ω), E(ω), and V(ω) were measured from the in-phase and out-of-phase portions of the tension response to length perturbation (25). A complex modulus, Y(ω), was defined as E(ω) + iV(ω), where i = √−1. Fitting Eq. 1 to the entire frequency range of moduli values provided estimates of six model parameters (A, k, B, 2πb, C, 2πc).
| (2) |
The A term in Eq. 1 reflects the viscoelastic mechanical response of passive elements in the muscle. The B and C terms of Eq. 1 reflect enzymatically driven myosin cross-bridge formation in activated muscle. Parameters 2πb and 2πc reflect cross-bridge-dependent rates that are sensitive to biochemical perturbations affecting enzymatic activity, such as MgATP, MgADP, or Pi concentrations (35). The B term reflects force generation, such that 2πb describes cross-bridge recruitment rate and should be directly related to rate of force development, kf,dev (31). The rate 2πb has also been described as the rate of myosin isomerization including Pi-dependent cross-bridge detachment (32, 36, 37). C term reflects processes pertaining to cross-bridge detachment or force decay, such that 2πc describes to the rate of cross-bridge detachment and should be related to rate of force release, kf,rel (33).
Statistical Analysis
All values are means ± SE, using n = 9, 8, 10, and 10 individual skinned myocardial strips for NTGβ, NTGβ,PKA, t/tβ, and t/tβ,PKA, respectively. Constrained nonlinear least squares fitting of Eq. 2 to moduli were performed using sequential quadratic programming methods in MATLAB (v 7.9.0, The MathWorks, Natick, MA). Statistical analyses were performed using SPSS (v. 27, IBM, Chicago, IL), using repeated measures ANOVA with Pi concentration as the repeated measure. The sensitivity of measured variables to Pi was calculated as the slope of a linear regression between the variable value and Pi conditions 0–5 mM. This sensitivity was then subject to two-way ANOVA to ascertain whether the absence of cMyBP-C and/or PKA differentially affected the sensitivity of these variables to Pi. The terms 2πb and 2πc are expected to directly represent the same physiological processes as kf,dev and kf,rel, respectively, and were compared by correlation. Statistical significance is reported at P < 0.05 and P < 0.01 levels.
RESULTS
Myosin Isoform and Protein Phosphorylation Status
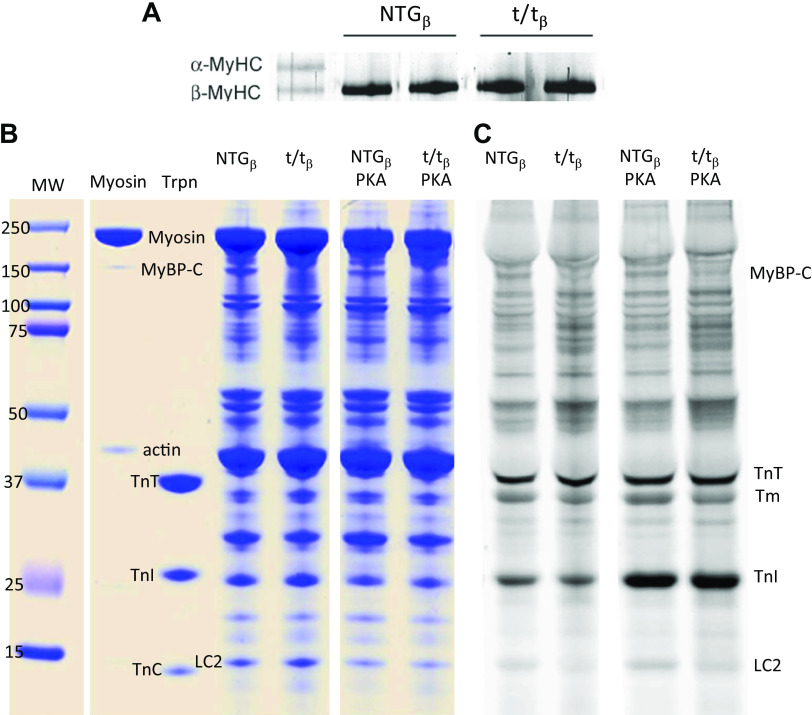
Hypothyroidism resulted in 100% expression of β-cardiac myosin in both the NTGβ and t/tβ groups (Fig. 1A). The application of PKA following alkaline phosphatase treatment clearly enhanced phosphorylation status of troponin-I, as indicated by Pro-Q diamond phospho-staining (Fig. 1B). PKA-mediated phosphorylation of cMyBP-C was quantified at four phosphorylation sites, Ser-273, Ser-282, Ser-302, and Ser-307, by LC-MS (Table 1). PKA treatment resulted in elevated overall phosphorylation status from 50% to 62% taken as an average phosphorylation of these four sites, and most notably, phosphorylation of Ser-307 was elevated from 3% to 27%.
Figure 1.
Cardiac myosin isoforms and PKA-induced phosphorylation of sarcomeric proteins. A: mice were hypothyroid after 12 wk of PTU diet and expressed 100% β-cardiac myosin in the LV at time of harvest. B: gels show comparable protein loads. The lane marked myosin provides a reference for myosin and its contaminants of MyBP-C and actin. The lane loaded with isolated troponin (Trpn) with its three components provides reference for TnT, TnC, and particularly phosphorylatable TnI at ∼24 kDa. C: Pro-Q diamond phospho-stain demonstrates that PKA treatment led to significant phosphorylation of TnI in both genotypes. Phosphorylation of cMyBP-C was not as visually apparent and was quantified instead by mass spectrometry (see Table 1). PKA, protein kinase A; PTU, 6-propyl-2-thiouracil.
Table 1.
Percentage of phosphorylated peptides as observed by mass spectrometry after AP and subsequent PKA treatments
| Phosphorylated Amino Acid | Phosphopeptide Observed | %Phosphorylation |
|
|---|---|---|---|
| AP | PKA | ||
| Ser-273 | 272TSpLAGAGR279 | 55 ± 11 | 68 ± 11 (13) |
| 271RTSpLAGAGR279 | |||
| Ser-282 | 281TSpDSHEDAGTLDFSSLLK298 | 84 ± 8 | 91 ± 5 (7) |
| 280RTSpDSHEDAGTLDFSSLLK298 | |||
| Ser-302 | 299KRDSpFR304 | 56 ± 12 | 63 ± 12 (7) |
| Ser-307 | 305RDSpKLEAPAEEDVWEILR322 | 3 ± 2 | 27 ± 6 (24) |
| Average | 50 ± 2 | 62 ± 13 (12) | |
Values are means ± SD (%change in phosphorylation due to PKA treatment). Average refers to average phosphorylated percentage and associated SD calculated as square root of the sum of variances to reflect error propagation. AP, alkaline phosphatase; PKA, protein kinase A.
Developed Tension Dependence on cMyBP-C, Pi, and PKA
The absence of cMyBP-C led to a reduced maximum developed tension (Tdev) at pCa 4.8 (Table 2, P = 0.020) as seen by overall lower maximum Tdev in the t/tβ compared with NTGβ when Pi and PKA conditions are controlled. Similar trends for a reduction in maximum Tdev with the loss of cMyBP-C in the β-cardiac myosin background have been reported previously (17, 25).
Table 2.
Results for maximum developed tension (Tdev) at pCa 4.8
| Tdev (kPa) | NTGβ | NTGβ,PKA | t/tβ | t/tβ,PKA | ANOVA |
|---|---|---|---|---|---|
| n | 9 | 8 | 10 | 10 | |
| Pi (mM) = 0 | 25.33 ± 1.60 | 14.98 ± 2.37 | 17.52 ± 1.76 | 13.38 ± 1.26 | Main effects |
| 1 | 21.49 ± 1.21 | 13.90 ± 2.41 | 16.34 ± 1.64 | 12.67 ± 1.11 | *MYBPC3, †Pi, †PKA |
| 2 | 20.34 ± 1.01 | 13.14 ± 2.23 | 15.10 ± 1.45 | 11.68 ± 1.03 | |
| 3 | 19.20 ± 1.03 | 11.98 ± 2.07 | 13.87 ± 1.27 | 10.66 ± 0.92 | Interactions |
| 4 | 18.38 ± 0.80 | 11.23 ± 1.89 | 13.05 ± 1.19 | 9.90 ± 0.91 | *Pi×PKA |
| 5 | 17.74 ± 0.72 | 10.53 ± 1.71 | 12.36 ± 1.20 | 9.09 ± 0.79 | |
| 8 | 16.37 ± 0.43 | 9.22 ± 1.46 | 10.72 ± 1.11 | 7.63 ± 0.72 | |
| 12 | 14.18 ± 0.73 | 7.88 ± 1.22 | 9.05 ± 1.01 | 6.02 ± 0.68 | |
| ΔTdev/ΔPi | −1.38 ± 0.17 | −0.90 ± 0.18 | −1.05 ± 0.16 | −0.88 ± 0.14 | *PKA |
Values are means ± SE; n, number of strips. Statistical results reflect repeated-measures ANOVA and demonstrate statistical significance for the main effects of MYBPC3, Pi, PKA, and for interactions Pi × MYBPC3, PKA × MYBPC3, Pi × PKA, Pi × PKA × MYBPC3. The sensitivity of Tdev to Pi was calculated by linear regression using values measured at 0–5 mM Pi and denoted here as ΔTdev/ΔPi. Two-way ANOVA was then used to determine whether ΔTdev/ΔPi was influenced by the absence of cMyBP-C and/or PKA. *P < 0.05, †P < 0.01. NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
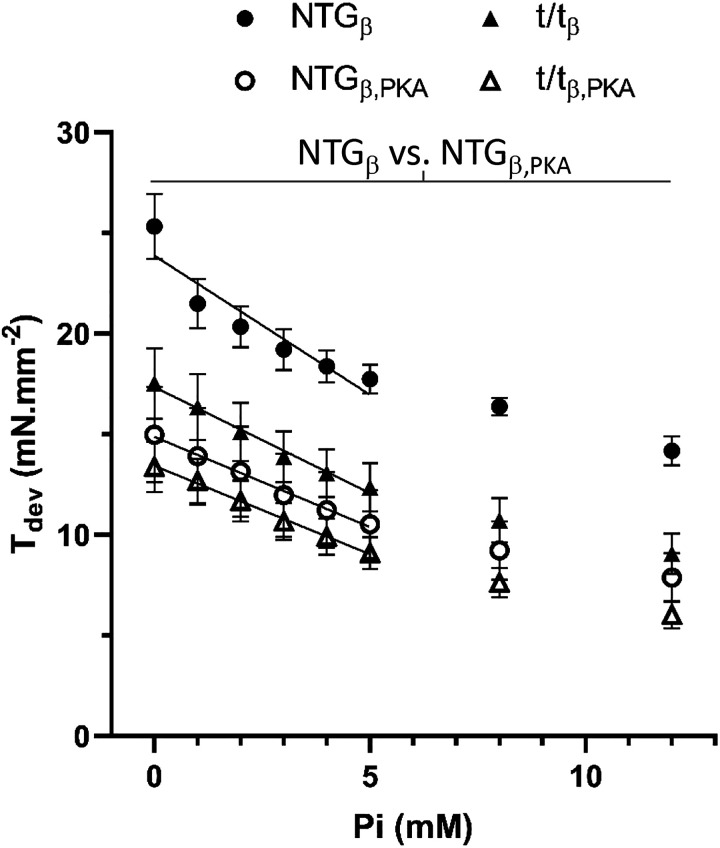
Increasing concentrations of Pi would be expected to reduce Tdev by biasing the myosin cross-bridge to a prepower state and reducing the number of cross-bridges in the force-producing postpower stroke state (22, 23). We likewise found maximum Tdev depressed with increasing Pi at maximum calcium activation (Fig. 2). Statistical analysis verified a highly significant Pi main effect independent of absence of cMyBP-C at both pCa 4.8 and 5.75 (Table 2, P < 0.01). We did not find a significant Pi × MYBPC3 interaction for maximum developed tension or a significant MYBPC3 main effect for Pi sensitivity ΔTdev/ΔPi (Table 2). Therefore, the effects of Pi on maximum developed tension were not significantly dependent upon cMyBP-C.
Figure 2.
Effects of Pi and protein kinase A (PKA) on maximum developed isometric tension in skinned myocardial strips. A: maximum developed isometric tension (Tdev) from NTGβ and t/tβ myocardial strips at pCa 4.8 was reduced with PKA and with increasing Pi. The Pi sensitivity of Tdev was calculated as ΔTdev/ΔPi over 0–5 mM Pi and depicted as the slope of the line drawn over the data points. This Pi sensitivity was reduced (shallower slope) with PKA treatment but was not found to be dependent upon presence or absence of cMyBP-C (see Table 2). Means ± SE. P < 0.01 for NTGβ vs. NTGβ,PKA. cMyBP-C, cardiac myosin binding protein-C; NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
PKA is known to reduce the sensitivity of the thin filament to calcium activation via phosphorylation of TnI and would be expected to reduce maximal developed tension (38). We found PKA treatment reduced maximal developed tension values in both the NTGβ with cMyBP-C and the t/tβ without cMyBP-C (Fig. 2) as affirmed by the highly significant PKA main effects (Table 2, P < 0.01). It is noteworthy that the drop in maximum Tdev with PKA was on the order of 20%, which we attribute to pretreatment with AP. The AP-treated muscle would possess dephosphorylated myofilament proteins TnI, titin, and myosin regulatory light chain.
A significant Pi × PKA interaction was detected for maximum developed tension, indicating that PKA treatment reduced the sensitivity of isometric tension to Pi. This is evident in the shallower slope of the Pi-reduced tension after PKA treatment in Fig. 2. This slope, indicative of the sensitivity of maximum Tdev to Pi and given as ΔTdev/ΔPi in Table 2, demonstrated a significant PKA main effect, meaning that the loss of Tdev with Pi was significantly reduced after PKA treatment. Upon noting the lack of a significant Pi × PKA × MYBPC3 interaction for Tdev and a lack of PKA × MYBPC3 interaction for ΔTdev/ΔPi, the Pi × PKA interaction for maximum Tdev was affected similarly both with and without cMyBP-C, suggesting that the effect of PKA on TnI and not cMyBP-C was likely the underlying cause of PKA treatment enhancing the Pi sensitivity of Tdev.
Step Response Sensitivity to cMyBP-C, Pi, and PKA
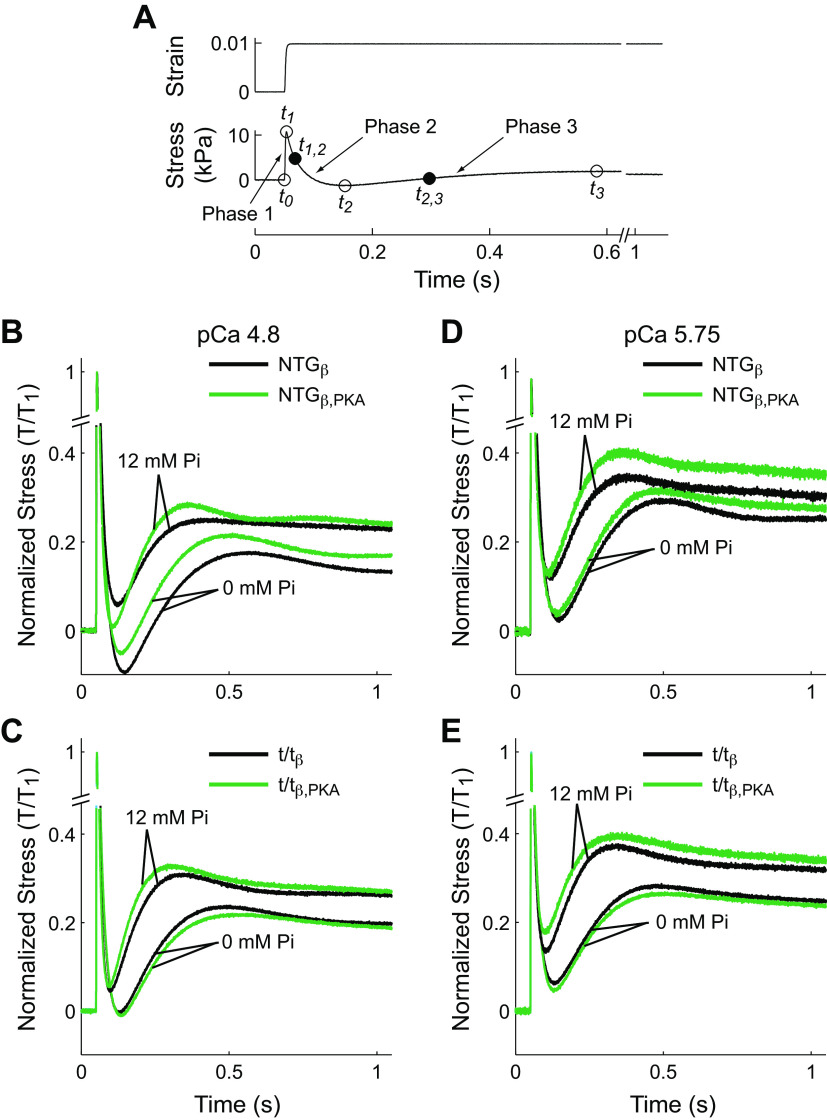
The effects of Pi and PKA on β-cardiac myosin cross-bridge rates of detachment and recruitment in the presence and absence of cMyBP-C were measured from the force response following a 1% length step change (Fig. 3A). Strain, defined as the fractional change in length, was rapidly applied at time t0. The typical stress response included a rapid rise until t1 (the end of phase 1), a decay from t1–t2 (phase 2), and finally a slower rise from t2–t3 (phase 3) (30).
Figure 3.
Step stretch response of skinned mouse myocardium and summary of effects of Pi and protein kinase A. A: measured values of strain and stress are plotted against time for a step length stretch of 1% muscle length. Open circles illustrate time points (t1–t3) bounding phases 1–3. Filled circles represent the time of half-maximal force for phase 2 (t1,2, the phase of relaxation or cross-bridge detachment) and phase 3 (t2,3, the phase of force redevelopment or cross-bridge recruitment). B: representative stress responses for NTGβbearing cMyBP-C at maximally activated pCa 4.8. Temporal characteristics of the stress response were significantly shortened by Pi and PKA. C: representative stress responses for t/tβ lacking cMyBP-C at maximally activated pCa 4.8. When compared against A, responses when cMyBP-C is absent were faster than those when cMyBP-C was present. Pi significantly shortened temporal characteristics of the response, but PKA did not significantly influence these characteristics when cMyBP-C was absent. D: stress responses for NTGβ at pCa 5.75, near-half maximally activation, in the presence of cMyBPC. Again, Pi significantly accelerated the response. PKA also accelerated the response although more subtly and can be seen by a shorter time to the nadir between phases 2 and 3. E: stress responses for t/tβ at pCa 5.75 were accelerated by Pi, whereas PKA did not. cMyBP-C, cardiac myosin binding protein-C; NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
The rates of force release and development, kf,rel and kf,dev, were consistently higher in the t/tβ myocardium lacking cMyBP-C compared with NTGβ bearing cMyBP-C when the comparison accounts for Pi and PKA conditions as indicated by a highly significant MYBPC3 effect at both maximal and submaximal calcium activations (Tables 3 and 4, MYBPC3 main effect P < 0.01). Values for kf,rel and kf,dev were noticeably higher in t/tβ versus NTGβ and in t/tβ,PKA versus NTGβ,PKA comparisons over all Pi (Fig. 4). These results demonstrate faster force transient dynamics when cMyBP-C is absent from the sarcomere as reported previously (14–17, 25).
Table 3.
Results for rates of force release, kf,rel, at maximal calcium activation pCa 4.8 and submaximal pCa 5.75
| kf,rel (s−1) at pCa 4.8 | NTGβ | NTGβ,PKA | t/tβ | t/tβ,PKA | ANOVA |
|---|---|---|---|---|---|
| n | 9 | 8 | 10 | 10 | |
| Pi (mM) = 0 | 46.96 ± 1.16 | 56.86 ± 2.74 | 63.31 ± 2.40 | 61.70 ± 1.26 | Main effects |
| 1 | 49.01 ± 2.06 | 62.68 ± 2.76 | 67.39 ± 2.19 | 70.74 ± 2.47 | †MYBPC3, †Pi, †PKA |
| 2 | 50.39 ± 2.15 | 64.55 ± 3.70 | 71.23 ± 2.38 | 77.41 ± 2.78 | |
| 3 | 51.50 ± 2.24 | 69.78 ± 3.73 | 78.09 ± 2.05 | 82.30 ± 2.26 | Interactions |
| 4 | 55.78 ± 2.40 | 74.02 ± 4.40 | 80.59 ± 2.31 | 87.29 ± 2.99 | †Pi × MYBPC3 |
| 5 | 60.05 ± 2.12 | 76.68 ± 3.90 | 85.63 ± 2.52 | 92.70 ± 2.64 | †Pi × PKA |
| 8 | 61.17 ± 2.72 | 80.35 ± 3.29 | 93.04 ± 2.41 | 99.86 ± 3.19 | |
| 12 | 63.60 ± 3.35 | 86.86 ± 3.25 | 96.71 ± 3.25 | 106.46 ± 3.54 | |
| Δkf,rel/ΔPi | 2.15 ± 0.24 | 3.27 ± 0.48 | 4.35 ± 0.37 | 5.51 ± 0.30 | †MYBPC3, †PKA |
| kf,rel (s-1) at pCa 5.75 | |||||
| Pi (mM) = 0 | 50.59 ± 1.87 | 64.13 ± 3.88 | 73.30 ± 3.31 | 70.59 ± 2.45 | Main effects |
| 1 | 52.22 ± 1.84 | 65.87 ± 2.21 | 76.84 ± 3.33 | 72.80 ± 2.45 | †MYBPC3, †Pi, †PKA |
| 2 | 54.76 ± 2.41 | 66.53 ± 2.20 | 80.43 ± 3.67 | 79.32 ± 2.93 | |
| 3 | 57.71 ± 2.26 | 68.76 ± 2.31 | 83.87 ± 2.73 | 82.54 ± 2.60 | Interactions |
| 4 | 60.26 ± 2.18 | 75.74 ± 2.12 | 88.13 ± 3.46 | 84.86 ± 2.46 | †PKA × MYBPC3 |
| 5 | 63.81 ± 2.19 | 79.94 ± 2.70 | 89.95 ± 3.24 | 89.34 ± 2.60 | |
| 8 | 66.37 ± 2.35 | 85.50 ± 2.96 | 93.21 ± 3.45 | 95.84 ± 3.19 | |
| 12 | 69.76 ± 2.42 | 83.84 ± 3.60 | 93.66 ± 3.74 | 94.29 ± 2.19 | |
| Δkf,rel/ΔPi | 2.64 ± 0.25 | 2.68 ± 0.54 | 3.45 ± 0.33 | 3.81 ± 0.37 | *MYBPC3 |
Values are means ± SE; n, number of strips. The sensitivity of kf,rel to Pi, Δkf,rel/ΔPi, was calculated as the slope of linear regression using values measured at 0–5 mM Pi. Statistical results reflect repeated-measures ANOVA. Two-way ANOVA was then used to determine whether Δkf,rel/ΔPi was influenced by absence of cMyBP-C and/or PKA. *P < 0.05, †P < 0.01. NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
Table 4.
Results for rate of force development, kf,dev, at maximal pCa 4.8 and submaximal pCa 5.75
| kf,dev (s-1) at pCa 4.8 | NTGβ | NTGβ,PKA | t/tβ | t/tβ,PKA | ANOVA |
|---|---|---|---|---|---|
| n | 9 | 8 | 10 | 10 | |
| Pi (mM) = 0 | 5.59 ± 0.24 | 6.12 ± 0.39 | 6.69 ± 0.41 | 6.37 ± 0.21 | Main Effects |
| 1 | 5.37 ± 0.37 | 6.45 ± 0.25 | 6.80 ± 0.43 | 7.57 ± 0.41 | MYBPC3, †Pi, *PKA |
| 2 | 5.98 ± 0.41 | 6.75 ± 0.27 | 7.19 ± 0.42 | 8.22 ± 0.53 | |
| 3 | 6.02 ± 0.44 | 7.12 ± 0.33 | 7.84 ± 0.41 | 9.08 ± 0.48 | Interactions |
| 4 | 6.69 ± 0.70 | 7.45 ± 0.31 | 8.20 ± 0.50 | 9.62 ± 0.58 | Pi × MYBPC3 |
| 5 | 6.94 ± 0.31 | 8.07 ± 0.46 | 8.87 ± 0.51 | 9.91 ± 0.48 | *Pi × PKA × MYBPC3 |
| 8 | 7.51 ± 0.44 | 8.23 ± 0.38 | 9.60 ± 0.50 | 10.67 ± 0.66 | |
| 12 | 8.42 ± 0.61 | 8.09 ± 0.27 | 9.82 ± 0.49 | 11.52 ± 0.59 | |
| Δkf,dev/ΔPi | 0.33 ± 0.07 | 0.40 ± 0.05 | 0.52 ± 0.06 | 0.68 ± 0.05 | †MYBPC3, *PKA |
| kf,dev (s-1) at pCa 5.75 | |||||
| Pi (mM) = 0 | 5.48 ± 0.24 | 6.10 ± 0.42 | 6.30 ± 0.39 | 6.48 ± 0.37 | Main effects |
| 1 | 5.90 ± 0.30 | 6.36 ± 0.44 | 6.93 ± 0.40 | 6.81 ± 0.71 | †MYBPC3, †Pi |
| 2 | 6.78 ± 0.53 | 6.36 ± 0.24 | 7.50 ± 0.36 | 8.38 ± 0.59 | |
| 3 | 6.81 ± 0.35 | 7.09 ± 0.36 | 7.80 ± 0.42 | 8.60 ± 0.48 | |
| 4 | 7.28 ± 0.20 | 7.38 ± 0.20 | 8.22 ± 0.36 | 9.05 ± 0.81 | |
| 5 | 7.53 ± 0.28 | 7.98 ± 0.44 | 8.80 ± 0.47 | 9.10 ± 0.45 | |
| 8 | 8.48 ± 0.51 | 8.24 ± 0.63 | 9.83 ± 0.62 | 10.10 ± 0.45 | |
| 12 | 8.69 ± 0.31 | 7.93 ± 0.57 | 9.67 ± 0.50 | 10.51 ± 0.98 | |
| Δkf,dev/ΔPi | 0.39 ± 0.03 | 0.23 ± 0.10 | 0.48 ± 0.04 | 0.64 ± 0.07 | †MYBPC3 *PKA × MYBPC3 |
Values are means ± SE; n, number of strips. The sensitivity of kf,dev to Pi, Δkf,dev/ΔPi, was calculated as the slope by linear regression using values measured at 0–5 mM Pi. Statistical results reflect repeated-measures ANOVA. Two-way ANOVA was then applied to Δkf,dev/ΔPi. *P < 0.05, †P < 0.01. NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
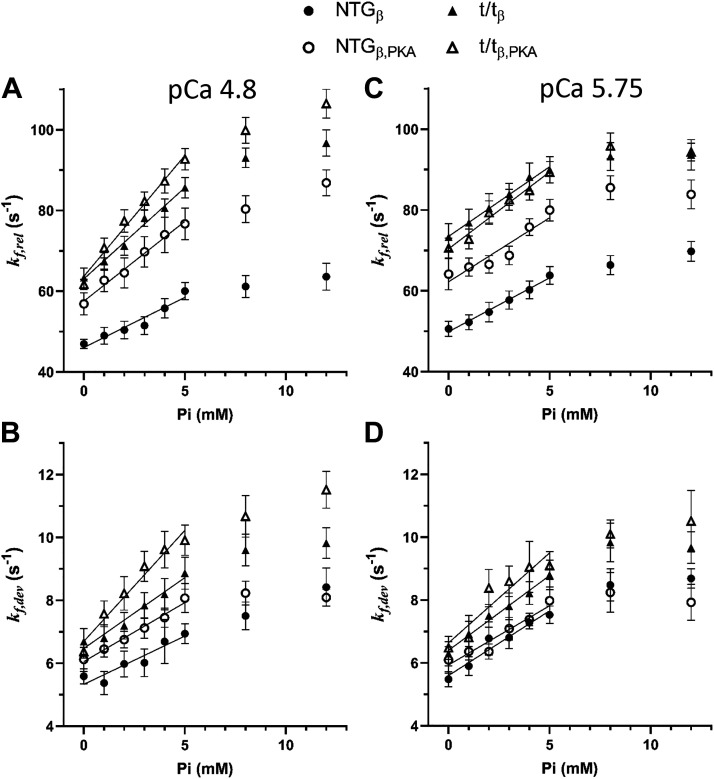
Figure 4.
Effects of Pi and PKA on rates of force release and force development from step responses. A: the rate of force release, kf,rel, at pCa 4.8 was significantly higher in the t/tβ and t/tβ,PKA compared with NTGβ and NTGβ,PKA and was accelerated with increasing Pi. PKA also accelerated kf,rel in both populations, but significantly more so in the NTGβ bearing cMyBP-C than in the t/tβ lacking cMyBP-C. B: rate of force development, kf,dev, at pCa 4.8 demonstrated similar trends to kf,rel including enhanced in the t/tβ and t/tβ,PKA lacking cMyBP-C. The rate kf,dev was also accelerated by Pi and PKA. C: at submaximal calcium activation, pCa 5.75, kf,rel was enhanced by lack of cMyBP-C and also by Pi and PKA. The enhancement of kf,rel by PKA was dependent upon the presence of cMyBP-C and implicates cMyBP-C as the primary mediator of PKA effects on β-cardiac myosin cross-bridge detachment rate. D: at pCa 5.75, kf,dev was also enhanced by lack of cMyBP-C and increasing Pi, but not by PKA. Means ± SE. cMyBP-C, cardiac myosin binding protein-C; NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
Pi effects and Pi × MYBPC3 interaction.
The rates kf,rel and kf,dev were highly sensitive to Pi at both maximal and submaximal calcium activations (Tables 3 and 4, Pi main effect P < 0.01). This sensitivity to Pi can be seen in the dramatic shortening of the durations in phases 2 and 3 as Pi is elevated from 0 to 12 mM (Fig. 3, B–E). The calculated values for kf,rel and kf,dev were clearly enhanced with increasing Pi (Fig. 4) and therefore consistent with results reported by others that myosin-dependent force dynamics are enhanced with Pi availability (22, 39, 40).
A significant Pi × MYBPC3 interaction was observed for both kf,rel and kf,dev (Tables 3 and 4, P < 0.01). These interactions may be better recognized upon considering the Pi sensitivity of the variables, Δkf,rel/ΔPi and Δkf,dev/ΔPi. These Pi sensitivities were consistently higher in the t/tβ lacking cMyBP-C compared with NTGβ bearing cMyBP-C as demonstrated by a significant MYBPC3 main effect for both Δkf,rel/ΔPi and Δkf,dev/ΔPi under both calcium activation conditions (Tables 3 and 4) and can be recognized in Fig. 4, A and B, where values for t/tβ show a greater rate of rise with Pi compared with NTGβ independent of the PKA condition. These results suggest that the Pi-sensitive β-myosin detachment rate and recruitment rate are slowed by the presence of cMyBP-C.
PKA effects and PKA × MYBPC3 interaction.
The rate of force release, kf,rel, was highly sensitive to PKA at both maximal and submaximal calcium activations (Table 3, P < 0.01). This rate, which characterizes the steepness of phase 2, is thought to reflect the myosin detachment rate (31, 33). Our findings suggest that PKA enhances myosin detachment rate and can be seen clearly as higher values for kf,rel in the NTGβ,PKA group bearing phosphorylated myofilament proteins including cMyBP-C compared with the NTGβ group in Fig. 4, A and C. Conversely, any effect of PKA was not apparent in the t/tβ lacking cMyBP-C. In fact, the significant PKA × MYBPC3 interaction for kf,rel at pCa 5.75 (Fig. 4C) indicates that the presence of cMyBP-C in the NTGβ group was statistically more sensitive to the effects of PKA over the entire range of Pi conditions. These results suggest that PKA enhances myosin detachment rate over the entire range of Pi when cMyBP-C is present.
The effect of PKA on the rate of force development, kf,dev, was subtler than that on kf,rel. There was a significant PKA main effect on kf,dev only at maximum calcium activation (Table 4, P = 0.025). The enhancement of kf,dev by PKA can be visualized in Fig. 4B where values in both NTGβ,PKA and t/tβ,PKA groups are generally higher than their respective non-PKA counterparts over the entire range of Pi conditions.
Pi × PKA and Pi × PKA × MYBPC3 interactions.
Whether PKA activity modifies the Pi sensitivity of myosin-dependent rate constants can be answered with the Pi × PKA interaction statistic. We found a highly significant Pi × PKA interaction for the rate of force release, kf,rel, at maximum calcium activation (Table 3, P < 0.01), which indicated that PKA modifies the Pi sensitivity of myosin detachment rate. As can be visualized in Fig. 4A, values for kf,rel in the PKA-treated myocardium were more sensitive to Pi and rose higher with each successively increasing Pi condition compared with non-PKA-treated myocardium either bearing or lacking cMyBP-C. This sensitivity, quantified as Δkf,rel/ΔPi, similarly demonstrated a PKA main effect at pCa 4.8 affirming that PKA-mediated phosphorylation of the myofilaments enhances Pi-dependent myosin detachment rate.
The presence or absence of cMyBP-C did not affect the PKA effect on Pi sensitivity, as indicated by the lack of a Pi × PKA × MYBPC3 interaction for kf,rel (Table 3, P = 0.853). These two results for kf,rel, a significant Pi × PKA interaction without a significant Pi × PKA × MYBPC3 interaction, suggest that the effect of PKA on enhancing the Pi sensitivity of myosin detachment rate is not mediated by cMyBP-C.
Although we did not find a significant Pi × PKA interaction for the rate of force development, kf,dev, at maximum calcium activation (Table 4), we did find a significant three-way interaction Pi × PKA × MYBPC3 (Table 4, P = 0.032). As can be visualized in Fig. 4B, values for kf,dev in the PKA-treated t/tβ,PKA myocardium lacking cMyBP-C were more sensitive to Pi compared with AP-treated t/tβ myocardium, whereas conversely values for kf,dev in the PKA-treated NTGβ,PKA myocardium bearing cMyBP-C were less sensitive to Pi compared with AP-treated NTGβ myocardium. This result, while statistically significant, appears a subtle effect of cMyBP-C phosphorylation on the Pi sensitivity of this characteristic of force development. More specifically, PKA phosphorylation of cMyBP-C reduced the Pi sensitivity of force development characterized by kf,dev.
Complex Modulus Sensitivity to cMyBP-C, Pi, and PKA
Example elastic and viscous moduli are presented in Fig. 5. All moduli were fit to Eq. 2 to assess cross-bridge rate of force development 2πb and cross-bridge detachment rate 2πc at pCa 4.8 and pCa 5.75. We present below results and statistical tests for 2πb and 2πc.
Figure 5.
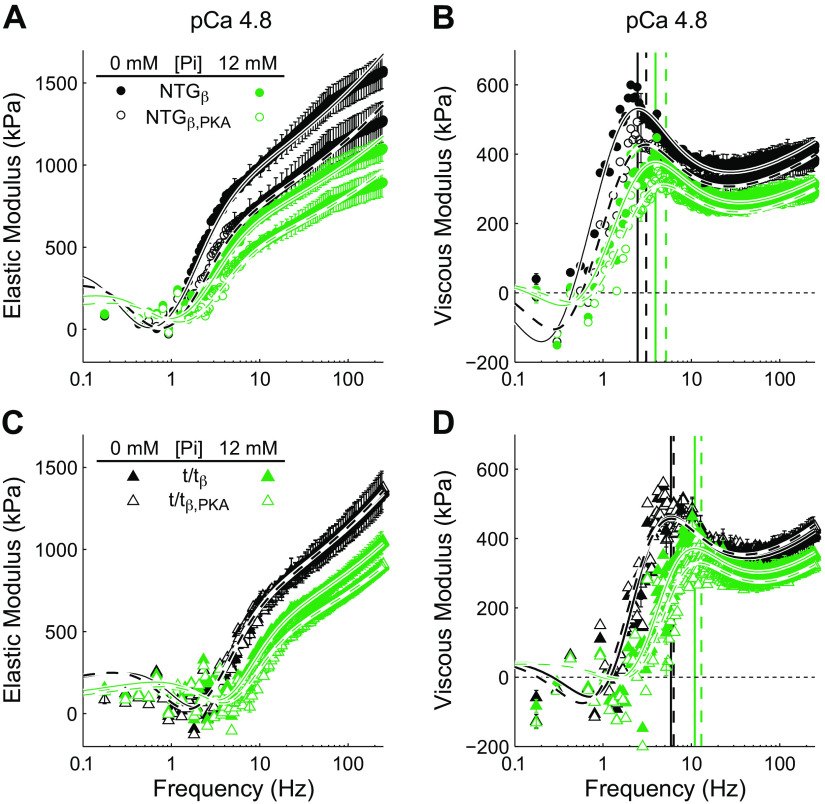
Effect of cMyBP-C, Pi, and PKA on elastic and viscous moduli measured by stochastic length perturbation analysis at pCa 4.8. Elastic (A and C) and viscous (B and D) moduli plotted against oscillatory frequency for NTGβ and t/tβ mice. Black and green lines indicate 0 and 12 mM Pi conditions, respectively. Solid and dashed lines over the data represent fits to Eq. 2 after AP or PKA treatment, respectively. Vertical lines in B and D indicate the frequencies of the peak viscous moduli and are black for 0 mM Pi, green for 12 mM Pi, solid for AP, and dashed for PKA. Characteristics of the moduli were shifted to higher frequencies with lack of cMyBP-C in the t/tβ, increasing Pi, and PKA treatment. AP, alkaline phosphatase; cMyBP-C, cardiac myosin binding protein-C; NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
The rates 2πb and 2πc were higher in myocardium lacking cMyBP-C compared with that bearing cMyBP-C as indicated by significant MYBPC3 main effects (Tables 5 and 6, P < 0.05). These effects can be visualized in Fig. 5, B and D, where the peak frequency of the viscous moduli, which best corresponds to 2πc, was higher in t/tβ compared with NTGβ.
Table 5.
Results for rate of force development, 2πb, at maximal pCa 4.8 and submaximal pCa 5.75
| 2πb (s-1) at pCa 4.8 | NTGβ | NTGβ,PKA | t/tβ | t/tβ,PKA | ANOVA |
|---|---|---|---|---|---|
| n | 9 | 8 | 10 | 10 | |
| Pi (mM) = 0 | 7.98 ± 0.59 | 7.98 ± 0.63 | 10.98 ± 0.61 | 8.64 ± 0.62 | Main effects |
| 1 | 8.27 ± 0.54 | 9.60 ± 0.63 | 12.79 ± 0.79 | 11.49 ± 0.89 | †MYBPC3, †Pi |
| 2 | 8.46 ± 0.60 | 10.87 ± 0.73 | 14.51 ± 0.79 | 13.15 ± 1.10 | |
| 3 | 9.23 ± 0.62 | 11.66 ± 1.03 | 16.54 ± 0.83 | 15.91 ± 1.31 | Interactions |
| 4 | 9.73 ± 0.84 | 12.61 ± 0.99 | 18.18 ± 0.84 | 18.60 ± 1.29 | †Pi × MYBPC3 |
| 5 | 9.90 ± 0.84 | 13.70 ± 1.12 | 19.32 ± 0.84 | 19.78 ± 1.59 | *Pi × PKA |
| 8 | 10.72 ± 1.38 | 14.36 ± 1.28 | 21.70 ± 1.24 | 23.12 ± 1.63 | |
| 12 | 12.71 ± 1.71 | 15.32 ± 1.40 | 23.48 ± 1.60 | 26.37 ± 1.69 | |
| Δ2πb/ΔPi | 0.55 ± 0.10 | 0.92 ± 0.18 | 1.62 ± 0.17 | 2.23 ± 0.33 | †MYBPC3, *PKA |
| 2πb (s-1) at pCa 5.75 | |||||
| Pi (mM) = 0 | 8.01 ± 0.52 | 7.95 ± 0.84 | 9.85 ± 0.86 | 7.47 ± 0.66 | Main effects |
| 1 | 8.09 ± 0.54 | 9.71 ± 1.09 | 12.05 ± 1.04 | 8.92 ± 0.85 | †MYBPC3, †Pi |
| 2 | 9.58 ± 0.55 | 9.75 ± 1.34 | 13.17 ± 0.86 | 10.45 ± 0.92 | |
| 3 | 9.48 ± 0.72 | 10.31 ± 1.51 | 14.57 ± 1.11 | 11.85 ± 1.01 | Interactions |
| 4 | 10.42 ± 0.61 | 11.66 ± 1.59 | 15.71 ± 1.42 | 12.84 ± 0.91 | †Pi × MYBPC3 |
| 5 | 11.15 ± 0.66 | 12.20 ± 1.71 | 17.51 ± 1.35 | 13.09 ± 1.32 | |
| 8 | 12.41 ± 1.00 | 15.00 ± 1.81 | 19.85 ± 1.37 | 16.43 ± 1.45 | |
| 12 | 13.31 ± 1.26 | 16.88 ± 2.03 | 21.78 ± 1.64 | 16.61 ± 1.51 | |
| Δ2πb/ΔPi | 0.68 ± 0.07 | 0.79 ± 0.19 | 1.41 ± 0.23 | 1.18 ± 0.20 | †MYBPC3 |
Values are means ± SE; n, number of strips. The sensitivity of 2πb to Pi was calculated and denoted here as Δ2πb/ΔPi. Statistical results reflect repeated-measures ANOVA. Two-way ANOVA was then applied to Δ2πb/ΔPi. *P < 0.05, †P < 0.01. NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
Table 6.
Results for rate of myosin detachment rate, 2πc, at maximal pCa 4.8 and submaximal pCa 5.75
| 2πc (s-1) at pCa 4.8 | NTGβ | NTGβ,PKA | t/tβ | t/tβ,PKA | ANOVA |
|---|---|---|---|---|---|
| n | 9 | 8 | 10 | 10 | |
| Pi (mM) = 0 | 15.75 ± 1.08 | 20.24 ± 1.44 | 20.63 ± 1.43 | 21.35 ± 1.66 | Main effects |
| 1 | 16.77 ± 1.56 | 21.29 ± 1.54 | 21.17 ± 1.01 | 24.36 ± 1.91 | *MYBPC3, †Pi, *PKA |
| 2 | 17.98 ± 1.69 | 22.07 ± 1.71 | 22.28 ± 0.92 | 26.08 ± 1.82 | |
| 3 | 19.17 ± 1.70 | 23.82 ± 1.76 | 23.33 ± 0.80 | 27.76 ± 1.84 | Interactions |
| 4 | 20.63 ± 1.74 | 24.55 ± 1.85 | 24.62 ± 0.77 | 28.80 ± 2.01 | †Pi × MYBPC3 |
| 5 | 21.98 ± 1.70 | 26.30 ± 1.90 | 26.39 ± 0.95 | 31.07 ± 1.96 | |
| 8 | 24.20 ± 1.78 | 29.02 ± 1.87 | 28.99 ± 1.19 | 34.25 ± 2.04 | |
| 12 | 27.07 ± 1.84 | 31.06 ± 1.81 | 32.16 ± 1.75 | 37.81 ± 1.95 | |
| Δ2πc/ΔPi | 1.15 ± 0.12 | 1.20 ± 0.18 | 1.32 ± 0.13 | 1.71 ± 0.17 | *MYBPC3 |
| 2πc (s-1) at pCa 5.75 | |||||
| Pi (mM) = 0 | 17.11 ± 1.34 | 19.56 ± 1.08 | 22.33 ± 1.55 | 24.99 ± 1.72 | Main effects |
| 1 | 19.01 ± 1.65 | 20.92 ± 1.12 | 23.22 ± 1.51 | 27.46 ± 2.09 | †MYBPC3, †Pi |
| 2 | 19.74 ± 1.64 | 22.62 ± 1.00 | 24.40 ± 1.49 | 29.60 ± 2.45 | |
| 3 | 22.14 ± 1.83 | 24.67 ± 1.19 | 25.84 ± 1.58 | 31.20 ± 2.68 | Interactions |
| 4 | 23.25 ± 1.86 | 26.10 ± 1.52 | 27.79 ± 1.81 | 33.03 ± 2.55 | †Pi × MYBPC3 |
| 5 | 24.75 ± 1.89 | 26.71 ± 1.25 | 29.24 ± 1.85 | 35.77 ± 2.73 | †Pi × PKA × MYBPC3 |
| 8 | 27.63 ± 2.06 | 27.95 ± 1.44 | 31.46 ± 1.81 | 38.87 ± 3.09 | |
| 12 | 29.44 ± 2.23 | 28.49 ± 1.59 | 34.43 ± 2.04 | 42.71 ± 2.97 | |
| Δ2πc/ΔPi | 1.56 ± 0.13 | 1.52 ± 0.16 | 1.48 ± 0.13 | 2.29 ± 0.31 | *PKA × MYBPC3 |
Values are means ± SE; n, number of strips. The sensitivity of 2πc to Pi was calculated and denoted here as Δ2πc/ΔPi. Statistical results reflect repeated-measures ANOVA. Two-way ANOVA was then applied to Δ2πb/ΔPi. *P < 0.05, †P < 0.01. NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
Pi effects and Pi × MYBPC3 interaction.
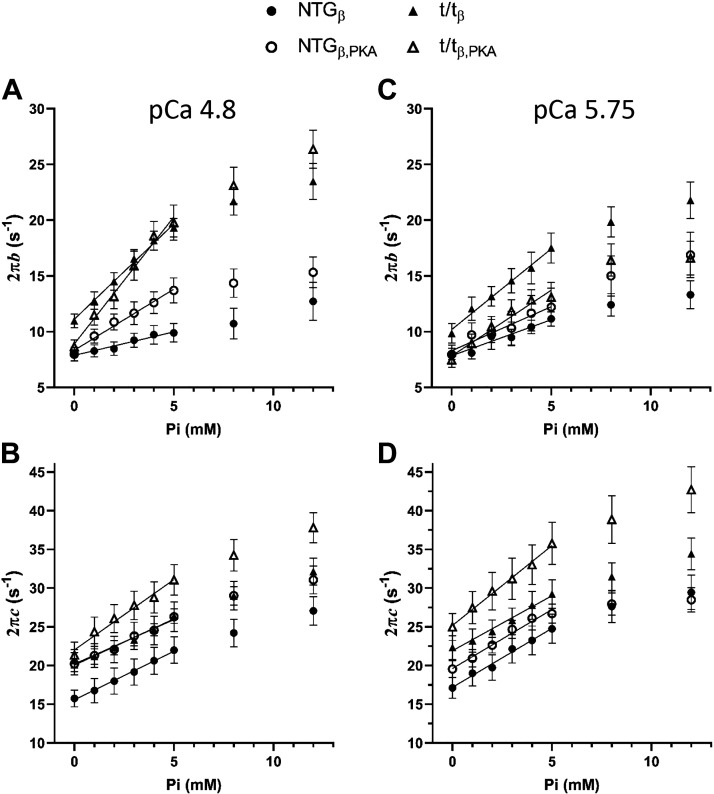
Moduli were also shifted to higher frequencies with higher concentrations of Pi as indicated by a highly significant Pi main effect for 2πb and 2πc (Tables 5 and 6, P < 0.01). Furthermore, the moduli of the myocardium lacking cMyBP-C were more sensitive to the effects of Pi than the that bearing cMyBP-C as indicated by a highly significant Pi × MYBPC3 interaction for both 2πb and 2πc at both pCa 4.8 and pCa 5.75 (Tables 5 and 6, P < 0.01). As an example of these results, the peak in the viscous modulus in the NTGβ (Fig. 5C) was shifted from ∼2 to ∼4 Hz with 12 mM Pi, whereas the peak in the t/tβ (Fig. 5D) was shifted from ∼5 to ∼10 Hz. These data demonstrate that the frequency of the peak viscous modulus, which reflects the myosin detachment rate, was shifted to higher frequencies by Pi, and more so when cMyBP-C was absent from the sarcomere.
This differential sensitivity to Pi due to the presence or absence of cMyBP-C is affirmed with the statistical analysis of Δ2πb/ΔPi and Δ2πb/ΔPi (Tables 5 and 6). The Pi sensitivity of the rate of force development, Δ2πb/ΔPi, was significantly enhanced by the absence of cMyBP-C at both maximal and submaximal calcium activations (Table 5, MYBPC3 main effect P < 0.01). The Pi sensitivity of the myosin detachment rate, Δ2πc/ΔPi, was significantly enhanced by the absence of cMyBP-C at maximal calcium activation (Table 6, MYBPC3 main effect P < 0.05).
These results collectively suggest that, whereas cMyBP-C plays a role in slowing cross-bridge detachment rate and associated rate of force development, cMyBP-C also suppresses Pi-dependent enhancement of cross-bridge detachment rate and rate of force development.
PKA effects and PKA × MYBPC3 interaction.
PKA treatment enhanced the frequency characteristic 2πc at pCa 4.8 (Table 4, PKA main effect P = 0.043). This is a subtle effect at 0 Pi but is clearer at 12 mM Pi where the peak frequency of the viscous modulus is higher in PKA-treated myocardium (Fig. 5, B and D). We did not observe a significant PKA × MYBPC3 interaction for 2πc with this data set, which would have implicated a cMyBP-C dependence in the PKA effect on 2πc.
Pi × PKA and Pi × PKA × MYBPC3 interactions.
Although PKA treatment did not result in statistically significant effects on 2πb, PKA treatment did enhance the Pi sensitivity of 2πb as indicated by a significant Pi × PKA interaction at pCa 4.8 (Table 4, P = 0.014). This sensitivity represented as Δ2πb/ΔPi demonstrated a significant PKA effect, affirming that the Pi-dependent enhancement of the rate of force development was further elevated by PKA. This effect is also illustrated in Fig. 6A where the values of 2πb are enhanced with increasing Pi and more so following treatment with PKA.
Figure 6.
Effect of cMyBP-C, Pi, and PKA on myosin cross-bridge kinetics in skinned myocardial strips. A: the rate of force development (2πb) at maximum calcium activation (pCa 4.8) was significantly enhanced by a lack of cMyBP-C and by increasing Pi. The sensitivity of 2πb to Pi was furthermore enhanced by the absence of cMyBP-C. These data suggest that cMyBP-C inhibits β-cardiac myosin cross-bridge kinetics including those dependent upon Pi. PKA also enhanced 2πb at pCa 4.8 although subtly. B: myosin cross-bridge detachment rate (2πc) at pCa 4.8 was enhanced by a lack of cMyBP-C and by increasing Pi. The Pi sensitivity of 2πc was further enhanced by a lack of cMyBP-C. C and D: results for 2πb and 2πc at submaximal calcium activation mirrored those at pCa 4.8 with enhancement due to lack of cMyBP-C and with increasing Pi. The sensitivity of 2πb and 2πc to Pi was further enhanced when cMyBP-C was absent. Means ± SE. cMyBP-C, cardiac myosin binding protein-C; NTGβ, nontransgenic; t/tβ, transgenic lacking cMyBP-C; PKA, protein kinase A.
Although we did not find a Pi × PKA interaction for 2πc, we did find a significant Pi × PKA × MYBPC3 interaction for 2πc at pCa 5.75 (Table 6, P < 0.01), which indicates that PKA phosphorylation of cMyBP-C influences the sensitivity of myosin detachment rate to Pi. The Pi sensitivity of myosin detachment rate, Δ2πc/ΔPi, demonstrated a PKA × MYBPC3 interaction, which indicates that cMyBP-C influences the PKA-dependent changes to Pi-sensitive myosin detachment. The values of Δ2πc/ΔPi among the four populations examined suggest that, when cMyBP-C is present, there is virtually no change in Pi sensitivity of myosin detachment caused by PKA, and when cMyBP-C is absent, there is a significant enhancement of the Pi sensitivity of myosin detachment caused by PKA. This result is illustrated in Fig. 6D where values for 2πc are shown to rise with increasing Pi but increase more so after PKA treatment in the t/tβ lacking cMyBP-C and less so after PKA treatment in the NTGβ bearing cMyBP-C. These data indicate that PKA phosphorylation of cMyBP-C does not affect the Pi sensitivity of the myosin detachment rate characterized by 2πc.
DISCUSSION
This study examined the role of cMyBP-C phosphorylation by PKA in Pi-sensitive β-cardiac myosin-dependent mechanics, as reflected in rates of detachment and recruitment, in an intact myofilament lattice. Our results confirmed that β-cardiac myosin detachment rate and recruitment rate are enhanced when cMyBP-C is removed from the sarcomere (14–17). The most straightforward interpretation includes a role for cMyBP-C in normally slowing myosin cross-bridge detachment rate by inhibiting MgADP release and extending the cross-bridge lifetime (15, 17, 34). We also found that β-cardiac myosin kinetics were elevated with increasing Pi concentrations in myocardial strips both with and without cMyBP-C. This finding was expected as it recapitulates reports of elevated myosin cross-bridge detachment rate or myosin-dependent muscle function, e.g., velocity of shortening, with increasing Pi (22, 41). The mechanism by which Pi influences the force-producing myosin cross bridge, however, is still debated and may include Pi-induced inhibition of myosin cross-bridge formation (42, 43), reversal of the myosin power stroke (20, 23, 24), and detachment of the myosin cross-bridge without reversal (21). Despite not knowing the specific action of Pi on the myosin cross bridge, we found that the absence of cMyBP-C further augmented the sensitivity of β-cardiac myosin to Pi. Our data provide evidence in support of the idea that cMyBP-C in the intact sarcomere normally inhibits the Pi effects on myosin detachment rate, likely through suppressing Pi-dependent detachment or reversal, in addition to its slowing MgADP release rate.
We also found that PKA treatment led to a faster rate of β-cardiac myosin cross-bridge detachment, as reflected in the rate kf,rel and 2πc, both with and without cMyBP-C, but was significantly more pronounced in the presence of cMyBP-C. This result was consistent across a range of Pi concentrations and agrees with the enhanced rate of force release observed following the quick stretch attributable to PKA phosphorylation of cMyBP-C at low Pi (9, 10, 40, 44). Our results at both maximal and submaximal activations substantiate the idea that Pi sensitivity of β-cardiac myosin cross-bridge detachment rate, reflected in kf,rel and 2πc, in the absence of cMyBP-C is mimicked by PKA-mediated phosphorylation of cMyBP-C. Thus, PKA-phosphorylation of cMyBP-C effectively removes cMyBP-C inhibition of Pi-dependent power stroke reversal.
We found cMyBP-C phosphorylation also enhances the rate of force development, kf,dev, across the full range of Pi examined at maximum calcium activation (Figs. 4B). However, we did not detect any effect of PKA phosphorylation of cMyBP-C on Pi sensitivity of kf,dev and its frequency analog 2πb. Other studies in an α-cardiac myosin background, most notably Stelzer et al. (9, 10) and Tong et al. (45), do report PKA-induced enhancement of the rate of force development due to cMyBP-C. Differences may reside in the cardiac myosin isoform, but further confirmation in the β-cardiac myosin is warranted.
Inferring cMyBP-C Function
How can cMyBP-C slow myosin detachment in the intact sarcomere? Given that cMyBP-C is not a kinase or phosphatase and is not obviously some other regulator of protein post-translational modification, it is presumed that cMyBP-C must act through its binding to nearby sarcomeric structures. We have already demonstrated that the C-terminus of cMyBP-C affects longitudinal thick filament stiffness (46) and its NH2-terminus effects transverse stiffness of the myofilament lattice (25). The concept that the lifetime, unitary force, or any other attribute of myosin cross-bridge can be affected by structural elements far from the myosin head is exemplified by the development of cardiomyopathies due to point mutations in cMyBP-C or in the myosin rod (47). Similarly, cMyBP-C may influence myosin cross-bridge force production by effectively providing a stabilizing scaffold on the lattice and thereby on the formed myosin cross-bridge. The result is an inhibition of MgADP release and Pi-dependent detachment and thereby prolongs the cross-bridge lifetime (33). A role for cMyBP-C in mechanically stabilizing myosin heads in a super-relaxed state has been described (48), where ablation of cMyBP-C removes this stabilization and myosin becomes more active. We would presume that phosphorylation of cMyBP-C would again mimic ablation, thereby releasing the super-relaxed myosin and increasing the active myosin population.
The many observations so far related to enhanced myosin-dependent rates through cMyBP-C phosphorylation (9, 10, 44, 45) suggest that the unbinding of the NH2-terminus from actin and myosin S2 (4, 7, 8) is sufficient to augment stiffness of the myofilament lattice and influence myosin cross-bridge rate of MgADP release or Pi-dependent power stroke reversal. Based on data from Michalek et al. (49), the phosphorylation of the cMyBP-C cardiac-specific motif leads to a more stable and less extensible state of the C0-C3 fragment of cMyBP-C. The structural attributes of cMyBP-C after phosphorylation might then diminish its interaction with S2 or actin, whichever is slowing detachment rate, and thus result in mechanical characteristics that appear more like an effective loss of the NH2-terminus of the molecule from the sarcomere. Because of the low MyBP-C:myosin ratio in the sarcomere C-zone and the absence of MyBP-C in the D-zone, we are currently biased against the idea that cMyBP-C phosphorylation affects myosin cross-bridge cycling through direct interactions with neighboring myosin molecules. Although our measurements cannot definitely rule out either of these possible mechanisms, we favor the idea of a S2-cMyBP-C interaction that indirectly stabilizes the β-cardiac myosin cross-bridge through a more stable myofilament lattice, in part because our findings were largely consistent between maximal and submaximal thin filament activations, and therefore relatively independent of Ca2+-activation level.
The NH2-terminus also plays a significant role in modulating actin velocity (2, 6, 8, 50) and must not be discounted as a contributing factor in the intact lattice. It is possible that an interaction of cMyBP-C NH2-terminus with the thin filament would slow or inhibit movement of the thin filament relative to the thick filament and thereby influence detachment of any attached cross-bridge (2, 6, 8, 50). Although this viscous-like interaction is possible in settings of significant sarcomere shortening or lengthening, we suspect this possible structural influence in our measurements. In particular, the 0.125% amplitude of the stochastic length perturbation analysis translates to only ∼1.2 nm movement of thin filaments relative to thick filaments in both directions, and therefore, the viscous-like load attributable to cMyBP-C would be negligible in our assays.
Phosphorylation of Other Sarcomeric Proteins
In addition to cMyBP-C, PKA also phosphorylates the sarcomeric proteins troponin-I (TnI) and titin. PKA-dependent phosphorylation of TnI at Ser-23/24 reduces myofilament Ca2+ sensitivity and increases cross-bridge cycling rate, which is thought to increase myocardial power output and facilitate faster relaxation during diastole (reviewed in 35 and 51). As noted earlier, PKA treatment of our myocardial strips also led to faster rates of cross-bridge detachment, which could contribute to accelerated relaxation during diastole. However, we also observed that PKA treatment produced a minimal effect on cross-bridge rates of force development and detachment in the absence of cMyBP-C, and this minimal effect of PKA persisted at both pCa levels. These relatively small changes in PKA-dependent cross-bridge cycling kinetics in the absence of cMyBP-C suggest that phosphorylation of TnI and titin contributes minimally to β-cardiac myosin kinetics, as suggested previously for α-cardiac myosin (9, 10, 45). Thus, we attribute the PKA-dependent increases in cross-bridge cycling kinetics in the NTGβ measurements to cMyBP-C phosphorylation.
PKA-dependent phosphorylation of titin’s N2B region reduces titin-dependent passive longitudinal stiffness of the sarcomere, which is thought to promote more rapid and complete ventricular filling during diastole (52–54). However, cMyBP-C is also responsible for ∼50% of longitudinal stiffness of the thick filament and the sarcomere (46). Titin runs the whole length of the sarcomere, and the effective myofilament lattice stiffness would be ranked NTGβ > NTGβ,PKA > t/tβ > t/tβ,PKA, which interestingly coincides with the ranked order of increasing rates of cross-bridge detachment among these populations (Figs. 4 and 6). These data could be interpreted to suggest that the mechanical consequences of titin phosphorylation by PKA require the presence of cMyBP-C. More specifically, the reduced titin stiffness as occurs with PKA phosphorylation may influence myosin kinetics only when cMyBP-C is abundant enough to contribute to a relatively high baseline level of sarcomere stiffness from which sarcomere stiffness can be reduced. This argument would suggest that contributions to sarcomeric stiffness due to cMyBP-C ablation from the sarcomere are greater than those due to titin phosphorylation by PKA. As hearts do not function well without cMyBP-C, these data would support the idea that phosphorylation of titin upon β-adrenergic stimulation contributes to faster cross-bridge detachment that can accelerate diastolic relaxation.
Limitations
The use of hypothyroidism to induce β-myosin expression in rodent hearts presents limitations. The myocardium develops some fibrosis, and myocytes develop a degree of disarray not seen before hypothyroidism (27). Furthermore, hypothyroidism could possibly exacerbate the cardiomyopathy already established in the t/t. However, a transgenic mouse expressing β-myosin without cMyBP-C is not available. We believe the use of hypothyroidism is justified given the usefulness of the β-myosin background to be relevant to the myosin isoform found in the human ventricle.
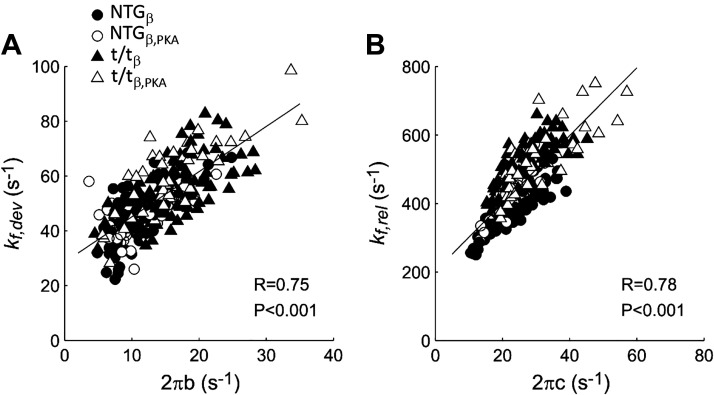
We calculated rates of force development (2πb) and myosin cross-bridge detachment (2πc) using stochastic length perturbations, which provides a frequency spectrum of the force response. We have not thoroughly examined the limitations of each method, but we do recognize that 2πb of the stochastic length perturbation assay is estimated less precisely than 2πc due to the comparatively fewer number of frequencies represented in the spectrum near the b-frequency. Nevertheless, both measures were precise enough for statistical analysis and inference of underlying myosin function.
The rates kf,dev and 2πb should theoretically correspond to each other if the force response demonstrates characteristics of a linear system (Fig. 7A). Likewise, results for kf,rel and 2πc should also correspond (Fig. 7B). We found high correlation coefficients (0.75 ≤ R ≤ 0.91) for kf,dev versus 2πb and for kf,rel versus 2πc. These correlations support the idea that the paired variables reflect similar phenomena. We also found that values of kf,dev and kf,rel from the quick stretch assay were ∼2.5 times and 8 times larger than estimates of 2πb and 2πc from stochastic-length perturbation analysis. This discrepancy may have arisen from estimating kf,dev and kf,rel indirectly using time durations. It is noteworthy that the oscillations of phase 4 in the raw data suggest that, whereas first-order rate constants kf,dev and kf,rel are reasonable characteristics, they do not describe the phenomenon thoroughly. Further work on fully characterizing the force response to length perturbations, whether in the time domain or frequency domain, is warranted.
Figure 7.
Comparison between cross-bridge rate constants from force response to a quick length step (Eq. 1) and stochastic perturbation analysis (Eq. 2). Rates of cross-bridge force development (A) and detachment (B) estimated step analysis are plotted against those estimated by stochastic perturbation analyses. Data represent estimated rates pooled from all measurements as pCa and Pi varied plus pre- and post-PKA treatment. Correlation coefficients (R) and P values are listed within each panel. Solid lines represent linear fits to the data. PKA, protein kinase A.
Conclusions
Our results suggest that PKA-mediated phosphorylation of cMyBP-C in the context of an intact myofilament lattice of the sarcomere elicits a cMyBP-C structural conformation that results in higher rates of β-cardiac myosin cross-bridge detachment, including Pi-dependent detachment, that would typically be inhibited or slowed at lower phosphorylation levels. These effects of cMyBP-C phosphorylation mimic those resulting from an absence of cMyBP-C. However, the influence of cMyBP-C phosphorylation specifically on the Pi dependency of myosin cross-bridge kinetics underlying the rate of force development does not fully mimic that of an absence of cMyBP-C. Although our use of mouse myocardium lacking cMyBP-C points to the phosphorylation of cMyBP-C as the primary PKA-sensitive influence on myosin cross-bridge kinetics, we recognize that it is still possible that effects of PKA-mediated phosphorylation on other proteins may require the presence of cMyBP-C to transmit their influence.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grants P01-HL-59408 (to B.C.W.T., Y.W., J.R., and B.M.P.) and R01-HL-150953 (to B.M.P.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.C.W.T., M.J.P., J.R., and B.M.P. conceived and designed research; B.C.W.T., M.J.P., and Y.W. performed experiments; B.C.W.T., M.J.P., and Y.W. analyzed data; B.C.W.T., M.J.P., Y.W., J.R., and B.M.P. interpreted results of experiments; B.C.W.T., M.J.P., and B.M.P. prepared figures; B.C.W.T. and B.M.P. drafted manuscript; B.C.W.T., M.J.P., J.R., and B.M.P. edited and revised manuscript; B.C.W.T., M.J.P., Y.W., J.R., and B.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of B. C. W. Tanner: Dept. of Integrative Physiology and Neuroscience, Washington State Univ., Pullman, WA 99164.
REFERENCES
- 1.Bhuiyan MS, Gulick J, Osinska H, Gupta M, Robbins J. Determination of the critical residues responsible for cardiac myosin binding protein C’s interactions. J Mol Cell Cardiol 53: 838–847, 2012. doi: 10.1016/j.yjmcc.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razumova MV, Shaffer JF, Tu A-Y, Flint GV, Regnier M, Harris SP. Effects of the N-terminal domains of myosin binding protein-C in an in vitro motility assay: evidence for long-lived cross-bridges. J Biol Chem 281: 35846–35854, 2006. doi: 10.1074/jbc.m606949200. [DOI] [PubMed] [Google Scholar]
- 3.Squire JM, Luther PK, Knupp C. Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol 331: 713–724, 2003. doi: 10.1016/s0022-2836(03)00781-2. [DOI] [PubMed] [Google Scholar]
- 4.Gruen M, Gautel M. Mutations in β-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol 286: 933–949, 1999. doi: 10.1006/jmbi.1998.2522. [DOI] [PubMed] [Google Scholar]
- 5.Ratti J, Rostkova E, Gautel M, Pfuhl M. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J Biol Chem 286: 12650–12658, 2011. doi: 10.1074/jbc.M110.156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Previs MJ, Previs SB, Gulick J, Robbins J, Warshaw DM. Molecular mechanics of cardiac myosin-binding protein-C in native thick filaments. Science 337: 1215–1218, 2012. doi: 10.1126/science.1223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem 284: 12318–12327, 2009. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weith A, Sadayappan S, Gulick J, Previs MJ, Vanburen P, Robbins J, Warshaw DM. Unique single molecule binding of cardiac myosin binding protein-C to actin and phosphorylation-dependent inhibition of actomyosin motility requires 17 amino acids of the motif domain. J Mol Cell Cardiol 52: 219–227, 2012. doi: 10.1016/j.yjmcc.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res 99: 884–890, 2006. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 10.Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res 101: 503–511, 2007. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- 11.Carrier L, Knöll R, Vignier N, Keller DI, Bausero P, Prudhon B, Isnard R, Ambroisine M-L, Fiszman M, Ross J, Schwartz K, Chien KR. Asymmetric septal hypertrophy in heterozygous cMyBP-C null mice. Cardiovasc Res 63: 293–304, 2004. doi: 10.1016/j.cardiores.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res 90: 594–601, 2002. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- 13.McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, Turnbull DH, Georgakopoulos D, Kass D, Bond M, Niimura H, Schoen FJ, Conner D, Fischman DA, Seidman CE, Seidman JG, Fischman DH. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest 104: 1235–1244, 1999. [Erratum in J Clin Invest 104: 1771, 1999]. doi: 10.1172/JCI7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korte FS, McDonald KS, Harris SP, Moss RL. Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ Res 93: 752–758, 2003. doi: 10.1161/01.RES.0000096363.85588.9A. [DOI] [PubMed] [Google Scholar]
- 15.Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ Res 98: 1212–1218, 2006. doi: 10.1161/01.RES.0000219863.94390.ce. [DOI] [PubMed] [Google Scholar]
- 16.Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J 90: 4119–4127, 2006. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer BM, Noguchi T, Wang Y, Heim JR, Alpert NR, Burgon PG, Seidman JG, Seidman CE, Maughan DW, LeWinter MM. Effect of cardiac myosin binding protein-C on mechanoenergetics in mouse myocardium. Circ Res 94: 1615–1622, 2004. doi: 10.1161/01.RES.0000132744.08754.f2. [DOI] [PubMed] [Google Scholar]
- 18.Rosas PC, Liu Y, Abdalla MI, Thomas CM, Kidwell DT, Dusio GF, Mukhopadhyay D, Kumar R, Baker KM, Mitchell BM, Powers PA, Fitzsimons DP, Patel BG, Warren CM, Solaro RJ, Moss RL, Tong CW. Phosphorylation of cardiac myosin-binding protein-C is a critical mediator of diastolic function. Circ Heart Fail 8: 582–594, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci USA. 103: 16918–16923, 2006. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dantzig JA, Goldman YE, Millar NC, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol 451: 247–278, 1992. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debold EP, Walcott S, Woodward M, Turner MA. Direct observation of phosphate inhibiting the force-generating capacity of a miniensemble of myosin molecules. Biophys J 105: 2374–2384, 2013. doi: 10.1016/j.bpj.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranatunga KW. Effects of inorganic phosphate on endothermic force generation in muscle. Proc Biol Sci 266: 1381–1385, 1999. doi: 10.1098/rspb.1999.0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DA, Geeves MA. Strain-dependent cross-bridge cycle for muscle. Biophys J 69: 524–537, 1995. doi: 10.1016/S0006-3495(95)79926-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woody MS, Winkelmann DA, Capitanio M, Ostap EM, Goldman YE. Single molecule mechanics resolves the earliest events in force generation by cardiac myosin. eLife 8: e49266, 2019. doi: 10.7554/eLife.49266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer BM, Sadayappan S, Wang Y, Weith AE, Previs MJ, Bekyarova T, Irving TC, Robbins J, Maughan DW. Roles for cardiac MyBP-C in maintaining myofilament lattice rigidity and prolonging myosin cross-bridge lifetime. Biophys J 101: 1661–1669, 2011. doi: 10.1016/j.bpj.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpert NR, Brosseau C, Federico A, Krenz M, Robbins J, Warshaw DM. Molecular mechanics of mouse cardiac myosin isoforms. Am J Physiol Heart Circ Physiol 283: H1446– H14–54., 2002. [Erratum in Am J Physiol Heart Circ Physiol 283: following table of contents, 2002]. doi: 10.1152/ajpheart.00274.2002. [DOI] [PubMed] [Google Scholar]
- 27.Tanner BC, Wang Y, Robbins J, Palmer BM. Kinetics of cardiac myosin isoforms in mouse myocardium are affected differently by presence of myosin binding protein-C. J Muscle Res Cell Motil 35: 267–278, 2014. doi: 10.1007/s10974-014-9390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80: 279–297, 1982. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Previs MJ, VanBuren P, Begin KJ, Vigoreaux JO, LeWinter MM, Matthews DE. Quantification of protein phosphorylation by liquid chromatography-mass spectrometry. Anal Chem 80: 5864–5872, 2008. doi: 10.1021/ac800337v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature 233: 533–538, 1971. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- 31.Campbell KB, Chandra M, Kirkpatrick RD, Slinker BK, Hunter WC. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol 286: H1535–H1545, 2004. doi: 10.1152/ajpheart.01029.2003. [DOI] [PubMed] [Google Scholar]
- 32.Kawai M, Halvorson HR. Two step mechanism of phosphate release and the mechanisms of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys J 59: 329–342, 1991. doi: 10.1016/S0006-3495(91)82227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer BM, Suzuki T, Wang Y, Barnes WD, Miller MS, Maughan DW. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J 93: 760–769, 2007. doi: 10.1529/biophysj.106.101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanner BC, Wang Y, Maughan DW, Palmer BM. Measuring myosin cross-bridge attachment time in activated muscle fibers using stochastic vs. sinusoidal length perturbation analysis. J Appl Physiol (1985) 110: 1101–1108, 2011. doi: 10.1152/japplphysiol.00800.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry 10: 4617–4624, 1971. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Muthu P, Szczesna-Cordary D, Kawai M. Diversity and similarity of motor function and cross-bridge kinetics in papillary muscles of transgenic mice carrying myosin regulatory light chain mutations D166V and R58Q. J Mol Cell Cardiol 62: 153–163, 2013. doi: 10.1016/j.yjmcc.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Kawai M. The effect of the lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J 64: 197–210, 1993. doi: 10.1016/S0006-3495(93)81357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66: 12–21, 2005. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Hibberd MG, Dantzig JA, Trentham DR, Goldman YE. Phosphate release and force generation in skeletal muscle fibers. Science 228: 1317–1319, 1985. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- 40.Tesi C, Colomo F, Nencini S, Piroddi N, Poggesi C. The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys J 78: 3081–3092, 2000. doi: 10.1016/S0006-3495(00)76845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinken AC, McDonald KS. Inorganic phosphate speeds loaded shortening in rat skinned cardiac myocytes. Am J Physiol Cell Physiol 287: C500– C50–7., 2004. doi: 10.1152/ajpcell.00049.2004. [DOI] [PubMed] [Google Scholar]
- 42.Houdusse A, Sweeney HL. Myosin motors: missing structures and hidden springs. Curr Opin Struct Biol 11: 182–194, 2001. doi: 10.1016/s0959-440x(00)00188-3. [DOI] [PubMed] [Google Scholar]
- 43.Pate E, Cooke R. Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflugers Arch 414: 73–81, 1989. doi: 10.1007/BF00585629. [DOI] [PubMed] [Google Scholar]
- 44.Mamidi R, Gresham KS, Verma S, Stelzer JE. Cardiac myosin binding protein-C phosphorylation modulates myofilament length-dependent activation. Front Physiol 7: 38, 2016. doi: 10.3389/fphys.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res 103: 974–982, 2008. doi: 10.1161/CIRCRESAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyland LR, Palmer BM, Chen Z, Maughan DW, Seidman CE, Seidman JG, Kreplak L, Vigoreaux JO. Cardiac myosin binding protein-C is essential for thick-filament stability and flexural rigidity. Biophys J 96: 3273–3280, 2009. doi: 10.1016/j.bpj.2008.12.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alpert NR, Mohiddin SA, Tripodi D, Jacobson-Hatzell J, Vaughn-Whitley K, Brosseau C, Warshaw DM, Fananapazir L. Molecular and phenotypic effects of heterozygous, homozygous, and compound heterozygote myosin heavy-chain mutations. Am J Physiol Heart Circ Physiol 288: H1097– H1–102., 2005. doi: 10.1152/ajpheart.00650.2004. [DOI] [PubMed] [Google Scholar]
- 48.McNamara JW, Li A, Smith NJ, Lal S, Graham RM, Kooiker KB, van Dijk SJ, Remedios CGD, Harris SP, Cooke R. Ablation of cardiac myosin binding protein-C disrupts the super-relaxed state of myosin in murine cardiomyocytes. J Mol Cell Cardiol 94: 65–71, 2016. doi: 10.1016/j.yjmcc.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalek AJ, Howarth JW, Gulick J, Previs MJ, Robbins J, Rosevear PR, Warshaw DM. Phosphorylation modulates the mechanical stability of the cardiac myosin-binding protein C motif. Biophys J 104: 442–452, 2013. doi: 10.1016/j.bpj.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walcott S, Docken S, Harris SP. Effects of cardiac myosin binding protein-C on actin motility are explained with a drag-activation-competition model. Biophys J 108: 10–13, 2015. doi: 10.1016/j.bpj.2014.11.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res 94: 146–158, 2004. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 52.Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Arch 449: 449–457, 2005. doi: 10.1007/s00424-004-1354-6. [DOI] [PubMed] [Google Scholar]
- 53.Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil 27: 435–444, 2006. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90: 1181–1188, 2002. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 55.Sadayappan S, Gulick J, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Robbins J. Cardiac myosin binding protein-C phosphorylation in a β-myosin heavy chain background. Circulation 119: 1253–1262, 2009. doi: 10.1161/CIRCULATIONAHA.108.798983. [DOI] [PMC free article] [PubMed] [Google Scholar]