Abstract
Cardiovascular disease (CVD) is the leading cause of death globally. Current treatment options include lifestyle changes, medication, and surgical intervention. However, many patients are unsuitable candidates for surgeries due to comorbidities, diffuse coronary artery disease, or advanced stages of heart failure. The search for new treatment options has recently transitioned from cell-based therapies to stem-cell-derived extracellular vesicles (EVs). A number of challenges remain in the EV field, including the effect of comorbidities, characterization, and delivery. However, recent revolutionary developments and insight into the potential of personalizing EV contents by bioengineering methods to alter specific signaling pathways in the ischemic myocardium hold promise. Here, we discuss the past limitations of cell-based therapies and recent EV studies involving in vivo, in vitro, and omics, and future challenges and opportunities in EV-based treatments in CVD.
Keywords: bioengineering, comorbidities, extracellular vesicles, myocardial ischemia, stem cells
INTRODUCTION
The American Heart Association reports startling statistics on the mortality and prevalence of cardiovascular disease (CVD) nationally and globally. CVD continues to be the leading cause of death, accounting for nearly 850,000 deaths in the United States and 17.6 million deaths across the world in 2016 as reported by the American Heart Association. Furthermore, an estimated 18 million Americans over the age of 20 yr have coronary heart disease, whereas the annual incidence of new and recurrent myocardial infarctions (MIs) is estimated at over 800,000 (1). As Dr. Gunnar Biörck described in his article five decades earlier, “The Biology of the Myocardial Infarction”, MI is an acute event in a chronic disease (2). Heart disease describes a variety of conditions that may be vascular, rhythmic, and/or muscular in root—coronary artery disease, arrhythmias, cardiomyopathies, etc. Early treatment options include lifestyle changes that emphasize diet and exercise, as well as medication such as vasodilators, β blockers, and anticoagulants. Late treatment options include a combination of medication and surgical interventions such as coronary artery bypass surgery, valve repair/replacements, and septal myectomies. As many patients with heart failure are unsuitable for lifesaving and invasive surgical procedures due to age, disease state, and comorbidities, new treatments are required to diversify and advance current treatment modalities.
Stem cell-based therapies have been heavily investigated in improving cardiac function following ischemia and infarction in human clinical trials due to their regenerative capacity and potential for autologous transplantation. Such trials include bone marrow transfer to enhance ST-elevation infarct regeneration (BOOST), cardiosphere-derived autologous stem cells to reverse ventricular dysfunction (CADUCEUS), cardiopoietic stem cell therapy in heart failure (C-CURE), and congestive heart failure cardiopoietic regenerative therapy (CHART-1) (3–7). These studies have demonstrated a key limitation of cell-based therapies through their short-lasting clinical improvements. Recently, in the midst of stem cell research, an exciting new approach has emerged via the extracellular vesicles they secrete. Here, we discuss a promising treatment option for patients with myocardial ischemia using mesenchymal-stem cell (MSC)-derived extracellular vesicles (EVs). Although a variety of stem-cell types and sources have been studied in preclinical and clinical studies, MSCs have been shown to be multipotent stem cells with low risk of tumorigenicity and immunogenicity (8). This review describes cardiovascular pathophysiology and the discovery, classification, and recent studies using MSC-derived EVs in animal models. It also discusses the present challenges and future potential of preconditioned and bioengineered EVs in the pursuit of translational applications in CVD with and without comorbidities.
CARDIAC REMODELING
Obstructive blood flow through cases such as atherosclerosis, thrombosis, and/or coronary spasms depletes oxygen supply to the myocardium leading to chronic myocardial ischemia, followed by cardiomyocyte death, cardiac remodeling, and subsequent heart failure. Cardiac remodeling is characterized by maladaptive genetic, molecular, cellular, and structural changes to the heart that adversely affect its function. These changes most notably correspond to energy metabolism, ion homeostasis, inflammatory response, and oxidative stress as described in this section and summarized in Table 1. This discussion emphasizes the broad effects of cardiac remodeling, while also highlighting crucial pathways. This section is subdivided into metabolism and ion channel dysregulation, innate and adaptive immune responses, and oxidative stress.
Table 1.
Pathophysiological processes involved in cardiac remodeling in the ischemic heart
| Processes | Ischemic Heart | Pathophysiology | Outcome | |
|---|---|---|---|---|
| Energy metabolism (9–11) | Fatty acid oxidation | Reduced | Low ATP and high acidosis | Apoptosis |
| Oxidative phosphorylation | Reduced | |||
| Glycolysis | Increased | |||
| Ion homeostasis (12–16) | H+ | Increased | Mitochondrial dysfunction | Necrosis Excitation-contraction uncoupling Hypertrophy |
| Na+ | Increased | |||
| Ca2+ | Increased | |||
| Immune response (17–23) | DAMPs | Increased | ROS, MMP, and cytokine release | Necrosis Fibrosis |
| Leukocyte infiltration | Increased | |||
| Redox regulation (20, 23–29) | Mitochondrial ETC ROS | Increased | DNA damage Lipid peroxidation MMP upregulation |
Apoptosis Necrosis Fibrosis |
| Fenton reaction | Increased | |||
| Nox | Increased |
DAMPs, danger-associated molecular patterns; ETC, electron transport chain; MMP, matrix metalloproteinase; NOX, NADPH oxidase; ROS, reactive oxygen species.
Metabolism and Ion Channel Dysregulation
Cardiomyocytes heavily rely on fatty acid oxidation for their energy production, whereas endothelial cells use glycolysis (11). During remodeling, cardiomyocyte fuel preference switches to glucose due to insufficient oxygen supply, resulting in lipotoxicity, acidosis, and depleted adenosine 5′-triphosphate (ATP). Low fatty acid oxidation coupled with the stimulation of the glycolytic pathway leads to lipotoxicity and lactate accumulation (9). These conditions directly interfere with cardiomyocyte ion homeostasis, which has been proposed to indirectly affect that of endothelial cells through monocarboxylate transporters. Furthermore, hypoxia-acidosis conditions have been shown to result in extensive cardiomyocyte cell death (10). In addition, the drop in cell pH that accompanies anaerobic metabolism forces hydrogen excretion and sodium influx via the Na+/H+ exchanger. Insufficient ATP supply affects the function of ATPases such as Na+/K+ ATPase, plasma membrane Ca2+ ATPase (PMCA), and sarcoplasmic reticulum Ca2+ transport ATPase (SERCA). Altogether, intracellular Na+ and Ca2+ become overloaded and adversely affect excitation-contraction coupling and mitochondrial function of cardiomyocytes, leading to cell hypertrophy and mitochondrial-dependent necrosis (12, 14, 15). In addition, accumulated lactate and H+ are thought to be cotransported through monocarboxylate transporters to exit cardiomyocytes and subsequently enter endothelial cells. Interestingly, this rise in acidity in endothelial cells has been associated with a rise in intracellular Ca2+ resulting in similar morbidities (13, 16).
Innate and Adaptive Immune Responses
The primary modes of cardiomyocyte death in myocardial ischemia and infarction are necrosis and apoptosis; cell death by necrosis results in the release of intracellular contents, otherwise referred to as damage-associated molecular patterns (DAMPs), which activate the innate immune system to clear the dead cells (22). DAMPs include DNA, nucleotides, high-mobility group box 1 (HMGB1), among many more intracellular protein and nonprotein sources. Pattern recognition receptors, such as toll-like receptors (TLRs) and receptor for advanced glycation end products (RAGE), are implicated in the failing heart and linked to the nuclear factor-κB (NF-κB) pathway, a regulator of cell adhesion molecule, chemokine, and cytokine expression to recruit leukocytes to the site of injury (17, 19). Furthermore, TLR stimulants were found to activate the myeloid differentiation factor (MyD88)/interleukin (IL)-1 axis resulting in cardiac fibrosis and heart failure (18). As in any immune response, macrophage and neutrophil infiltration are well characterized in cardiac remodeling and result in cytokine amplification, complement activation, oxidative stress, and release of matrix metalloproteinases (MMPs) that, when extended, become damaging to the heart (20, 23). Adaptive immunity has also been proposed to play a role in remodeling as well (21).
Oxidative Stress
Under ischemic conditions, reactive oxygen species (ROS) homeostasis is disrupted resulting in oxidative stress. Despite reduced oxygen levels, ischemia paradoxically increases ROS (28). Although there are a variety of potential sources for ROS production, the mitochondrial electron transport chain (ETC) has largely been found responsible in the setting of myocardial ischemia (24–26, 29). One study in particular, evaluated the effects of metabolic inhibitors on ROS production in cardiomyocytes during ischemia; although little to no effects were found with nitric oxide synthase (NOS), NADPH oxidase (NOX), nor xanthine oxidase inhibitors, they pinpointed complex I and complex III of the mitochondrial ETC as most significant (24). Levraut et al. (26) conducted a series of experiments implicating ROS generation in membrane depolarization during ischemia, which was correlated with extent of cell death during reperfusion. In addition, they demonstrated the mitochondrial ETC and Fenton reaction as sources of ROS generation, leading to increased lipid peroxidation (26). The inflammatory cascade also contributes significantly to oxidative stress and dysregulation of antioxidant systems; peroxinitrite-mediated nitrosylation at Tyrosine 34 of manganese superoxide dismutase (MnSOD) results in inactivation of this critical mitochondrial antioxidant enzyme (20, 23, 27).
PAST: MESENCHYMAL STEM-CELL THERAPY
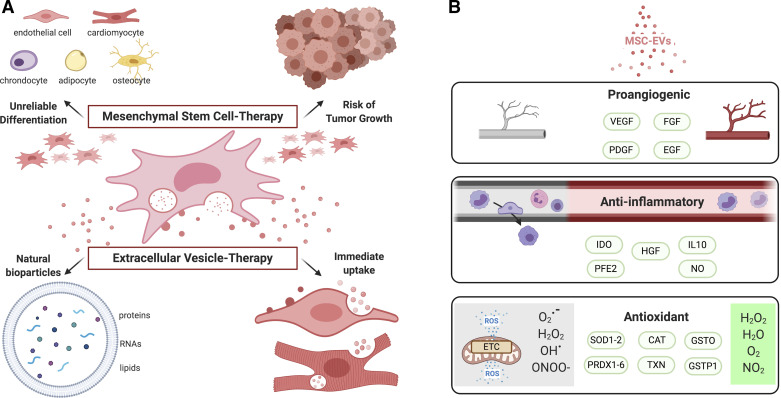
As ventricular remodeling is caused by drastic and diverse pathophysiological changes, treatments must target a variety of mechanisms to provide an adequate impact. As such, stem cells have been of great clinical interest in many diseases for their regenerative properties and potential in autologous transplantations. MSCs are multipotent and well known to have chondrogenic, osteogenic, and adipogenic potential. Many preclinical studies have explored the effect of MSC-based therapies in cardiovascular diseases, detailing the antifibrotic, anti-apoptotic, anti-inflammatory, proangiogenic effects that result in cardioprotection (30–32). Several studies have, furthermore, exhibited their ability to differentiate into cardiomyocytes and endothelial cells, subsequently leading to extensive research in MSCs’ ability to replace damaged myocardial tissue (33–35). This research has shown promising short-term results; most significantly, however, we have developed a better understanding of the limitations in stem-cell based therapies, while attributing the underlying effects of MSCs to paracrine factors and EVs (Fig. 1A). A number of clinical studies investigating MSC-based therapies showed improvement in early cardiac recovery, but was not sustained in the long-term (3, 7). Furthermore, favorable differentiation of MSCs is a rare event in situ and holds the risk of tumorigenicity as observed in several studies (36, 37).
Figure 1.
Mesenchymal stem cell (MSC)- and extracellular vesicle (EV)-based therapies. A: stem cell-based treatments in models of myocardial infarction have led to short-lasting clinical improvements with unreliable or less defined differentiation and risk of tumor growth, contrary to immediate and natural paracrine effects that EV contents may provide. B: MSC-EVs have been shown to have proangiogenic, anti-inflammatory, and antioxidant properties that result in cardioprotection. CAT, catalase; EGF, epidermal growth factor; FGF, fibroblast growth factor; GSTO, glutathione S-transferase O; GSTP1, glutathione S-transferase P1; HGF, hepatocyte growth factor; IDO, indoleamine 2,3 dioxygenase; IL-10, interleukin 10; NO, nitric oxide; PDGF, platelet-derived growth factor; PFE2, prostaglandin E2; PRDX1-6, peroxiredoxin 1-6; SOD1-2, superoxide 1-2; TXN, thioredoxin; VEGF, vascular endothelial growth factor. Created with BioRender.com and published with permission.
The paracrine actions of MSCs were first discovered by protein kinase B (Akt)-overexpression MSCs, which prevented ventricular remodeling and reestablished heart function less than 72 h following MI (38, 39). This speed of recovery led investigators to question the differentiation pathway of MSCs, and Gnecci et al. proceeded to inject Akt-MSC-conditioned medium into infarcted hearts. They observed similar results in recovery and upregulation of potential mediators such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF)-2, and hepatocyte growth factor (HGF) (39). The emergence of EVs was further supported by intravenous and intracoronary injections of MSC-conditioned medium containing products greater than 1,000 kDa in size, which resulted in restoration of ventricular performance in a porcine model of ischemia reperfusion injury (40). The size suggested that paracrine signaling functions not as individual molecules, but as a complex.
PRESENT: STEM CELL-DERIVED EXTRACELLULAR VESICLE THERAPY
Classification
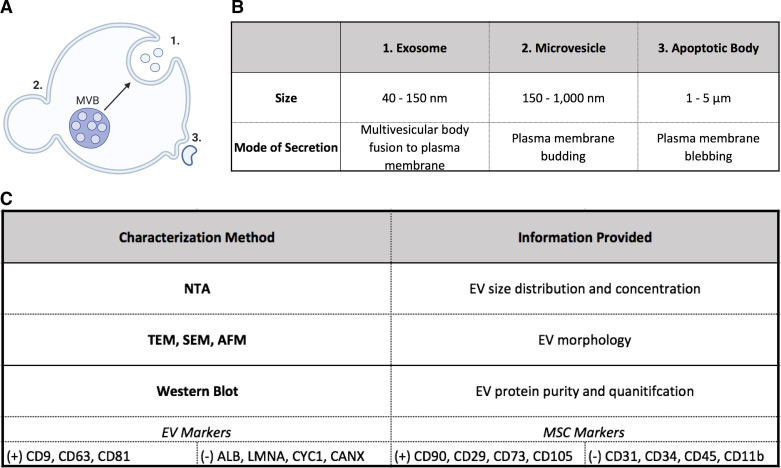
Classification of EVs as exosomes, microvesicles, or apoptotic bodies has traditionally been according to size and mode of secretion. Exosomes range from ∼40 to 150 nm and are released by fusion of the multivesicular body (MVB) to the plasma membrane, whereas microvesicles are ∼150 nm to 1 μm and form through budding from the cell membrane. Apoptotic bodies are between 1 μm and 5 μm and released by membrane blebbing of apoptotic cells (Fig. 2) (41). Oftentimes, however, current isolation methods result in a heterogeneous population of EV subtypes that researchers typically refer to as small EVs (sEVs) when in the range of 40–200 nm. Figure 2 shows the three vesicle subtypes and typical characterization methods of MSC-EVs. It is necessary to verify the presence and absence of tissue-specific markers in addition to EV markers. Altogether, the three classes of vesicles carry a diverse set of cargo that includes microRNA (miRNA), small interfering RNA (siRNA), proteins, lipids, cytokines, and growth factors (41). An important feature of EVs in translational applications is decreased immunogenicity in comparison to cell-based treatments, which could be due to a limited amount of DNA and MHC content (41).
Figure 2.
Characteristics of extracellular vesicles (EV) subtypes and characterization methods. A: schematic showing mode of secretion of EV subtypes. B: summary of size and secretion mode of each class of EVs. C: characterization approaches, which include examples of classic EV (+) positive and (−) negative markers in addition to mesenchymal stem cell (MSC)-specific markers. AFM, atomic force microscopy; MVB, micro vesicular body; NTA, nanoparticle tracking analysis; SEM, scanning electron microscopy; TEM, transmission electron microscopy. Created with BioRender.com and published with permission.
Preclinical Studies
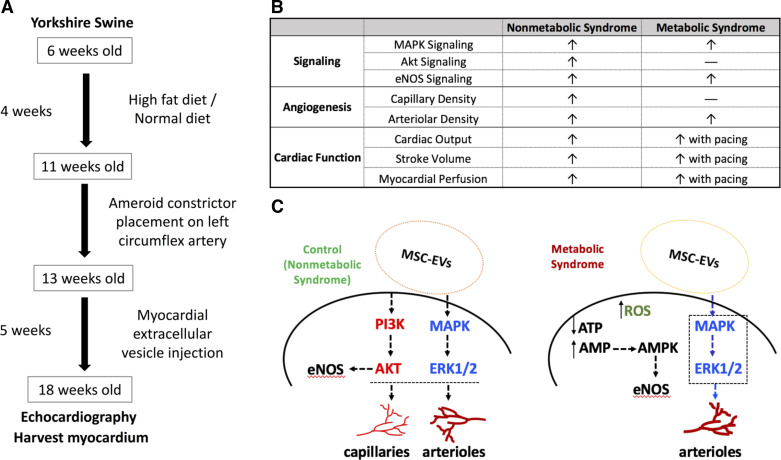
MSC-derived EV therapies in the setting of myocardial infarction have been extensively reviewed and summarized by Alibhai et al. (42) in rat and mouse models. Our laboratory has studied the effect of human bone-marrow MSC-derived EVs in the setting of chronic myocardial ischemia in Yorkshire swine. Swine that received intramyocardial EV injection had improved cardiac output and blood flow as well as increased capillary and arteriolar density (43). These results were accompanied by an increase in expression of proangiogenic signaling molecules regarding the mitogen-activated protein kinase (MAPK) and Akt/endothelial nitric oxide synthase (eNOS) pathways (43).
In a subsequent study, swine were given high-fat diets for 4 wk, followed by surgical intervention to induce chronic ischemia and myocardial injection of EVs 2 wk postsurgery (44). Only with ventricular pacing, cardiac output and stroke volume were significantly improved, as was perfusion in ischemic and nonischemic myocardium. Interestingly, arteriolar density increased in this metabolic syndrome setting, whereas capillary density did not in both ischemic and nonischemic areas of the heart in these animals (44). Perhaps related, only MAPK and eNOS signaling pathways appeared to be activated, whereas Akt phosphorylation remained unchanged unlike the results obtained using the nonmetabolic syndrome animals. Taken together, the interaction between the host environment and preconditioned EVs appears to determine activation of the signaling pathways, angiogenesis, and functional outcomes in chronically ischemic myocardium as described in Fig. 3.
Figure 3.
Comparison of extracellular vesicles (EV) treatment outcomes in chronic myocardial ischemia using a porcine model with or without metabolic syndrome. A: flow diagram of swine model development with or without metabolic syndrome, followed by induction of chronic myocardial ischemia and EV treatment. B: table comparing the signaling, angiogenic, and functional effects of EV injection in ischemic hearts of swine with or without metabolic syndrome. C: schematic of signaling events following EV injection in ischemic hearts with and without metabolic syndrome. AKT, protein kinase B; AMPK, AMP-activated protein kinase; ATP, adenosine 5′-triphosphate; ERK1/2, extracellular signal-regulated kinases ½; eNOS, endothelial nitric oxide synthase; MAPK, mitogen-activated protein kinase; MSC-EVs, mesenchymal-stem cell-derived extracellular vesicles; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species.
In a more recent study, our laboratory further explored the effects of metabolic stress, that is, dyslipidemia and hyperglycemia, on EV treatment in a porcine model of chronic myocardial ischemia. Most interestingly, we found paradoxical molecular signaling in the high-fat diet cohort with increased expression of phosphorylated eNOS, but deficient phosphorylated vascular endothelial growth factor receptor 2 (VEGFR2) (45). These results, combined with cardiac function, perfusion, and vascular density, suggested that metabolic stress impairs revascularization of the ischemic myocardium. Interestingly, gene expression statistics, heat map, and pathway enrichment found that EVs had a disparate effect on global gene expression in chronically ischemic myocardium between normal diet versus high-fat diet animals (45).
EV Contents and Actions
Davidson and Yellon (46) detail the cardioprotective effects of exosomes derived from various cell sources; MSC-exosomes have been shown to improve cardiac systolic function, angiogenesis, and perfusion as well as reduce cardiac apoptosis, infarct size, and fibrosis in animal models of cardiovascular disease. Indeed, proteomic profiling has supported the therapeutic potential of MSC-EVs through many pathways, including metabolic processes, redox control, collagen-fibril organization; all of which may mediate the widespread effects of cardiac remodeling described previously (47). In this section, we describe the proangiogenic, antifibrotic, immunosuppressive, and antioxidant properties of EVs, with an accompanying illustration in Fig. 1B. These topics are described further in an excellent recent review by Öztürk et al. (48).
The proangiogenic effects of these exosomes have been corroborated in vitro, stimulating endothelial cell tube formation, migration, and proliferation (47, 49). Furthermore, study of the proteome has revealed enrichment in pathways supporting angiogenesis, including cell adhesion and migration (49). Anderson et al. (49) performed a comprehensive proteomic analysis of MSC-EVs that were preconditioned under expansion or ischemic-tissue-stimulated conditions, and reported that differentially expressed proteins demonstrated enrichment of PDGF, EGF, FGF, and NF-κB signaling pathways. Their in vitro inhibition studies suggested an essential role for NF-κB signaling in mediating MSC-exosome-induced angiogenesis in endothelial cells (49). The underlying mechanisms for the antifibrotic actions of MSC-EVs in the heart have not been well understood; it is plausible that this may be an indirect effect of EVs’ immunomodulatory capabilities (50, 51). A plethora of in vivo and in vitro studies have reported an immunosuppressive effect of MSC-EVs on innate and adaptive immune cells, which have been attributed to indoleamine 2,3 dioxygenase (IDO), prostaglandin E2 (PFE2), IL-10, nitric oxide (NO), and HGF (52). Meanwhile, the antioxidant effects have been reported to be mediated by mitochondrial antioxidant MnSOD and nuclear factor erythroid 2-related factor 2 (Nrf2) pathway in models of I/R hepatic injury and acute kidney injury, respectively (53, 54). Notably, MSC exosomes containing SOD1, SOD2, peroxiredoxin 1–6 (PRDX1-6), catalase (CAT), thioredoxin (TXN), glutathione S-transferase (GSTO), and GSTP1 antioxidant enzymes have been shown to reduce hepatic oxidative stress and cell death in vitro (53).
Interestingly, a study of miRNA, mRNA, proteomic cargoes, and their interactions reported significant enrichment in nucleotide binding, regulation of transcription, and glycoproteins (55). This finding suggests a critical regulatory role for EVs in recipient cells. In an elegant miRNA study of MSC-exosomes conducted by Ferguson et al. (56), miRNA-targeted genes were overrepresented in cardiovascular development and angiogenesis, cell death and growth, and fibrotic pathways. MSC-exosomes were then examined in vitro and found to reduce collagen production by cardiac fibroblast, cardiomyocyte death, and increased EC angiogenesis. Proteomic and transcriptomic analyses have provided key insights into the molecular mechanisms underlying cellular processes. Pinpointing essential molecules and determining integral pathways will, however, require further studies involving well-designed experiments, bioinformatics, and omics studies using recipient cells. We discuss these further in the next section.
FUTURE: QUESTIONS TO ANSWER
Vesicle and Tissue-Specific Characterization
As the study of EVs transitions to clinical applications, several major questions loom before the field can evolve. Perhaps the most prominent obstacle regarding EVs is the lack of precision in their characterization across research laboratories due to variables such as cell-source, cell-type, and isolation method. Without certain constants, much like in a mathematical formula, the identification of crucial factors in optimizing EV concentration, contents, stability, localization, etc. becomes difficult. The Minimal Information for Studies of EVs (MISEV2014) was an effort by the International Society for Extracellular Vesicles (ISEV) to address this matter and establish a set of guidelines for defining and characterizing EVs, which was later updated to MISEV2018 (57, 58). Notably, the article outlines several categories of markers supplemented with specific examples to analyze for EV presence and purity. It emphasizes the importance of demonstrating that functional outcomes are associated with EVs rather than serum-free media, EV-depleted culture, or coisolates, accompanied by potential assays and also recommends the use of EV-TRACK for submission of isolation and characterization protocols (58).
As MISEV provides a broad framework for all EVs, so as not to restrain the field, all studies should also refer to existing literature for the characterization of specific EV populations on the basis of cell source and type. In regard to the focus of our article, MSC-EVs, the review by Witwer et al. (59) provides necessary insight on their characterization, purity, integrity, activity, among other topics of discussion. A key point the article highlights is the unlikelihood of regulating MSC-EV production and, therefore, the need to define these EVs, or any EVs really, by standardized and quantifiable physical, biochemical, and functional assays. The authors propose and describe the future development of an MSC-EV reference sample as a universal benchmark (59). Separately, a sample of 10 MSC-EV proteomic profiles were analyzed and 15 unique proteins were identified; furthermore, a comparison between MSCs from a variety of sources determined a 22-protein signature for bone marrow derived-MSC-EVs (60). Although the sample size of this study is limited, the results are encouraging in establishing future standardization and comparison measures.
Preconditioning
In discussing the future of EVs, it is also of importance to delve into methods of optimizing and even customizing their cargo. One of the earliest and most popular techniques is referred to as preconditioning, or priming stem cells with a given stimuli during the culturing stage of MSCs to modulate EV contents. The possibilities in the realm of preconditioning include cytokine exposure, oxygen level control, drug administration, nutrient addition, or starvation, etc.—all of which are comprehensively explored in the review by de Cássia Noronha et al., (61) which is specific to MSC-based therapies but can also be applicable to their EVs.
Hypoxic conditions resemble the niche environment of MSCs and are therefore thought to preserve or even improve their therapeutic potential. Consistently, priming MSCs under low levels of oxygen improves angiogenesis in vivo through induction of the HIF-1α and Akt pathways that promote the expression of proangiogenic and prosurvival genes (62). A particularly relevant and hallmark study conducted by Gonzalez-King et al. (63), furthermore, showed increased secretion of exosomes by HIF-1α overexpressing MSCs. Beegle et al. (64) also report an effect on metabolism, where hypoxia-preconditioned (HP) MSCs were enriched with amino acids, pyrophosphate, cholesterol, and lactic acid. In a murine model of myocardial infarction, HP-MSCs improved cardiac function, fibrosis, and cardiomyocyte survival. These cardioprotective effects were abolished upon autophagy inhibition, supporting a profound impact on metabolic processes and nutrient recycling (65). It is important to note that in vivo oxygen levels often differ from in vitro culturing conditions; maintaining consistency in hypoxia, normoxia, and hyperoxia compared with in vivo will be important in attributing the effect of changes in EVs and their actions to increased or decreased oxygen level.
The immunomodulatory capabilities of MSCs have been little explored in the context of CVD. As previously described, the innate and adaptive immune responses further progress cardiac remodeling to heart failure through oxidative stress, ECM remodeling, and cell death. Preconditioning through cytokine exposure, therefore, presents an opportunity to tune MSCs toward an anti-inflammatory state. Transcriptome profiling with interleukin (IL)-17 preconditioned BM-MSCs found similar class I and class II MHC expression levels, indicating no effect on the cells’ immunogenicity and increased array of immunosuppressive and chemokine genes thought to promote the generation and recruitment of regulatory T cells (66). Separately, IFN-γ priming resulted in an MSC-mediated inhibition of IFN-γ from natural killer (NK) cells in vitro (67).
Chemical agents, hormones, and new techniques have also been used to prime MSCs toward a given profile (68–71). Among these, recent techniques include the application of the concept of cold therapy, wherein ex vivo cold storage of MSC-EVs were found to preserve donor hearts before transplantation in mice (71). All together, these findings suggest that there are many opportunities to explore through preconditioning techniques. This large-scale approach has great potential to mediate the widespread changes that occur during cardiac remodeling. In the path to translational applications, proteomics, transcriptomics, lipidomics are necessary in evaluating the ontology of each family and pinpointing essential molecules through various preconditioning methods and even between MSC types and sources. One interesting question is whether one family of macromolecules in EVs is more important and critical than another? EV depletion studies and techniques may, therefore, present valuable insights and influence the way we think about EVs and their contents (72, 73). In addition, direct stochastic optical reconstruction microscopy (dSTORM) is a novel technique to visualize and quantify EV contents—proteins, DNA, RNA, lipids, etc.—at 20-nm resolution.
Bioengineering
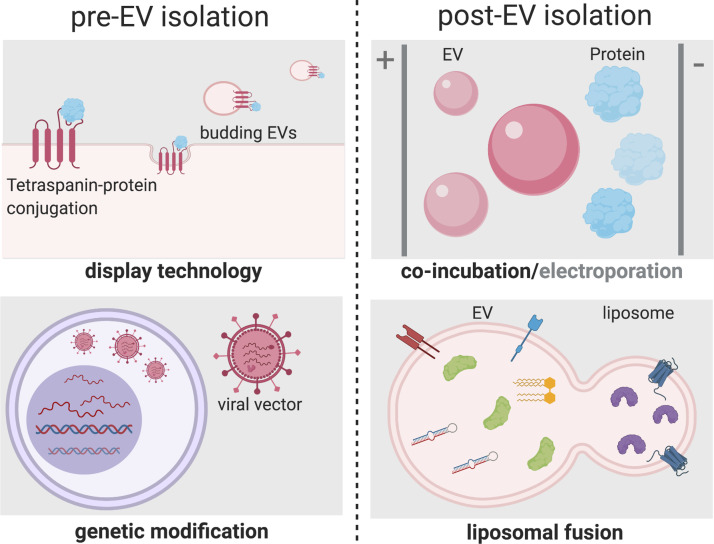
Overall, the advantage in the preconditioning approach is its scale. Essentially, an entire EV profile is influenced as opposed to small-scale techniques that focus on one molecule. On the contrary, bioengineering of vesicles has its own benefits that may include identification of crucial molecules, their mechanisms of action, and customizing EVs as drug delivery systems. Methods of bioengineering of EVs available are pre- or post-EV isolation; genetic modification and surface display technology are popular choices of modifying parent cells, whereas coincubation, electroporation, and liposomal fusion can be used to modify individual or a group of EVs specifically (Fig. 4) (74).
Figure 4.
Schemes of currently available extracellular vesicles (EV) bioengineering methods. Modes of modification before EV isolation (pre-EV isolation) include display technology, where tetraspanins are conjugated with a protein of interest, which are subsequently incorporated onto the membrane of budding EVs. In addition, genetic modification by viral vectors using transfection/transduction method provides an opportunity to overexpress or silence proteins of interest. Post-EV isolation methods include simple co-incubation and electroporation. Liposomal fusion is an emerging method to engineer incorporation of both soluble and membrane components with EVs. Created with BioRender.com and published with permission.
Overall, the significance of customizing EVs has been increasingly demonstrated. Injection of GATA binding protein 4 (GATA-4), a zinc finger transcription factor involved in myocardial development, or Akt overexpressing MSC-derived exosomes into rat models of myocardial infarction resulted in improved functional outcomes compared with sham, saline, and untreated exosome controls (75, 76). More specifically, GATA-4-MSC-EVs had increased intensity in the heart and resulted in reduced cardiomyocyte apoptosis, increased vascular density, and altered expression of proteins related to cell differentiation and apoptosis (75). Akt-MSC-EVs improved endothelial proliferation, migration and tube formation; these results were abrogated with short interfering platelet-derived growth factor D (siPDGFD)-MSC-EVs, suggesting PDGFD mediates the angiogenic effects of Akt-modified EVs (76). Hypoxia inducible factor-1α (HIF-1α)-overexpressing MSC-derived exosomes similarly increased angiogenesis in in vitro and in vivo models more significantly than non-HIF-1α overexpressing exosomes, which was found to be dependent on Jagged-1 activation in the Notch pathway (63).
Micro RNA (miR) overexpression has also been studied; exosomes enriched with miR-126, an important regulator of angiogenesis, indeed improved angiogenic and functional outcomes (77). Micro RNA-93-5p overexpressing suppressed hypoxia-induced autophagy by autophagy related (Atg7) and inflammatory cytokine expression by TLR4, and thereby attenuated post-MI damage. Interestingly, this group further showed that autophagy can indirectly activate the inflammatory response (78). Exosomal miR-21-5p was shown to improve cardiac tissue contractility in vitro through the increased expression of calcium handling genes and activation of the PI3K signaling pathway (79). This study employed partial least square regression (PLSR) analysis examining the top cardiac-related human MSC exosomal miRNAs across several parent cells matched to a number of gene expression responses, namely developmental force, calcium handling, and apoptosis (79). This method enabled investigators to identify miRNAs of interest, for example, miRNAs correlated with positive expression of calcium handling genes but negative for those associated with apoptosis.
The study of bioengineered factors in extracellular vesicles in models of CVD is necessary in the optimization and potential personalization of EV cargo. Furthermore, the continued identification of essential molecules is also important and must be characterized through large-scale clinical transcriptome, proteome, metabolome, and epigenome profiling in patients with heart failure (80). Bioinformatic analysis methods such as PLSR will be of great value in synthesizing, translating, and standardizing EV contents with desired outcomes in CVD.
EV Delivery
Our studies showed that intravenous injection in the auricular vein had insignificant physiological effects in our chronic ischemia porcine models, in contrast to direct intramyocardial injection (43). In the pursuit of developing noninvasive administration methods with organ specificity, further research on their stability, localization, and cellular uptake is required. In a comparative study, in vivo biodistribition of EVs varied between cell source, dose, and route of administration (81). A similar study found a short circulation half-life of EVs, 1.2–1.3 min, with relatively immediate tissue uptake; furthermore, investigators conducted real-time EV biodistribution analysis, finding rapid distribution to the lung, followed by liver then spleen and eventually throughout the whole body (82). Indeed the underlying pathological processes of the disease state of the recipient may also affect tissue targeting. Intravenous delivery of MSC-EVs in a model of acute kidney injury (AKI) found that EVs accumulated in the kidneys of AKI mice and lasted for over 5 h, whereas healthy mice saw fluorescence signal in the spleen that decreased rapidly (83). Recruitment of MSCs to injured kidneys has been attributed to CD44 and hyaluronic acid interactions, and could reasonably be extended to their EVs (84). In a radiation injury bone marrow model, intravenously administered MSC-EVs accumulated predominantly in the liver and spleen before radiation; postradiation saw a significant increase in spleen and bone marrow (tibia and femur) accumulation (85). This study further found that accumulation was dependent on the dose of EV and dose of radiation exposure, and determined the CD11b+ and F4/80+ cell populations in the spleen and bone-marrow as accounting for EV uptake (85).
Bioengineering will also play an important role in improving the delivery and biodistribution of EVs to target tissues. In regards to the cardiovascular system, Zahid et al. (86) identified a cardiac-specific protein transduction domain, cardiac homing peptide (CHP), by incubating a cardiomyoblast cell line with a peptide phage display library, which was recovered and subjected to in vivo biopanning. Following this discovery, cardiac stem-cell-derived exosomes were conjugated with CHP via a linker and injected into rat models of MI. In comparison to controls, cardiomyocyte exosome uptake was increased and functional outcomes were improved in regards to infarct size, cellular proliferation, and angiogenesis (87). Using a similar in vivo biopanning approach discussed previously, Kanki et al. (88) identified several targeting peptides specific for ischemic myocardium as opposed to both healthy and injured heart tissue. The fusion of an ischemic myocardium-targeting peptide (IMTP) with an exosomal membrane protein, and the injection of these bioengineered MSC-exosomes into an MI model resulted in increased accumulation in the ischemic myocardium and improved therapeutic effects (89). These discoveries and findings will be instrumental in improving EV functionality in CVD.
The use of hydrogels to encapsulate and release EVs over a prolonged period of time is also quite intriguing. Chen et al. (90) used bioengineering methods to incorporate endothelial progenitor cell-derived EVs into a hyaluronic acid hydrogel. Released EV fractions from the sheer-thinning gel (STG) were collected over 21 days and showed a steady cumulative release pattern in vitro. The STG-EVs significantly further augmented vessel density and reduced infarct size compared to EV treatment control in a rat model of myocardial infarction (90). Zhang et al. (91) found similar results with a chitosan hydrogel encapsulated with MSC-derived exosomes and tested in a mouse model of hindlimb ischemia with augmented retention, stability, and therapeutic effects.
Clinical Trials and Comorbidities
Although MSC-based therapies have been heavily investigated clinically in the context of cardiovascular diseases, the field of MSC-derived EVs remains in its infancy. Encouragingly, however, clinical trials have begun in similar disease states. In a phase I study, patients with acute ischemic stroke were intravenously treated with transfected miR-124 MSC-derived exosomes, which has now transitioned to phase II (NCT03384433). Separately, MSC-derived exosomes are being studied in the prevention of multiple organ dysfunction syndrome (MODS) postsurgical repair for acute type A aortic dissection (ATAAD) NCT04356300. Although these referenced clinical trials are ongoing and yet without any available results, they signal the onset of a new era of clinical applications of EVs.
As the field of EVs makes this transition, it is necessary to evaluate their effects on cardiovascular disease with underlying comorbidities. However, successful preclinical studies have failed to produce positive results in a clinical setting; the reason may be due to underlying conditions and disease processes in humans that are unaccounted for in laboratory animal models, such as diabetes, obesity, alcoholism, aging, etc. The Centers for Disease Control and Prevention (CDC) reports the prevalence of diabetes in the United States population at 10.5% and predicts 20% are unaware that they are living with this condition; meanwhile obesity rates are above 40% (92, 93). Recent studies by our group, investigating EVs in porcine models of chronic myocardial ischemia with and without administration of a high-fat diet that was associated with hyperglycemia and hypercholesterolemia, found an impaired angiogenic capacity, paradoxical molecular signaling, and disparate gene expression profiles in ischemic myocardium due to high-fat diet (44, 45). Another comorbidity model of renovascular disease with diet-induced metabolic syndrome and MSC-EV treatment was examined. Notably, intrarenal delivery of MSC-EVs into this metabolic renovascular disease state did in fact improve kidney function, reduce proinflammatory cytokines, and improve cardiac function compared to controls (94).
Future studies aimed at understanding these disparate effects of EV treatment in the setting of cardiovascular disease with and without metabolic stress are necessary to fine-tune and/or “personalize” EVs. In vitro studies with diabetic endothelial cells to evaluate proliferation, wound healing, tube formation, and so forth by EVs may provide further insight into the angiogenic outcomes. It may be of further value to explore the effect of EVs on endothelial cell and cardiomyocyte metabolism by examining mitochondrial reactive oxygen species (ROS), membrane potential, oxygen consumption, oxidative phosphorylation compared to glycolysis, etc. to determine the effects of comorbidities on mitochondrial respiration and glycolysis. These studies will be especially interesting under hypoxic and/or starvation conditions to mimic myocardial ischemia. Finally, should the field progress to autologous transfer of stem-cell derived EVs, it will be of critical importance to evaluate their effects and profile from various disease states. A proteomic comparison of nonmetabolic and metabolic syndrome (MS)-derived MSC-EVs from pigs, for example, showed enrichment of proteins related to proinflammatory pathways in EVs derived from MS animals (95).
CONCLUSIONS
Myocardial ischemia is a prevalent illness that results in widespread pathophysiological changes in the myocardium during remodeling. As discussed, a combination of maladaptation in energy metabolism, ion homeostasis, inflammatory response, and oxidative stress accelerate chronic ischemia to heart failure. Recent evidence has shown the limits of cell-based therapies and the promise of EV-based therapies. In fact, the malleability of MSC-derived EVs for treatment purposes is both a blessing and a curse. They present a unique opportunity to treat a variety of diseases, however, characterization and standardization become increasingly difficult with confounding variables. Overcoming this limitation may be the key determinant of a successful future for EV therapies. Furthermore, EVs have been manipulated through overexpression studies, but their success likely resides in their broad effects in altering the environmental milieu due to their vast miRNA, siRNA, protein, lipid, cytokine, and growth factor contents. Optimizing EV content and delivery through preconditioning and/or bioengineering methods will pave the way for translational applications. EV studies in animal models of CVD with comorbidities will be of immense value in the development of successful treatment modalities for the clinical setting.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants 1R01HL133624 (to M.R.A.) and R01HL128831 and R01HL46716 (to F.W.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.K. and M.R.A. designed the manuscript; C.K. wrote the manuscript; C.K. and M.R.A. prepared figures; M.R.A. and F.W.S. edited the manuscript; C.K., F.W.S., and M.R.A. revised the manuscript; F.W.S. and M.R.A. provided funding support; C.K., F.W.S., and M.R.A. approved the final version of the manuscript.
REFERENCES
- 1.Emelia BJ, Paul M, Alonso A, Bittencourt MS, Callaway CW, Carson AP , et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Biörck G. The biology of myocardial infarction. Circulation 37: 1071–1085, 1968. doi: 10.1161/01.CIR.37.6.1071. [DOI] [PubMed] [Google Scholar]
- 3.Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B , et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J 38: 648–660, 2017. doi: 10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet (London, England) 379: 895–904, 2012. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 63: 110–122, 2014. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113: 1287–1294, 2006. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer A, Zwadlo C, Fuchs M, Meyer GP, Lippolt P, Wollert KC, Drexler H. Long-term effects of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: 5-year results from the randomized-controlled BOOST trial – an echocardiographic study. Eur J Echocardiogr 11: 165–171, 2010. doi: 10.1093/ejechocard/jep191. [DOI] [PubMed] [Google Scholar]
- 8.Müller P, Lemcke H, David R. Stem cell therapy in heart diseases – cell types, mechanisms and improvement strategies. Cell Physiol Biochem 48: 2607–2655, 2018. doi: 10.1159/000492704. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo PS, Minicucci MF, Santos PP, Paiva SAR, Zornoff LAM. Energy metabolism in cardiac remodeling and heart failure. Cardiol Rev 21: 135–140, 2013. doi: 10.1097/CRD.0b013e318274956d. [DOI] [PubMed] [Google Scholar]
- 10.Graham RM, Frazier DP, Thompson JW, Haliko S, Li H, Wasserlauf BJ, Spiga MG, Bishopric NH, Webster KA. A unique pathway of cardiac myocyte death caused by hypoxia-acidosis. J Exp Biol 207: 3189–3200, 2004. doi: 10.1242/jeb.01109. [DOI] [PubMed] [Google Scholar]
- 11.Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P. Endothelial cell metabolism in health and disease. Trends Cell Biol 28: 224–236, 2018. doi: 10.1016/j.tcb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 12.David AE, Jessica L, Kornél K, Andrew WT. Calcium and excitation-contraction coupling in the heart. Circ Res 121: 181–195, 2017. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladilov Y, Schäfer C, Held A, Schäfer M, Noll T, Piper HM. Mechanism of Ca2+ overload in endothelial cells exposed to simulated ischemia. Cardiovasc Res 47: 394–403, 2000. doi: 10.1016/S0008-6363(00)00108-5. [DOI] [PubMed] [Google Scholar]
- 14.Molkentin JD, Lu J-R, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998. doi: 10.1016/S0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BHL, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 117: 2431–2444, 2007. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol Cell Physiol 264: C761–C782, 1993. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 17.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbäurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117: 3216–3226, 2008. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 18.Blyszczuk P, Kania G, Dieterle T, Marty RR, Valaperti A, Berthonneche C, Pedrazzini T, Berger CT, Dirnhofer S, Matter CM, Penninger JM, Lüscher TF, Eriksson U. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res 105: 912–920, 2009.doi: 10.1161/CIRCRESAHA.109.199802. [DOI] [PubMed] [Google Scholar]
- 19.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 104: 271–280, 1999. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 102: 240–248, 2014. doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilatovskaya DV, Halade GV, DeLeon-Pennell KY. Adaptive immunity-driven inflammation and cardiovascular disease. Am J Physiol Heart Circ Physiol 317: H1254–H1257, 2019. doi: 10.1152/ajpheart.00642.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289, 2008. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 6: 11, 2013. doi: 10.1186/1755-1536-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker LB, Vanden Hoek TL, Shao Z-H, Li C-Q, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol Heart Circ Physiol 277: H2240–H2246, 1999. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 25.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 114: 524–537, 2014. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levraut J, Iwase H, Shao ZH, Vanden HT, Schumacker PT. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am J Physiol Heart Circ Physiol 284: 549–558, 2003. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 27.Surmeli NB, Litterman NK, Miller A-F, Groves JT. Peroxynitrite mediates active site tyrosine nitration in manganese superoxide dismutase. Evidence of a role for the carbonate radical anion. J Am Chem Soc 132: 17174–17185, 2010. doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol 29: 2571–2583, 1997. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 29.Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol 29: 2441–2450, 1997. doi: 10.1006/jmcc.1997.0481. [DOI] [PubMed] [Google Scholar]
- 30.Gen S, Vijay I, Te-Chung L, John MC. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res 109: 1044–1054, 2011. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis 11: 349, 2020. doi: 10.1038/s41419-020-2542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weil BR, Suzuki G, Leiker MM, Fallavollita JA, Canty JM. Comparative efficacy of intracoronary allogeneic mesenchymal stem cells and cardiosphere-derived cells in swine with hibernating myocardium. Circ Res 117: 634–644, 2015. doi: 10.1161/CIRCRESAHA.115.306850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. J Chem Inf Model 53: 1689–1699, 2019. doi: 10.1017/CBO9781107415324.004. [DOI] [PubMed] [Google Scholar]
- 34.Shim WSN, Jiang S, Wong P, Tan J, Chua YL, Seng Tan Y, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun 324: 481–488, 2004. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 229: 623–631, 2004. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez ME, Martin EE, Anwar T, Arellano-Garcia C, Medhora N, Lama A, Chen Y-C, Tanager KS, Yoon E, Kidwell KM, Ge C, Franceschi RT, Kleer CG. Mesenchymal stem cell induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Rep 18: 1215–1228, 2017. doi: 10.1016/j.celrep.2016.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He L, Zhao F, Zheng Y, Wan Y, Song J. Loss of interactions between p53 and survivin gene in mesenchymal stem cells after spontaneous transformation in vitro. Int J Biochem Cell Biol 75: 74–84, 2016. doi: 10.1016/j.biocel.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368, 2005. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 39.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 40.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DPV. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 1: 129–137, 2007. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cell 177: 428–445, 2019. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alibhai FJ, Tobin SW, Yeganeh A, Weisel RD, Li R-K. Emerging roles of extracellular vesicles in cardiac repair and rejuvenation. Am J Physiol Heart Circ Physiol 315: H733–H744, 2018. doi: 10.1152/ajpheart.00100.2018. [DOI] [PubMed] [Google Scholar]
- 43.Potz BA, Scrimgeour LA, Pavlov VI, Sodha NR, Ruhul Abid M, Sellke FW. Extracellular vesicle injection improves myocardial function and increases angiogenesis in a swine model of chronic ischemia. J Am Heart Assoc 7: e008344, 2018. doi: 10.1161/JAHA.117.008344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scrimgeour LA, Potz BA, Aboul GA, Shi G, Stanley M, Zhang Z, Sodha NR, Ahsan N, Abid MR, Sellke FW. Extracellular vesicles promote arteriogenesis in chronically ischemic myocardium in the setting of metabolic syndrome. J Am Heart Assoc 8: 1–14, 2019. doi: 10.1161/jaha.119.012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aboulgheit A, Potz BA, Scrimgeour LA, Karbasiafshar C, Shi G, Zhang Z, Machan JT, Schorl C, Brodsky AS, Braga K, Pfeiffer M, Gao M, Cummings O, Sodha NR, Abid MR, Sellke FW. Effects of high fat versus normal diet on extracellular vesicle-induced angiogenesis in a swine model of chronic myocardial ischemia. J Am Heart Assoc 10: e017437, 2021. doi: 10.1161/JAHA.120.017437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson SM, Yellon DM. Exosomes and cardioprotection – a critical analysis. Mol Aspects Med 60: 104–114, 2018. doi: 10.1016/j.mam.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H-S, Choi D-Y, Yun SJ, Choi S-M, Kang JW, Jung JW, Hwang D, Kim KP, Kim D-W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 11: 839–849, 2012. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 48.Öztürk S, Elçin AE, Elçin YM. Functions of mesenchymal stem cells in cardiac repair. Adv Exp Med Biol, 2020. Dec 17 (Epub ahead of print). doi: 10.1007/5584_2020_598. [DOI] [PubMed] [Google Scholar]
- 49.Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtiö J, Nolta JA. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor-KappaB signaling. Stem Cells 34: 601–613, 2016. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao F, Chiu SM, Motan DAL, Zhang Z, Chen L, Ji H-L, Tse H-F, Fu Q-L, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 7: e2062, 2016. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novokreshchenova AN, Butorina NN, Payushina OV, Sheveleva ON, Evtushenko EG, Domaratskaya EI. Mesenchymal stromal cell-derived extracellular vesicles: their features and impact on fibrosis and myogenesis in vitro. Biochemistry (Moscow) 14: 289–297, 2020. doi: 10.1134/S1990747820100013. [DOI] [Google Scholar]
- 52.Gomzikova MO, James V, Rizvanov AA. Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. Front Immunol 10: 2663, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, Li S, Li H, Chen L, He L, Chen H, Fu H, Zhang Q, Chen G, Yang Y, Zhang Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J 33: 1695–1710, 2019. doi: 10.1096/fj.201800131RR. [DOI] [PubMed] [Google Scholar]
- 54.Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G, Chen M, Zhu Y. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press Res 41: 119–128, 2016. doi: 10.1159/000443413. [DOI] [PubMed] [Google Scholar]
- 55.Eirin A, Zhu X-Y, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One 12: e0174303, 2017. doi: 10.1371/journal.pone.0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep 8: 1419, 2018. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin I V, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3: 26913, 2014. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R , et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, Hill AF, De Kleijn D, Koh M, Lai RC, Mitsialis SA, Ortiz LA, Rohde E, Asada T, Toh WS, Weiss DJ, Zheng L, Giebel B, Lim SK. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell vesicles 8: 1609206, 2019. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Balkom BWM, Gremmels H, Giebel B, Lim SK. Proteomic signature of mesenchymal stromal cell-derived small extracellular vesicles. Proteomics 19: 1800163, 2019. doi: 10.1002/pmic.201800163. [DOI] [PubMed] [Google Scholar]
- 61.Noronha N de C, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Correction to: priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther 10: 132, 2019. doi: 10.1186/s13287-019-1259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li B, Li C, Zhu M, Zhang Y, Du J, Xu Y, Liu B, Gao F, Liu H, Cai J, Yang Y. Hypoxia-induced mesenchymal stromal cells exhibit an enhanced therapeutic effect on radiation-induced lung injury in mice due to an increased proliferation potential and enhanced antioxidant ability. Cell Physiol Biochem 44: 1295–1310, 2017.doi: 10.1159/000485490. [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 35: 1747–1759, 2017.doi: 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 64.Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 33: 1818–1828, 2015. doi: 10.1002/stem.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z, Yang C, Shen M, Yang M, Jin Z, Ding L, Jiang W, Yang J, Chen H, Cao F, Hu T. Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction. Stem Cell Res Ther 8: 89, 2017. doi: 10.1186/s13287-017-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sivanathan KN, Rojas-Canales D, Grey ST, Gronthos S, Coates PT. Transcriptome profiling of IL-17A preactivated mesenchymal stem cells: a comparative study to unmodified and IFN-γ modified mesenchymal stem cells. Stem Cells Int 2017: 1025820, 2017.doi: 10.1155/2017/1025820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noone C, Kihm A, English K, O'Dea S, Mahon BP. IFN-γ stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev 22: 3003–3014, 2013. doi: 10.1089/scd.2013.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan I, Ali A, Akhter MA, Naeem N, Chotani MA, Mustafa T, Salim A. Preconditioning of mesenchymal stem cells with 2,4-dinitrophenol improves cardiac function in infarcted rats. Life Sci 162: 60–69, 2016. doi: 10.1016/j.lfs.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 69.Liu X-B, Wang J-A, Ji X-Y, Yu SP, Wei L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res Ther 5: 111, 2014.doi: 10.1186/scrt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamura Y, Kita S, Tanaka Y, Fukuda S, Obata Y, Okita T, Nishida H, Takahashi Y, Kawachi Y, Tsugawa-Shimizu Y, Fujishima Y, Nishizawa H, Takakura Y, Miyagawa S, Sawa Y, Maeda N, Shimomura I. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol Ther 28: 2203–2219, 2020. doi: 10.1016/j.ymthe.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang M, Yan L, Li Q, Yang Y, Turrentine M, March K, Wang I-W. Mesenchymal stem cell secretions improve donor heart function following ex vivo cold storage. J Thorac Cardiovasc Surg S0022-5223: 32487–32489, 2020. doi: 10.1016/j.jtcvs.2020.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ibrahim AG-E, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2: 606–619, 2014. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lang JK, Young RF, Ashraf H, Canty JM. Inhibiting extracellular vesicle release from human cardiosphere derived cells with lentiviral knockdown of nSMase2 differentially effects proliferation and apoptosis in cardiomyocytes, fibroblasts and endothelial cells in vitro. PLoS One 11: e0165926, 2016. doi: 10.1371/journal.pone.0165926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.García-Manrique P, Matos M, Gutiérrez G, Pazos C, Blanco-López MC. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles 7: 1422676, 2018. doi: 10.1080/20013078.2017.1422676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He J-G, Li H-R, Han J-X, Li B-B, Yan D, Li H-Y, Wang P, Luo Y. GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Sci Rep 8: 9047, 2018. doi: 10.1038/s41598-018-27435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med 6: 51–59, 2017. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem 44: 2105–2116, 2017. doi: 10.1159/000485949. [DOI] [PubMed] [Google Scholar]
- 78.Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, Jin M, Wang H. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther Nucleic Acids 11: 103–115, 2018. doi: 10.1016/j.omtn.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mayourian J, Ceholski DK, Gorski PA, Mathiyalagan P, Murphy JF, Salazar SI, Stillitano F, Hare JM, Sahoo S, Hajjar RJ, Costa KD. Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res 122: 933–944, 2018. doi: 10.1161/CIRCRESAHA.118.312420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kiran M, Erik I, Myriam F, Peter L, Ma M, Kristin NL, Christopher N-C, Pm V, Deepak V, Daniel W. The Expressed genome in cardiovascular diseases and stroke: refinement, diagnosis, and prediction: a scientific statement from the American Heart Association. Circ Cardiovasc Genet 10: e000037, 2017. doi: 10.1161/HCG.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 81.Wiklander OPB, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CIE, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJA, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316, 2015. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta D, Liang X, Pavlova S, Wiklander OPB, Corso G, Zhao Y, Saher O, Bost J, Zickler AM, Piffko A, Maire CL, Ricklefs FL, Gustafsson O, Llorente VC, Gustafsson MO, Bostancioglu RB, Mamand DR, Hagey DW, Görgens A, Nordin JZ, EL Andaloussi S. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J Extracell Vesicles 9: 1800222, 2020. doi: 10.1080/20013078.2020.1800222. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med 33: 1055–1063, 2014. doi: 10.3892/ijmm.2014.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int 72: 430–441, 2007. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 85.Wen S, Dooner M, Papa E, Del Tatto M, Pereira M, Borgovan T, Cheng Y, Goldberg L, Liang O, Camussi G, Quesenberry P. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a radiation injury bone marrow murine model. Int J Mol Sci 20: 5468, 2019. doi: 10.3390/ijms20215468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zahid M, Phillips BE, Albers SM, Giannoukakis N, Watkins SC, Robbins PD. Identification of a cardiac specific protein transduction domain by in vivo biopanning using a M13 phage peptide display library in mice. PLoS One 5: e12252, 2010. doi: 10.1371/journal.pone.0012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, Qian L, Cheng K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 8: 1869–1878, 2018. doi: 10.7150/thno.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanki S, Jaalouk DE, Lee S, Yu AYC, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J Mol Cell Cardiol 50: 841–848, 2011. doi: 10.1016/j.yjmcc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, Yang Z, Chen Y, Li J, Ma T, Liu H, Li Z, Yang J, Shen Z. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc 7: e008737, 2018. doi: 10.1161/JAHA.118.008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen CW, Wang LL, Zaman S, Gordon J, Arisi MF, Venkataraman CM, Chung JJ, Hung G, Gaffey AC, Spruce LA, Fazelinia H, Gorman RC, Seeholzer SH, Burdick JA, Atluri P. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc Res 114: 1029–1040, 2018. doi: 10.1093/cvr/cvy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang Y, Liu L, Zhao W, Han Z, Kong D, Zhao Q, Guo Z, Han Z, Liu N, Ma F, Li Z. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interfaces 10: 30081–30091, 2018. doi: 10.1021/acsami.8b08449. [DOI] [PubMed] [Google Scholar]
- 92.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief: 1–8, 2020. [PubMed] [Google Scholar]
- 93.U.S. Department of Health and Human Services. National Diabetes Statistics Report, 2020. (Online). https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. [2021 Apr 19].
- 94.Zhang L, Zhu X-Y, Zhao Y, Eirin A, Liu L, Ferguson CM, Tang H, Lerman A, Lerman LO. Selective intrarenal delivery of mesenchymal stem cell-derived extracellular vesicles attenuates myocardial injury in experimental metabolic renovascular disease. Basic Res Cardiol 115: 16, 2020. doi: 10.1007/s00395-019-0772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eirin A, Zhu X-Y, Woollard JR, Tang H, Dasari S, Lerman A, Lerman LO. Metabolic syndrome interferes with packaging of proteins within porcine mesenchymal stem cell-derived extracellular vesicles. Stem Cells Transl Med 8: 430–440, 2019.doi: 10.1002/sctm.18-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]