Abstract
The prevalence of preeclampsia and obesity have increased. Although obesity is a major risk factor for preeclampsia, the mechanisms linking these morbidities are poorly understood. Circulating leptin levels increase in proportion to fat mass. Infusion of this adipokine elicits hypertension in nonpregnant rats, but less is known about how hyperleptinemia impacts blood pressure during placental ischemia, an initiating event in the pathophysiology of hypertension in preeclampsia. We tested the hypothesis that hyperleptinemia during reduced uterine perfusion pressure (RUPP) exaggerates placental ischemia-induced hypertension. On gestational day (GD) 14, Sprague–Dawley rats were implanted with osmotic mini-pumps delivering recombinant rat leptin (1 µg/kg/min iv) or vehicle concurrently with the RUPP procedure to induce placental ischemia or Sham. On GD 19, plasma leptin was elevated in Sham + Leptin and RUPP + Leptin. Leptin infusion did not significantly impact mean arterial pressure (MAP) in Sham. MAP was increased in RUPP + Vehicle vs. Sham + Vehicle. In contrast to our hypothesis, placental ischemia-induced hypertension was attenuated by leptin infusion. To examine potential mechanisms for attenuation of RUPP-induced hypertension during hyperleptinemia, endothelial-dependent vasorelaxation to acetylcholine was similar between Sham and RUPP; however, endothelial-independent vasorelaxation to the nitric oxide (NO)-donor, sodium nitroprusside, was increased in Sham and RUPP. These findings suggest that NO/cyclic guanosine monophosphate (cGMP) signaling was increased in the presence of hyperleptinemia. Plasma cGMP was elevated in Sham and RUPP hyperleptinemic groups compared with vehicle groups but plasma and vascular NO metabolites were reduced. These data suggest that hyperleptinemia during placental ischemia attenuates hypertension by compensatory increases in NO/cGMP signaling.
NEW & NOTEWORTHY Ours is the first study to examine the impact of hyperleptinemia on the development of placental ischemia-induced hypertension using an experimental animal model.
Keywords: blood pressure, blood vessel, obesity, RUPP, women’s health
INTRODUCTION
The prevalence of hypertensive disorders of pregnancy continues to rise around the world (1–4). One of these disorders is preeclampsia, which is defined as new-onset hypertension and multiorgan dysfunction occurring after the twentieth week of pregnancy (5). The rising rates of preeclampsia are thought to be partly driven by the increasing number of overweight and obese women (6). The number of women that are overweight or obese during pregnancy is increasing, especially in low income groups (7). However, the mechanistic role of obesity-related metabolic factors in the development of hypertension during preeclampsia has not yet been elucidated.
One such obesity-related metabolic factor is the adipokine, leptin, which is a satiety hormone that is elevated during the leptin-resistant state of obesity. Adipose tissue expression and circulating leptin levels parallel adiposity (8). When infused into nonpregnant lean animal models, it reduces food intake but increases blood pressure via crossing the blood brain barrier and activating the sympathetic nervous system and downstream adrenergic receptor signaling in cardiovascular-relevant tissues (9, 10). However, experimental studies have yet to be performed to examine the impact of hyperleptinemia on blood pressure regulation during preeclampsia. Although studies have reported that elevated leptin levels can be found in preeclamptic women with either increased body mass (11) or normal body weight (12–14), there are limited data on how hyperleptinemia influences blood pressure regulation during normal pregnancy and preeclampsia. One study found that the ability of acute leptin infusion into the intracerebroventricular space to elicit sympathetic outflow was attenuated in normal late-pregnant versus nonpregnant anesthetized female rats (15), whereas systemic infusion of leptin from gestational day (GD) 14 to 19 into normal pregnant rats increased blood pressure levels (16). However, it is unknown whether hyperleptinemia impacts the development of hypertension in response to placental ischemia, which is an important initiating event in the pathophysiology of hypertension during preeclampsia (17, 18). Therefore, the purpose of our study was to test the hypothesis that hyperleptinemia during reduced uterine perfusion pressure (RUPP) exaggerates placental ischemia-induced hypertension. For this purpose, on GD 14, Sprague–Dawley rats were implanted with osmotic mini-pumps to deliver recombinant rat leptin or vehicle at the same time as conducting the RUPP procedure to induce placental ischemia or Sham surgery. At GD 19, maternal blood pressure, ex vivo vascular function in small arteries, and systemic NO-cGMP signaling was assessed by measuring circulating cGMP concentrations as well as plasma and vascular nitrite + nitrate (NOx) content. Fetal and placental biometrics were also assessed.
METHODS
Animals
All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals with all animal-use protocols approved by The University of Mississippi Medical Center’s Institutional Animal Care and Use Committee. Timed-pregnant SAS Sprague–Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Rats were delivered to our animal facility between GD 11 and 12 and placed on Envigo 8640 diet.
RUPP Procedure and Leptin Infusion Protocols
On GD 14, the RUPP procedure was performed as previously described (19). Briefly, rats were placed under isoflurane anesthesia (Butler Schein Animal Health, Dublin, OH). An abdominal incision was made and a silver clip (0.203 mm, internal diameter) was placed around the subrenal abdominal aorta above the uterine arteries along with a clip (0.1 mm, internal diameter) on each branch of the ovarian arteries in both uterine horns. The control group consisted of rats that underwent a Sham surgery on GD 14, with similar abdominal incision and suturing without clip placement.
At the same time of RUPP or Sham surgeries, an osmotic mini-pump (model 2ML1, Alzet, Cupertino, CA) was implanted into the dorsal subcutaneous space. Pumps had been loaded and primed with an attached catheter containing recombinant rat leptin (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA) for infusion at a rate of 1 µg/kg/min or vehicle (Tris·HCl, pH 8.0). The catheter was tunneled and inserted into the jugular vein. The dorsal incision was then closed with staples. All rats were singly housed following surgeries. Pregnant animals were weighed on GD 14 and randomly assigned to one of our four study groups. They were weighed again at the end of the experimental protocol on GD 19. Cumulative food intake was measured from GD 14 to 19. The number of rats in each group were as follows: Sham + Vehicle (N = 13), Sham + Leptin (N = 10), RUPP + Vehicle (N = 11), and RUPP + Leptin (N = 7).
Measurement of Maternal Mean Arterial Blood Pressure and Heart Rate
On GD 18, rats were placed under isoflurane anesthesia, and indwelling catheters were implanted in the left carotid artery and exposed at the nape of the neck using aseptic techniques. Catheters consisted of V/1 tubing attached to V/3 tubing (Scientific Commondities Inc., Lake Havasu City, AZ). Approximately 2.5 cm of the V/3 end of the catheter was inserted into the carotid. Catheters were filled with sterile heparin-0.9% saline solution (300 mg/mL; Pfizer, New York City, NY) and stoppered with a stainless-steel catheter plug (SP22/12; Instech Laboratories, Inc., Plymouth Meeting, PA) to maintain patency. On GD 19, rats were placed in restraint cages and catheters connected to pressure transducers (MLT0699; ADInstruments, Colorado Springs, CO) coupled to a computerized data acquisition system (PowerLab, ADInstruments). Once hemodynamic readings stabilized (∼1 h), mean arterial blood pressure (MAP) and heart rate (HR) data were collected and averaged over a 10-min period.
Pregnancy Biometrics and Tissue Harvest
On GD 19, rats were placed under isoflurane anesthesia, a midline incision was made, and uterine horns with fetuses were exteriorized. Blood was collected from the abdominal aorta into Vacutainer K2EDTA tubes or Serum Blood Collection Tubes (BD, Franklin Lakes, NJ), spun at 2,500 rpm for 12 min at 4°C, and plasma and serum were separated and stored at −20°C. The number of viable and reabsorbed fetuses in each animal was recorded along with individual viable fetus and placenta weights. Data presented are the combined averages along with total fetal and placental weights for each pregnant rat per group. Placental sufficiency was calculated as fetal weight divided by placental weight for each fetal-placental unit and then averaged for each pregnant rat; this is similar to the calculation for this measurement in humans (20). Visceral adipose tissue, including the fat from around the kidneys, adrenals and the retroperitoneal fat, was weighed and recorded.
Vasorelaxation Studies
On GD 19, mesenteric artery arcades were collected for vasorelaxation studies from a subset of rats in each group. Third-order mesenteric arteries were cleaned of perivascular adipose tissue. Vascular rings of ∼2.5 mm in length were mounted on chucks in a wire myograph (model 620 M, Danish Myo Technology, A/S, Aarhus, Denmark) containing 5 mL of physiological saline solution (PSS, concentration in mmol/L: 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 11.1 dextrose; Sigma, St. Louis, MO) warmed to 37°C and bubbled with 95% O2-5% CO2. In all, 4 mN of preload was placed on the rings, as previously described (21). Blood vessel viability was examined with a bolus dose (2 µM) of phenylephrine (Phe, Sigma) to produce vasoconstriction followed by a bolus (200 µM) of acetylcholine (ACh, Sigma) to induce vasorelaxation. Segments were washed with PSS and equilibrated at 4 mN for 15 min. Rings were constricted with Phe (2 µM) then cumulative concentration–response curves were generated to increasing concentrations to the endothelium-dependent vasorelaxation substance, ACh (1 × 10−10 to 1 × 10−4.5). Segments were washed, equilibrated, constricted with Phe (2 µM), and endothelium-independent vasorelaxation responses to the nitric oxide (NO)-donor, sodium nitroprusside (SNP; 1 × 10−10 to 1 × 10−4.5) were recorded. Data are presented as percent relaxation from Phe constriction.
Vascular NOx Extraction and Quantification
The mesenteric artery arcade was snap frozen in liquid N2 and stored at −80°C until processed. Samples were homogenized using 350 µL of the radioimmunoprecipitation assay (RIPA) Lysis Buffer System (Santa Cruz Biotechnology, Dallas, TX) loaded into MP Lysing Matrix D (500 × 2 mL) tubes (MP Biochemicals, Santa Ana, CA). Tubes were placed in a FastPrep-24 5 G machine (MP Biomedicals) and tissue homogenized at a setting of 2 × 40 s at a speed of 6.0 m/s. Homogenates were quickly centrifuged at 13,200 rpm at room temperature. The supernatant fraction was used to quantify total protein levels via the bicinchoninic acid (BCA) method (Thermo Scientific, Waltham, MA). The remaining supernatant was transferred to a Qiagen Mini Spin Column (Qiagen, mat no. 1011708) and centrifuged at 10,000 g for 20 min at 4°C, and NOx was quantified in the eluted solution using a commercially available kit (Cayman Chemicals, Ann Arbor, MI). NOx content was normalized to total protein for each mesenteric arterial bed from respective rats.
Plasma Biochemistry
Circulating levels of leptin and placental growth factor-2 (PlGF) were quantified in plasma using Quantikine enzyme-linked immunosorbent assays (ELISAs) per manufacturers’ instructions (R&D Systems, Minneapolis, MN). Circulating NO bioavailability was estimated by measuring the NO metabolites, NOx, using a colorimetric assay, and cyclic guanosine monophosphate (cGMP) was assayed by ELISA (Cayman Chemicals). Serum levels of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP-32) were quantified using ELISAs from Abcam (Cambridge, MA).
Statistical Analysis
All data are expressed as means ± SE. Data were graphed and analyzed using GraphPad Prism 8 (La Jolla, CA). For the vasorelaxation studies, effective maximal concentration (Emax) was calculated with the equation: Emax = (maximum constriction to Phe – relaxation at 1E-4.5 ÷ maximum constriction to Phe – baseline tension pre-Phe) × 100. Area under the curve (AUC) was calculated to assess total vasorelaxation responses, and sensitivity (LogEC50) was determined using the least-squares curve fitting method of nonlinear regression provided by GraphPad Prism. Data were analyzed using a two-way ANOVA to assess statistical significance between the two factors of Leptin × RUPP. Statistical significance was considered as P < 0.05. If the two-way ANOVA detected an interaction, Tukey’s multiple comparisons post hoc tests were performed. Symbols of statistical significance were placed in the scattered-dot plots to designate specific differences between the groups only if an interaction was detected.
RESULTS
Effects of Leptin Infusion on Its Circulating Levels in Sham and RUPP Rats
The RUPP or Sham surgeries were conducted on GD 14 along with beginning the intravenous infusion of recombinant rat leptin (1 µg/kg/min) or vehicle until GD 19. Figure 1A presents the data for circulating plasma levels of leptin at GD 19 following this protocol. The two-way ANOVA detected a main effect for increased leptin levels in the RUPP groups (PRUPP < 0.05); even though the post hoc analysis did not reveal a specific difference (P > 0.05) between plasma leptin levels in Sham + Vehicle and RUPP + Vehicle rats, these levels were numerically higher in the latter group (Fig. 1A). Leptin levels were significantly increased (PLeptin < 0.05) in Sham + Leptin and RUPP + Leptin groups—although the same dose of leptin was infused into both pregnant groups, the post hoc analysis indicated that leptin was slightly higher (P < 0.05) in the Sham + Leptin versus RUPP + Leptin animals (Fig. 1A).
Figure 1.
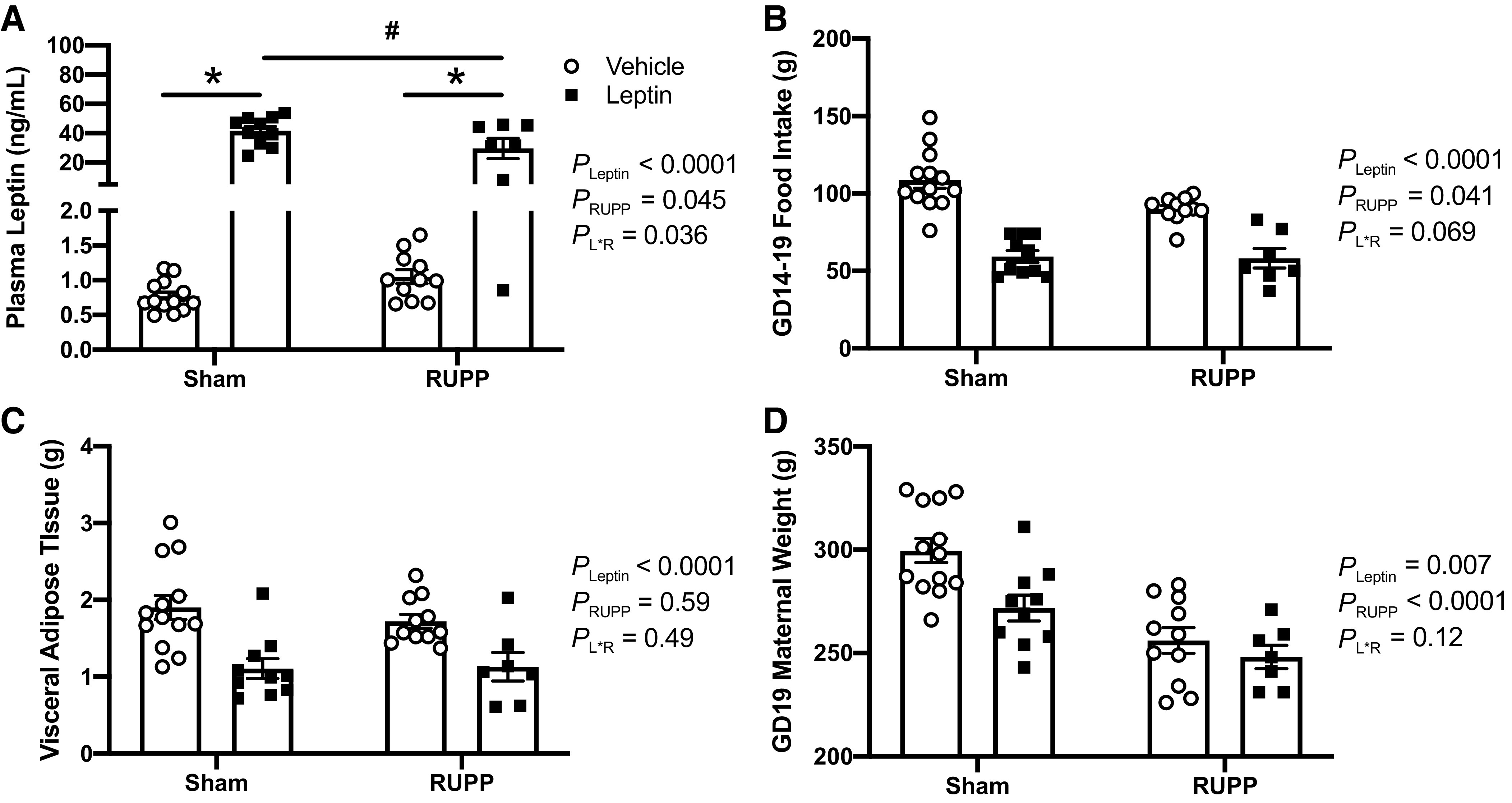
Effects of leptin infusion on its circulating levels (A), cumulative food intake (B), maternal visceral fat mass (C), and maternal body weight (D) in Sham and RUPP rats. Sham or RUPP procedure plus leptin or vehicle infusion were started on GD 14 and measurements collected on GD 19. Data are presented as scattered-dot plots with mean ± SE. The results from the two-way ANOVA are inset. *P < 0.0001 for leptin effects within Sham or RUPP groups; #P < 0.0001 for differences between leptin-infused Sham and RUPP groups. ANOVA, analysis of variance; GD, gestational day; RUPP, reduced uterine perfusion pressure. Sham + Vehicle (N = 13), Sham + Leptin (N = 10), RUPP + Vehicle (N = 11), and RUPP + Leptin (N = 7).
Effects of Leptin Infusion on Food Intake and Body Weight in Sham and RUPP Rats
Although there was variation in the circulating levels of leptin following the infusion protocol, the apparent uniform effect of leptin to reduce food intake in both Sham + Leptin and RUPP + Leptin groups (Fig. 1B) is supportive evidence of successful leptin delivery. Cumulative food intake was measured from GD 14 to 19 (Fig. 1B). The two-way ANOVA detected a main effect for both RUPP (PRUPP < 0.05) and leptin (PLeptin < 0.05) to reduce food intake. As food intake was reduced in both Sham and RUPP leptin-treated groups, maternal fat mass and body mass were weighed. The two-way ANOVA revealed that there was no significant effect of RUPP on maternal visceral white adipose tissue (PRUPP > 0.05), but leptin infusion resulted in parallel reductions (PLeptin < 0.05) in fat mass in both of Sham and RUPP groups (Fig. 1C). Regarding maternal body mass, the two-way ANOVA signified a main effect of leptin to reduce (PLeptin < 0.05) this parameter (Fig. 1D). Following post hoc analysis, it was found that leptin infusion resulted in lower (P < 0.05) body mass specifically in the Sham group, but did not further lower (P > 0.05) the body mass in the RUPP dams (Fig. 1D). Thus, the reduced body mass in the RUPP + Vehicle versus Sham + Vehicle rats (P < 0.05; Fig. 1D) was likely due to fetal absorptions and reduced fetal weights caused by the RUPP procedure, as demonstrated below.
Effects of Hyperleptinemia on Fetal and Placental Biometrics in Sham and RUPP Rats
Fetal and placental biometrics were measured at GD 19 in response to combined RUPP and leptin infusion. The two-way ANOVA indicated that RUPP led to reduced average fetal weights (Fig. 2A) and total fetal weights (Fig. 2B) (PRUPP < 0.05). The two-way ANOVA also detected a main effect for leptin infusion to reduce (PLeptin < 0.05) total fetal weight (Fig. 2B). Regarding the number of live fetuses (Fig. 2C) and percentage of fetal absorptions (Fig. 2D), the two-way ANOVA revealed a main effect for both leptin (PLeptin < 0.05) and RUPP (PRUPP < 0.05) to reduce and increase these fetal outcomes, respectively. However, the post hoc analysis did not denote any specific differences in how leptin impacted fetal biometrics in Sham versus RUPP groups.
Figure 2.
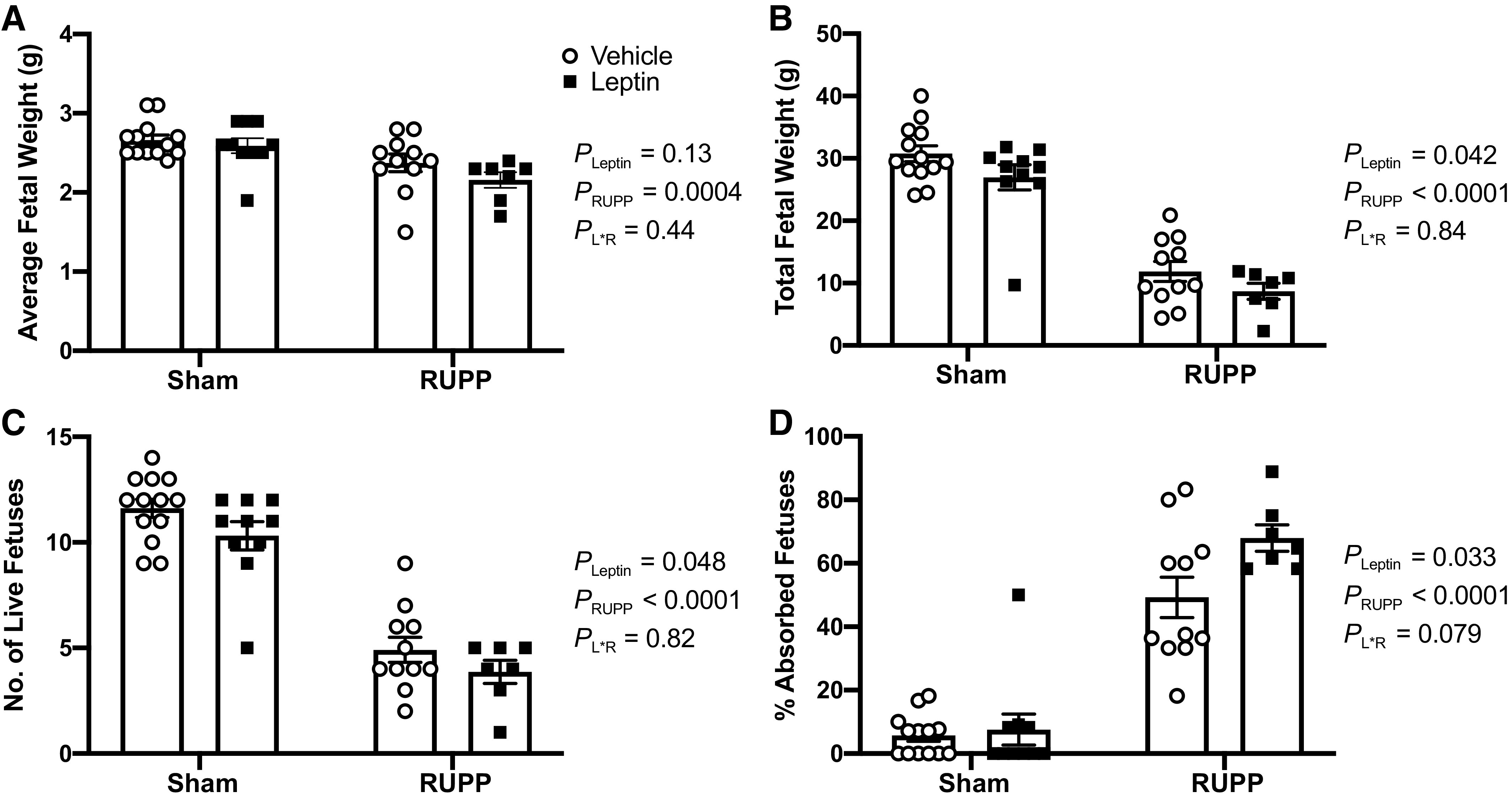
Effects of hyperleptinemia on fetal biometrics in Sham and RUPP rats. Sham or RUPP procedure plus leptin or vehicle infusion were conducted on GD 14 and measurements collected on GD 19. Average fetal weight (A), total fetal weight (B), number of live fetuses (C), and percent absorbed fetuses (D). Data are presented as scattered-dot plots with mean ± SE. The results from the two-way ANOVA are inset. ANOVA, analysis of variance; GD, gestational day; RUPP, reduced uterine perfusion pressure. Sham + Vehicle (N = 13), Sham + Leptin (N = 10), RUPP + Vehicle (N = 11), and RUPP + Leptin (N = 7).
Average placental weights were similar (P > 0.05) between all groups (Fig. 3A). As for total placental weights, the two-way ANOVA identified a main effect for RUPP to reduce (PRUPP < 0.05) this parameter, but it was not altered (PLeptin > 0.05) by leptin infusion (Fig. 3B). Plasma levels of PlGF mirrored the changes in total placental weight for Sham + Vehicle: 69 ± 10 pg/mL; Sham + Leptin: 60 ± 13 pg/mL; RUPP + Vehicle: 23 ± 4 pg/mL; and RUPP + Leptin: 28 ± 6 pg/mL (PLeptin = 0.8, PRUPP = 0.0003, PL×R = 0.05). Placental sufficiency was similar between all groups (Fig. 3C).
Figure 3.
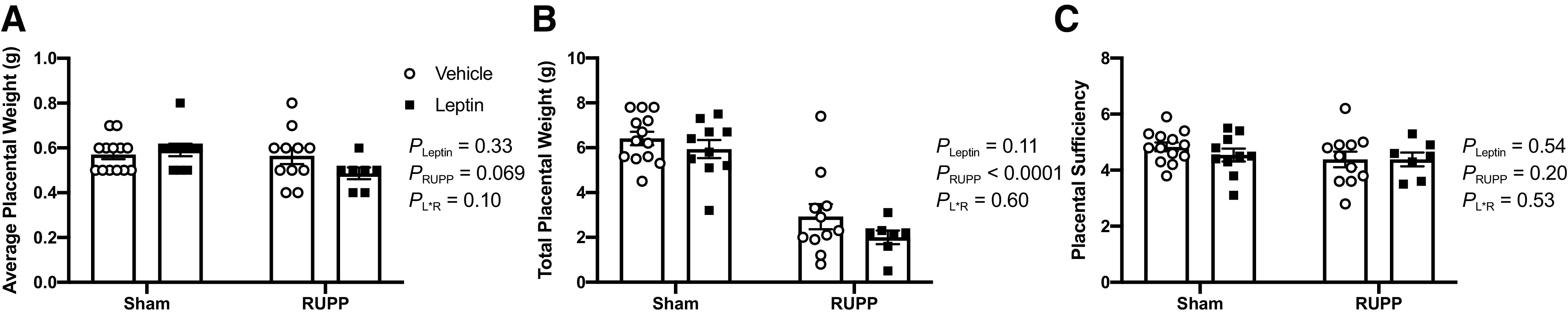
Effects of hyperleptinemia on placental biometrics in Sham and RUPP rats. Average placental weight (A), total placental weight (B), and placental sufficiency (C). Sham or RUPP procedure plus leptin or vehicle infusion were conducted on GD 14 and measurements collected on GD 19. Data are presented as scattered-dot plots with mean ± SE. The results from the two-way ANOVA are inset. ANOVA, analysis of variance; GD, gestational day; RUPP, reduced uterine perfusion pressure. Sham + Vehicle (N = 13), Sham + Leptin (N = 10), RUPP + Vehicle (N = 11), and RUPP + Leptin (N = 7).
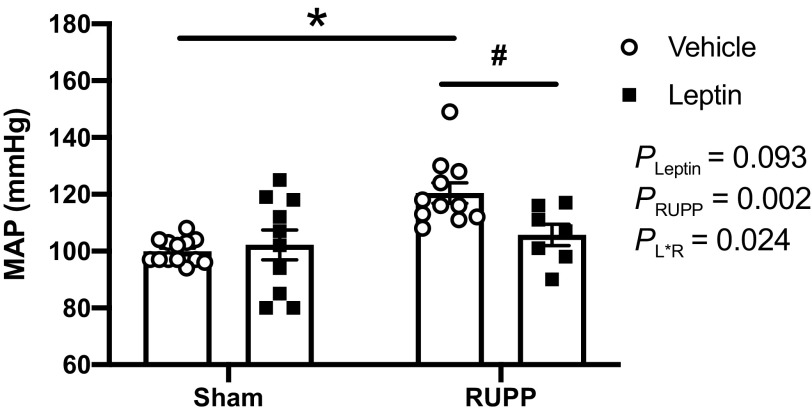
Effects of Hyperleptinemia on MAP and HR in Sham and RUPP Rats
Figure 4 illustrates the maternal MAP that was assessed at GD 19 in all groups. The post hoc analysis indicated that MAP was significantly increased (P < 0.05) in the RUPP + Vehicle versus Sham + Vehicle rats. Leptin infusion did not have a significant impact (P > 0.05) on average MAP values in the Sham group. In contrast, leptin infusion attenuated (P < 0.05) placental ischemia-induced hypertension in the RUPP group. HR was similar (P > 0.05) between all groups: Sham + Vehicle (397 ± 21 beats/min), Sham + Leptin (403 ± 18 beats/min), RUPP + Vehicle (412 ± 13 beats/min), and RUPP + Leptin (432 ± 16 beats/min).
Figure 4.
Effects of hyperleptinemia on mean arterial blood pressure (MAP) levels in Sham and RUPP rats. Sham or RUPP procedure plus leptin or vehicle infusion were conducted on GD 14 and measurements collected on GD 19 in conscious rats. Data are presented as scattered-dot plots with mean ± SE. The results from the two-way ANOVA are inset. *P < 0.0004 for Sham + Vehicle and RUPP + Vehicle groups; #P = 0.048 for differences between RUPP + Vehicle and RUPP + Leptin groups. ANOVA, analysis of variance; GD, gestational day; RUPP, reduced uterine perfusion pressure. Sham + Vehicle (N = 13), Sham + Leptin (N = 10), RUPP + Vehicle (N = 11), and RUPP + Leptin (N = 7).
Effects of Hyperleptinemia on Vascular Function in Small Arteries from Sham and RUPP Rats
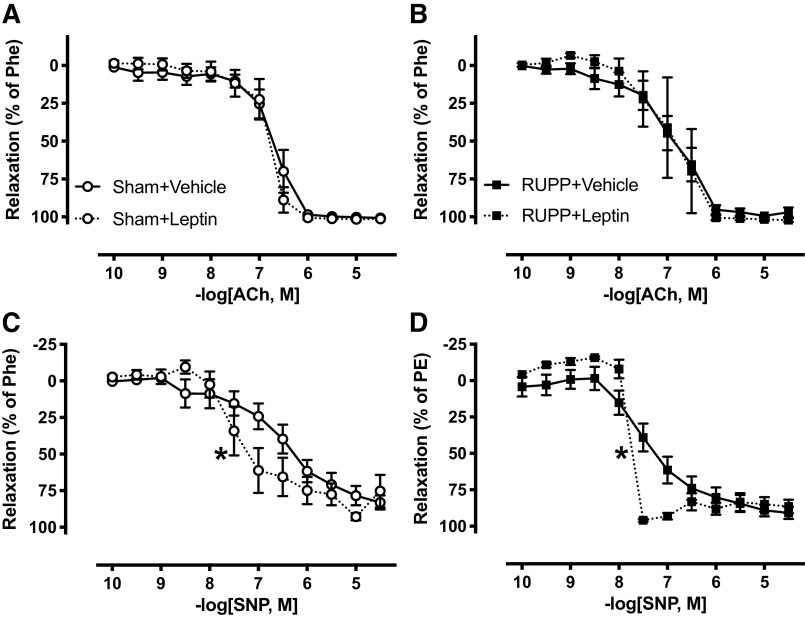
We assessed whether leptin infusion altered endothelium-dependent vasorelaxation in third-order mesenteric arteries. These vessels were isolated at GD 19 and prepared for wire myography. Endothelium-dependent vasorelaxation was evaluated in response to increasing concentrations of ACh. When assessed by Emax, AUC, and LogEC50, these values were not altered (P > 0.05) by leptin infusion in Sham + Leptin versus Sham + Vehicle (Fig. 5A, Table 1) or RUPP + Leptin versus RUPP + Vehicle (Fig. 5B, Table 1) rats.
Figure 5.
Effects of hyperleptinemia on vascular function in small arteries from Sham and RUPP rats. Sham or RUPP procedure plus leptin or vehicle infusion were conducted on GD 14, and third-order mesenteric arteries isolated and their function tested on GD 19. Endothelial-dependent vasorelaxation in response to acetylcholine (ACh) was assessed in Sham + Vehicle vs. Sham + Leptin (A) and RUPP + Vehicle vs. RUPP + Leptin (B), and endothelium-independent vasorelaxation in response to the NO-donor, sodium nitroprusside (SNP) in Sham + Vehicle vs. Sham + Leptin (C) and RUPP + Vehicle vs. RUPP + Leptin (D). Phe, phenylephrine; EC50, 50% effective concentration. Data are presented as connecting lines with mean ± SE. Each data set was analyzed by a two-way ANOVA: *P < 0.05 for differences in sensitivity (M, − LogEC50) of leptin-infused Sham or RUPP rats compared with their vehicle-infused counterparts. ANOVA, analysis of variance; GD, gestational day; RUPP, reduced uterine perfusion pressure. Sham + Vehicle (N = 10), Sham + Leptin (N = 5), RUPP + Vehicle (N = 11), and RUPP + Leptin (N = 4). N = individual rats.
Table 1.
Vascular function studies
| Vascular Function Parameter | Sham + Vehicle | Sham + Leptin | RUPP + Vehicle | RUPP + Leptin | Two-Way ANOVA |
|---|---|---|---|---|---|
| N | 10 | 5 | 11 | 4 | |
| ACh, Emax, % | 101 ± 1 | 101 ± 1 | 97 ± 3 | 102 ± 1 |
PLeptin = 0.4 PRUPP = 0.6 PL×R = 0.5 |
| ACh, LogEC50, M | −6.8 ± 0.1 | −6.9 ± 0.2 | −7.0 ± 0.2 | −7.0 ± 0.3 |
PLeptin = 0.9 PRUPP = 0.5 PL×R = 0.8 |
| ACh, AUC, AU | 250 ± 9 | 260 ± 14 | 264 ± 21 | 272 ± 27 |
PLeptin = 0.7 PRUPP = 0.6 PL×R = 1.0 |
| SNP, Emax, % | 85 ± 5 | 75 ± 11 | 91 ± 4 | 87 ± 5 |
PLeptin = 0.3 PRUPP = 0.2 PL×R = 0.7 |
| SNP, LogEC50, M | −6.0 ± 0.2 | −6.8 ± 0.3 | −6.9 ± 0.2 | −7.6 ± 0.007 |
PLeptin = 0.02 PRUPP = 0.01 PL×R = 1.0 |
| SNP, AUC, AU | 178 ± 18 | 238 ± 37 | 260 ± 22 | 308 ± 18 |
PLeptin = 0.1 PRUPP = 0.02 PL×R = 0.9 |
Values are means ± SE; N, number of rats/group. ACh, acetylcholine; ANOVA, analysis of variation; AU, arbitrary units; AUC, total response as area under the curve; Emax, %maximum response; LogEC50, sensitivity of response in molar concentration (M); RUPP, reduced uterine perfusion pressure; SNP, sodium nitroprusside. Results from the two-way ANOVA are detailed in the rightmost column.
Endothelium-independent vasorelaxation was evaluated in response to increasing concentrations of the NO-donor, SNP. The Emax response was not altered (P > 0.05) by leptin infusion in Sham (Fig. 5C, Table 1) or RUPP (Fig. 5D, Table 1) groups. However, the two-way ANOVA detected a main effect of RUPP to enhance AUC and LogEC50 responses to SNP (PRUPP < 0.05; Table 1). Moreover, the two-way ANOVA revealed a main effect of leptin to increase the LogEC50 response to SNP-induced relaxation (PLeptin < 0.05; Table 1).
Although there was no statistically significant difference in the Emax response to SNP-induced vasorelaxation in small mesenteric arteries between any of the four groups, the SNP responses in each of the groups were numerically less than ACh (Table 1). We are not certain why the maximal ACh responses were greater, but similar findings were reported in previous studies where ex vivo perfusion pressure of the mesenteric arterial bed was assessed in pregnant Sprague–Dawley rats (22). Although that study did not directly compare or focus on this difference, the SNP-induced reduction in perfusion pressure was numerically less than that of ACh in this vessel bed from pregnant rats, which is similar to our findings.
Effects of Hyperleptinemia on NOx, cGMP, ANP, and BNP Levels in Sham and RUPP Rats
Vascular NOx content was quantified in mesenteric arteries (Fig. 6A). The two-way ANOVA detected a main effect for leptin (PLeptin < 0.05) and RUPP (PRUPP < 0.05) to reduce vascular NOx content, but there was no significant interaction (PL×R > 0.05) between these two factors. Plasma levels of NOx were assessed as a marker of circulating NO bioavailability (Fig. 6B). Although plasma NOx levels were not statistically different between RUPP + Vehicle and Sham + Vehicle groups (PRUPP > 0.05), these values were numerically lower in the former group. Regarding the impact of leptin, the two-way ANOVA detected a main effect for leptin to reduce (PLeptin < 0.05) plasma NOx.
Figure 6.
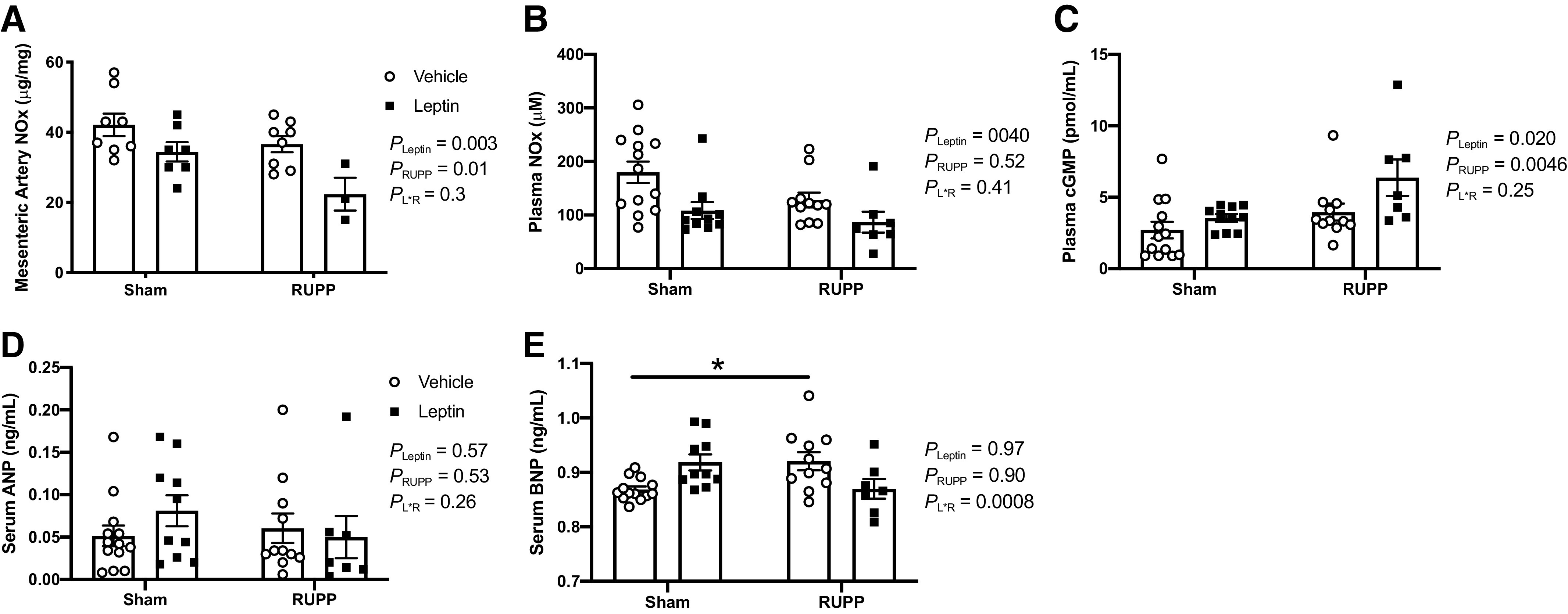
Effects of hyperleptinemia on mesenteric artery NOx content (A) and circulating NOx (B), cGMP (C), ANP (D), and BNP (E) levels in Sham and RUPP rats. Sham or RUPP procedure plus leptin or vehicle infusion were conducted on GD 14, and measurements were assessed in plasma or serum on GD 19. Data are presented as scattered-dot plots with mean ± SE. *P = 0.033 for differences in BNP levels between Sham + Vehicle vs. RUPP + Vehicle. The results from the two-way ANOVA are inset. ANOVA, analysis of variance; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; cGMP, cyclic guanosine monophosphate; GD, gestational day; NOx, nitrite + nitrate; RUPP, reduced uterine perfusion pressure. The number (N) of rats in each group were as follows: Sham + Vehicle (N = 8–13), Sham + Leptin (N = 5–10), RUPP + Vehicle (N = 8–11), and RUPP + Leptin (N = 3–7).
Because we found increased NO-donor-induced vasorelaxation in both Sham + Leptin and RUPP + Leptin groups at GD 19, this suggested that NO/cGMP signaling was greater in the circulatory system of both Sham and RUPP groups following the 5 days of leptin infusion. Therefore, we assessed circulating bioavailable levels of cGMP (Fig. 6C). The two-way ANOVA detected a main effect of RUPP to increase (PRUPP < 0.05) plasma cGMP levels. Moreover, the two-way ANOVA revealed a main effect for leptin to increase (PLeptin < 0.05) plasma concentrations of cGMP (Fig. 6C). No interaction of RUPP and leptin was detected.
To probe for changes in circulating factors that could potentially explain the increases in NO-donor induced vasorelaxation or the elevated circulating cGMP levels in RUPP rats and the leptin-infused groups, serum ANP and BNP levels were assessed. These natriuretic peptides have long been known to activate soluble Guanylate Cyclase (sGC) and stimulate production of cGMP (23). No significant differences were detected in ANP levels among the four groups (P > 0.05; Fig. 6D). The two-way ANOVA and post hoc analyses denoted that serum concentrations of BNP were greater (P < 0.05) in RUPP + Vehicle versus Sham + Vehicle groups (Fig. 6E). Although the mean BNP levels were numerically higher in Sham + Leptin versus Sham + Vehicle groups and lower in RUPP + Leptin versus RUPP + Vehicle groups, the post hoc analysis did not indicate any explicit statistically significant differences between either of these two comparisons (Fig. 6E).
DISCUSSION
We report that experimental hyperleptinemia during the last gestational trimester of rats does not lead to the development of hypertension during pregnancy and actually attenuates the development of placental ischemia-induced hypertension. We found that blood pressure levels at GD 19 were similar between Sham + Leptin and Sham + Vehicle rats and that placental ischemia-induced hypertension was significantly lowered in the RUPP + Leptin group compared to the RUPP + Vehicle group.
The rationale for examining whether hyperleptinemia affects blood pressure regulation during pregnancy comes from the vast amount of epidemiological information that overweight and obesity are major risk factors for hypertensive disorders of pregnancy, including preeclampsia, which has been reviewed elsewhere (24). This suggests that obesity-related metabolic factors play an important role in the risk for hypertension during pregnancy. Leptin is an obesity-related metabolic factor, and its circulating levels increase in parallel with body fat mass. Associative studies have found that leptin is elevated in the circulation of pregnancies complicated by preeclampsia. Indeed, leptin was noted to be elevated at the time of admission and further elevated by time of delivery in preeclamptic patients (25–29). Moreover, it has been demonstrated that these elevated leptin levels in preeclampsia are independent of maternal body weight. However, these human studies did not provide evidence for causality of excessive leptin-induced high blood pressure during pregnancy. Thus, the aim of this study was to determine whether hyperleptinemia only during RUPP exaggerates placental ischemia-induced hypertension during this time period later in gestation mimicking the clinical manifestation of increased blood pressure during preeclampsia.
Placental ischemia/reperfusion events are thought to play a role in the pathogenesis of hypertension in preeclampsia (30). Our experimental animal model consisted of the RUPP procedure to induce placental ischemia-induced hypertension on GD 14, and results were compared with the Sham procedure. At the same time, i.v. infusion of recombinant rat leptin was begun. All pregnancy outcomes were assessed on GD 19. The dose of leptin was chosen based on previous studies in male rodents where it led to increased MAP and HR after 5 days of infusion by increasing sympathetic drive and adrenergic receptor activity (31). We first evaluated the degree to which this infusion protocol for 5 consecutive days elevated circulating leptin levels in the Sham and RUPP groups. Plasma leptin was raised to 30–40 ng/mL in Sham + Leptin or RUPP + Leptin rats, which is similar to circulating levels observed in obese preeclamptic women (32, 33).
We subsequently assessed how hyperleptinemia impacted blood pressure levels and vascular function during normal pregnancy. The leptin infusion protocol from GD 14 to 19 did not alter MAP in Sham + Leptin rats compared to Sham + Vehicle rats. This finding is in contrast to our previous study where leptin infusion for the same time period led to increased blood pressure in pregnant rats (16). The reasons for our conflicting results are unclear at this time, but may relate to the substrain of Sprague–Dawley rat used. While our previous study used timed-pregnant Sprague–Dawley rats from Envigo, the current study used timed-pregnant Sprague–Dawley rats from Charles River Laboratories. Studies directly comparing non-pregnant rats obtained from both of these vendors indicated that Charles River rats may have more nitric oxide synthase (NOS) capacity compared to Envigo rats (34). NOS-mediated signaling is a vasodilatory pathway that is increased during normal pregnancy (35). It might be that our timed-pregnant rats from Charles River have greater NOS signaling and NO-dependent blood pressure regulation than timed-pregnant rats from Envigo in which our previous studies were performed.
We also examined whether hyperleptinemia would alter the development of placental ischemia-induced hypertension. Based on the findings that preeclampsia is associated with elevated leptin levels (25–29), we initially proposed that leptin infusion would further increase blood pressure levels in RUPP + Leptin rats versus RUPP + Vehicle rats. However, we discovered that leptin infusion for the duration of the RUPP protocol attenuated the development of placental ischemia-induced hypertension.
What is known about the impact of chronic hyperleptinemia on vascular function and blood pressure regulation largely comes from studies in males, thus not females and especially not during pregnancy. In male rats, chronic infusion of leptin, similar to the dose used in our studies, not only increased blood pressure but also exaggerated the hypertensive response to NOS inhibition (36). This implicates that the NOS/NO signaling pathway, which includes downstream activation of cGMP, compensates to buffer the vascular dysfunction and blood pressure response during leptin-induced hypertension. We determined vascular function in third-order mesenteric arteries and not in large vessels, like the aorta, that are typically targeted and damaged by hypertension to result in classical endothelial dysfunction, as determined by ACh-induced vasorelaxation (37). It is noteworthy that the RUPP rats did not present with this classical endothelial dysfunction in small mesenteric arteries, which is a similar observation made by others (38–40). However, these findings do not mean that the contribution of NO to vasorelaxation is preserved. It was found by Morton et al. (40) that, although on the surface, endothelial-dependent vasorelaxation was similar in mesenteric arteries between RUPP and Sham rats, the contribution of NOS to this response was reduced in placental ischemic rats. Here, we quantitated a biochemical marker of NO bioavailability (NOx) in small mesenteric arteries. Foremost, there was a main effect of RUPP to reduce vascular NOx content, but we found maintained ACh-induced vasorelaxation in these rats; this suggests other compensatory pathways that may help maintain their vasorelaxation capacity, as discussed in the next paragraph. Whereas, in the leptin-infused groups, it was found that, although hyperleptinemia did not affect ACh-induced vasorelaxation in either Sham or RUPP rats, circulating levels and mesenteric artery content of NOx were reduced with a main effect of leptin. NO activates sGC to produce the second messenger, cGMP, which subsequently targets and activates pathways that mediate vasorelaxation of the smooth muscle (41). Here, circulating cGMP levels were increased in leptin-infused Sham and RUPP rats, with the two-way ANOVA detecting main effects for leptin. The vasorelaxation response to the NO-donor, SNP, was enhanced by leptin in both RUPP and Sham rats. Overall, our data suggest that mesenteric arteries from pregnant rats with hyperleptinemia have a reduction in NO bioavailability, which is counteracted by increases in vascular smooth muscle cell responsiveness to NO/cGMP signaling. Future studies should assess ACh-induced NOx and cGMP production in various isolated vessel beds from these experimental groups.
As discussed above, we detected increased NO-donor responsiveness in mesenteric arteries from RUPP versus Sham rats. Studies in preeclamptic humans have assessed circulating factors that are known to activate sGC-cGMP signaling, including natriuretic peptides. Preeclamptic women have increased circulating BNP (42). Moreover, they have increased circulating cGMP but reduced circulating nitrite levels. We assessed both circulating ANP and BNP levels in this study. Circulating levels of BNP were found to be greater in RUPP + Vehicle versus Sham + Vehicle groups along with increased and reduced circulating cGMP and NOx, respectively. Therefore, we suggest that the increased NO responsiveness of the vasculature in RUPP versus Sham rats is a compensatory mechanism in these hypertensive pregnant rats that is mediated by BNP.
We examined the impact of hyperleptinemia on maternal food intake. Leptin is a satiety hormone by acting in the brain to reduce food intake (43). Although circulating leptin levels following the infusion protocol were significantly less in the RUPP + Leptin versus Sham + Leptin groups, there was similar hypophagia as well as reduced body weight and fat mass. In contrast, placental weights were numerically lowest in the RUPP + Leptin group. Perhaps the RUPP-induced reductions in placental weight in combination with exogenous infusion of leptin attenuated its endogenous production in the placenta. Future studies should assess the impact of exogenous leptin infusion on endogenous leptin content and release from the placenta.
We also examined whether hyperleptinemia in preeclampsia may negatively impact the fetus. In our previous study, leptin reduced both average placental and fetal weights in timed-pregnant rats derived from the Envigo Corporation (16). It was determined from that study the reduction in pregnancy biometrics was due to leptin-induced decreases in food intake. However, in the present study, we did not observe significant alterations in either average placental or fetal weights. When estimating food intake between these two studies, it seems that the degree of leptin-mediated decrease in food intake was ∼65% in our previous study but only ∼50% in the current study. Perhaps the lesser leptin-mediated alterations in food intake led us to find no impact of leptin on average placental or fetal weights. Nonetheless, our current findings show that hyperleptinemia reduced total fetal weights in both Sham + Leptin and RUPP + Leptin groups. It has been reported that hyperleptinemia during the perinatal period developmentally programs for detrimental neurologic and cardiovascular alterations (44). Future experiments should examine how hyperleptinemia affects the neurological and cardiovascular health in offspring derived from RUPP + Leptin dams and Sham + Leptin dams.
Limitations
There was a potent vasorelaxation to SNP between 10−8 and 10−7 M in the RUPP + Leptin rats, which seems to have driven the increased sensitivity to SNP in this group. Perhaps if we had performed these curves in quarter-molar increments, instead of half-molar increments in this study, we would have observed a more gradual, sigmoidal response. Furthermore, it is known that normal pregnancy is a state of leptin resistance with progressive rises in leptin levels in the circulation throughout gestation (45, 46). Increasing maternal adipose tissue and placental production contribute to the elevated circulating levels of leptin during normal pregnancy (47). Although we did not account for changes in endogenous leptin levels during normal pregnancy between gestational days 14 and 19, the aim of this study was to determine whether hyperleptinemia during RUPP exaggerates placental ischemia-induced hypertension. To our knowledge, this was the first experimental animal study that probed the impact of hyperleptinemia on blood pressure during placental ischemia-induced hypertension. However, we only performed leptin infusion during the RUPP protocol of placental ischemia. Elevated leptin levels in humans have been detected before 20 wk of gestation, when the symptoms of preeclampsia begin to present, and are associated with increased risk for the development of this hypertensive disorder (48). In contrast, another study conducted between gestational ages of 8 and 15 wk found that leptin was not associated with preeclampsia (49). We propose future studies to perform leptin infusion experiments beginning before, or during early pregnancy, when its endogenous levels are lower to assess the impact of more chronic hyperleptinemia on blood pressure regulation during normal pregnancy or in RUPP preeclamptic rats.
PERSPECTIVES AND SIGNIFICANCE
Although increased circulating leptin levels have been associated with preeclamptic pregnancies in numerous studies, it had yet to be examined how hyperleptinemia impacted placental ischemia-induced hypertension. We used a controlled experimental animal model to explore this, which is the novelty of our study. Foremost, we found that hyperleptinemia during the last gestational trimester in rats does not significantly alter blood pressure levels during normal pregnancy. Furthermore, we provide evidence that hyperleptinemia only during the manifestation of preeclampsia attenuates the hypertensive response to placental ischemia. Future studies should address the molecular mechanisms whereby leptin regulates maternal blood pressure and how combined hyperleptinemia and placental insufficiency influence the long-term health of the offspring from preeclamptic pregnancies.
GRANTS
This work was funded by National Institutes of Health Grants R00HL130577 and P20GM121334 (to F. T. Spradley) and P01HL051971, P20GM104357, and U54GM115428 (to J. P. Granger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C.P., J.P.G., and F.T.S. conceived and designed research; A.C.P., H.L.M., B.A.W., and F.T.S. performed experiments; A.C.P. and F.T.S. analyzed data; A.C.P., H.L.M., J.P.G., and F.T.S. interpreted results of experiments; A.C.P., H.L.M., and F.T.S. prepared figures; A.C.P., B.A.W., and F.T.S. drafted manuscript; A.C.P., H.L.M., B.A.W., C.D.A., J.P.G., and F.T.S. edited and revised manuscript; A.C.P., H.L.M., B.A.W., C.D.A., J.P.G., and F.T.S. approved final version of manuscript.
REFERENCES
- 1.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 25: 124–132, 2013. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- 2.Noubiap JJ, Bigna JJ, Nyaga UF, Jingi AM, Kaze AD, Nansseu JR, Fokom DJ. The burden of hypertensive disorders of pregnancy in Africa: a systematic review and meta-analysis. J Clin Hypertens 21: 479–488, 2019. doi: 10.1111/jch.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong T, Mu Y, Liang J, Zhu J, Li X, Li J, Liu Z, Qu Y, Wang Y, Mu D. Hypertensive disorders in pregnancy and stillbirth rates: a facility-based study in China. Bull World Health Organ 96: 531–539, 2018. doi: 10.2471/BLT.18.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc 7: e009382, 2018. doi: 10.1161/JAHA.118.009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 6.Mbah AK, Kornosky JL, Kristensen S, August EM, Alio AP, Marty PJ, Belogolovkin V, Bruder K, Salihu HM. Super-obesity and risk for early and late pre-eclampsia. BJOG 117: 997–1004, 2010. doi: 10.1111/j.1471-0528.2010.02593.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS One 13: e0202183, 2018. doi: 10.1371/journal.pone.0202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chilliard Y, Bonnet M, Delavaud C, Faulconnier Y, Leroux C, Djiane J, Bocquier F. Leptin in ruminants. Gene expression in adipose tissue and mammary gland, and regulation of plasma concentration. Domest Anim Endocrinol 21: 271–295, 2001. doi: 10.1016/s0739-7240(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 9.da Silva AA, do Carmo JM, Hall JE. Role of leptin and central nervous system melanocortins in obesity hypertension. Curr Opin Nephrol Hypertens 22: 135–140, 2013. doi: 10.1097/MNH.0b013e32835d0c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva AA, Pinkerton MA, Spradley FT, Palei AC, Hall JE, do Carmo JM. Chronic CNS-mediated cardiometabolic actions of leptin: potential role of sex differences. Am J Physiol Regul Integr Comp Physiol 320: R173–R181, 2021. doi: 10.1152/ajpregu.00027.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molvarec A, Szarka A, Walentin S, Beko G, Karadi I, Prohaszka Z, Rigo J , Jr. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol 9: 124, 2011. doi: 10.1186/1477-7827-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charkiewicz K, Jasinska E, Goscik J, Koc-Zorawska E, Zorawski M, Kuc P, Raba G, Kluz T, Kalinka J, Sakowicz A, Laudanski P. Angiogenic factor screening in women with mild preeclampsia - new and significant proteins in plasma. Cytokine 106: 125–130, 2018. doi: 10.1016/j.cyto.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Eleuterio NM, Palei AC, Rangel MJ, Tanus-Santos JE, Cavalli RC, Sandrim VC. Correlations between circulating levels of adipokines and anti-angiogenic factors in women with BMI <30 and a late-onset preeclampsia. Hypertens Pregnancy 33: 72–80, 2014. doi: 10.3109/10641955.2013.837174. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy JF, Misra DN, Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am J Obstet Gynecol 180: 731–736, 1999. doi: 10.1016/S0002-9378(99)70280-2. [DOI] [PubMed] [Google Scholar]
- 15.Shi Z, Hansen KM, Bullock KM, Morofuji Y, Banks WA, Brooks VL. Resistance to the sympathoexcitatory effects of insulin and leptin in late pregnant rats. J Physiol 597: 4087–4100, 2019. doi: 10.1113/JP278282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palei AC, Spradley FT, Granger JP. Chronic hyperleptinemia results in the development of hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol 308: R855–R861, 2015. doi: 10.1152/ajpregu.00286.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol 159: 1031–1043, 2001. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol 213: S9.e1, 2015. doi: 10.1016/j.ajog.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 20.Risnes KR, Romundstad PR, Nilsen TI, Eskild A, Vatten LJ. Placental weight relative to birth weight and long-term cardiovascular mortality: findings from a cohort of 31,307 men and women. Am J Epidemiol 170: 622–631, 2009. doi: 10.1093/aje/kwp182. [DOI] [PubMed] [Google Scholar]
- 21.Spradley FT, Sasser JM, Musall JB, Sullivan JC, Granger JP. Nitric oxide synthase-mediated blood pressure regulation in obese melanocortin-4 receptor-deficient pregnant rats. Am J Physiol Regul Integr Comp Physiol 311: R851–R857, 2016. doi: 10.1152/ajpregu.00285.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ralevic V, Burnstock G. Mesenteric arterial function in the rat in pregnancy: role of sympathetic and sensory-motor perivascular nerves, endothelium, smooth muscle, nitric oxide and prostaglandins. Br J Pharmacol 117: 1463–1470, 1996. doi: 10.1111/j.1476-5381.1996.tb15307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohno M, Yokokawa K, Horio T, Yasunari K, Murakawa K, Takeda T. Atrial and brain natriuretic peptides inhibit the endothelin-1 secretory response to angiotensin II in porcine aorta. Circ Res 70: 241–247, 1992. doi: 10.1161/01.res.70.2.241. [DOI] [PubMed] [Google Scholar]
- 24.Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Mitra A, Terzidou V, Bennett P, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ 359: j4511, 2017. doi: 10.1136/bmj.j4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anim-Nyame N, Sooranna SR, Steer PJ, Johnson MR. Longitudinal analysis of maternal plasma leptin concentrations during normal pregnancy and pre-eclampsia. Hum Reprod 15: 2033–2036, 2000. doi: 10.1093/humrep/15.9.2033. [DOI] [PubMed] [Google Scholar]
- 26.El Shahat AM, Ahmed AB, Ahmed MR, Mohamed HS. Maternal serum leptin as a marker of preeclampsia. Arch Gynecol Obstet 288: 1317–1322, 2013. doi: 10.1007/s00404-013-2915-8. [DOI] [PubMed] [Google Scholar]
- 27.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Umbilical cord plasma leptin is increased in preeclampsia. Am J Obstet Gynecol 186: 427–432, 2002. doi: 10.1067/mob.2002.120486. [DOI] [PubMed] [Google Scholar]
- 28.Taylor BD, Ness RB, Olsen J, Hougaard DM, Skogstrand K, Roberts JM, Haggerty CL. Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women. Hypertension 65: 594–599, 2015. doi: 10.1161/HYPERTENSIONAHA.114.03979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teppa RJ, Ness RB, Crombleholme WR, Roberts JM. Free leptin is increased in normal pregnancy and further increased in preeclampsia. Metabolism 49: 1043–1048, 2000. doi: 10.1053/meta.2000.7707. [DOI] [PubMed] [Google Scholar]
- 30.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol 38: 139–145, 2014. doi: 10.1053/j.semperi.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 39: 496–501, 2002. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 32.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, Cotton DB. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol 193: 979–983, 2005. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Vernini JM, Moreli JB, Costa RA, Negrato CA, Rudge MV, Calderon IM. Maternal adipokines and insulin as biomarkers of pregnancies complicated by overweight and obesity. Diabetol Metab Syndr 8: 68, 2016. doi: 10.1186/s13098-016-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol Regul Integr Comp Physiol 275: R1719–R1723, 1998. doi: 10.1152/ajpregu.1998.275.5.R1719. [DOI] [PubMed] [Google Scholar]
- 35.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 31: 1065–1069, 1998. doi: 10.1161/01.hyp.31.5.1065. [DOI] [PubMed] [Google Scholar]
- 36.Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension 37: 670–676, 2001. [Erratum in Hypertension 44: e2, 2004]. doi: 10.1161/01.hyp.37.2.670. [DOI] [PubMed] [Google Scholar]
- 37.Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase–dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49: 893–901, 2007. doi: 10.1161/01.HYP.0000259669.40991.1e. [DOI] [PubMed] [Google Scholar]
- 38.Anderson CM, Lopez F, Zhang HY, Shirasawa Y, Pavlish K, Benoit JN. Mesenteric vascular responsiveness in a rat model of pregnancy-induced hypertension. Exp Biol Med (Maywood) 231: 1398–1402, 2006. doi: 10.1177/153537020623100813. [DOI] [PubMed] [Google Scholar]
- 39.Brennan L, Morton JS, Quon A, Davidge ST. Postpartum vascular dysfunction in the reduced uteroplacental perfusion model of preeclampsia. PLoS One 11: e0162487, 2016. doi: 10.1371/journal.pone.0162487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton JS, Levasseur J, Ganguly E, Quon A, Kirschenman R, Dyck JRB, Fraser GM, Davidge ST. Characterisation of the selective reduced uteroplacental perfusion (sRUPP) model of preeclampsia. Sci Rep 9: 9565, 2019. doi: 10.1038/s41598-019-45959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA 91: 7583–7587, 1994. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandrim VC, Palei AC, Sertorio JT, Amaral LM, Cavalli RC, Tanus-Santos JE. Alterations in cyclic GMP levels in preeclampsia may reflect increased B-type natriuretic peptide levels and not impaired nitric oxide activity. Clin Biochem 44: 1012–1014, 2011. doi: 10.1016/j.clinbiochem.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 43.da Silva AA, Spradley FT, Granger JP, Hall JE, do Carmo JM. Brain-mediated antidiabetic, anorexic, and cardiovascular actions of leptin require melanocortin-4 receptor signaling. J Neurophysiol 113: 2786–2791, 2015. doi: 10.1152/jn.00911.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor PD, Samuelsson AM, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol (Oxf) 210: 508–523, 2014. doi: 10.1111/apha.12223. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A new mouse model to explore therapies for preeclampsia. PLoS One 5: e13663, 2010. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briffa JF, McAinch AJ, Romano T, Wlodek ME, Hryciw DH. Leptin in pregnancy and development: a contributor to adulthood disease? Am J Physiol Endocrinol Metab 308: E335–E350, 2015. doi: 10.1152/ajpendo.00312.2014. [DOI] [PubMed] [Google Scholar]
- 47.Perez-Perez A, Toro A, Vilarino-Garcia T, Maymo J, Guadix P, Duenas JL, Fernandez-Sanchez M, Varone C, Sanchez-Margalet V. Leptin action in normal and pathological pregnancies. J Cell Mol Med 22: 716–727, 2018. doi: 10.1111/jcmm.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeboah FA, Ngala RA, Bawah AT, Asare-Anane H, Alidu H, Hamid AM, Wumbee JDK. Adiposity and hyperleptinemia during the first trimester among pregnant women with preeclampsia. Int J Womens Health 9: 449–454, 2017. doi: 10.2147/IJWH.S134088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thagaard IN, Hedley PL, Holm JC, Lange T, Larsen T, Krebs L, Christiansen M. Leptin and adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertens 15: 78–83, 2019. doi: 10.1016/j.preghy.2018.12.002. [DOI] [PubMed] [Google Scholar]