Abstract
The actin-binding sarcomeric nebulette (NEBL) protein provides efficient contractile flexibility via interaction with desmin intermediate filaments. NEBL gene mutations affecting the nebulin repeat (NR) domain are known to induce cardiomyopathy. The study aimed to explore the roles of NEBL in exercise and biomechanical stress response. We ablated exon3 encoding the first NR of Nebl and created global Neblex3-/ex3- knockout mice. Cardiac function, structure, and transcriptome were assessed before and after a 4-wk treadmill regimen. A Nebl-based exercise signaling network was constructed using systems genetics methods. H9C2 and neonatal rat cardiomyocytes (NRCs) expressing wild-type or mutant NEBL underwent cyclic mechanical strain. Neblex3-/ex3- mice demonstrated diastolic dysfunction with preserved systolic function at 6 mo of age. After treadmill running, 4-mo-old Neblex3-/ex3- mice developed concentric cardiac hypertrophy and left ventricular dilation compared with running Nebl+/+ and sedentary Neblex3-/ex3- mice. Disturbance of sarcomeric Z-disks and thin filaments architecture and disruption of intercalated disks and mitochondria were found in exercised Neblex3-/ex3- mice. A Nebl-based exercise signaling network included Csrp3, Des, Fbox32, Jup, Myh6, and Myh7. Disturbed expression of TM1, DES, JUP, β-catenin, MLP, α-actinin2, and vinculin proteins was demonstrated. In H9C2 cells, NEBL was recruited into focal adhesions at 24-h poststrain and redistributed along with F-actin at 72-h poststrain, suggesting time-dependent redistribution of NEBL in response to strain. NEBL mutations cause desmin disorganization in NRCs upon stretch. We conclude that Nebl's NR ablation causes disturbed sarcomere, Z-disks, and desmin organization, and prevents NEBL redistribution to focal adhesions in cardiomyocytes, weakening cardiac tolerance to biomechanical stress.
NEW & NOTEWORTHY We demonstrate that ablation of first nebulin-repeats of sarcomeric nebulette (Nebl) causes diastolic dysfunction in Neblex3-/ex3- mice. Exercise-induced development of diastolic dysfunction, cardiac hypertrophy and ventricular dilation in knockouts. This was associated with sarcomere disturbance, intercalated disks disruption, and mitochondrial distortion upon stress and altered expression of genes involved in Nebl-based stress network. We demonstrate that G202R and A592 mutations alter actin and desmin expression causing disorganization of desmin filaments upon cyclic strain.
Keywords: desmin intermediate filament, exercise, focal adhesion, nebulette, sarcomere
INTRODUCTION
Inherited cardiomyopathies, major causes of morbidity and mortality, commonly result from mutated genes encoding cytoskeletal and sarcomeric proteins (1). Individuals carrying gene mutations including patients and their apparently healthy relatives who may have latent, early, or undiagnosed disease aspire to a physically active lifestyle. However, a large number of those individuals have progressed, with an increased risk of accelerated disease severity and/or sudden death (2). In particular, young patients may not present clinical signs of cardiomyopathy until adulthood due to a time-related mechanism associated with mutation-induced abnormal mechanosensation and the response to physical activity, leading to overt cardiomyopathy (3).
Mutations in sarcomeric nebulette (NEBL, MN_006393.2, isoform1), encoding an actin-binding protein, has been reported to cause various forms of familial cardiomyopathy (4–6). Sarcomeric NEBL, a 107-kDa protein, is abundantly expressed in the Z-disks and surrounding I band of cardiomyocytes, extending through the F-actin filament (Fig. 1A). It belongs to the nebulin family of proteins that are highly conserved across most vertebrates and play key scaffolding functions in cytoskeleton and focal adhesion dynamics (7, 8). The universal characteristics of nebulin family proteins include a capacity to bind actin filaments via nebulin repeats (NRs). Each NR contains a SZXXYK motif forming an α-helix that binds a single actin subunit (9). Four distinct domains are enriched in NEBL (Fig. 1A): actin binding domain (ABD), 23 NRs, linker domain (LD), and SH3 domain (10). ABD and NRs, located at the NH2 terminus, play crucial roles in binding F-actin, desmin, tropomyosin-troponin T (TM-TnT) complex, and filamin C (11–13). Located inside the Z-line lattice, the LD and SH3 domains are involved in the Z-disk architecture and tyrosine signaling (14, 15) by interacting with α-actinin2 (16), desmin (17), myopalladin (18), zyxin (19), and cadherins (20). Recently, NEBL has been identified as a strong “cytolinker” in the heart that reinforces and temporally fine-tunes cardiac muscle relaxation-contraction cycles via regulation of interactions between actin and desmin intermediate filaments (17).
Figure 1.
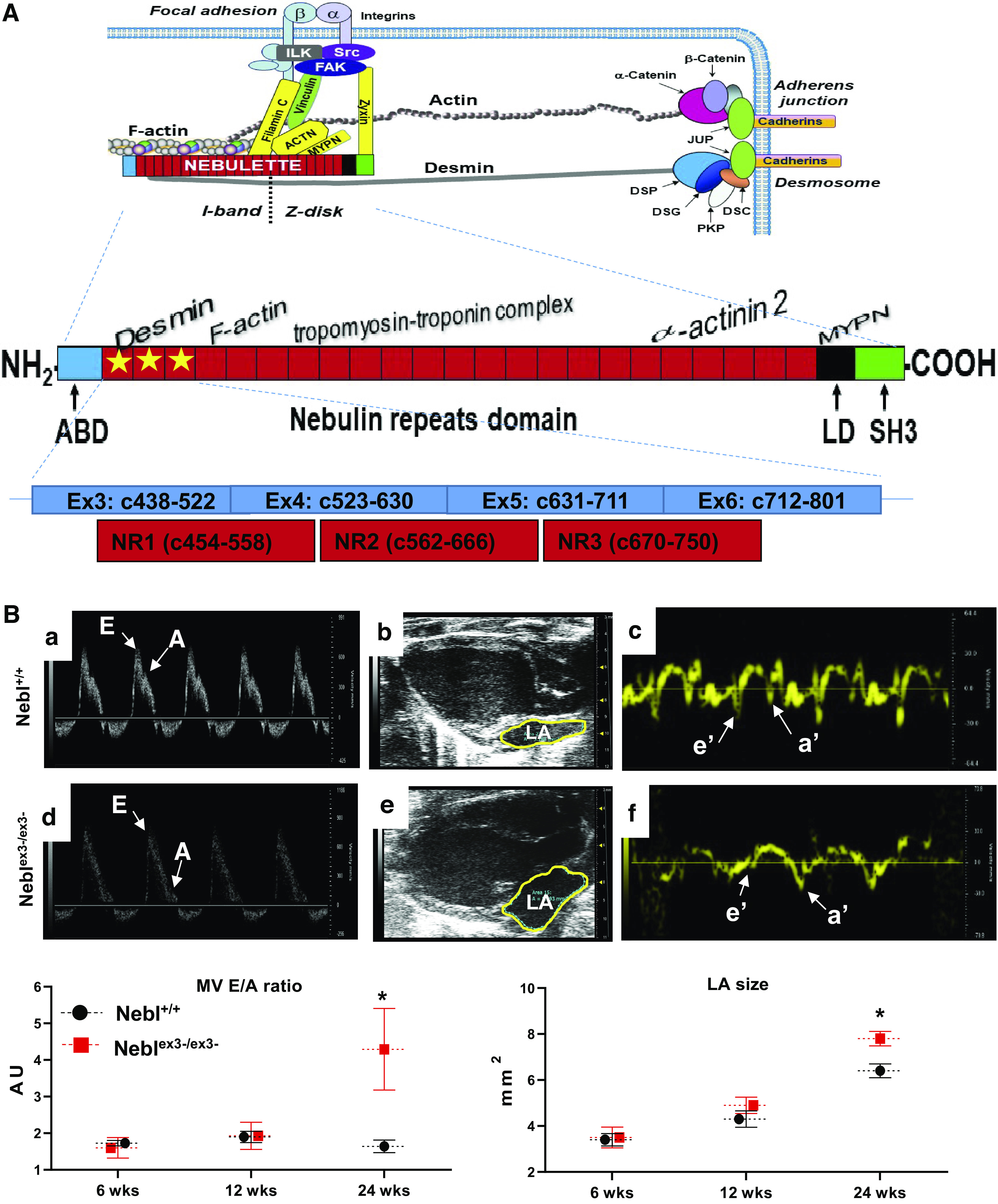
Structure and interactions of sarcomeric nebulette (NEBL) protein and cardiac function assessment of Neblex3-knockout (KO) mice. A: structure of NEBL protein and its interacting partners. The NH2-terminal portion of NEBL is located at the I-band, expending across the F-actin filaments. The COOH-terminal end of NEBL is bound to the Z-disks. The functional domains of NEBL include F-actin binding domain (ABD; orange), 23 modules of nebulin repeat (NR; red), linker domain (LD; black), and SH3 domain (green). Interacting proteins are shown to the corresponding binding NRs and domains. The ablated first 3 NRs encoded by exons3-6 of Nebl is indicated by yellow stars. B: results of serial echocardiography in 6-, 12- and 24-week-old Neblex3-KO mice (n > 5/genotype). Ba-–Bf: echocardiographic images of mitral valve (MV) E and A movements in M-mode (a and d), outlined left atrium (LA) in 4-chamber view (b and e), and interventricular septum (IVS) movements detected by tissue Doppler imaging (c and f) of hearts of Nebl+/+ (a–c) and Neblex3-/ex3- (d–f) mice. B, bottom: comparative analysis of MV E/A ratio (left) and area of LA (right). *P < 0.05, significant difference between age-matched Nebl+/+ (black circle) and Neblex3-/ex3- (red square) groups. AU, arbitrary units.
Interestingly, most NEBL mutations identified in patients with cardiomyopathy are located in the NR domain, suggesting an importance of NRs in disease pathogenesis. We have previously shown that the K60N and Q128R mutations caused embryonic lethality in transgenic mutant mice due to severe cardiac abnormalities (6). The mutations G202R and A592E caused postnatal occult diastolic dysfunction in a preclinical stage of dilated cardiomyopathy (DCM) in vivo due to altered calcium handling (21). During aging, the mutant G202R and A592E mice developed overt DCM with impaired systolic function and this prevented NEBL translocation to the focal adhesions during mechanical cyclic stretch of cardiomyocytes (6). Recently, Mastrototaro et al. (22) developed a global knockout of Nebl-exon1 with total ablation of sarcomeric NEBL in the heart. Intriguingly, these knockout mice lacked a strong cardiac phenotype while sedentary but displayed Z-line widening and upregulation of cardiac stress markers, suggesting the potential that NEBL plays a role in biomechanical stress regulation.
In this study, we hypothesized that NRs are important in cardiac stress physiology and ablation of NRs of NEBL may reflect the functional significance of NEBL. To test this hypothesis, we ablated exon3 of Nebl, encoding the first NR residues that are responsible for binding F-actin and desmin filaments (Fig. 1A, stars) and created a global Neblex3 knockout (Neblex3-KO) mouse. An acute and controlled moderately intense running regimen was applied in animals and cardiac performance, morphology, and gene expression was quantitatively assessed. Based on the results, a putative Nebl-based stress signaling network was constructed using a systems genetics approach. In addition, NEBL’s intracellular translocation and its roles regarding desmin and actin filament organization were tested in vitro under mechanical strain.
METHODS
Generation of Neblex3-KO Mice
To generate a Neblex3-KO mouse, exon3 of the mouse Nebl (NM_028757.3) gene was targeted using recombineering-based method as reported previously (23). The complete map and sequence of the targeting construct is provided in the Supplemental Materials at https://doi.org/10.6084/m9.figshare.c.5388611.v1. Briefly, 10.3 kb-long genomic fragment, containing the exon3 of Nebl was excised from the mouse BAC clone. The targeting vector was composed of 9.4-kb long homology arm with the exon3 flanked with LoxP site upstream from 5′-end, a neomycin resistance cassette (Neo) flanked with another LoxP site downstream from 3′-end, and a short homology arm of 0.9 kb. The LoxP recognition site was inserted 664 bp upstream of exon3. A Neo-LoxP site was inserted 333 bp downstream from the exon3. Thymidine kinase gene (HSV-TK) was used for negative selection (Supplemental Fig. S1A). Homologous recombination in Embryomax 129/SVEV embryonic stem cells (Millipore, Billerica, MA) was performed by electroporating the linearized targeting vector. Neomycin- and gancyclovir-resistant ES cell clones were screened by a PCR-based method using primers p1 and p2. Clones that were confirmed to be correctly targeted were injected into C57BL/6 blastocysts at the Transgenic Mouse Core Facility of Cincinnati Children’s Hospital Medical Center (CCHMC; Cincinnati, OH). Mouse colonies were maintained under barrier conditions. Mice were genotyped by PCR using LongAmp PCR mastermix (NEB). Chimeric animals were bred with C57/Bl6 mice (Charles River) or backcrossed to obtain mice with floxed exon3. Heterozygote (Nebl+/ex3-) and homozygote (Neblex3-/ex3-) mice were obtained by breeding mice of “floxed” Nebl exon3 with the transgenic line that globally expresses a CMV-Cre-recombinase (JAX) and genotyped using Nebl-KO-glo-F and Nebl-KO-glo-R primers (Supplemental Fig. S1B). Prediction of molecular weights of proteins was done using a SerialCloner 2-6 software (www.serialbasics.com). All animal protocols were approved by the CCHMC and University of Tennessee Health Science Center Institutional Animal Care and Use Committee and performed in compliance with National Institutes of Health guidelines.
Echocardiographic Evaluation of Heart Function
Serial transthoracic two-dimensional and Doppler echocardiography was performed in male and female littermate mice (n > 5 animals/group/sex) at 6, 12, and 24 wk of age to assess cardiac function using a Vevo2100 Micro-Imaging System (VisualSonics Inc., Toronto, Canada) with a 30-mHz transducer. The chests of the mice, which were anesthetized with 1–2% oxygenated isoflurane, were treated with a chemical hair remover to reduce ultrasound attenuation. Normothermic mouse core temperatures were maintained using a heated platform. Images were stored in the ultrasound system hard drive and transferred to an image server for offline analysis. Measurements including left ventricular (LV) and left atrial (LA) dimensions as well as LV mass (LVM) and wall thickness were performed by two echocardiography technicians blinded to genotype.
Electrocardiograms and Blood Pressure Recording
Mice were anesthetized by oxygenated 1.5% isoflurane, and core temperatures were maintained using a heated platform set at 37°C. Subcutaneous needle electrodes were placed in both front and rear left legs in all mice in similar positions to obtain consistent recordings across all mice. A single lead electrocardiogram (ECG) tracing was recorded for 5 min at a sampling rate of 200 Hz on a BIOPAC (Goleta, CA) using Acknowledge 3.9.2 software. The gain was set at 1,000 with 1 mv/division voltage and duration of 0.7 s/division. The noninvasive CODA system was used to measure blood pressure (BP) in conscious mice placed in a mouse restrainer via tail cuff using volume pressure recording. As the occlusion cuff is deflated, the volume pressure recording measures the physiological values for systolic and diastolic BP, heart rate, tail blood flow, and volume. All recorded ECG and BP values were imported into the software for further evaluation.
Acute Running Stress and Endurance Regimen
Male Neblex3-/ex3- mice and wild-type (WT) littermate controls were divided randomly into two groups, sedentary and exercise (n = 12). Age- and sex-matched littermates without exercise were kept sedentary as a control group for the entire study duration. Animals had free access to standard laboratory rodent chow and water. All mice were subjected to their first baseline echocardiograms, ECG, and BP recordings at 12 wk of age. Before the stress test, animals from the exercise group were allowed 1-wk exercise acclimatization with 5–10 min running at the 5 m/min speed. Starting at 12 wk of age, mice underwent an exercise regimen using an Exer3/6 treadmill (Columbus Instruments, Columbus, OH). An acute stress test was obtained by running of mice on a treadmill with continuously increasing speed from 5 m/min to 20 m/min until mouse exhaustion. Clear endpoints for quantifying running time-to-exhaustion for each mouse were defined and reflected in behavioral signs of exhaustion, such as splayed posture and labored breathing and lack of willingness to run after a few gentle pushes with the tongue depressor. Total running distance and time were recorded.
After a week of rest, the mice underwent 5 days/wk chronic stress testing with steadily increased exercise intensity by increasing the speed, distance, and inclination of the treadmill. Speed was gradually increased from 5 m/min for 5 min with treadmill inclination at 0° on day 1 of exercise regimen up to 12 m/min for 20 min at 15° inclination on day 21 (Supplemental Table S2). An initial 5-min warm-up period was incorporated in each exercise session. After 4 wk of running or staying sedentary, second echocardiograms, ECG and BP recordings were performed and hearts were obtained under isoflurane anesthesia.
Histology and Electron Microscopy
Under deep anesthesia, mouse hearts were continuously perfused via the left ventricular (LV) apex with cardioplegic solution containing 3.73 g/L KCl and nifedipine and fixed in 10% formalin (Sigma-Aldrich) for a minimum of 48 h. Perfused hearts then were paraffin-embedded and cut into 5-µm sections and stained with hematoxylin and eosin or Masson’s trichrome. Ultrastructural analysis was performed on glutaraldehyde-perfused hearts with 3.5% glutaraldehyde in cardioplegic solution for 2 min, followed by 3.5% glutaraldehyde in 100 mM cacodylate solution (pH 7.3) for 2 min. Hearts were dissected into fragments and fixed into 1% glutaraldehyde/2% paraformaldehyde overnight. Tissue fragments were postfixed on ice in 1% cacodylate solution, dehydrated in acetone, and embedded in a Poly/Bed 812 resin mixture. Sections were counterstained with uranyl acetate and lead citrate, and transmission electron microscopy (TEM) was performed using a Zeiss912 microscope.
RNA Isolation, Microarray, and Quantitative RT-PCR
After isolation of total RNA with a Qiagen RNA isolation kit from snap frozen ventricular tissues collected at baseline and after mechanical stress (n = 4/group), 1 µg of purified RNA was used to synthesize cDNA using qScript Supermix (Quanta Biosciences). The quality and the purity of the extracted total RNA were checked using an Agilent Bioanalyzer 2100 system to assess the relative quantities of 18S and 28S RNA as well as RNA integrity. A minimum RNA integrity score of 8 was required to proceed. Total RNA samples then were hybridized to Affymetrix Mouse Gene 2.0 ST Arrays according to manufacturer's protocols. Raw expression data were normalized using the robust multichip average method with default analysis parameters, and modified z-score was defined as described previously (24). Differential expression analysis between groups was examined by t test, and genes with multiple hypothesis corrected P < 0.05 were defined as differentially expressed genes (DEGs). Candidate DEGs were validated by quantitative (q)RT-PCR using a Quant Studio 6 Flex (ThermoFisher) and Eva-Green master mix (Bio-Rad). All samples were assayed in triplicate, and the average value was used for quantification. The data were analyzed using the comparative ΔCt method (ΔΔCt method), and the results are expressed in fold changes related to WT. In all experiments, Gapdh was used as the housekeeping gene. Five animals per group were used.
Genetic Correlation Analysis
To identify Nebl genetically correlated genes, we compared cardiac Nebl expression to all genes across the mouse transcriptomes in mouse BXD strains used as a murine genetic reference population (GRP). This cardiac transcriptome data set was generated from our previous study and consists of 40 BXD strains and their two parental strains C57BL/6J and DBA/2J (25). Genetic correlation analysis was carried out using Pearson correlation analysis through the tool on the Genenetwork website (http://genenetwork.org). P < 0.05 was used as the significance threshold. Array platform and data normalization were the same as the aforementioned and can be accessed at the http://genenetwork.org (Accession No. GN485).
Construction and Validation of NEBL-Based Exercise Stress Signaling Network
For generation of a predictive Nebl-based exercise stress signaling network, genes with significant correlation with Nebl (P < 0.05) and implicated in the Z-disk gene ontology (GO:0030018) were used to construct the Nebl-based stress gene network. The nodes in the network represent genes, and the edge between two nodes represents their genetic correlation. Gene perturbations (differential expression) with both, Nebl+/+ and Neblex3-/ex3- at sedentary and exercised, were included in the network.
Protein Isolation, Expression, and Localization Analysis
Mouse hearts were dissected, minced into small pieces and protein isolates were prepared using the T-PER (ThermoFisher) with phenyl methanesulfonylfluoride (Sigma) and protease inhibitors (Roche) according to the manufacturer’s instructions. For collection of soluble phase, the tissue mixed with stainless steel beads was disrupted using TissueLyser II (Qiagen), centrifuged at 10,000 g for 5 minutes and the supernatant was collected. Proteins were quantified using the Pierce BCA Protein Assay Kit, and 25 µg of total protein were applied on 4–12% BT gels (Invitrogen) and transferred to a PVDF membrane for application of primary and secondary antibodies followed by detection using the LICOR system. Primary antibodies against nebulette (NEBL) and myopalladin (MYPN) were provided by Dr. S. Labeit as previously reported (20), and antibodies against NEBL (Invitrogen), α-actinin2 (ACTN, Upstate), muscle LIM protein (MLP), N- and E-cadherin, desmin (DES; Abcam), tropomyosin (TM), cardiac troponin T (TnT), FAK, and p-FAK (Cell Signaling), filamin C, cardiac ankyrin repeat protein (CARP), vinculin (VCL, Santa Cruz), and desmoplakin (DSP, Fitzgerald) were used as recommended by the manufacturer. GAPDH (Santa Cruz) was used as a control. Relative intensity was carried out for protein quantification using a Kodak Image system.
To visualize protein localization, immunohistochemistry was performed on snap-frozen OCT embedded 5-µm heart tissues. Multiple sections from at least three mice/group were analyzed. After primary antibody application, samples were incubated with Alexa Fluor-conjugated secondary antibodies or phalloidin for F-actin (Molecular Probes) for 30 min, counterstained with DAPI (2.5 μg/ml, Roche Diagnostics), and subsequently mounted with mounting media (Vector Laboratories).
Cell Culture, Transient Expression of NEBL, and Cyclic Mechanical Strain
To study the effects of NEBL during mechanical stretch, H9C2 cells and primary rat neonatal cardiomyocytes (NRCs) plated on BioFlex plates (Flexcell International Corporation) were used. The H9C2 cells were transfected with human NEBL cDNA expressed by pcDNA3.1NT-GFP-TOPO vector (Invitrogen, Carlsbad, CA) using Effectine (Qiagen, Valencia, CA) to visualize NEBL’s intracellular redistribution during stretch. To assess effects of NEBL on actin and desmin filament organization, the NRCs were infected with adenoviral vectors containing human NEBL (WT, G202R, and A592E mutant) as described previously (26). After 48 h of vector delivery, the cells were subjected to 10% Eulerian biaxial strain at a frequency of 1 Hz for 1 h/day using the FX-5000T Tension system or kept in static conditions. The H9C2 cells were fixed at 24- and 72-h and stained with anti-green fluorescent protein (GFP) and phalloidin-594 (Invitrogen). The NRCs were fixed after 72 h of strain, stained with DES or phalloidin, and visualized using a Zeiss710 confocal microscope. To determine DES and phalloidin colocalization, Pearson correlation coefficient was used to measure colocalization of two fluorescent probes using ImageJ Coloc2 plugin (>Analyze > Colocalization > Coloc2). The same size regions of interest (n = 10) were cropped by freehand selections from confocal images obtained by red and green channels separately, and correlations between fluorophores were quantified by Pearson scoring expressed in arbitrary units.
Statistical Analyses
All variables are reported as means ± SD. Continuous variables were compared by use of the Student’s t test. Two-way ANOVA was performed to determine the effect of genotype and exercise (before/after stress) on functional parameters using GraphPad Prism 8 software. P < 0.05 was considered as statistically significant.
RESULTS
Exon3 Knockout Resulted in Ablation of First Three NRs of NEBL
Many transcripts are expressed in murine heart. Thus, we first confirmed transcription of all Nebl exons in Neblex3-KO mouse hearts using quantitative real-time PCR (qRT-PCR) with primers specific to murine Nebl (Supplemental Table S1). Analysis of mRNA levels demonstrated increased exon1 transcripts, a significant decrease exons2-3, and a total ablation of exon3 in the Neblex3-/ex3- heart. Although expression of exons4-28 encoding all NRs were significantly decreased (Supplemental Fig. S1B), we detected a spike increase of exon7 that corresponds to Nebl isoform2 (NM_001362722.1), suggestive for a splice variant expression. We also detected increase in exons28-30 followed by normal levels of exons29-32, showing normal expression of LD and SH3 regions of NEBL. Therefore, to precisely detect ablated transcripts, we sequenced exons1-7 cDNA and found only exons3-6 knocked-out that encode NRs1-3 (Fig. 1A, stars). To validate, we performed Western blot analysis of the heart (soluble and insoluble tissue fractions) using two different antibodies, PA5-78571 (ThermoFisher) that recognizes the 107-kDa NEBL and LIM-NEBL (∼50 kDa) and NBP1-45223 (Novus Biologicals) that recognizes another isoform LASP2 of ∼31 kDa (Supplemental Fig. S1C). The results demonstrated that knockouts have similar molecular weights NEBL as WT mice (red arrow), although NEBL levels were decreased in the heart of Neblex3-/ex3- mice versus Nebl+/ex3- (P = 0.04) and Nebl+/+ (no significance) mice. No LIM-NEBL or other isoforms (asterisks) were detected in the heart of any Nebl-KO mice. In addition, we found no sarcomeric NEBL expressed in the kidney, liver, or skeletal muscle. These results suggested reduced NRs1-3 null NEBL in Neblex3-/ex3- hearts despite preserved exons7-28 transcribing most of NRs.
Neblex3-/ex3- Mice Exhibit Primary Diastolic Dysfunction with Aging
In order to determine effects of NRs1-3 ablation on heart function, we performed serial echocardiography in the same cohort of littermate mice at 6-, 12-, and 24 wk of age and found normal ejection fraction (EF%) and fractional shortening (FS%), demonstrating no impairment in systolic function (Supplemental Table S3). No chamber dilation or increase in interventricular septum (IVS) or LV myocardial posterior wall (PW) thickness was found in any mice. Interestingly, we found signs of diastolic dysfunction in 24-wk-old Neblex3-/ex3- mice compared with age-matched heterozygous Nebl+/ex3- and Nebl+/+ mice, evidenced by a significant increase in E/A ratio of mitral valve (MV) movement due to decrease in A-wave amplitude (Fig. 1Bd), increase in LA size (Fig. 1Be), and abnormal IVS longitudinal strain rate on tissue Doppler imaging (Fig. 1Bf). These data suggested that Neblex3-/ex3- mice exhibit primary diastolic dysfunction (impaired LV relaxation) with aging, similar to mutant NeblG202R and NeblA592E transgenic mice reported previously (21). Insufficient LV filling due to impaired LV relaxation and abnormal increase in LA size and pressure, consistent with restrictive physiology, are reported as important contributors to exercise intolerance in patients with primary diastolic dysfunction with preserved systolic function (27).
Effects of Acute Treadmill and Chronic Endurance Exercise on Cardiac Function in Neblex3-KO Mice
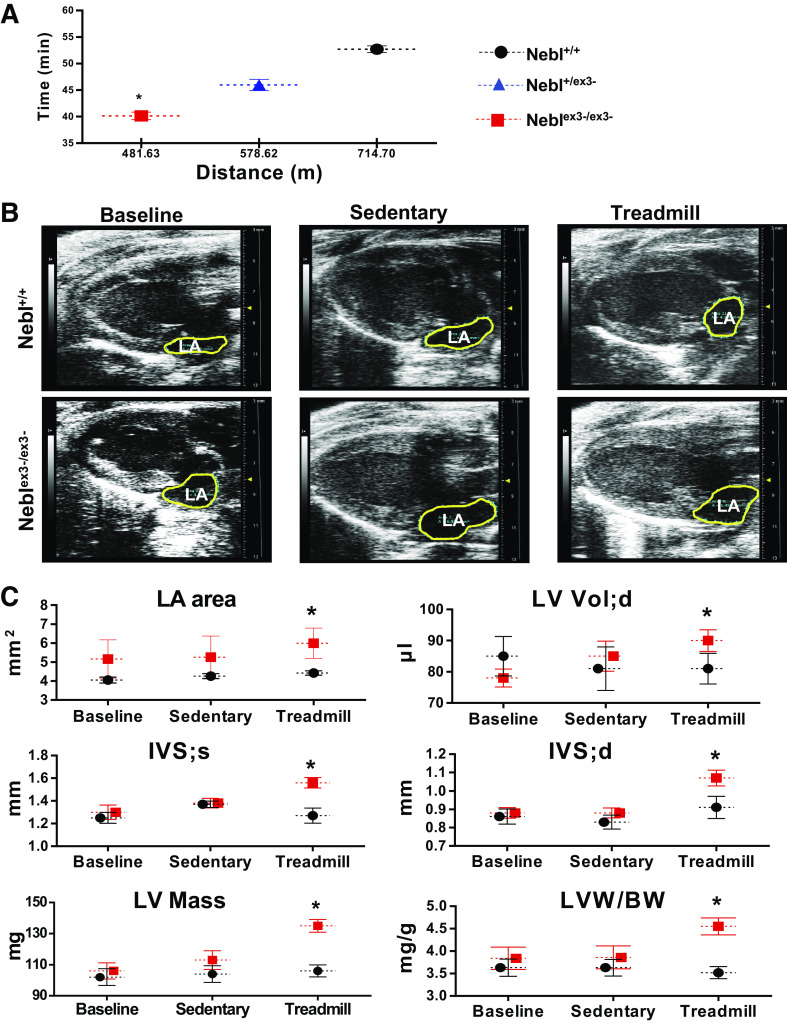
We next tested if NRs1-3 ablation plays a functional role during treadmill exercise stress. For stress tolerance tests, we used 12-wk-old animals (n = 11) when Neblex3-/ex3- mice exhibited normal diastolic function. During acute stress running, Neblex3-/ex3- mice ran a significantly shorter distance and running time was significantly reduced (P = 0.04) before reaching exhaustion compared with Nebl+/+ littermates (Fig. 2A), showing significant intolerance of Neblex3-/ex3- mice to acute running.
Figure 2.
Response of Neblex3-knockout (KO) mice to an acute and chronic running regimens. Nebl, nebulette. A: total running distance and time during acute treadmill testing in Nebl+/+ (black circles), heterozygous Nebl+/ex3- (blue triangles) and Neblex3-/ex3- (red squares) mice. *P < 0.05, significant difference between age-matched groups. B and C: results of echocardiography in Neblex3-KO mice at baseline (12-wk-old), while sedentary or after 4-wk treadmill running (16-wk-old). B: representative 4-chamber echocardiographic images with outlined left atrium (LA). C: echocardiographic parameters demonstrating LA area (mm2), left ventricular (LV) volume (Vol, µl), dimentions of interventricular septum (IVS) at end-diastole (d) or end-systole (s), LV mass (g), and LV mass and body weight (LVM/BW) ratios. *P < 0.05, significant difference compared with age-matched stressed and sedentary Nebl+/+ or sedentary Neblex3-/ex3- groups.
We next ran the mice for 4 consecutive wk on a controlled moderately increased exercise regimen. All groups of mice tolerated endurance exercise well, showing no signs of distress. To compare cardiac function between exercised and sedentary mice, echocardiography was performed in 16-wk-old mice under anesthesia. As expected, systolic function was preserved in all mice (normal EF% and FS%, Supplemental Table S4). However, exercised Neblex3-/ex3- mice showed significantly dilated LA compared with that from other groups (Fig. 2, B and C), consistent with diastolic dysfunction. End-diastolic LV volume was significantly larger in exercised Neblex3-/ex3- mice as well. We also found significantly (P < 0.05) increased LVM (134.9 ± 13.8 mg) and thickened IVS at end-systole (1.56 ± 0.16 mm) and end-diastole (1.07 ± 0.11 mm) in exercised Neblex3-/ex3- mice compared with sedentary Neblex3-/ex3- or Nebl+/+ controls (Fig. 2C). Collectively, the data demonstrated that after 4 wk of running, Neblex3-/ex3- mice developed diastolic dysfunction and eccentric hypertrophy (increased LVM, IVS hypertrophy, and LV chamber dilation) commonly associated with chronic volume overload, suggesting that NEBL is important for maintaining normal LV relaxation to provide sufficient LV filling during diastole in the biomechanical stress response.
Effects of Endurance Exercise on Heart Weight, Cardiac Rhythm, and Blood Pressure in Neblex3-KO Mice
Endurance exercise places distorted strain on cardiac performance and morphology in patients and animal models with cardiomyopathy, which may promote arrhythmias and cardiovascular remodeling (28). To detect these abnormalities, we compared LVM (obtained by echocardiography), body weight (BW), BP, and ECG tracings in Nebl mice before and after exercise. As expected, the LVM/BW ratio was significantly higher in exercised Neblex3-/ex3- mice compared with that in other groups (Fig. 2C). We also noticed mild enlargement of the whole heart and increase in cardiomyocyte surface area in exercised Neblex3-/ex3- mice compared with that in the other mice (Supplemental Fig. S2A). Histology revealed no significant fibrosis or infiltration in mouse hearts from any groups, suggesting minimal changes at the tissue level.
No significant difference in systolic and diastolic BP values between groups was identified (data not shown). Frequency of arrhythmias [premature atrial contractions (PACs), premature ventricular contractions (PVCs), and bradycardia] calculated per 1,000 heartbeats was higher in Neblex3-/ex3- mice compared with WT mice; however, the difference did not reach a statistical significance (Supplemental Fig. S2B). Remarkably, exercised Neblex3-/ex3- mice displayed PVCs and bradycardia compared with exercised Nebl+/+ controls, which displayed solo PACs in response to exercise.
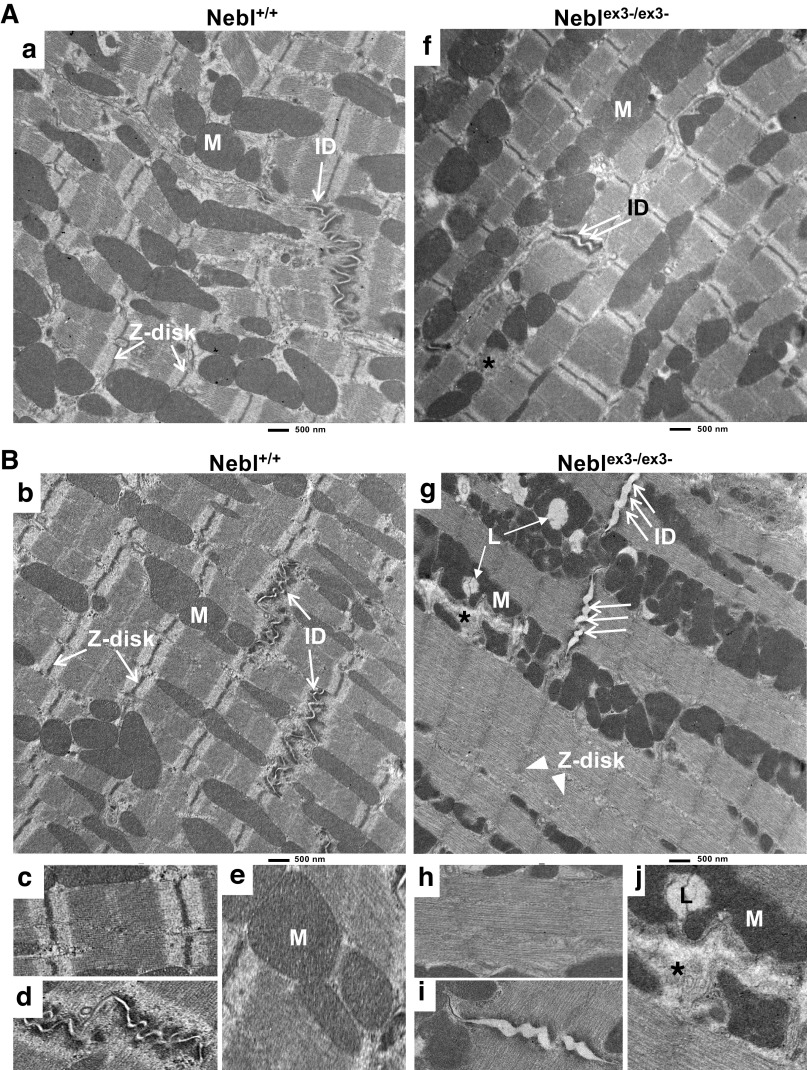
Chronic Exercise Leads to Ultrastructural Abnormalities in Neblex3-/ex3- Mice
To determine cardiomyocyte subcellular organization, we performed TEM in 16-wk-old Neblex3--KO mice while sedentary (Fig. 3A) and after exercise stress (Fig. 3B). TEM revealed that both sedentary and exercised control Nebl+/+ mice had well organized sarcomeres with clear Z-I-A-M bands (Fig. 3Aa, Bb, and Bc) and intercalated disks (ID; Fig. 3Bd) were narrow and normally folded with distinct adherens and desmosomal junction “plaques” at the cell-cell connections (arrows). Mitochondria (Fig. 3Be) had normal ellipsoid shape and cristae aligning through myofibrils. Sedentary Neblex3-/ex3- mice also demonstrated well defined Z-I-A-M lines similar to WT mice (Fig. 3Af), however, the IDs were focally disrupted and cell-cell contacts were mildly widened (double arrow). Mitochondria with blurry cristae were abnormally shaped and some mitochondria were damaged (Supplemental Fig. S3, Ab and Ac, asterisks). In contrast, exercised Neblex3-/ex3- mice had diffusely disturbed sarcomere architecture (Fig. 3Bg, arrowheads) with substantially lower density of crosslinked F-actin fibers and smeared Z-I-A-M bands (Fig. 3Bh). Disruption of IDs was vast and diffuse (triple arrows, Fig. 3Bg and Bi) and loss of cell-cell contacts became pronounced (mean ID width of 170 ± 54 nm vs. 58 ± 13 nm in WT mice, P value = 1.20867E-06). Stressed knockouts further demonstrated abnormal proliferation and irregular size, arrangement and shape of mitochondria. Diffusely damaged mitochondria formed large necrotic clusters (Fig. 3Bj) and lysosomal inclusions. Taken together, these results showed that actin cytoskeleton, sarcomere, the Z-disks, IDs, and overall cardiomyocyte morphology were significantly distorted by mechanical stress in Neblex3-/ex3- mice.
Figure 3.
Electron microscopy analysis of Neblex3-knockout (KO) mouse hearts while sedentary (A) or after exercise (B). Nebl, nebulette. Scale bar = 500 μm. Bc–Be and Bh-Bj: higher magnifications of the sarcomere, intercalated disks (IDs), and mitochondria (M) in Nebl+/+ (Bc–Be from Aa and Bb) or Neblex3-/ex3- (Bh-Bj from Af and Bg) mice. L, lysosome. Arrows point to normal Z-disks and IDs, double arrows indicate focal mild ID widening, and triple arrows indicate diffuse severe ID disruptions. *Mitochodrial distortion.
Nebl-Based Exercise Signaling Network in the Heart
Based on the effects of exercise on cardiac morphology and function in Neblex3-/ex3- mice, we hypothesized that Nebl may modulate downstream signaling in response to endurance exercise and therefore performed cardiac microarray analysis in Nebl mice before and after exercise to define associated DEGs. In control Nebl+/+ mice, we found 198 DEGs between sedentary and stressed groups (Fig. 4A). In Neblex3-/ex3- mice, 204 DEGs were found between sedentary and exercised groups. Out of those DEGs, Mir23a, Mir1904, Olfr1036, Olfr547, Olfr1093, and Spin2d were differentially expressed between Nebl+/+ and Neblex3-/ex3- mice. Of those genes, Mir23a has been shown to be one of the stress-responsive miRNAs that induce cardiac hypertrophy and heart failure in humans (29). Further unsupervised clustering analysis of selected top 100 DEGs involved in cardiac structure, physiology and disease pathogenesis demonstrated that Bcl2, Bcl7c, Il21r, Mapk11, Mybpc1, Myh7, Nppa, and Pgf were differentially expressed between sedentary Nebl+/+ and Neblex3-/ex3- mice (Fig. 4B). In the exercised group, Atp2a1, Jph3, Kcna1, Kcnh1, Kcnv2, Mapk6, and Myom2 were the DEGs expressed between Nebl+/+ and Neblex3-/ex3- mice (Supplemental Table S5). We performed principal component analysis and found both exercised Nebl+/+ and exercised Neblex3-/ex3- groups can be clearly distinguished from the sedentary groups (Fig. 4C). We then performed the Kyoto Encyclopedia of Genes and Genomes (KEGG) and GO pathway analysis; however, only one KEGG pathway (Olfactory transduction) and nine GO terms not related to cardiovascular characteristics were overrepresented in those DEGs (data not shown).
Figure 4.
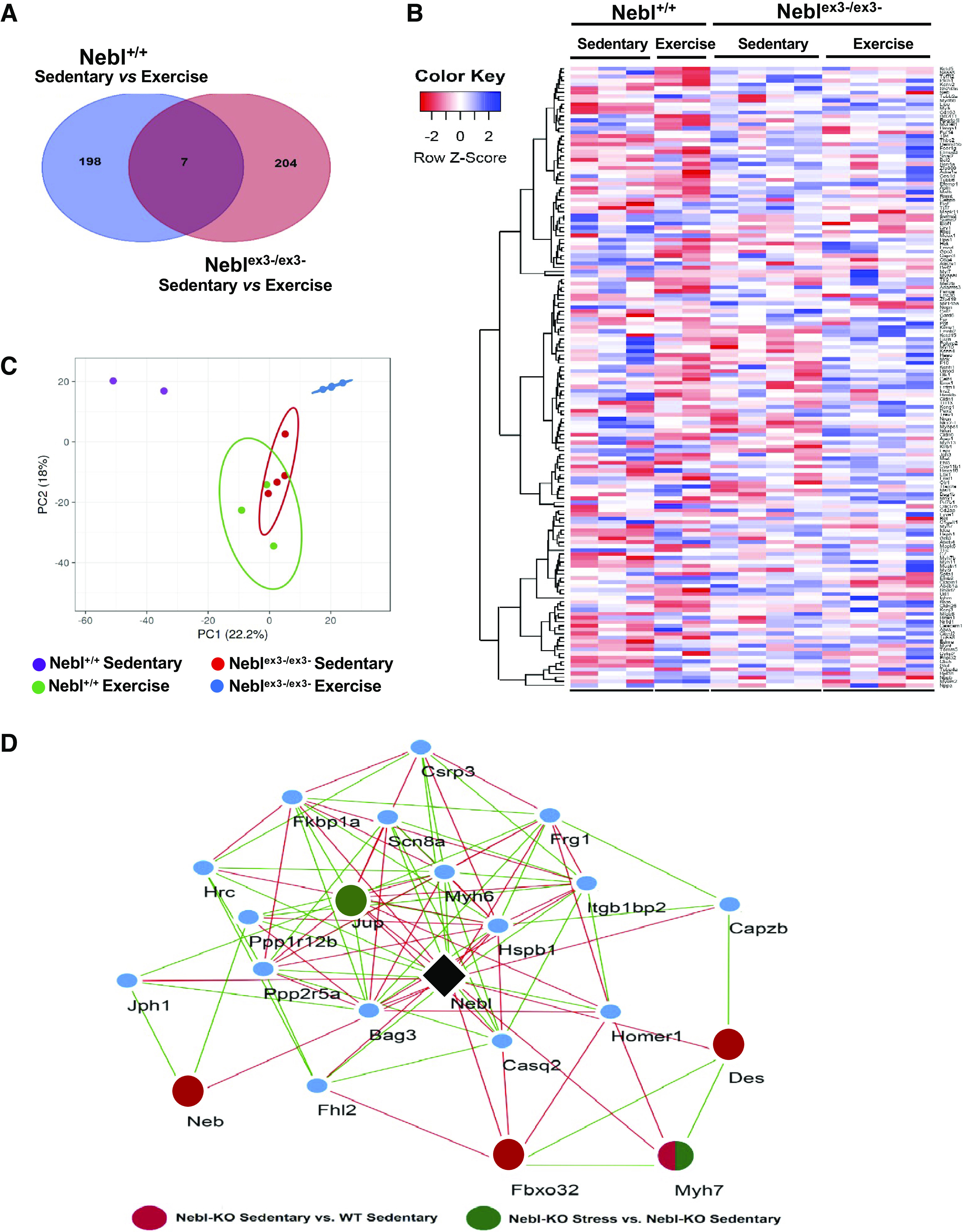
Cardiac gene expression in Neblex3-knockout (KO) mice and nebulette (Nebl)-based putative stress network. A and B: Venn diagram and heat-map of differentially expressed genes (DEGs) between sedentary and exercised Nebl+/+ and Neblex3-/ex3- mice. C: results of principal component analysis (PCA), showing distinguished clustering of exercised mice from the sedentary groups. D: putative cardiac Nebl-based Z-disk regulatory network constructed based on a genetic correlation analysis of cardiac Nebl (black) expression with cardiac transcriptomes across 40 BXD strains and their two parental strains. WT, wild type. Twenty-two Z-disk related genes significantly (P < 0.05) correlated with cardiac Nebl and with each other were identified. Red nodes represent DEGs with a significance between Neblex3-/ex3- sedentary and Nebl+/+ sedentary. Green nodes represent DEGs with a significance between Neblex3-/ex3- stress and Neblex3-/ex3- sedentary. Negative genetic correlation between genes is indicated by red edges, and green edges indicate positive correlation (P < 0.05).
To construct Nebl-induced exercise signaling pathways and discover distinct modules and transcriptional regulators, we performed genetic correlation analysis for Nebl against a cardiac gene expression database of BXD strains representing a murine GRP as we reported previously (25). This allowed us to identify ∼3,200 Nebl genetically correlated transcripts (P < 0.05), which could be functionally related and involved in the same biological and functional processes. Twenty-two genes, including all Nebl’s interacting genes from the GO category of the Z-disk, were used to construct Nebl-based exercise signaling gene network depicted in Fig. 4D. Pearson correlation analysis demonstrated that Csrp3, Des, Jup, Myh6, and Myh7 are highly correlated with each other, with 172 gene pairs showing significant correlation (P < 0.05). Additionally, four DEGs (Des, Fbox32, Myh7, and Neb) were identified in this network being upregulated (P < 0.05) in sedentary Neblex3-/ex3- versus sedentary Nebl+/+ (red circle), with two DEGs (Jup and Myh7) being also upregulated in sedentary Neblex3-/ex3- versus exercise stress Neblex3-/ex3- mice (green circle).
Validation of Proteins Involved in Cardiac Nebl-Stress Signaling
We first confirmed NEBL expression in the heart using Western blotting and demonstrated that levels of NEBL are decreased in sedentary Neblex3-/ex3- mice compared with other groups; however, the difference did not reach a statistical significance. Interestingly, expression of NEBL in exercised Neblex3-/ex3- mice increased to the similar level seen in Nebl+/+ animals (Fig. 5A), suggesting that an increase of NRs1-3 null NEBL was insufficient to adequately respond to treadmill stress. As NRs of NEBL interact with the TM-TnT complex, we quantitatively evaluated expression of TM1/TM2 and cTnT and found significantly increased TM1 levels in exercised Neblex3-/ex3- animals. We also confirmed that ratios in expression of TM1 to cTnT are significantly altered in knockout mice while sedentary versus with exercise, suggesting disturbance in TM-TnT complex. The NRs1-5 of NEBL are the binding regions to desmin intermediate filaments. Levels of DES were significantly increased in Neblex3-/ex3- versus Nebl+/+ mice while sedentary (Fig. 5A). In contrast, levels of junctional plakoglobin (JUP) were significantly decreased in Neblex3-/ex3- versus Nebl+/+ hearts while sedentary, while no JUP alteration was found in exercised Neblex3-/ex3- and Nebl+/+ controls. As JUP is reported to be involved in Wnt/β-catenin signaling, we also confirmed expression of β-catenin that was altered reciprocally compared with JUP. Expression of MLP (a Z-disk mechanosensitive protein encoded by Csrp3) and VCL (focal adhesion protein) was significantly (P < 0.05) decreased in sedentary Neblex3-/ex3- compared with exercised Nebl+/+ and Neblex3-/ex3- mice.
Figure 5.
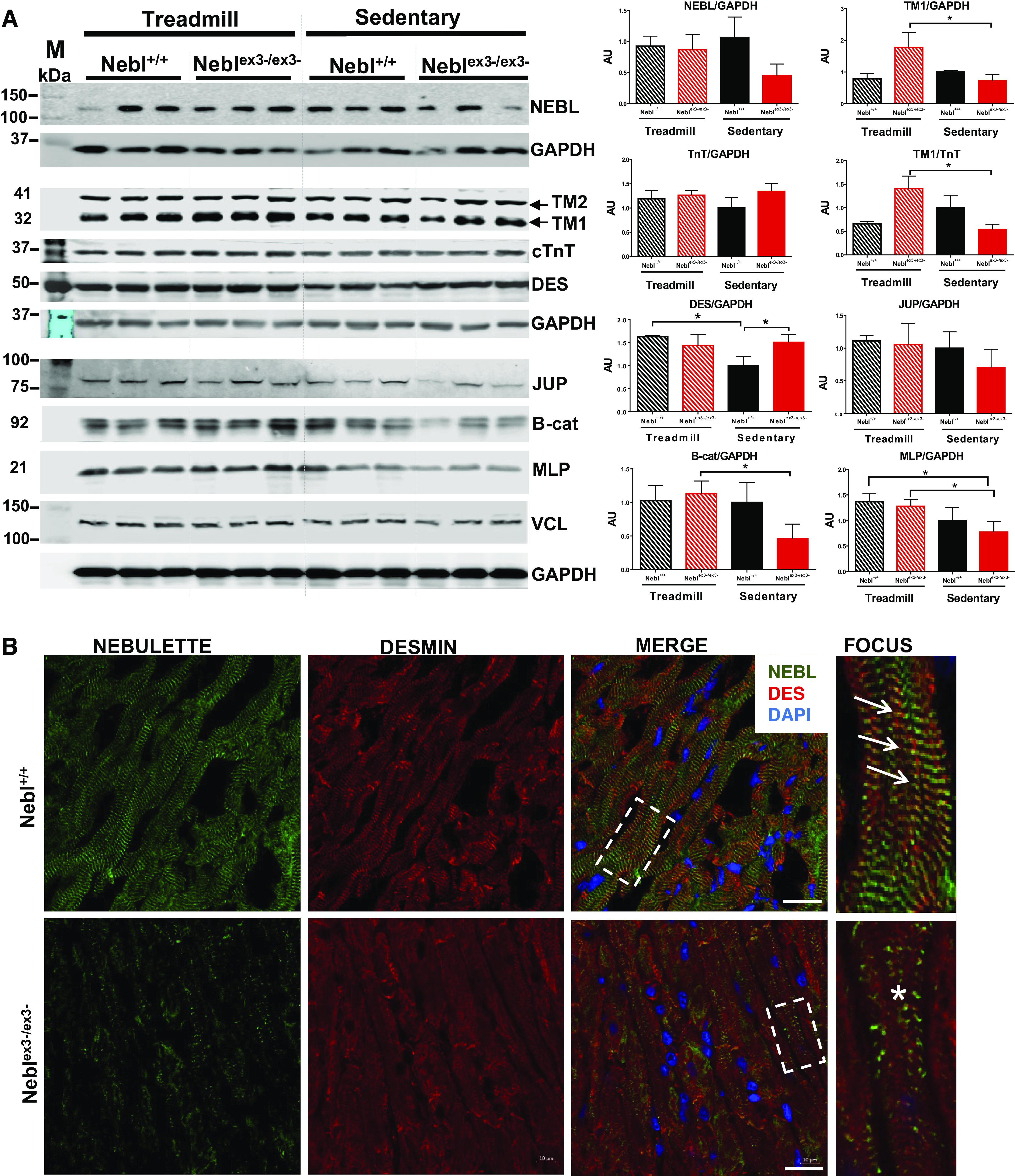
Protein analysis of Neblex3-knockout (KO) mouse hearts. A: Western blots showing relative expression of proteins in Nebl+/+ and Neblex3-/ex3- mouse hearts (n = 3). GAPDH was used as a reference protein for each protein gel. Graphs on the right indicate rations of proteins/reference. Nebl+/+ sedentary group was used as an expression reference for other groups. *P < 0.05, significant difference between groups. AU, arbitrary units. B: immunohistochemistry and confocal microscopy of mouse hearts after exercise. In Nebl+/+ mice, arrows indicate normal expression of nebulette (NEBL; green) and desmin (DES; red) localized at the Z-disks. Yellow spots indicate overlapped NEBL and DES in merged images. Fourth column: focuses on the details of the outlined squares. In Neblex3-/ex3- hearts, NEBL is scarcely expressed in sarcomeres, while DES is disorganized and diffusely expressed in the sarcoplasm (asterisks). Bar = 10 μm.
Next, we analyzed localization of the proteins using immunohistochemistry in exercised mice. Coimmunofluorescent staining of NEBL and DES demonstrated that Nebl+/+ hearts expressed NEBL at the Z-disks overlapping with a well-developed grid-like DES arrangement (Fig. 5B, arrows). In contrast, Neblex3-/ex3- mice had greatly diminished and scattered NEBL expression, presumably due to splice expression of Nebl. Arrangement of DES was greatly disturbed and disorganized (asterisks) as well. Furthermore, disarranged DES had lesser overlap with VCL (Fig. 6A, asterisks) at the fewer and smaller focal adhesions seen in the Neblex3-/ex3- myocardium. In contrast, Nebl+/+ hearts had abundant VCL expression forming yellow spots at the focal adhesions, where each spot overlapped with grid-like DES expression (Fig. 6A, double-headed arrows). Actin expression was largely unaffected in exercised mice (Supplemental Fig. S4A). In addition, disruption of α-actinin2, a Z-disk crosslinking protein, in Neblex3-/ex3- cardiomyocytes was seen. Further, disruption of DSP (binding partner of DES) was notable in IDs of Neblex3-/ex3- compared with Nebl+/+ mice (Supplemental Fig. S4A), while JUP and β-catenin location was unaffected (data not shown). Collectively, our results suggest important roles of NEBL’s NRs1-3 in stabilizing the sarcomere, desmin filaments, the Z-disk, IDs, as well as focal adhesion structure during biomechanical stress.
Figure 6.
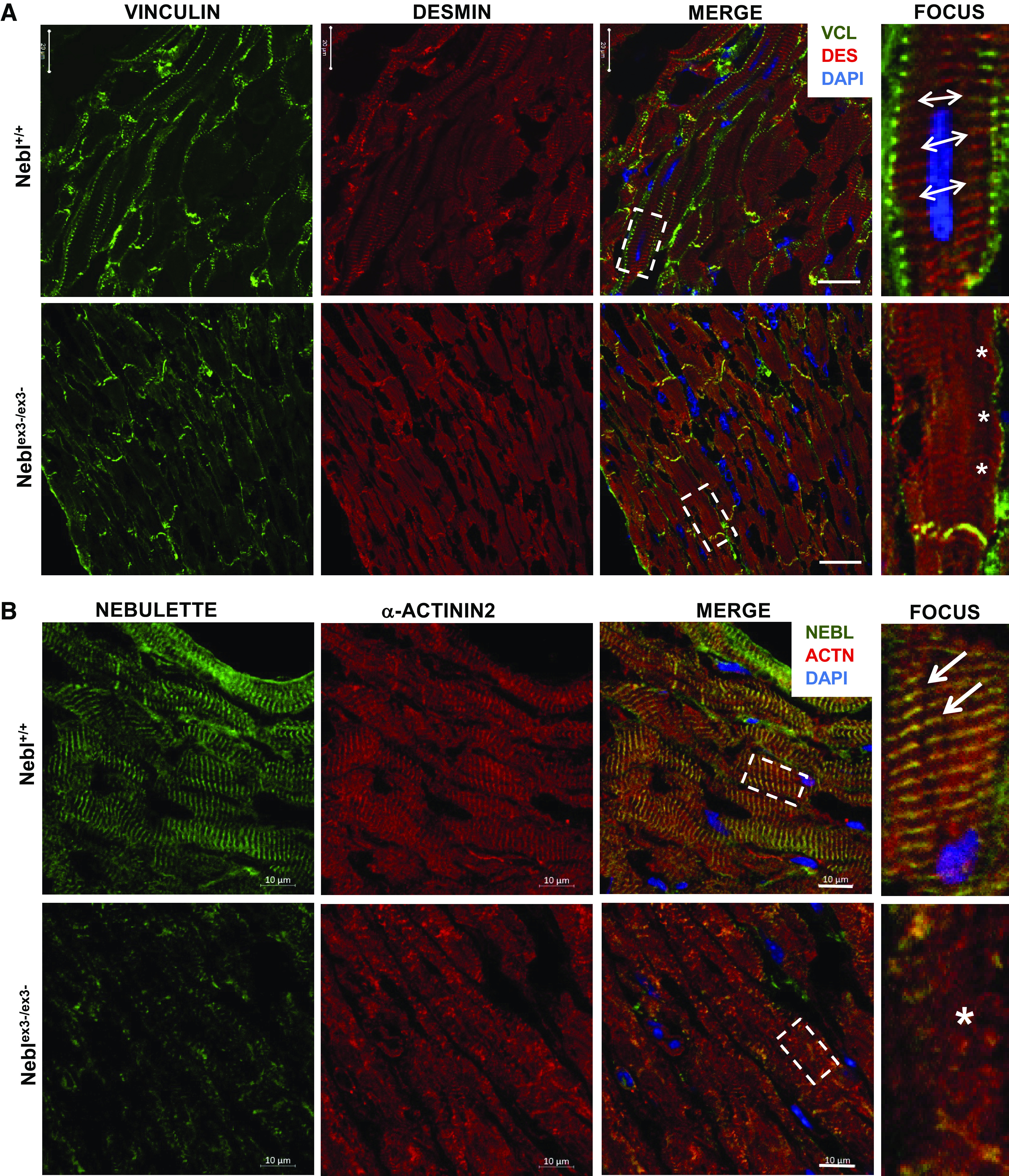
Coimmunohistochemistry and confocal microscopy of Neblex3-knockout (KO) hearts after exercise. A: vinculin (VCL; green) and DES (red) coexpression. In Nebl+/+ mice, double-headed arrows indicate well-organized DES and VCL at focal adhesions adjacent to Z-disks (4th column). In Neblex3-/ex3- hearts, diminished VCL (asterisks) is seen at fewer focal adhesions. Bar = 20 μm. B: nebulette (NEBL; green) and α-actinin2 (red) coexpression. In Nebl+/+ mice, arrows indicate α-actinin2 and NEBL overlap (yellow) in well-organized Z-disks (4th column). In Neblex3-/ex3- heart, disruption of α-actinin2 and NEBL is indicated by the asterisk. Bar = 10 μm.
Temporal Recruitment of NEBL to Focal Adhesions is Essential in Mechanical Strain Response
We hypothesized that NEBL plays important roles in maintaining structural resilience of cardiomyocytes by instantly translocating to the focal adhesions during biomechanical stress conditions and thereby recruiting crucial adhesion molecules. To test our hypothesis, we transfected H9C2 cells with NEBL-GFP, cultured them on fibronectin-coated stretchable silicone membranes, and observed intracellular translocation of GFP with no stretch (static conditions) or under 10% Eulerian strain at a frequency of 1-Hz stretch at 24-h (Fig. 7A) and 72-h (Fig. 7B) time points using immunofluorescence. At 24-h static conditions, NEBL-GFP was expressed in the perinuclear cytosol of H9C2 cells (Fig. 7, Aa–Ac). When cells were stretched, NEBL was strongly recruited to focal adhesions (Fig. 7, Ad–Af, arrows), while its crosslinks with the actin cytoskeleton were clearly visible (arrowhead). After 72 h without strain, NEBL remained in perinuclear regions in the static cells (Fig. 7, Bh–Bj). In cells under strain, NEBL was diffusely and uniformly distributed along the F-actin filaments across the cardiomyocyte including the focal adhesions (Fig. 7, Bk–Bm). These results further suggest that, while NEBL may be redundant during baseline conditions, it is functionally critical in stretch or biomechanical stress.
Figure 7.
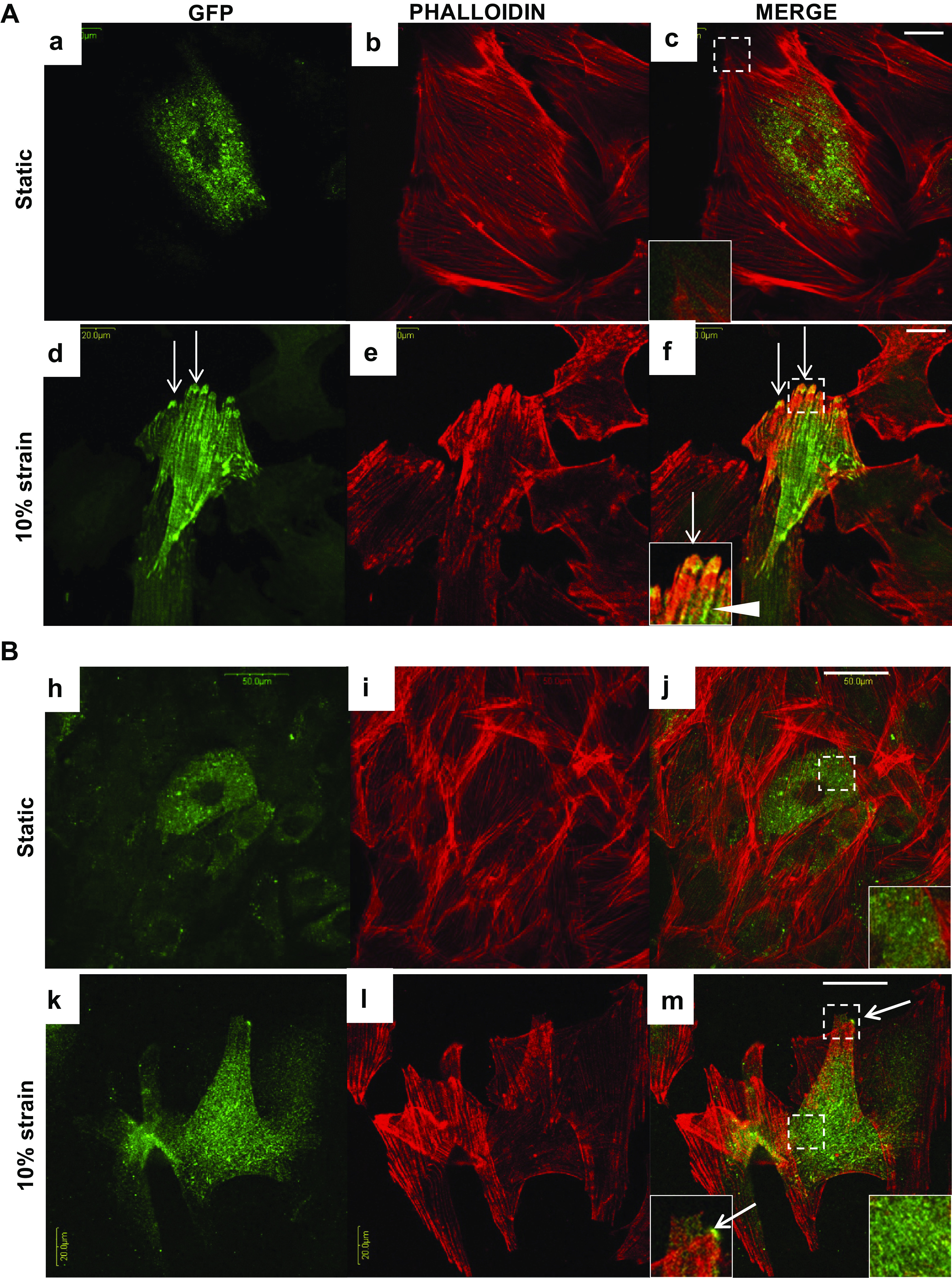
Analysis of nebulette (NEBL)-green fluorescent protein (GFP) destribution in H9C2 cells at 24-h and 72-h under cyclic strain. Representative images of coimmunocytochemical analysis of GFP (green) and phalloidin (red) in H9C2 cells at 24-h (Aa–Ac and Bh–Bj, bar = 20 μm) and at 72-h (Ad–Af and Bk–Bm, bar = 50 μm) at the static or under strain conditions. Bottom left insets in the merged images represent focused detals of the squares outlining NEBL-GFP (arrows) at the focal adhesions, and bottom right insets represent NEBL-GFP expression in the cytosol.
NEBL is Critical for Organization of Desmin Intermediate Filaments under Cyclic Stretch
Finally, we tested the hypothesis whether NEBL maintains actin and desmin filament organization during mechanical strain and whether the mutations G202R and A592E diminish NEBL’s scaffolding functions. As shown in Fig. 8, desmin and actin filaments were well organized and seen overlapped in control uninfected or expressing NEBLWT NRCs (arrows). In mutant NEBLG202R or NEBLA592E cells, actin was still visible in a striation-like pattern, while DES was more disarrayed (asterisks) and less organized and significantly less overlapped with actin filaments compared with that in control or NEBLWT cells (Supplemental Fig. S4B). In addition, mutant cells had reduced sarcomere structures, but more “stress fibers” were visible (arrowheads) compared with NEBLWT cells, further suggesting an essential role of NEBL in maintaining sarcomere structural resilience during stress conditions.
Figure 8.
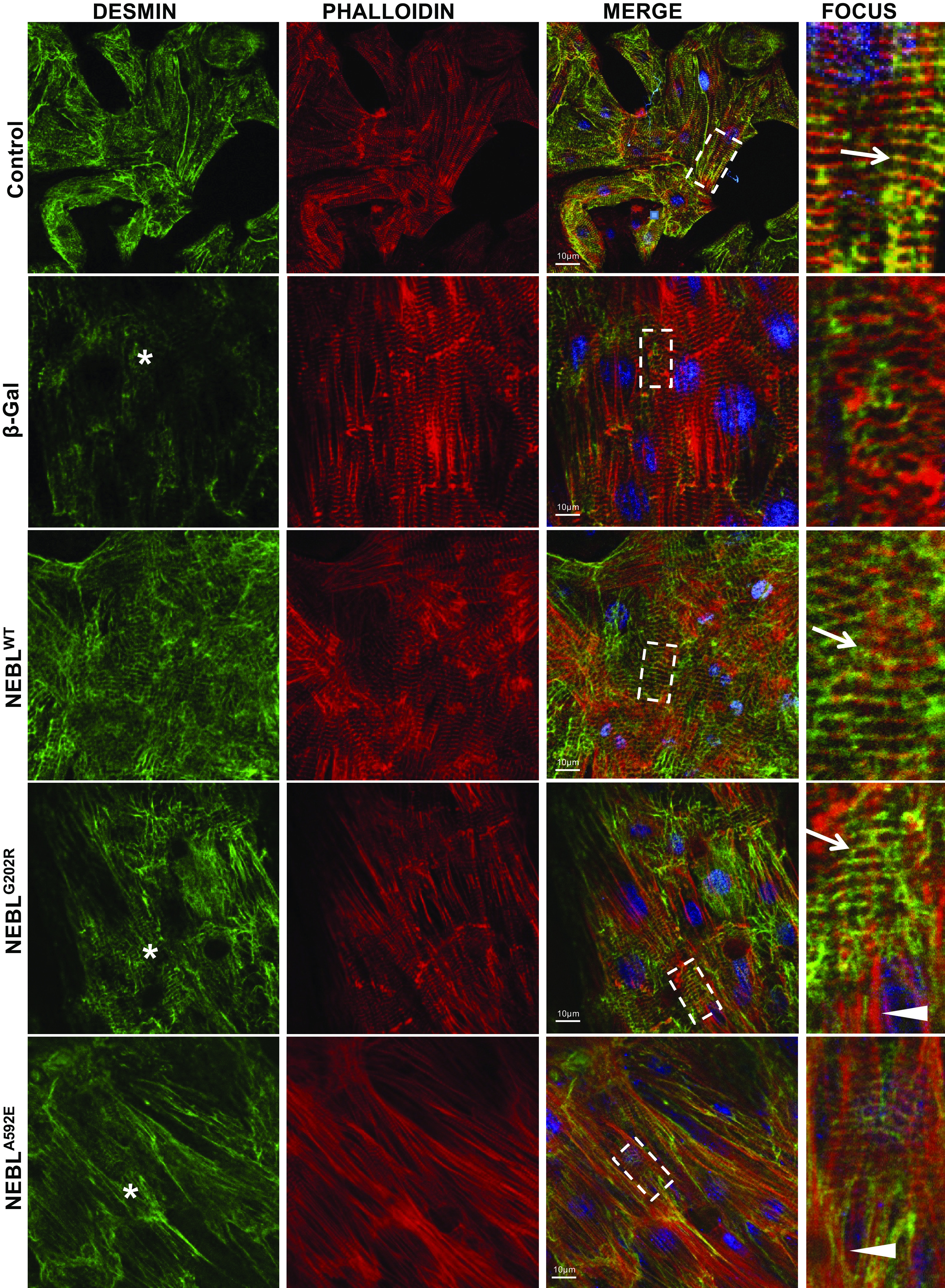
Immunohistochemical analysis of desmin and actin structure in neonatal rat cardiomyocytes (NRCs) expressing wild-type (WT) or mutant nebulette (NEBL) under strain. Representative images of coimmunocytochemical analysis of desmin (green) and actin (phalloidin, red) in NRCs, uninfected (control), expressing β-gal, human NEBLWT, mutant NEBLG202R, or NEBLA592E delivered using adenoviral vectors under strain conditions. Scale bar = 10 μm. Fourth column: details of the square outlined in the merged images. Arrows indicate striation-like overlap of desmin and actin (yellow), and asterisks indicate disrupted desmin.
DISCUSSION
While it is well known that mechanical overload or genetic defects in the proteins enabling acto-myosin interactions alter force generation and contractility in the heart and lead to systolic dysfunction, a significant lack in understanding of the roles for actin-binding proteins in a resilient adaptive diastolic response against biomechanical stress persists. Sarcomeric NEBL has been shown to be one such protein in the actin-binding machinery that senses and dynamically responds to mechanical and pathological stimuli in the heart (8, 9). Besides the fact that NEBL mutations located in the NR domain cause domain-specific disturbances in NEBL function resulting in heterogeneous cardiomyopathy phenotypes (5, 6), the mechanisms by which NEBL contributes to the adaptive response to biomechanical strain continues to be completely unexplored. Recently, Hernandez et al (17) identified NEBL as a powerful “cytolinker” that organizes and stabilizes desmin to actin filaments suggesting that NRs1-5 via which NEBL interacts with desmin may carry on important functions. This cross-linking stabilization role had recently been demonstrated in another isoform of NEBL, namely LIM-nebulette, whereby silencing of Nebl in kidney epithelia has been shown to result in reduced cytoskeletal stability among actin fibers and intermediate filaments as measured by atomic force microscopy (30). Thus we hypothesized that NR domain of NEBL is important in cardiac biomechanical physiology and ablation of NRs of NEBL may reflect the functional significance of NEBL. To test, we created Neblex3-/ex3- knockout mice with ablation of exon3 of Nebl that resulted in ablation of first three NRs and applied acute and 4-wk strenuous treadmill exercise in mice to explore the roles of sarcomeric NEBL in the physiology and molecular signaling of the heart against increased biomechanical workload. Previously reported exon1 Nebl knockouts had a total ablation of sarcomeric 107-kDa NEBL in the heart. Although ablation of targeted exon3 resulted in ablation of exons3-6, we detected a splice transcription of exons7 and 28–32, which resulted in ablation of NRs1-3 and partial NEBL deficiency in the heart of our knockouts. Nonetheless, Neblex3-/ex3- animals showed primary diastolic dysfunction with preserved systolic function by the age of 24 wk after birth while sedentary, similar to the phenotype reported previously in mutant NEBLG202R and NEBLA592E transgenic mice (21). No major baseline abnormalities were seen in Neblex3-/ex3- cardiomyocytes aside from isolated focal IDs widening and mitochondrial damage while sedentary.
A large cross-sectional study has shown that resting diastolic dysfunction is the strongest echocardiographic predictor of exercise intolerance and the magnitude of the decrease in exercise capacity increases with age independent of sex (31). When an exercise regimen was applied in Neblex3- knockouts, we analyzed for stress tolerance, structural destabilization of actin and desmin filaments, sarcomere, focal adhesions and desmosomes, and finally modulation of downstream signaling in response to mechanical stress. In contrast to WT mice, exercise in Neblex3-/ex3- mice induced development of LA enlargement (sign of diastolic dysfunction) and thickening of the IVS, increase in LV mass, and dilation of the LV chamber (signs of eccentric hypertrophy). These phenotypic disparities observed in exercised Neblex3-/ex3- mice were uncoupled with increased levels of NR1-3 null NEBL, underscoring the dominant negative effect of NRs1-3 ablation in protecting the heart against biomechanical stress.
Interestingly, exercise-induced diastolic dysfunction and hypertrophy was found despite absence of cardiac fibrosis and minimal tissue deformation assessed by DTI in exercised Neblex3-/ex3- mice. This phenomenon has been also noted in NEBL-Tg mice (6). Similar to Neblex3-/ex3- mice, pediatric patients with hypertrophic cardiomyopathy with diastolic dysfunction consistent with restrictive physiology are at highest risk of developing progressive IVS hypertrophy and poor clinical outcomes associated with LA enlargement (32). Adult patients with primary diastolic dysfunction with preserved systolic function have shown a deficiency in augmenting LV filling rates without an abnormal increase in LA pressure and treatment with angiotensin II receptor blockers or verapamil may improve exercise tolerance in some patients with primary diastolic dysfunction (27). Ogut et al. (33) demonstrated that NRs significantly increase the assembly of the TM-TnT complex to F-actin maintaining the biomechanical efficiency of contractile myofilaments. Thus we suggest that altered expression of TM1 and TM1/TnT ratios, the essential regulators in actin-myosin interactions, may underlie a diastolic dysfunction, eccentric hypertrophy, and intolerance of Neblex3-/ex3- hearts against biomechanical stress. Supporting this, NEBLG202R transgenic mice demonstrated occult diastolic dysfunction in a preclinical stage of DCM due to enhanced cTnI phosphorylation and increased calcium decay rate (21). Mutations in TM1 are associated with different types of cardiomyopathies (dilated or hypertrophic), the mechanisms of which have been supported by dose-dependent and opposing effects of TM1 on calcium sensitivity of myofilaments (34). Therefore, our future studies will be directed toward assessing calcium transients and contractility in Neblex3-/ex3- hearts in response to exercise and biomechanical stress.
On a subcellular level, ablation of NRs1-3 strongly correlated with diffuse structural sarcomere disturbance, ID disruption, and mitochondrial distortion upon stress, suggesting that NEBL is important during increased biomechanical stress. This is also in agreement with prior observations of decreased cellular elastic modulus in nebulette silenced cells (30). On a transcription level, we found that Mir23a is one of the cardiac genes differentially expressed in Nebl+/+ and Neblex3-/ex3- mice under sedentary and stressed conditions, suggesting that future studies to elucidate the role(s) of Mir23a in exercise physiology should be pursued. Using a systems genetics approach, we constructed a Nebl-based exercise stress network which included Casq2 (calcium binding), Csrp3 (Z-disk mechanosensor), Capzb (Z-disk actin cap), Des (intermediate filament), Itgb1bp2 (focal adhesions), Jup and Jph1 (desmosome and adherens junction), Myh7 and Myh6 (thick filament), Scn8a (sodium channel), and Fhl2 and Bag3 (scaffolding chaperone) genes (21).
It has been previously reported that the main role of NEBL is for anchoring actin filaments to the Z-disks (13). NEBL-actin interactions increase cardiomyocyte tolerance against mechanical stress and the biomechanical efficiency of contractile myofilaments. This present study defined that NEBL is not only expressed at the Z-disks along the F-actin filaments, it also time dependently translocates to the focal adhesions, and that expression of vinculin to the focal adhesions is greatly disrupted in cardiomyocytes of Neblex3-/ex3- deficient mouse under biomechanical stress. Ablation of NRs1-3 of NEBL was sufficient to destabilize desmin filaments and disrupt DSP expression at the desmosomes, resulting in rupture of cell-cell connections. We have also shown that NRs1-3 ablation is detrimental for maintaining sarcomere structure by disrupting α-actinin2 at the Z-disks that provides additional tolerance and flexibility against biomechanical stress. Since cardiomyocytes operate in a system of complex interactions and this environment is mechanically compromised in inherited cardiomyopathy, we also tested whether mutations in NEBL alter actin and desmin expression and found that G202R and A592 mutations cause disorganization of desmin intermediate filaments in response to cyclic strain.
In summary, our study has identified the dominant negative mechanisms through which sarcomeric Z-disks, desmin intermediate filaments, focal adhesions, and IDs, as well as mitochondrial structure and function, are lost in vivo during biomechanical stress when NRs1-3 of murine NEBL was ablated. We conclude that scaffolding and signaling functions of NEBL’s NRs1-3 are necessary for preserving the tolerance and resilience of the sarcomere and maintaining structural integrity of cardiomyocytes under biomechanical stress conditions. Therefore, mechanistic studies elucidating roles of NRs1-3 in myocardial contraction and relaxation, calcium handling, and mitochondrial function during biomechanical stress should to be pursued. These studies may have a major translational impact on understanding of actin-binding machinery function during early cardiac disease pathogenesis.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-HL-53392 and R01-HL-087000 (to J.A.T.), R01-HL-128350 (to L.L.), and R01-DK-118222 (to E.U.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS CONTRIBUTIONS
E.P. conceived and designed research; R.M.V., B.-O.O., N.R.A., N.L., U.M., Z.K., R.M., and E.P. performed experiments; R.M.V., B.-O.O., N.R.A., N.L., U.M., F.X., L.L., and E.P. analyzed data; B.O.-O, L.L., J.A.T., F.X., and E.P. interpreted results of experiments; R.M.V., B.-O.O., N.L., U.M., Z.K., F. X., and E.P. prepared figures; R.M.V. and Z.K. drafted manuscript; B.O.-O, N.L., E.U.A., L.L., J.A.T., and E.P. edited and revised manuscript; R.M.V., B.O.-O., N.R.A., N.L., U.M., Z.K., R.M., E.U.A., F.X., L.L., and E.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge S. Labeit (Department of Integrative Pathophysiology, University of Heidelberg) for providing an antibody against myopalladin and Dr. N. Gong (Cincinnati Children’s Hospital Medical Center) for technical assistance.
REFERENCES
- 1.Towbin JA. Inherited cardiomyopathies. Circ J 78: 2347–2356, 2014. doi: 10.1253/circj.CJ-14-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman JM, Burr JF, Banks L, Thomas SG. The acute risks of exercise in apparently healthy adults and relevance for prevention of cardiovascular events. Can J Cardiol 32: 523–532, 2016. doi: 10.1016/j.cjca.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Chaitman BR, Ackerman MJ, Bayes de Luna A, Corrado D, Crosson JE, Deal BJ, Driscoll DJ, Estes NA 3rd, Araujo CG, Liang DH, Mitten MJ, Myerburg RJ, Pelliccia A, Thompson PD, Towbin JA, Van Camp SP, Working Groups of the American Heart Association Committee on Exercise Cardiace Rehabilitation, Prevention; Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 109: 2807–2816, 2004. doi: 10.1161/01.CIR.0000128363.85581.E1. [DOI] [PubMed] [Google Scholar]
- 4.Arimura T, Nakamura T, Hiroi S, Satoh M, Takahashi M, Ohbuchi N, Ueda K, Nouchi T, Yamaguchi N, Akai J, Matsumori A, Sasayama S, Kimura A. Characterization of the human nebulette gene: a polymorphism in an actin-binding motif is associated with nonfamilial idiopathic dilated cardiomyopathy. Hum Genet 107: 440–451, 2000. doi: 10.1007/s004390000389. [DOI] [PubMed] [Google Scholar]
- 5.Perrot A, Tomasov P, Villard E, Faludi R, Melacini P, Lossie J, Lohmann N, Richard P, De Bortoli M, Angelini A, Varga-Szemes A, Sperling SR, Simor T, Veselka J, Ozcelik C, Charron P. Mutations in NEBL encoding the cardiac Z-disk protein nebulette are associated with various cardiomyopathies. Arch Med Sci 12: 263–278, 2016. doi: 10.5114/aoms.2016.59250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purevjav E, Varela J, Morgado M, Kearney DL, Li H, Taylor MD, Arimura T, Moncman CL, McKenna W, Murphy RT, Labeit S, Vatta M, Bowles NE, Kimura A, Boriek AM, Towbin JA. Nebulette mutations are associated with dilated cardiomyopathy and endocardial fibroelastosis. J Am Coll Cardiol 56: 1493–1502, 2010. doi: 10.1016/j.jacc.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang ML, Chen J. Roles of nebulin family members in the heart. Circ J 79: 2081–2087, 2015. doi: 10.1253/circj.CJ-15-0854. [DOI] [PubMed] [Google Scholar]
- 8.Pappas CT, Bliss KT, Zieseniss A, Gregorio CC. The Nebulin family: an actin support group. Trends Cell Biol 21: 29–37, 2011. doi: 10.1016/j.tcb.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Millevoi S, Trombitas K, Kolmerer B, Kostin S, Schaper J, Pelin K, Granzier H, Labeit S. Characterization of nebulette and nebulin and emerging concepts of their roles for vertebrate Z-discs. J Mol Biol 282: 111–123, 1998. doi: 10.1006/jmbi.1998.1999. [DOI] [PubMed] [Google Scholar]
- 10.Moncman CL, Wang K. Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil Cytoskeleton 32: 205–225, 1995. doi: 10.1002/cm.970320305. [DOI] [PubMed] [Google Scholar]
- 11.Holmes WB, Moncman CL. Nebulette interacts with filamin C. Cell Motil Cytoskeleton 65: 130–142, 2008. doi: 10.1002/cm.20249. [DOI] [PubMed] [Google Scholar]
- 12.McElhinny AS, Kolmerer B, Fowler VM, Labeit S, Gregorio CC. The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J Biol Chem 276: 583–592, 2001. doi: 10.1074/jbc.M005693200. [DOI] [PubMed] [Google Scholar]
- 13.Moncman CL, Wang K. Functional dissection of nebulette demonstrates actin binding of nebulin-like repeats and Z-line targeting of SH3 and linker domains. Cell Motil Cytoskeleton 44: 1–22, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 14.Moncman CL, Wang K. Targeted disruption of nebulette protein expression alters cardiac myofibril assembly and function. Exp Cell Res 273: 204–218, 2002. doi: 10.1006/excr.2001.5423. [DOI] [PubMed] [Google Scholar]
- 15.Politou AS, Millevoi S, Gautel M, Kolmerer B, Pastore AS. in muscles: solution structure of the SH3 domain from nebulin. J Mol Biol 276: 189–202, 1998. doi: 10.1006/jmbi.1997.1521. [DOI] [PubMed] [Google Scholar]
- 16.Esham M, Bryan K, Milnes J, Holmes WB, Moncman CL. Expression of nebulette during early cardiac development. Cell Motil Cytoskeleton 64: 258–273, 2007. doi: 10.1002/cm.20180. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez DA, Bennett CM, Dunina-Barkovskaya L, Wedig T, Capetanaki Y, Herrmann H, Conover GM. Nebulette is a powerful cytolinker organizing desmin and actin in mouse hearts. Mol Biol Cell 27: 3869–3882, 2016. doi: 10.1091/mbc.E16-04-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol 153: 413–427, 2001. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Zhuang L, Trueb B. Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J Biol Chem 279: 20401–20410, 2004. doi: 10.1074/jbc.M310304200. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Neilson LJ, Zhong H, Murray PS, Zanivan S, Zaidel-Bar R. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci Signal 7: rs7, 2014. doi: 10.1126/scisignal.2005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiellaro-Rafferty K, Wansapura JP, Mendsaikhan U, Osinska H, James JF, Taylor MD, Robbins J, Kranias EG, Towbin JA, Purevjav E. Altered regional cardiac wall mechanics are associated with differential cardiomyocyte calcium handling due to nebulette mutations in preclinical inherited dilated cardiomyopathy. J Mol Cell Cardiol 60: 151–160, 2013. doi: 10.1016/j.yjmcc.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastrototaro G, Liang X, Li X, Carullo P, Piroddi N, Tesi C, Gu Y, Dalton ND, Peterson KL, Poggesi C, Sheikh F, Chen J, Bang ML. Nebulette knockout mice have normal cardiac function, but show Z-line widening and up-regulation of cardiac stress markers. Cardiovasc Res 107: 216–225, 2015. doi: 10.1093/cvr/cvv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13: 476–484, 2003. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, Hsu HC, Mountz JD, Baldwin NE, Langston MA, Threadgill DW, Manly KF, Williams RW. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet 37: 233–242, 2005. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- 25.Gu Q, Mendsaikhan U, Khuchua Z, Jones BC, Lu L, Towbin JA, Xu B, Purevjav E. Dissection of Z-disc myopalladin gene network involved in the development of restrictive cardiomyopathy using system genetics approach. World J Cardiol 9: 320–331, 2017. doi: 10.4330/wjc.v9.i4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirono K, Saito K, Munkhsaikhan U, Xu F, Wang C, Lu L, Ichida F, Towbin JA, Purevjav E. Familial left ventricular non-compaction is associated with a rare p.v407i variant in bone morphogenetic protein 10. Circ J 83: 1737–1746, 2019. doi: 10.1253/circj.CJ-19-0116. [DOI] [PubMed] [Google Scholar]
- 27.Little WC, Kitzman DW, Cheng CP. Diastolic dysfunction as a cause of exercise intolerance. Heart Fail Rev 5: 301–306, 2000. doi: 10.1023/A:1026503028065. [DOI] [PubMed] [Google Scholar]
- 28.Martherus R, Jain R, Takagi K, Mendsaikhan U, Turdi S, Osinska H, James JF, Kramer K, Purevjav E, Towbin JA. Accelerated cardiac remodeling in desmoplakin transgenic mice in response to endurance exercise is associated with perturbed Wnt/beta-catenin signaling. Am J Physiol Heart Circ Physiol 310: H174–H187, 2016. doi: 10.1152/ajpheart.00295.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 103: 18255–18260, 2006. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge X, Zhang T, Yu X, Muwonge AN, Anandakrishnan N, Wong NJ, Haydak JC, Reid JM, Fu J, Wong JS, Bhattacharya S, Cuttitta CM, Zhong F, Gordon RE, Salem F, Janssen W, Hone JC, Zhang A, Li H, He JC, Gusella GL, Campbell KN, Azeloglu EU. LIM-nebulette reinforces podocyte structural integrity by linking actin and vimentin filaments. J Am Soc Nephrol 31: 2372–2391, 2020. doi: 10.1681/asn.2019121261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA 301: 286–294, 2009. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maskatia SA, Decker JA, Spinner JA, Kim JJ, Price JF, Jefferies JL, Dreyer WJ, Smith EO, Rossano JW, Denfield SW. Restrictive physiology is associated with poor outcomes in children with hypertrophic cardiomyopathy. Pediatr Cardiol 33: 141–149, 2012. doi: 10.1007/s00246-011-0106-6. [DOI] [PubMed] [Google Scholar]
- 33.Ogut O, Hossain MM, Jin JP. Interactions between nebulin-like motifs and thin filament regulatory proteins. J Biol Chem 278: 3089–3097, 2003. doi: 10.1074/jbc.M205853200. [DOI] [PubMed] [Google Scholar]
- 34.Dorsch LM, Kuster DW, Jongbloed JD, Boven LG, van Spaendonck-Zwarts KY, Suurmeijer AJ, Vink A, Du Marchie Sarvaas GJ, van den Berg MP, van der Velden J, Brundel B, van der Zwaag PA. The effect of tropomyosin variants on cardiomyocyte function and structure that underlie different clinical cardiomyopathy phenotypes. Int J Cardiol 323: 251–258, 2021. doi: 10.1016/j.ijcard.2020.08.101. [DOI] [PubMed] [Google Scholar]