Abstract
African American (AA) individuals are at a greater risk for the development of cardiovascular complications, such as hypertension, compared with European Americans (EAs). Higher vagally mediated heart rate variability (HRV) is typically associated with lower blood pressure (BP) and total peripheral resistance (TPR). However, research has yet to examine the differential impact of HRV on longitudinal hemodynamic activity between AAs and EAs. We sought to rectify this in a sample of 385 normotensive youths (207 AAs, 178 EAs; mean age 23.16 ± 2.9 yr). Individuals participated in two laboratory evaluations spanning approximately 6 yr. Bioimpedance was used to assess HRV at time 1 and cardiac output at both time 1 and time 2. Mean arterial pressure (MAP) was measured at both time points via an automated BP machine. TPR was calculated as MAP divided by cardiac output. Results showed AAs to have higher BP and higher TPR at time 2 compared with EAs, independent of several important covariates. Also, higher HRV at time 1 significantly predicted both lower TPR and BP at time 2 among EAs only; these associations were attenuated and not significant in AAs. HRV did not significantly predict cardiac output at time 2 in the full sample or split by ethnicity. Our findings highlight that AAs show TPR mediated long-term increases in BP irrespective of resting HRV, providing a physiological pathway linking AAs with a greater risk for mortality and morbidity from hypertension and potentially other cardiovascular disease.
NEW & NEWSWORTHY African Americans and European Americans differ in hemodynamics underlying long-term blood pressure regulation. Over 6 yr, African Americans show total peripheral resistance-mediated increases in blood pressure compared with European Americans. Higher heart rate variability predicts lower blood pressure and total peripheral resistance 6 yr later in European Americans but not African Americans.
Keywords: blood pressure, ethnicity, health disparities, heart rate variability, total peripheral resistance
INTRODUCTION
African American (AA) individuals are at a greater risk for the development of cardiovascular complications, such as hypertension, compared with European Americans (EAs) (1). Elevated blood pressure (BP) is a risk factor for increased cardiovascular morbidity and mortality. Compared with EAs, AAs typically show higher resting blood pressure (2) marked by higher total peripheral resistance (TPR) (3). TPR-mediated increases in BP are particularly deleterious, as elevated TPR is associated with end-organ damage. However paradoxically, AAs also show higher resting vagally mediated heart rate variability (hereafter simply called HRV) (4). Given the cardioprotective nature of higher HRV (5), this paradoxical pattern of increased resting α-adrenergic sympathetic and parasympathetic activity in AAs has been termed the “cardiovascular conundrum” (3, 4). Interestingly, studies have shown that greater doses of acetylcholine are needed for AAs to produce the same degree of vasodilation as in EAs (6, 7). Another study showed AAs to have increased vascular α-adrenergic responsivity and suggest increased TPR is more easily achieved in AAs compared with EAs (8). Finally, pregnancy is a potent vasodilator; we recently showed pregnant AAs had greater TPR compared with pregnant EAs (9). These findings serve as direct evidence for impaired vasodilation in AAs relative to EAs.
There is growing interest in the role of the vagus nerve in cardiovascular disease (10, 11). Heart rate variability is a widely accepted method to assess autonomic activity with high-frequency HRV used to index vagally mediated HRV. We have recently shown that HRV was prospectively associated with both systolic BP and diastolic BP (12). It has also been reported that HRV is positively associated with endothelial function as indexed by brachial artery flow-mediated dilation in humans (13). The role of the vagus nerve in regulating heart rate (HR) and cardiac contractility (e.g., stroke volume), and thus cardiac output, has been extensively documented (14). Whereas the vagal innervation of the heart is well established, there is no evidence of direct vagal innervation of the vessels. Thus, the mechanism by which vagal activity might regulate BP and endothelial function and how this might differ between EAs and AAs is not established.
Therefore, research aimed at understanding the differential impact of vagal activity on longitudinal hemodynamic activity between AAs and EAs is warranted. In the present study, we sought to understand how vagal activity, as indexed by HRV, may predict hemodynamics underlying “long-term” blood pressure regulation differentially as a function of ethnicity. Mean arterial pressure (MAP) is determined by the product of cardiac output (CO) and TPR (MAP = CO × TPR). Therefore, although MAP typically increases with age, the hemodynamics (TPR or CO) underlying higher MAP may differ between AAs and EAs. Using secondary analysis of archival data from an empirical prospective study, we investigated hemodynamic patterns over the course of approximately 6 yr, with physiological measures taken at two time points: 1) baseline/initial visit (time 1); and 2) follow-up visit on an average of 6 yr later (time 2).
To our knowledge, this will be the first study to investigate if HRV may predict long-term hemodynamic activity differentially based on ethnicity. We aim to highlight how the impact of vagal activity on long-term blood pressure regulation may differ between AAs and EAs. Such results would have important implications for the course of elevated blood pressure in AA individuals.
METHOD
Participants and Experimental Design
In this study, 385 normotensive youths were enrolled in the Augusta Heart Study (15–17). Participants were initially between the ages of 15–32 yr old (mean age 23.16 ± 2.9 yr). There were 207 AAs (127 women) and 178 EAs (83 women). Individuals were participants in longitudinal studies of the development of cardiovascular risk factors in children with verified family histories of cardiovascular diseases (i.e., essential hypertension and/or premature myocardial infarction) (18–19). All individuals participated in two separate laboratory evaluations separated by an average of 6.32 yr (minimum of 2.1 yr, maximum of 8.2 yr). Smoking, medication histories, parents’ education levels, and physical activity were obtained via participant interview.
Subject’s height (in centimeters) and weight (in kilograms) were measured without shoes with a Health-O-Meter medical scale, which was calibrated daily. Body mass index (BMI) was calculated as weight/height2. The study was approved by the institutional review committee, and informed consent was obtained from all participants on each study visit. All subjects were escorted to a quiet temperature-controlled room (20°C–22°C) and fitted with equipment for recording BP (Dinamap model 1846 SX, Critikon Inc.) as previously described (18–19). Blood pressure was assessed via the same arm for each participant both at baseline and the follow-up. The subject was placed in a supine position and given standardized instructions to relax as completely as possible for 15 min. Three hemodynamic measurements (at min 11, 13, and 15) throughout the 15-min baseline period were obtained and the final two measurements (at min 13 and 15) averaged to provide one reading of blood pressure per evaluation to derive mean arterial pressure (MAP; mmHG).
Heart rate (HR), cardiac output (CO; l/min), and stroke volume (SV; ml) were recorded using a BioZ impedance monitor (Cardio-Dynamics, San Diego, CA) as previously described (18–19). The raw RR interval data were first screened using the following two criteria: 1) RR intervals between 300 and 2,000 ms and 2) successive RR-interval ratios between 0.8 and 1.2. Kubios; HRV analysis software was used to generate HRV parameters from the recorded RR intervals (20). For the present study, we employed a frequency domain measure, high-frequency (HF) power (defined as the power between 0.15 and 0.40 Hz, using Fast Fourier Transformation), for all analyses1. HF-HRV is a valid measure of predominantly parasympathetic (i.e., vagal) cardiac influence (21). To account for skewness, HRV was transformed using a natural logarithm. HRV measures were available at time 1 only; hemodynamic measures assessed via bio-impedance were available for both time points. Total peripheral resistance (TPR) was calculated as MAP divided by CO. Cardiac index (CI) and total peripheral index (TPI) we calculated by dividing CO and TPR by body surface area, respectively. Body surface area was calculated using the Du Bois and Du Bois (21a) calculation: body surface area = 0.007184 × weight0.425 × height0.725. CI and TPI were used as the primary measures of CO and TPR, respectively.
Statistical Analyses
Independent sample t tests were used to determine group differences in demographic and cardiovascular variables at time 1 (T1) and time 2 (T2) as a function of ethnicity independently. In addition, independent sample t tests were used to test the change in MAP, TPI, and CI between T1 and T2. To examine our primary question, a series of hierarchical regression models were conducted to examine whether HRV predicted MAP, CO, and TPR from T1 to T2 both in the whole sample and stratified by ethnicity. Age, sex, ethnicity, BMI, father’s education (proxy measure for socioeconomic status for our relatively young sample), and smoking were entered in model 1. For respective models, T1 MAP, CO, and TPR were added in the following step (model 2), followed by a final step including T1 HRV (model 3). Dependent measures at T2 included MAP, CO, and TPR. All analyses were conducted using SPSS (v. 20, IBM Armonk, NY); significance was set at P < 0.05. Unstandardized βs (b) and standard errors (in brackets), partial r coefficients, and P values are provided in the text for primarily associations. Standardized βs (b), t values, P values, zero-order correlations, and partial r correlations for all variables in all regression models are presented in Tables 2–4.
RESULTS
Means and standard deviations for the full sample, and stratified by ethnicity, are presented in Table 1. At both T1 and T2, AAs were significantly older by ∼6 mo compared with EAs (each P < 0.01) and had higher BMI (each P < 0.01), higher MAP (each P < 0.001), TPR (each P < 0.01), and TPI (each P = 0.001). AAs reported significantly lower father’s education (P < 0.001) at T1 compared with EAs. AAs also had significantly lower CO, CI, and SV at T2 (each P < 0.01). Finally, the increase in MAP and TPI from T1 to T2 was significantly greater in AAs compared with EAs (each P < 0.05). The increase in CI from T1 to T2 was significantly greater in EAs compared to AAs (P = 0.037).
Table 1.
Sample characteristics and ethnic comparisons
| Full Sample |
EAs (n = 178) |
AAs (n = 207) |
|||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | P | |
| Age, yr T1 | 23.16 | 2.85 | 22.66 | 2.88 | 23.58 | 2.77 | 0.001 |
| Age, yr T2 | 29.47 | 2.96 | 28.93 | 2.94 | 29.93 | 2.91 | 0.001 |
| Body mass index, kg/m2 T1 | 28.77 | 8.45 | 27.06 | 6.77 | 30.23 | 9.44 | <0.001 |
| Body mass index, kg/m2 T2 | 30.93 | 8.74 | 29.30 | 7.55 | 32.33 | 9.44 | 0.001 |
| Father’s education, yr) T1 | 13.45 | 2.37 | 14.05 | 2.44 | 12.93 | 2.18 | <0.001 |
| Smoking T1 | 0.35 | 0.48 | 0.38 | 0.49 | 0.32 | 0.47 | 0.195 |
| Physical activity T1 | 0.14 | 0.34 | 0.14 | 0.35 | 0.13 | 0.34 | 0.758 |
| HF-HRV T1 | 7.17 | 1.06 | 7.13 | 1.11 | 7.21 | 1.00 | 0.475 |
| MAP T1 | 81.63 | 8.40 | 78.95 | 7.26 | 83.94 | 8.64 | <0.001 |
| TPR T1 | 16.82 | 4.69 | 16.03 | 4.09 | 17.49 | 5.06 | 0.002 |
| TPI T1 | 32.16 | 8.06 | 30.35 | 6.65 | 33.73 | 8.83 | <0.001 |
| CO T1 | 5.20 | 1.36 | 5.24 | 1.33 | 5.16 | 1.39 | 0.596 |
| CI T1 | 2.67 | 0.58 | 2.71 | 0.55 | 2.63 | 0.61 | 0.184 |
| HR T1 | 64.64 | 9.40 | 63.90 | 9.06 | 65.27 | 9.66 | 0.154 |
| SV T1 | 80.97 | 20.07 | 82.31 | 18.97 | 79.82 | 20.95 | 0.226 |
| MAP T2 | 86.30 | 10.39 | 82.60 | 8.95 | 89.48 | 10.50 | <0.001 |
| TPR T2 | 16.25 | 4.13 | 14.79 | 3.32 | 17.49 | 4.34 | <0.001 |
| TPI T2 | 32.24 | 8.10 | 29.18 | 6.22 | 34.87 | 8.61 | <0.001 |
| CO T2 | 5.60 | 1.34 | 5.82 | 1.26 | 5.42 | 1.38 | 0.003 |
| CI T2 | 2.79 | 0.54 | 2.92 | 0.49 | 2.69 | 0.56 | <0.001 |
| HR T2 | 64.60 | 9.48 | 63.93 | 9.86 | 65.17 | 9.13 | 0.199 |
| SV T2 | 87.88 | 21.16 | 92.26 | 20.47 | 84.11 | 21.06 | <0.001 |
| MAPΔ | 4.66 | 8.88 | 3.65 | 8.83 | 5.54 | 8.85 | 0.024 |
| TPIΔ | 0.08 | 10.01 | −1.16 | 8.31 | 1.15 | 11.17 | 0.043 |
| CIΔ | 0.13 | 0.72 | 0.21 | 0.66 | 0.06 | 0.77 | 0.037 |
This table depicts sample characteristics for the full sample (n = 385) and stratified by ethnicity. Means and standard deviations are presented on variables at both time 1 (subscript denoted as T1) and time 2 (subscript denoted as T2). Significant differences between African Americans (AAs) and European Americans (EAs) were evaluated and corresponding P values are presented. CO, cardiac output; CI, cardiac index; HF-HRV, high frequency heart rate variability; HR, heart rate; MAP, mean arterial pressure; SV, stroke volume; TPR, total peripheral resistance; TPI, total peripheral index. Δ Change. Significant P values (P < 0.05) are bold.
Predicting Mean Arterial Pressure at Time 2
Regression results for MAP at T2 are presented in Table 2A for the full sample. Model 1 accounted for 19.1% of the variance in T2 MAP with age, sex, and ethnicity emerging as significant predictors (each P < 0.01). In model 2, T1 MAP significantly accounted for an additional 19.1% of the variance [B = 0.60 (0.06), rpartial = 0.486, P < 0.001]. In model 3, HRV emerged as a significant inverse predictor [B = −1.34 (0.40), rpartial = −0.170, P = 0.001], explaining an additional 1.8% of the variance in T2 MAP.
Table 2.
Hierarchical regression models predicting time 2 mean arterial pressure for full sample and stratified by ethnicity
| b | t | P | r | rpartial | b | t | P | r | rpartial | b | t | P | r | rpartial | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: Full sample | Model 1 (R2 = 0.191) | Model 2 (R2change = 0.191) | Model 3 (R2change = 0.018) | ||||||||||||
| Age T1 | 0.148 | 3.04 | 0.003 | 0.218 | 0.154 | 0.053 | 1.22 | 0.223 | 0.218 | 0.063 | 0.033 | 0.75 | 0.452 | 0.218 | 0.039 |
| Sex | −0.208 | −4.32 | <0.001 | −0.146 | −0.217 | −0.106 | −2.46 | 0.014 | −0.146 | −0.126 | −0.111 | −2.59 | 0.010 | −0.146 | −0.133 |
| Ethnicity | 0.315 | 6.47 | <0.001 | 0.330 | 0.316 | 0.168 | 3.76 | <0.001 | 0.330 | 0.190 | 0.181 | 4.08 | <0.001 | 0.330 | 0.206 |
| BMI T1 | 0.094 | 1.88 | 0.061 | 0.170 | 0.096 | 0.123 | 2.81 | 0.005 | 0.170 | 0.143 | 0.119 | 2.76 | 0.006 | 0.170 | 0.141 |
| Father’s education T1 | −0.032 | −0.66 | 0.511 | −0.147 | −0.034 | −0.015 | −0.34 | 0.734 | −0.147 | −0.017 | −0.015 | −0.36 | 0.719 | −0.147 | −0.019 |
| Smoking T1 | 0.049 | 1.02 | 0.307 | 0.078 | 0.053 | 0.011 | 0.25 | 0.800 | 0.078 | 0.013 | 0.005 | 0.13 | 0.899 | 0.078 | 0.007 |
| MAP T1 | 0.484 | 10.81 | <0.001 | 0.571 | 0.486 | 0.472 | 10.66 | <0.001 | 0.571 | 0.482 | |||||
| HF-HRV T1 | −0.137 | −3.35 | 0.001 | −0.184 | −0.170 | ||||||||||
| B: EAs | Model 1 (R2 = 0.092) | Model 2 (R2change = 0.140) | Model 3 (R2change = 0.039) | ||||||||||||
| Age T1 | 0.030 | 0.38 | 0.703 | 0.078 | 0.029 | −0.032 | −0.44 | 0.657 | 0.078 | −0.034 | −0.055 | −0.76 | 0.446 | 0.078 | −0.058 |
| Sex | −0.222 | −2.96 | 0.003 | −0.184 | −0.220 | −0.086 | −1.17 | 0.243 | −0.184 | −0.089 | −0.094 | −1.31 | 0.193 | −0.184 | −0.100 |
| BMI T1 | 0.203 | 2.52 | 0.013 | 0.195 | 0.189 | 0.224 | 3.01 | 0.003 | 0.195 | 0.224 | 0.207 | 2.83 | 0.005 | 0.195 | 0.212 |
| Father’s education T1 | −0.056 | −0.73 | 0.464 | −0.101 | −0.056 | −0.040 | −0.56 | 0.573 | −0.101 | −0.043 | −0.052 | −0.76 | 0.447 | −0.101 | −0.058 |
| Smoking T1 | 0.036 | 0.49 | 0.623 | 0.091 | 0.037 | 0.005 | 0.07 | 0.947 | 0.091 | 0.005 | −0.009 | −0.13 | 0.894 | 0.091 | −0.010 |
| MAP T1 | 0.403 | 5.58 | <0.001 | 0.422 | 0.393 | 0.394 | 5.57 | <0.001 | 0.422 | 0.393 | |||||
| HF-HRV T1 | −0.201 | −3.01 | 0.003 | −0.232 | −0.225 | ||||||||||
| C: AAs | Model 1 (R2 = 0.116) | Model 2 (R2change = 0.266) | Model 3 (R2change = 0.008) | ||||||||||||
| Age T1 | 0.229 | 3.35 | 0.001 | 0.254 | 0.230 | 0.091 | 1.55 | 0.123 | 0.254 | 0.109 | 0.075 | 1.26 | 0.208 | 0.254 | 0.089 |
| Sex | −0.211 | −3.06 | 0.003 | −0.229 | −0.211 | −0.148 | −2.53 | 0.012 | −0.229 | −0.176 | −0.149 | −2.57 | 0.011 | −0.229 | −0.179 |
| BMI T1 | 0.052 | 0.76 | 0.451 | 0.077 | 0.053 | 0.090 | 1.56 | 0.120 | 0.077 | 0.110 | 0.091 | 1.58 | 0.116 | 0.077 | 0.111 |
| Father’s education T1 | −0.005 | −0.08 | 0.940 | −0.057 | −0.005 | 0.022 | 0.38 | 0.705 | −0.057 | 0.027 | 0.026 | 0.46 | 0.647 | −0.057 | 0.032 |
| Smoking T1 | 0.048 | 0.70 | 0.482 | 0.119 | 0.050 | 0.000 | 0.00 | 0.999 | 0.119 | 0.000 | 0.000 | 0.00 | 0.999 | 0.119 | 0.000 |
| MAP T1 | 0.544 | 9.28 | <0.001 | 0.588 | 0.549 | 0.534 | 9.10 | <0.001 | 0.588 | 0.542 | |||||
| HF-HRV T1 | −0.093 | −1.63 | 0.105 | −0.192 | −0.115 | ||||||||||
This table depicts hierarchical regression models predicting mean arterial pressure (MAP) at time 2 (T2) in all individuals. Table 2A give regression statistics for the full sample, and Table 2, B and C present regression statistics in European Americans (EAs) and African Americans (AAs). Age is measured in years. BMI, body mass index, kg/m2; HF-HRV, natural log transformed high frequency heart rate variability; T1, time 1. Significant predictors (P < 0.05) are bold at each step.
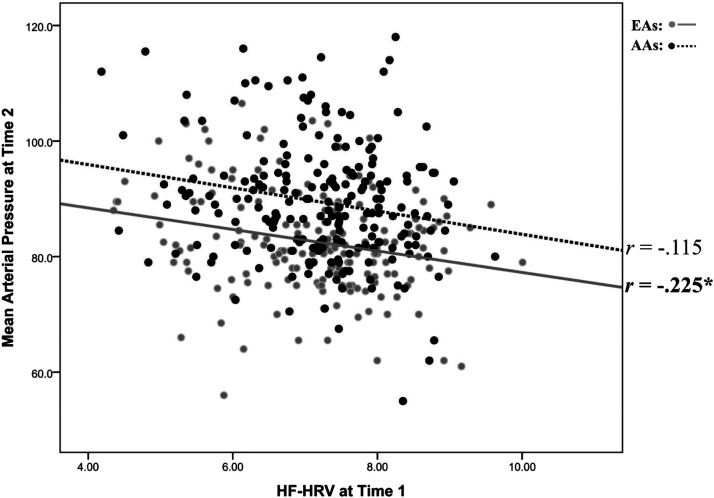
Regression results for MAP at T2 are presented in Table 2B for EAs. Model 1 accounted for 9.2% of the variance in T2 MAP with sex and BMI emerging as significant predictors (each P < 0.01). In model 2, T1 MAP significantly accounted for an additional 14.0% of the variance [B = 0.50 (0.09), rpartial = 0.393, P < 0.001]. In model 3, HRV emerged as a significant inverse predictor (B = −1.61 (0.54), rpartial = −0.225, P = 0.003), explaining an additional 3.9% of the variance in T2 MAP in EAs. Regression results for MAP at T2 are presented in Table 2C for AAs. Model 1 accounted for 11.6% of the variance in T2 MAP with sex and age emerging as significant predictors (each P < 0.01). In model 2, T1 MAP significantly accounted for an additional 26.6% of the variance [B = 0.50 (0.09), rpartial = 0.393, P < 0.001]. In model 3, HRV explained only an additional 0.8% of the variance and did not significantly predict T2 MAP in AAs [B = −0.97 (0.60), rpartial = −0.115, P = 0.105]. Figure 1 depicts a scatterplot of HRV at T1 and MAP at T2 stratified by ethnicity.
Figure 1.
Scatterplot between heart rate variability (HRV) at time 1 and mean arterial pressure at time 2. Note: This figure depicts a scatterplot between high-frequency heart rate variability (HF-HRV) at time 1 and mean arterial pressure (MAP) at time 2. Regression results showed HF-HRV to significantly predict MAP for European Americans (n = 178) (EAs; rpartial = −0.225, P = 0.003) but not African Americans (n = 207) (AAs; rpartial = −0.115, P = 0.105). *P < 0.05.
Predicting Total Peripheral Index at Time 2
Regression results for TPI at T2 are presented in Table 3A for the full sample. Model 1 accounted for 21.9% of the variance in T2 TPI with only BMI and ethnicity emerging as significant predictors (each P < 0.01). In model 2, T1 TPI significantly accounted for an additional 2.7% of the variance [B = 0.17 (0.05), rpartial = 0.187, P < 0.001]. In model 3, HRV emerged as a significant inverse predictor [B = −0.74 (0.35), rpartial = −0.107, P = 0.038), explaining an additional 1.8% of the variance in T2 TPI.
Table 3.
Hierarchical regression models predicting time 2 total peripheral resistance index for the full sample and stratified by ethnicity
| b | t | P | r | rpartial | b | t | P | r | rpartial | b | t | P | r | rpartial | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: Full sample | Model 1 (R2 = 0.219) | Model 2 (R2change = 0.027) | Model 3 (R2change = 0.009) | ||||||||||||
| Age T1 | 0.088 | 1.83 | 0.068 | 0.212 | 0.094 | 0.083 | 1.75 | 0.080 | 0.212 | 0.090 | 0.066 | 1.39 | 0.165 | 0.212 | 0.072 |
| Sex | −0.042 | −0.90 | 0.370 | 0.039 | −0.046 | −0.019 | −0.41 | 0.683 | 0.039 | −0.021 | −0.018 | −0.38 | 0.702 | 0.039 | −0.020 |
| Ethnicity | 0.286 | 5.98 | <0.001 | 0.351 | 0.294 | 0.248 | 5.14 | <0.001 | 0.351 | 0.256 | 0.250 | 5.21 | <0.001 | 0.351 | 0.260 |
| BMI T1 | 0.264 | 5.38 | <0.001 | 0.347 | 0.267 | 0.268 | 5.56 | <0.001 | 0.347 | 0.275 | 0.266 | 5.55 | <0.001 | 0.347 | 0.275 |
| Father’s education T1 | −0.043 | −0.90 | 0.368 | −0.186 | −0.046 | −0.040 | −0.84 | 0.402 | −0.186 | −0.043 | −0.039 | −0.84 | 0.403 | −0.186 | −0.043 |
| Smoking T1 | 0.047 | 1.00 | 0.316 | 0.053 | 0.052 | 0.049 | 1.07 | 0.285 | 0.053 | 0.055 | 0.045 | 0.98 | 0.326 | 0.053 | 0.051 |
| TPI T1 | 0.171 | 3.69 | <0.001 | 0.233 | 0.187 | 0.189 | 4.04 | <0.001 | 0.233 | 0.204 | |||||
| HF-HRV T1 | −0.096 | −2.08 | 0.038 | −0.081 | −0.107 | ||||||||||
| B: EAs | Model 1 (R2 = 0.131) | Model 2 (R2change = 0.034) | Model 3 (R2change = 0.024) | ||||||||||||
| Age T1 | 0.044 | 0.57 | 0.567 | 0.149 | 0.044 | 0.047 | 0.63 | 0.532 | 0.149 | 0.048 | 0.029 | 0.39 | 0.701 | 0.149 | 0.030 |
| Sex | −0.028 | −0.38 | 0.703 | 0.019 | −0.029 | −0.013 | −0.17 | 0.862 | 0.019 | −0.013 | −0.014 | −0.19 | 0.847 | 0.019 | −0.015 |
| BMI T1 | 0.326 | 4.13 | <0.001 | 0.344 | 0.300 | 0.332 | 4.27 | <0.001 | 0.344 | 0.311 | 0.320 | 4.15 | <0.001 | 0.344 | 0.303 |
| Father’s education T1 | 0.010 | 0.13 | 0.897 | −0.091 | 0.010 | 0.017 | 0.23 | 0.816 | −0.091 | 0.018 | 0.008 | 0.12 | 0.907 | −0.091 | 0.009 |
| Smoking T1 | 0.099 | 1.37 | 0.173 | 0.136 | 0.104 | 0.107 | 1.50 | 0.137 | 0.136 | 0.114 | 0.097 | 1.37 | 0.174 | 0.136 | 0.104 |
| TPI T1 | 0.185 | 2.64 | 0.009 | 0.167 | 0.198 | 0.214 | 3.03 | 0.003 | 0.167 | 0.226 | |||||
| HF-HRV T1 | −0.159 | −2.22 | 0.028 | −0.166 | −0.168 | ||||||||||
| C: AAs | Model 1 (R2 = 0.110) | Model 2 (R2change = 0.029) | Model 3 (R2change = 0.003) | ||||||||||||
| Age T1 | 0.121 | 1.77 | 0.079 | 0.186 | 0.124 | 0.112 | 1.66 | 0.099 | 0.186 | 0.116 | 0.100 | 1.45 | 0.150 | 0.186 | 0.102 |
| Sex | −0.050 | −0.72 | 0.475 | −0.035 | −0.050 | −0.020 | −0.28 | 0.777 | −0.035 | −0.020 | −0.018 | −0.26 | 0.795 | −0.035 | −0.018 |
| BMI T1 | 0.258 | 3.73 | <0.001 | 0.288 | 0.254 | 0.261 | 3.83 | <0.001 | 0.288 | 0.261 | 0.262 | 3.84 | <0.001 | 0.288 | 0.263 |
| Father’s education T1 | −0.080 | −1.18 | 0.240 | −0.133 | −0.083 | −0.079 | −1.18 | 0.238 | −0.133 | −0.083 | −0.076 | −1.14 | 0.257 | −0.133 | −0.080 |
| Smoking T1 | 0.012 | 0.17 | 0.865 | 0.048 | 0.012 | 0.012 | 0.18 | 0.857 | 0.048 | 0.013 | 0.012 | 0.17 | 0.864 | 0.048 | 0.012 |
| TPI T1 | 0.174 | 2.61 | 0.010 | 0.179 | 0.182 | 0.186 | 2.72 | 0.007 | 0.179 | 0.190 | |||||
| HF-HRV T1 | −0.057 | −0.83 | 0.406 | −0.059 | −0.059 | ||||||||||
This table depicts hierarchical regression models predicting total peripheral index at time 2 (T2) in all individuals. Table 3A give regression statistics for the full sample, and Table 3, B and C present regression statistics in European Americans (EAs) and African Americans (AAs). Age measured in years, BMI, body mass index, kg/m2; HF-HRV, natural log transformed high frequency heart rate variability; TPI, total peripheral index; T1, time 1. Significant predictors (P < 0.05) are bold at each step.
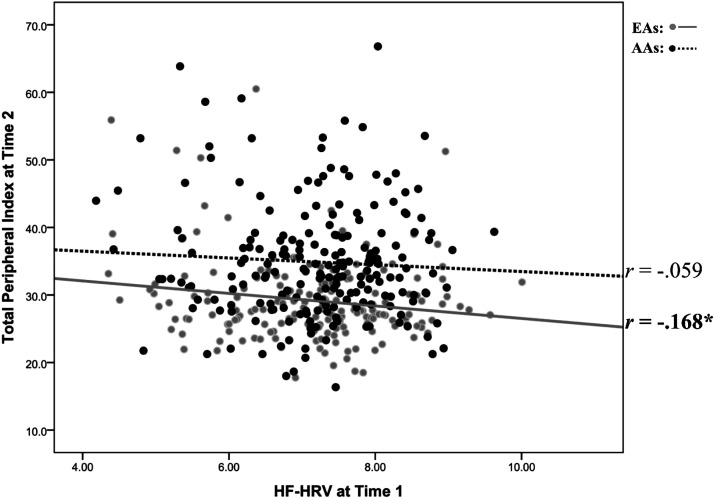
For EAs, regression results for TPI at T2 are presented in Table 3B. Model 1 accounted for 13.1% of the variance in T2 TPI with only BMI emerging as a significant predictor (P < 0.001). In model 2, T1 TPI significantly accounted for an additional 3.4% of the variance [B = 0.17 (0.06), rpartial = 0.198, P = 0.009]. In model 3, higher HRV significantly predicted lower T2 TPI [B = −0.89 (0.40), rpartial = −0.168, P = 0.028), explaining an additional 2.4% of the variance in T2 TPI in EAs. Regression results for TPI at T2 are presented in Table 3C for AAs. Model 1 accounted for 11.0% of the variance in T2 TPI with sex and age emerging as significant predictors (each P < 0.01). In model 2, T1 TPI significantly accounted for an additional 2.9% of the variance [B = 0.17 (0.07), rpartial = 0.182, P = 0.010]. In model 3, HRV explained only an additional 0.3% of the variance and did not significantly predict T2 TPI in AAs [B = −0.49 (0.59), rpartial = −0.059, P = 0.406). Figure 2 depicts a scatterplot of HRV at T1 and TPR at T2 stratified by ethnicity.
Figure 2.
Scatterplot between heart rate variability (HRV) at time 1 and total peripheral resistance at time 2. Note: This figure depicts a scatterplot between high frequency heart rate variability (HF-HRV) at time 1 and total peripheral resistance (TPR) at time 2. Regression results showed HF-HRV to significantly predict TPR for European Americans (n = 178) (EAs; rpartial = −0.168, P = 0.028) but not African Americans (n = 207) (AAs; rpartial = −0.059, P = 0.406). *P < 0.05.
Predicting Cardiac Index at Time 2
Regression results for CI at T2 are presented in Table 4A for the full sample. Model 1 accounted for 10.5% of the variance in T2 CI with ethnicity and BMI emerging as significant predictors (each p < 0.01). In model 2, T1 CI significantly accounted for an additional 2.7% of the variance (B = 0.15 (0.05), rpartial = .173, p = 0.001). In model 3, HRV did not significantly predict T2 CI (B = -0.12 (0.03), rpartial = −.023, p = 0.655), explaining virtually no variance in T2 CI. Regression results for CI at T2 are presented in Table 4B for EAs. Model 1 accounted for 6.7% of the variance in T2 CI with BMI emerging as a significant predictor (P = 0.002). In model 2, T1 CI significantly accounted for an additional 2.3% of the variance [B = 0.18 (0.07), rpartial = 0.206, P < 0.001]. In model 3, HRV did not significantly predict T2 CI [B = 0.01 (0.03), rpartial = 0.017, P = 0.826), explaining virtually no variance in T2 CI. Regression results for CI at T2 are presented in Table 4C for AAs. Model 1 accounted for 6.5% of the variance in T2 CI with BMI as a significant predictor (each P = 0.003). In model 2, T1 CI significantly accounted for an additional 2.3% of the variance [B = 0.14 (0.06), rpartial = 0.158, P = 0.025]. In model 3, HRV explained only an additional 0.4% of the variance and did not significantly predict T2 CI [B = −0.36 (0.04), rpartial = −0.063, P = 0.371] in AAs.
Table 4.
Hierarchical regression models predicting time 2 cardiac index for full sample and stratified by ethnicity
| b | t | P | r | rpartial | b | t | P | r | rpartial | b | t | P | r | rpartial | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: Full sample | Model 1 (R2 = 0.105) | Model 2 (R2change = 0.027) | Model 3 (R2change = 0.000) | ||||||||||||
| Age T1 | −0.005 | −0.11 | 0.916 | −0.102 | −0.005 | −0.020 | −0.39 | 0.700 | −0.102 | −0.020 | −0.023 | −0.44 | 0.657 | −0.102 | −0.023 |
| Sex | −0.063 | −1.24 | 0.214 | −0.121 | −0.064 | −0.073 | −1.46 | 0.145 | −0.121 | −0.075 | −0.073 | −1.46 | 0.146 | −0.121 | −0.075 |
| Ethnicity | −0.152 | −2.97 | 0.003 | −0.211 | −0.151 | −0.139 | −2.74 | 0.006 | −0.211 | −0.140 | −0.138 | −2.72 | 0.007 | −0.211 | −0.139 |
| BMI T1 | −0.225 | −4.29 | 0.000 | −0.272 | −0.215 | −0.220 | −4.26 | 0.000 | −0.272 | −0.214 | −0.221 | −4.27 | 0.000 | −0.272 | −0.215 |
| Father’s education T1 | 0.030 | 0.59 | 0.555 | 0.120 | 0.030 | 0.029 | 0.57 | 0.569 | 0.120 | 0.029 | 0.029 | 0.57 | 0.569 | 0.120 | 0.029 |
| Smoking T1 | −0.007 | −0.14 | 0.885 | 0.003 | −0.007 | −0.018 | −0.37 | 0.711 | 0.003 | −0.019 | −0.019 | −0.38 | 0.702 | 0.003 | −0.020 |
| CI T1 | 0.165 | 3.42 | 0.001 | 0.172 | 0.173 | 0.160 | 3.19 | 0.002 | 0.172 | 0.162 | |||||
| HF-HRV T1 | −0.023 | −0.45 | 0.655 | −0.051 | −0.023 | ||||||||||
| B: EAs | Model 1 (R2 = 0.067) | Model 2 (R2change = 0.040) | Model 3 (R2change = 0.000) | ||||||||||||
| Age T1 | 0.018 | 0.23 | 0.821 | −0.075 | 0.017 | 0.003 | 0.03 | 0.973 | −0.075 | 0.003 | 0.004 | 0.06 | 0.956 | −0.075 | 0.004 |
| Sex | −0.052 | −0.69 | 0.492 | −0.090 | −0.052 | −0.045 | −0.61 | 0.545 | −0.090 | −0.046 | −0.045 | −0.60 | 0.551 | −0.090 | −0.046 |
| BMI T1 | −0.252 | −3.08 | 0.002 | −0.253 | −0.229 | −0.253 | −3.15 | 0.002 | −0.253 | −0.235 | −0.252 | −3.12 | 0.002 | −0.253 | −0.233 |
| Father’s education T1 | −0.008 | −0.10 | 0.918 | 0.063 | −0.008 | −0.012 | −0.16 | 0.871 | 0.063 | −0.012 | −0.011 | −0.15 | 0.881 | 0.063 | −0.011 |
| Smoking T1 | −0.009 | −0.12 | 0.905 | −0.028 | −0.009 | −0.029 | −0.39 | 0.696 | −0.028 | −0.030 | −0.028 | −0.38 | 0.705 | −0.028 | −0.029 |
| CI T1 | 0.201 | 2.75 | 0.007 | 0.192 | 0.206 | 0.204 | 2.73 | 0.007 | 0.192 | 0.205 | |||||
| HF-HRV T1 | 0.017 | 0.22 | 0.826 | 0.003 | 0.017 | ||||||||||
| C: AAs | Model 1 (R2 = 0.065) | Model 2 (R2change = 0.023) | Model 3 (R2change = 0.004) | ||||||||||||
| Age T1 | −0.020 | −0.28 | 0.779 | −0.067 | −0.020 | −0.036 | −0.51 | 0.609 | −0.067 | −0.036 | −0.047 | −0.66 | 0.510 | −0.067 | −0.047 |
| Sex | −0.076 | −1.07 | 0.284 | −0.095 | −0.076 | −0.098 | −1.38 | 0.171 | −0.095 | −0.097 | −0.096 | −1.34 | 0.180 | −0.095 | −0.095 |
| BMI T1 | −0.217 | −3.06 | 0.003 | −0.238 | −0.211 | −0.209 | −2.97 | 0.003 | −0.238 | −0.206 | −0.209 | −2.97 | 0.003 | −0.238 | −0.206 |
| Father’s education T1 | 0.058 | 0.84 | 0.403 | 0.085 | 0.059 | 0.060 | 0.87 | 0.385 | 0.085 | 0.061 | 0.063 | 0.92 | 0.361 | 0.085 | 0.065 |
| Smoking T1 | −0.003 | −0.04 | 0.965 | 0.002 | −0.003 | −0.010 | −0.14 | 0.887 | 0.002 | −0.010 | −0.010 | −0.14 | 0.888 | 0.002 | −0.010 |
| CI T1 | 0.154 | 2.26 | 0.025 | 0.141 | 0.158 | 0.136 | 1.90 | 0.059 | 0.141 | 0.133 | |||||
| HF-HRV T1 | −0.065 | −0.90 | 0.371 | −0.084 | −0.063 | ||||||||||
This table depicts hierarchical regression models predicting cardiac index (CI) at time 2 (T2) in all individuals. Table 4A give regression statistics for the full sample, and Table 4, B C present regression statistics in European Americans (EAs) and African Americans (AAs). Age is measured in years. BMI, body mass index, kg/m2; HF-HRV, natural log transformed high frequency heart rate variability; T1, time 1. Significant predictors (P < 0.05) are bold at each step.
DISCUSSION
Results from the current study showed AAs to have higher MAP marked by higher TPR at T2 compared with EAs, independent of several important covariates including BMI, sex, socioeconomic status (fathers’ education), and respective hemodynamic measures at T1. The major novel findings of this study showed that higher HRV at T1 significantly predicted both lower TPR and MAP at T2 among EAs only; these associations were attenuated and not significant in AAs, especially HRV predicting TPR at T2. HRV did not significantly predict CO in the full sample or split by ethnicity.
Overall, the current investigation is the first to investigate how cardiac vagal tone (i.e., HRV) may differentially predict hemodynamics underlying long-term BP regulation based on ethnicity. Recent research showed HRV to predict long-term systolic and diastolic BP in EAs but not AAs (22), and these findings are in line with the current results; here, we show HRV to predict long-term MAP in EAs however this association was attenuated in AAs. Novel results in the current investigation extend prior findings: HRV differentially predicts the “hemodynamics” underlying such BP elevations over time between AAs and EAs. This is important, as BP elevations via increased TPR are particularly deleterious and associated with end organ damage. In all, we highlight that greater vagal activity may be beneficial in reducing BP (MAP) via reductions in TPR in EAs but not in AAs. This result in EAs is consistent with the finding that HRV was associated with endothelial function as indexed by brachial artery flow mediated dilation in humans (13).
There is increasing interest in the role of the vagus in cardiovascular disease in general and hypertension in particular (10, 11, 23, 24). Whereas there is ample evidence for direct vagal innervation of the heart, there is currently no evidence of direct vagal innervation of the vessels (14, 25). Therefore, the exact way in which the vagus is related to peripheral resistance is not clear. It has been reported that in rodents, activation of preganglionic vagal neurons in the dorsal motor nucleus of the vagus is associated with decreased ventricular contractility (26). This has led to the speculation that the impact on peripheral resistance may be secondary to prolonged vagally meditated decreases in CO (25). However in the current study, we found that HRV was not a significant predictor of CO in either EAs or AAs in our sample of young adult humans. Another speculation is that, at least in rodent models, acetylcholine released from the vagus nerve and carried through the bloodstream may act on muscarinic receptors on endothelial cells to produce vasodilation (27, 28). However, to date, there is no evidence for this mechanism in humans.
The most promising hypothesis put forward to explain the effects of vagal activity on the peripheral vasculature involves the cholinergic anti-inflammatory pathway (29–31). It has been proposed that this pathway is dysregulated in hypertension (30). Rodent models have shown that vagal activation leads to down regulation of proinflammatory cytokines in the serum and vasculature (11, 32). In humans, we have reported in both cross-sectional and prospective studies, that greater HRV is associated with lower levels of inflammatory markers (33–35). Of special relevance to the current findings, it has also been reported that AAs have both greater HRV and greater levels of proinflammatory cytokines relative to EAs (4, 36). Thus, the lack of association between HRV and TPR in the current study may be due to a dysregulation in the cholinergic anti-inflammatory pathway. However, additional research is needed to fully explore this potential mechanism.
Previous cross-sectional studies have examined ethnic differences in HRV and TPR (e.g., 3, 4, 9, 37), however the present study is the first investigation to examine ethnic differences in the longitudinal association between HRV and TPR 6 yr later. Sherwood and Hinderliter (8) showed that in response to the α-adrenergic agonist, phenylephrine, AAs had greater vasoconstriction (i.e., greater TPR) compared with EAs. Moreover, as mentioned, pregnancy is a potent vasodilator, and yet, pregnant AA women showed higher resting TPR and higher HRV compared with EA women, suggesting impaired vasodilation in AAs (9). Taken together, evidence suggests impaired vasodilation in response to both exogenous (e.g., Ref. 8) and endogenous (e.g., Ref. 9) vasodilatory agents. The current results are in line with these data, as higher HRV is not associated with lower TPR in AAs. Novel results from the current investigation suggest that this HRV and TPR association is evident longitudinally in EAs, but not AAs, thereby providing a physiological mechanism linking AAs with increased risk for cardiovascular disease.
Indeed, these data have major implications for health disparities research working to understand physiological mechanisms underpinning poorer cardiovascular health in AAs compared with EAs. To put this into a clinical context, the magnitude of the difference between AAs and EAs in TPR is equivalent to the difference between dippers and nondippers reported by Sherwood and colleagues (38). Nondipping is known to be associated with greater left ventricular hypertrophy, myocardial infarction, and stroke. Thus, the reported magnitude of the effect in the present study could be associated with the greater incidence of these adverse factors in AAs.
As early as 1948, the baroreflex was thought to play a role in both long-term and short-term BP regulation (39). Despite some conflicting empirical studies (e.g., Ref. 40) due to potential methodological issues (41), decades of research suggest that the baroreflex, mediated by the vagus and associated brain regions, is a major determinant of long-term BP regulation (see Ref. 42 for review). Therefore, given the current results regarding less effective vagal activity in AAs in decreasing TPR, the baroreflex may play an important role in the differential hemodynamics underlying long-term BP regulation between AAs and EAs. Overall, the current data support the idea of long-term BP regulation by the vagally mediated baroreflex (41, 42) and this influence of the baroreflex may differ between AAs and EAs; however future studies are needed to examine this directly.
The current study is not without its limitations. Although there was a high prevalence of smoking among our sample, smoking did not emerge as a significant predictor in any of our analyses. Also, the level of regular physical activity was generally low across the entire sample, however importantly, there were no significant differences in physical activity as a function of ethnicity. Results remain the same when controlling for the change in BMI from T1 to T2 (not a significant predictor in any model). In addition, whereas father’s education, as a proxy for socioeconomic status in this young adult sample, was significantly higher in EAs, it was not a significant predictor in any of the regression models. In the current study, impedance cardiography was used to provide an indirect measure of peripheral blood flow. Future studies should examine more direct measures such as brachial artery flow mediated dilation as used by Pinter et al. (13).
In summary, the current results suggest that in normotensive individuals, higher HRV predicts decreased MAP and TPR 6 yr later in EAs. In contrast, there was no association between HRV and hemodynamics 6 yr later in AAs. HRV did not predict CO in either EAs or AAs. Thus, it is unlikely that the effect of HRV on TPR was secondary to HRV effects on CO. In closing, our data highlight that AAs show TPR mediated long-term increases in BP irrespective of resting HRV, providing a physiological pathway linking AAs with a greater risk for mortality and morbidity from hypertension and potentially other cardiovascular disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.P.W., J.D.H., X.W., and G.K. conceived and designed research; J.D.H. performed experiments; D.P.W. analyzed data; D.P.W., J.F.T., and G.K. interpreted results of experiments; D.P.W. prepared figures; D.P.W. drafted manuscript; D.P.W., J.F.T., J.D.H., X.W., and G.K. edited and revised manuscript; D.P.W., J.F.T., J.D.H., X.W., and G.K. approved final version of manuscript.
Footnotes
We also estimated a time domain measure of HRV, the root mean square of successive differences of normal RR intervals (RMSSD). HF-HRV and RMSSD were highly correlated in our sample (r = 0.90, P < 0.001). Because all results were virtually identical when analyses were conducted using RMSSD, only results for HF-HRV are reported.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi N, McKeigue PM, Marmot MG. Resting and ambulatory blood pressure differences in Afro-Caribbeans and Europeans. Hypertension 22: 90–96, 1993. doi: 10.1161/01.hyp.22.1.90. [DOI] [PubMed] [Google Scholar]
- 3.Brownlow BN, Williams DP, Kapuku G, Vasey MW, Anderson NB, Koenig J, Thayer JF, Hill LK. Ethnic differences in resting total peripheral resistance: a systematic review and meta-analysis. Psychosomatic Med 82: 548–560, 2020. doi: 10.1097/PSY.0000000000000820. [DOI] [PubMed] [Google Scholar]
- 4.Hill LK, Hu DD, Koenig J, Sollers JJ, Kapuku G, Wang X, Snieder H, Thayer JF. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosom Med 77: 16–25, 2015. doi: 10.1097/PSY.0000000000000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141: 122–131, 2010. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 6.Jones DS, Andrawis NS, Abernethy DR. Impaired endothelial–dependent forearm vascular relaxation in black Americans. Clin Pharmacol Thers 65: 408–412, 1999. doi: 10.1016/S0009-9236(99)70135-9. [DOI] [PubMed] [Google Scholar]
- 7.Taherzadeh Z, Brewster LM, Van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J Clin Hypertens (Greenwich) 12: 431–438, 2010. doi: 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwood A, Hinderliter AL. Responsiveness to α-and β-adrenergic receptor agonists effects of race in borderline hypertensive compared to normotensive men. Am J Hypertens 6: 630–635, 1993. doi: 10.1093/ajh/6.7.630. [DOI] [PubMed] [Google Scholar]
- 9.Christian LM, Koenig J, Williams DP, Kapuku G, Thayer JF. Impaired vasodilation in pregnant African Americans: preliminary evidence of potential antecedents and consequences. Psychophysiology 58: e13699, 2021. doi: 10.1111/psyp.13699. [DOI] [PubMed] [Google Scholar]
- 10.He X, Zhao M, Bi X, Sun L, Yu X, Zhao M, Zang W. Novel strategies and underlying protective mechanisms of modulation of vagal activity in cardiovascular diseases. Br J Pharmacol 172: 5489–5500, 2015. doi: 10.1111/bph.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Zhao M, Yu X, Zang W. Pharmacological modulation of vagal nerve activity in cardiovascular diseases. Neurosci Bull 35: 156–166, 2019. doi: 10.1007/s12264-018-0286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill LK, Thayer JF, Williams DP, Halbert JD, Hao G, Robinson V, Harshfield G, Kapuku G. Ethnic and sex differences in the longitudinal association between heart rate variability and blood pressure. Blood Pressure : 1–7, 2021. doi: 10.1080/08037051.2021.1876517. [DOI] [PubMed] [Google Scholar]
- 13.Pinter A, Horvath T, Sarkozi A, Kollai M. Relationship between heart rate variability and endothelial function in healthy subjects. Auton Neurosci 169: 107–112, 2012. doi: 10.1016/j.autneu.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Levy MN. Neural control of cardiac function. Ballieres Clin Neurol 6: 227–244, 1997. [PubMed] [Google Scholar]
- 15.Hao G, Halbert JD, Su S, Bagi Z, Robinson V, Thayer J, Harshfield G, Kapuku GK. Rapid decline of resting heart rate trajectories from childhood to young adulthood is paradoxically associated with increased cardiac mass. Acta Cardiologica : 1–7, 2021. doi: 10.1080/00015385.2020.1871262. [DOI] [PubMed] [Google Scholar]
- 16.Kapuku G, Howie M, Ghosh S, Doshi V, Bykhovsky M, Ange B, Halbert J, Robinson V, Bagi Z, Harshfield G, George V. Effects of race, cardiac mass and cardiac load on myocardial function trajectories from childhood to young adulthood - The Augusta Heart Study. J Am Heart Assoc 10: e015612, 2021. doi: 10.1161/JAHA.119.015612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapuku G, Ghosh S, Doshi V, Hall P, Strong W, Treiber F, George V. Journal of Environment and Health Science. ISSN : 2378–6841, 2019. doi: 10.15436/2378-6841.19.2039. [DOI] [Google Scholar]
- 18.Dysart JM, Treiber FA, Pflieger K, Davis H, Strong WB. Ethnic differences in the myocardial and vascular reactivity to stress in normotensive girls. Am J Hypertens 7: 15–22, 1994. doi: 10.1093/ajh/7.1.15. [DOI] [PubMed] [Google Scholar]
- 19.Treiber FA, Davis H, Musante L, Raunikar RA, Strong WB, McCaffrey F, Meeks MC, Vandernoord R. Ethnicity, gender, family history of myocardial infarction, and hemodynamic responses to laboratory stressors in children. Health Psychol 12: 6–15, 1993. doi: 10.1037/0278-6133.12.1.6. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Thayer JF, Treiber F, Snieder H. Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol 96: 1166–1172, 2005. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 21a.Du Bois D, Du Bois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17: 863–871, 1916. doi: 10.1001/archinte.1916.00080130010002. [DOI] [PubMed] [Google Scholar]
- 22.Hill LK, Thayer JF, Williams DP, Halbert JD, Hao G, Robinson V, Harshfield G, Kapuku G. (in press). Ethnic and sex differences in the longitudinal association between heart rate variability and blood pressure. Blood Press: 1–7, 2021. doi: 10.1080/08037051.2021.1876517. [DOI] [PubMed] [Google Scholar]
- 23.Chapleau MW, Rotella DL, Reho JJ, Rahmouni K, Stauss HM. Chronic vagal nerve stimulation prevents high-salt diet-induced endothelial dysfunction and aortic stiffening in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 311: H276–H285, 2016. doi: 10.1152/ajpheart.00043.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petkovich BW, Vega J, Thomas S. Vagal modulation of hypertension. Curr Hypertens Rep 17: 26, 2015. doi: 10.1007/s11906-015-0532-6. [DOI] [PubMed] [Google Scholar]
- 25.Moreira TS, Antunes VR, Falquetto B, Marina N. Long-term stimulation of cardiac vagal preganglionic neurons reduces blood pressure in the spontaneously hypertensive rat. J Hypertens 36: 2444–2452, 2018. doi: 10.1097/HJH.0000000000001871. [DOI] [PubMed] [Google Scholar]
- 26.Machhada A, Ang R, Ackland GL, Ninkina N, Buchman VL, Lythgoe MF, Trapp S, Tinker A, Marina N, Gourine AV. Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm 12: 2285–2293, 2015. doi: 10.1016/j.hrthm.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bény JL, Nguyen MN, Marino M, Matsui M. Muscarinic receptor knockout mice confirm involvement of M3 receptor in endothelium-dependent vasodilatation in mouse arteries. J Cardiovasc Pharmacol 51: 505–512, 2008. doi: 10.1097/FJC.0b013e31816d5f2f. [DOI] [PubMed] [Google Scholar]
- 28.Bi XY, He X, Zhao M, Yu XJ, Zang WJ. Role of endothelial nitric oxide synthase and vagal activity in the endothelial protection of atorvastatin in ischemia/reperfusion injury. J Cardiovasc Pharmacol 61: 391–400, 2013. doi: 10.1097/FJC.0b013e318286baf3. [DOI] [PubMed] [Google Scholar]
- 29.Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 30.Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res 59: 243–253, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 59: 755–762, 2012. doi: 10.1161/HYPERTENSIONAHA.111.186833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao M, He X, Bi XY, Yu XJ, Wier WG, Zang WJ. Vagal stimulation triggers peripheral vascular protection through the cholinergic anti-inflammatory pathway in a rat model of myocardial ischemia/reperfusion. Basic Res Cardiol 108: 345, 2013. doi: 10.1007/s00395-013-0345-1. [DOI] [PubMed] [Google Scholar]
- 33.Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C–reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med 265: 439–447, 2009. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 34.Jarczok MN, Koenig J, Mauss D, Fischer JE, Thayer JF. Lower heart rate variability predicts increased level of C–reactive protein 4 years later in healthy, nonsmoking adults. J Intern Med 276: 667–671, 2014. doi: 10.1111/joim.12295. [DOI] [PubMed] [Google Scholar]
- 35.Williams DP, Koenig J, Carnevali L, Sgoifo A, Jarczok MN, Sternberg EM, Thayer JF. Heart rate variability and inflammation: a meta-analysis of human studies. Brain Behav Immun 80: 219–226, 2019. doi: 10.1016/j.bbi.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Heffernan KS, Jae SY, Vieira VJ, Iwamoto GA, Wilund KR, Woods JA, Fernhall B. C-reactive protein and cardiac vagal activity following resistance exercise training in young African-American and white men. Am J Physiol Regul Integr Comp Physiol 296: R1098–R1105, 2009. doi: 10.1152/ajpregu.90936.2008. [DOI] [PubMed] [Google Scholar]
- 37.Carnevali L, Ottaviani C, Williams DP, Kapuku G, Thayer JF, Hill LK. Hemodynamic profile and compensation deficit in African and European Americans during physical and mental stress. Biol Psychol 141: 17–24, 2019. doi: 10.1016/j.biopsycho.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherwood A, Hill LK, Blumenthal JA, Hinderliter AL. Circadian hemodynamics in men and women with high blood pressure: dipper vs. nondipper and racial differences. J Hyperten 36: 250–258, 2018. doi: 10.1097/HJH.0000000000001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volhard F. On the pathogenesis of red essential arterial hypertension and of malignant nephrosclerosis. Stanford Med Bull 6: 13–28, 1948. [PubMed] [Google Scholar]
- 40.Cowley AW Jr, Liard JF, Guyton AC. Role of the baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res 32: 564–576, 1973. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- 41.Thrasher TN. Arterial baroreceptor input contributes to long-term control of blood pressure. Curr Hypertens Rep 8: 249–254, 2006. doi: 10.1007/s11906-006-0058-z. [DOI] [PubMed] [Google Scholar]
- 42.Victor RG. Carotid baroreflex activation therapy for resist hypertension. Nat Rev Cardiol 12: 451–463, 2015. doi: 10.1038/nrcardio.2015.96. [DOI] [PubMed] [Google Scholar]