Abstract
Fine particulate matter (PM2.5) air pollution exposure increases the risk of developing cardiovascular disease (CVD). Although the precise mechanisms by which air pollution exposure increases CVD risk remain uncertain, research indicates that PM2.5-induced endothelial dysfunction contributes to CVD risk. Previous studies demonstrate that concentrated ambient PM2.5 (CAP) exposure induces vascular inflammation and impairs insulin and vascular endothelial growth factor (VEGF) signaling dependent on pulmonary oxidative stress. To assess whether CAP exposure induces these vascular effects via plasmatic factors, we incubated aortas from naïve mice with plasma isolated from mice exposed to HEPA-filtered air or CAP (9 days) and examined vascular inflammation and insulin and VEGF signaling. We found that treatment of naïve aortas with plasma from CAP-exposed mice activates NF-κBα and induces insulin and VEGF resistance, indicating transmission by plasmatic factor(s). To identify putative factors, we exposed lung-specific ecSOD-transgenic (ecSOD-Tg) mice and wild-type (WT) littermates to CAP at concentrations of either ∼60 µg/m3 (CAP60) or ∼100 µg/m3 (CAP100) and measured the abundance of plasma metabolites by mass spectrometry. In WT mice, both CAP concentrations increased levels of fatty acids such as palmitate, myristate, and palmitoleate and decreased numerous phospholipid species; however, these CAP-induced changes in the plasma lipidome were prevented in ecSOD-Tg mice. Consistent with the literature, we found that fatty acids such as palmitate are sufficient to promote endothelial inflammation. Collectively, our findings suggest that PM2.5 exposure, by inducing pulmonary oxidative stress, promotes unique lipidomic changes characterized by high levels of circulating fatty acids, which are sufficient to trigger vascular pathology.
NEW & NOTEWORTHY We found that circulating plasma constituents are responsible for air pollution-induced vascular pathologies. Inhalation of fine particulate matter (≤PM2.5) promotes a unique form of dyslipidemia that manifests in a manner dependent upon pulmonary oxidative stress. The air pollution-engendered dyslipidemic phenotype is characterized by elevated free fatty acid species and diminished phospholipid species, which could contribute to vascular inflammation and loss of insulin sensitivity.
Keywords: air pollution, cardiovascular disease, free fatty acids, plasma metabolome, pulmonary oxidative stress
INTRODUCTION
Exposure to ambient air pollution is a leading cause of death worldwide and has been linked to 7 million premature deaths globally (1). Exposure to fine particulate matter (PM2.5) air pollution appears particularly detrimental to cardiovascular health. In the United States, exposure to elevated levels of PM2.5 is associated with 200,000 excessive deaths per year, of which 60%–80% are attributable to cardiovascular disease (CVD) (2–4). Because of this, PM2.5 exposure is now recognized as a modifiable risk factor that contributes to cardiovascular morbidity and mortality.
Acute PM2.5 exposure increases the risk of myocardial infarction, arrhythmias, and stroke (2), whereas chronic exposure contributes to the development of hypertension, atherosclerosis, and diabetes (2, 4–6). In utero and early life exposure is associated with cardiac dysfunction and the induction of transcriptional as well as metabolic changes in cardiomyocytes (7–10). Because PM2.5 exposure affects several aspects of CVD, it is likely that it alters processes that are common to several manifestations of CVD. One such unifying mechanism that could contribute to multiple aspects of CVD is vascular dysfunction. Changes in endothelial reactivity affect not only blood pressure regulation but also atherogenesis, heart failure, and arrhythmogenesis (11). Thus, PM2.5-induced endothelial dysfunction could potentially explain several cardiac effects of PM2.5 exposure. Nevertheless, the specific processes that trigger PM2.5-induced cardiovascular injury and mortality remain unclear.
Several studies (12–15), including our own (16–21), demonstrate that the endothelium is a sensitive target for PM2.5 exposure. Chronic PM2.5 exposure is associated with persistent endothelial dysfunction (13, 14), and even acute exposure of healthy adults to PM2.5 promotes conduit artery vasoconstriction (12), increases blood pressure (15, 22), impairs the levels of circulating endothelial progenitor cells, and induces inflammation and endothelial injury (21, 23). Because the endothelium regulates key processes that contribute to CVD-related pathologies such as atherosclerosis, platelet activation, and thrombosis (11), it is possible that PM2.5 exposure increases the risk of developing CVD by impairing endothelial health.
Our previous studies demonstrate that exposure to concentrated ambient PM2.5 (CAP) induces vascular inflammation, characterized by activation of the NF-κBα/inflammasome pathway and triggers the development of insulin or VEGF resistance in the blood vessel (17–20). Although PM2.5-induced vascular inflammation and insulin resistance occurred in the absence of other metabolic defects, we found that exposure to CAP exacerbates diet-induced systemic insulin resistance and impairs vascular repair mechanisms (17, 20). These findings support the idea that induction of vascular inflammation and growth factor resistance by PM2.5 contribute, at least in part, to vascular injury, and could promote the development and progression of type 2 diabetes and CVD. Interestingly, lung-specific overexpression of the antioxidant enzyme extracellular superoxide dismutase (ecSOD) that protects against PM2.5-induced pulmonary oxidative stress, preserves vascular VEGF and insulin sensitivity, and prevents vascular inflammation (17, 20), suggesting that pulmonary oxidative stress is a key mediator of PM2.5-induced vascular pathologies.
It remains unclear how oxidative stress in the lungs transmits pathological signals that impact blood vessels. A possible means of transmission could be plasmatic factors, which could convey PM2.5-induced pulmonary oxidative stress to the circulation, where they trigger vascular inflammation and growth factor resistance. In support of this general idea, exposure to diesel exhaust particles was found to activate the endothelium via a plasmatic factor (24). Nevertheless, the identity of the plasmatic factors that contribute to air pollution-induced vascular inflammation and growth factor resistance remain unknown.
Circulating metabolites may be effective transducers of stress in response to air pollution exposure as likewise shown for other pathologies. For example, free fatty acids have been shown to promote vascular inflammation and insulin resistance in the context of metabolic disease (25, 26). In this study, we examined whether CAP-induced vascular inflammation and vascular growth factor resistance could be mediated by a plasmatic factor. Our findings show that plasmatic factors are sufficient to promote vascular inflammation and impair vascular endothelial growth factor (VEGF) and insulin signaling in the blood vessel. To identify potential plasmatic factors responsible for these vascular defects, we assessed the abundance of plasma metabolites after CAP exposure and found that CAP has robust effects on circulating lipids. Exposure to CAP increased circulating fatty acids, which are known to be sufficient to induce vascular inflammation and insulin resistance. Interestingly, we found that CAP exposure increased free fatty acids and decreased circulating phospholipids, sphingomyelins, and cholesterol, and these changes to the plasma lipidome were completely prevented by pulmonary overexpression of ecSOD. Collectively, these findings indicate that PM2.5 exposure alters the plasma metabolome in a manner dependent on the redox state of the lungs and that distinct classes of metabolites, such as fatty acids, may directly impact the health of blood vessels and their signaling responses to growth factors such as insulin and VEGF.
MATERIALS AND METHODS
Animals and Exposures
For plasma incubation experiments, 12-wk-old male C57BL/6J mice (Jackson Laboratories, exposures 1 and 2) were randomly assigned to three experimental groups (group 1: aorta donors, no exposure, naïve; group 2: plasma donor, HEPA-filtered air exposure, air; group 3: plasma donor, CAP exposure, CAP) and acclimated to our housing conditions for 1 wk. For the metabolomics experiments (exposures 3 and 4), male, age-matched, in-house-bred ecSOD-Tg mice (17, 20, 27), and wild-type (WT) littermates were assigned randomly to air and CAP exposure groups. The mice were exposed for 9 consecutive days to either HEPA-filtered air (6 h/day) or CAP (6 h/day) using our versatile aerosol enrichment system (VACES), as described before (17–20). The specific exposure concentrations are summarized in Table 1. On day 9, aortas were collected from naïve (unexposed) mice for the plasma incubation experiments. Immediately after the final exposure, blood glucose was measured (Accu-Check glucometer, Aviva, Roche) in the donor mice. Mice were euthanized with sodium pentobarbital (150 mg/kg). Blood was collected via cardiac puncture using Na4·EDTA as an anticoagulant, and organs and tissues were excised. Separated plasma and collected tissues were either used immediately (for plasma incubation experiments) or snap-frozen and stored at −80°C. All animal experiments were performed in accordance with the APS’s “Guiding Principles in the Care and Use of Animals,” following protocols approved by the University of Louisville Institutional Animal Care and Use Committee.
Table 1.
Gravimetric assessment of the exposure concentration
| Exposure | Experiment | Duration, days | HEPA, μg/m3 | Ambient, μg/m3 | CAP, μg/m3 | Enrichment Factor |
|---|---|---|---|---|---|---|
| 1 | Plasma incubation I | 9 | 1.9 | 8.8 | 83.7 | 5.3-fold |
| 2 | Plasma incubation II | 9 | 1.6 | 14.1 | 74.6 | 5.3-fold |
| 3 | Metabolome I, CAP60 | 9 | 1.9 | 8.3 | 61.8 | 7.4-fold |
| 4 | Metabolome II, CAP100 | 9 | 8.9 | 19.0 | 97.0 | 5.1-fold |
Data for the fine particulate matter (PM2.5) concentration in the ambient air, the HEPA-filtered air, and the concentrated ambient PM2.5 (CAP) chambers during the specific 9-day exposures. Data are based on gravimetric filter measurements defined as mass divided by air flow (L/min) and the enrichment factor indicates the fold increase by which the versatile aerosol enrichment system (VACES) concentrates PM2.5 from the ambient air.
Plasma and Free Fatty Acid Incubations
To assess whether CAP exposure induces vascular inflammation and impairs vascular VEGF and insulin signaling via a circulating factor, we performed ex vivo plasma incubation experiments following a protocol that was adapted from studies by others (24, 28, 29) and our own experience with isolated aorta experiments (17, 19, 20). For this, we isolated aortas from naïve animals, removed periadventitial connective tissue, and then incubated (1 h; standard cell culture conditions: 5% CO2, 37°C) the aortas with plasma immediately after its isolation from air or CAP-exposed mice. The aortas were then stimulated with vehicle (HBSS), insulin (100 nM, 10 min; Humulin-RP, Eli-Lilly), or VEGF (20 ng/mL, 10 min; recombinant murine VEGF165, Peprotech). Aortas were washed in ice-cold PBS, snap-frozen, and stored at −80°C until their analysis by Western blot.
To test for the induction of vascular inflammation by free fatty acids (FFA), we incubated human umbilical vein cells [HUVECs, Promocell, cultured as described (30)] with bovine serum albumin (BSA, vehicle, Sigma-Aldrich) or 100 µM palmitic acid (Sigma-Aldrich, complexed with BSA, PA/BSA) for 1 h. HUVEC incubated with TNF-α (10 ng/mL, 15 min) were used as a positive control. After the treatment, the cells were washed in ice-cold PBS and lysed; lysates were stored at −80°C until their analysis by Western blot, as in Ref. (30).
Western Blot Analyses
Aortic inflammation and vascular insulin and VEGF signaling were analyzed by performing Western blots to examine IκBα degradation, a marker of NF-κBα activation, and insulin or VEGF-induced Akt phosphorylation (17–20, 31). In addition, IκBα degradation was examined to test for the activation of the NF-κBα pathway by FFA in HUVECs. As described before (17–20, 30, 31), tissue and cells were lysed, and proteins were separated by SDS-PAGE, transferred to PVDF membranes (Bio-Rad, Hercules, CA), and probed with antibodies against IκBα and actin or phospho-Akt (Ser473) and Akt. Antibodies against IκBα, phospho-Akt (Ser473), and Akt (1:1,000) were obtained from Cell Signaling Technology (Danvers, MA), and the actin (1:2,000) antibody was purchased from Sigma-Aldrich. The blots were developed with ECL plus (Amersham Biosciences, Piscataway, NJ; or Pierce, Thermo Fisher, Waltham, MA), and band intensities (Typhoon 9400 variable mode imager Amersham Biosciences; or myECL Imager, Thermo Fisher) were quantified using Image Quant TL software (Amersham Biosciences, Piscataway, NJ; or myImageAnalysis Software, Thermo Fisher).
Plasma Parameter
Circulating levels of insulin (ALPCO, Salem, NH) and vascular growth factor (VEGF; R&D Systems, Minneapolis, MN) were determined by immunoassay. HOMA-IR and HOMA-β scores were calculated from fasting blood glucose and plasma insulin levels according to the homeostatic model assessment (HOMA).
Real-Time PCR
Pulmonary mRNA was isolated using the RNeasy Mini Kit (Qiagen), and cDNA was prepared with the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative real-time PCR (qRT-PCR) was performed as described (30) using the following primer sets: soluble superoxide dismutase 1 (Sod1): forward primer: 5′- GATGAAGAGAGGCATGTTGGA-3′, reverse primer: 5′- TGTACGGCCAATGATGGAATG-3′; mitochondrial superoxide dismutase 2 (Sod2): forward primer: 5′- GCGGTCGTGTAAACCTCAT-3′, reverse primer: 5′- CCAGAGCCTCGTGGTACTTC-3′; extracellular superoxide dismutase 3 (Sod3): forward primer: 5′- CTGAGGACTTCCCAGTGAC-3′, reverse primer: 5′- GGTGAGGGTGTCAGAGTGT-3′; catalase (Cat): forward primer: 5′- AGCGACCAGATGAAGCAGTG-3′, reverse primer: 5′- TCCGCTCTCTGTCAAAGTGTG-3′; heme oxygenase-1 (Hmox1): forward primer: 5′- CACGCATATACCCGCTACCT-3′, reverse primer: 5′- CCAGAGTGTTCATTCGAGA-3′; nuclear factor (erythroid-derived 2)-like 2 (Nrf2): forward primer: 5′- CTCGCTGGAAAAAGAAGTG-3′, reverse primer: 5′- CCGTCCAGGAGTTCAGAGG-3′; glutathione S-transferase-A (Gsta): forward primer: 5′- TGATTGCCGTGGCTCCATTTA-3′, reverse primer: 5′- CAACGAGAAAAGCCTCTCCGT-3′; glutathione S-transferase-M (Gstm): forward primer: 5′- AGCTCACGCTATTCGGCTG-3′, reverse primer: 5′- GCTCCAAGTATTCCACCTTCAGT-3′; glutathione S-transferase-P (Gstp): forward primer: 5′- ATGCCACCATACACCATTGTC-3′, reverse primer: 5′- GGGAGCTGCCCATACAGAC-3′; tumor necrosis factor-α (Tnf): forward primer: 5′- GCATGATCCGCGACGTGGAA-3′, reverse primer: 5′- AGATCCATGCCGTTGGCCAG-3′; macrophage inflammatory protein-1α (Ccl3): forward primer: 5′- ACTGACCTGGAACTGAATGCCTGA-3′, reverse primer: 5′- ATGTGGCTACTTGGCAGCAAACAG-3′; monocyte chemotactic protein-1 (Ccl2): forward primer: 5′- ATGCAGGTCCCTGTCATG-3′, reverse primer: 5′- GCTTGAGGTGGTTGTGGA-3′; interleukin-1β (Il1): forward primer: 5′- CTCCATGAGCTTTGTACAAGG-3′, reverse primer: 5′- TGCTGATGTACCAGTTGGGG-3′; and glyceraldehyde 3-phosphate dehydrogenase (Gapdh): forward primer: 5′- AGGTCATCCCAGAGCTGAACG-3′, reverse primer: 5′- GGAGTTGCTGTTGAAGTCGCA-3′. The primer set for interleukin-6 (Il6) was purchased from SA Bioscience (SABioscience, Qiagen, Valencia, CA).
Metabolomics
Plasma was stored frozen until metabolite extraction by Metabolon, Inc., and subjected to metabolic profiling by ultrahigh-performance liquid chromatography/tandem mass spectrometry. To remove protein, dissociate small molecules bound to protein or trapped in the precipitated protein matrix, and to recover chemically diverse metabolites, proteins were precipitated with methanol under vigorous shaking for 2 min (Glen Mills GenoGrinder 2000) followed by centrifugation. For quality control, recovery standards were added before the first step in the extraction process. The extracts were divided into several fractions. Two fractions were used for reverse-phase (RP)/UPLC-MS/MS comprising positive ion mode electrospray ionization (ESI) and negative ion mode ESI; one fraction was used for HILIC/UPLC-MS/MS with negative ion mode ESI; and one fraction was reserved for potential later analyses. A cocktail of standards known not to interfere with the measurement of endogenous compounds was spiked into every sample, which helps monitor instrument performance and aids in chromatographic alignment.
All methods used a Waters ACQUITY UPLC and a Thermo Scientific Q-Exactive high-resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. The sample extract was reconstituted in solvents compatible with each MS/MS method. Each reconstitution solvent contained a series of standards at fixed concentrations to ensure injection and chromatographic consistency. One aliquot was analyzed using acidic positive ion conditions, chromatographically optimized for more hydrophilic compounds. In this method, the extract was gradient eluted from a C18 column (Waters UPLC BEH C18-2.1 × 100 mm, 1.7 µm) using water and methanol, containing 0.05% perfluoropentanoic acid (PFPA) and 0.1% formic acid (FA). Another aliquot was also analyzed using acidic positive ion conditions; however, it was chromatographically optimized for more hydrophobic compounds. In this method, the extract was gradient eluted from the same aforementioned C18 column using methanol, acetonitrile, water, 0.05% PFPA, and 0.01% FA and was operated at an overall higher organic content. Another aliquot was analyzed using basic negative ion optimized conditions using a separate dedicated C18 column. The basic extracts were gradient-eluted using methanol and water, with 6.5 mM ammonium bicarbonate at pH 8. The fourth aliquot was analyzed via negative ionization following elution from a HILIC column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 µm) using a gradient consisting of water and acetonitrile, with 10 mM ammonium formate, pH 10.8. The MS analysis alternated between MS and data-dependent MSn scans using dynamic exclusion. The scan range varied slightly between methods but covered 70–1,000 m/z.
Compounds were identified by comparison to library entries of purified, authenticated standards or recurrent unknown entities, with known retention indices (RI), mass to charge ratios (m/z), and chromatographic signatures (including MS/MS spectral data) (Metabolon, Inc.). Biochemical identifications were based on three criteria: retention index within a narrow RI window of the proposed identification, accurate mass match to the library ±10 ppm, and the MS/MS forward and reverse scores between experimental data and authentic standards. Proprietary visualization and interpretation software (Metabolon, Inc.) was used to confirm the consistency of peak identification among the various samples. Library matches for each compound were checked for each sample and corrected, if necessary. Area under the curve was used for peak quantification.
Metabolomic data from two independent exposure studies were analyzed using Metaboanalyst 4.0 software (http://www.metaboanalyst.ca/). Data were analyzed following imputation of values and interquartile range filtering, which identified and removed variables unlikely to be of use when modeling the data. The data were then log-transformed and auto-scaled (mean-centered and divided by the standard deviation of each variable), followed by univariate (e.g., ANOVA contrasts, volcano plots), multivariate (e.g., PLS-DA, importance measures), and cluster (heatmap and dendrogram) analyses.
Statistical Analyses
Data are reported as means ± SE, unless indicated otherwise. Unpaired Student’s t test or ANOVA are used for two group or multiple group comparisons, where appropriate, and P < 0.05 was considered significant. For Volcano plot analyses, a raw P value <0.05 and a fold change of 1.25 were used as cut-offs. To gain a broad picture of the analytes affected by CAP, metabolites with an adjusted P value (false discovery rate, FDR) of P < 0.10 were considered statistically significant, and Fisher’s least significant difference test was used for multiple comparisons.
RESULTS
Exposure to CAP Induces Vascular Inflammation and Impairs Vascular Growth Factor Signaling via a Plasmatic Factor
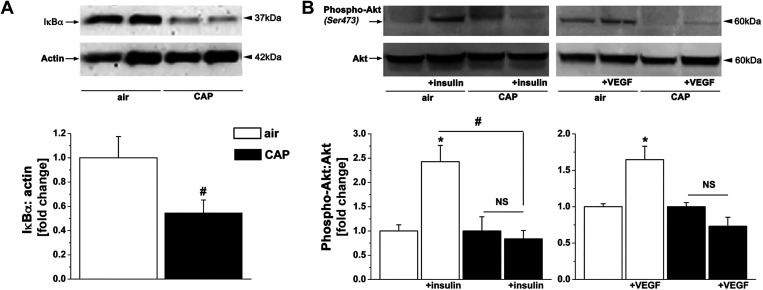
Our previous studies suggest that PM2.5 stimulates vascular inflammation and impairs vascular insulin and VEGF signaling by inducing pulmonary oxidative stress (17, 20). However, it is still unclear how the induction of pulmonary oxidative stress transmits systemically to induce vascular injury. To determine whether a circulating factor might be responsible for the vascular effects of PM2.5 inhalation, we investigated whether ex vivo treatment of naïve aortas with plasma isolated from CAP-exposed mice induces inflammation (exposure 1) and insulin/VEGF resistance (exposure 2). Plasma was isolated from mice exposed to a cumulative CAP dose of 83.7 or 74.6 μg/m3 in exposure 1 or 2, respectively (Table 1). Of note, CAP exposure did not affect circulating levels of insulin or VEGF (Table 2). Naïve aortas were incubated with freshly isolated plasma from air- or CAP-exposed mice for 1 h and then stimulated with either insulin or VEGF for 10 min. Western blot analysis showed that CAP exposure decreased the abundance of IκBα (Fig. 1A) and reduced insulin- and VEGF-induced Akt phosphorylation (Fig. 1B), indicating activation of the NκBα pathway as well as the induction of insulin and VEGF resistance. These findings support the idea that a plasmatic factor is responsible for the induction of vascular inflammation and insulin and VEGF resistance that manifests with CAP exposure.
Table 2.
Physiological and plasma parameter in mice used for the plasma incubation experiment (exposure 2)
| Insulin |
VEGF |
||||||
|---|---|---|---|---|---|---|---|
| Naïve | Air | CAP | P | Air | CAP | P | |
| BW, g | 28.1 ± 0.4 | 27.3 ± 1.2 | 26.9 ± 0.3 | 0.719 | 28.3 ± 0.7 | 28.5 ± 1.0 | 0.815 |
| Heart:BW, % | 0.48 ± 0.01 | 0.48 ± 0.01 | 0.920 | 0.50 ± 0.01 | 0.52 ± 0.02 | 0.139 | |
| Lung:BW, % | 0.51 ± 0.01 | 0.49 ± 0.02 | 0.287 | 0.52 ± 0.03 | 0.52 ± 0.01 | 0.919 | |
| Glucose, mg/dL | 182 ± 4 | 184 ± 15 | 170 ± 9 | 0.435 | 189 ± 11 | 165 ± 10 | 0.142 |
| Insulin, ng/mL | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.637 | 0.42 ± 0.01 | 0.40 ± 0.01 | 0.109 | |
| HOMA-IR | 5.4 ± 0.5 | 4.9 ± 0.2 | 0.393 | 5.6 ± 0.3 | 4.8 ± 0.3 | 0.100 | |
| HOMA-β, % | 36.6 ± 3.9 | 40.2 ± 3.3 | 0.516 | 35.3 ± 3.3 | 41.4 ± 3.1 | 0.225 | |
| VEGF, pg/mL | 15.3 ± 2.8 | 15.4 ± 3.7 | 0.978 | 19.8 ± 2.0 | 13.14 ± 2.1 | 0.065 | |
Values are means ± SE, n = 4. Physiological parameters measured in naïve mice (aorta donor) and in mice exposed for 9 days (exposure 2) to air or CAP (plasma donor) for the plasma incubation experiment (see Fig. 1B). BW, body weight; HOMA-IR, fasting blood glucose (mmol/L) × fasting plasma insulin levels (mU/L)/22.5; HOMA-β, 20× fasting plasma insulin levels (mU/L)/fasting blood glucose (mmol/L)–3.5, %; VEGF, vascular growth factor.
Figure 1.
CAP exposure induces vascular injury via a plasmatic factor. Western blot analysis of inflammation (A) and insulin/VEGF resistance (B) in naïve aortas incubated with plasma isolated from mice exposed for 9 days to HEPA-filtered air or CAP. The aortas were treated with vehicle or stimulated with either insulin (100 nM) or VEGF (20 ng/mL) for 10 min. Western blot data are normalized to the vehicle or air controls. Data are means ± SE (*P < 0.05, insulin/VEGF vs. vehicle; #P < 0.05, air vs. CAP, n = 4). CAP, concentrated ambient PM2.5; NS, not significant; VEGF, vascular endothelial growth factor.
It has been proposed that PM2.5-induced oxidative stress is linked mechanistically to cardiac and vascular injury (2, 32–37). Moreover, our previous findings suggest that CAP exposure promotes vascular inflammation and impairs vascular signaling dependent on the induction of pulmonary oxidative stress (17, 20). To confirm CAP-induced pulmonary oxidative stress and adaptive responses, we measured the expression of several antioxidant response genes in the lungs from air and CAP-exposed mice. We found that CAP exposure increased, similar to our previous findings, the mRNA levels of Sod2 and Sod3 in lungs (Table 3). We observed no upregulation of inflammatory genes in lungs from CAP-exposed mice (Table 3).
Table 3.
Expression of antioxidant defense and inflammatory genes in lungs of plasma donor mice that have been exposed for 9 days to air or CAP (exposure 2)
| Relative mRNA: Gapdh (Fold Change) | Air | CAP | P |
|---|---|---|---|
| Sod1 | 1.00 ± 0.32 | 0.78 ± 0.28 | 0.571 |
| Sod2 | 1.00 ± 0.19 | 4.24 ± 1.36 | 0.015 |
| Sod3 | 1.00 ± 0.34 | 11.10 ± 7.11 | 0.017 |
| Cat | 1.00 ± 0.43 | 0.80 ± 0.32 | 0.512 |
| Hmox1 | 1.00 ± 0.25 | 0.87 ± 0.24 | 0.678 |
| Nrf2 | 1.00 ± 0.36 | 0.88 ± 0.44 | 0.571 |
| Gsta | 1.00 ± 0.53 | 0.54 ± 0.27 | 0.212 |
| Gstm | 1.00 ± 0.25 | 0.94 ± 0.26 | 0.910 |
| Gstp | 1.00 ± 0.56 | 0.78 ± 0.23 | 0.623 |
| Tnfa | 1.00 ± 0.22 | 1.52 ± 0.73 | 0.970 |
| Il1b | 1.00 ± 0.45 | 0.84 ± 0.19 | 0.678 |
| Il6 | 1.00 ± 0.23 | 1.10 ± 0.23 | 0.791 |
| Ccl3 | 1.00 ± 0.16 | 0.92 ± 0.14 | 0.721 |
| Ccl2 | 1.00 ± 0.27 | 0.63 ± 0.27 | 0.212 |
Values are means ± SE normalized to air controls; n = 8. Levels of pulmonary mRNA were measured in lungs of mice exposed for 9 days to air or CAP (exposure 2, plasma donor). Sod1, soluble superoxide dismutase 1; Sod2, mitochondrial superoxide dismutase 2; Sod3, extracellular superoxide dismutase 3, aka ecSOD; Cat, catalase; Hmox1, heme oxygenase-1; Nrf2, nuclear factor (erythroid-derived 2)-like 2; Gsta, glutathione S-transferase-A; Gstm, glutathione S-transferase-M; Gstp, glutathione S-transferase-P; Tnfa, tumor necrosis factor-α; Il1b, interleukin-1b; Il6, interleukin-6; Ccl3, macrophage inflammatory protein-1α, aka mip-1α; Ccl2, monocyte chemotactic protein-1, aka mcp-1.
Pulmonary Overexpression of ecSOD Prevents CAP-Induced Changes in the Circulating Metabolome
Our results from plasma incubation experiments, combined with findings from our previous studies that demonstrate that PM2.5 by inducing pulmonary oxidative stress stimulates vascular inflammation and insulin/VEGF resistance (17, 20), suggest that PM2.5-induced vascular inflammation and insulin/VEGF resistance is caused by a circulating factor and that the generation of this circulating factor is dependent on pulmonary oxidative stress. Hence, we tested how pulmonary oxidative stress contributes to CAP-induced changes in the plasma metabolome. For this, we exposed ecSOD-Tg mice and their WT littermates to HEPA-filtered air or CAP in two independent exposure experiments. Mice were exposed to a cumulative CAP dose (Table 1) of either 61.8 μg/m3 (exposure 3, Metabolome I, CAP60) or 97.0 μg/m3 (exposure 4, Metabolome II, CAP100). Immediately after the final exposure, we collected plasma and assessed the relative concentrations of circulating metabolites via untargeted metabolomic analysis. Our previous studies confirmed the overexpression of ecSOD in the lungs and established that this protects from CAP-induced pulmonary oxidative stress (17, 20).
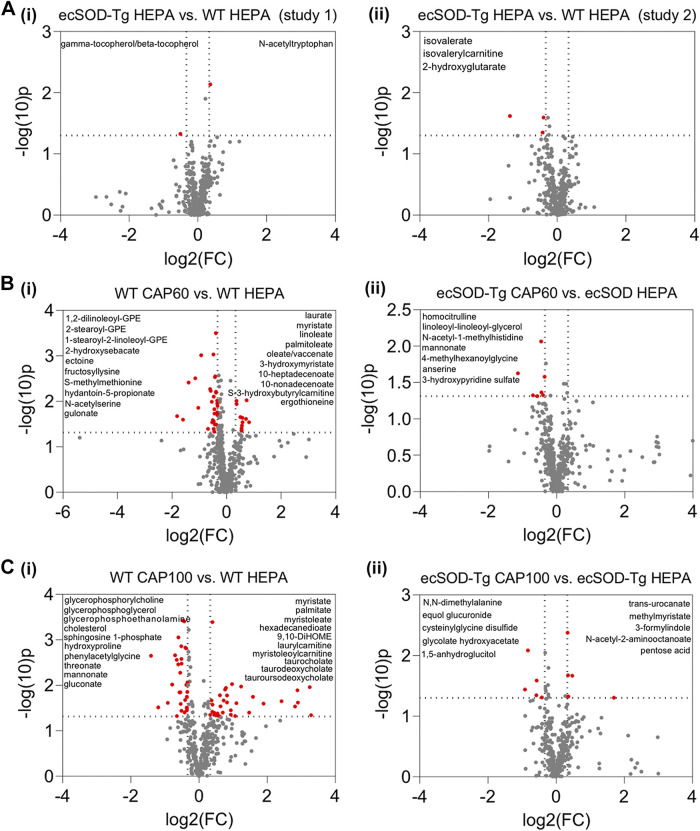
To gain insight into metabolic changes caused by PM2.5 exposure in WT mice, we assessed the relative concentrations of circulating metabolites via untargeted metabolomic analyses. We then compared the metabolomic changes that occurred in the plasma collected from WT and ecSOD-Tg mice exposed to either HEPA-filtered air, CAP60, or CAP100. Overexpression of ecSOD in the lungs had minimal effects on the plasma metabolome in control mice inhaling HEPA-filtered air, with only five metabolites differing from WT controls (Fig. 2A). In WT mice, we found 45 plasma metabolites that were elevated by CAP60 exposure by at least 1.25-fold (raw P value ≤ 0.05). Of the metabolites elevated in WT mice exposed to CAP60, 16 were in the lipid superfamily, with numerous saturated (e.g., laurate, myristate, 3-hydroxymyristate) and unsaturated (e.g., palmitoleate, linoleate, oleate/vaccinate) fatty acids increasing with CAP exposure. Significantly decreased by CAP60 exposure were several GPE species as well as other metabolites (e.g., 2-hydroxysebacate, ectoine, fructosyllysine, and gulonate) (Fig. 2Bi). In ecSOD-Tg mice exposed to CAP60, only seven metabolites were significantly different in plasma from CAP60-exposed mice (Fig. 2Bii).
Figure 2.
Overexpression of ecSOD in the lung prevents CAP-induced changes in the plasma metabolome. Volcano plots comparing plasma metabolite changes in ecSOD-Tg and WT mice under control conditions (HEPA-filtered air) and after CAP exposure: Mice were subjected to 9 days of exposure (6 h/day) to HEPA-filtered air, ∼60 µg/m3 CAP (CAP60, exposure 3), or ∼100 µg/m3 CAP (CAP100, exposure 4). Relative metabolite abundances in plasma were measured by unbiased metabolomic profiling. Metabolites that changed at least 1.25-fold with a raw P value <0.05 were considered statistically significant (indicated in red). The identities of some of the metabolites that changed significantly with CAP exposure are provided in the upper left and/or right quadrants of each volcano plot. A: plasma metabolite changes caused by ecSOD overexpression alone in metabolomics study I (i; n = 15 mice, 9 WT mice and 6 ecSOD-Tg mice) and metabolomics study II (ii; n = 19 mice, 10 WT mice and 9 ecSOD-Tg mice). B: plasma metabolite changes caused by CAP60 exposure in WT mice (i; n = 17 mice, 8 WT CAP60, 9 WT HEPA) and ecSOD-Tg mice (ii; n = 15 mice, 9 ecSOD-Tg CAP60 and 6 ecSOD-Tg HEPA). C: plasma metabolite changes caused by CAP100 exposure in WT mice (i; n = 19 mice, 9 WT CAP100 and 10 WT HEPA) and ecSOD-Tg mice (ii; n = 19 mice, 10 ecSOD-Tg CAP100 and 9 ecSOD-Tg HEPA). CAP, concentrated ambient PM2.5; ecSOD-Tg, extracellular superoxide dismutase-transgenic; WT, wild type.
Exposure to a higher CAP concentration (CAP100) significantly altered the abundance of 63 metabolites in WT mice (Fig. 2Ci), with 29 significantly different metabolites derived from the lipid family. In particular, CAP100 increased saturated fatty acid species (e.g., myristate, palmitate, 3-hydroxylaurate, 3-hydroxystearate, and 3-hydroxymyristate) as well as augmented levels of several unsaturated fatty acids (e.g., palmitoleate, myristoleate, stearidonate, and tetradecadienoate); increased concentrations of dicarboxylate fatty acid species (e.g., hexadecanedioate, octadecenedioate, and 9,10-diHOME); augmented laurylcarnitine, myristoleoylcarnitine, and palmitoleoylcarnitine levels; and elevated the abundance of circulating bile acid metabolites (e.g., taurocholate, taurodeoxycholate, taurochenodeoxycholate, tauroursodeoxycholate, 12-ketolithocholate, and among others). Exposure to CAP100 diminished cholesterol and several phospholipid species including glycerophosphorylcholine, glycerophosphoglycerol, and glycerophosphorylethanolamine. Other metabolites diminished by CAP100 include sphingosine 1-phosphate, hydroxyproline, and several sugar acid species (e.g., threonate, mannonate, and gluconate). Similar to the CAP60 study results, the metabolic features of the CAP-induced dyslipidemia found in WT mice exposed to CAP100 were largely absent in ecSOD-Tg mice: in ecSOD-Tg mice, only 10 metabolites were significantly altered by CAP100 (Fig. 2Cii). Collectively, these findings indicate that oxidative stress in the lungs reproducibly drives the acquisition of CAP-induced dyslipidemia, which is characterized by high levels of circulating fatty acids, acylcarnitines and bile acids, and low levels of circulating phospholipids.
High Concentrations of CAP Drive More Severe Changes in the Circulating Lipidome
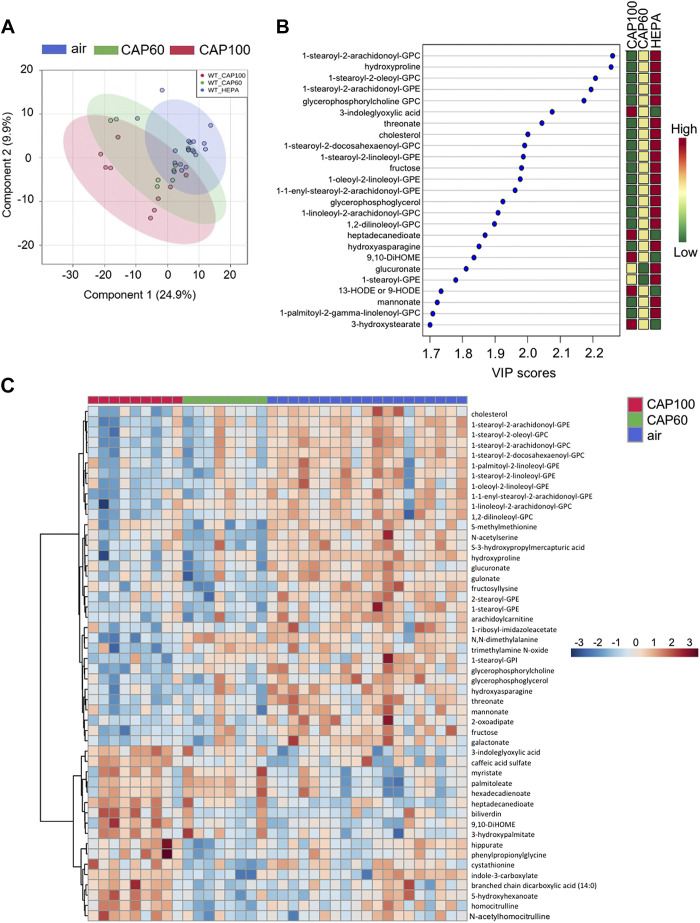
Metabolomics analysis of merged data from the CAP60 and CAP100 exposure studies revealed that CAP exposure significantly altered the levels of 96 out of the 620 metabolites (Table 4). Partial least squared discriminant analysis (PLS-DA) implies separation of the groups based on the level of CAP exposure (Fig. 3A), and variable importance in projection (VIP) scoring indicated that many of the metabolites driving group separation were lipid species (Fig. 3B). Heatmap analysis also demonstrated that lipids are the most prominent metabolite species altered by CAP exposure. Cholesterol and numerous phospholipids were lower in both the CAP60 and CAP100 groups, whereas fatty acids and hydroxylated fatty acids (e.g., myristate, oleate, palmitoleate, and 3-hydroxypalmitate) were higher. In addition, bioactive mono- and dihydroxy fatty acids derived from linoleic acid such as 9- or 13-HODE, 9,10-diHOME, and 12,13-diHOME were elevated in plasma from CAP-exposed mice (Figs. 3C and 4 and Table 4).
Table 4.
Significant CAP-exposure induced changes in plasma metabolites in WT mice
| Metabolite | F Value | P Value | −10 log(p) | FDR | Fisher’s LSD |
|---|---|---|---|---|---|
| Hydroxyproline | 15.722 | 1.60E-05 | 4.7959 | 0.007439 | air vs. CAP100; air vs. CAP60 |
| 1-Stearoyl-2-arachidonoyl-GPC 18:0/20:4 | 13.347 | 5.66E-05 | 4.2473 | 0.009064 | air vs. CAP100; air vs. CAP60 |
| 1-Stearoyl-2-oleoyl-GPC 18:0/18:1 | 12.374 | 9.78E-05 | 4.0099 | 0.009064 | air vs. CAP100; air vs. CAP60 |
| 1-stearoyl-2-arachidonoyl-GPE 18:0/20:4 | 12.062 | 0.000117 | 3.9319 | 0.009064 | air vs. CAP100; air vs. CAP60 |
| Homocitrulline | 11.736 | 0.000141 | 3.8497 | 0.009064 |
CAP100 vs. CAP60 air vs. CAP100; air vs. CAP60 |
| N,N-dimethylalanine | 11.628 | 0.000151 | 3.8224 | 0.009064 | air vs. CAP100; air vs. CAP60 |
| Glycerophosphorylcholine (GPC) | 11.478 | 0.000164 | 3.7841 | 0.009064 |
CAP100 vs. CAP60 air vs. CAP100; air vs. CAP60 |
| 2-Stearoyl-GPE 18:0 | 11.342 | 0.000178 | 3.7492 | 0.009064 | air vs. CAP100; air vs. CAP60 |
| Glucuronate | 11.196 | 0.000194 | 3.7114 | 0.009064 | air vs. CAP100; air vs. CAP60 |
| Fructosyllysine | 11.182 | 0.000196 | 3.7078 | 0.009064 |
CAP100 vs. CAP60 air vs. CAP100; air vs. CAP60 |
| N-acetylserine | 11.032 | 0.000214 | 3.6687 | 0.009064 |
CAP100 vs. CAP60 air vs. CAP100; air vs. CAP60 |
| 1-Stearoyl-GPE 18:0 | 10.727 | 0.000258 | 3.5889 | 0.009986 | air vs. CAP100; air vs. CAP60 |
| Threonate | 10.165 | 0.000363 | 3.4396 | 0.012482 | air vs. CAP100; air vs. CAP60 |
| 1-Stearoyl-2-linoleoyl-GPE 18:0/18:2 | 10.065 | 0.000387 | 3.4126 | 0.012482 | air vs. CAP100; air vs. CAP60 |
| S-methylmethionine | 9.9783 | 0.000408 | 3.3892 | 0.012482 |
CAP100 vs. CAP60 air vs. CAP100; air vs. CAP60 |
| 3-Indoleglyoxylic acid | 9.8966 | 0.000429 | 3.3671 | 0.012482 | air vs. CAP100; air vs. CAP60 |
| Trimethylamine N-oxide | 9.0477 | 0.000737 | 3.1328 | 0.019097 |
CAP100 vs. CAP60 air vs. CAP100 |
| Cholesterol | 8.9272 | 0.000796 | 3.0989 | 0.019097 | air vs. CAP100; air vs. CAP60 |
| 1-Stearoyl-2-docosahexa enoyl-GPC 18:0/22:6 | 8.9252 | 0.000797 | 3.0984 | 0.019097 | air vs. CAP100; air vs. CAP60 |
| Palmitoleate 16:1n7 | 8.8794 | 0.000821 | 3.0855 | 0.019097 |
CAP100 vs. CAP60 air vs. CAP100; air vs. CAP60 |
| Glycerophosphoglycerol | 8.591 | 0.000992 | 3.0036 | 0.021015 | air vs. CAP100; air vs. CAP60 |
| 1-Linoleoyl-2-arachidonoyl-GPC 18:2/20:4n6 | 8.5232 | 0.001037 | 2.9842 | 0.021015 |
CAP100 vs. CAP60 air vs. CAP100 |
| Fructose | 8.4745 | 0.001071 | 2.9702 | 0.021015 | air vs. CAP100; air vs. CAP60 |
| 1-Oleoyl-2-linoleoyl-GPE 18:1/18:2 | 8.4553 | 0.001085 | 2.9647 | 0.021015 | air vs. CAP100; air vs. CAP60 |
| Gulonate | 8.3262 | 0.001182 | 2.9275 | 0.021978 | air vs. CAP100; air vs. CAP60 |
| 1-1-Enyl-stearoyl-2-arachidonoyl-GPE P-18:0/20:4 | 8.2655 | 0.00123 | 2.91 | 0.022003 | air vs. CAP100; air vs. CAP60 |
| 1,2-Dilinoleoyl-GPC 18:2/18:2 | 7.9573 | 0.001513 | 2.8203 | 0.025873 | air vs. CAP100; air vs. CAP60 |
| Cystathionine | 7.9136 | 0.001558 | 2.8074 | 0.025873 |
CAP100 vs. CAP60 air vs. CAP60 |
| 9,10-DiHOME | 7.4997 | 0.002066 | 2.6849 | 0.033124 |
CAP100 vs. CAP60 air vs. CAP100 |
| Heptadecanedioate C17-DC | 7.3354 | 0.002314 | 2.6357 | 0.034457 | air vs. CAP100; air vs. CAP60 |
| Indole-3-carboxylate | 7.312 | 0.002352 | 2.6287 | 0.034457 |
CAP100 vs. CAP60 air vs. CAP60 |
| Hydroxyasparagine | 7.3 | 0.002371 | 2.625 | 0.034457 |
CAP100 vs. CAP60 air vs. CAP60 |
| N-acetylcitrulline | 7.1256 | 0.002677 | 2.5723 | 0.037723 |
CAP100 vs. CAP60 air vs. CAP100 |
| 5-Hydroxyhexanoate | 6.8747 | 0.003193 | 2.4958 | 0.043283 |
CAP100 vs. CAP60 air vs. CAP100 |
| N-acetylhomocitrulline | 6.8462 | 0.003258 | 2.4871 | 0.043283 |
CAP100 vs. CAP60 air vs. CAP100 |
| Arachidoylcarnitine C20 | 6.7083 | 0.003592 | 2.4446 | 0.046399 | air vs. CAP100; air vs. CAP60 |
| 1-Palmitoyl-2-linoleoyl-GPE 16:0/18:2 | 6.5395 | 0.004052 | 2.3923 | 0.050927 | air vs. CAP100; air vs. CAP60 |
| 1-Ribosyl-imidazoleacetate | 6.4757 | 0.004242 | 2.3724 | 0.051906 | air vs. CAP100; air vs. CAP60 |
| Mannonate | 6.2803 | 0.004884 | 2.3113 | 0.058228 | air vs. CAP100; air vs. CAP60 |
| Biliverdin | 6.2295 | 0.005067 | 2.2953 | 0.058902 |
CAP100 vs. CAP60 air vs. CAP100 |
| Galactonate | 6.1126 | 0.005517 | 2.2583 | 0.061149 | air vs. CAP100; air vs. CAP60 |
| 3-Hydroxypalmitate | 6.1111 | 0.005523 | 2.2578 | 0.061149 | air vs. CAP100; air vs. CAP60 |
| Oleate/vaccenate 18:1 | 6.0668 | 0.005705 | 2.2438 | 0.06169 |
CAP100 vs. CAP60 air vs. CAP60 |
| Myristate 140 | 5.9848 | 0.006058 | 2.2177 | 0.064022 | air vs. CAP100; air vs. CAP60 |
| 1-Stearoyl-2-oleoyl-GPG 18:0/18:1 | 5.9103 | 0.006399 | 2.1939 | 0.066123 |
CAP100 vs. CAP60 air vs. CAP60 |
| 13-HODE or 9-HODE | 5.8315 | 0.006782 | 2.1687 | 0.068554 | air vs. CAP100; air vs. CAP60 |
| 12,13-DiHOME | 5.7508 | 0.007199 | 2.1427 | 0.0695 |
CAP100 vs. CAP60 air vs. CAP100 |
| 1-Stearoyl-2-docosahexa enoyl-GPE 18:0/22:6 | 5.7489 | 0.007209 | 2.1421 | 0.0695 | air vs. CAP100; air vs. CAP60 |
| 10-Heptadecenoate 17:1n7 | 5.7277 | 0.007324 | 2.1353 | 0.0695 |
CAP100 vs. CAP60 air vs. CAP60 |
| 1-Palmitoyl-2-γ-linolenoyl-GPC 16:0/18:3n6 | 5.6091 | 0.007999 | 2.0969 | 0.074017 | air vs. CAP100 |
| 3-Hydroxystearate | 5.5838 | 0.008152 | 2.0887 | 0.074017 |
CAP100 vs. CAP60 air vs. CAP100 |
| 2-Oxoadipate | 5.5634 | 0.008277 | 2.0821 | 0.074017 | air vs. CAP100; air vs. CAP60 |
| 10-Nonadecenoate 19:1n9 | 5.5289 | 0.008494 | 2.0709 | 0.074519 | air vs. CAP60 |
| Taurocholate | 5.4693 | 0.008882 | 2.0515 | 0.076485 |
CAP100 vs. CAP60 air vs. CAP100 |
| Taurodeoxycholate | 5.4181 | 0.00923 | 2.0348 | 0.078039 |
CAP100 vs. CAP60 air vs. CAP100 |
| S-3-hydroxypropyl mercapturicacid (HPMA) | 5.3892 | 0.009434 | 2.0253 | 0.078333 | air vs. CAP100; air vs. CAP60 |
| 3-Phenylpropionate (hydrocinnamate) | 5.2757 | 0.010279 | 1.988 | 0.083856 |
CAP100 vs. CAP60 air vs. CAP100 |
| 3-Hydroxymyristate | 5.2001 | 0.010886 | 1.9631 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| S-3-hydroxybutyrylcarnitine | 5.1533 | 0.011281 | 1.9477 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| 2-Methylbutyrylcarnitine C5 | 5.1156 | 0.01161 | 1.9352 | 0.086605 |
CAP100 vs. CAP60 air vs. CAP100 |
| 1-Linoleoyl-2-linolenoyl-GPC 18:2/18:3 | 5.1087 | 0.011671 | 1.9329 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Malonate | 5.0925 | 0.011816 | 1.9275 | 0.086605 |
CAP100 vs. CAP60 air vs. CAP60 |
| 3-Hydroxylaurate | 5.0628 | 0.012088 | 1.9176 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Lyxonate | 5.0218 | 0.012474 | 1.904 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| 5-Hydroxylysine | 5.0204 | 0.012487 | 1.9035 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Linoleate 18:2n6 | 5.013 | 0.012558 | 1.9011 | 0.086605 |
CAP100 vs. CAP60 air vs. CAP60 |
| 10-Undecenoate 11:1n1 | 4.9814 | 0.012866 | 1.8905 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Dodecadienoate 12:2 | 4.973 | 0.012949 | 1.8877 | 0.086605 | air vs. CAP100 |
| Hexadecadienoate16:2n6 | 4.9416 | 0.013266 | 1.8772 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| N2,N2-dimethylguanosine | 4.8918 | 0.013785 | 1.8606 | 0.086605 |
CAP100 vs. CAP60 air vs. CAP100 |
| Sphingomyelin d18:2/18:1 | 4.8889 | 0.013816 | 1.8596 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Linolenate 18:3n3 or 3n6 | 4.8772 | 0.013942 | 1.8557 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Glycerophosphoethanolamine | 4.8671 | 0.014051 | 1.8523 | 0.086605 | air vs. CAP100 |
| 1-Stearoyl-GPI 18:0 | 4.86 | 0.014127 | 1.8499 | 0.086605 | air vs. CAP100 |
| 16-Hydroxypalmitate | 4.8524 | 0.014211 | 1.8474 | 0.086605 | air vs. CAP100 |
| N-formylmethionine | 4.8456 | 0.014285 | 1.8451 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Carotene diol 1 | 4.8339 | 0.014415 | 1.8412 | 0.086605 |
CAP100 vs. CAP60 air vs. CAP60 |
| Myristoleate 14:1n5 | 4.8131 | 0.01465 | 1.8342 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| 3-Hydroxydecanoate | 4.8074 | 0.014714 | 1.8323 | 0.086605 | air vs. CAP100; air vs. CAP60 |
| Hippurate | 4.754 | 0.015336 | 1.8143 | 0.089138 |
CAP100 vs. CAP60 air vs. CAP100 |
| Eicosenoate 20:1n9 or 1n11 | 4.7034 | 0.015951 | 1.7972 | 0.091568 | air vs. CAP60 |
| Cinnamate | 4.6483 | 0.016651 | 1.7786 | 0.092252 |
CAP100 vs. CAP60 air vs. CAP100 |
| Stearidonate 18:4n3 | 4.6429 | 0.016722 | 1.7767 | 0.092252 | air vs. CAP100; air vs. CAP60 |
| 2-Hydroxyoctanoate | 4.6185 | 0.017043 | 1.7685 | 0.092252 |
CAP100 vs. CAP60 air vs. CAP100 |
| Phenylpyruvate | 4.6169 | 0.017064 | 1.7679 | 0.092252 |
CAP100 vs. CAP60 air vs. CAP100 |
| Laurylcarnitine C12 | 4.5871 | 0.017467 | 1.7578 | 0.092252 | air vs. CAP100; air vs. CAP60 |
| Hexadecanedioate C16 | 4.5828 | 0.017525 | 1.7563 | 0.092252 | air vs. CAP100; air vs. CAP60 |
| Palmitate 160 | 4.5802 | 0.017561 | 1.7554 | 0.092252 | air vs. CAP100; air vs. CAP60 |
| N-acetyl-β-alanine | 4.5712 | 0.017686 | 1.7524 | 0.092252 |
CAP100 vs. CAP60 air vs. CAP60 |
| Gluconate | 4.5466 | 0.018029 | 1.744 | 0.092252 | air vs. CAP100; air vs. CAP60 |
| 2- or 3-Decenoate 10:1n7 or n8 | 4.5449 | 0.018054 | 1.7434 | 0.092252 | air vs. CAP100 |
| Palmitoleoylcarnitine C16:1 | 4.5184 | 0.018433 | 1.7344 | 0.093169 | air vs. CAP100; air vs. CAP60 |
| Myristoleoylcarnitine C14:1 | 4.4885 | 0.01887 | 1.7242 | 0.094351 | air vs. CAP100; air vs. CAP60 |
| Formiminoglutamate | 4.4746 | 0.019079 | 1.7194 | 0.09438 |
CAP100 vs. CAP60 air vs. CAP60 |
| 3-Methoxytyrosine | 4.438 | 0.019636 | 1.7069 | 0.096114 |
CAP100 vs. CAP60 air vs. CAP60 |
| N-acetylglutamate | 4.4233 | 0.019865 | 1.7019 | 0.09622 | air vs. CAP60 |
Metabolites extracted from plasma of male wild-type mice inhaling CAP or HEPA-filtered air for 6 h/day for 9 days. Metabolites were subjected to LC-MS analysis. Metabolites with an adjusted P value (FDR) cutoff of P < 0.1 were considered significantly different. Fisher’s least significant difference (LSD) method was used for multiple comparisons. CAP, concentrated ambient PM2.5; FDR, false discovery rate.
Figure 3.
Merged plasma metabolomic data reveal lipid species that are strongly influenced by CAP. Multivariate and heatmap metabolomic analyses of plasma from WT mice exposed to HEPA-filtered air, 60 µg/m3 CAP (CAP60) or 100 µg/m3 CAP (CAP100) for 9 days (6 h/day). Data from metabolomics study 1 (exposure 3) and metabolomics study 2 (exposure 4) were merged and each biochemical was rescaled to set the median equal to 1. Then, missing values were imputed with the minimum value for each biochemical. A: partial least squared discriminant analysis. B: variable importance in projection (VIP) analysis. C: heatmap analysis showing the 50 most significantly changed plasma metabolites in CAP-exposed mice (ANOVA). An FDR of <0.10 was considered statistically significant (n = 36 WT mice: 19 HEPA, 8 CAP60, and 9 CAP100). CAP, concentrated ambient PM2.5; FDR, false discovery rate; WT, wild type.
Figure 4.
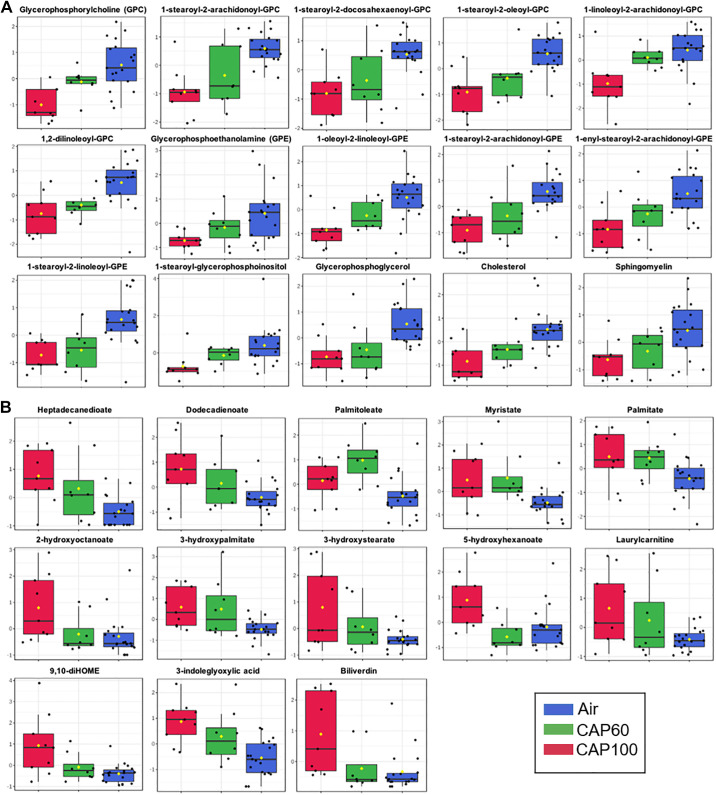
CAP concentration influences the circulating metabolome. Box plots of circulating metabolites that were significantly different in WT mice exposed to HEPA-filtered air, 60 µg/m3 CAP (CAP60) or 100 µg/m3 CAP (CAP100) for 9 days (6 h/day). Data from metabolomics study 1 (exposure 3) and metabolomics study 2 (exposure 4) were merged and each biochemical was rescaled to set the median equal to 1. Then, missing values were imputed with the minimum value for each biochemical. A: lipid species that decreased progressively with increasing CAP exposure. B: lipid species that increased progressively with increasing CAP exposure. ANOVA: an FDR of <0.10 was considered statistically significant (n = 36 WT mice: 19 HEPA, 8 CAP60, and 9 CAP100). CAP, concentrated ambient PM2.5; FDR, false discovery rate; WT, wild type.
Higher concentrations of CAP augmented metabolite changes in plasma. For instance, exposure to either CAP60 or CAP100 decreased the abundance of GPC, GPE, and glycerophosphoinositol (GPI) species as well as cholesterol and sphingomyelin, an effect that appears stronger with CAP100 than with CAP60 exposure (Fig. 4A). Nonhydroxylated and hydroxylated fatty acids, inflammatory lipids, and acylcarnitine species increased with the higher CAP dose (Fig. 4A). Collectively, these data suggest that CAP concentration influences the magnitude by which CAP increases levels of circulating fatty acids and decreases levels of phospholipids, cholesterol, and sphingomyelins.
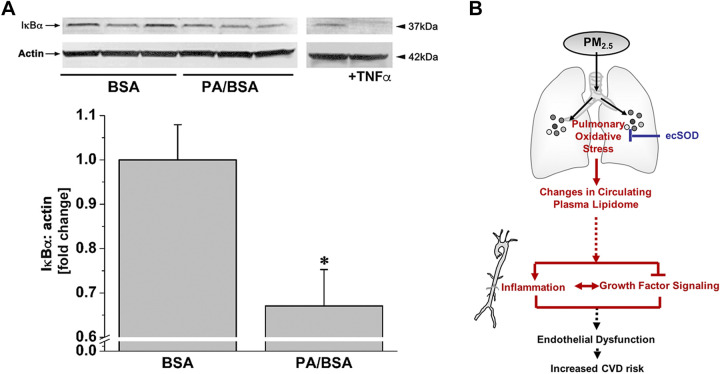
Treatment with Fatty Acids Induces Endothelial Inflammation
Previous studies indicate that circulating free fatty acids promote vascular inflammation and impair insulin signaling (38–42). The influence of fatty acids on vascular inflammation was confirmed by our experiment in endothelial cells, which demonstrated that incubation with palmitic acid (PA) decreases the abundance of IκBα (Fig. 5A).
Figure 5.
Free fatty acids are sufficient to cause vascular pathology. Western blot analysis of IκBα in endothelial cells (A) incubated for 1 h with either bovine serum albumin (BSA, vehicle) or 100 µM palmitic acid (Sigma-Aldrich, complexed with BSA, PA/BSA). HUVEC incubated with TNF-α (10 ng/mL, 15 min) was used as a positive control. Western blot data are normalized to the vehicle controls. Data are means ± SE (*P < 0.05, PA/BSA vs. BSA, n = 3). B: inhalation of PM2.5 air pollution promotes a unique form of dyslipidemia that manifests in a manner dependent on pulmonary oxidative stress. This dyslipidemic phenotype is characterized by diminished phospholipid species and elevated free fatty acid species. Because elevated free fatty acids are sufficient to cause vascular inflammation and insulin or VEGF resistance, it is likely that PM2.5-induced dyslipidemia contributes to the increased CVD risk associated with air pollution. BSA, bovine serum albumin; HUVEC, human umbilical vein cell.
Taken together, exposure to PM2.5 induces changes to the plasma metabolome that are dependent on pulmonary oxidative stress. This CAP-induced dyslipidemic phenotype is characterized by an increase in free fatty acids and a decrease in phospholipid. As elevated free fatty acids have been demonstrated as sufficient to cause vascular inflammation and growth factor resistance, it is likely that PM2.5-induced dyslipidemia contributes to the increased CVD risk associated with air pollution exposure as summarized in Fig. 5B.
DISCUSSION
Our results indicate that exposure to PM2.5 air pollution induces vascular inflammation as well as vascular insulin and VEGF resistance via a plasmatic factor. We find that PM2.5 exposure leads to the development of a dyslipidemic phenotype dependent on CAP-induced oxidative stress in the lungs. This pollution-induced form of dyslipidemia is characterized by high levels of circulating FFAs and acylcarnitines and low levels of phospholipid species. We also find that exposure of endothelial cells to heightened levels of fatty acids, such as occurs in vivo with PM2.5 exposure, is sufficient to promote inflammation. Taken as a whole, these findings suggest that PM2.5-induced elevations in circulating fatty acids may contribute to vascular pathology caused by air pollution.
These findings add to our understanding of the mechanisms by which air pollution promotes vascular injury and dysfunction. Our previous studies demonstrate that exposure to CAP activates NF-κB and inflammasome pathways in blood vessels and impairs vascular insulin and VEGF signaling (17–20). Our findings also suggest that pulmonary oxidative stress contributes to the CAP-induced vascular pathology because lung-specific overexpression of ecSOD prevents PM2.5-induced vascular inflammation and growth factor resistance (17, 20). That CAP induces pulmonary expression of Sod2 and Sod3, which encodes the mitochondrial and extracellular isoforms of SOD, in the absence of upregulation of genes encoding other sensitive antioxidant enzyme such as HO-1 and catalase suggests a unique antioxidant response caused by air pollution. This unique response seems to have translational relevance as exposure to elevated levels of PM2.5 upregulates ecSOD in human plasma (43).
Our studies also identify a unique form of dyslipidemia caused by PM2.5. We find that CAP diminishes levels of numerous phospholipid species, cholesterol, and sphingomyelin, which is similar to the serum metabolomic changes reported after airway administration of a water-soluble PM2.5 extract (44). CAP also increased the levels of circulating FFAs such as myristate, palmitate, and palmitoleate, among others, in a manner dependent on CAP concentration. This is interesting given the fact that many fatty acid species have been shown to be sufficient causes of vascular and endothelial inflammation and insulin resistance (38–42, 45, 46). That these metabolic changes in plasma could contribute to vascular inflammation and growth factor resistance are demonstrated by our ex vivo incubation experiments in which plasma from CAP-exposed mice promoted NF-κB activation and insulin and VEGF resistance. Such findings are congruent with those that find that particulate air pollution from diesel exhaust activates the endothelium via a plasmatic factor (24). This study demonstrated that a 24-h treatment of ECs with plasma of individuals exposed to diesel emission induces inflammation. Studies from the same group demonstrated that infusion of serum from ozone-exposed rats for 30 min impaired Ach-mediated vasodilation, indicating that short-term treatment with plasma isolated from air pollution-exposed rodents triggers vascular dysfunction (29, 47). The results of our isolated aorta study similarly indicate that the induction of vascular inflammation and insulin resistance is an immediate response to a PM2.5-induced plasmatic factor (e.g., FFA). This is further supported by a study showing that short-term FFA incubation (3 h) induces vascular inflammation and insulin resistance (28). However, processes contributing to PM2.5-induced generation and plasmatic accumulation of FFA in vivo might take longer (e.g., days) and require further investigation. Collectively, our findings help develop a working model suggesting that CAP-induced vascular toxicity is initiated via oxidative stress in the lungs, which elevates levels of circulating FFAs and promotes vascular inflammation and growth factor resistance (Fig. 5B).
It remains unclear how PM2.5-induced oxidative stress in the lungs elevates circulating fatty acids and diminishes phospholipids, cholesterol, and sphingomyelins. Possibilities supported by the literature include activation of lipolysis, dysregulation of lipid metabolism in the liver, and/or mitochondrial dysfunction. Supporting the idea that lipolysis may be involved are studies showing that exposure to air pollution elevates catecholamines (48–50), which are potent activators of lipolysis enzymes (51) and could lead to the development of CAP-induced dyslipidemia. The potential involvement of the liver is supported by numerous studies showing that PM2.5 promotes liver steatosis and fibrosis (52–55). In addition, we found that biliverdin was elevated in mice exposed to the higher level of CAP, which may suggest some degree of liver damage. The idea that mitochondrial damage occurs with CAP exposure is supported by in vivo stable isotope metabolomics data, which indicates decreased liver Krebs cycle activity in mice exposed to CAP (56). That circulating acylcarnitines—known markers of defects in fatty acid oxidation (57)—were elevated by CAP in our study further attests to the plausibility of this mechanism of CAP-induced dyslipidemia. Future studies are required to delineate the contribution of each of these possibilities to CAP-induced changes in the circulating lipidome.
Beyond changes in simple saturated and unsaturated fatty acids, phospholipids, and cholesterol, several other metabolites were found to be significantly altered by CAP. For example, toxic hydroxylated fatty acids such as 9,10-diHOME were elevated by CAP, which could influence the onset or progression of PM2.5-induced pathology; 9,10-diHOME is particularly interesting because it is formed from a cytochrome P450-derived epoxide via soluble epoxide hydrolase, a metabolic conversion that has been suggested to occur in neutrophils (58, 59). Thus, it is possible that neutrophils, which are increased in the lungs with PM2.5 exposure (53), may stimulate pulmonary redox changes that provoke CAP-induced vascular pathologies. In mice exposed to higher levels of CAP, we also found an elevation in numerous bile acid species. These species were not changed at the lower level of CAP exposure, which suggests a threshold effect of CAP on bile acids. Bile acids may be important for regulating systemic lipid metabolism and the development of insulin resistance (60). Decreases in sugar acids were also prominent at the higher level of CAP exposure; it remains unclear why these metabolites decrease with CAP and what their significance to particle toxicity may be, if any. Regardless, the fact that ecSOD overexpression blocked CAP-induced dyslipidemia and other changes in the plasma metabolome suggests that pulmonary oxidative stress is a nidus for the systemic metabolic effects of PM2.5.
Our study has several limitations. Although it is known that elevated saturated fatty acids are sufficient causes of endothelial inflammation and growth factor resistance, it is possible that other circulating factors contribute to PM2.5-induced vascular toxicity. One possibility is that circulating cytokines could contribute to vascular injury caused by CAP; however, in past studies, we have not found changes in circulating cytokines such as TNF-α and IL-6 in the plasma from CAP-exposed mice (20). In contrast, our studies and investigations by others indicate that PM2.5 exposure increases local inflammation in vascular and cardiac tissue (10, 19). Another possibility is that circulating microparticles or exosomes could deliver cargo to the vasculature that contributes to pathology; few published reports have addressed this possibility (23, 61, 62). Nevertheless, our findings suggest that PM2.5 promotes a unique form of dyslipidemia that is dependent on pulmonary oxidative stress which could contribute to the etiology and progression of vascular disease.
GRANTS
This work was supported by grants from the National Institute of Health: ES027881, ES028268, and GM127607.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.G.H. and P.H. conceived and designed research; B.R., A.R., and P.H. performed experiments; B.G.H., B.R., A.R., and P.H. analyzed data; B.G.H. and P.H. interpreted results of experiments; B.G.H., A.R., and P.H. prepared figures; B.G.H. and P.H. drafted manuscript; B.G.H. and P.H. edited and revised manuscript; B.G.H., B.R., A.R., and P.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Animal Models and Phenotypic Core of the Diabetes and Obesity Center as well as the staff of the Inhalation Facility for technical assistance and thank Jason Clark, Jin Jiu-Zhen, Deanna Davis, and Zimple Kurlawala for technical support.
REFERNCES
- 1.World Health Organization (WHO). https://www.who.int/health-topics/air-pollution#tab=tab_1.
- 2.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109: 71–77, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res 116: 108–115, 2015. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 5.Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes care 33: 2196–2201, 2010. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao X, Montresor-Lopez J, Puett R, Rajagopalan S, Brook RD. Ambient air pollution: an emerging risk factor for diabetes mellitus. Curr Diab Rep 15: 603, 2015. doi: 10.1007/s11892-015-0603-8. [DOI] [PubMed] [Google Scholar]
- 7.Goodson JM, MacDonald JW, Bammler TK, Chien WM, Chin MT. In utero exposure to diesel exhaust is associated with alterations in neonatal cardiomyocyte transcription, DNA methylation and metabolic perturbation. Part Fibre Toxicol 16: 17, 2019. doi: 10.1186/s12989-019-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorr MW, Velten M, Nelin TD, Youtz DJ, Sun Q, Wold LE. Early life exposure to air pollution induces adult cardiac dysfunction. Am J Physiol Heart Circ Physiol 307: H1353–H1360, 2014. doi: 10.1152/ajpheart.00526.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Weldy CS, Chin MT. Neonatal diesel exhaust particulate exposure does not predispose mice to adult cardiac hypertrophy or heart failure. IJERPH 13: 1178, 2016. doi: 10.3390/ijerph13121178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanwar V, Adelstein JM, Grimmer JA, Youtz DJ, Sugar BP, Wold LE. PM2.5 exposure in utero contributes to neonatal cardiac dysfunction in mice. Environ Pollut 230: 116–124, 2017. doi: 10.1016/j.envpol.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation 109: II27–II33, 2004. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 12.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105: 1534–1536, 2002. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O'Neill MS, Herrington DM, Polak JF, Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA air (multi-ethnic study of atherosclerosis and air pollution). J Am Coll Cardiol 60: 2158–2166, 2012. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pope CA 3rd, Hansen JC, Kuprov R, Sanders MD, Anderson MN, Eatough DJ. Vascular function and short-term exposure to fine particulate air pollution. J Air Waste Manag Assoc 61: 858–863, 2011. doi: 10.3155/1047-3289.61.8.858. [DOI] [PubMed] [Google Scholar]
- 15.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect 113: 1052–1055, 2005. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abplanalp W, Haberzettl P, Bhatnagar A, Conklin DJ, O'Toole TE. Carnosine supplementation mitigates the deleterious effects of particulate matter exposure in mice. J Am Heart Assoc 8: e013041, 2019. doi: 10.1161/JAHA.119.013041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberzettl P, Conklin DJ, Abplanalp WT, Bhatnagar A, O'Toole TE. Inhalation of fine particulate matter impairs endothelial progenitor cell function via pulmonary oxidative stress. Arterioscler Thromb Vascul Biol 38: 131–142, 2018. doi: 10.1161/ATVBAHA.117.309971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberzettl P, Lee J, Duggineni D, McCracken J, Bolanowski D, O’Toole TE, Bhatnagar A, Conklin DJ. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ Health Perspect 120: 848–856, 2012. doi: 10.1289/ehp.1104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haberzettl P, McCracken JP, Bhatnagar A, Conklin DJ. Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am J Physiol Heart Circ Physiol 1310: H1423–H1438, 2016. doi: 10.1152/ajpheart.00369.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberzettl P, O’Toole TE, Bhatnagar A, Conklin DJ. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect 124: 1830–1839, 2016. doi: 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, Bhatnagar A, Pope CA 3rd.. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res 107: 200–203, 2010. doi: 10.1161/CIRCRESAHA.110.222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens 3: 332–350, 2009. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O'Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119: 1204–1214, 2016. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol Sci 127: 179–186, 2012. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clerk LH, Rattigan S, Clark MG. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51: 1138–1145, 2002. doi: 10.2337/diabetes.51.4.1138. [DOI] [PubMed] [Google Scholar]
- 26.de Jongh RT, Serne EH, Ijzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 53: 2873–2882, 2004. doi: 10.2337/diabetes.53.11.2873. [DOI] [PubMed] [Google Scholar]
- 27.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest 103: 1055–1066, 1999. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 29.Paffett ML, Zychowski KE, Sheppard L, Robertson S, Weaver JM, Lucas SN, Campen MJ. Ozone inhalation impairs coronary artery dilation via intracellular oxidative stress: evidence for serum-borne factors as drivers of systemic toxicity. Toxicol Sci 146: 244–253, 2015. doi: 10.1093/toxsci/kfv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haberzettl P, Vladykovskaya E, Srivastava S, Bhatnagar A. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol Appl Pharmacol 234: 14–24, 2009. doi: 10.1016/j.taap.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheat LA, Haberzettl P, Hellmann J, Baba SP, Bertke M, Lee J, McCracken J, O'Toole TE, Bhatnagar A, Conklin DJ. Acrolein inhalation prevents vascular endothelial growth factor-induced mobilization of Flk-1+/Sca-1+ cells in mice. Arterioscler Thromb Vascul Biol 31: 1598–1606, 2011. doi: 10.1161/ATVBAHA.111.227124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation 109: 2655–2671, 2004.doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 33.Carll AP, Haykal-Coates N, Winsett DW, Hazari MS, Ledbetter AD, Richards JH, Cascio WE, Costa DL, Farraj AK. Cardiomyopathy confers susceptibility to particulate matter-induced oxidative stress, vagal dominance, arrhythmia and pulmonary inflammation in heart failure-prone rats. Inhal Toxicol 27: 100–112, 2015. doi: 10.3109/08958378.2014.995387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davel AP, Lemos M, Pastro LM, Pedro SC, de Andre PA, Hebeda C, Farsky SH, Saldiva PH, Rossoni LV. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 295: 39–46, 2012. doi: 10.1016/j.tox.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Riggs DW, Zafar N, Krishnasamy S, Yeager R, Rai SN, Bhatnagar A, O'Toole TE. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ Res 180: 108890, 2020. doi: 10.1016/j.envres.2019.108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider A, Neas LM, Graff DW, Herbst MC, Cascio WE, Schmitt MT, Buse JB, Peters A, Devlin RB. Association of cardiac and vascular changes with ambient PM2.5 in diabetic individuals. Part Fibre Toxicol 7: 14, 2010. doi: 10.1186/1743-8977-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wauters A, Dreyfuss C, Pochet S, Hendrick P, Berkenboom G, van de Borne P, Argacha JF. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension 62: 352–358, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00991. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Li H, Bao Y, Zhang X, Yu Y. Free fatty acids induce endothelial dysfunction and activate protein kinase C and nuclear factor-kappaB pathway in rat aorta. Int J Cardiol 152: 218–224, 2011. doi: 10.1016/j.ijcard.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Pillon NJ, Azizi PM, Li YE, Liu J, Wang C, Chan KL, Hopperton KE, Bazinet RP, Heit B, Bilan PJ, Lee WL, Klip A. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. Am J Physiol Endocrinol Metab 309: E35–E44, 2015. doi: 10.1152/ajpendo.00611.2014. [DOI] [PubMed] [Google Scholar]
- 40.Spigoni V, Fantuzzi F, Fontana A, Cito M, Derlindati E, Zavaroni I, Cnop M, Bonadonna RC, Dei Cas A. Stearic acid at physiologic concentrations induces in vitro lipotoxicity in circulating angiogenic cells. Atherosclerosis 265: 162–171, 2017. doi: 10.1016/j.atherosclerosis.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Sun L, Liu YL, Ye F, Xie JW, Zeng JW, Qin L, Xue J, Wang YT, Guo KM, Ma MM, Tang YB, Li XY, Gao M. Free fatty acid-induced H2O2 activates TRPM2 to aggravate endothelial insulin resistance via Ca(2+)-dependent PERK/ATF4/TRB3 cascade in obese mice. Free Radic Biol Med 143: 288–299, 2019. doi: 10.1016/j.freeradbiomed.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes 55: 2301–2310, 2006. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Wang B, Yang D, Wei H, Li H, Pan L, Huang J, Wang X, Qin Y, Zheng C, Shima M, Deng F, Guo X. Ambient particulate air pollution and circulating antioxidant enzymes: a repeated-measure study in healthy adults in Beijing. Environ Pollut 208: 16–24, 2016. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C, Niu M, Song S, Li J, Su Z, Wang Y, Gao Q, Wang H. Serum metabolomics analysis of mice that received repeated airway exposure to a water-soluble PM2.5 extract. Ecotoxicol Environ Saf 168: 102–109, 2019. doi: 10.1016/j.ecoenv.2018.10.068. [DOI] [PubMed] [Google Scholar]
- 45.Artwohl M, Roden M, Waldhausl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J 18: 146–148, 2004. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- 46.Staiger H, Staiger K, Stefan N, Wahl HG, Machicao F, Kellerer M, Haring HU. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes 53: 3209–3216, 2004. doi: 10.2337/diabetes.53.12.3209. [DOI] [PubMed] [Google Scholar]
- 47.Robertson S, Colombo ES, Lucas SN, Hall PR, Febbraio M, Paffett ML, Campen MJ. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci 134: 304–311, 2013. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiarella SE, Soberanes S, Urich D, Morales-Nebreda L, Nigdelioglu R, Green D, Young JB, Gonzalez A, Rosario C, Misharin AV, Ghio AJ, Wunderink RG, Donnelly HK, Radigan KA, Perlman H, Chandel NS, Budinger GR, Mutlu GM. β(2)-Adrenergic agonists augment air pollution-induced IL-6 release and thrombosis. J Clin Invest 124: 2935–2946, 2014. doi: 10.1172/JCI75157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hajat A, Diez Roux AV, Castro-Diehl C, Cosselman K, Golden SH, Hazlehurst MF, Szpiro A, Vedal S, Kaufman JD. The association between long-term air pollution and urinary catecholamines: evidence from the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect 127: 57007, 2019. doi: 10.1289/EHP3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heusser K, Tank J, Holz O, May M, Brinkmann J, Engeli S, Diedrich A, Framke T, Koch A, Grosshennig A, Jan Danser AH, Sweep F, Schindler C, Schwarz K, Krug N, Jordan J, Hohlfeld JM. Ultrafine particles and ozone perturb norepinephrine clearance rather than centrally generated sympathetic activity in humans. Sci Rep 9: 3641, 2019. doi: 10.1038/s41598-019-40343-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang A, Mottillo EP. Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics. Biochem J 477: 985–1008, 2020. doi: 10.1042/BCJ20190468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge CX, Qin YT, Lou DS, Li Q, Li YY, Wang ZM, Yang WW, Wang M, Liu N, Wang Z, Zhang PX, Tu YY, Tan J, Xu MX. iRhom2 deficiency relieves TNF-alpha associated hepatic dyslipidemia in long-term PM2.5-exposed mice. Biochem Biophys Res Commun 493: 1402–1409, 2017. doi: 10.1016/j.bbrc.2017.09.152. [DOI] [PubMed] [Google Scholar]
- 53.Jeong S, Park SA, Park I, Kim P, Cho NH, Hyun JW, Hyun YM. PM2.5 exposure in the respiratory system induces distinct inflammatory signaling in the lung and the liver of mice. J Immunol Res 2019: 3486841, 2019. doi: 10.1155/2019/3486841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C, Xu X, Bai Y, Wang TY, Rao X, Wang A, Sun L, Ying Z, Gushchina L, Maiseyeu A, Morishita M, Sun Q, Harkema JR, Rajagopalan S. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect 122: 17–26, 2014. doi: 10.1289/ehp.1306841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Z, Zhang X, Wang J, Dandekar A, Kim H, Qiu Y, Xu X, Cui Y, Wang A, Chen LC, Rajagopalan S, Sun Q, Zhang K. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J Hepatol 63: 1397–1404, 2015. doi: 10.1016/j.jhep.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reyes-Caballero H, Rao X, Sun Q, Warmoes MO, Lin P, Sussan TE, Park B, Fan TW, Maiseyeu A, Rajagopalan S, Girnun GD, Biswal S. Air pollution-derived particulate matter dysregulates hepatic Krebs cycle, glucose and lipid metabolism in mice. Sci Rep 9: 17423, 2019. [Erratum in Sci Rep 10: 5082, 2020]. doi: 10.1038/s41598-019-53716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol 64: 477–502, 2002. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- 58.Greene JF, Williamson KC, Newman JW, Morisseau C, Hammock BD. Metabolism of monoepoxides of methyl linoleate: bioactivation and detoxification. Arch Biochem Biophys 376: 420–432, 2000. doi: 10.1006/abbi.2000.1753. [DOI] [PubMed] [Google Scholar]
- 59.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med 3: 562–566, 1997. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling—mechanisms and research needs. Nat Rev Endocrinol 15: 701–712, 2019. doi: 10.1038/s41574-019-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benedikter BJ, Wouters EFM, Savelkoul PHM, Rohde GGU, Stassen FRM. Extracellular vesicles released in response to respiratory exposures: implications for chronic disease. J Toxicol Environ Health B Crit Rev 21: 142–160, 2018. doi: 10.1080/10937404.2018.1466380. [DOI] [PubMed] [Google Scholar]
- 62.Macchi C, Ferri N, Favero C, Cantone L, Vigna L, Pesatori AC, Lupo MG, Sirtori CR, Corsini A, Bollati V, Ruscica M. Long-term exposure to air pollution raises circulating levels of proprotein convertase subtilisin/kexin type 9 in obese individuals. Eur J Prevent Cardiol 26: 578–588, 2019. doi: 10.1177/2047487318815320. [DOI] [PubMed] [Google Scholar]