Abstract
On average, black individuals are widely believed to be more sensitive than white individuals to blood pressure (BP) effects of changes in salt intake. However, few studies have directly compared the BP effects of changing salt intake in black versus white individuals. In this narrative review, we analyze those studies and note that when potassium intake substantially exceeds the recently recommended US dietary goal of 87 mmol/day, black adults do not appear more sensitive than white adults to BP effects of short-term or long-term increases in salt intake (from an intake ≤50 mmol/day up to 150 mmol/day or more). However, with lower potassium intakes, racial differences in salt sensitivity are observed. Mechanistic studies suggest that racial differences in salt sensitivity are related to differences in vascular resistance responses to changes in salt intake mediated by vasodilator and vasoconstrictor pathways. With respect to cause and prevention of racial disparities in salt sensitivity, it is noteworthy that 1) on average, black individuals consume less potassium than white individuals and 2) consuming supplemental potassium bicarbonate, or potassium rich foods can prevent racial disparities in salt sensitivity. However, the new US dietary guidelines reduced the dietary potassium goal well below the amount associated with preventing racial disparities in salt sensitivity. These observations should motivate research on the impact of the new dietary potassium guidelines on racial disparities in salt sensitivity, the risks and benefits of potassium-containing salt substitutes or supplements, and methods for increasing consumption of foods rich in nutrients that protect against salt-induced hypertension.
Keywords: hypertension, nitrate, race, salt-sensitive, sodium
INTRODUCTION
Salt (sodium chloride) sensitivity of blood pressure has been defined as a physiological trait by which the blood pressure (BP) in some people changes in the same direction as changes in salt intake (1, 2). Salt sensitivity diagnosed with a properly validated dietary protocol, but not with other methods, has been found to be a highly reproducible phenotype and is associated with increased risk for adverse cardiovascular events (3, 4). Thus, there is considerable interest in the demographic and mechanistic aspects of dietary salt sensitivity (1, 2).
It has been reported that black Americans have a higher prevalence of hypertension and greater risk for adverse consequences of hypertension than white Americans (5, 6). Dietary factors including the dietary sodium to potassium ratio appear to be associated with racial disparities in risk for hypertension and cardiovascular disease (7). As discussed by Kris-Etherton et al. (8), a host of social, environmental, economic, and cultural determinants may mediate racial disparities in diet quality and cardiovascular risk. Recently, a scientific statement from the American Heart Association proposed that “on average, blacks compared with whites have a greater BP response to a change in salt intake and that this finding is independent of baseline BP level” (1). However, only a small number of studies have directly compared the changes in BP induced by short-term or long-term changes in salt intake in black individuals versus white individuals. In this narrative review, we focus on two main questions: 1) In hypertensive or normotensive study participants, are black individuals more sensitive than white individuals to the blood pressure effects of short-term or long-term changes in dietary salt intake? 2) If so, what are the major determinants of racial differences in salt sensitivity and how should they be addressed?
IS SALT SENSITIVITY MORE COMMON IN BLACK INDIVIDUALS THAN IN WHITE INDIVIDUALS?
Methods of Classifying Study Participants as Black or White
In evaluating studies of salt sensitivity in black and in white individuals, it is first necessary to consider the methods used for classifying study participants as black, white, and salt sensitive. Because there is no objective scientific method that is accepted for classifying people as black or white, studies of salt sensitivity have typically relied on self-identification of race/ethnicity (SIRE) in the recruitment and racial classification of study participants. In the studies discussed in this analysis, including those where the method of racial classification was not described, we assume that SIRE was used in classifying participants as black or white unless stated otherwise.
As emphasized by Wolf et al. (9), race is an ill-defined concept. Race itself is not a genetic or biological category. Rather, race is considered a social construction, largely defined by region-specific cultural and historical ideas rather than inherent biological characteristics (10, 11). Furthermore, self-identification of race “embodies myriad social, biological, and morphological factors” (9). Notwithstanding the limitations of using SIRE in physiological studies, Wolf et al. (9) note that “research aimed at understanding differences in disease processes between self-identified groups does provide important information.” They indicate that the value of such information can be enhanced by “considering the specific biological, social, and/or environmental foundations from which these differences arise” (9). Such information could lead to improved approaches to improving health in various population groups. Accordingly, in the current analysis, we discuss the extent to which black and white individuals differ with respect to salt sensitivity. We also examine the studies that have investigated the role of specific factors in mediating racial differences in salt sensitivity.
Although the current analysis focuses on studies comparing BP salt sensitivity between groups of black study participants and white study participants, future studies that include comparisons with other racial groups would also be of interest. Some studies have attempted to investigate a genetic basis for salt sensitivity in various racial groups (12–14). In the present analysis, the categorization by race is not intended to imply that race membership itself is a mechanistic risk factor for salt sensitivity or that the degree of salt sensitivity is an innate feature of any race however race is defined. Rather, race is a sociological construct and individuals in different races may tend to have different experiences (e.g., differences in dietary and lifestyle habits, differences in exposure to various types and degrees of psychosocial stress, etc.) that may alter physiological mechanisms regulating blood pressure and other factors that influence human health.
Methods of Classifying Individuals for Sensitivity to the Blood Pressure Effects of Changes in Dietary Salt Intake
A variety of different protocols have been used to investigate the prevalence and pathogenesis of salt-sensitivity (3). Study participants are typically classified as salt-sensitive if the testing protocol causes mean arterial pressure to change by more than an arbitrary cutoff chosen by the investigators (1, 3). However, as emphasized by de Leeuw and Kroon (15), “the magnitude of the response above which pressure is considered to be salt-sensitive varies enormously among studies.” Thus, instead of treating salt sensitivity as a discrete trait, some investigators study BP salt sensitivity as a continuous trait and directly compare salt-induced increases in BP between different experimental groups (16, 17). The methods of salt loading have also varied substantially, and many studies have employed intravenous (IV) infusions of saline and loop diuretic, furosemide-based protocols as surrogate tests for dietary salt sensitivity (3). These saline infusion and furosemide-based protocols are not considered in the present analysis because they have shown poor reproducibility and accuracy for identifying study participants who are sensitive to the blood pressure effects of changes in dietary salt intake (3).
Design of the Review
In this narrative review, we primarily focus on protocols that directly compare black adults versus white adults with respect to the absolute numerical changes in blood pressure that occur in response to a given change in oral salt intake. We conducted a narrative review rather than a meta-analysis and meta-regression analysis because of extensive heterogeneity in the trial designs and because of the large number of factors that may moderate blood pressure responses to changes in sodium intake [e.g., age, sex, low-salt dose, high-salt dose, baseline BP, obesity/body mass index, socioeconomic status, dietary factors, study duration, method of BP measurement, and experimental setting (inpatient, outpatient)]. The studies discussed in this analysis were identified from the systematic review by Huang et al. (18) of trials of dietary sodium on blood pressure and isolating on the trials in which blood pressure responses to changes in salt intake were reported in both black and white study participants. Additional studies were identified using keyword searches of PubMed to identify experiments in which black individuals were compared with white individuals with respect to blood pressure responses to changes in salt intake. The keyword searches were based on “salt sensitive and black,” or “sodium sensitive and black,” or “sodium and black and blood pressure,” or “salt and black and blood pressure.” The search identified publications reporting experiments with a wide variety of designs and conditions in which BP responses to changes in salt intake were compared between black and white study participants (17, 19–37).
Given evidence suggesting that changes in salt intake have greater effects on blood pressure among individuals with higher blood pressure (18), we analyzed studies of hypertensive individuals separately from those of normotensive individuals and those which included adjustment for baseline blood pressure. Unless otherwise stated, hypertension was defined as blood pressure equal to or greater than 140 mmHg systolic and or 90 mmHg diastolic.
We focused the analysis on studies that reported potassium intake as well as sodium intake because of data indicating that 1) potassium intake can modify blood pressure responses to salt intake (29, 38) and 2) racial disparities in the incidence of hypertension are associated with racial differences in the dietary sodium to potassium ratio (7). Of the 11 studies identified that reported potassium intake and numerical BP responses (in mmHg) to changes in salt intake, nine were conducted in normotensive individuals and are shown in Table 1 and Figs. 1 and 2. In studies in hypertensive individuals, eight reported numerical BP responses to changes in salt intake but only two of them reported potassium intake. Because of the limited number of studies in hypertensive individuals that reported potassium intake, the results of all studies in hypertensive individuals with numerical BP responses to changes in salt intake are shown in Table 2 and Fig. 3. Finally, some publications reported the frequency of salt sensitivity based on arbitrarily defined cutoffs, with or without reporting absolute numerical BP responses to changes in salt intake (21, 26, 29, 32, 36, 37). Results showing the frequency of salt sensitivity in the different racial groups are included in the Supplemental Figures (all Supplemental Figures are available at https://doi.org/10.6084/m9.figshare.14444654). Because of the limitations of arbitrarily defining the frequency of salt sensitivity in nonstandardized testing protocols, we focus the discussion on the numerical BP responses to salt loading in the different racial groups.
Table 1.
Studies that reported potassium intake and numerical blood pressure data in normotensive subjects
| Racial Group | n | Sex | Mean Age | Baseline Weight, kg | NaCl/K+ Intake, (mmol/day)/(mmol/day) Low-NaCl Phase | NaCl/K+ Intake, (mmol/day)/(mmol/day) High-NaCl Phase | K+ intake in High-NaCl Phase | Full Diet Control | Change in MAP, mmHg | % Salt Sensitive |
|---|---|---|---|---|---|---|---|---|---|---|
| B (29) | 24 | M | 39 | 79.1 | 15/30, 14 days | 250/30, 7 days | 30 mmol/day | Y | 6.6* | 79* |
| W | 14 | M | 38 | 74.3 | 15/30, 14 days | 250/30, 7 days | 30 mmol/day | Y | 2.0 | 36 |
| B (31) | 9 | M | 38 | 77.3 | 15/30, 14 days | 250/30, 7 days | 30 mmol/day | Y | 5.0* | ? |
| W | 6 | M | 42 | 73.9 | 15/30, 14 days | 250/30, 7 days | 30 mmol/day | Y | 1.0 | ? |
| AA (34) | 115 | 78F/37M | 49? | 85.6? | 50/44, 30 days | 150/44, 30 days | 44 mmol/day | Y | 5.7* | ? |
| Non-AA | 89 | 33F/56M | 49? | 85.6? | 50/44, 30 days | 150/44, 30 days | 44 mmol/day | Y | 3.2 | ? |
| B (31) | 9 | M | 38 | 77.3 | 15/30, 14 days | 250/70, 7–21 days | 70 mmol/day | Y | 4.0* | ? |
| W | 6 | M | 42 | 73.9 | 15/30, 14 days | 250/70, 7–21 days | 70 mmol/day | Y | 1.0 | ? |
| B (29) | 12 | M | ? | ? | 15/30, 14 days | 250/70, 7 days | 70 mmol/day | Y | 5.0** | 68** |
| W | 14 | M | 38 | ? | 15/30, 14 days | 250/70, 7 days | 70 mmol/day | Y | 0.5 | 20 |
| B (26) | 7 | M | 32? | ? | 10/80, 7 days | 300/80, 3 days | 80 mmol/day | Y | 3.4* | 43 |
| W | 7 | M | 32? | ? | 10/80, 7 days | 300/80, 3 days | 80 mmol/day | Y | −0.1 | 0 |
| B (22) | 23 | M | 32 | ? | 10/100, 5 days | 200/100, 4 days | 100 mmol/day | Y | −3.2 | ? |
| W | 19 | M | 32 | ? | 10/100, 5 days | 200/100, 4 days | 100 mmol/day | Y | −3.2 | ? |
| B (29) | 10 | M | ? | ? | 15/30, 14 days | 250/120, 7 days | 120 mmol/day | Y | 0.0 | 20 |
| W | 14 | M | 38 | ? | 15/30, 14 days | 250/70, 7 days | 70 mmol/day | Y | 0.5 | 20 |
| AA (34) | 119 | 86F/33M | 47? | 82.8 ? | 50/120, 30 days | 150/120, 30 days | 120 mmol/day | Y | 2.5 | ? |
| Non-AA | 89 | 37F/52M | 47? | 82.8 ? | 50/120, 30 days | 150/120, 30 days | 120 mmol/day | Y | 1.6 | ? |
Numbers in parentheses correspond to the citations of the study publications. AA, African American; B, black; BP, blood pressure; F, female; M, male; MAP, average change in mean arterial pressure obtained by subtracting the blood pressure in the low-salt diet phase from the blood pressure in the high-salt diet phase; n, sample size; W, white. Question marks indicate data not available or results are for all subjects combined when racial subgroup data were not available. The DASH-Sodium study (34) included a mixture of hypertensive and normotensive subjects and the data were adjusted for baseline systolic BP. The study from Ref. 37 with 101 normotensive female participants is not included in this table because it did not provide information on K+ intake. There were no racial differences in salt sensitivity in the normotensive females.
Significant difference reported for MAP or SBP between black subjects versus white subjects.
Significant difference compared with whites consuming 70 mmol/day potassium and to blacks consuming 120 mmol/day potassium.
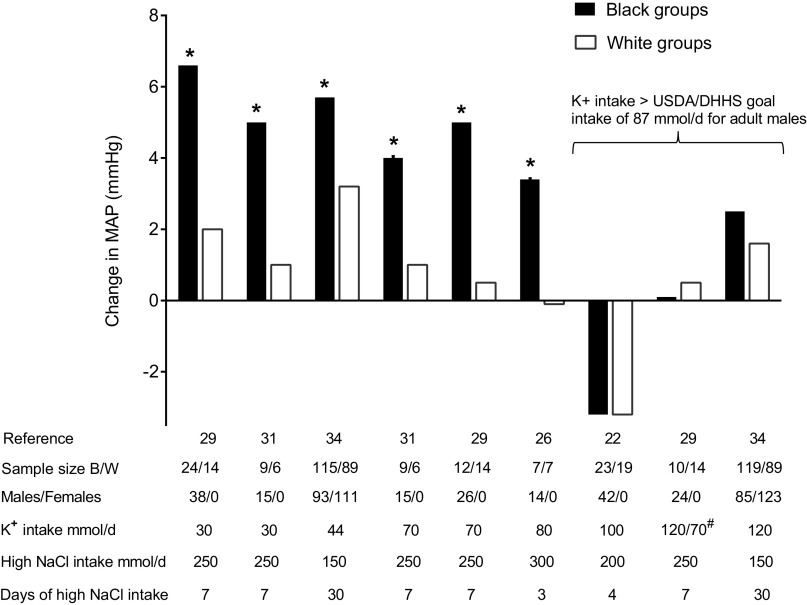
Figure 1.
Average changes in mean arterial pressure (MAP) in black (B) and white (W) normotensive groups induced by switching from low NaCl intake to high NaCl intake. Subjects were normotensive or results were adjusted for baseline systolic blood pressures in study (Ref. 34) that included both normotensive and hypertensive subjects and where order of administration of the low- and high-salt diets was randomized. *Significant difference between B and W groups. #B group given K+ intake of 120 mmol/day and W group 70 mmol/day. Target NaCl intake in the low-salt phase was less than or equal to 50 mmol/day in all studies. DHHS, Department of Health and Human Services; USDA, US Department of Agriculture.
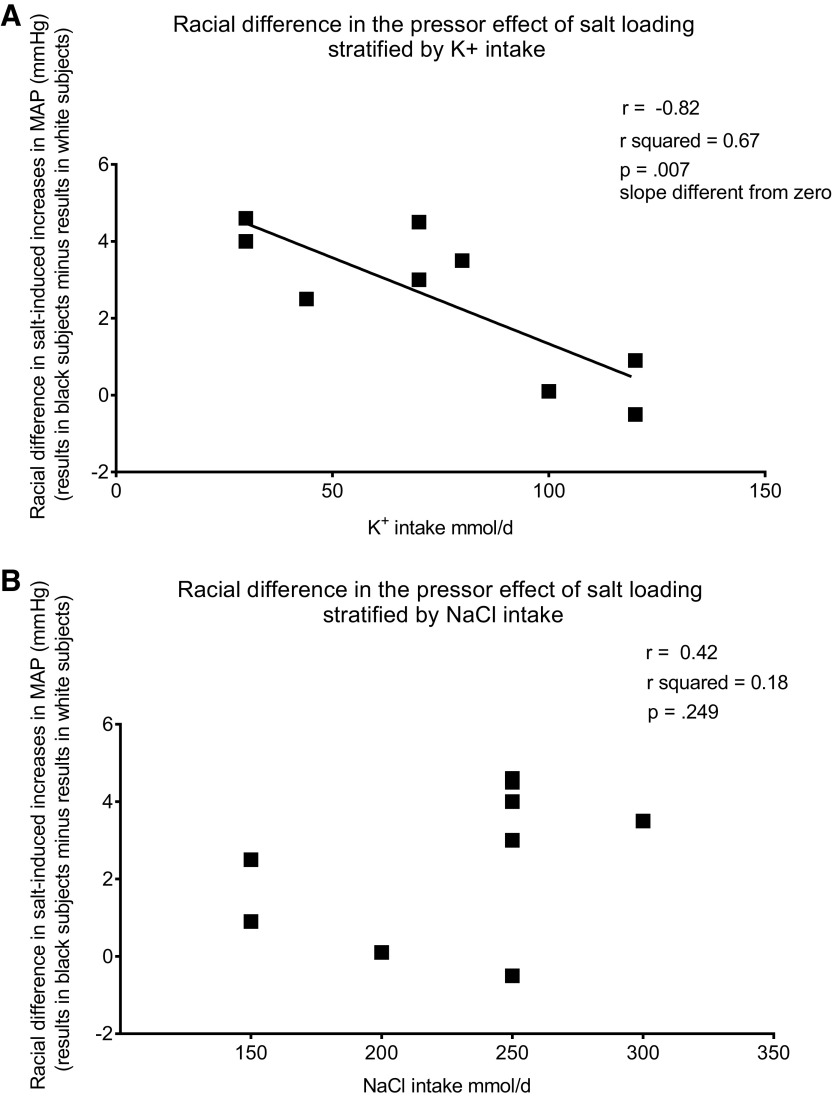
Figure 2.
Relationship between racial differences in salt-induced changes in mean arterial pressure (MAP) and K+ intake (A) or NaCl intake (B).
Table 2.
Studies that reported numerical blood pressure data in hypertensive subjects
| Racial Group | n | Sex | Mean Age | Baseline Weight, kg | NaCl/K+ Intake, (mmol/day)/(mmol/day) Low-NaCl phase | NaCl/K+ Intake, (mmol/day)/(mmol/day)High-NaCl phase | K+ Intake in High-NaCl Phase | Full Diet Control | Change in MAP, mmHg | % Salt Sensitive |
|---|---|---|---|---|---|---|---|---|---|---|
| B (30) | 22 | M | 41 | 29.3 BMI | 40/80, 5 days | ∼150/80, 5 days | 80 mmol/day | Y | 6 | ? |
| W | 59 | M | 43 | 27.7 BMI | 40/80, 5 days | ∼150/80, 5 days | 80 mmol/day | Y | 7 | ? |
| B (22) | 16 | M | 33 | ? | 10/100, 5 days | 200/100, 5 days | 100 mmol/day | Y | 1 | ? |
| W | 17 | M | 34 | ? | 10/100, 5 days | 200/100, 5 days | 100 mmol/day | Y | −1 | ? |
| AA (19) | 66 | 46F/20M | 66? | ? | 80 mmol/day, 3.5 mo | Usual diet | Not reported | N | 3.1 | ? |
| Non AA | 250 | 108F/142M | 66? | ? | 80 mmol/day, 3.5 mo | Usual diet | Not reported | N | 3.0 | ? |
| B (24) | 69 | 35F/34M | 50 | 31 BMI | 116 mmol/day, 1.5 mo | 162 mmol/day, 1.5 mo | Not reported | N | 3.1 | ? |
| W | 71 | 15F/56M | 52 | 28 BMI | 104 mmol/day, 1.5 mo | 163 mmol/day, 1.5 mo | Not reported | N | 3.0 | ? |
| B (28) | 7 | ? | 49? | ? | ∼80 mmol/day, 30 days | ∼160 mmol/day, 30 days | Not reported | N | 7.8 | ? |
| W | 12 | ? | 49? | ? | ∼80 mmol/day, 30 days | ∼160 mmol/day, 30 days | Not reported | N | 5.5 | ? |
| B (23) | 35 | 9F/26M | 21 | 82.8 | Usual | Usual diet | Not reported | N | −1.8 | ? |
| W | 8 | 2F/6M | 20 | 78.2 | Diet | +170, 14 days | Not reported | N | 1.1 | ? |
| B (37) | 50 | F | 55 | 77.7* | <20 mmol/day, 7 days | >200 mmol/day, 7 days | Not reported | Y | 12.6* | 64 |
| W | 48 | F | 58 | 72.3 | <20 mmol/day, 7 days | >200 mmol/day, 7 days | Not reported | Y | 8.2 | 52 |
| B (25) | 33 | 18F/15M | 44 | 74.6* | 10 mmol/day, 5 days | ∼350 mmol/day, 5 days | Not reported | N | 14.0* | ? |
| W | 71 | 38F/33M | 49 | 70.8 | 10 mmol/day, 5 days | ∼350 mmol/day, 5 days | Not reported | N | 9.7 | ? |
Numbers in parentheses correspond to the citations of the study publications.
Average changes in mean arterial pressure (MAP) in black (B) and white (W) hypertensive individuals induced by changing salt intake. Changes in MAP calculated by subtracting MAP in low-salt phase from MAP in high-salt phase. AA, African American; BMI, body mass index; F, female; M, male; N, no; Y, yes. Target NaCl intake in the low-salt phase was less than 50 mmol/day except for Refs. 19, 23, 24, and 28 where it was 80–110 mmol/day or higher. Question marks indicate data not available.
Significant difference between responses of B and W subjects to the intervention.
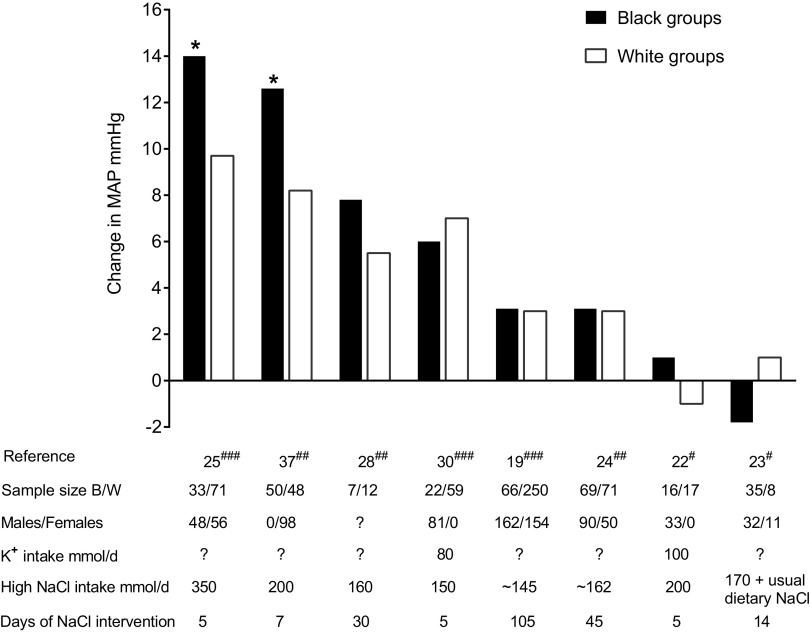
Figure 3.
Average changes in mean arterial pressure (MAP) in black (B) and white (W) hypertensive groups induced by changing salt intake. Changes in MAP calculated by subtracting MAP in low-salt phase from MAP in high-salt phase. #Salt loading studies where low NaCl phase preceded high-salt intervention. ##Studies where low-salt and high-salt interventions were randomized and of equal duration. ###Salt restriction studies where high-salt phase preceded low-salt intervention. *Significant difference between B and W groups. Target NaCl intake in the low-salt phase was less than 50 mmol/day except Refs. 19, 23, 24, and 28 where it was 80–110 mmol/day or higher.
Because of a possible dose-response relationship between changes in salt intake and changes in blood pressure (18), we provide information on the amount of dietary salt given to the study participants. We excluded experiments that employed changes in salt intake outside the dietary range of salt intake reported in humans (a range of ≈10–350 mmol/day) (39). According to Huang et al. (18), the blood pressure lowering effect of reducing sodium intake may be greater in studies longer than 2-wk duration than in shorter studies. Here, we indicate study duration for all experiments. We identified only one publication with salt loading of more than 2-wk duration that reported both sodium intake and potassium intake together with BP responses to changes in salt intake (34). Finally, because dietary patterns of black individuals may differ from those of white individuals, and because multiple dietary factors besides potassium (e.g., chloride, nitrate, sodium density, fiber, etc.) may affect blood pressure, we specify whether the studies tightly controlled all nutrient intake of the participants during the study.
Blood Pressure Effects of Changes in Salt Intake in Black Normotensive versus White Normotensive Study Participants
We identified nine studies from five publications reporting numerical blood pressure responses to changes in salt intake in black normotensive versus white normotensive individuals in which salt intake and potassium intake were specified (Table 1) (22, 26, 29, 31, 34). In one of the publications (report of the DASH Sodium trial), the analysis was performed on data from a mix of normotensive and hypertensive study participants (34). The results of the DASH Sodium trial were adjusted for baseline systolic blood pressure in the statistical modeling of interactions between the racial groupings and the salt-induced changes in blood pressure (34). In all studies, salt intake during the low-salt diet phase was 2.9 g/day (50 mmol/day) or less. Salt intake during the high-salt phase was 8.7 g/day (150 mmol/day) or more (between 150 mmol/day and 300 mmol/day; Table 1). Potassium intake ranged from 30 mmol/day to 120 mmol/day (Table 1). Seven of the studies involved male participants and two of the studies (both from the DASH Sodium trial) involved a combination of male and female participants (Table 1).
In the six studies in which potassium intake was less than the goal intake of ∼87 mmol/day (3,400 mg/day) recommended for adult males in the latest US dietary guidelines (40), salt loading increased mean arterial pressure significantly more in black individuals than white individuals (Table 1 and Fig. 1). In sharp contrast, in the three studies in which potassium intake was 100 mmol/day or more, salt loading caused little or no increase in mean arterial pressure and significant racial differences in salt sensitivity were not apparent (Table 1 and Fig. 1). In studies that reported systolic and diastolic blood pressures, the results were consistent with those for mean arterial pressure (data not shown).
In four of the nine studies in normotensive individuals in Table 1, the investigators reported data that also allowed estimating the percentage of participants that would be classified as salt sensitive when using fixed BP cutoffs for diagnosing salt sensitivity as a discrete trait. The blood pressure cutoffs used for diagnosis were close to those recommended for classifying normotensive individuals as salt sensitive (∼3–5 mmHg, mean arterial pressure) (3). More black individuals were classified as salt sensitive than white individuals except in one study where potassium intake exceeded the amount recommended by the 2020–2025 US dietary guidelines (Table 1, Supplemental Fig. S1).
Figure 2 (top) shows a plot of the relationship between potassium intake and the racial difference in salt-induced changes in mean arterial pressure. Racial differences in salt-induced changes in mean arterial pressure were calculated by subtracting the changes in mean arterial pressure for the white participants from the changes in mean arterial pressure for the black participants (Fig. 1 and Table 1). In a linear regression analysis, the racial differences in salt-induced increases in blood pressure correlated inversely with potassium intake (r = −0.82, r2 = 0.67, P = 0.007 for slope significantly different from zero; Fig. 2, top). Although the magnitude of salt loading varied among the studies, the racial differences in salt-induced changes in blood pressure were not significantly correlated with the magnitude of salt loading (Fig. 2, bottom). In all of these studies, food intake was carefully controlled and the racial differences in salt sensitivity are unlikely to be related to racial differences in the intake of other nutrients during the study. However, these experiments do not exclude the possibility that the racial differences in salt sensitivity could have been influenced by racial differences in nutrient intake that might have been present before the studies.
Overall, the results indicate that when consuming ≤80 mmol/day of potassium, black normotensive adults are more sensitive than white normotensive adults to the pressor effects of increasing salt intake from 2.9 g/day (50 mmol/day) or less to 8.7 g/day (150 mmol/day) or more. Racial differences in salt sensitivity are not apparent when potassium intake is 100 mmol/day or more, a level that substantially exceeds the intake recommended for men (87 mmol/day, 3,400 mg/day) and for women (67 mmol/day, 2,600 mg/day) in the latest US dietary guidelines for Americans (40).
It is important to note that there is a paucity of studies on racial differences in salt sensitivity in normotensive females and in study participants over 65 yr of age. The data are not sufficient to assess the extent to which the racial differences in salt sensitivity are influenced by sex, age, or body weight of the study participants. There is also a paucity of long-term studies of salt loading with only one study of more than 14 days duration in individuals consuming a low-potassium diet (44 mmol/day), and one study in individuals consuming a high-potassium diet (120 mmol/day). Both of these studies were part of the DASH-Sodium trial (17, 20, 34).
Blood Pressure Effects of Changes in Salt Intake in Black Hypertensive versus White Hypertensive Study Participants
Hypertensive individuals are considered to be more sensitive to the blood pressure effects of changes in salt intake than normotensive individuals (1, 41). We identified only two studies reporting numerical blood pressure responses to changes in salt intake in black hypertensive versus white hypertensive study participants in which salt intake and potassium intake were specified (Table 2) (22, 30). Six studies in hypertensive individuals were identified that reported numerical BP responses to changes in salt intake that did not report potassium intake (Table 2) (19, 23–25, 28, 37). Because of the paucity of studies reporting potassium intake in hypertensive individuals, we have listed both the studies that did not report potassium intake (n = 6 studies), as well as those that did report potassium intake (n = 2 studies) in Table 2 and in Fig. 3. In only two of the eight studies in hypertensive participants, the salt-induced changes in blood pressure were significantly greater in black hypertensive individuals than in white hypertensive individuals (Table 2 and Fig. 3). Neither of these studies reported potassium intake. Both were short-term studies with one conducted in females and the other conducted in females and males.
In the six studies in hypertensive participants that did not show racial differences in salt-induced changes in blood pressure, only two reported potassium intake (80 mmol/day and 100 mmol/day) and both were short-term studies in males (Table 2). The other four studies did not report potassium intake and three of them were long-term studies and one was a short-term study (Table 2). Table 2 does not include the results from a subgroup analysis of the long-term DASH Sodium study, which suggested that salt-induced changes in blood pressure might be greater in hypertensive African Americans consuming a low potassium diet (44 mmol/day) than in hypertensive non-African Americans (∼90% non-Hispanic whites) consuming the same diet (20). However, the post hoc subanalysis did not report statistically significant differences between the two groups and there was considerable overlap in the 95% confidence intervals for the mean changes in systolic blood pressure in the African American hypertensive participants (7.2–11.5 mmHg) versus the non-African American hypertensive participants (4.1–9.4 mmHg) in the DASH-Sodium study (20).
We identified four dietary studies (Supplemental Fig. S2) comparing black hypertensive individuals and white hypertensive individuals with respect to the frequency of salt sensitivity diagnosed using blood pressure cutoffs close to those recommended for classifying hypertensive study participants as salt sensitive (3). Only two of the four studies reported potassium intake. The frequency of salt sensitivity appeared greater in black hypertensive versus white hypertensive individuals but was statistically significant in only one of the four studies (Supplemental Fig. S2). In that study, potassium intake was 80 mmol/day and the numbers of males and females were not specified.
In summary, studies of racial differences in salt sensitivity in hypertensive individuals have given mixed results. Because of wide variation in study design, it is difficult to draw conclusions about racial disparities in salt sensitivity in hypertensive individuals. It is possible that when compared with white hypertensive individuals, black hypertensive individuals are more sensitive to the blood pressure effects of salt restriction, but not more sensitive to the blood pressure effects of salt loading. In studies in hypertensive participants, the sodium content of the low-salt diets varied between 10 mmol/day and 100 mmol/day or higher. The sodium content of the high-salt diets varied between 150 mmol/day and 350 mmol/day. Few studies reported potassium intake and the data are not sufficient to assess whether dietary potassium is a determinant of racial differences in salt sensitivity in hypertensive individuals. The data are also not sufficient to assess the extent to which racial differences in salt sensitivity in hypertensive individuals are influenced by body weight, age, or sex of the study participants.
DIETARY POTASSIUM INTAKE AS A DETERMINANT OF RACIAL DIFFERENCES IN SALT SENSITIVITY
The study by Morris et al. (29) and the analysis of the DASH-Sodium trial by Vollmer et al. (34) indicate that ingestion of 120 mmol/day of potassium may be sufficient to prevent racial differences in the BP responses to short-term and long-term increases in dietary salt intake. The DASH diet provided a potassium intake of 120 mmol/day and essentially prevented long-term increases in salt intake from causing greater increases in blood pressure in black individuals than in white individuals (34). However, while the DASH diet is rich in potassium, it should be recognized that the blood pressure benefits of the DASH diet are not necessarily related to potassium alone (42). For example, the impact of the DASH diet on salt sensitivity could reflect blood pressure effects of high levels of other nutrients such as inorganic nitrate in addition to those of potassium (43, 44). In contrast, the study by Morris et al. (29) directly tested the blood pressure effects of variations in potassium intake per se on racial differences in salt sensitivity. In that study, increasing potassium intake from 30 mmol/day or 70 mmol/day to 120 mmol/day by supplementing only with potassium bicarbonate, essentially abolished the greater BP response to short-term salt loading in black normotensive versus white normotensive individuals (29). These effects of potassium bicarbonate supplementation alone on salt sensitivity in black individuals (29) are of particular interest because of the evidence that on average, black individuals consume less potassium than white individuals, while consuming similar amounts of sodium as white individuals (45–49). Although low intake of potassium per se may have negative health consequences, it should be recognized that low-potassium consumption might be associated with suboptimal intake of other dietary components that also could contribute to adverse health outcomes.
Should the US Dietary Guidelines on Potassium Intake Be Revised?
The studies by Morris et al. (29) and the results of the DASH-Sodium trial (34) suggest that racial disparities in salt sensitivity could be eliminated by increasing potassium intake to the goal intake of 120 mmol/day (4,700 mg/day) recommended in the US dietary guidelines before 2020 (50). Even in the absence of any reductions in salt intake, increasing potassium intake to 120 mmol/day would substantially reduce the sodium to potassium ratio. Reducing the sodium to potassium ratio by increasing potassium intake to 120 mmol/day (4,700 mg/day) would be of interest for reducing the risk of hypertension in general, and for reducing racial disparities in the incidence of hypertension (7). However, in the latest US dietary guidelines (40), which were based on a recent committee report by the National Academy of Sciences (NAS) (46), the recommended goal for potassium intake was reduced from 4,700 mg/day (120 mmol/day) in adults to 2,600 mg/day (67 mmol/day) in adult females and 3,400 mg/day (87 mmol/day) in adult males (40). This raises the question: On what basis did the NAS committee establish these new recommendations for potassium intake that are substantially lower than the level of 4,700 mg/day previously recommended by the NAS and the US dietary guidelines (50, 51)?
The new recommendations of the NAS on potassium intake relied heavily on a commissioned analysis by the Agency for Healthcare Research and Quality (AHRQ) that did not address the impact of potassium intake on salt sensitivity (46). Thus, the role of potassium in preventing salt sensitivity and racial disparities in cardiovascular risk received little attention in the 2019 NAS report or the new US dietary guidelines (40, 46). The 2019 NAS report defined the adequate intake (AI) of potassium based on the highest median potassium intake of two national surveys in normotensive males and females without a self-reported history of cardiovascular disease (46).
In contrast to the 2019 NAS recommendations on potassium intake (46), previous NAS recommendations in 2005 were influenced by studies indicating that high-potassium intakes could protect against salt sensitivity in African Americans as well as reduce blood pressure in certain individuals (51). The 2005 NAS report specifically stated that “Because African Americans have lower intakes of potassium and a higher prevalence of elevated blood pressure and salt sensitivity, this subgroup of the population would especially benefit from an increased intake of potassium” (51). The 2005 NAS recommendations in turn influenced the previous US Dietary Guidelines that specifically recommended that black individuals, middle-aged, and older adults, and hypertensive individuals aim to meet a potassium intake goal of 4,700 mg/day (120 mmol/day) (50). The present analysis raises questions about the recent decision of the NAS committee and the US dietary guidelines to set adequate intake for potassium at levels substantially below 4,700 mg/day. We believe this decision will not help, and might impede, efforts to reduce racial disparities in salt sensitivity and efforts to reduce blood pressure in the general population. It should be noted that fewer than 2.5% of black or white individuals in the United States are achieving a potassium intake of 120 mmol/day (4,700 mg/day) (45, 46).
Impact of Dietary Potassium on Mechanisms Mediating Blood Pressure Reponses to Changes in Salt Intake
Before discussing mechanisms whereby a low-potassium intake promotes greater salt sensitivity in black individuals than in white individuals, it is helpful to understand the mechanistic pathways that mediate initiation of salt-induced increases in blood pressure. The historical “natriuretic dysfunction” theory championed by Arthur C. Guyton and others holds that salt sensitivity is usually caused by abnormally large increases in sodium retention and cardiac output mediated by an impaired ability to excrete sodium in response to increases in salt intake (52–57). According to Guyton’s theory, individuals with normal kidney function are salt-resistant because they “rapidly eliminate the excess salt and blood volume is hardly altered” (55). However, as we have discussed in detail elsewhere (58–60), this historical natriuretic dysfunction theory was based on studies that did not include measurements of sodium retention and cardiac output in salt-loaded normal controls (salt resistant, normotensive controls). When Guyton’s model was tested for its ability to predict sodium balance and hemodynamic responses to increases in salt intake in normal salt-resistant controls, it failed validation testing (61, 62). Because Guyton’s model/theory did not accurately reflect the normal renal and hemodynamic responses to salt loading in salt-resistant controls, it could not accurately identify which responses to salt loading were abnormal in salt-sensitive individuals.
In contrast to the historical natriuretic dysfunction theory, the contemporary “vasodysfunction” theory is based on studies in humans that included assessment of sodium retention, cardiac output, and vascular resistance in both salt-sensitive individuals and appropriate normal controls (35, 58, 60, 63–66). The vasodysfunction theory holds that in response to salt loading, increases in blood pressure are usually initiated by a failure to normally vasodilate and decrease vascular resistance, together with normal increases in sodium balance and cardiac output (Fig. 4) (58, 60). Thus, preventing either the abnormal hemodynamic response to increased salt intake (i.e., preventing the abnormal vascular resistance response), or preventing the normal hemodynamic response to increased salt intake (i.e., preventing the normal salt-induced increases in cardiac output), should be sufficient to attenuate salt-induced increases in blood pressure.
Figure 4.
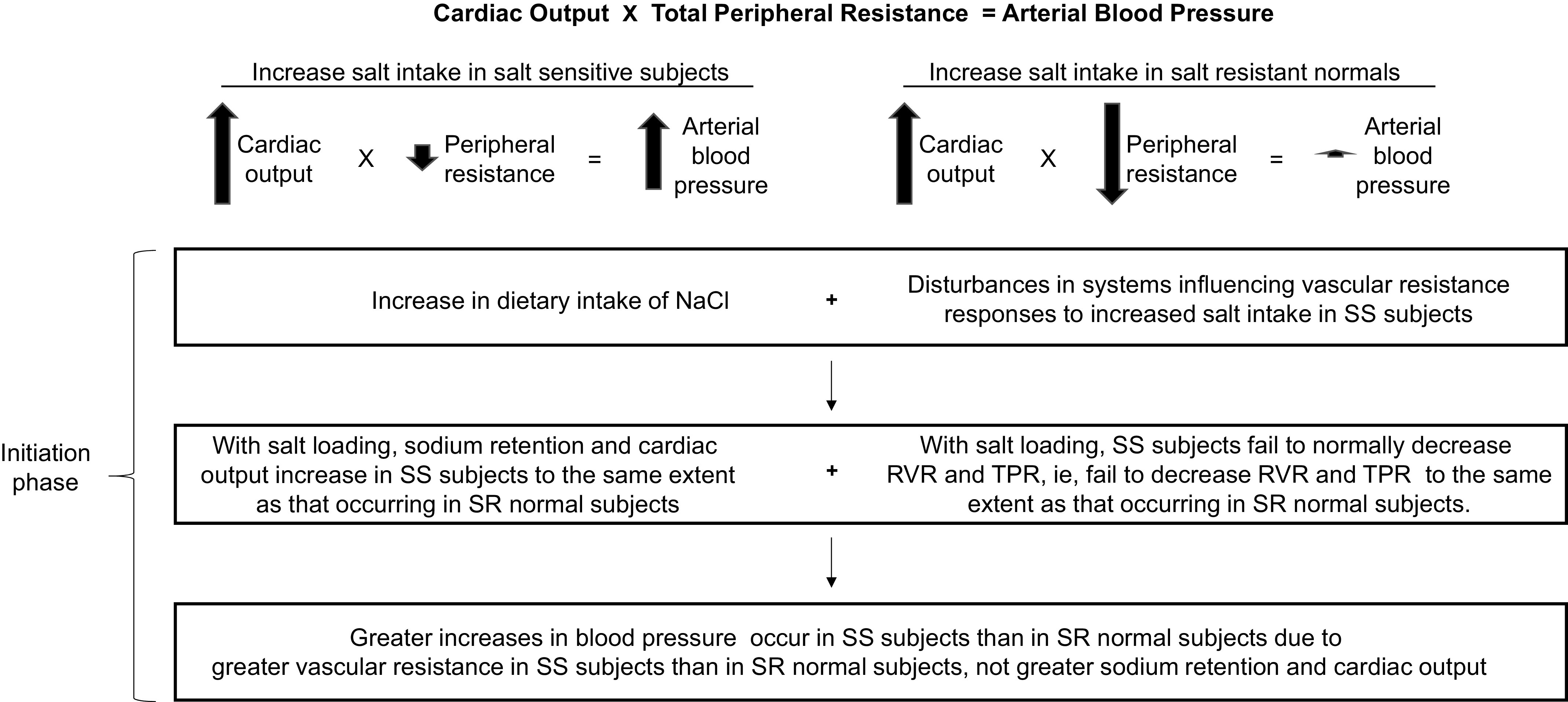
The vasodysfunction theory for the usual pathogenesis of salt-induced increases in blood pressure. The initiation of salt-induced increases in blood pressure is hemodynamically mediated by an abnormal vascular resistance response to increases in salt intake, together with normal salt-induced increases in cardiac output. RVR, renal vascular resistance; SR, salt resistant; SS, salt sensitive; TPR, total peripheral resistance. Adapted from Kurtz et al. (2), with permission.
Many mechanistic pathways have been proposed to mediate racial differences in salt sensitivity (67, 68), including those that influence blood pressure by affecting vascular resistance and or sodium balance and cardiac output. However, only a few studies have investigated the mechanisms mediating salt sensitivity during experiments in which the changes in salt intake caused greater changes in blood pressure in black individuals than in white individuals undergoing the same study protocol. Such studies have mainly involved measurements to assess renal sodium handling and activity of systems regulating vascular tone. Here, we discuss those studies while taking into consideration two issues: First, it is important to recognize that the mechanistic pathways which mediate pressor responses to dietary salt loading are not necessarily the same as those that mediate the blood pressure lowering effects of salt restriction. For example, increased blood pressure during salt loading might be largely mediated by a subnormal increase in nitric oxide activity in response to increased salt intake. In contrast, decreased blood pressure during salt restriction might be largely mediated by a subnormal increase in plasma renin activity in response to decreased salt intake. Second, the mechanistic pathways that mediate changes in blood pressure in response to changes in salt intake may differ between hypertensive study participants and normotensive study participants. Therefore, we distinguish between studies that involve dietary salt loading and those that involve dietary salt restriction, and we also distinguish between studies involving normotensive individuals and those involving hypertensive individuals.
Do Racial Differences in Renal Sodium Retention Mediate Racial Differences in Salt Sensitivity?
As previously noted, black individuals on average consume less potassium than white individuals, while consuming similar amounts of sodium as white individuals (45–49). A low-potassium intake has been reported to induce increases in renal sodium retention (69). It has been proposed that in response to increases in salt intake, black individuals may retain more sodium than white individuals (67) and this may contribute to racial differences in salt sensitivity by causing greater increases in blood volume and cardiac output in black individuals than in white individuals. However, few studies have directly investigated whether greater BP responses to increased dietary salt in black groups versus white groups are caused by greater sodium retention or cardiac output secondary to lower renal sodium excretion.
We identified three studies in which urinary excretion of sodium was measured when increases in dietary salt caused significantly greater increases in BP in black normotensive than in white normotensive individuals (26, 29, 31). One study found that in individuals ingesting a low-potassium diet (30 mmol/day), increasing salt intake from 15 mmol/day to 250 mmol/day for 7 days caused greater increases in BP in black normotensive versus white normotensive study participants that were associated with smaller increases in sodium excretion in the black individuals versus the white individuals (29). However, measurements of cardiac output were not reported and the extent to which reduced sodium excretion contributed to the greater salt sensitivity in black normotensive versus white normotensive individuals was not determined. In another study in participants ingesting a low-potassium diet (30 mmol/day), increasing salt intake from 15 mmol/day to 250 mmol/day for 7 days caused greater increases in BP in black normotensive versus white normotensive individuals that were not associated with significant differences in sodium excretion between the black individuals and white individuals (31). In a third study, potassium intake was 80 mmol/day and the greater BP response to increasing salt intake from 10 mmol/day to 300 mmol/day for 3 days in black normotensive versus white normotensive individuals was not associated with racial differences in either urinary sodium excretion or cardiac index (26). As stated by Luft et al. (26), “since cardiac index was not different in black and white subjects, the differences in blood pressure must be attributed to differing responses in peripheral vascular resistance.”
We identified one study in which urinary excretion of sodium was measured while changes in dietary salt caused significantly greater changes in blood pressure in black hypertensive than in white hypertensive individuals (25). The study specifically investigated the effects of salt restriction on blood pressure and therefore, the high-salt diet phase preceded the low-salt diet phase. The fall in blood pressure with salt restriction was significantly greater in the black hypertensive group than in the white hypertensive group (25). However, the reductions in sodium excretion, blood volume, and body weight in response to salt restriction were not significantly different between the black hypertensive and white hypertensive groups (25). In summary, there is little or no direct evidence demonstrating that in response to changes in dietary salt intake, greater BP salt sensitivity in black individuals than in white individuals is due to greater renal sodium retention in black individuals.
Impact of Potassium Intake on Racial Differences in Sodium Retention and Salt Sensitivity
Given that a low-potassium intake can cause potassium depletion and increase renal sodium retention (69), it is conceivable that replenishment of potassium stores by supplemental dietary potassium might reduce racial differences in salt sensitivity by decreasing renal sodium retention in black individuals. The study by Morris et al. (29) demonstrated that in normotensive study participants, consuming a low-potassium diet delivering 30 mmol/day of potassium, increasing salt intake induced significant increases in the average blood pressure of the black normotensive individuals but not of the white normotensive individuals. Salt loading also induced greater renal retention of sodium in the black normotensive individuals than in the white normotensive individuals (29). Because black individuals on average consume less potassium than white individuals (45–49), it is possible that at the time of enrollment in the study by Morris et al. (29), potassium stores in some tissues may have been lower in the black participants than in the white participants. Oral administration of 120 mmol/day of potassium bicarbonate eliminated the salt-induced increase in blood pressure in normotensive black individuals while causing a mean weight loss of 1.1 ± 0.5 (SD) kilograms (29). This suggests that the supplemental potassium bicarbonate eliminated salt-induced increases in blood pressure in the normotensive black individuals by causing increases in renal sodium excretion that resulted in reductions in sodium balance, blood volume, and cardiac output. However, effects on blood volume, cardiac output, and vascular resistance were not reported and the mechanisms whereby potassium supplementation reversed the salt-induced increases in blood pressure require further investigation.
Racial Differences in Nitric Oxide Activity, Potassium Intake, and Salt Sensitivity
The greater blood pressure response to salt loading on average in black salt-sensitive individuals than in black salt-resistant normal controls usually involves a comparatively reduced ability to vasodilate and decrease vascular resistance in the black salt-sensitive group (60, 65). In salt-sensitive humans and in animals, impaired vasodilator responses to salt loading often involve a reduced ability to increase nitric oxide (NO) activity in response to increases in salt intake (38, 60, 65, 70–77). According to Brothers et al. (5), NO bioavailability appears reduced in black individuals compared with white individuals and is associated with impaired endothelial function as evidenced by blunted vasodilatory responses to a variety of stimuli. The reduced NO bioavailability in black individuals may be related in part to increased oxidative stress (5). These observations are consistent with the possibility that racial differences in oxidative stress and NO activity in response to increases in salt intake might contribute to racial differences in salt sensitivity. High-salt intake has been reported to reduce flow-mediated endothelial dependent vasodilation in the brachial artery (78, 79). However, this effect did not appear to be greater in a salt-sensitive normotensive group than in the salt-resistant normotensive group (7 white participants, 2 black participants, and 1 Asian participant in each group) (79). It remains to be determined whether racial differences in salt sensitivity are mediated by racial differences in oxidative stress, NO bioavailability, and endothelial-dependent vasodilation in response to increased salt intake. It would be of particular interest to study this in the renal vasculature because salt-sensitive individuals are characterized by subnormal renal vasodilatory responses to salt loading (58). It is noteworthy that a high-salt diet impairs the ability of the NO precursor l-arginine to induce renal vasodilation in salt-sensitive hypertensive Japanese individuals but not in salt-resistant hypertensive Japanese individuals (80).
The vasodilatory properties of potassium (81) and its effects on the NO system (38, 82) may contribute to the protective effect of high-potassium intake on salt sensitivity. Studies by Oberleithner et al. (82) have shown that an increase of potassium in the physiological range can reduce endothelial cell stiffness and increase release of NO. Thus, chronically lower potassium intake in black than in white individuals (45–49) could theoretically contribute to endothelial stiffness, impaired endothelial function, and reduced bioavailability of NO in response to increased salt intake in black versus white individuals. Smiljanec et al. (83) recently reported that a high-potassium intake (120 mmol/day) can prevent salt-induced reductions in flow-mediated dilation in the brachial artery. In addition, studies by Fang et al. (38) in normotensive salt-sensitive humans in Northern China indicate that the capacity of supplemental potassium to protect against salt-induced increases in blood pressure may be related to its capacity to prevent salt-induced increases in the NO synthesis inhibitor asymmetrical dimethylarginine (ADMA), and prevent decreases in plasma levels of nitrate and nitrite. These findings raise the possibility that supplemental potassium intake might attenuate greater salt sensitivity in black populations by improving NO bioavailability and vasodilator responses to a high-salt diet. Additional studies will be necessary to directly investigate the blood pressure effects of potassium intake and changes in NO activity during salt loading in groups of black individuals and white individuals.
Are Racial Differences in Salt Sensitivity Mediated by Racial Differences in Plasma Renin Activity?
Plasma renin activity (PRA) is a critical regulator of plasma levels of the potent vasoconstrictor angiotensin II. In cross-sectional studies without salt loading, normotensive black individuals have been reported to have lower PRA levels than normotensive white individuals (84, 85). We identified two studies in which PRA was measured while increases in dietary salt caused greater increases in blood pressure in black normotensive than in white normotensive individuals (26, 29). PRA was not significantly different between black normotensive individuals and white normotensive individuals during salt loading. Thus, the greater pressor response to oral salt loading in black normotensive versus white normotensive individuals was not associated with greater or lower levels of PRA in the black participants versus the white participants during salt loading (26, 29). In these studies, the dietary intake of potassium was below the previously recommended amount of 120 mmol/day.
We identified one study in which PRA was measured while changes in dietary salt intake caused significantly greater changes in blood pressure in the black hypertensive group than in the white hypertensive group (25). In this study, all participants were switched from a high-salt diet to a low-salt diet to specifically investigate the blood pressure lowering effects of salt restriction. The dietary intake of potassium was not reported. In response to salt restriction, blood pressure decreased more and PRA increased less in the black hypertensive group than in the white hypertensive group (25). As noted by He et al. (25), the results suggest that the greater fall in blood pressure with salt restriction in black hypertensive than in white hypertensive individuals is due at least in part to an impaired ability to increase PRA during salt restriction. However, there is little or no evidence that the greater pressor response to salt loading in black individuals versus white individuals involves differences in the ability to suppress PRA during salt loading. Other investigators have noted that sensitivity to the pressor effects of a high-salt diet is usually not associated with an impaired ability to suppress PRA during salt loading (29, 66, 86).
In summary, when considering whether alterations in PRA or other vasoactive pathways play a role in determining BP responses to changes in salt intake, it is important to distinguish between studies investigating the BP effects of salt restriction versus those investigating the BP effects of salt loading. A greater fall in blood pressure with salt restriction in black hypertensive than in white hypertensive individuals appears related to racial differences in PRA levels during salt restriction (25). However, the greater rise in blood pressure with salt loading in black normotensive than in white normotensive individuals does not appear related to racial differences in PRA levels during salt loading.
Are Racial Differences in Salt Sensitivity Mediated by Racial Differences in Sympathetic Nervous System Activity?
According to a recent analysis by Brothers et al. (5), a “possible mechanism for elevated cardiovascular disease risk in the black population could be related to overactivity of the sympathetic nervous system (SNS), which would result in a number of physiological alterations including increased peripheral vascular resistance and arterial blood pressure.” In hypertensive humans, it has been proposed that salt sensitivity may be related to abnormally high SNS activity in response to increased salt intake (86). In addition, Vranish et al. (87) recently demonstrated that young healthy black men have greater vasoconstriction and pressor responses to spontaneous bursts of sympathetic outflow than do white men. However, only few studies have investigated whether racial differences in salt sensitivity are mediated by racial differences in SNS activity or in the transduction of SNS activity to changes in blood pressure.
In studies of black normotensive and hypertensive groups, and white normotensive and hypertensive groups, Dimsdale et al. (22) measured the effects of changing salt intake on blood pressure, plasma norepinephrine levels, and the blood pressure response to infusion of norepinephrine. The sequence of administration of the low-salt and high-salt diets was randomized and the diet provided a potassium intake of 100 mmol/day. In black hypertensive but not in white hypertensive individuals, administration of the high-salt diet significantly increased the systolic blood pressure response to norepinephrine infusion (22). However, ANOVA did not show a statistically significant effect of race on BP salt sensitivity or a significant interaction of race with hypertension status on salt sensitivity (22). According to Dimsdale et al. (22), salt sensitivity of SBP was associated with a failure to decrease plasma norepinephrine levels in response to salt loading. However, plasma levels of norepinephrine were not separately reported in the black hypertensive versus the white hypertensive individuals. It should also be noted that changes in plasma norepinephrine levels reflect both the rate of norepinephrine release, and the rate that norepinephrine is removed from the circulation (88). In addition, BP responses to norepinephrine infusions assess vascular reactivity not SNS activity (89). Accordingly, neither changes in plasma norepinephrine levels nor BP responses to norepinephrine infusions are considered reliable indicators of changes in SNS activity. In summary, there is little or no information available on the role of the SNS in mediating racial differences in salt sensitivity. It also remains to be determined whether a high-potassium intake eliminates racial differences in salt sensitivity through effects on SNS activity or in the transduction of SNS activity to changes in blood pressure.
Do Racial Differences in Psychosocial Stress Influence the Blood Pressure Responses to Changes in Salt Intake?
It has been noted that groups of black individuals have greater blood pressure responses than groups of white individuals to mental stress and other stimuli, and that more mechanistic studies are needed on the role of psychosocial stress in explaining population differences in the development of vascular dysfunction (5, 9, 90). According to Wolf et al. (9), “those who identify as belonging to a minority group are more likely to experience daily psychosocial stress.” It has been proposed that a “differential burden of psychological stress may help explain the greater prevalence of salt sensitivity in blacks” (67). However, we are not aware of any studies that have assessed the “burden of psychological stress” in studies of salt sensitivity comparing black individuals and white individuals and whether it contributes to racial differences in salt sensitivity.
In studies in black or white normotensive and hypertensive individuals, Falkner and Kushner (23) investigated the BP responses to the stress of mental arithmetic before and after increasing dietary salt intake for 14 days. The change in mean arterial pressure induced by mental stress was significantly greater in the white study participants than in the black study participants and was not altered by increasing salt intake (23). In these studies, the level of potassium intake was not reported.
Sudhir et al. (31) found that changes in systolic and diastolic pressure in response to mental arithmetic stress were not significantly different in black normotensive and white normotensive men ingesting a low-potassium/low-salt diet (30 mmol/day K+/15 mmol/day Na+) or a low-potassium/high-NaCl diet (30 mmol/day K+/250 mmol/day Na+). However, in the black normotensive men but not in the white normotensive men ingesting a high-salt diet, increasing potassium intake from 30 mmol/day to 70 mmol/day attenuated the elevations in systolic and diastolic pressure induced by mental stress (31). Increasing potassium intake from 30 mmol/day to 70 mmol/day also abolished both the increases in systolic and diastolic pressure induced by cold stress in the normotensive black individuals and the increases in systolic pressure induced by cold stress in the normotensive white individuals (31). Based on the aforementioned findings, Sudhir et al. (31) speculated that in black normotensive men, the vasopressor response to mental arithmetic and cold stress might be enhanced by low-potassium intake and reduced by high-potassium intake.
WHAT IS THE SCIENTIFIC OR CLINICAL IMPORTANCE OF STUDIES THAT HAVE SHOWN RACIAL DIFFERENCES IN SALT SENSITIVITY?
Summary and Future Directions
Studies of racial disparities in salt sensitivity have many limitations including the use of questionable methods for categorizing individuals according to race and blood pressure salt sensitivity. Furthermore, Huang et al. (18) specifically noted that “short term studies of sodium reduction are not a sound basis for drawing conclusions about the effects of sodium reduction on blood pressure and are not helpful in formulating policy recommendations for public health.” The DASH-Sodium study arm that involved a low-potassium diet is the only long-term study showing that black individuals are more sensitive than white individuals to the blood pressure effects of a change in dietary salt intake (34). However, the racial difference in salt sensitivity was demonstrated only when changing salt intake between 50 mmol/day (2.9 g/day NaCl, 1,150 mg/day Na+) and 150 mmol/day (8.8 g/day NaCl, 3,450 mg/day Na+), i.e., only when changing to or from a salt intake of 50 mmol/day which is far below the goal intake recommended by US authorities of 100 mmol/day (5.85 g/day NaCl, 2,300 mg/day Na+)(40, 46). When salt intake was changed between 100 mmol/day and 150 mmol/day, the DASH-Sodium trial showed little or no racial difference in salt sensitivity (20, 29, 34).
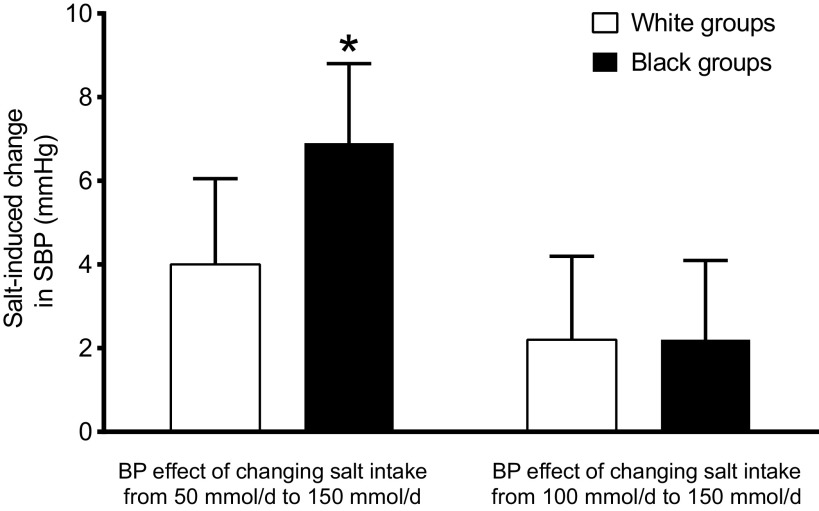
Figure 5 shows that changing salt intake from 100 mmol/day to 150 mmol/day for 30 days does not cause greater changes in systolic blood pressure in African Americans than in non-African Americans even when consuming a low-potassium diet. This is important because a salt intake of 100 mmol/day is the amount of dietary salt recommended by the US dietary guidelines, and a salt intake of 150 mmol/day is close to the mean and median levels of salt intake in the United States (91). Thus, there is little or no direct evidence showing that black individuals are more sensitive than white individuals to the blood pressure effects of long-term changes in dietary salt intake within the range that is practical to achieve in real life. This raises the question: what is the medical value of the data in the DASH-Sodium trial showing racial differences in blood pressure responses to changing salt intake to or from a level of 50 mmol/day, i.e., to or from a goal salt intake that is not likely to be achieved outside of a research study?
Figure 5.
Changes in systolic blood pressure (SBP) in normotensive subjects induced by changing salt intake from 50 mmol/day to 150 mmol/day, or from 100 mmol/day to 150 mmol/day for 30 days. Note that a salt intake of 100 mmol/day is the maximum recommended in most guidelines. The results show the mean changes and 95% confidence intervals. *The salt-induced increase in blood pressure is significantly greater in the black group. Results are from the analysis of Bray et al. (20) of the control diet arm of the DASH-Sodium trial in which potassium intake was targeted to be 44 mmol/day.
Given that reducing mean population salt intake to less than 100 mmol/day has not proven feasible (92), the medical value of knowing that black individuals on average are more sensitive than white individuals to the blood pressure lowering effects of reducing salt intake to 50 mmol/day is open to question (because of the difficulty in achieving a salt intake of 50 mmol/day in real life). However, the observations that supplemental potassium bicarbonate or administration of a DASH-type diet can reduce blood pressure in black groups consuming a high-salt diet are of considerable medical interest (20, 29, 34). These findings should motivate further research on the risks and benefits of using potassium-containing salt substitutes (93) or potassium supplements while keeping in mind the potential hazards of hyperkalemia in individuals with chronic kidney disease. They should also stimulate research on methods for increasing the consumption of DASH-type diets and foods rich in nutrients such as potassium and nitrate that may protect against salt-induced increases in blood pressure (43, 44, 92, 94, 95). The recent requirement by the Food and Drug Administration to include potassium content on food labels reflects the medical importance of this nutrient and should be helpful for guiding consumers in making healthy food choices in the future.
Conclusions
There is mounting evidence that racial disparities in risk for incident hypertension and cardiovascular disease involve racial differences in diet composition (7). In the present analysis, we discuss evidence showing that on average, normotensive black individuals are more sensitive to the BP effects of changes in salt intake than normotensive white individuals. However, in hypertensive individuals, the evidence for racial disparities in salt sensitivity is less clear. In normotensive individuals, potassium intake in excess of the amount recommended by the US dietary guidelines appears to prevent racial disparities in salt sensitivity. Black individuals generally consume less potassium than white individuals, while consuming similar amounts of sodium as white individuals. Thus, racial differences in dietary potassium intake might contribute to racial disparities in salt sensitivity. It should be noted that racial differences in dietary habits and diet composition are potentially mediated by multiple social, environmental, economic, and cultural factors (8). The determinants of dietary patterns are complex and further research will be needed to overcome barriers to consumption of diets rich in potassium and other nutrients considered beneficial to human health.
Finally, the present analysis raises concerns about the recent decision by the NAS committee and the authors of the 2020–2025 US Dietary Guidelines to reduce the recommended goal for potassium intake (40, 46). It appears that this decision did not take into consideration the potential impact of potassium intake on racial disparities in salt sensitivity. Thus, we suggest that future research should also examine the impact of reducing the goal for dietary potassium intake on racial disparities in salt sensitivity and risk for hypertension and cardiovascular disease in vulnerable populations.
GRANTS
This work was funded by National Center for Research Resources Grant M0 RR-00079, National Heart, Lung, and Blood Institute Grant RO1-HL64230, Praemium Academiae Award AP1502 of the Czech Academy of Sciences (to M. Pravenec), and gifts from the Saw Island Foundation, the Antel Foundation, and the Maier Family Foundation.
DISCLOSURES
S. E. DiCarlo, M. Pravenec, and R. C. Morris have nothing to disclose. T. W. Kurtz is a cofounder and stockholder of Mission Salt, Inc., that has filed patents for salty food compositions including nitrate-rich vegetable ingredients. The company goal is to develop methods to prevent salt-induced hypertension. T. W. Kurtz does not receive any financial support from Mission Salt and no funds from Mission Salt were used for the submitted work.
AUTHOR CONTRIBUTIONS
T.W.K. and R.C.M. conceived and designed research; T.W.K., S.E.D., and R.C.M. analyzed data; T.W.K., S.E.D., M.P., and R.C.M. interpreted results of experiments; T.W.K. prepared figures; T.W.K. drafted manuscript; T.W.K., S.E.D., M.P., and R.C.M. edited and revised manuscript; T.W.K., S.E.D., M.P., and R.C.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the multiple colleagues, trainees, and staff who have contributed to various aspects of the scientific research presented in this review.
REFERENCES
- 1.Elijovich F, Weinberger MH, Anderson CAM, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; Stroke Council. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension 68: e7–e46, 2016. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC Jr.. The American Heart Association Scientific Statement on salt sensitivity of blood pressure: prompting consideration of alternative conceptual frameworks for the pathogenesis of salt sensitivity? J Hypertens 35: 2214–2225, 2017. doi: 10.1097/HJH.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. An appraisal of methods recently recommended for testing salt sensitivity of blood pressure. J Am Heart Assoc 6: e005653, 2017. doi: 10.1161/JAHA.117.005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350: 1734–1737, 1997. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 5.Brothers RM, Fadel PJ, Keller DM. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol 317: H777–H789, 2019. doi: 10.1152/ajpheart.00126.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright JT Jr.Profile of systemic hypertension in black patients. Am J Cardiol 61: 41H–45H, 1988. doi: 10.1016/0002-9149(88)91104-6. [DOI] [PubMed] [Google Scholar]
- 7.Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, Manly JJ, Flaherty ML, Judd SE, Wadley VG, Long DL, Howard VJ. Association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA 320: 1338–1348, 2018. doi: 10.1001/jama.2018.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kris-Etherton PM, Petersen KS, Velarde G, Barnard ND, Miller M, Ros E, O'Keefe JH, Williams K Sr, Horn LV, Na M, Shay C, Douglass P, Katz DL, Freeman AM. Barriers, opportunities, and challenges in addressing disparities in diet-related cardiovascular disease in the United States. J Am Heart Assoc 9: e014433, 2020. doi: 10.1161/jaha.119.014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf ST, Jablonski NG, Kenney WL. Examining “race” in physiology. Am J Physiol Heart Circ Physiol 319: H1409–H1413, 2020. doi: 10.1152/ajpheart.00698.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman AH. Why genes don’t count (for racial differences in health). Am J Public Health 90: 1699–1702, 2000. doi: 10.2105/ajph.90.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobles M. History counts: a comparative analysis of racial/color categorization in US and Brazilian censuses. Am J Public Health 90: 1738–1745, 2000. doi: 10.2105/ajph.90.11.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly TN, He J. Genomic epidemiology of blood pressure salt sensitivity. J Hypertens 30: 861–873, 2012. doi: 10.1097/HJH.0b013e3283524949. [DOI] [PubMed] [Google Scholar]
- 13.Mei H, Gu D, Hixson JE, Rice TK, Chen J, Shimmin LC, Schwander K, Kelly TN, Liu DP, Chen S, Huang JF, Jaquish CE, Rao DC, He J. Genome-wide linkage and positional association study of blood pressure response to dietary sodium intervention: the GenSalt Study. Am J Epidemiol 176, Suppl 7: S81–S90, 2012. doi: 10.1093/aje/kws290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svetkey LP, McKeown SP, Wilson AF. Heritability of salt sensitivity in black Americans. Hypertension 28: 854–858, 1996. doi: 10.1161/01.hyp.28.5.854. [DOI] [PubMed] [Google Scholar]
- 15.de Leeuw PW, Kroon AA. Salt and sensitivity. Hypertension 62: 461–462, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01831. [DOI] [PubMed] [Google Scholar]
- 16.GenSalt Collaborative Research Group. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens 21: 639–646, 2007. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 344: 3–10, 2001. doi: 10.1056/nejm200101043440101. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NRC, Li Q, Lackland DT, Leung AA, Anderson CAM, MacGregor GA, He FJ. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ 368: m315, 2020. doi: 10.1136/bmj.m315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 161: 685–693, 2001. doi: 10.1001/archinte.161.5.685. [DOI] [PubMed] [Google Scholar]
- 20.Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 94: 222–227, 2004. doi: 10.1016/j.amjcard.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 21.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 18: 805–812, 1991. doi: 10.1161/01.hyp.18.6.805. [DOI] [PubMed] [Google Scholar]
- 22.Dimsdale JE, Ziegler M, Mills P, Berry C. Prediction of salt sensitivity. Am J Hypertens 3: 429–435, 1990. doi: 10.1093/ajh/3.6.429. [DOI] [PubMed] [Google Scholar]
- 23.Falkner B, Kushner H. Effect of chronic sodium loading on cardiovascular response in young blacks and whites. Hypertension 15: 36–43, 1990. doi: 10.1161/01.hyp.15.1.36. [DOI] [PubMed] [Google Scholar]
- 24.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 54: 482–488, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133223. [DOI] [PubMed] [Google Scholar]
- 25.He FJ, Markandu ND, Sagnella GA, MacGregor GA. Importance of the renin system in determining blood pressure fall with salt restriction in black and white hypertensives. Hypertension 32: 820–824, 1998. doi: 10.1161/01.HYP.32.5.820. [DOI] [PubMed] [Google Scholar]
- 26.Luft FC, Rankin LI, Bloch R, Weyman AE, Willis LR, Murray RH, Grim CE, Weinberger MH. Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation 60: 697–706, 1979. doi: 10.1161/01.cir.60.3.697. [DOI] [PubMed] [Google Scholar]
- 27.Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens 8: 663–670, 1990. doi: 10.1097/00004872-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 28.MacGregor GA, Markandu ND, Best FE, Elder DM, Cam JM, Sagnella GA, Squires M. Double-blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet 351–355, 1982. doi: 10.1016/s0140-6736(82)91389-7. [DOI] [PubMed] [Google Scholar]
- 29.Morris RC Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt sensitivity—effects of race and dietary potassium. Hypertension 33: 18–23, 1999. doi: 10.1161/01.HYP.33.1.18. [DOI] [PubMed] [Google Scholar]
- 30.Parmer RJ, Stone RA, Cervenka JH. Renal hemodynamics in essential hypertension. Racial differences in response to changes in dietary sodium. Hypertension 24: 752–757, 1994. doi: 10.1161/01.hyp.24.6.752. [DOI] [PubMed] [Google Scholar]
- 31.Sudhir K, Forman A, Yi S-L, Sorof J, Schmidlin O, Sebastian A, Morris RC. Reduced dietary potassium reversibly enhances vasopressor response to stress in African Americans. Hypertension 29: 1083–1090, 1997. doi: 10.1161/01.hyp.29.5.1083. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension 17: I61–I68, 1991. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 33.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med 157: 657–667, 1997. [PubMed] [Google Scholar]
- 34.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N; DASH-Sodium Trial Collaborative Research Group. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med 135: 1019–1028, 2001. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 35.Wedler B, Brier ME, Wiersbitzky M, Gruska S, Wolf E, Kallwellis R, Aronoff GR, Luft FC. Sodium kinetics in salt-sensitive and salt-resistant normotensive and hypertensive subjects. J Hypertens 10: 663–669, 1992. [PubMed] [Google Scholar]
- 36.Weir MR, Dengel DR, Behrens MT, Goldberg AP. Salt-induced increases in systolic blood pressure affect renal hemodynamics and proteinuria. Hypertension 25: 1339–1344, 1995. doi: 10.1161/01.hyp.25.6.1339. [DOI] [PubMed] [Google Scholar]
- 37.Wright JT Jr, Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, Islam M, Eissa M, White S, Douglas JG. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension 42: 1087–1092, 2003. doi: 10.1161/01.HYP.0000101687.89160.19. [DOI] [PubMed] [Google Scholar]
- 38.Fang Y, Mu J-J, He L-C, Wang S-C, Liu Z-Q. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive Asians. Hypertension 48: 724–729, 2006. doi: 10.1161/01.HYP.0000238159.19614.ce. [DOI] [PubMed] [Google Scholar]
- 39.Elliott P, Brown I. Sodium Intakes Around the World. Geneva: World Health Organization, 2007. [Google Scholar]
- 40.United States Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2020–2025. Washington, DC: US Department of Health and Human Services and US Department of Agriculture, 2020. https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf [Google Scholar]
- 41.Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease—a delicate balance. N Engl J Med 368: 1229–1237, 2013. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 42.Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fiber. J Hum Hypertens 24: 237–246, 2010. doi: 10.1038/jhh.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90: 1–10, 2009. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 44.Morris RC Jr, Pravenec M, Silhavy J, DiCarlo SE, Kurtz TW. Small amounts of inorganic nitrate or beetroot provide substantial protection from salt-induced increases in blood pressure. Hypertension 73: 1042–1048, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cogswell ME, Zhang Z, Carriquiry AL, Gunn JP, Kuklina EV, Saydah SH, Yang Q, Moshfegh AJ. Sodium and potassium intakes among US adults: NHANES 2003-2008. Am J Clin Nutr 96: 647–657, 2012. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press, 2019. https://www.nap.edu/read/25353/chapter/1#ii. [PubMed] [Google Scholar]
- 47.Newby PK, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region are associated with nutrient intakes among black and white men in the United States. J Nutr 141: 296–303, 2011. doi: 10.3945/jn.110.130583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newby PK, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region have independent and synergistic effects on dietary intakes in black and white women. Nutr J 11: 25, 2012. doi: 10.1186/1475-2891-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Va P, Dodd KW, Zhao L, Thompson-Paul AM, Mercado CI, Terry AL, Jackson SL, Wang C-Y, Loria CM, Moshfegh AJ, Rhodes DG, Cogswell ME. Evaluation of measurement error in 24-hour dietary recall for assessing sodium and potassium intake among US adults—National Health and Nutrition Examination Survey (NHANES), 2014. Am J Clin Nutr 109: 1672–1682, 2019. doi: 10.1093/ajcn/nqz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.United States Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2005. Washington, DC: US Department of Health and Human Services and US Department of Agriculture, 2004. https://www.dietaryguidelines.gov/about-dietary-guidelines/previous-editions/2005-dietary-guidelines-americans. [Google Scholar]
- 51.National Academies of Sciences Engineering Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: The National Academies Press, 2005. doi: 10.17226/10925. [DOI] [Google Scholar]
- 52.Brands MW. Chronic blood pressure control. Compr Physiol 2: 2481–2494, 2012. doi: 10.1002/cphy.c100056. [DOI] [PubMed] [Google Scholar]
- 53.Guyton AC. Arterial pressure and hypertension. In: Circulatory Physiology III. Philadelphia: WB Saunders, 1980. https://ci.nii.ac.jp/naid/10009568978/. [Google Scholar]
- 54.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol Regul Integr Comp Physiol 259: R865–R877, 1990. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 55.Hall JE. Guyton and Hall Textbook of Medical Physiology. Philadelphia: Elsevier, 2015. https://www.elsevier.com/books/guyton-and-hall-textbook-of-medical-physiology/hall/978-1-4557-7005-2. [Google Scholar]
- 56.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 57.Schrier RW. Renal and Electrolyte Disorders. Philadelphia: Wolters Kluwer, 2018. [Google Scholar]
- 58.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC Jr.. The pivotal role of renal vasodysfunction in salt sensitivity and the initiation of salt-induced hypertension. Curr Opin Nephrol Hypertens 27: 83–92, 2018. doi: 10.1097/mnh.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 59.Kurtz TW, DiCarlo SE, Pravenec M, Schmidlin O, Tanaka M, Morris RC. An alternative hypothesis to the widely held view that renal excretion of sodium accounts for resistance to salt-induced hypertension. Kidney Int 90: 965–973, 2016. doi: 10.1016/j.kint.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris RC Jr, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation 133: 881–893, 2016. doi: 10.1161/CIRCULATIONAHA.115.017923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beard DA. Assessing the validity and utility of the Guyton model of arterial blood pressure control. Hypertension 72: 1272–1273, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurtz TW, DiCarlo SE, Pravenec M, Jezek F, Silar J, Kofránek J, Morris RC Jr.. Testing computer models predicting human responses to a high-salt diet. Hypertension 72: 1407–1416, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishii M, Atarashi K, Ikeda T, Hirata Y, Igari T, Uehara Y, Takagi M, Matsuoka H, Takeda T, Murao S. Role of the aldosterone system in the salt-sensitivity of patients with benign essential hypertension. Jpn Heart J 24: 79–90, 1983. doi: 10.1536/ihj.24.79. [DOI] [PubMed] [Google Scholar]
- 64.Laffer CL, Scott RC 3rd, Titze JM, Luft FC, Elijovich F. Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension 68: 195–203, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC Jr.. Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension 58: 380–385, 2011. doi: 10.1161/hypertensionaha.111.170175. [DOI] [PubMed] [Google Scholar]
- 66.Schmidlin O, Sebastian AFA, Morris RC Jr.. What initiates the pressor effect of salt in salt-sensitive humans? Observations in normotensive blacks. Hypertension 49: 1032–1039, 2007. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flack JM, Ensrud KE, Mascioli S, Launer CA, Svendsen K, Elmer PJ, Grimm RH Jr.. Racial and ethnic modifiers of the salt-blood pressure response. Hypertension 17: I115–I121, 1991. doi: 10.1161/01.hyp.17.1_suppl.i115. [DOI] [PubMed] [Google Scholar]
- 68.Richardson SI, Freedman BI, Ellison DH, Rodriguez CJ. Salt sensitivity: a review with a focus on non-Hispanic blacks and Hispanics. J Am Soc Hypertens 7: 170–179, 2013. doi: 10.1016/j.jash.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishna GG, Chusid P, Hoeldtke RD. Mild potassium depletion provokes renal sodium retention. J Lab Clin Med 109: 724–730, 1987. [PubMed] [Google Scholar]
- 70.Bragulat E, de la Sierra A. Salt intake, endothelial dysfunction, and salt-sensitive hypertension. J Clin Hypertens (Greenwich) 4: 41–46, 2002. doi: 10.1111/j.1524-6175.2002.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Y, Mu J-J, Fang Y, Yuan Z-Y, Liu F-Q. Impact of high salt independent of blood pressure on PRMT/ADMA/DDAH pathway in the aorta of Dahl salt-sensitive rats. Int J Mol Sci 14: 8062–8072, 2013. doi: 10.3390/ijms14048062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest 88: 1559–1567, 1991. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension 33: 1008–1012, 1999. doi: 10.1161/01.HYP.33.4.1008. [DOI] [PubMed] [Google Scholar]
- 74.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation 101: 856–861, 2000. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 75.Manning RD Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol Scand 179: 243–250, 2003. doi: 10.1046/j.0001-6772.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 76.Matsuoka H, Itoh S, Kimoto M, Kohno K, Tamai O, Wada Y, Yasukawa H, Iwami G, Okuda S, Imaizumi T. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension 29: 242–247, 1997. doi: 10.1161/01.HYP.29.1.242. [DOI] [PubMed] [Google Scholar]
- 77.Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens 29: 415–424, 2011. doi: 10.1097/HJH.0b013e328341d19e. [DOI] [PubMed] [Google Scholar]
- 78.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matthews EL, Brian MS, Ramick MG, Lennon-Edwards S, Edwards DG, Farquhar WB. High dietary sodium reduces brachial artery flow-mediated dilation in humans with salt-sensitive and salt-resistant blood pressure. J Appl Physiol 118: 1510–1515, 2015. doi: 10.1152/japplphysiol.00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Higashi Y, Oshima T, Watanabe M, Matsuura H, Kajiyama G. Renal response to l-arginine in salt-sensitive patients with essential hypertension. Hypertension 27: 643–648, 1996. doi: 10.1161/01.HYP.27.3.643. [DOI] [PubMed] [Google Scholar]
- 81.Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep 13: 309–317, 2011. doi: 10.1007/s11906-011-0197-8. [DOI] [PubMed] [Google Scholar]
- 82.Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmuller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci USA 106: 2829–2834, 2009. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smiljanec K, Mbakwe A, Ramos Gonzalez M, Farquhar WB, Lennon SL. Dietary potassium attenuates the effects of dietary sodium on vascular function in salt-resistant adults. Nutrients 12: 1206, 2020. doi: 10.3390/nu12051206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He J, Klag MJ, Appel LJ, Charleston J, Whelton PK. The renin-angiotensin system and blood pressure: differences between blacks and whites. Am J Hypertens 12: 555–562, 1999. doi: 10.1016/s0895-7061(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 85.Rifkin DE, Khaki AR, Jenny NS, McClelland RL, Budoff M, Watson K, Ix JH, Allison MA. Association of renin and aldosterone with ethnicity and blood pressure: the multi-ethnic study of atherosclerosis. Am J Hypertens 27: 801–810, 2014. doi: 10.1093/ajh/hpt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt-sensitive patients with essential hypertension. Kidney Int 21: 371–378, 1982. doi: 10.1038/ki.1982.32. [DOI] [PubMed] [Google Scholar]
- 87.Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Keller DM, Fadel PJ. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young black men. Hypertension 71: 192–198, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Esler M, Jennings G, Korner P, Blombery P, Sacharias N, Leonard P. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol Endocrinol Metab 247: E21–E28, 1984. doi: 10.1152/ajpendo.1984.247.1.E21. [DOI] [PubMed] [Google Scholar]
- 89.Vlachakis ND, Frederics R, Velasquez M, Alexander N, Singer F, Maronde RF. Sympathetic system function and vascular reactivity in hypercalcemic patients. Hypertension 4: 452–458, 1982. doi: 10.1161/01.hyp.4.3.452. [DOI] [PubMed] [Google Scholar]
- 90.Calhoun DA. Hypertension in Blacks—socioeconomic stress and sympathetic nervous-system activity. Am J Med Sci 304: 306–311, 1992. doi: 10.1097/00000441-199211000-00008. [DOI] [PubMed] [Google Scholar]
- 91.Cogswell ME, Loria CM, Terry AL, Zhao L, Wang C-Y, Chen T-C, Wright JD, Pfeiffer CM, Merritt R, Moy CS, Appel LJ. Estimated 24-hour urinary sodium and potassium excretion in US adults. JAMA 319: 1209–1220, 2018. doi: 10.1001/jama.2018.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurtz TW, Pravenec M, Dicarlo SE. Strategies are needed to prevent salt-induced hypertension that do not depend on reducing salt intake. Am J Hypertens 33: 116–118, 2020. doi: 10.1093/ajh/hpz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greer RC, Marklund M, Anderson CAM, Cobb LK, Dalcin AT, Henry M, Appel LJ. Potassium-enriched salt substitutes as a means to lower blood pressure: benefits and risks. Hypertension 75: 266–274, 2020. doi: 10.1161/HYPERTENSIONAHA.119.13241. [DOI] [PubMed] [Google Scholar]
- 94.Keller RM, Beaver L, Prater MC, Hord NG. Dietary nitrate and nitrite concentrations in food patterns and dietary supplements. Nutr Today 55: 218–226, 2020. doi: 10.1097/NT.0000000000000253. [DOI] [Google Scholar]
- 95.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC. Functional foods for augmenting nitric oxide activity and reducing the risk for salt-induced hypertension and cardiovascular disease in Japan. J Cardiol 72: 42–49, 2018. doi: 10.1016/j.jjcc.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]