Abstract
Extracellular vesicles (EVs) are nanosized lipid bilayer-delimited particles released from cells that mediate intercellular communications and play a pivotal role in various physiological and pathological processes. Subtypes of EVs may include plasma membrane ectosomes or microvesicles and endosomal origin exosomes, although functional distinctions remain unclear. EVs carry cargo proteins, nucleic acids (RNA and DNA), lipids, and metabolites. By presenting or transferring this cargo to recipient cells, EVs can trigger cellular responses. We summarize contemporary understanding of EV biogenesis, composition, and function, with an emphasis on the role of EVs in the cardiovascular system. In addition, we outline the functional relevance of EVs in cardiovascular pathophysiology, further highlighting their potential for diagnostic and therapeutic applications.
Keywords: cardiovascular disease, ectosome, exosome, exosomal cargo, extracellular vesicles
INTRODUCTION
Extracellular vesicles (EVs) are released by a variety of cell types and are today known as critical mediators of intercellular communication. Defined by their lipid bilayer membrane, cytoplasmic content, and an inability to replicate (1), EVs have recently shown promise as biomarkers and biovehicles for drug delivery. Famously called “platelet dust” by Wolf in 1967 (2), EVs had a long but sporadic research history over several decades before a critical mass was reached to form a dedicated field (1). Early investigations nevertheless gave tantalizing hints for roles of EVs in coagulation (2), bone calcification (3, 4), microbe mating (5), endocrine signaling (6), and cancer (7). In the 1980s, EVs were found to be vehicles for shedding of membrane enzymes and receptors (8–13) from both the cell surface and endosomal compartments. However, rapid growth of the field was spurred by the discovery that EVs could package and transport functional RNA (14–16), with implications for clinical exploitation. Multiple signaling functions of EVs were thus added to previously appreciated roles in cellular disposal of toxic or excess materials. The goals of this review are to: 1) provide an overview on the recent classification of extracellular vesicles; 2) discuss the role of EVs released by different cardiac cell types in diverse cardiac pathophysiology; 3) identify the ways by which different cell-derived EVs exert beneficial effect(s) in preclinical and clinical cardiovascular studies; and finally, 4) discuss the limitations and the future directions of EV-based therapies.
Diversity of EVs
RNA and other cargo of EVs are among the many determinants of a vast heterogeneity of EVs (17–19). A diverse array of biological molecules is found within the EV lumen (corresponding to cytoplasmic content), embedded in the lipid membrane, and tightly or loosely associated with the EV surface. Luminal content in particular is generally stable under numerous physiochemical conditions, protected from degrading enzymes by the EV lipid bilayer (20). EV molecular biotypes include proteins, nucleic acids, lipids, and metabolites, and each can be used to classify EVs by composition. Furthermore, EVs are released from all studied cell types. Markers from the parent cell classify EVs by tissue or cell of origin. In mammals, released EVs are abundant in biofluids like blood, lymph, tears, cerebrospinal fluid, saliva, breast milk, urine, gastric acid, and more (21–24). This heterogeneous group of EVs has been implicated in processes including cell-to-cell communication, immunomodulation, growth and development, aging, and various pathologies (20, 25). In turn, EV composition and categorization may be influenced by pathologic conditions, such as cancer, cardiovascular diseases (CVDs), oxidative stress, and aging (26–28). EVs can also be separated and classified based on physical characteristics such as density and stiffness. However, EVs have most frequently been classified by presumed biogenesis or size, as ectosomes or exosomes (29).
Ectosomes: Microvesicles and More
Ectosomes are vesicles that bud directly from the plasma membrane to the extracellular space. They form a heterogeneous population of EVs varying in both size (from the lower limits dictated by membrane physics up to microns in diameter) and molecular composition (30). To date, microvesicles (MVs), apoptotic bodies, exophers, and oncosomes are among the classes of ectosomes that have been identified and defined. Microvesicles (MVs) are often described as having diameters up to around 1,000 nm (31). However, larger ectosomes have also been described. Apoptotic bodies, for example, are sometimes listed as a third type of EV, but they are formed from apoptotic cells by blebbing of the plasma membrane (32, 33). Apoptotic bodies have a wide range of sizes, from around 100 nm to 5 microns in diameter. Exophers were first described as massive (4 microns or larger) vesicles that release protein aggregates and mitochondria from neurons under stress (34), and they have recently been reported to eject dysfunctional mitochondria from cardiomyocytes (35, 36). Similarly, MVs derived from mesenchymal stem cells (MSCs) subjected to oxidative stress were also found to contain mitochondrial particles like mtDNA (37). Large oncosomes are another category of ectosome, shed from malignant cells, and up to tens of microns in diameter (38). Yet, ectosomes can also be quite small, presenting as one type of “small EVs” (sEVs) less than 200 nm in diameter (39–41).
Exosomes
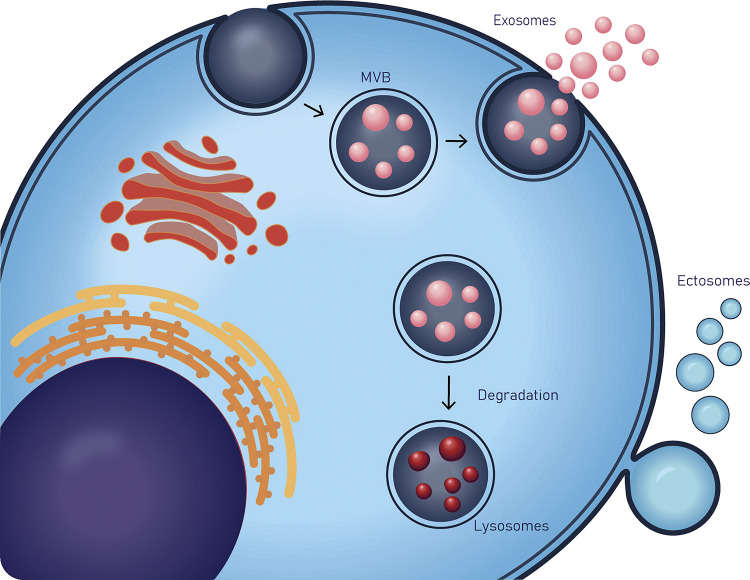
Vesicles released from exocytosis of multivesicular bodies form the second broad category of EVs, termed exosomes (42). Their sizes range from around 30 nm to 200 nm in diameter, and they arise from and are limited in size by the endosomal system. Intraluminal vesicles (ILVs) pinch off into endosomes, thus defining the multivesicular body (MVB). If the MVB fuses with the plasma membrane, the ILVs are expelled into the extracellular space as exosomes (33). Formation in the endosome includes processes that depend on the endosomal sorting complex required for transportation (ESCRT) and processes that are ESCRT-independent. In addition to ESCRT proteins, other factors in exosome biogenesis include but are not limited to small integral membrane protein of the lysosome/late endosome (SIMPLE), tetraspanins CD9 and CD82, enzymes such as neutral sphingomyelinase 2 (nSMase 2), diacylglycerol kinase, and phospholipase D2, and GTPases Rab27a and Rab27b (43). The entire process involving biogenesis of the exosomes and ectosomes is summarized in Fig. 1.
Figure 1.
Biogenesis and release of extracellular vesicles (EVs). Extracellular vesicles can be broadly classified as: ectosomes that are produced by outward budding and fission of the plasma membrane; and exosomes that are formed within the endosomal network and released upon fusion of multivesicular bodies (MVBs) with the plasma membrane. EVs, extracellular vesicles; MVBs, multivesicular bodies.
Ectosomes and Exosomes: Differences and Similarities
Besides their subcellular origin, what are the differences between ectosomes and exosomes, and do they include functional differences? The lipid bilayer that delimits EVs is enriched with glycosphingolipids, cholesterol (44, 45), phosphatidylserine (46), and ceramide. The smaller the EV, the more these membrane components will predominate. In contrast, the larger the EV, the more of the cellular interior it can sample and carry away, including organelles and active cytoskeleton components in some cases. It has become clear, though, that size alone cannot be used to separate ectosomes from exosomes since both include sEVs that are smaller than 200 nm in diameter (1). It is also not at all straightforward to use surface markers or internal content to trace an EV back to a specific biogenesis pathway after it has left the cell. For this reason, even though use of the term “exosome” remains popular in the literature, most studies on exosomes are actually studies of mixed populations of sEVs, and functional differences of EVs from the plasma membrane versus endocytic system remain largely unclear. Thus, we will use the term “EV” in this review unless biogenesis pathway was clearly established by a cited report. In any case, both ectosomes and exosomes are potent carriers of proteins, RNAs (mRNAs, miRNAs, and other ncRNAs), as well as short DNA sequences. Functions of EVs include cytotoxicity, apoptosis, antigen presentation, inflammation, and immunomodulation. In particular, the role of EVs in cardiac development and physiology has been of recent interest and will be expanded upon. These points are summarized in Table 1.
Table 1.
Ectosomes and exosomes: similarities and differences
| Characteristics | Ectosomes | Exosomes |
|---|---|---|
| Formation | By budding of plasma membrane | By invagination into and subsequent exocytosis from multivesicular bodies |
| Diameter | ∼30 nm to 1 micron or larger | ∼30 nm to ∼200 nm |
| Markers | Proposed markers include plasma membrane integrins and selectins | Proposed markers include tetraspanins (CD63, CD81), Alix, TSG 101, but overlap with ectosomes has been widely observed |
| Timing of release | More rapid | More delayed |
| Cargo | mRNA, miRNA, proteins; may be more diverse than exosomes simply because of maximum size | mRNA, miRNA, proteins |
| Vesicle population | Wider range of sizes | Size limited by multivesicular body |
EXTRACELLULAR VESICLES IN CARDIAC PHYSIOLOGY
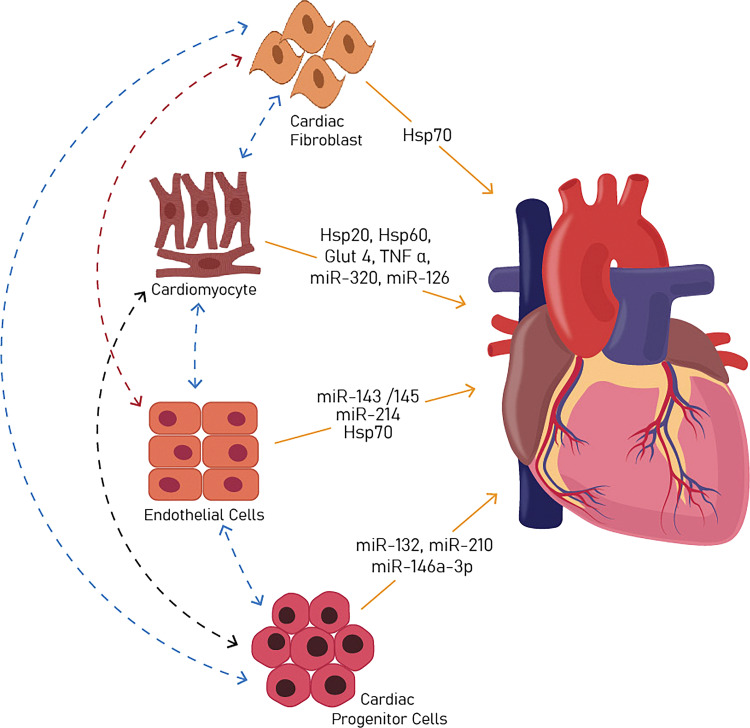
As discussed, EVs mediate both short- and long-range communications between cells. The concentration of EVs in the plasma of a healthy individual has been estimated ∼1010/mL (47) (although estimates vary depending on technique), which is highly suggestive of their physiological importance. The one objective of this review is cardiac cell-derived EVs and their physiological functions, which are introduced and summarized in Fig. 2.
Figure 2.
EV-mediated intercellular communication in the heart. EVs released by various cells in the heart act as messengers by transferring their cargo between cells of the myocardium and help maintain normal physiology. EVs, extracellular vesicles; Hsp, heat shock protein; miR, microRNA.
Cardiomyocyte-Derived EVs
EVs derived from cardiomyocytes have been found to harbor a large variety of biomolecules, their composition being influenced by the origin, microenvironment, and pathophysiologic state of the cell (48). Of note, these EVs are enriched with heat shock proteins (Hsp) like Hsp20, Hsp60, and Hsp70, which play critical roles in cardiomyocyte growth and survival (49). Furthermore, these EVs are known to carry IL-6 and TNF-α, inflammatory factors responsible for cardiac remodeling (50). Other biomolecules known to be found within cardiac cell-derived exosomes include GLUT4, GLUT1, and lactate dehydrogenase, which function in endothelial cells (ECs) for glucose transport and metabolism (51), and miRNAs, namely miR-320 and miR-126 (52). Notably, miR-320 targets genes in endothelial cells and regulates angiogenesis.
Fibroblast-Derived Exosomes
Fibroblasts constitute less than 20% of the noncardiomyocyte cells in murine and human hearts (53) and play a major role in the production of extracellular matrix and the regulation of cardiomyocyte proliferation during development (54). EVs separated from in vitro culture of neonatal cardiac fibroblasts (CF) under normoxic conditions were found to carry proteins associated with the extracellular matrix, cytoskeleton, mitochondrial, and nucleotide-binding (55). Furthermore, EVs from cardiac fibroblasts in neonatal hearts expressed protein markers like vimentin, CD9, CD63, and Hsp70 (56). In vitro studies have found that these EVs entered cardiomyocytes within 5 min and activated mitogen-activated protein kinases (MAPKs), namely extracellular signal-regulated kinase (ERK), p38, c-Jun N-terminal kinase (JNK), and protein kinase B (Akt), which may further trigger the renin-angiotensin system (RAS) pathway in cardiomyocytes, which is pro-hypertrophic. In summary, these studies imply that CF EVs are active paracrine signaling molecules within the myocardium.
Endothelial Cell-Derived EVs
Many studies have shown that surface biomarkers of endothelial cell-derived EVs, like CD144+, CD31+/CD42+, correlated with cardiometabolic risk factors (57). One study by van Balkom et al. found that EV miR-214 from endothelial cells stimulated migration, angiogenesis, and signaling between endothelial cells. Surprisingly, in vitro studies found that EVs derived from miR-214-depleted endothelial cells failed to do so. These EVs may also inhibit senescence by targeting the ataxia telangiectasia gene (58). One study by Hergenreider et al. observed that EV-associated miRNAs, namely miR-143 and miR-145, mediated intercellular communication between endothelial and smooth muscle cells and exerted an atheroprotective effect (59). These miRNAs were also found to regulate Krüppel-like Factor 2 (KLF2), a critical mediator of vasculoprotection. Another important protein associated with cardiac endothelial cell-derived EVs is Hsp70, which was observed to regulate communication between endothelial cells and monocytes (60). In summary, miRNAs and Hsp70 of endothelium-derived EVs are known to act as intercellular communication signals between cardiac endothelial cells, smooth muscle cells, and monocytes, highlighting potential implications between these biomarkers and cardiometabolic risk factors.
Cardiac Progenitor Cell-Derived EVs
Though cardiac progenitor cells (CPCs), Sca1+ cells, comprise only a small percentage of all cardiac cells, they exhibit the ability to differentiate into all major cell types within the myocardium. CPC-derived EVs reportedly contain miRNAs like miR-146a-3p, miR-132, and miR-210, which stimulate angiogenesis (61). In fact, when injected after myocardial infarction (MI), CPC EVs replete with these miRs to improve left ventricular ejection fraction, likely by enhancing angiogenesis (62). Depending on the microenvironment, CPC EVs differentially carry other miRNAs, like miR-17, miR-210, miR-292, and miR-133a (63). Of these, miR-133a has been shown experimentally to improve cardiac function and delay fibrosis in MI models (64). Furthermore, CPC EV-associated miR-21 exhibited an anti-apoptotic effect by targeting programmed cell death protein 4 (PDCD4) (65). Gene ontology term enrichment analysis of EVs released by cardiomyocytes under ischemic conditions has revealed miRNA targets associated with heart development, although whether these EVs contribute significantly to the development of the heart tube remains unknown (66). Nonetheless, the relevance of cardiac EVs as therapeutics for congenital heart disease and abnormalities of heart development is increasingly recognized, as discussed below.
EXTRACELLULAR VESICLES IN CARDIAC PATHOLOGY
As mentioned, EVs derived from various cardiac cell types have been implicated in physiological functions like angiogenesis, cellular proliferation, and cellular maturation. Moreover, the composition of the EVs is greatly influenced by the pathophysiological state of the cells, as probably most frequently reported for miRNAs. In total, these data strongly suggest that alterations in EV cargo contribute to the development of various cardiovascular pathologies and are summarized in Table 2.
Table 2.
Involvement of EV cargo in the development of cardiovascular pathology
| Cardiovascular Anomaly | EV Cargo | Detrimental Effects | References |
|---|---|---|---|
| Congenital cardiac defects | Differentially expressed miR-133, miR-30, miR-99, miR-23 (maternal blood exosomes) | Affect fetal cardiac development | (67) |
| Adults with congenital cardiac defects | Increased endothelial microparticles | Activate inflammatory pathways | (68) |
| Hypertensive heart disease | Upregulated miR-21* (cardiac fibroblast exosomes) Differentially expressed miR-486, miR-122-5p (plasma exosomes) | Induce cardiac hypertrophy | (69, 70) |
| Diabetic cardiomyopathy | Differentially expressed miR-126, miR-26, miR-320, miR-29b, miR-455 (circulating exosomes) | Adversely affect endothelial function and angiogenesis Regulate the remodeling enzymes like MMP9 |
(52, 71, 72) |
| Myocardial infarction | Differentially expressed miR-30a (hypoxic cardiomyocyte exosomes), miR-208a (cardiomyocyte exosomes), miR-133a (serum exosomes) | Development of myocardial fibrosis, affect autophagy | (73–75) |
EV, extracellular vesicle.
Congenital Cardiac Defects
The incidence of congenital heart defects varies from 4 in 1,000 to 50 in 1,000 live births, with increased frequency in maternal pregestational diabetes mellitus. Studies have found that high glucose and cellular stress alter both the concentration and cargo of EVs (76). EVs may also move from maternal tissues directly to the fetus and vice versa, promoting alterations to intercellular communication (77). One study by Shi et al. investigated the EV content of diabetic pregnant mice and found significant differences in miRNA abundance compared with control mice. EV miRNAs like miR-133, miR-30, miR-99, and miR-23 were differentially abundant, and these miRNAs are, unsurprisingly, implicated in cardiac development. When EVs from diabetic mice were injected into control pregnant mice, the embryos exhibited cardiac defects, indicative of the role of these maternal EV miRNAs (or perhaps another EV component) in cardiac development and development of congenital heart disease (67). In addition, numbers of large, flow cytometry-detectable EVs termed “endothelial microparticles” (EMPs) were elevated in adults with congenital heart defects, like atrial and ventricular septal defects. These EMPs reportedly disrupted the endothelial nitric oxide synthase (eNOS) pathway and activated inflammatory pathways, leading to endothelial dysfunction, in a p38 MAPK-dependent manner. These data suggest that inhibiting p38 MAPK by depleting or blocking EMPs has potential as a therapeutic strategy for controlling inflammation in congenital heart disease (68).
Hypertensive Heart Disease and Cardiac Hypertrophy
Uncontrolled hypertension can result in cardiac remodeling, leading to left ventricular hypertrophy, a major contributor to the development of heart failure. Cardiac fibroblasts play a critical key role in cardiac fibrosis by secreting extracellular matrix proteins and proinflammatory cytokines (78). Recent studies have shown that miRNA-containing EVs released by fibroblasts during acute myocardial infarction induce hypertrophic gene expression in cardiomyocytes in vitro (69). In vivo studies have found that miR-21* was upregulated in the pericardial fluid of mice with cardiac hypertrophy. This miRNA was also found in EVs released by cardiac fibroblasts in hypertrophied hearts, which are subsequently shuttled to cardiomyocytes. miR-21* targets signaling pathways involved in hypertrophy in cardiomyocytes, namely Sorbin and SH3 domain containing 2 (SORBS2) and PDZ and LIM domain 5 (PDLIM5). Treatment with miR-21* antagonists protected the murine heart from ANG II-induced cardiac hypertrophy, highlighting its potential use as a therapeutic target in heart failure. Compared with EVs from normal rats, EVs of hypertensive rats included differentially expressed miRNAs that were involved in hypertension-specific signaling pathways (70). One study found that cardiac hypertrophy stimulated the release of EVs from cardiac fibroblasts, leading to pathologic effects. CF-derived EVs upregulated RAS in cardiomyocytes via activation of MAPKs and Akts. Inhibition of EV release from CFs, in contrast, attenuated ANG II-induced cardiac hypertrophy and reduced cardiac fibrosis in vivo (56).
Diabetic Cardiomyopathy
Dysregulation of the endothelial function in hyperglycemia is the primary cause of cardiovascular diseases in diabetes. The involvement of EVs in mediating diabetic cardiomyopathy has been reviewed elsewhere, in detail (79). Here, we provide a brief overview of miRNAs implicated in diabetic cardiomyopathy. In diabetes, large circulating EVs (pelleted at relatively low centrifugation speeds) were found to express low levels of miR-126 and miR-26, which are pro-angiogenic (72). In addition, cardiomyocyte-derived EVs have been shown to accumulate in endothelial cells and regulate their function in vitro (80). EVs from diabetic rats adversely affected the migration and proliferation of ECs, whereas those from control rats promoted their function (52). Furthermore, cardiomyocyte-derived EVs from diabetic patients contained higher levels of miR-320, which suppresses Hsp20 in endothelial cells, exerting an anti-angiogenic function, but lower levels of miR-126. EVs from diabetic heart tissue were found to be enriched with miR-29b, miR-133-5p, miR-455, and miR-466 and to regulate the expression of extracellular matrix remodeling enzymes, like matrix metalloproteinase 9 (MMP9) (71). These studies suggest that the pathologic role of EVs involves transfer of miRNAs that target pathways contributing to the development of diabetic cardiomyopathy.
Myocardial Infarction
EVs released from hypoxic cardiomyocytes contain miR-30a, which regulates autophagy by altering the expression of Beclin-1 and autophagy-related 12 (ATG12) (75). miR-208a contained in cardiomyocyte EVs is transferred to cardiac fibroblasts and may contribute to the development of myocardial fibrosis (74). miR-133a, which was elevated in the infarcted and peri-infarcted myocardium of patients with acute coronary syndrome (73), was also found in EVs released by stimulated H9c2 cardiomyocytes. Consequently, a few proteins identified in plasma EVs have the potential to be used as biomarkers and therapeutic agents for cardiovascular diseases (81).
Role of Circulating EVs in Cardiovascular Pathophysiology
EVs of varying sizes and cellular origin are released during normal cellular physiology as well as during cellular stress and, importantly, in disease. At least in healthy individuals, the observed EVs are mostly released from immune cells, platelets, and erythrocytes (82). Although cell-specific origin of EVs can be challenging to identify in an in vivo system, ongoing development of tools that identify the cell of origin of EVs may permit even more powerful biomarker tools in the near future. At the present time, total circulating EVs may offer a relatively noninvasive means to assess cardiovascular disease states (83). The role of circulating EVs in specific cardiovascular pathologies, namely ischemic cardiomyopathy, valvular heart disease, and ischemia-reperfusion injury, will be discussed here. Plasma EVs have a role as biomarkers and as therapeutic targets for inhibition, particularly in renal dysfunction post-cardiac surgery, whereas EV miRNAs may be therapeutic targets for upregulation, particularly in ischemia-reperfusion injury.
A differential proteomic analysis performed on plasma EVs isolated from ST-elevation myocardial infarction (STEMI) patients identified upregulation of α2-macroglobulin isoforms, fibrinogen, and viperin, among other proteins involved in inflammatory responses (84). This protein network constitutes a promising source of biomarkers, highlighting the importance of EVs in assessing myocardial injury beyond serum troponins. Circulating EVs from patients with valvular heart disease were found to contain inflammatory components, which are known to impair endothelial function (85). The potential role of EVs extends beyond biomarkers in ischemic cardiomyopathy and valvular heart disease into the realm of therapeutics. For example, the protective role of dexmedetomidine against renal dysfunction in cardiac surgery was attributed to the inhibition of circulating EV-induced neutrophil chemotaxis (86).
Conversely, miRNAs in circulating EVs have been shown to exert cardioprotection. For instance, miR-342-5p protected the myocardium of long-term exercised rats against myocardial ischemia/reperfusion injury by inhibiting apoptotic signaling and activating survival signaling (87). Supporting this observation, others have reported intramyocardial injection of circulating EVs from exercised mice to offer protection against acute ischemia/reperfusion (88). These studies attribute the protective effect of exercise on cardiometabolic health, at least in part, to circulating EVs. However, validation of these findings in larger cohorts is needed to further explore the potential clinical applications of circulating EVs in cardiovascular disease.
EFFECT OF EV THERAPY ON CARDIOVASCULAR PHYSIOLOGY
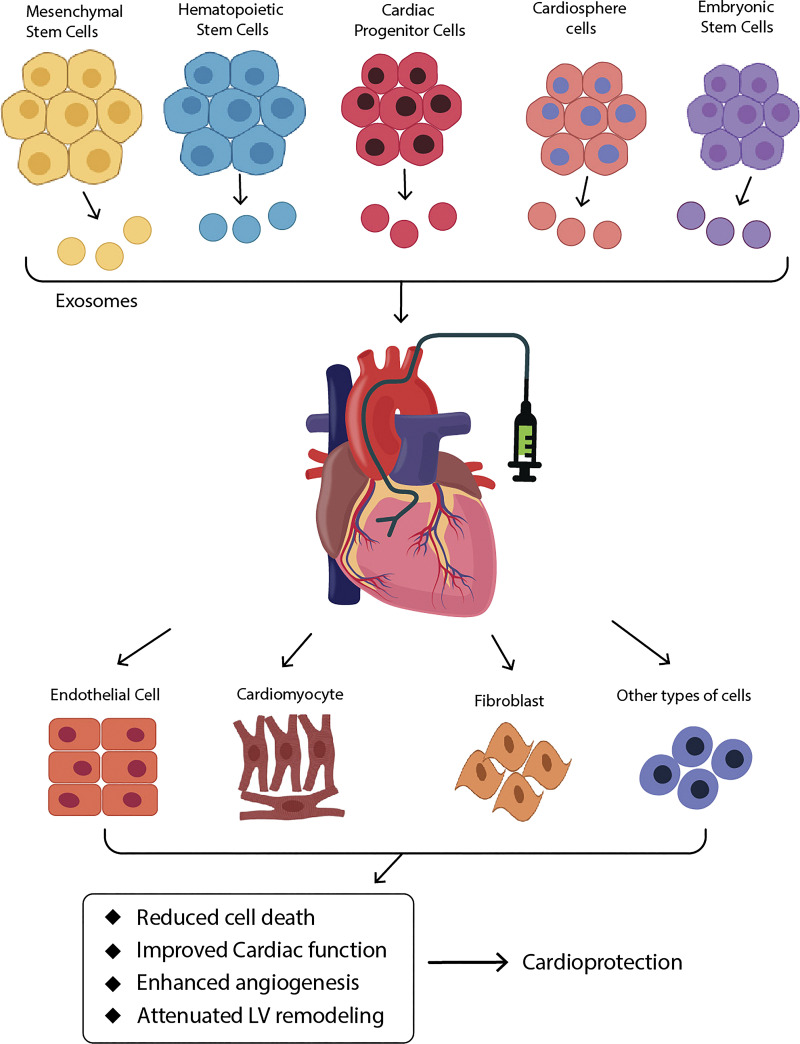
Studies have established that EVs derived from different types of stem cells provide cardiac protection by decreasing cardiomyocyte apoptosis, enhancing angiogenesis, limiting inflammation, and reducing fibrosis (Fig. 3). The functional components in stem cell-derived EVs are summarized in Table 3 and are discussed in detail below.
Figure 3.
Cardiac protection by stem cell-derived EVs. EVs derived from mesenchymal stem cells, cardiac progenitor cells, cardiosphere cells, hematopoietic stem cells, and embryonic stem cells exert cardioprotective effects. EVs, when injected into pathologic heart, interact with cardiac cells and help restore cardiac structure and function. EVs, extracellular vesicles.
Table 3.
Stem cell-derived extracellular vesicles in cardiac protection
| Source of Nanovesicles | Model | Protective Molecule in EVs | Mechanisms | References |
|---|---|---|---|---|
| Mesenchymal stem cells | Mice | Enriched with miR-22, miR-19a, miR-221 | Reduce fibrosis, decrease apoptosis Cardioprotective effects Preserve ejection fraction |
(89–91) |
| Cardiac progenitor cells | In vitro (HL1, H9c2 cell line) In vivo mice |
Enriched with miR-210, miR-132 | Prevent apoptosis in cardiomyocyte cell lines Reduce scar size and improve cardiac function |
(61, 62, 65) |
| Cardiosphere cells | In vivo mice, porcine | Enriched with miR-146a, ncRNA like Y-RNA | Reduce fibrosis, improve cardiac function Decrease scar size Reduce inflammation and hypertrophy |
(92–94) |
| Hematopoietic stem cells | In vitro In vivo mice |
Enriched with miR-126, miR-130a Sonic hedgehog protein |
Induce angiogenesis Increase capillary density, preserve ejection fraction |
(95, 96) |
| Embryonic stem cells | In vivo mice | Enriched in miR-294 | Promote cardiomyocyte survival Decrease oxidative stress, reverse cardiac remodeling |
(97–100) |
EV, extracellular vesicle.
Mesenchymal Stem Cell-Derived EVs
Mesenchymal stem cells (MSCs) are multipotent stem cells that are found in almost every tissue and have the ability to differentiate into various cell types. However, recent data show that the protective effects of MSCs can be attributed at least in part to paracrine factors released by stem cells, including EVs and their cargo. Therapeutic benefits have indeed been confirmed in animal models of MI (101). Bone marrow-derived MSC-EVs enriched with miR-22 were found to reduce fibrosis and decrease apoptosis in mouse models of MI (89). miR-19a and miR-221 from GATA-4 overexpressing MSCs were also found to have cardioprotective effects (91). Reperfusion therapy can lead to cardiomyocyte cell death and functional deterioration. However, intravenous injection of conditioned medium from MSCs reduced infarct size, preserved cardiac function, and reduced oxidative stress after reperfusion (102). Injection of MSCs (intravenously) along with MSC-derived EVs (intramyocardially) into a rat model of acute MI preserved ejection fraction (90), possibly by improving the cardiac microenvironment. It is important to note, however, that in some studies the relative contributions of EVs to effects are difficult to determine.
Cardiac Progenitor Cell-Derived EVs
Adults have a limited population of stem and progenitor cells, which are known to exert cardiac protection via both regeneration and paracrine mechanisms. CPCs in the murine and human heart express markers including c-kit, Sca-1, Islet-1, and platelet-derived growth factor receptor-alpha (PDGFRα+) (103). A study by Sharma et al. (104) on adult and neonatal c-kit+ CPCs found that the conditioned medium from neonatal CPCs was more effective in recovering cardiac function, stimulating neovascularization, and promoting cardiac remodeling when compared with adult CPCs. In addition, CPC-derived EVs exhibit anti-apoptotic, pro-angiogenic, and antifibrotic properties. In vitro studies using CPC-derived EVs prevented stress-induced apoptosis in HL1 cardiomyocytes; these EVs were found to be enriched in miR-210 and miR-132 (62). Another study found that CPC-derived EVs inhibited oxidative stress-related apoptosis in H9c2 cells by restoring miR-21/PDCD4 pathway (65). These miRNAs, miR-210 and miR-132, reportedly play an important role in improving angiogenesis and cardiac function in MI models (105, 106). Intramyocardial injection of CPC EVs in rat models of acute MI reduced cardiomyocyte apoptosis, reduced scar size, and improved vascular density. The functional benefits of CPC EVs included improved left ventricular ejection fraction (LVEF), reduced LV diameter, and enhanced systolic LV wall thickness (61).
Cardiosphere-Derived EVs
Cardiosphere-derived cells (CDCs) are of intrinsic cardiac origin. CDCs comprise a heterogeneous population of cells and express mesenchymal stem cell markers CD90 and CD105, stem cell marker c-kit, and pluripotency markers Sox2 and Oct4 (107). The underlying benefit of CDC therapy is thought to be due in part to the transfer of EVs and their payload to target cells. Studies using CDCs in animal models for acute MI, nonischemic cardiomyopathy, and Duchenne muscular dystrophy have all demonstrated improvement in their respective conditions, which has been attributed to the specific cargo of EVs. One study by Marban et al. established that CDC EVs induce angiogenesis, reduce fibrosis, and improve cardiac function (94, 108). The therapeutic benefit of allogeneic CDCs in patients, however, is currently unclear. Although the CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction (CADUCEUS) trial has proved the cardioprotective effect of CDC, the ALLSTAR trial showed that despite the safety of the therapeutic, allogeneic CDCs in patients with post-MI LV dysfunction did not reduce scar size (109). Other studies by Ibrahim et al. observed that the beneficial effects were specifically due to EVs released by CDCs, perhaps due to the presence of miR-146a. Furthermore, global cardiac function, including improved ejection fraction, decreased scar mass, decreased chamber dilation, and reduced myocyte hypertrophy, improved in animals injected with CDC EVs (93). A study by Gallet et al. investigated the beneficial effects of intramyocardial injection of CDC EVs into porcine models of MI. Following treatment, the animals exhibited reduced infarct size, preserved ejection fraction, increased capillary density, reduced collagen fibrosis, and reduced cardiomyocyte hypertrophy (110). Beyond miRNAs, other noncoding RNAs (ncRNAs) may also have effects. For example, Y-RNAs are abundant in CDC EVs; EV-YF1, an YRNA fragment, alters the phenotype of macrophages to induce cardioprotection through enhanced release of IL-10. Furthermore, EV-YF1 provides protection against myocardial hypertrophy and inflammation (92).
Hematopoietic Stem Cell-Derived EVs
Hematopoietic stem cells (HSCs) have the ability to self-renew and differentiate into all lineages of blood cells. Because of poor reported survival of transplanted HSCs, their cardioprotection has been called into question. Nevertheless, HSCs have been shown to be cardioprotective in both human and animal models (111, 112), likely due to paracrine mechanisms. EVs from CD34+ cells induced angiogenesis in endothelial cells in vitro (96); these vesicles were found to be enriched with miR-126 and miR-130a. In addition, sonic hedgehog (Shh)-modified CD34+ cells were found to elicit cardioprotection following intramyocardial injection (95). In this study, Shh protein was stored and transferred to other cell types via EVs, which increased capillary density, reduced infarct size, and preserved cardiac function.
Embryonic Stem Cell-Derived EVs
Embryonic stem cells (ESCs) are undifferentiated cells derived from the inner cell mass. Although ESCs have the highest regenerative potential, their use remains controversial due to both ethical concerns and high risk of tumor formation. ESCs exert their beneficial effects primarily by engraftment and differentiation into specific cell types. ESCs also release EVs; for example, EVs that are enriched with miR-294, which has been shown to promote cardiomyocyte survival and reduce fibrosis following intramyocardial delivery in murine MI models (99). In addition, ESC-derived EVs exhibited cardioprotective effects by promoting cardiomyocyte proliferation and neovascularization and exerting anti-apoptotic and antifibrotic effects. Other studies have shown that conditioned medium collected from human ESC-derived MSCs and cardiovascular progenitors exert cardioprotective effects (98, 100). Yet, another study found that ESC-derived MSC EVs decreased oxidative stress, decreased scar burden, and halted adverse remodeling in a murine model of MI/reperfusion injury (97). In summary, both ESCs and ESC-derived cells exhibit analogous cardioprotection, primarily through paracrine mechanisms that include EVs.
HARNESSING THE THERAPEUTIC POTENTIAL OF EVs: BENEFITS AND BARRIERS
EVs carry and deliver a multitude of molecules, and, in specific contexts, they are able to mediate crosstalk in a multifunctional and multidirectional manner. EVs can target local cells via paracrine mechanisms or more distal cells via the circulation. EVs can affect target cells by interacting with surface molecules and by delivering cargo into the recipient cell. Cells respond via stimulated or inhibited pathways (surface interactions) or as a result of the interactions of delivered molecules like proteins or miRNAs with counterparts in the recipient cell. As discussed above, EV cargo is a potential mediator of cell survival and homeostasis and shows promise as a cardiovascular biomarker (Table 4). Furthermore, mechanisms for targeting of dysfunctional EVs are being developed for the treatment of cardiovascular disease (113). Essential to the development of improved therapeutic strategies is a mechanistic understanding of EV-mediated cell-cell communications.
Table 4.
EV miRNAs and proteins as biomarkers for cardiovascular diseases
| Biomarker (miRNAs/Proteins) | EV Source | Cardiovascular Pathology | Reference |
|---|---|---|---|
| miR-1, miR-133a | Serum of humans | Myocardial damage, acute coronary syndrome | (100) |
| miR-208a | Plasma of humans | Coronary artery occlusion | (29) |
| miR-208b, miR-499-5p | Plasma of humans | Increased risk for heart failure | (63) |
| miR-192, has-miR-194, miR-34a | Serum of humans | Heart failure following acute myocardial infarction | (34) |
| Apolipoprotein D, C-III; complement C1q, C5; platelet basic protein | Plasma of humans | Indicator of thrombosis, vascular inflammation, myocardial injury | (81) |
EV, extracellular vesicle.
In addition to circulating throughout the body, EVs are thought to be able to cross barriers such as the blood-brain barrier, highlighting their potential for carrying therapeutic entities. EVs generated from different cardiac cells may have either beneficial or detrimental effects on target cells, depending in part on the status of the parent cell. Therefore, it is imperative to identify and develop strategies to either inhibit harmful mediators or enhance beneficial ones. In the context of cardiovascular disease, harnessing the therapeutic potential of EVs is particularly important for cardioprotection. Several molecules, including GW4869 and amiloride, have previously been identified as inhibitors of the biogenesis and/or formation of endosomal origin EVs (114, 115), although next-generation inhibitors may be needed to enhance efficiency and limit off-target effects (116). An alternative approach to enhance efficacy involves loading EVs with non-native cargo postproduction by passive or active cargo encapsulation methods (117, 118). Artificial EVs (EV mimetics) can also be engineered to correct molecular defects. While promising, more work is required to develop cost-effective and scalable protocols for EV separation, production, and quality control before implementation. Furthermore, a more detailed understanding of EV kinetics after various administration pathways is required to maximize the benefits of EVs engineered for cardiovascular therapies.
CONCLUSIONS
EVs, including exosomes and ectosomes, are not only important in normal physiology but also implicated as mediators of many pathophysiological conditions. Recently, EVs have been shown to be attractive candidates for novel biomarkers, although the small cohorts of patients studied necessitate validation in multicenter clinical cohorts (26). EVs have been identified as promising agents for the treatment of a variety of cardiovascular pathologies. Intricate mechanisms for EV-mediated cellular crosstalk in the cardiovascular system have been of significant interest, and mechanisms are beginning to be unraveled.
Challenges to the successful implementation of EV therapeutics can be summarized as challenges to administering the “right EVs” to the “right place” in the “right dose.” Different modes of EV production and separation may affect EV contents, and thus the “rightness” of the administered EVs (119), in addition to influencing cost. Simple, cost-effective technologies to produce, separate, and characterize consistent batches of EVs will be key to advancing the field, as indeed recommended by the standardization efforts of the International Society for Extracellular Vesicles (120). Once EVs are prepared and delivered, the pharmacokinetics in vivo remain incompletely described, which impedes therapeutic development as well. Fine-tuning of retention by desired cells, and avoidance of accumulation in undesired sites, will be needed to maximize bioavailability and site specificity, thereby enhancing benefit. Finally, dosing and toxicity studies are required to assess the safety, including possible immunogenicity, of higher doses of EVs. To our knowledge, few such studies exist. There are thus multiple challenges for the research community to address in coming years.
Despite these challenges, the remarkable characteristics of EVs to influence target cell function have encouraged scientists to engineer or genetically modify vesicles to harness their potential. Improved understanding of the mechanisms of EV uptake, circulation, and systemic clearance will maximize the use of EVs as novel clinical therapies. To further advance clinical translation, several questions regarding EV biology, and mechanisms associated with the origin and function of EVs need to be addressed. In particular,
-
1.
The specific origin of circulating EVs in vivo is largely unknown, and this continues to be a major limitation in our understanding.
-
2.
EV-mediated regulation of cardiomyocytes and noncardiomyocytes within the healthy heart remains elusive.
-
3.
A detailed, mechanistic understanding of multifactorial EV function is needed to develop new and personalized EV-based therapies for CVD.
Nonetheless, EVs are a highly flexible platform, allowing for the engineering of efficient, targeted, and nonimmunogenic delivery systems. As a cell-derived but cell-free releasate, EVs and their associated molecules have proven to be efficient in promoting cardiac repair and continue to hold promise as a safe and effective therapy for cardiovascular patients.
GRANTS
K. W. Witwer is supported in part by UG3CA241694 (NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and V.P.J. drafted manuscript; S.S., V.P.J., K.W.W., and S.K. edited and revised manuscript; S.S., V.P.J., K.W.W., and S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Fuad Zain and BioRender software for assistance in preparing the figures.
REFERENCES
- 1.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R , et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7: 1535750, 2018. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 13: 269–288, 1967. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson HC. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol 35: 81–101, 1967. doi: 10.1083/jcb.35.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res 20: 33–50, 1967. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- 5.Snell WJ. Mating in Chlamydomonas: a system for the study of specific cell adhesion. I. Ultrastructural and electrophoretic analyses of flagellar surface components involved in adhesion. J Cell Biol 68: 48–69, 1976. doi: 10.1083/jcb.68.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunez EA, Wallis J, Gershon MD. Secretory processes in follicular cells of the bat thyroid. III. The occurrence of extracellular vesicles and colloid droplets during arousal from hibernation. Am J Anat 141: 179–201, 1974. doi: 10.1002/aja.1001410203. [DOI] [PubMed] [Google Scholar]
- 7.Feller WF, Chopra HC. A small virus-like particle observed in human breast cancer by means of electron microscopy. J Natl Cancer Inst 40: 1359–1373, 1968. [PubMed] [Google Scholar]
- 8.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97: 329–339, 1983. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 35: 256–263, 1984. [PubMed] [Google Scholar]
- 10.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262: 9412–9420, 1987. [PubMed] [Google Scholar]
- 11.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33: 967–978, 1983. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 12.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101: 942–948, 1985. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trams EG, Lauter CJ, Salem N, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645: 63–70, 1981. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 14.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, Greer D, Pimentel J, Kolankiewicz LM, Puente N, Faradyan S, Ferland P, Bearer EL, Passero MA, Adedi M, Colvin GA, Quesenberry PJ. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells 25: 2245–2256, 2007. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20: 847–856, 2006. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 16.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 17.Lässer C, Jang SC, Lötvall J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol Aspects Med 60: 1–14, 2018. doi: 10.1016/j.mam.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci 373: 20160479, 2018. doi: 10.1098/rstb.2016.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yáñez-Mó M, Siljander PR-M, Andreu Z, Zavec AB, Borràs FE, Buzas EI, , et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066, 2015. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 8: 307, 2019. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton A, Buschmann D, Byrd JB, Carter DRF, Cheng L, Compton C, , et al. Summary of the ISEV workshop on extracellular vesicles as disease biomarkers, held in Birmingham, UK, during December 2017. J Extracell Vesicles 7: 1473707, 2018. doi: 10.1080/20013078.2018.1473707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2: 20360, 2013. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, Odenthal M, Fries JWU. Exosomes as miRNA carriers: formation-function-future. Int J Mol Sci 17: 2028, 2016. doi: 10.3390/ijms17122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zebrowska A, Skowronek A, Wojakowska A, Widlak P, Pietrowska M. Metabolome of exosomes: focus on vesicles released by cancer cells and present in human body fluids. Int J Mol Sci 20: 3461, 2019. doi: 10.3390/ijms20143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release Off J Control Release Soc 219: 278–294, 2015. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Neupane YR, Pavon MV, Zharkova O, Pastorin G, Wang J-W. Extracellular vesicles in cardiovascular diseases: alternative biomarker sources, therapeutic agents, and drug delivery carriers. Int J Mol Sci 20: 3272, 2019. doi: 10.3390/ijms20133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picca A, Guerra F, Calvani R, Bucci C, Lo Monaco MR, Bentivoglio AR, Coelho-Júnior HJ, Landi F, Bernabei R, Marzetti E. Mitochondrial dysfunction and aging: insights from the analysis of extracellular vesicles. Int J Mol Sci 20: 805, 2019. doi: 10.3390/ijms20040805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins PD. Extracellular vesicles and aging. Stem Cell Investig 4: 98, 2017. doi: 10.21037/sci.2017.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 8: 1648167, 2019. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocucci E, Cocucci E, Meldolesi J. Ectosomes. Curr Biol 21: R940–R941, 2011. [Erratum in Curr Biol 22: 1359, 2012]. doi: 10.1016/j.cub.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 31.van der Pol E, Coumans F. A W, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, Sturk A, van Leeuwen TG, Nieuwland R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost 12: 1182–1192, 2014. doi: 10.1111/jth.12602. [DOI] [PubMed] [Google Scholar]
- 32.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36: 301–312, 2016. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30: 255–289, 2014. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 34.Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, Hall DH, Driscoll M. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542: 367–371, 2017. doi: 10.1038/nature21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartelt A, Weber C. Mitochondrial ejection for cardiac protection: the macrophage connection. Cell Metab 32: 512–513, 2020. doi: 10.1016/j.cmet.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Nicolás-Ávila JA, Lechuga-Vieco AV, Esteban-Martínez L, Sánchez-Díaz M, Díaz-García E, Santiago DJ, , et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183: 94–109, 2020. doi: 10.1016/j.cell.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 37.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DWH, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6: 8472, 2015. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meehan B, Rak J, Di Vizio D. Oncosomes - large and small: what are they, where they came from? J Extracell Vesicles 5: 33109, 2016. doi: 10.3402/jev.v5.33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathieu M, Névo N, Jouve M, Valenzuela JI, Maurin M, Verweij F, Palmulli R, Lankar D, Dingli F, Loew D, Rubinstein E, Boncompain G, Perez F, Théry C. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking and synchronized extracellular vesicle release of CD9 and CD63 (Preprint). bioRxiv :323766, 2020. doi: 10.1101/2020.10.27.323766. [DOI]
- 40.Russell AE, Sneider A, Witwer KW, Bergese P, Bhattacharyya SN, Cocks A, , et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J Extracell Vesicles 8: 1684862, 2019. doi: 10.1080/20013078.2019.1684862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, , et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20: 332–343, 2018. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25: 364–372, 2015.doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75: 193–208, 2018. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Llorente A, Skotland T, Sylvänne T, Kauhanen D, Róg T, Orłowski A, Vattulainen I, Ekroos K, Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 1831: 1302–1309, 2013. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Morelli AE, Larregina AT, Shufesky WJ, Sullivan MLG, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104: 3257–3266, 2004. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 46.Laulagnier K, Grand D, Dujardin A, Hamdi S, Vincent-Schneider H, Lankar D, Salles J-P, Bonnerot C, Perret B, Record M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett 572: 11–14, 2004. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 47.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P, Carr B, Redman CWG, Harris AL, Dobson PJ, Harrison P, Sargent IL. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7: 780–788, 2011. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H, Wang Z. Cardiomyocyte-derived exosomes: biological functions and potential therapeutic implications. Front Physiol 10: 1049, 2019. doi: 10.3389/fphys.2019.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292: H3052–H3056, 2007. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 50.Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, Liao Y. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1α, presented by exosomes. J Mol Cell Cardiol 53: 848–857, 2012. doi: 10.1016/j.yjmcc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res 109: 397–408, 2016. doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan G-C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 74: 139–150, 2014. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res 118: 400–409, 2016. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong T-T, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev Cell 16: 233–244, 2009. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cosme J, Guo H, Hadipour-Lakmehsari S, Emili A, Gramolini AO. Hypoxia-induced changes in the fibroblast secretome, exosome, and whole-cell proteome using cultured, cardiac-derived cells isolated from neonatal mice. J Proteome Res 16: 2836–2847, 2017. doi: 10.1021/acs.jproteome.7b00144. [DOI] [PubMed] [Google Scholar]
- 56.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 89: 268–279, 2015. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berezin AE. Impaired phenotype of circulating endothelial-derived microparticles: novel marker of cardiovascular risk. J Cardiol Ther 2: 365–370–370, 2015. http://www.ghrnet.org/index.php/jct/article/view/1316/1454. [Google Scholar]
- 58.van Balkom BWM, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MAJ, Pegtel DM, Stoorvogel W, Würdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 121: 3997–4006, 2013. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 59.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14: 249–256, 2012. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 60.Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L, Wang L, Wang Y, Wang X, Qian L-J. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun 387: 229–233, 2009. doi: 10.1016/j.bbrc.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 61.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 103: 530–541, 2014. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 62.Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V, Bolis S, Altomare C, Matteucci M, Di Silvestre D, Brambilla F, Fertig TE, Torre T, Demertzis S, Mauri P, Moccetti T, Vassalli G. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc Res 114: 992–1005, 2018. doi: 10.1093/cvr/cvy055. [DOI] [PubMed] [Google Scholar]
- 63.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 116: 255–263, 2015. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izarra A, Moscoso I, Levent E, Cañón S, Cerrada I, Díez-Juan A, Blanca V, Núñez-Gil I-J, Valiente I, Ruíz-Sauri A, Sepúlveda P, Tiburcy M, Zimmermann W-H, Bernad A. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Reports 3: 1029–1042, 2014. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis 7: e2277, 2016. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, Batista-Almeida D, Pereira R, Coelho-Santos V, Silva AP, Fernandes R, Zuzarte M, Enguita FJ, Costa MC, Pinto-do-Ó P, Pinto MT, Gouveia P, Ferreira L, Mason JC, Pereira P, Kwak BR, Nascimento DS, Girão H. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res 113: 1338–1350, 2017. doi: 10.1093/cvr/cvx118. [DOI] [PubMed] [Google Scholar]
- 67.Shi R, Zhao L, Cai W, Wei M, Zhou X, Yang G, Yuan L. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem Biophys Res Commun 483: 602–608, 2017. doi: 10.1016/j.bbrc.2016.12.097. [DOI] [PubMed] [Google Scholar]
- 68.Lin Z-B, Ci H-B, Li Y, Cheng T-P, Liu D-H, Wang Y-S, Xu J, Yuan H-X, Li H-M, Chen J, Zhou L, Wang Z-P, Zhang X, Ou Z-J, Ou J-S. Endothelial microparticles are increased in congenital heart diseases and contribute to endothelial dysfunction. J Transl Med 15: 4, 2017. doi: 10.1186/s12967-016-1087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X, Yuan W, Yang L, Li J, Cai J. miRNA Profiling of exosomes from spontaneous hypertensive rats using next-generation sequencing. J Cardiovasc Transl Res 12: 75–83, 2019. doi: 10.1007/s12265-017-9784-7. [DOI] [PubMed] [Google Scholar]
- 71.Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med 19: 2153–2161, 2015. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107: 810–817, 2010. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 73.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 4: 446–454, 2011. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Yu X, Xue F, Li Y, Liu W, Zhang S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am J Transl Res 10: 4350–4366, 2018. [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Y, Li Y, Chen X, Cheng X, Liao Y, Yu X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J Mol Med (Berl) 94: 711–724, 2016. doi: 10.1007/s00109-016-1387-2. [DOI] [PubMed] [Google Scholar]
- 76.Müller G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab Syndr Obes Targets Obes 5: 247–282, 2012. doi: 10.2147/DMSO.S32923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Record M. Intercellular communication by exosomes in placenta: a possible role in cell fusion? Placenta 35: 297–302, 2014. doi: 10.1016/j.placenta.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 78.Oka T, Komuro I. Molecular mechanisms underlying the transition of cardiac hypertrophy to heart failure. Circ J Off J 72 Suppl A: A13–A16, 2008. doi: 10.1253/circj.cj-08-0481. [DOI] [PubMed] [Google Scholar]
- 79.Salem ESB, Fan G-C. Pathological effects of exosomes in mediating diabetic cardiomyopathy. Adv Exp Med Biol 998: 113–138, 2017. doi: 10.1007/978-981-10-4397-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 24: 199–206, 2015. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 81.Cheow ESH, Cheng WC, Lee CN, D de K, Sorokin V, Sze SK. Plasma-derived extracellular vesicles contain predictive biomarkers and potential therapeutic targets for myocardial ischemic (MI) injury. Mol Cell Proteomics 15: 2628–2640, 2016. doi: 10.1074/mcp.M115.055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sluijter JPG, Davidson SM, Boulanger CM, Buzás EI, Kleijn D, Victor DP, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Laake V, Wilhelmina L, Ytrehus K, Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the working group on cellular biology of the heart of the European Society of Cardiology. Cardiovasc Res 114: 19–34, 2018. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jansen F, Nickenig G, Werner N. Extracellular vesicles in cardiovascular disease. Circ Res 120: 1649–1657, 2017. doi: 10.1161/CIRCRESAHA.117.310752. [DOI] [PubMed] [Google Scholar]
- 84.Vélez P, Parguiña AF, Ocaranza-Sánchez R, Grigorian-Shamagian L, Rosa I, Alonso-Orgaz S, de la Cuesta F, Guitián E, Moreu J, Barderas MG, González-Juanatey JR, García AI. Identification of a circulating microvesicle protein network involved in ST-elevation myocardial infarction. Thromb Haemost 112: 716–726, 2014. doi: 10.1160/TH14-04-0337. [DOI] [PubMed] [Google Scholar]
- 85.Jian Y-P, Yuan H-X, Hu K-H, Chen C, Li Y-Q, Li Y, Yang T-X, Ou Z-J, Ou J-S. Protein compositions changes of circulating microparticles in patients with valvular heart disease subjected to cardiac surgery contribute to systemic inflammatory response and disorder of coagulation. Shock 52: 487–496, 2019. doi: 10.1097/SHK.0000000000001309. [DOI] [PubMed] [Google Scholar]
- 86.Yuan H-X, Chen C-Y, Li Y-Q, Ning D-S, Li Y, Chen Y-T, Li S-X, Fu M-X, Li X-D, Ma J, Jian Y-P, Liu D-H, Mo Z-W, Peng Y-M, Xu K-Q, Ou Z-J, Ou J-S. Circulating extracellular vesicles from patients with valvular heart disease induce neutrophil chemotaxis via FOXO3a and the inhibiting role of dexmedetomidine. Am J Physiol Endocrinol Metab 319: E217–E231, 2020. doi: 10.1152/ajpendo.00062.2020. [DOI] [PubMed] [Google Scholar]
- 87.Hou Z, Qin X, Hu Y, Zhang X, Li G, Wu J, Li J, Sha J, Chen J, Xia J, Wang L, Gao F. Longterm exercise-derived exosomal miR-342-5p. Circ Res 124: 1386–1400, 2019. doi: 10.1161/CIRCRESAHA.118.314635. [DOI] [PubMed] [Google Scholar]
- 88.Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, Tigges J, Toxavidis V, Ghiran I, Shah R, Li Y, Zhang Y, Das S, Xiao J. Exercise-induced circulating extracellular vesicles protect against cardiac ischemia–reperfusion injury. Basic Res Cardiol 112: 38, 2017. [Erratum in Basic Res Cardiol 114: 44, 2019]. doi: 10.1007/s00395-017-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PloS One 9: e88685, 2014. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang P, Wang L, Li Q, Xu J, Xu J, Xiong Y, Chen G, Qian H, Jin C, Yu Y, Liu J, Qian L, Yang Y. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res Ther 10: 300, 2019. doi: 10.1186/s13287-019-1353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 182: 349–360, 2015. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cambier L, de Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, Kreke M, Smith RR, Marbán L, Marbán E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL‐10 expression and secretion. EMBO Mol Med 9: 337–352, 2017. doi: 10.15252/emmm.201606924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibrahim AG-E, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2: 606–619, 2014. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marbán E. The secret life of exosomes: what bees can teach us about next-generation therapeutics. J Am Coll Cardiol 71: 193–200, 2018. doi: 10.1016/j.jacc.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW. Sonic hedgehog modified human CD34+ cells preserve cardiac function following acute myocardial infarction. Circ Res 111: 312–321, 2012. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 109: 724–728, 2011. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10: 301–312, 2013. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Kervadec A, Bellamy V, El Harane N, Arakélian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Périer M-C, Toeg HD, Richart A, Lemitre M, Yin M, Loyer X, Larghero J, Hagège A, Ruel M, Boulanger CM, Silvestre J-S, Menasché P, Renault NKE. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 35: 795–807, 2016. doi: 10.1016/j.healun.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 99.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117: 52–64, 2015. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DPV, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4: 214–222, 2010. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 92: 387–397, 2014. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 102.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DPV. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 1: 129–137, 2007. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 103.Amini H, Rezaie J, Vosoughi A, Rahbarghazi R, Nouri M. Cardiac progenitor cells application in cardiovascular disease. J Cardiovasc Thorac Res 9: 127–132, 2017. doi: 10.15171/jcvtr.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L, Tulapurkar ME, Taylor BS, Yang P, Karathanasis S, Goodlett DR, Kaushal Sunjay A. Deep proteome analysis identifies the complete secretome as the functional unit of human cardiac progenitor cells. Circ Res 120: 816–834, 2017. doi: 10.1161/CIRCRESAHA.116.309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, Wu JC. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 122: S124–S131, 2010. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res 109: 894–906, 2011. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carr CA, Stuckey DJ, Tan JJ, Tan SC, Gomes RSM, Camelliti P, Messina E, Giacomello A, Ellison GM, Clarke K. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks – an MRI study. PLoS One 6: e25669, 2011. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ibrahim A, Marbán E. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol 78: 67–83, 2016. doi: 10.1146/annurev-physiol-021115-104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Makkar RR, Kereiakes DJ, Aguirre F, Kowalchuk G, Chakravarty T, Malliaras K, Francis GS, Povsic TJ, Schatz R, Traverse JH, Pogoda JM, Smith RR, Marbán L, Ascheim DD, Ostovaneh MR, Lima JAC, DeMaria A, Marbán E, Henry TD. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-controlled, double-blinded trial. Eur Heart J 41: 3451–3458, 2020. doi: 10.1093/eurheartj/ehaa541. [DOI] [PubMed] [Google Scholar]
- 110.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38: 201–211, 2017. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balmer GM, Riley PR. Harnessing the potential of adult cardiac stem cells: lessons from haematopoiesis, the embryo and the niche. J Cardiovasc Transl Res 5: 631–640, 2012. doi: 10.1007/s12265-012-9386-3. [DOI] [PubMed] [Google Scholar]
- 112.Balsam LB, Robbins RC. Haematopoietic stem cells and repair of the ischaemic heart. Clin Sci (Lond) 109: 483–492, 2005. doi: 10.1042/CS20050087. [DOI] [PubMed] [Google Scholar]
- 113.Sahoo S, Emanueli C. Exosomes in diabetic cardiomyopathy: the next-generation therapeutic targets? Diabetes 65: 2829–2831, 2016. doi: 10.2337/dbi16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin J-P, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rébé C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 120: 457–471, 2010. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247, 2008. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 116.Stepanek O, Hin N, Thomas AG, Dash RP, Alt J, Rais R, Rojas C, Slusher BS, Tsukamoto T. Neutral sphingomyelinase 2 inhibitors based on the 4-(1H-imidazol-2-yl)-2,6-dialkoxyphenol scaffold. Eur J Med Chem 170: 276–289, 2019. doi: 10.1016/j.ejmech.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205: 35–44, 2015. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 118.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18: 1606–1614, 2010. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amosse J, Martinez MC, Le S L. Extracellular vesicles and cardiovascular disease therapy. Stem Cell Investig 4: 102, 2017. doi: 10.21037/sci.2017.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nieuwland R, Falcón-Pérez JM, Théry C, Witwer KW. Rigor and standardization of extracellular vesicle research: paving the road towards robustness. J Extracell Vesicles 10: e12037, 2020. doi: 10.1002/jev2.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]