Abstract
High altitude-related excessive erythrocytosis (EE) is associated with increased cardiovascular risk. The experimental aim of this study was to determine the effects of microvesicles isolated from Andean highlanders with EE on endothelial cell inflammation, oxidative stress, apoptosis, and nitric oxide (NO) production. Twenty-six male residents of Cerro de Pasco, Peru (4,340 m), were studied: 12 highlanders without EE (age: 40 ± 4 yr; BMI: 26.4 ± 1.7; Hb: 17.4 ± 0.5 g/dL, Spo2: 86.9 ± 1.0%) and 14 highlanders with EE (43 ± 4 yr; 26.2 ± 0.9; 24.4 ± 0.4 g/dL; 79.7 ± 1.6%). Microvesicles were isolated, enumerated, and collected from plasma by flow cytometry. Human umbilical vein endothelial cells were cultured and treated with microvesicles from highlanders without and with EE. Microvesicles from highlanders with EE induced significantly higher release of interleukin (IL)-6 (89.8 ± 2.7 vs. 77.1 ± 1.9 pg/mL) and IL-8 (62.0 ± 2.7 vs. 53.3 ± 2.2 pg/mL) compared with microvesicles from healthy highlanders. Although intracellular expression of total NF-κB p65 (65.3 ± 6.0 vs. 74.9 ± 7.8.9 AU) was not significantly affected in cells treated with microvesicles from highlanders without versus with EE, microvesicles from highlanders with EE resulted in an ∼25% higher (P < 0.05) expression of p-NF-κB p65 (173.6 ± 14.3 vs. 132.8 ± 12.2 AU). Cell reactive oxygen species production was significantly higher (76.4.7 ± 5.4 vs. 56.7 ± 1.7% of control) and endothelial nitric oxide synthase (p-eNOS) activation (231.3 ± 15.5 vs. 286.6 ± 23.0 AU) and NO production (8.3 ± 0.6 vs. 10.7 ± 0.7 μM/L) were significantly lower in cells treated with microvesicles from highlanders with versus without EE. Cell apoptotic susceptibility was not significantly affected by EE-related microvesicles. Circulating microvesicles from Andean highlanders with EE increased endothelial cell inflammation and oxidative stress and reduced NO production.
NEW & NOTEWORTHY In this study, we determined the effects of microvesicles isolated from Andean highlanders with excessive erythrocytosis (EE) on endothelial cell inflammation, oxidative stress, apoptosis, and NO production. Microvesicles from highlanders with EE induced a dysfunctional response from endothelial cells characterized by increased cytokine release and expression of active nuclear factor-κB and reduced nitric oxide production. Andean highlanders with EE exhibit dysfunctional circulating extracellular microvesicles that induce a proinflammatory, proatherogenic endothelial phenotype.
Keywords: endothelial cells, erythrocytosis, highlanders, microvesicles
INTRODUCTION
More than 140 million people habitually reside at high altitude (>2,500 m) (1). One of the pathological consequences of living at high altitude is chronic mountain sickness (CMS), a maladaptation to high altitude characterized by excessive erythrocytosis (EE; hemoglobin concentration in males: ≥21 g/dL and in females: ≥19 g/dL) and hypoxemia (2). In Peru, where one-third of the population live at or above 4,000 m, nearly one in five Andean highlanders have EE and other symptoms of CMS (3). Chronic EE is not a benign condition; it is associated with a myriad of cardiovascular comorbidities such as endothelial dysfunction, hypertension, coronary artery disease, and thrombosis (4–7). The mechanisms underlying the increased cardiovascular risk in Andean highlanders with EE are not well understood. Animal studies suggest that EE induces a proatherogenic, prothrombotic vascular phenotype increasing the propensity for cardiovascular diseases and thrombotic events (8–10). Circulating extracellular microvesicles are key mediators of cardiovascular health and disease owing to their constant interaction with the vascular endothelium. It is currently unknown if EE influences the functional phenotype of circulating microvesicles.
Microvesicles are small (0.1–1.0 µM in diameter), anucleate, extracellular vesicles that bud from the plasma cell membrane of almost all cell types in response to various stressors that trigger either cellular activation or apoptosis (11, 12). Microvesicles play an important role in homeostatic cell-to-cell communication in the regulation of normal biological processes such as appropriate immune responses (13, 14). However, in pathological conditions, the numerical and functional characteristics of circulating microvesicles often change, imparting detrimental effects particularly on the vascular endothelium. Microvesicles stemming from pathological conditions can induce a proatherosclerotic endothelial phenotype characterized by excessive inflammation and oxidative stress as well as impaired nitric oxide (NO) production (15–18). For example, microvesicles isolated from patients with coronary artery disease (19) and end-stage renal disease (20) have been shown to suppress nitric oxide-mediated endothelium-dependent relaxation. We recently demonstrated that microvesicles isolated from HIV-1-seropositive adults cause severe endothelial cell damage and dysfunction and increase the propensity for premature cell death (21). Thus, clinical interest in circulating microvesicles has increased given their direct involvement in the development and progression of vascular disease (22).
Accordingly, the experimental aim of this study was to determine the effects of microvesicles isolated from Andean highlanders with EE on endothelial cell inflammation, oxidative stress, apoptosis, and nitric oxide (NO) production. We tested the hypothesis that microvesicles from Andean highlanders with EE would increase endothelial cell inflammation and oxidative stress, enhance endothelial apoptotic susceptibility, and diminish endothelial nitric oxide synthase (eNOS) activation and NO production compared with microvesicles from healthy Andean highlanders. EE-related microvesicle-induced changes in these key endothelial cell characteristics, which are known to be etiologically involved in the development of atherosclerosis and thrombosis, may underlie the increased cardiovascular risk and event burden in Andean highlanders with CMS (4).
MATERIALS AND METHODS
Subjects
Twenty-six male highlanders residing in Cerro de Pasco, Peru (4,340 m), were studied: 12 Andean highlanders without EE and 14 Andean highlanders with EE (Hb range: 21.6–27.0 g/dL). EE disproportionally affects male compared with female Andean highlanders. All of the males were nonobese, normotensive, normolipidemic, and nondiabetic. Before participation in the study, all subjects had the research study and its potential risks and benefits explained fully in Spanish by a Spanish-speaking research assistant before providing written informed consent in Spanish. The experimental procedures and protocols were approved by the University of British Columbia Clinical Research Ethics Board (H17-02687 and H18-01404) and local Peruvian ethics committee for the Universidad Peruana Cayetano Heredia (No. 101686) in adherence with the principles of the Declaration of Helsinki. This study was conducted as part of the Global Research Expedition on Altitude-related Chronic Health (REACH) to Cerro de Pasco, Peru. Details of this research expedition and the study population have been published elsewhere (23). Given the nature of the research expedition, some participants took part in multiple studies associated with REACH. However, the experimental aims and associated data presented herein are novel and address independent, a priori research questions.
Body Composition and Metabolic and Hematological Measures
Body mass was measured to the nearest 0.1 kg and height to nearest cm. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques. Blood samples to analyze Hb and hematocrit (Hct) were collected from an antecubital vein. Hb was analyzed using an ABL90 Flex blood analyzer (Radiometer, Copenhagen, Denmark). Hct was measured in triplicate using standard microhematocrit techniques and corrected for trapped plasma volume within trapped erythrocytes (24). Peripheral oxygen saturation (Spo2) was measured using a standard pulse oximeter (Choice Mmed, MD300C2, Beijing Choice Electric Technology Co. Ltd, Beijing, China). Chronic mountain sickness (CMS) score was assessed using the Qinghai CMS score, which is based on the sum of scores of the following series of signs and symptoms: breathlessness/palpitations, sleep disturbance, cyanosis, venodilation, paresthesia, headache, and tinnitus (25). Each symptom is assigned a score of 0 (i.e., sign absent) to 3 (i.e., severe) and summed, giving the resulting CMS severity as absent (0–5), mild (6–10), moderate (11–14), and severe (≥15) (25).
Plasma Cytokine Concentration
Plasma concentrations of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α were determined by ELISA (R&D Systems, Minneapolis, MN). Intraassay coefficient of variation for each assay was <8%.
Microvesicle Identification and Isolation
Microvesicle isolation was performed as previously described by our laboratory (21, 26). Briefly, venous blood from an antecubital vein was collected in sodium citrate tubes and centrifuged at 1,500 g for 10 min at room temperature; thereafter, plasma was collected and stored at −80°C for batch analysis and microvesicle isolation. To harvest microvesicles from each sample for use in cell experiments, plasma was centrifuged at 13,000 g for 2 min to remove cellular debris and then recentrifuged at 20,500 g for 50 min at 4°C to pellet microvesicles. Pelleted microvesicles were then resuspended in media. To determine the concentration of the isolated microvesicles, media samples were centrifuged at 13,000 g for 2 min, and 100 µL was transferred to a TruCount tube (BD Biosciences, Franklin Lakes, NJ). Samples were then fixed with 2% paraformaldehyde (ChemCruz Biochemicals, Santa Cruz, CA) and diluted with PBS. All samples were analyzed using a FACS Aria I flow cytometer (BD Biosciences). Microvesicle size threshold was established using Megamix-Plus SSC calibrator beads (Biocytex, Marseille, France), and only events >0.16 µM and <1 µM in size were counted. The concentration of microvesicles was determined using the formula: [number of events in region containing microvesicles/number of events in absolute count bead region] × [total number of beads per test/total volume of sample] (27). All samples were run on a BD FACSAria instrument. The event rate was set to 10,000 events per second up to 1,000,000 events (27). These methods were in accordance with the most current guidelines set by the International Society for Extracellular Vesicles (28) available at the time of our study; microvesicles were isolated by ultracentrifugation techniques of platelet-free plasma and quantified by flow cytometry using size gating specific to microvesicle size parameters.
Endothelial Cell Culture and Microvesicle Treatment
Human umbilical vein endothelial cells (HUVECs; Life Technologies, Thermo Fisher Scientific, Waltham, MA,) were cultured in endothelial growth media (ATCC, Manassas, VA) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin under standard cell culture conditions (37°C and 5% CO2) as previously described (26, 29). Briefly, cells were serially passaged after reaching 80%–90% confluence and harvested for experimentation after reaching ∼90% confluence on the third passage. HUVECs were seeded into six-well tissue culture plates with media containing an equal concentration of microvesicles from Andean highlanders without and with EE for 24 h. Cells were treated with microvesicles on a 2:1 (microvesicle:endothelial cell) ratio (21). To block NF-κB activation, IκB kinase (IKK) inhibitor was used. For these experiments, cells were co-treated with 400 nM IKK-16 (IKK inhibitor VII) (MilliporeSigma, Burlington, MA) along with microvesicles for 24 h. Initial IKK-16 titrations were based on reported half maximal inhibitory concentrations (IC50) of the ATP-competitive inhibitor of the IKK complex and its subunits and optimized for cell survivability by MTT assay (30). After all treatment conditions, cells and media were harvested for the determination of cellular protein expression, microRNA (miRNA) expression, cytokine release, and nitric oxide production.
Endothelial Cell Cytokine Release and Nitric Oxide Production
Release of interleukin (IL)-6 and IL-8 into cell supernatant from all microvesicle-treated conditions was determined using chemiluminescent ELISA (R&D Systems, Minneapolis, MN). To assess NO production, total nitrite in the supernatant was determined using the Total Nitric Oxide and Nitrate/Nitrite Parameters Assay Kit (R&D Systems, Minneapolis, MN). Intraassay coefficient of variation for the media-based ELISAs was <10% for each assay.
Endothelial Cell Protein Expression
Whole cell lysates were obtained from microvesicle-treated HUVECs for the quantification of intracellular proteins. As previously described (26), microvesicle-treated cells were washed in ice-cold PBS and incubated in ice-cold RIPA buffer containing protease and phosphatase inhibitors (Thermo Fisher Scientific) for 10 min. Cell lysates were then sonicated for 20 s (four 5-s cycles spaced by 90 s between each cycle), incubated on ice for an additional 20 min, and then centrifuged at 13,000 g at 4°C for 10 min. After centrifugation, the supernatant was collected, and protein concentration was determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA). Protein expression was measured by capillary electrophoresis immunoassay (Wes, ProteinSimple, San Clara, CA). (26, 29) Briefly, 2–3 ng of cell lysate was combined with a provided sample master mix (ProteinSimple, Santa Clara, CA) containing 1× sample buffer, 1× fluorescent molecular weight markers, and 40 mM DTT. Samples were vortexed and heated at 95°C for 5 min before combining with blocking solution, primary antibodies, horseradish peroxidase-conjugated secondary antibody, chemiluminescent substrate, and separation and stacking matrices for automated electrophoresis (375 V for 25 min) and immunodetection using the Wes system. Protein expression was normalized to total protein in the sample and presented as arbitrary units (AU). Rabbit primary antibodies against nuclear factor-κB (NF-κB) p65 (No. D14E12), phospho (p)-NF-κB p65 (Ser536) (No. 93H1), superoxide dismutase (SOD)1 (No. 2770), catalase (D5N7V) (No. 14097), caspase-3 (C3) (No. 9665S), and cleaved-C3 (No. 9664S) (all dilutions 1:250) (Cell Signaling Technologies, Danvers, MA) and endothelial nitric oxide synthase (eNOS) (No. PA1-037), p-eNOS (Ser1177) (No. PA5-35879), and p-eNOS (Thr495) (No. PA5-17706) (diluted 1:50, 1:250, and 1:250, respectively) (Thermo Fisher Scientific, Waltham, MA) were used. Initial titrations were performed to optimize antibody and total protein concentration for each protein target of interest. Representative protein histograms are shown in Fig. 1.
Figure 1.
Representative protein histograms of immunodetection using the Wes system for p-NF-κB p65(Ser536) (A), SOD1 (B), catalase (C), active caspase 3 (D), and p-eNOS (Ser1177) (E). eNOS, endothelial nitric oxide synthase; SOD1, superoxide dismutase 1.
Endothelial Cell Reactive Oxygen Species
To determine cellular reactive oxygen species (ROS), HUVECs were seeded in 96-well tissue culture plates (Thermo Fisher Scientific, Waltham, MA) and allowed to adhere for at least 1 h. Adherent cells were washed and treated with 2′,7′-dichlorofluorescin diacetate (Abcam, Cambridge, MA) stain (25 µmol/L) for 45 min. After 2′,7′-dichlorofluorescin diacetate treatment, cells were washed twice and stimulated with media or media containing microvesicles for 3 h. Immediately thereafter, fluorescence was measured using a GEMINI EM microplate reader (Molecular Devices, Sunnyvale, CA) and reported as the percentage relative to control (31).
Endothelial Cell miRNA Expression
Cellular miRNAs-associated inflammation (miR-146a and miR-181b), oxidative stress (miR-200c), and eNOS (miR-126 and miR-Let-7a) were determined by RT-PCR (32). After microvesicle treatment, 1.0 × 105 cells were collected and total cellular RNA was isolated using the miRVANA RNA isolation kit (Exiqon, Vedbake, Denmark). RNA concentration was determined using a Nanodrop Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Thereafter, 150 ng of RNA was reverse transcribed using the miScript II Reverse Transcription Kit (Qiagen, Hilden, German). RT-PCR was performed using BioRad CFX96 RT-PCR with the miScript SYBR green PCR kit (Qiagen, Hilden, Germany) and primers for miR-146a (Hs_miR-146a_1), miR-181b (Hs_miR-181b_1), miR-200c (Hs_miR-200c), miR-126 (Hs_miR-126), miR-Let-7a (Hs_miR-Let-7a), and U6 (Hs_RNU6-2_11) (Qiagen, Hilden, Germany). Table 1 lists all miRNA primers. All samples were assayed in duplicate. miRNA expression was quantified using the comparative Ct method and normalized to U6 (21). Relative expression of each transcript was calculated as the 2−ΔCt, where 2−(Ct[miR]−Ct[RNU6]) and presented as AU.
Table 1.
miRNA primers
| Product Name | Human miRNA Name | Targeted Human miRNA Sequence |
|---|---|---|
| Hs_miR-146a_1 | hsa-miR-146a | hsa-miR-146a:UGAGAACUGAAUUCCAUGGGUU |
| Hs_miR-181b_1 | hsa-miR-181b | hsa-miR-181b:AACAUUCAUUGCUGUCGGUGGGU |
| Hs_miR-200c_1 | hsa-miR-200c | hsa-miR-200c:UAAUACUGCCGGGUAAUGAUGGA |
| Hs_miR_126_1 | hsa-miR-126 | hsa-miR-126:UCGUACCGUGAGUAAUAAUGCG |
| Hs_miR_let-7a_1 | hsa-miR-let-7a | hsa-miR-let-7a:UGAGGUAGUAGGUUGUAUAGUU |
miRNA, microRNA.
Statistical Analysis
The distribution of the data was assessed by the Shapiro–Wilk test, and the homogeneity of variances was determined by the Levene test. Differences in subject characteristics and all cellular outcome variables were determined by Student’s t test unpaired or one-way analysis of variance (ANOVA) (normally distributed data) or the Mann–Whitney U test (nonnormally distributed data). Data are presented as means ± SE for normally distributed variables and as the median (interquartile range) for nonnormally distributed variables. Statistical significance was set a priori at P < 0.05.
RESULTS
Subject characteristics are presented in Table 2. There were no significant group differences in age, anthropometric, or metabolic variables. However, Hb, Hct, and CMS score as well as plasma concentrations of IL-6, IL-8, and TNF-α were significantly higher and Spo2 was significantly lower in the Andean highlanders with EE. Circulating microvesicle number (median [IQR]: 27,909 [11,571–42,711] vs. 15,258 [10,583–37,150] AU; P = 0.48) was not significantly different between the highlanders with and without EE.
Table 2.
Select subject characteristics
| Variable | Healthy Highlanders | Highlanders with EE |
|---|---|---|
| n | 12 | 14 |
| Age, yr | 40 ± 4 | 43 ± 4 |
| Body mass, kg | 69.8 ± 4.1 | 68.3 ± 3.0 |
| BMI, kg/m2 | 26.4 ± 1.7 | 26.2 ± 0.9 |
| Systolic BP, mmHg | 116 ± 4.1 | 112 ± 3.6 |
| Diastolic BP, mmHg | 74 ± 3.6 | 74 ± 2.8 |
| Hemoglobin, g/dL | 17.4 ± 0.5 | 23.4 ± 0.4* |
| Hematocrit, % | 53.0 ± 2 | 71.3 ± 1.1* |
| CMS score | 0.8 ± 0.1 | 7.1 ± 1.0* |
| Spo2, % | 86.9 ± 1.0 | 79.7 ± 1.6* |
| Total cholesterol, mg/dL | 124.8 ± 11.7 | 121.6 ± 5.7 |
| LDL-cholesterol, mg/dL | 73.7 ± 8.7 | 74.4 ± 4.2 |
| HDL-cholesterol, mg/dL | 34.8 ± 1.7 | 33.6 ± 1.4 |
| Triglycerides, mg/dL | 150.7 ± 43.8 | 133.8 ± 16.8 |
| Glucose, mg/dL | 83.3 ± 4.9 | 81.4 ± 4.8 |
| Insulin, µU/mL | 9.0 ± 1.3 | 7.0 ± 1.3 |
| IL-6, pg/mL | 2.7 + 0.4 | 4.7 + 0.7* |
| IL-8, pg/mL | 8.3 + 0.5 | 10.7 + 0.9* |
| TNF-α, pg/mL | 5.4 + 0.2 | 6.7 + 0.4* |
Values are means ± SE. BMI, body mass index; BP, blood pressure; CMS, chronic mountain sickness; EE, excessive erythrocytosis; HDL, high-density lipoprotein; IL-6, interleukin-6; IL-8, interleukin-8; LDL, low-density lipoprotein; Spo2, oxygen saturation; TNF-α, tumor necrosis factor-α. *P < 0.05.
Endothelial Cell Inflammation
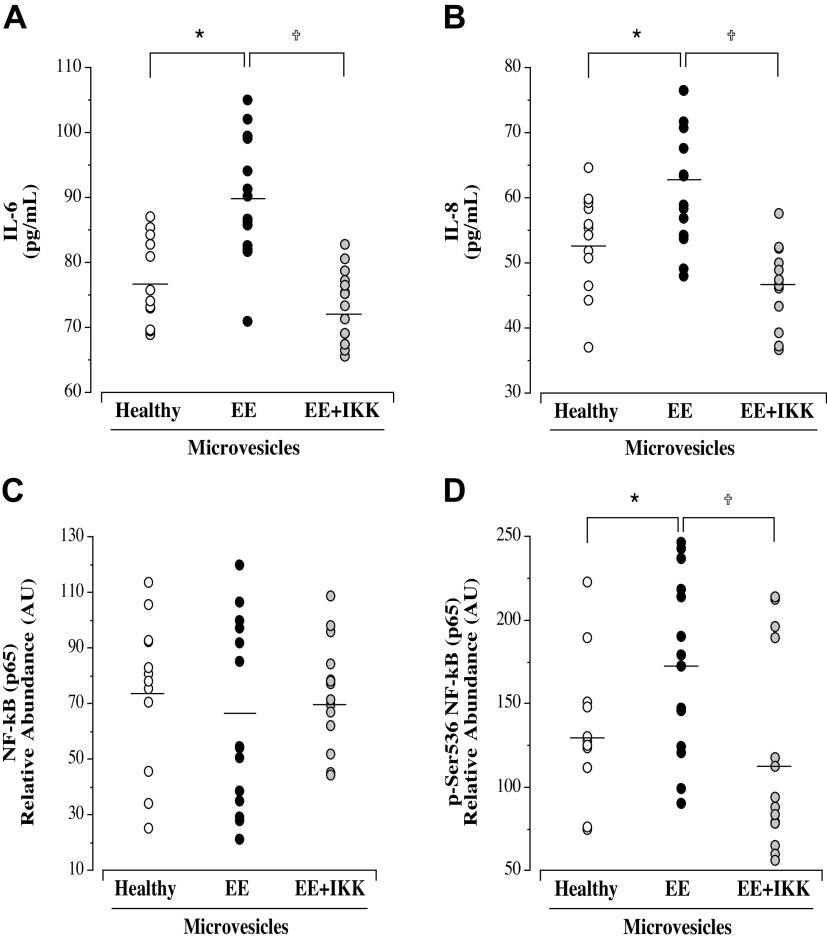
Microvesicles from Andean highlanders with EE induced significantly greater release of both IL-6 (means ± SE: 89.8 ± 2.7 vs. 77.1 ± 1.9 pg/mL; P = 0.01) and IL-8 (means ± SE: 62.0 ± 2.7 vs. 53.3 ± 2.2 pg/mL; P = 0.02) from endothelial cells compared with microvesicles from healthy highlanders (Fig. 2). Intracellular expression of total NF-κB p65 (means ± SE: 65.3 ± 6.0 vs. 74.9 ± 7.8.9 AU; P = 0.44) was not significantly different in HUVECs treated with microvesicles from highlanders without and with EE. However, microvesicles from the highlanders with EE resulted in an ∼25% higher expression of p-NF-κB p65 (Ser536; active NF-κB) (means ± SE: 173.6 ± 14.3 vs. 132.8 ± 12.2 AU; P = 0.03) (Fig. 2). In addition, intracellular expression of miR-146a (P = 0.004) and miR-181b (P = 0.004) was significantly lower in cells treated with microvesicles from the Andean highlanders with EE compared with those from healthy highlanders (Table 3).
Figure 2.
Endothelial cell release of IL-6 (A) and IL-8 (B) and intracellular expression of NF-κB p65 (C) and p-NF-κB p65(Ser536) (D) in response to treatment with microvesicles from healthy highlanders (n = 12) and highlanders with EE (n = 14) in the presence or absence of IKK 16. Mean value is denoted for IL-6, IL-8, NF-κB, and p-NF-κB. Analysis by one-way ANOVA. *P < 0.05 vs. healthy; †P < 0.05 vs. EE. EE, excessive erythrocytosis; IKK, IκB kinase.
Table 3.
Effects of microvesicles from healthy and EE highlanders on cell expression of inflammation- and eNOS-related miRNAs
| Variable | Healthy Microvesicles | EE Microvesicles |
|---|---|---|
| Inflammation-related miRs | ||
| miR-146a, AU | 4.4 ± 0.4 | 2.9 ± 0.2* |
| miR-181b, AU | 1.1 ± 0.1 | 0.8 ± 0.1* |
| eNOS-related miRs | ||
| miR-126, AU | 2.5 ± 0.2 | 1.7 ± 0.1* |
| miR-Let-7a, AU | 2.6 ± 0.2 | 1.8 ± 0.1* |
Values are means ± SE. *P < 0.05 vs. healthy. AU, arbitrary unit; EE, excessive erythrocytosis; eNOS, endothelial nitric oxide synthase; miRNA, microRNA.
The NF-κB inhibitor IKK 16 significantly blunted the EE-related microvesicle-induced production of IL-6 (means ± SE: 73.5 ± 1.6 vs. 89.8 ± 2.7 pg/mL; P = 0.003) and IL-8 (means ± SE: 46.6 ± 1.71 vs. 62.0 ± 2.7 pg/mL; P = 0.001) (Fig. 2). IKK 16 had no significant effect on total NF-κB expression (means ± SE: 68.8 ± 7.4 vs. 65.3 ± 6.0 AU; P = 0.66); however, p-NF-κB (Ser536) expression was ∼40% lower (means ± SE: 117.6 ± 15.7 vs. 173.6 ± 14.3 AU; P = 0.0001) in the cells co-treated with microvesicles from the EE highlanders and IKK 16 (Fig. 2).
Endothelial Cell Oxidative Stress
ROS production was significantly higher in HUVECs treated with microvesicles from the highlanders with EE (means ± SE: 76.4.7 ± 5.4 vs. 56.7 ± 1.7% of control; P = 0.003). However, cellular expression of superoxide dismutase 1 (median [IQR]: 133.0 [123.7–148.2] vs. 139.5 [107.0–159.1] AU; P = 0.75) and catalase (median [IQR]: 419.2 [381.2–453.9] vs. 417.4 [326.0–461.4] AU; P = 0.59) was not significantly different between cells treated with microvesicles from healthy highlanders compared with those from highlanders with EE (Fig. 3).
Figure 3.
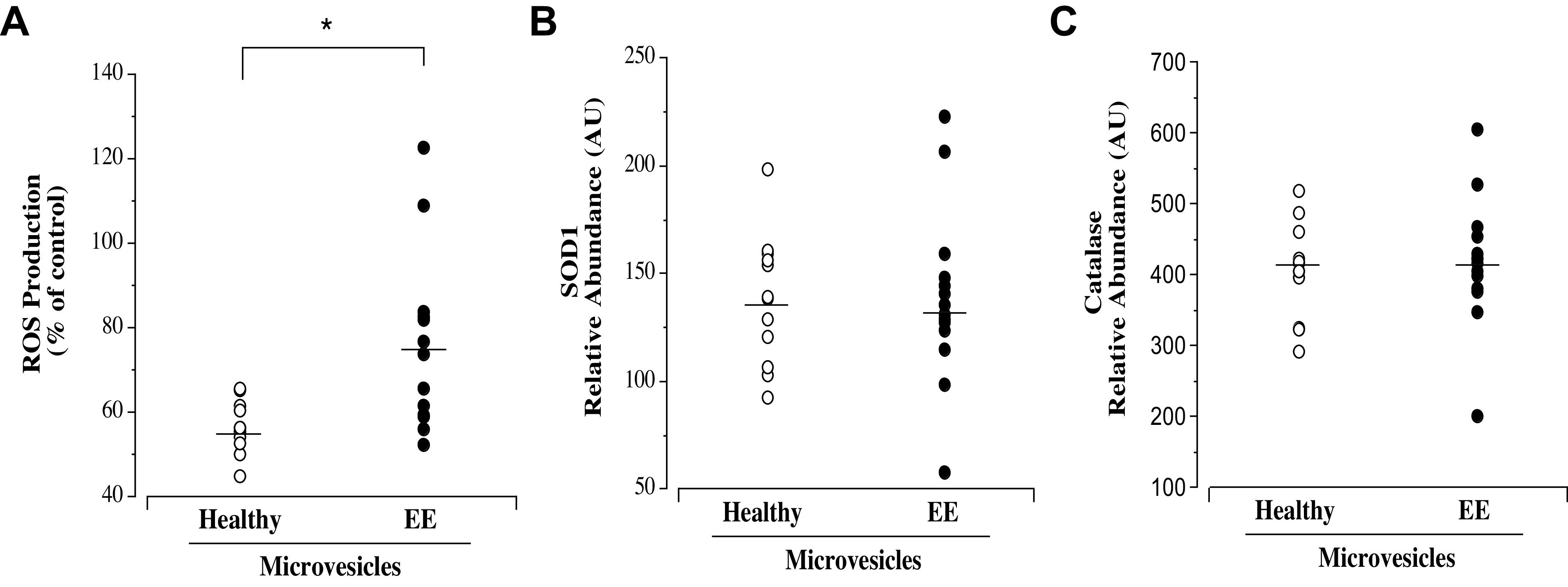
Endothelial cell reactive oxygen species (ROS) production (A) and superoxide dismutase 1 (SOD1) (B) and catalase (C) expression in response to treatment with microvesicles from healthy highlanders (n = 12) and highlanders with EE (n = 14). Mean value is denoted for ROS production, and analysis by independent t test. Median value is denoted for SOD1 and catalase data; analysis by Mann–Whitney U test. *P < 0.05 vs. healthy. EE, excessive erythrocytosis.
Endothelial Cell Apoptosis
There were no significant differences in cellular expression of total caspase-3 (means ± SE: 204.4 ± 15.7 vs. 188.9 ± 19.7 AU; P = 0.54) or active caspase-3 (means ± SE: 147.3 ± 11.7 vs. 128.3 ± 8.8 AU; P = 0.22) in cells treated with microvesicles from healthy highlanders compared with those from highlanders with EE.
Endothelial Nitric Oxide Production and eNOS Expression
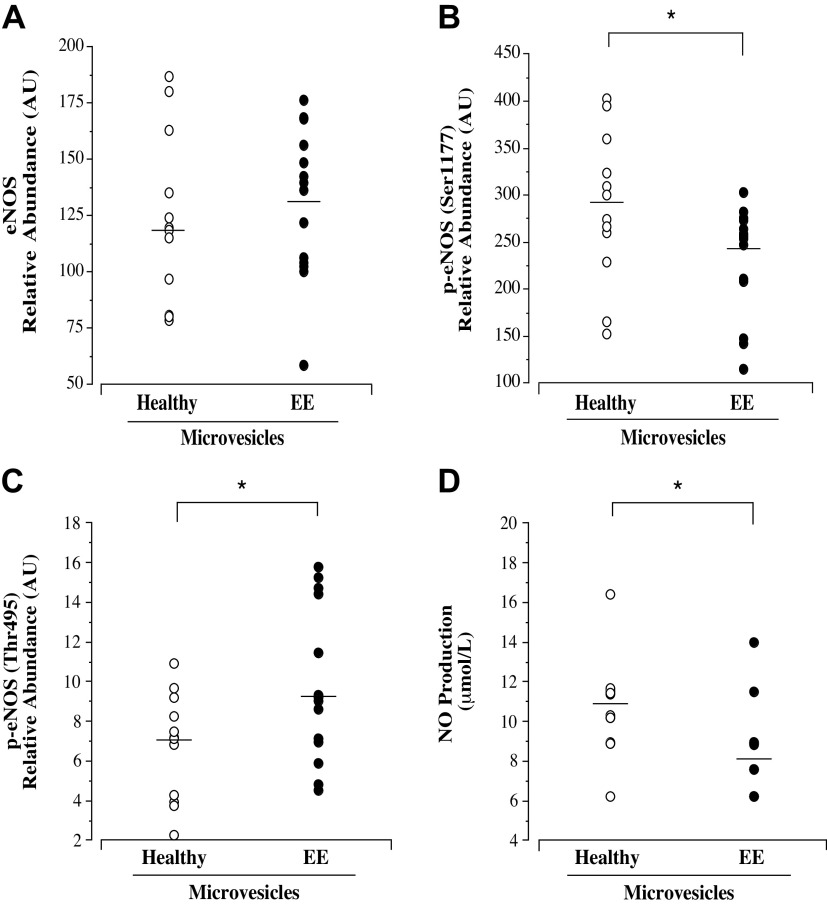
Cellular expression of e-NOS, p-eNOS (Ser1177), and p-eNOS (Thr495) and NO production are shown in Fig. 4. Total eNOS (means ± SE: 130.8 ± 8.9 vs. 123.3 ± 10.8 AU; P = 0.59) was not significantly different between cells treated with microvesicles from highlanders without and with EE. However, p-eNOS (Ser1177) (median [IQR]: 254.7 [208.2–272.8] vs. 287.3 [244.8–341.8] AU; P = 0.03) expression was significantly lower and p-eNOS (Thr495) (median [IQR]: 9.1 [7.0–13.6] vs. 7.2 [4.0–8.9] AU; P = 0.04) expression was significantly higher in cells treated with microvesicles from highlanders with EE. Consistent with changes in p-eNOS, NO production was ∼30% lower (median [IQR]: 8.2 [6.2–8.9] vs. 10.8 [9.6–11.5] µmol/L; P = 0.007) in cells treated with microvesicles from highlanders with EE compared with those from highlanders without EE. Cell expression of miR-126 and miR-Let-7a was significantly lower (∼30%; P = 0.0001 and ∼35%; P = 0.0004, respectively) in cells treated with microvesicles from the Andean highlanders with EE (Table 3).
Figure 4.
Endothelial cell expression of total eNOS (A), p-eNOS (Ser1177) (B), and p-eNOS (Thr495) (C) and NO production (D) in response to treatment with microvesicles from healthy highlanders (n = 12) and highlanders with EE (n = 14). Mean value is denoted for eNOS; analysis by independent t test. Median value is denoted for p-eNOS and NO production; analysis by Mann–Whitney U test. *P < 0.05 vs. healthy. EE, excessive erythrocytosis; eNOS, endothelial nitric oxide synthase; NO, nitric oxide.
DISCUSSION
The seminal, novel findings of this study are that microvesicles isolated from Andean highlanders with EE increase inflammation and oxidative stress as well as diminish eNOS activation and NO production in endothelial cells. Heightened endothelial cell inflammation and oxidative burden as well as impaired NO production are hallmark features of an atherogenic-prone endothelium (33, 34). Although the number of circulating microvesicles was similar in Andean highlanders with EE compared with healthy highlanders, the microvesicle phenotype in Andean highlanders with EE is dysfunctional with pathogenic endothelial effects. Microvesicle-mediated disruption of endothelial cell function has been shown to contribute to cardiovascular disease development, progression, and severity (12, 19). Thus, circulating microvesicles may be an important mechanistic factor underlying endothelial dysfunction and increased cardiovascular risk in Andean highlanders with EE.
Several studies have reported chronic low-grade systemic inflammation (35, 36) in Andean highlanders with EE. For example, Heinrich et al. (36) observed significantly higher plasma IL-6 concentrations in Andean males with EE compared with their healthy peers. The results of this study confirm and significantly extend these findings by demonstrating that in addition to elevations in systemic inflammatory cytokine concentrations (IL-6, IL-8, and TNF-α), circulating microvesicles isolated from Andean highlanders with EE induced significantly greater release of IL-6 and IL-8 from endothelial cells compared with microvesicles from healthy highlanders. Microvesicle-induced release of IL-6 and IL-8 may underlie the markedly higher systemic levels of IL-6 and IL-8 in the Andean highlanders with EE, providing novel mechanistic insight for the chronic low-grade inflammation observed in this population and a potential therapeutic target to mitigate inflammation (35, 36). Consistent with greater IL-6 and IL-8 production, cellular NF-κB activation was upregulated in cells treated with microvesicles from the Andean highlanders with EE. NF-κB is the main transcription factor regulating the production and release of IL-6 and IL-8 (37–39). To establish that the increase in IL-6 and IL-8 production induced by the microvesicles from the highlanders with EE was due to NF-κB activation, we inhibited NF-κB activation using an IκB kinase (IKK) inhibitor. IKK is an enzyme that frees NF-κB from its IκB subunit in the cytoplasm, allowing NF-κB translocation to the nucleus for phosphorylation resulting in protein activation (40). When IKK is blocked, NF-κB release for IκB submit is inhibited, preventing translocation to the nucleus and ultimately activation. Inhibiting NF-κB activation abolished the increase in endothelial cell IL-6 and IL-8 production induced by the microvesicles from the Andean highlanders with EE. Thus, the proinflammatory endothelial effects of microvesicles from Andean highlanders with EE appear to be largely NF-κB mediated.
Microvesicle-induced changes in NF-κB activation may be due, at least in part, to alterations in cellular miRNA (41–43). The expression of miR-146a and miR-181b, two miRNAs involved in the regulation of NF-κB activation, was significantly lower in cells treated with microvesicles from the Andean highlanders with EE, suggesting a potential mechanism for the EE-related microvesicle effect on NF-κB activation. miR-146a suppresses NF-κB activation through IRAK1 and TRAF6 inhibition, key adaptor molecules involved in NF-κB phosphorylation (41), whereas miR-181b inhibits importin-α-3, the protein responsible for translocation of NF-κB to the nucleus (42, 43). Future studies are needed using miRNA mimics to determine whether reduced bioavailability of these miRNAs played a role in augmented NF-κB activation. Nevertheless, taken together, these findings indicate that circulating microvesicles from Andean highlanders with EE exert profound proinflammatory effects on endothelial cells mediated by enhanced NF-κB activation. Increased endothelial cell inflammation as a result of NF-κB activation is a primary trigger for atherosclerosis and thrombosis (44–47) and a key contributor to the low-grade systemic inflammation associated with EE in Andean highlanders.
Like inflammation, oxidative stress renders the endothelium prone to accelerated atherosclerosis (48). Although a natural byproduct of cellular metabolism, excessive generation and/or deficient clearance and buffering of reactive oxygen species (ROS) causes endothelial cell dysfunction (49). Microvesicles from the Andean highlanders with EE significantly increased endothelial cell ROS production without triggering a compensatory antioxidant response, as the cellular expression of SOD-1 and catalase was unchanged. Circulating microvesicles provide a novel mechanism for the exaggerated oxidative stress with EE and, in turn, CMS. In a formative study, Bailey et al. (29) reported heightened systemic oxidative-nitrosative stress in Bolivian high-altitude dwellers with chronic mountain sickness. It is reasonable to postulate that a prooxidative microvesicle phenotype contributes to EE-related oxidative stress.
Reduced eNOS activation and, in turn, diminished NO production represent the most prominent indicator of endothelial cell dysfunction and a primary initiating cause in the development of atherosclerosis and thrombosis (50–52). eNOS is expressed constitutively in endothelial cells and is involved in not only the regulation of vascular tone via NO production and endothelin-1 inhibition but also the control of smooth muscle proliferation, platelet aggregation, and leukocyte adhesion to the endothelial surface as well as various cellular repair processes (33, 53, 54). As such, eNOS activity is paramount to normal endothelial cell function and disease prevention. In this study, microvesicles from Andean highlanders with EE significantly reduced eNOS activation and endothelial cell NO production. eNOS activity is dictated predominantly by phosphorylation of the enzyme at various sites (55). For example, phosphorylation at Ser1177 induces the strongest activation of eNOS, whereas phosphorylation at Thr495 inhibits eNOS activity (56, 57). The expression of p-eNOS(Ser1177) was significantly lower and the expression of p-eNOS(Thr495) significantly higher in cells treated with microvesicles from the Andean highlanders with EE compared with those of heathy Andean highlanders. Thus, microvesicles from the Andean highlanders with EE disrupted eNOS activation at two critical regulatory sites. Consistent with a reduction in eNOS activity, NO production was markedly lower in cells treated with microvesicles from the Andean highlanders with EE. From a clinical perspective, it is plausible that microvesicle-induced reduction in eNOS activity and NO production is a causative factor for the impairment in endothelial vasodilator function noted in Andean highlanders with EE (7, 58, 59).
The mechanisms underlying the EE-related microvesicle disruption in eNOS activation are not clear. The expression of both miR-126 and miR-Let-7a was lower in the cells treated with EE-related microvesicles. Highly expressed in endothelial cells, miR-126 and miR-Let-7a affect two distinct, independent regulatory pathways involved in eNOS activation (60, 61). miR-126 sustains the activity of the phosphatidylinositol 3-kinase/protein kinase B/eNOS signaling pathway critical for maintenance and upregulation of eNOS activation (61), whereas miR-Let-7a protects eNOS by suppressing the expression of lectin-like low-density lipoprotein receptor 1 (LOX-1), an antagonist of eNOS and NO production (60). It is conceivable that EE-related microvesicle-induced reduction in the expression of miR-126 and miR-Let-7a exposed the enzyme to unfavorable disruptions in signaling pathways that directly affect its activation potential and function.
Interestingly, cellular apoptosis was unaffected by microvesicles from Andean highlanders with EE. Total and active caspase-3 expression was not different in cells treated with microvesicles from the healthy Andeans and Andeans with EE. Intercellular caspase-3, particularly the active form of caspase-3, is a specific and sensitive indicator of the apoptotic status of a cell. The fact that the microvesicle phenotype associated with EE did not induce an apoptotic response is in line with data from Zhao et al. (62) demonstrating that erythroblast apoptosis is downregulated in Chinese highlanders with EE. Hypoxia-induced activation of the PI3/Akt signaling pathway has been suggested to play a central role in conferring this antiapoptosis protection (63, 64). Although we did not assess PI3/Akt signaling in this study, activation of this pathway is a potent inhibitor of caspase-3 activity (65), providing a viable explanation for the null effect of microvesicles from the Andean highlanders with EE on caspase-3, regardless of the presence of a proapoptotic cellular environment.
There are a few experimental considerations regarding this study that deserve mention. First, our study involved only Andean males. Although EE is far more prevalent in Adean men, we cannot, and should not, assume similar functional microvesicle phenotypes in Andean females with and without EE. Indeed, there is evidence of sex-related differences in circulating microvesicle number, structure, and function across physiological and pathological states (66–68). Second, the results of this study should be viewed with specific reference to Andean highlanders and not generalized to other high-altitude-dwelling populations such as Ethiopian Amhara and Oromo highlanders from the Simien and Bale mountains, respectively, or the Sherpa of Tibetan descent. Geographically distinct highlander populations represent different evolutionary-driven phenotypes with unique physiological adaptations to high altitude (69, 70). Future studies are needed to determine the numerical and functional phenotype of circulating microvesicles in these populations to allow for cross-comparison between high-altitude dwellers. These types of studies are central to the overall mission of the REACH program (and its investigators) to better understand physiological and pathological similarities and differences among Peruvian, Tibetan, and Ethiopian highlanders (23). Third, we did not determine the cellular source of increase in ROS production induced by microvesicles from the highlanders with EE. Future studies are needed to distinguish whether cytosolic components or mitochondria dysfunction underlie the microvesicle-stimulated increase in ROS production. Finally, given the cross-sectional study design and the in vitro nature of our experiments, we are unable to make definitive translational statements regarding microvesicle function in Andean highlanders with EE and clinical risk. However, the changes in endothelial cell function noted in response to microvesicles isolated from Andean highlanders with EE are key precipitating events in the development of endothelial dysfunction, atherosclerosis, and thrombosis (71–75). Moreover, the use of cultured endothelial cells and explanted vessel preparations to understand the influence of circulating microvesicles on vascular health and function has provided novel mechanistic insight on microvesicles as biomarkers and mediators of vascular disease development, progression, severity, and outcome (19, 76–78) as well as potential therapeutic targets to treat atherosclerosis (79, 80).
In conclusion, the results of this study demonstrate that circulating microvesicles from Andean highlanders with EE adversely affect endothelial cells promoting a pathological endothelial phenotype characterized by increased inflammation and oxidative stress and impaired NO production. Circulating microvesicles may contribute to increased cardiovascular events such as myocardial infarction and stroke as well as development of peripheral artery disease in Andean highlanders with EE.
GRANTS
This study was supported, at least in part, by the Natural Sciences and Engineering Research Council of Canada (to P. N. Ainslie), Canada Research Chairs program (to P. N. Ainslie), and National Heart, Lung, and Blood Institute Grants HL077450 and HL107715 (to C. A. DeSouza).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.M.B., F.C.V., P.N.A., and C.A.D. conceived and designed research; L.M.B., A.R.B., V.P.G., H.K.F., R.S., N.M.D., J.J.G., M.M.T., G.A.V-G., R.J.F-M., F.C.V., and P.N.A. performed experiments; L.M.B., V.P.G., H.K.F., and J.J.G. analyzed data; L.M.B., V.P.G., J.J.G., and C.A.D. interpreted results of experiments; V.P.G. and J.J.G. prepared figures; C.A.D. drafted manuscript; P.N.A. and C.A.D. edited and revised manuscript; L.M.B., R.J.F-M., A.R.B., V.P.G., H.K.F., R.S., N.M.D., J.J.G., M.M.T., G.A.V., F.C.V., P.N.A., and C.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the Andean males who participated in the study and the staff at the University of Colorado Anschutz Medical Campus ACI/ID Flow Core for technical assistance.
REFERENCES
- 1.West JB, Schoene RB, Luks AM, Milledge JS. High Altitude Medicine and Physiology. London: CRC Press, 2012. [Google Scholar]
- 2.Villafuerte FC, Corante N. Chronic mountain sickness: clinical aspects, etiology, management, and treatment. High Alt Med Biol 17: 61–69, 2016. doi: 10.1089/ham.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monge C, Leon-Velarde F, Arregui A. Increasing prevalence of excessive erythrocytosis with age among healthy high-altitude miners. N Engl J Med 321: 1271, 1989. doi: 10.1056/NEJM198911023211819. [DOI] [PubMed] [Google Scholar]
- 4.Corante N, Anza-Ramirez C, Figueroa-Mujica R, Macarlupu JL, Vizcardo-Galindo G, Bilo G, Parati G, Gamboa JL, Leon-Velarde F, Villafuerte FC. Excessive erythrocytosis and cardiovascular risk in Andean highlanders. High Alt Med Biol 19: 221–231, 2018. doi: 10.1089/ham.2017.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J 21: 515–520, 2000. doi: 10.1053/euhj.1999.1699. [DOI] [PubMed] [Google Scholar]
- 6.Rimoldi SF, Rexhaj E, Pratali L, Bailey DM, Hutter D, Faita F, Salinas Salmon C, Villena M, Nicod P, Allemann Y, Scherrer U, Sartori C. Systemic vascular dysfunction in patients with chronic mountain sickness. Chest 141: 139–146, 2012. doi: 10.1378/chest.11-0342. [DOI] [PubMed] [Google Scholar]
- 7.Tymko MM, Lawley JS, Ainslie PN, Hansen A, Hofstaetter F, Rainer SL, Amin S, Moralez G, Gasho C, Vizcardo-Galindo GA, Bermudez D, Villafuerte FC, Hearon CM Jr.. Global REACH 2018: heightened alpha-adrenergic signaling impairs endothelial function during chronic exposure to hypobaric hypoxia. Circ Res 127: e1–e13, 2020. doi: 10.1161/CIRCRESAHA.119.316053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, Butman JA, Jedlickova K, Prchal JT, Polyakova LA. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood 103: 3924–3932, 2004. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 9.Nadeem O, Gui J, Ornstein DL. Prevalence of venous thromboembolism in patients with secondary polycythemia. Clin Appl Thromb Hemost 19: 363–366, 2013. doi: 10.1177/1076029612460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quaschning T, Ruschitzka F, Stallmach T, Shaw S, Morawietz H, Goettsch W, Hermann M, Slowinski T, Theuring F, Hocher B, Luscher TF, Gassmann M. Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. FASEB J 17: 259–261, 2003. doi: 10.1096/fj.02-0296fje. [DOI] [PubMed] [Google Scholar]
- 11.Lovren F, Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin Chem 59: 1166–1174, 2013. doi: 10.1373/clinchem.2012.199711. [DOI] [PubMed] [Google Scholar]
- 12.Rautou P-E, Vion A-C, Amabile N, Chironi G, Simon A, Tedgui A, Boulanger CM. Microparticles, vascular function, and atherothrombosis. Circ Res 109: 593–606, 2011. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 13.Alexandru N, Costa A, Constantin A, Cochior D, Georgescu A. Microparticles: from biogenesis to biomarkers and diagnostic tools in cardiovascular disease. Curr Stem Cell Res Ther 12: 89–102, 2017. doi: 10.2174/1574888X11666151203224058. [DOI] [PubMed] [Google Scholar]
- 14.Santilli F, Marchisio M, Lanuti P, Boccatonda A, Miscia S, Davi G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost 116: 220–234, 2016. doi: 10.1160/TH16-03-0176. [DOI] [PubMed] [Google Scholar]
- 15.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol 173: 1210–1219, 2008. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost 100: 878–885, 2008. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky SV, Zhang F, Nasjletti A, Goligorsky MS. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol 286: H1910–H1915, 2004. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 18.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res 100: 7–18, 2013. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 19.Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 14: 259–272, 2017. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 20.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol 16: 3381–3388, 2005. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 21.Hijmans JG, Stockelman KA, Garcia V, Levy MV, Brewster LM, Bammert TD, Greiner JJ, Stauffer BL, Connick E, DeSouza CA. Circulating microparticles are elevated in treated HIV -1 infection and are deleterious to endothelial cell function. J Am Heart Assoc 8: e011134, 2019. doi: 10.1161/JAHA.118.011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meziani F, Tesse A, Andriantsitohaina R. Microparticles are vectors of paradoxical information in vascular cells including the endothelium: role in health and diseases. Pharmacol Rep 60: 75–84, 2008. [PubMed] [Google Scholar]
- 23.Tymko MM, Hoiland RL, Tremblay JC, Stembridge M, Dawkins TG, Coombs GB, Patrician A, Howe CA, Gibbons TD, Moore JP, Simpson LL, Steinback CD, Meah VL, Stacey BS, Bailey DM, MacLeod DB, Gasho C, Anholm JD, Bain AR, Lawley JS, Villafuerte FC, Vizcardo-Galindo G, Ainslie PN. The 2018 global research expedition on altitude related chronic health (Global REACH) to Cerro de Pasco, Peru: an experimental overview. Exp Physiol 106: 86–103, 2021. doi: 10.1113/EP088350. [DOI] [PubMed] [Google Scholar]
- 24.Chaplin H, Mollison P. Correction of plasma trapped in the red cell column of hematocrit. Blood 7: 1227–1238, 1952. doi: 10.1182/blood.V7.12.1227.1227. [DOI] [PubMed] [Google Scholar]
- 25.Leon-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, Zubieta-Calleja G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 6: 147–157, 2005. doi: 10.1089/ham.2005.6.147. [DOI] [PubMed] [Google Scholar]
- 26.Brewster LM, Coombs GB, Garcia VP, Hijmans JG, DeSouza NM, Stockelman KA, Barak OF, Mijacika T, Dujic Z, Greiner JJ, Phillips AA, Ainslie PN, DeSouza CA. Effects of circulating extracellular microvesicles from spinal cord-injured adults on endothelial cell function. Clin Sci (Lond) 134: 777–789, 2020. doi: 10.1042/CS20200047. [DOI] [PubMed] [Google Scholar]
- 27.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, Abu Hussein N, Kebschull M, Bedorf J, Franklin BS, Latz E, Nickenig G, Werner N. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol 32: 1925–1935, 2012. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 28.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3: 26913, 2014. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey DM, Brugniaux JV, Filipponi T, Marley CJ, Stacey B, Soria R, Rimoldi SF, Cerny D, Rexhaj E, Pratali L, Salmon CS, Murillo Jauregui C, Villena M, Smirl JD, Ogoh S, Pietri S, Scherrer U, Sartori C. Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J Physiol 597: 611–629, 2019. doi: 10.1113/JP276898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waelchli R, Bollbuck B, Bruns C, Buhl T, Eder J, Feifel R, Hersperger R, Janser P, Revesz L, Zerwes HG, Schlapbach A. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg Med Chem Lett 16: 108–112, 2006. doi: 10.1016/j.bmcl.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 31.Xu S, He Y, Vokurkova M, Touyz RM. Endothelial cells negatively modulate reactive oxygen species generation in vascular smooth muscle cells: role of thioredoxin. Hypertension 54: 427–433, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133983. [DOI] [PubMed] [Google Scholar]
- 32.Hijmans JG, Stockelman KA, Levy MV, Brewster LM, Bammert TD, Greiner JJ, Connick E, DeSouza CA. Effects of HIV-1 gp120 and TAT derived microvesicles on endothelial cell function. J Appl Physiol 126: 1242–1249, 2019. doi: 10.1152/japplphysiol.01048.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network On A; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 54: 2129–2138, 2009. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boos CJ, Woods DR, Varias A, Biscocho S, Heseltine P, Mellor AJ. High altitude and acute mountain sickness and changes in circulating endothelin-1, interleukin-6, and interleukin-17a. High Alt Med Biol 17: 25–31, 2016. doi: 10.1089/ham.2015.0098. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich E, Anza-Ramirez C, Macarlupu J, Corante N, Vizcardo-Galindo G, Djokic M, Villafuerte FC, Powell FL, Simonson TS. Interleukin 6 is elevated in Andean higlanders with chronic mountain sickness. Am J Respir Clin Care Med 197: A2365, 2019. [Google Scholar]
- 37.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab 13: 11–22, 2011. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brasier AR. The nuclear factor-κB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 86: 211–218, 2010. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue X, Xia W, Wenzhong H. A modeled dynamic regulatory network of NF-κB and IL-6 mediated by miRNA. Biosystems 114: 214–218, 2013. doi: 10.1016/j.biosystems.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther 2: 17023, 2017. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Campisi J, Benz CC. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene 27: 5643–5647, 2008. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma S, Tian XY, Zhang Y, Mu C, Shen H, Bismuth J, Pownall HJ, Huang Y, Wong WT. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep 6: 22910, 2016. doi: 10.1038/srep22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Registry M, Blackwell TS, Baron RM, Feinberg MW; MICU Registry. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest 122: 1973–1990, 2012. doi: 10.1172/jci61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins T, Cybulsky MI. NF-κB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 107: 255–264, 2001. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang Y, Li X, Zhang X, Li Z, Wang L, Sun Y, Liu Z, Ma X. Elevated levels of plasma TNF-α are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock 41: 275–281, 2014. doi: 10.1097/SHK.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 46.Ramkaran P, Khan S, Phulukdaree A, Moodley D, Chuturgoon AA. miR-146a polymorphism influences levels of miR-146a, IRAK-1, and TRAF-6 in young patients with coronary artery disease. Cell Biochem Biophys 68: 259–266, 2014. doi: 10.1007/s12013-013-9704-7. [DOI] [PubMed] [Google Scholar]
- 47.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 340: 115–126, 1999. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 48.Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 100: 1–19, 2018. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462, 2014. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balakumar P, Kathuria S, Taneja G, Kalra S, Mahadevan N. Is targeting eNOS a key mechanistic insight of cardiovascular defensive potentials of statins? J Mol Cell Cardiol 52: 83–92, 2012. doi: 10.1016/j.yjmcc.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Gimbrone M, Kume N, Cybulski M. Vascular endothelial dysfunction and the pathogenesis of atherosclerosis. In: Atherosclerosis Reviews, edited by Weber P, Leaf A.. New York: Raven Press, 1993, p. 1–9. [Google Scholar]
- 52.Matthys KE, Bult H. Nitric oxide function in atherosclerosis. Mediators Inflamm 6: 3–21, 1997. doi: 10.1080/09629359791875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heiss C, Rodriguez-Mateos A, Kelm M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxid Redox Signal 22: 1230–1242, 2015. doi: 10.1089/ars.2014.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab 20: 295–302, 2009. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heiss EH, Dirsch VM. Regulation of eNOS enzyme activity by posttranslational modification. Curr Pharm Des 20: 3503–3513, 2014. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: E68–E75, 2001. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 57.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 58.Tremblay JC, Coombs GB, Howe CA, Vizcardo-Galindo GA, Figueroa-Mujica RJ, Bermudez D, Tymko MM, Villafuerte FC, Ainslie PN, Pyke KE. Global REACH 2018: reduced flow-mediated dilation stimulated by sustained increases in shear stress in high-altitude excessive erythrocytosis. Am J Physiol Heart Circ Physiol 317: H991–H1001, 2019. doi: 10.1152/ajpheart.00316.2019. [DOI] [PubMed] [Google Scholar]
- 59.Tremblay JC, Hoiland RL, Howe CA, Coombs GB, Vizcardo-Galindo GA, Figueroa-Mujica RJ, Bermudez D, Gibbons TD, Stacey BS, Bailey DM, Tymko MM, MacLeod DB, Gasho C, Villafuerte FC, Pyke KE, Pn. Global Reach A. High blood viscosity and hemoglobin concentration contribute to reduced flow-mediated dilation in high-altitude excessive erythrocytosis. Hypertension 73: 1327–1335, 2019. doi: 10.1161/hypertensionaha.119.12780. [DOI] [PubMed] [Google Scholar]
- 60.Bao MH, Zhang YW, Lou XY, Cheng Y, Zhou HH. Protective effects of let-7a and let-7b on oxidized low-density lipoprotein induced endothelial cell injuries. PLoS One 9: e106540, 2014. doi: 10.1371/journal.pone.0106540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang HH, Chen Y, Gao CY, Cui ZT, Yao JM. Protective effects of MicroRNA-126 on human cardiac microvascular endothelial cells against hypoxia/reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cell Physiol Biochem 42: 506–518, 2017. doi: 10.1159/000477597. [DOI] [PubMed] [Google Scholar]
- 62.Zhao C, Li Z, Ji L, Ma J, Ge RL, Cui S. PI3K-Akt signal transduction molecules maybe involved in downregulation of erythroblasts apoptosis and perifosine increased its apoptosis in chronic mountain sickness. Med Sci Monit 23: 5637–5649, 2017. doi: 10.12659/msm.905739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier M, Nitschke M, Hocke C, Kramer J, Jabs W, Steinhoff J, Schutt M. Insulin inhibits caspase-3 activity in human renal tubular epithelial cells via the PI3-kinase/Akt pathway. Cell Physiol Biochem 21: 279–286, 2008. doi: 10.1159/000129386. [DOI] [PubMed] [Google Scholar]
- 64.Sun G, Zhou Y, Li H, Guo Y, Shan J, Xia M, Li Y, Li S, Long D, Feng L. Over-expression of microRNA-494 up-regulates hypoxia-inducible factor-1 alpha expression via PI3K/Akt pathway and protects against hypoxia-induced apoptosis. J Biomed Sci 20: 100, 2013. doi: 10.1186/1423-0127-20-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romorini L, Garate X, Neiman G, Luzzani C, Furmento VA, Guberman AS, Sevlever GE, Scassa ME, Miriuka SG. AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival. Sci Rep 6: 35660, 2016. doi: 10.1038/srep35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bammert TD, Hijmans JG, Kavlich PJ, Lincenberg GM, Reiakvam WR, Fay RT, Greiner JJ, Stauffer BL, DeSouza CA. Influence of sex on the number of circulating endothelial microparticles and microRNA expression in middle-aged adults. Exp Physiol 102: 894–900, 2017. doi: 10.1113/EP086359. [DOI] [PubMed] [Google Scholar]
- 67.Gustafson CM, Shepherd AJ, Miller VM, Jayachandran M. Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol Sex Differ 6: 10, 2015. doi: 10.1186/s13293-015-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunter LW, Jayachandran M, Miller VM. Sex differences in the expression of cell adhesion molecules on microvesicles derived from cultured human brain microvascular endothelial cells treated with inflammatory and thrombotic stimuli. Biol Sex Differ 10: 26, 2019. doi: 10.1186/s13293-019-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beall CM. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol 46: 18–24, 2006. doi: 10.1093/icb/icj004. [DOI] [PubMed] [Google Scholar]
- 70.Beall CM. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA 104(Suppl 1): 8655–8660, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, , et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 72.Badimon JJ, Meyer B, Feigen LP, Baron DA, Chesebro JH, Fuster V, Badimon L. Thrombosis triggered by severe arterial lesions is inhibited by oral administration of a glycoprotein IIb/IIIa antagonist. Eur J Clin Invest 27: 568–574, 1997. doi: 10.1046/j.1365-2362.1997.1480697.x. [DOI] [PubMed] [Google Scholar]
- 73.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 24: 1468–1474, 1994. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 74.Migliacci R, Becattini C, Pesavento R, Davi G, Vedovati MC, Guglielmini G, Falcinelli E, Ciabattoni G, Dalla Valle F, Prandoni P, Agnelli G, Gresele P. Endothelial dysfunction in patients with spontaneous venous thromboembolism. Haematologica 92: 812–818, 2007. doi: 10.3324/haematol.10872. [DOI] [PubMed] [Google Scholar]
- 75.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol 171: 1691–1704, 2007. doi: 10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen F, Nickenig G, Werner N. Extracellular vesicles in cardiovascular disease: potential applications in diagnosis, prognosis, and epidemiology. Circ Res 120: 1649–1657, 2017. doi: 10.1161/CIRCRESAHA.117.310752. [DOI] [PubMed] [Google Scholar]
- 77.Nomura S. Microparticle and atherothrombotic diseases. J Atheroscler Thromb 23: 1–9, 2016. doi: 10.5551/jat.32326. [DOI] [PubMed] [Google Scholar]
- 78.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med 379: 958–966, 2018. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- 79.Agrahari V, Agrahari V, Burnouf P-A, Chew CH, Burnouf T. Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol 37: 707–729, 2019. doi: 10.1016/j.tibtech.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Yin M, Loyer X, Boulanger CM. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol 763: 90–103, 2015. doi: 10.1016/j.ejphar.2015.06.047. [DOI] [PubMed] [Google Scholar]