Abstract
Thoracic aortic aneurysm and dissection (TAAD) is a deadly disease characterized by intimal disruption induced by hemodynamic forces of the circulation. The effect of exercise in patients with TAAD is largely unknown. β-Aminopropionitrile (BAPN) is an irreversible inhibitor of lysyl oxidase that induces TAAD in mice. The objective of this study was to investigate the effect of aerobic exercise on BAPN-induced TAAD. Upon weaning, mice were given either BAPN-containing water or standard drinking water and subjected to either conventional cage activity (BAPN-CONV) or forced treadmill exercise (BAPN-EX) for up to 26 wk. Mortality was 23.5% (20/85) for BAPN-CONV mice versus 0% (0/22) for BAPN-EX mice (hazard ratio 3.8; P = 0.01). BAPN induced significant elastic lamina fragmentation and intimal-medial thickening compared with BAPN-untreated controls, and aneurysms were identified in 50% (5/10) of mice that underwent contrast-enhanced CT scanning. Exercise significantly decreased BAPN-induced wall thickening, calculated circumferential wall tension, and lumen diameter, with 0% (0/5) of BAPN-EX demonstrating chronic aortic aneurysm formation on CT scan. Expression of selected genes relevant to vascular diseases was analyzed by qRT-PCR. Notably, exercise normalized BAPN-induced increases in TGF-β pathway-related genes Cd109, Smad4, and Tgfβr1; inflammation-related genes Vcam1, Bcl2a1, Ccr2, Pparg, Il1r1, Il1r1, Itgb2, and Itgax; and vascular injury- and response-related genes Mmp3, Fn1, and Vwf. Additionally, exercise significantly increased elastin expression in BAPN-treated animals compared with controls. This study suggests that moderate aerobic exercise may be safe and effective in preventing the most devastating outcomes in TAAD.
NEW & NOTEWORTHY Moderate aerobic exercise was shown to significantly reduce mortality, extracellular matrix degradation, and thoracic aortic aneurysm and dissection formation associated with lysyl oxidase inhibition in a mouse model. Gene expression suggested a reversal of TGF-β, inflammation, and extracellular matrix remodeling pathway dysregulation, along with augmented elastogenesis with exercise.
Keywords: aortic aneurysm, aortic dissection, BAPN, exercise, lysyl oxidase
INTRODUCTION
Estimates of the incidence of aortic dissection in the literature range from 0.5 to 4.7 cases per 100,000 people per year (1), with aortic aneurysm and dissection accounting for ∼10,000 deaths in the United States annually (2). An initial tear in the tunica intima causes blood to enter the tunica media resulting in a longitudinal separation of the vessel wall. Aortic dissection can have catastrophic acute consequences which include organ and limb malperfusion or death. In addition, chronic aneurysm formation and rupture complicates ∼10% of cases of medically treated Type B dissections (3). The pathophysiology that leads to thoracic aortic aneurysm and dissection (TAAD) and its progression can be broadly described as a degenerative process that compromises the structural integrity of the aortic wall. Characteristic findings include fragmentation and depletion of elastic fibers, vascular smooth muscle cell (vSMC) apoptosis, inflammatory cell infiltration, and replacement of areas of degeneration with proteoglycans and glycosaminoglycans (4).
Increases in systemic blood pressure and heart rate during aerobic exercise have a theoretical risk of worsening aortic dissection. Elevated patient pulse pressure is associated with an increased incidence of dissection (5). Increased wall shear stress is associated with aortic wall expansion leading to aneurysm formation (5, 6). Thus, while the benefit of aerobic exercise in most cardiovascular diseases including Type 2 diabetes (7), ischemic heart disease (8), heart failure (9), and peripheral artery disease (10) are well established, the safety and potential benefit in TAAD are largely unknown.
Literature exists in thoracic aortic disease suggesting moderate aerobic exercise is safe, and may, in fact, be beneficial. In a mouse model of Marfan syndrome, moderate exercise was shown to improve aortic root dilation, but the mechanism behind this benefit is unclear (11). Gibson et al. (12) showed a reduction of matrix metalloproteinases (MMPs) in the vessel wall of exercised Marfan syndrome mice, and attenuation of proteolytic processes, which damage the structural integrity of the aorta. Recent trials of exercise training in patients with small abdominal aortic aneurysm (AAA) have demonstrated that exercise is not associated with adverse events or excessive aneurysm growth (13) and may even protect against AAA expansion (14, 15). A pilot trial and registry study in patients discharged after operative repair for Type A aortic dissection reveals that moderate aerobic activity is feasible and may promote cardiovascular health and improved quality of life postoperatively (16, 17). However, the safety and effect of physical activity and exercise in aortic dissection, especially in nonoperatively treated aortic dissection, is still not established. As such, further animal and human studies investigating exercise in TAAD are clearly warranted.
Lysyl oxidase (LOX) is a copper and quinone-containing enzyme amine oxidase involved in the cross linking of collagen and elastin monomers in the extracellular matrix (ECM). These cross links provide tensile strength and elasticity in vessel walls and are essential to normal embryonic development, connective tissue function, and wound healing. Abnormal expression or function of LOX is implicated in numerous diseases, including scleroderma, Alzheimer’s disease, amyotrophic lateral sclerosis, cancer, AAA, and TAAD (18–24). LOX deficiency is embryonically lethal in mice or causes perinatal death from aortic aneurysm rupture (25). LOX inhibition by β-aminopropionitrile (BAPN) significantly alters the structure and integrity of the aortic wall, inducing TAAD in rodents (26–33). The constellation of histologic findings includes elastic fiber fragmentation, vSMC apoptosis, and inflammatory infiltration, recapitulating key findings found in human disease. BAPN treatment is also associated with significant mortality, primarily from aortic rupture, with rates as high as 36%–56% (29, 33, 34). Therefore, BAPN-induced TAAD has become an accepted and established animal model for the study of thoracic aortic disease.
Exercise alters blood vessel biomechanics such as wall tension and shear stress, which can modify disease progression in aortic dissection (35, 36). It also changes systemic hemodynamic factors relevant to aortic dissection pathophysiology such as blood pressure, heart rate, and pulse pressure. There is a significant knowledge gap in whether exercise therapy modulates the development and progression of aortic dissection and its sequelae. This absence of quantitative data has led to inadequate evidence-based guidance regarding activity recommendations for patients, for fear of acutely or chronically causing changes in blood pressures, which may exacerbate disease. The objective of this study was to test the hypothesis that moderate aerobic exercise is beneficial in the prevention of TAAD in the BAPN mouse model.
METHODS
Study Approval
The Institutional Animal Care and Use Committee at the University of Maryland, Baltimore, approved this study.
Exercise Protocol
Male mice on a mixed background (C56BL/6 SJL) were weaned at 3–4 wk of age onto a standard rodent chow diet (2018sx, Harlan Teklad, Madison, WI) and administered BAPN (Sigma-Aldrich, St. Louis, MO) dissolved in drinking water (3 g/L) or standard drinking water as adapted from described protocols (29, 33, 37). The exercise regimens were in accordance whenever possible with guidelines for animal exercise and training published by the American Journal of Physiology-Heart and Circulatory Physiology (38).
Upon weaning, BAPN-untreated and BAPN-treated mice were subjected to forced treadmill exercise (EX, BAPN-EX, respectively) or conventional cage activity (CONV, BAPN-CONV, respectively) for 5–6 mo. In the exercise group, treadmill acclimation occurred over 2 wk (5 days/wk) beginning with exposure to the stationary treadmill and advancing to the goal regimen of running at 5 m/min for 5 min, 13 m/min for 20 min, and 5 m/min for 5 min, 5 days each week. Based on published protocols, this was estimated to be 65%–75% Vo2 max, consistent with moderate intensity exercise (12). One BAPN-EX mouse was excluded from analysis due to poor adherence to the exercise protocol.
Tissue Collection
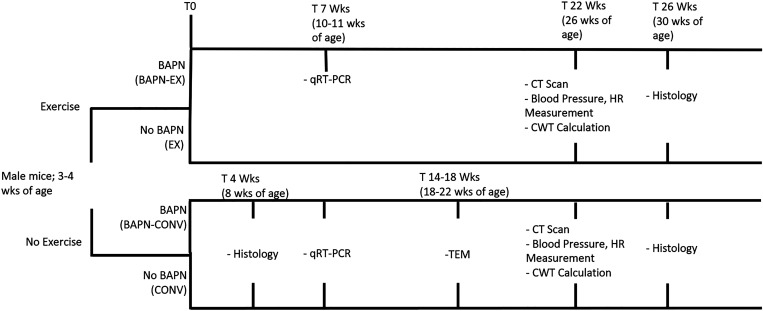
All tissues were collected with consistent euthenization methods and treatment conditions. Unless states otherwise, animals were euthanized with an average age of ∼30 wk for histology and 26 wk for CT. The range of ages for the CONV mice in these experiments ranged from 22 to 34 wk of age. Prior work in our laboratory has not demonstrated significant histologic or radiographic differences in CONV mice ranging from 8 wk to up to 50 wk of age. For qRT-PCR experiments, the exercise protocol and BAPN treatment were abbreviated to ∼7 wk, with animals ranging between 10 and 11 wk of age for analysis. There were no significant differences in ages among any of the groups in any experiment. Figure 1 summarizes the scheme and time line of the experiments, as well as the average ages of the animals for each experiment.
Figure 1.
Experimental scheme.
Surviving animals were euthanized at protocol completion by CO2 asphyxiation, and the ascending aorta (AscAo), descending thoracic aorta (DTA), and abdominal aorta (AbdAo) were collected for experiments. The adventitia and periadventitial fat from the aortas were removed for all experiments except when tissue was used for histologic analysis. After collection, tissue was snap-frozen and stored at −80°C for RNA analyses, fixed in 4% paraformaldehyde overnight for histologic analyses, or fixed in 3% glutaraldehyde, 0.1 M sodium cacodylate (pH 7.4) for transmission electron microscopy (TEM). Animals used for microcomputed tomography (micro-CT) underwent perfusion, image acquisition, and tissue collection within 24 h of death.
Micro-CT Analysis
Micro-CT was used to measure aortic lumen diameter, as previously described (39). Fourteen to eighteen hours before analysis, mice were perfused with 10 mL of heparinized phosphate-buffered saline through a left ventricular puncture. An incision in the right atrium drained the solutions. Animals were then perfused with Microfil contrast reagent (Flow Tech, Inc., Carver, MA) using a single syringe infusion pump (Cole-Parmer, Vernon Hills, IL) at a constant rate. Cross-sectional CT images were acquired the following day (Inveon, Siemens, Munich, Germany) and converted to Digital Imaging and Communications in Medicine format. Two independent observers (one blinded) analyzed the images utilizing OsiriX MD (v.8.0.2, Pixmeo Sarl, Bernex, Switzerland). Minor-axis diameters of the largest segment of ascending, descending thoracic, and abdominal aorta were measured with minimal interobserver variability. Mean lumen measurements of CONV mice established age-matched controls. An aneurysm was defined as a lumen diameter 50% greater than the mean diameter of the CONV controls.
Vessel Wall Histology, Morphometry, and Immunohistochemistry
Serial formalin-fixed, paraffin-embedded (FFPE) sections (5 µm) of AscAo, DTA, and AbdAo were subjected to hematoxylin and eosin (H&E), Masson’s trichrome, and Verhoeff Van Gieson (VVG) staining. Morphometric measurements were performed using EVOS FL Auto Imaging System software (Invitrogen, Carlsbad, CA). The average number of elastin fiber nicks and breaks per high power field per animal was calculated from VVG-stained circumferential aortic sections by two independent observers (one blinded) with minimal interobserver variability. To calculate the intima-media, adventitial, and total wall thickness, two independent observers (one blinded) measured the distance from the inner elastic lamina to external elastic lamina, the external elastic lamina to the outer edge of the adventitia, and the full length of the vessel wall, respectively. Twelve measurements per section were taken along a clock-face pattern and averaged for each animal using ImageJ software.
Survival Analysis
Animals from cohorts of each group were observed for survival. All mice that died prematurely were immediately autopsied and recorded as a mortality event in the survival analysis. Animals that were removed for the experiments described in these studies or for other purposes were included in the survival curve and censored from analysis at the time of removal.
Transmission Electron Microscopy
Sections of AscAo from young adult mice CONV and BAPN-CONV animals were fixed in 3% glutaraldehyde, 0.1 M sodium cacodylate (pH 7.4), and stained sequentially with osmium tetroxide, tannic acid, and uranyl acetate. Aortas were dehydrated through a graded methanol series, infiltrated with Epon, embedded in pure Epon, and polymerized. Sixty-nanometer sections were counterstained with uranyl acetate and lead citrate, and images captured using a Philips FEI Tecnai T12 G2 transmission electron microscope and advanced microscopy techniques CCD camera. Several grids were examined per each sample. There were consistent intragroup histologic findings identified within both the CONV and BAPN-CONV groups (animals were 18–22 wk of age). A representative slide is presented.
Blood Pressure Measurement and Circumferential Wall Tension
A tail-cuff method (40) (Coda Non-Invasive Blood Pressure System; Kent Scientific, Torrington, CT) was used to measure systolic and diastolic blood pressure (SBP and DBP, respectively) and heart rate (HR) at ∼26 wk of age; pulse pressure (PP = SBP − DBP) and mean arterial pressure (MAP = 1/3 × SBP + 2/3 × DBP) were calculated from these measurements. All mice were allowed to acclimate to the restrainer for 10–20 min per day over at least 3 days before obtaining measurements. During blood pressure measurement, five acclimation cycles preceded 20 measurement cycles. This was repeated over at least 2 days. Measurements were excluded if they had a poor waveform or the mice were agitated during data collection. The mean of these measurements was used for each animal.
For the random subset of animals that underwent both CT scanning and hemodynamic measurements, mean circumferential wall tension (CWT) was calculated with the following formula: CWT = MAP × (D/2), where CWT is expressed in dyne/cm, MAP is mean arterial pressure (dynes/cm2), and D is lumen diameter (cm) based on micro-CT measurement (41).
RNA Extraction
The frozen aorta was mechanically disrupted, and total RNA was extracted from CONV, BAPN-CONV, and BAPN-EX mice using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The quality and quantity of purified RNA were estimated using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Quantitative Real-Time Polymerase Chain Reaction
Ninety-two genes relevant to vascular diseases were examined using quantitative real-time polymerase chain reaction (qRT-PCR) (TaqMan Gene Expression Assays, Applied Biosystems, Foster, CA). A full list of gene names and abbreviations are included (Table 1). Total RNA was extracted as above. The resulting RNA was quantified, treated with ezDNase (Invitrogen, Carlsbad, CA), and reverse transcribed to cDNA (High-Capacity cDNA Reverse Transcription kit, Applied Biosystems). Ten nanogram of each sample was used per reaction. qRT-PCR was performed with TaqPath qPCR Master Mix, CG (Applied Biosystems) using a QuantStudio 3 RT-PCR System (Applied Biosystems) under the condition of 120 s at 50°C, 120 s at 95°C, 1 s at 95°C, and 20 s at 60°C for 40 cycles. Expression levels of each gene were determined using four biological replicates for each group and normalized against Gapdh using the 2−ΔCT method (42).
Table 1.
Gene names and gene symbols of qRT-PCR experiment
| Gene Symbol | Gene Name |
|---|---|
|
Abca1 Ace Acta2 Apoe Bax Bcl2 Bcl2a1(a,b,d) Bcl2l1 Bid Birc3 Ccl2 Ccl5 Ccr1 Ccr2 Cd109 Cd44 Cdh5 Cflar Col1a2 Col3a1 Ctgf Cxcl1 Eln Fas Fgf2 Fn1 Hbegf Icam1 Il1b Il1r1 Il1r2 Il5 Itga2 Itga5 Itgax Itgb2 Klf2 Ldlr Lif Lox Lpl Ltbp2 Mmp2 Mmp3 Mmp9 Msr1 Nfkb1 Npy Pdgfa Pdgfb Pdgfrb Plin2 Ppara Ppard Pparg Sele Sell Selp Selplg Serpinb2 Serpine1 Smad3 Smad4 Spp1 Tgfb1 Tgfb2 Tgfbr1 Timp1 Tnc Tnfaip3 Tnfrsf11b Ubc Vcam1 Vegfa Vwf |
ATP-binding cassette, subfamily A (ABC1), member 1 Angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 Actin, α2, smooth muscle, aorta Apolipoprotein E BCL2-associated X protein B-cell leukemia/lymphoma 2 B-cell leukemia/lymphoma 2 related protein A1 BCL2-like 1 BH3-interacting domain death agonist Baculoviral IAP repeat-containing 3 Chemokine (C-C motif) ligand 2 Chemokine (C-C motif) ligand 5 Chemokine (C-C motif) receptor 1 Chemokine (C-C motif) receptor 2 CD109 antigen CD44 antigen Cadherin 5 CASP8- and FADD-like apoptosis regulator Collagen, type I, α2 Collagen, type III, α1 Connective tissue growth factor Chemokine (C-X-C motif) ligand 1 Elastin Fas (TNF receptor superfamily member 6) Fibroblast growth factor 2 Fibronectin 1 Heparin-binding EGF-like growth factor Intercellular adhesion molecule 1 Interleukin 1β Interleukin 1 receptor Type 1 Interleukin 1 receptor Type 2 Interleukin 5 Integrin subunit α2 Integrin subunit α5 Integrin subunit α X Integrin subunit β 2 Kruppel-like factor 2 Low-density lipoprotein receptor LIF interleukin 6 family cytokine Lysyl oxidase Lipoprotein lipase Latent transforming growth factor β binding protein 2 Matrix metallopeptidase 2 Matrix metallopeptidase 3 Matrix metallopeptidase 9 Macrophage scavenger receptor 1 Nuclear factor κ B subunit 1 Neuropeptide Y Platelet-derived growth factor subunit A Platelet-derived growth factor subunit B Platelet-derived growth factor receptor β Perilipin 2 Peroxisome proliferator-activated receptor α Peroxisome proliferator-activated receptor δ Peroxisome proliferator-activated receptor γ Selectin E Selectin L Selectin P Selectin P ligand Serpin family B member 2 Serpin family E member 1 SMAD family member 3 SMAD family member 4 Secreted phosphoprotein 1 Transforming growth factor β1 Transforming growth factor β2 Transforming growth factor β receptor 1 TIMP metallopeptidase inhibitor 1 Tenascin C TNFα induced protein 3 TNF receptor superfamily member 11b Ubiquitin C Vascular cell adhesion molecule 1 Vascular endothelial growth factor A Von Willebrand factor |
Compiled from NCBI Gene Database.
Statistics
Unless specified, all values are presented as means ± SE and are analyzed for significance using either unpaired Student’s t test or two-way ANOVA with post hoc Tukey’s multiple comparisons test (Prism 8.0, GraphPad Software, San Diego, CA). For the qRT-PCR experiment, one-way ANOVA with post hoc Tukey’s tests was performed. For categorical data, Fischer’s exact tests were utilized. Sample sizes for each experiment are noted in the figure captions. Survival curves were used to analyze the survival proportions of BAPN-CONV and BAPN-EX mice. The mortality data are presented both as a Mantel–Haenszel hazard ratio with 95% confidence intervals (CI) and as a number of deaths over the total number of animals included for analysis for each group. The log-rank (Mantel–Cox) test was used to identify significant differences between the survival proportions of the BAPN-CONV and BAPN-EX mice. Significance for all experiments was defined as being met when the two-tailed α was ≤0.05.
RESULTS
LOX Inhibition with BAPN Causes Aortic Wall Remodeling
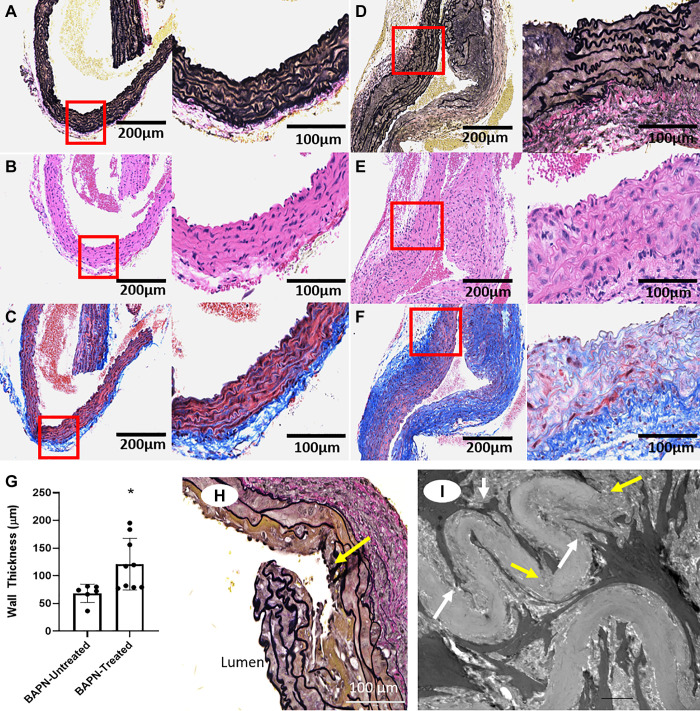
We examined the effects of irreversible LOX inhibition with BAPN on the vessel wall architecture. Histologic analysis identified abnormal ECM structure and remodeling in the ascending aorta of BAPN-treated mice as young as 8 wk of age (Fig. 2, A–G). These included increased elastin fragmentation (Fig. 2D), collagen deposition (Fig. 2F), and wall thickening (Fig. 2G). Elastin fragmentation and areas of dissection were found throughout the aorta. However, the most prominent findings were identified in the AscAo of BAPN-treated animals (Fig. 2H). Micro-CT evidence of dissection, chronic aneurysm formation, and quantitation of chronic aneurysm incidence have been published previously by our laboratory (39). TEM analysis of the AscAo from mice at 18–22 wk of age identified elastin fragmentation and reduced focal adhesions and dense bodies interacting with the ECM (Fig. 2I).
Figure 2.
LOX inhibition with BAPN causes aortic remodeling of the ascending aorta. A–F: representative histological sections of the ascending aorta (AscAo) of 8-wk-old mice without BAPN treatment (A–C) and with BAPN treatment (D–F); Verhoeff–Van Gieson (VVG) (A and D), hematoxylin and eosin (H&E) (B and E), and Masson’s Trichrome (C and F) stains. Remodeling is present in the arterial wall of BAPN-treated animals as highlighted by fragmentation of the elastin lamina (black-stained elastin layers in D compared with A) and increased collagen deposition (blue staining in F compared with C). Left panels of each subfigure are ×20 mag., right panels of each subfigure are ×40 mag. G: quantification of wall thickness in BAPN-untreated versus BAPN-treated mice (8 wk old; n = 6 BAPN-untreated, 9 BAPN-treated). *P = 0.02 by Student’s t test. H: representative dissection in a BAPN-treated mouse (8 wk old). Yellow arrow points to area of dissection; ×40 mag. I: transmission electron microscopy of AscAo section of a BAPN-treated mouse (22 wk old), demonstrating aberrant SMC cytoplasmic interaction with elastin (white arrow) and discontinuity of the elastin layer (yellow arrow). Scale bar represents 2 µm. AscAo, ascending aorta; BAPN, β-aminopropionitrile; LOX, lysyl oxidase; SMC, smooth muscle cell.
BAPN causes Aortic Rupture
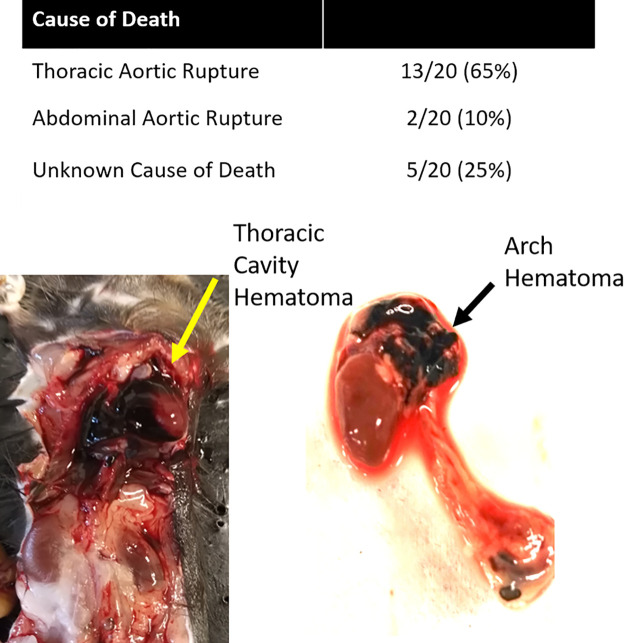
Twenty mice out of 85 unexercised mice treated with BAPN (BAPN-CONV) died during the time frame of these studies (mortality 23.5%). Thirteen animals died from rupture of the ascending or descending thoracic aorta, two from rupture of the abdominal aorta, and five from unknown cause based on necropsy (Fig. 3).
Figure 3.
LOX inhibition causes frequent aortic rupture. Top: necropsy of expired mice identified thoracic aortic ruptures as the most frequent cause of death with BAPN treatment. Bottom: thoracic cavity hematoma (left) and arch and descending thoracic aortic hematoma (right) from aortic rupture. BAPN, β-aminopropionitrile; LOX, lysyl oxidase.
Exercise Reduces BAPN-Associated Mortality
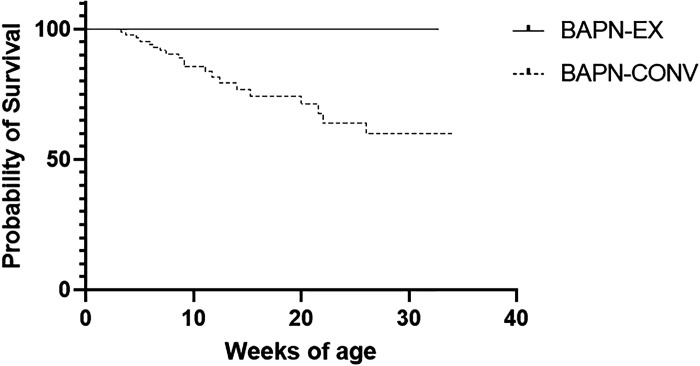
To investigate the effect of aerobic exercise on BAPN-induced TAAD, we subjected a cohort of mice treated with BAPN to forced treadmill exercise. Survival was observed for animals for the entirety of their life span during the time frame of these studies. Remarkably, zero animals died in the BAPN-EX group (n = 22) (vs. 20 deaths in the BAPN-CONV group). This survival benefit was statistically significant as compared with the survival of the BAPN-CONV animals (hazard ratio: 3.8; 95% CI 1.3–10.7; P = 0.01; Fig. 4). There were no deaths in mice untreated with BAPN (CONV: n = 31 or EX, n = 11), ranging from 8 to 30 wk of age depending on time euthanized for experiments. A secondary analysis of deaths only from aortic rupture identified the same significant survival advantage in BAPN-EX mice versus BAPN-CONV mice (data not shown).
Figure 4.
Exercise reduces mortality in BAPN-treated mice. Survival curve in BAPN-treated mice without and with exercise (n = 85 BAPN-CONV, n = 22 BAPN-EX). The survival benefit was statistically significant (Mantel–Haenszel hazard ratio = 3.8, P = 0.01 by log-rank test). There were no deaths in mice untreated with BAPN (CONV, n = 31 or EX, n = 11). Data from CONV and EX are not shown in graph due to overlying curve with BAPN-EX. BAPN, β-aminopropionitrile; BAPN-CONV, conventional cage activity; BAPN-EX, forced treadmill exercise.
Exercise Reduces BAPN-Associated Aortopathy
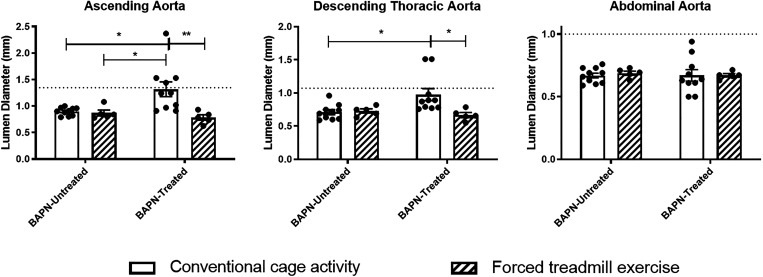
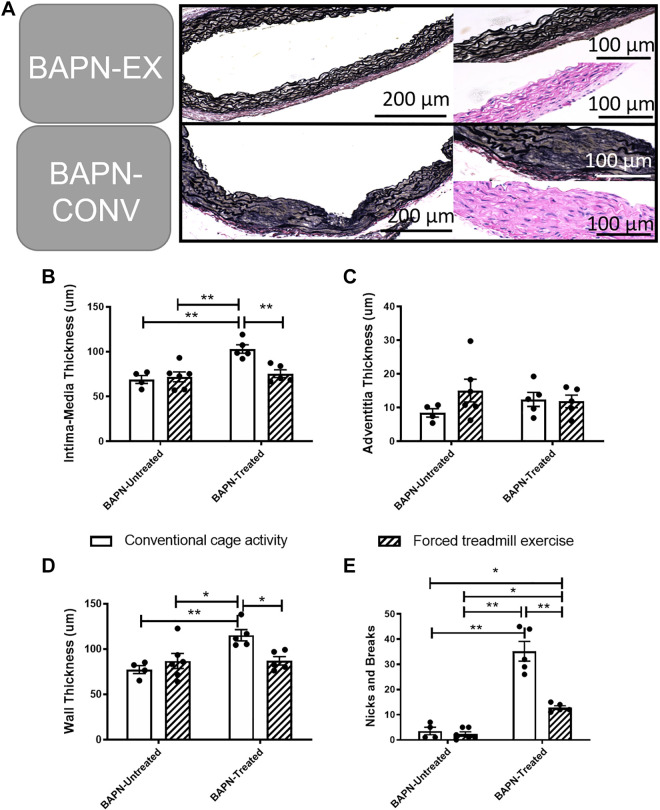
Unexercised BAPN-treated animals demonstrated significantly increased lumen diameters in the AscAo (P = 0.01) and the DTA (0 = 0.02) compared with unexercised BAPN-untreated animals based on micro-CTA imaging. In BAPN-treated animals, exercise resulted in a significant reduction in the lumen size of the AscAo (P < 0.01) and DTA (P = 0.03; Fig. 5) compared with those that were not exercised, with BAPN-EX mice demonstrating no difference in lumen diameter compared with BAPN-untreated CONV controls. There were no statistically significant differences in the luminal diameters of the AbdAo among any of the four conditions. Likewise, exercise had no effect on lumen size in any aortic segment in BAPN-untreated animals. In total, five out of the 10 BAPN-CONV animals (50%) developed six aneurysms (four AscAo and two DTA), and 0 of the 5 BAPN-EX animals (0%) developed an aneurysm by the completion of the exercise and treatment regimen (P = 0.1). One animal in the BAPN-CONV group had both an AscAo and DTA aneurysm. Histologic analysis of the AscAo demonstrated significantly less wall thickness BAPN-EX mice compared with BAPN-CONV mice (P = 0.037; Fig. 6, A–D). Similarly, there was a significant improvement in the overall elastin architecture in BAPN-EX mice with significantly fewer elastin nicks and breaks compared with BAPN-CONV mice (P < 0.001; Fig. 6E).
Figure 5.
Exercise reduces aortic aneurysmal dilatation in BAPN-treated mice. Quantification of aortic lumen diameters by micro-CT imaging demonstrates a decrease in AscAo and DTA lumen size in BAPN-EX mice compared with BAPN-CONV mice. Exercise did not affect lumen size in BAPN-untreated animals. Dashed lines represent 1.5× the mean diameter of the relevant aortic segment in BAPN-untreated, conventional cage activity animals. *P < 0.05, **P < 0.01 by two-way ANOVA with Tukey’s multiple comparisons test. n = 10 CONV, 5 EX, 10 BAPN-CONV, 5 BAPN-EX. AscAo, ascending aorta; BAPN, β-aminopropionitrile; BAPN-CONV, conventional cage activity; BAPN-EX, forced treadmill exercise; DTA, descending thoracic aorta.
Figure 6.
Exercise reduces aortic wall remodeling in BAPN-treated mice. A: representative section of AscAo demonstrating reduced elastin degradation in BAPN-EX mice compared with BAPN-CONV mice. Magnification in left panels (VVG) ×20 and in right panels (VVG and H&E) is ×40. B–D: quantification of intimal-medial thickness (B), adventitial thickness (C), and total wall thickness (D). E: decrease in nicks and breaks of the elastic lamina. *P < 0.05, **P < 0.01 by two-way ANOVA with Tukey’s multiple comparisons test. n = 4 CONV, 6 EX, 5 BAPN-CONV, 5 BAPN-EX. AscAo, ascending aorta; BAPN, β-aminopropionitrile; BAPN-CONV, conventional cage activity; BAPN-EX, forced treadmill exercise; VVG, Verhoeff–Van Gieson.
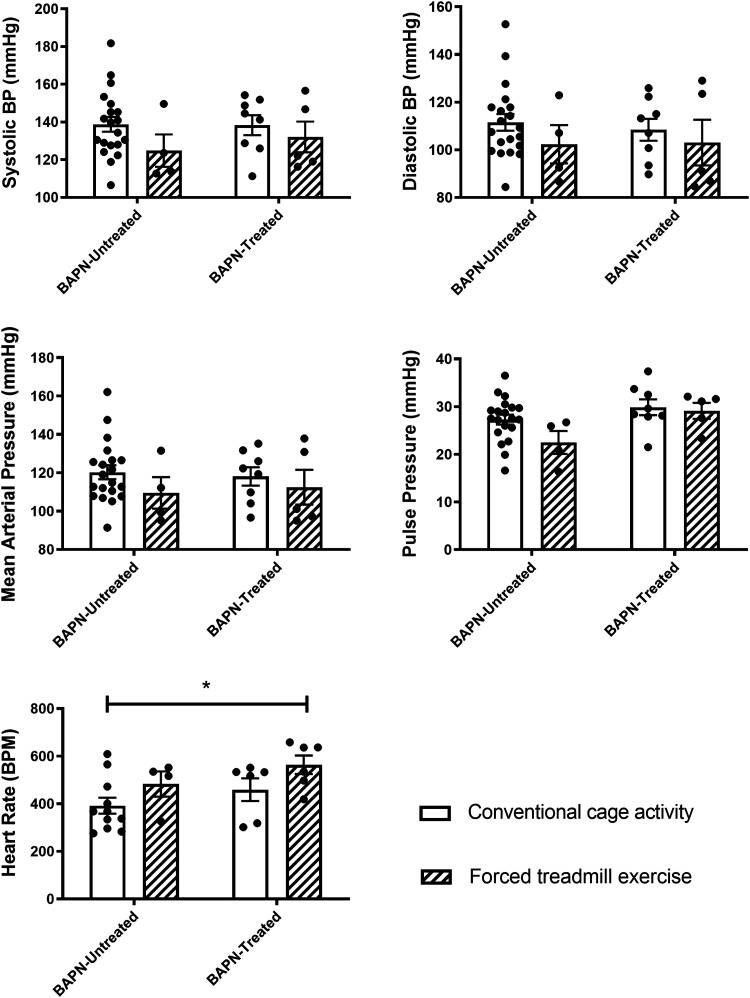
Systemic Hemodynamics
Systolic and diastolic pressures, mean arterial pressure, pulse pressures, and heart rates were measured at the completion of the exercise and/or treatment regimens as described above. Neither BAPN treatment nor exercise affected blood pressures, mean arterial pressures, or pulse pressures, with no significant differences demonstrated among any of the groups (Fig. 7). The heart rates of the BAPN-EX mice were significantly increased compared with the CONV mice (564 BPM vs. 392 BPM, respectively; P = 0.02).
Figure 7.
The effect of BAPN and exercise on systemic hemodynamics. There was no difference in systolic, diastolic, mean arterial blood pressure, or pulse pressure in BAPN-treated mice as compared with BAPN-untreated mice. Exercise significantly increased the heart rate of BAPN-EX mice versus CONV mice. *P < 0.05 by two-way ANOVA with Tukey’s multiple comparisons test. n = 20 CONV, 4 EX, 8 BAPN-CONV, 5 BAPN-EX. BAPN, β-aminopropionitrile; BAPN-CONV, conventional cage activity; BAPN-EX, forced treadmill exercise.
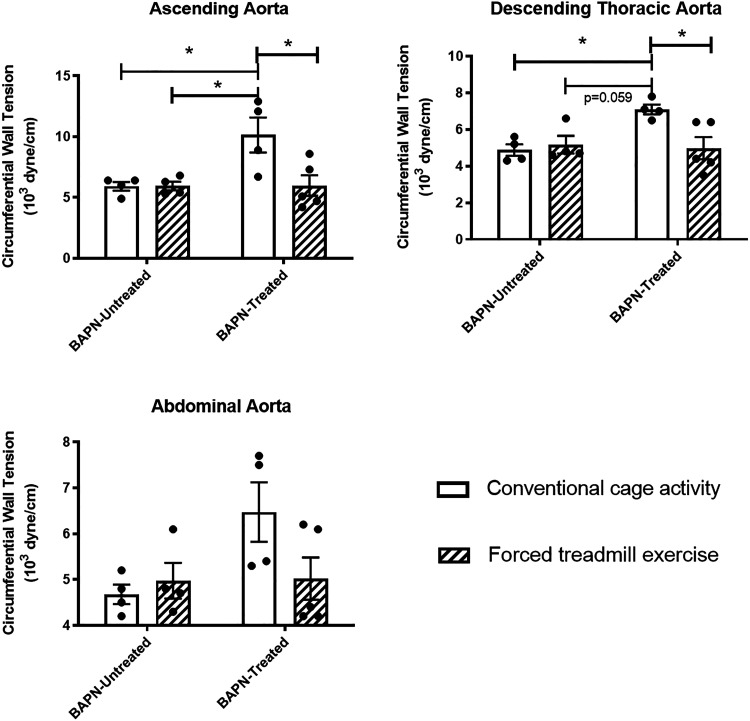
Exercise Reduces Circumferential Wall Tension
The circumferential wall tension was calculated in mice treated or untreated with BAPN with and without exercise (Fig. 8). There was a significant increase in the CWT of the ascending (P = 0.02) and descending thoracic aorta (P = 0.03) in BAPN-CONV animals compared with CONV animals. There was a significant reduction in the CWT at the end of the exercise protocol in BAPN-EX mice compared with BAPN-CONV mice in the ascending aorta (6.0 × 103 vs. 10.2 × 103 dyn/cm, respectively; P = 0.02) and in the descending thoracic aorta (5.0 × 103 vs. 7.1 × 103 dyn/cm, respectively; P = 0.03). There was no significant difference in the CWT of the abdominal aorta in BAPN-CONV mice versus CONV mice or in BAPN-EX mice compared with BAPN-CONV mice (P = 0.15).
Figure 8.
Exercise reduces circumferential wall tension in BAPN-treated mice. CWT in the ascending aorta and descending thoracic aorta was significantly reduced in BAPN-treated mice that underwent forced treadmill exercise. CWT was calculated from measured mean arterial pressures and lumen diameters on micro-CT. *P < 0.05 by two-way ANOVA with Tukey’s multiple comparisons test. n = 4 CONV, 4 EX, 4 BAPN-CONV, 5 BAPN-EX. BAPN, β-aminopropionitrile; BAPN-CONV, conventional cage activity; BAPN-EX, forced treadmill exercise; CWT, circumferential wall tension; micro-CT, microcomputed tomography.
Effects of BAPN Treatment and Exercise on Gene Expression in the Aortic Wall
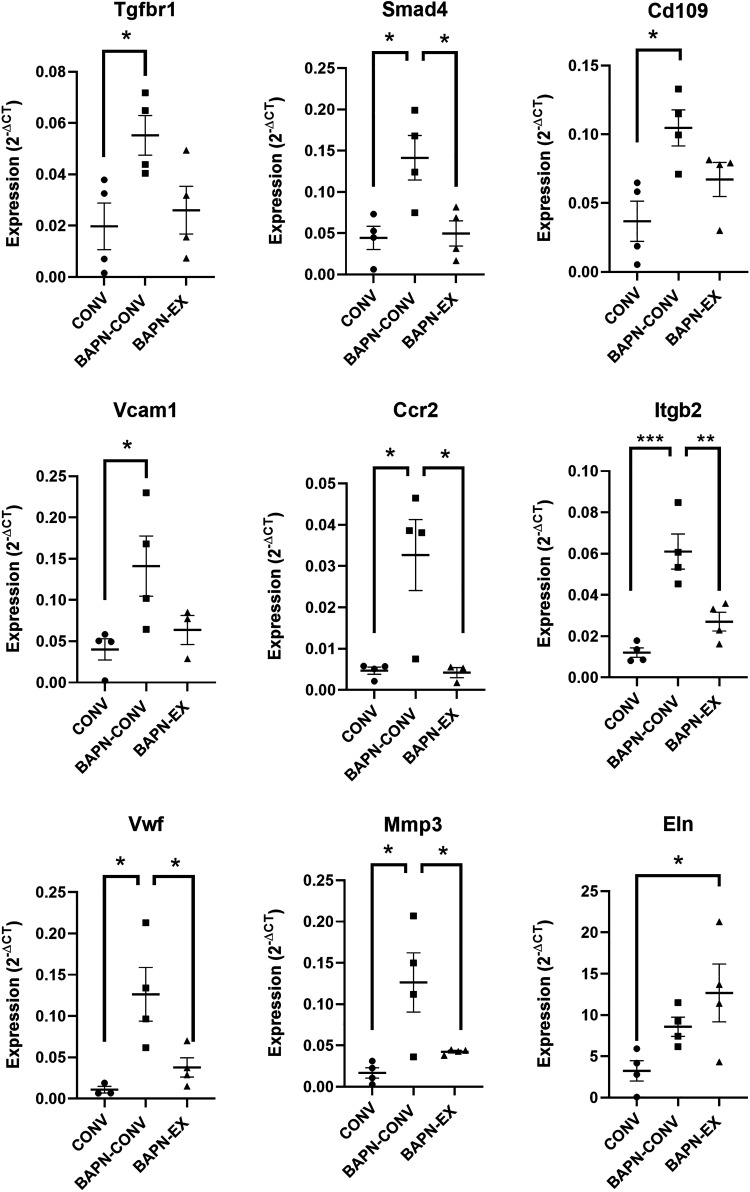
We next aimed to identify genes that were differentially expressed in BAPN-treated mice with and without exercise (Fig. 9 and Table 2). We examined mRNA abundance by qRT-PCR of selected genes involved in biological pathways involved in vascular diseases. Of the 92 gene array, 74 transcripts were detected. These analyses revealed BAPN treatment in unexercised mice significantly increased expression most notably of TGF-β pathway-related genes Cd109, Smad4, and Tgfβr1; inflammation-related genes Vcam1, Bcl2a1, Ccr2, Pparg, Il1r1, Il1r1, Itgb2, and Itgax; and vascular injury- and response-related genes Mmp3, Fn1, and Vwf (Fig. 9). Expression of these genes in BAPN-EX mice was either significantly reduced compared with BAPN-CONV mice or not significantly different than CONV controls. Elastin expression was significantly increased in BAPN-EX mice compared with CONV controls (P = 0.038) (Fig. 9).
Figure 9.
Differential gene expression in the aorta after BAPN treatment with and without exercise. Expression level (2−ΔCT) of selected genes involved in TGFβ pathway (top row), inflammation (middle row), and vascular injury and response (bottom row) related genes in CONV, BAPN-CONV, and BAPN-EX in 10–11-wk-old mice quantified by qRT-PCR. Gene expression is analyzed with one-way ANOVA with post hoc Tukey’s tests for multiple comparisons and is normalized to GAPDH. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. n = 4 biological replicates per group. Extreme outliers, missing data, and unamplified transcripts are excluded. BAPN, β-aminopropionitrile; BAPN-CONV, conventional cage activity; BAPN-EX, forced treadmill exercise.
Table 2.
Quantitative RT-PCR of AscAo using a customized vascular disease array.
| Gene | BAPN-CONV vs. CONVa | BAPN-EX vs. CONVa | BAPN-EX vs. BAPN-CONVa | P Valueb |
|---|---|---|---|---|
| Abca1 | 3.17 | −1.14 | −3.62 | 0.1002 |
| Ace | 3.59 | 1.40 | −2.56 | 0.0806 |
| Acta2 | 3.68 | 2.05 | −1.80 | 0.3080 |
| Apoe | 2.30 | 2.08 | −1.11 | 0.3830 |
| Bax | 2.02 | 2.73 | 1.35 | 0.1528 |
| Bcl2 | 1.92 | 2.06 | 1.07 | 0.3623 |
| Bcl2a1 | 7.53* | 1.87 | −4.02* | 0.0176 |
| Bcl2l1 | −1.31 | 1.29 | 1.69 | 0.5864 |
| Bid | −1.06 | 2.04 | 2.17 | 0.3367 |
| Birc3 | 2.09 | 1.08 | −1.94 | 0.1432 |
| Ccl2 | 4.00 | 5.29 | 1.32 | 0.4246 |
| Ccl5 | 1.89 | 1.75 | −1.08 | 0.5319 |
| Ccr1 | 4.76 | 2.90 | −1.64 | 0.4860 |
| Ccr2 | 6.14* | 1.31 | −4.68* | 0.0091 |
| Cd109 | 4.13* | 2.52 | −1.64 | 0.0184 |
| Cd44 | 2.36 | 1.90 | −1.24 | 0.1667 |
| Cdh5 | 2.17 | 1.19 | −1.83 | 0.3817 |
| Cflar | 2.45 | 1.70 | −1.44 | 0.5667 |
| Col1a2 | 2.94 | 1.55 | −1.89 | 0.1415 |
| Col3a1 | 2.78 | 1.56 | −1.78 | 0.2407 |
| Ctgf | 2.80 | 4.31 | 1.54 | 0.1009 |
| Cxcl1 | 3.56 | 6.46 | 1.82 | 0.3241 |
| Eln | 5.74 | 7.52* | 1.31 | 0.0454 |
| Fas | 1.86 | 2.94 | 1.58 | 0.2147 |
| Fgf2 | 2.28 | 7.81 | 3.43 | 0.0839 |
| Fn1 | 6.08* | 3.48 | −1.75 | 0.0018 |
| Hbegf | 2.41 | 2.65 | 1.10 | 0.2042 |
| Icam1 | 2.78 | 3.64 | 1.31 | 0.1823 |
| Il1b | 4.51 | 3.47 | −1.30 | 0.4836 |
| Il1r1 | 3.62* | 2.71 | −1.34 | 0.0460 |
| Il1r2 | 3.19 | 2.41 | −1.32 | 0.0569 |
| Itga2 | 2.99 | 2.19 | −1.37 | 0.1133 |
| Itga5 | 2.18 | 2.80 | 1.28 | 0.2873 |
| Itgax | 4.93* | 3.34 | −1.13 | 0.0464 |
| Itgb2 | 5.20* | 2.26 | −2.31* | 0.0006 |
| Klf2 | 4.59 | 2.58 | −1.78 | 0.1059 |
| Ldlr | −1.07 | 1.81 | 1.94 | 0.2661 |
| Lif | 1.75 | 1.63 | −1.07 | 0.5698 |
| Lox | 3.21 | 3.22 | 1.00 | 0.4708 |
| Lpl | 2.31 | 1.20 | −1.93 | 0.1168 |
| Ltbp2 | 3.54 | 5.58 | 1.58 | 0.2444 |
| Mmp2 | 5.00 | 4.79 | −1.04 | 0.3833 |
| Mmp3 | 8.80* | 3.52 | −2.50* | 0.0125 |
| Mmp9 | 1.94 | 1.01 | −1.92 | 0.6351 |
| Msr1 | 6.01 | 2.12 | −2.84 | 0.4889 |
| Nfkb1 | 2.77 | 1.00 | −2.75 | 0.3029 |
| Npy | 1.75 | 3.76 | 2.14 | 0.1863 |
| Pdgfa | 3.16 | 2.36 | −1.34 | 0.2446 |
| Pdgfb | 3.51 | 2.03 | −1.73 | 0.1578 |
| Pdgfrb | 3.89 | 3.07 | −1.27 | 0.3779 |
| Plin2 | 3.23 | 1.58 | −2.05 | 0.3364 |
| Ppara | 2.60 | −1.07 | −2.77 | 0.3303 |
| Ppard | 2.96 | 4.73 | 1.60 | 0.3926 |
| Pparg | 3.75* | 1.13 | −3.32* | 0.0286 |
| Sele | 10.23 | 6.49 | −1.58 | 0.1730 |
| Sell | 3.15 | 2.45 | −1.28 | 0.3232 |
| Selp | 4.98 | 1.16 | −4.28 | 0.3095 |
| Selplg | 5.36 | 2.40 | −2.24 | 0.3926 |
| Serpinb2 | −1.16 | −1.21 | −1.04 | 0.6956 |
| Serpine1 | 16.23 | 6.14 | −2.64 | 0.1291 |
| Smad3 | 3.25 | 4.51 | 1.39 | 0.3494 |
| Smad4 | 4.08* | 1.27 | −3.20* | 0.0113 |
| Spp1 | 29.11 | 51.46 | 1.77 | 0.2859 |
| Tgfb1 | 4.36 | 1.53 | −2.85 | 0.2354 |
| Tgfb2 | 4.79 | 1.23 | −3.88 | 0.1216 |
| Tgfbr1 | 4.96* | 1.91 | −2.60 | 0.0398 |
| Timp1 | 14.89 | 3.78 | −3.94 | 0.1009 |
| Tnc | 13.69 | 2.99 | −4.58 | 0.0893 |
| Tnfaip3 | 1.51 | 1.65 | 1.09 | 0.6457 |
| Tnfrsf11b | 5.12 | 4.50 | −1.14 | 0.4039 |
| Ubc | 1.22 | 2.07 | 1.70 | 0.5272 |
| Vcam1 | 5.21* | 3.65 | −1.43 | 0.0508 |
| Vegfa | 3.09 | 1.10 | −2.81 | 0.0766 |
| Vwf | 9.14* | 2.62 | −3.49* | 0.0149 |
Ninety-two genes related to vascular disease were analyzed by (TaqMan Gene Expression Assays, Applied Biosystems, Foster, CA) in 10-wk-old mice. Seventy-four transcripts were identified. Gapdh-normalized gene expression (2−ΔCT) is analyzed with one-way ANOVA with post hoc Tukey’s tests for multiple comparisons. The fold-change (2−ΔΔCT) versus comparison groups is presented. n = 4 mice per group. Extreme outliers, missing data, and unamplified transcripts are excluded. AscAo, ascending aorta; BAPN, β-aminopropionitrile; BAPN-CONV, conventional cage activity; BAPN-EX, forced treadmill exercise.
Fold-change (2−ΔΔCT) versus comparative group.
P value of gene expression (2−ΔCT) among three treatment groups determined by ANOVA.
*P value of gene expression (2−ΔCT) versus comparative group ≤0.05 by Tukey’s post test.
DISCUSSION
Our studies investigated how moderate aerobic exercise modulates the aortopathy caused by lysyl oxidase inhibition in mice using a well-established model of TAAD. The consequences of LOX inhibition with BAPN treatment were evidenced by significant elastin fragmentation, aortic wall remodeling, and identification of abnormal cell–matrix interactions, which recapitulate human disease. The mortality rate, aneurysm incidence, and histologic changes associated with BAPN treatment reported by our group are consistent with what others have published using this model (31, 33, 43–46). We demonstrate that moderate aerobic exercise dramatically reduces the incidence of thoracic aortic dissection and aneurysm, pathologic aortic wall remodeling, and associated aortic rupture and mortality.
The survival benefit of exercise in these studies is especially notable. BAPN-treated mice with conventional cage activity demonstrated significant mortality (23.5% overall), primarily from aortic rupture. This likely underestimates the true mortality in this model, as a number of mice were euthanized for experiments or other purposes before they were fully aged. Regardless, most remarkably, mice that underwent a forced treadmill exercise regimen initiated simultaneously with BAPN treatment demonstrated a mortality of 0%. A statistically significant and robust survival advantage from exercise was demonstrated by the survival analysis. Our results demonstrate that moderate aerobic exercise also reduces circumferential wall tension in mice treated with BAPN, which is consistent with the lower rupture rates observed in exercised mice. This finding was not the result of blood pressure changes, but more likely the result of aortic dilation. The potential translational benefit of this observation strongly argues for further study of the mechanism of this effect. Identification of biomarkers predictive of a beneficial response to exercise could provide insight into which patients would most benefit from cardiac rehabilitation after presentation with TAAD.
As noted above, we did not demonstrate any significantly deleterious chronic effects in the systemic hemodynamics associated with exercise. While the tail-cuff method has the known limitation of providing some degree of variability in blood pressure measurements, we demonstrate that mice undergoing a forced exercise regimen had similar blood pressures, pulse pressures, and mean arterial pressures compared with those that did not. There was a curious increase in the heart rate of exercised mice treated with BAPN compared with CONV controls, though the potential physiological significance of this is unclear. Acute changes in heart rate and blood pressures during exercise in our studies were not measured. Previous work in our laboratory has suggested that catheterization and ligation of carotid arteries in other mouse models of aortopathy can have artifactual effects on blood pressure and vascular remodeling. As such, intravascular measurements at the time of exercise were not performed. Regardless, since BAPN-EX mice demonstrated better outcomes than their unexercised BAPN-treated counterparts, the chronic changes in heart rate or any unmeasured acute changes in systemic hemodynamics during exercise were likely not harmful.
There are few studies that have evaluated the effect of exercise on aortic dissection, though evidence is accumulating for its benefits in aortic disease overall. Bailey et al. (47) recently published studies, which suggested that exercise affects endothelial function in patients with aortic aneurysms by increasing flow-mediated dilation at the level of the brachial artery. Two recently published studies demonstrate a benefit in a mouse model of Marfan disease, with the timing of exercise initiation during pathogenesis affecting the degree of benefit. Mas-Stachurska et al. (11) demonstrated a delayed exercise regimen initiated at 4 mo of age was associated with significantly less aortic root dilatation compared with sedentary animals, though there was no difference in aortic root stiffness, tunica media fibrosis, or elastic lamina ruptures. This contrasts with studies by Gibson et al. (12), which demonstrated that exercise initiated earlier (at 4-wk of age) in Marfan mice led to significant improvements in structural and functional aortic properties, including improved elastin architecture, increased aortic compliance to wall stretch, improved vSMC contractility, and reduced aortic diameter. The findings of the latter study are consistent with the results of our studies in a nongenetic TAAD mouse model, demonstrating improved elastin architecture with the early initiation of an exercise regimen at the time of lysyl oxidase inhibition with BAPN. Taken together, ours and the above-described studies suggest a beneficial effect of moderate aerobic exercise in TAAD when introduced early in the pathogenesis.
It is unclear how exercise prevents the phenotype associated with LOX inhibition. We do not demonstrate an associated increase in Lox gene expression in the aortic wall to suggest an increased abundance of LOX. Whether exercise augments LOX activity, inhibits BAPN activity, or directly influences cross linking of elastin and collagen and other LOX functions is unknown. There is a compensatory increase in elastin expression in our studies in both the BAPN-CONV and BAPN-EX groups, though only significantly so in the BAPN-EX group. Thus, one explanation for the observed effect is that a reparative mechanism in response to abnormal elastogenesis caused by lysyl oxidase inhibition may be augmented by a forced and supervised exercise regimen. Whether this directly contributes to the protective effects of exercise in the formation of TAAD remains to be explored. Additionally, it is possible that exercise compensates for inhibited LOX activity by directly impacting cross linking events normally catalyzed by LOX. Exercise has been shown to promote collagen cross linking in bones of mice, increase mature cross linking even with BAPN treatment, and prevent BAPN-induced morphologic changes of bone collagen (48, 49). Studies in murine tendons suggest that exercise has the effect of promoting repair of damaged extracellular matrix with aging, associated in part with increased Lox expression (50).
In addition to augmented elastin expression in our studies, several genes upregulated with LOX inhibition suggest ongoing vascular injury and reparative responses. Most of these genes were normalized in BAPN-treated mice that underwent an exercise regimen. Among these genes are Mmp3, Fn1, and Vwf, suggesting ongoing matrix degradation from proteolysis and a coagulation injury response that is induced with LOX inhibition. Interestingly, we have consistently shown no BAPN or exercise-induced changes in Mmp2 or Mmp9 expression, contrasting with some findings in other models of aortic disease (12, 51, 52). Some evidence in the literature in fact suggests the opposite interaction, with LOX inhibition preventing pathologic increases in MMP levels (53). While the link between LOX activity and MMP expression or activity is not fully elucidated in our studies, we provide histologic evidence of significant degradative processes and increased Mmp3 gene expression with LOX inhibition, which is prevented with exercise.
We did examine if LOX inhibition directly impacted inflammatory gene regulation in the aortic wall as either a causative or associated factor in the pathogenesis of TAAD in this model. Gene expression patterns in the aortic wall do suggest differential levels of inflammation in the BAPN-EX mice compared with BAPN-CONV mice. A number of genes involved in inflammation including Vcam1, Bcl2a1, Ccr2, Pparg, Il1r1, Il1r1, Itgb2, and Itgax were significantly upregulated in the aortic wall of BAPN-CONV mice at the 7-wk time point, but not in BAPN-EX mice. Prior published studies have shown that LOX inhibition with BAPN treatment leads to the accumulation of inflammatory mediators in the aortic wall, including IL-1β, IL-6, and the recruitment of neutrophils and macrophages (29, 33, 37, 54). Additionally, LOX has been shown to suppress monocyte chemoattractant protein-1 (MCP-1) (55). These results are consistent with prior published data in aortic aneurysms establishing a link to aortic disease and inflammation (56, 57). Thus, the identification of activated inflammatory genes in BAPN-CONV mice is consistent with the published literature, suggesting activated inflammation with BAPN treatment. Interestingly, both mouse and human studies have found exercise regimens attenuate inflammatory markers including CRP, TNF, NFκB, IL-1β, and IL-6 (58, 59), suggesting that exercise has an anti-inflammatory effect that may be beneficial in patients with vascular disease (47). This is consistent with the findings of our studies demonstrating reduced inflammatory gene expression in BAPN-EX mice. The role of inflammation in the pathogenesis of TAAD, and its possible mitigation with aerobic exercise, is worthy of further investigation.
The overexpression of TGF-β pathway genes Tgfβr1 and Smad4 as well as the overexpression of the TGF pathway negative regulator Cd109 with BAPN treatment (60) are highly suggestive of TGF-β pathway dysregulation with LOX inhibition. Interestingly, the expression of these genes is normalized with exercise. TGF-β signaling has a significant role in regulating vSMC phenotype and is required for vSMC differentiation. While global loss of TGF-β signaling, or selective ablation in vSMC during development, leads to severe vascular defects and embryonic lethality (61–64), the role of TGF-β signaling postnatally on aortic disease appears to be more complicated. Conditional deletion of Tgfbr2 (65) or Smad4 (66) in vSMC postnatally has been shown to result in TAAD in animal models, suggesting a protective role for basal TGF-β signaling in aneurysm and dissection. However, excess TGF-β signaling seems to promote pathologic features and aneurysm formation in fibrillin-1 deficient and Loeys–Dietz syndrome mice, possibly through noncanonical signaling pathways (67–70). Additionally, missense Smad4 variants have been identified in patients with sporadic, nonfamilial thoracic aortic dissection (71, 72), and have been associated with increased inflammation and activated TGF-β signaling (57). Taken together, these studies reveal that tight regulation of TGF-β signaling is essential for the development and maintenance of the vessel wall and that postnatal dysregulation is associated with aortic disease. Whether the TGF-β pathway is functioning as a causative molecular mediator of BAPN-induced aortopathy or a compensatory reparative mechanism is unclear. In addition, interactions between LOX and the TGF-β pathway, and the effect and mechanisms of exercise in modulating these pathways are areas in need of further study. Whole transcriptome analyses of animals treated or untreated with BAPN with or without exercise, and experiments studying the effect of TGF-β inhibition in this model are planned in our laboratory in the near future to address these questions.
Because exercise was introduced simultaneously to the initiation of BAPN treatment in our studies, one limitation of our findings is that they may translate most directly to a preventative role for aerobic exercise in patients predisposed to TAAD. Whether exercise initiated after presentation of acute dissection is protective is not directly addressed by this model. Experiments evaluating the benefits of late initiation of exercise and experiments testing the effects of exercise in an acute dissection model are planned in our laboratory. It is important to note, however, that even late initiation of exercise does appear to limit disease progression in animal models of aortic disease, as suggested by Mas-Stachurska et al. (11). This is consistent with findings in human studies, which demonstrate a functional benefit from exercise initiated well into adulthood for patients with abdominal aortic aneurysm (13–15, 73). A second limitation of these studies is that the great majority of the most severe pathology in the BAPN model is found in the ascending aorta. While the experiments described in this manuscript most directly address the effect of exercise in nonoperatively managed dissections, it should be noted that Type A dissections (those involving the ascending aorta and aortic arch) in humans are almost never treated nonoperatively. However, we believe the model can potentially translate to nonoperatively managed Type B dissections (those not involving the ascending aorta and arch) since many of the pathologic processes described in this model are universal to the pathogenesis in all aortic diseases.
In summary, we have demonstrated a robust benefit from moderate aerobic exercise in reducing mortality and pathologic aortic remodeling in a TAAD mouse model. Further investigation will be needed to understand the role of TGF-β signaling and inflammation in mediating this effect. We believe that elucidation of the basic biologic mechanisms that are affected by LOX inhibition with BAPN, and the modulatory effects of aerobic exercise on these mechanisms, may provide both for a window into the fundamental understanding of TAAD and for a novel therapeutic approach for patients with TAAD.
GRANTS
This work was supported by National Institutes of Health Grants K08 HL146893 and SVS Foundation/American College of Surgeons Mentored Clinical Scientist Research Career Development Award (to A.A.U.), F32 HL143910 (to B.O.A.), F30 HL145952 (to A.L.A.), T32 HL007698-18 (to D.K.S.), R35 HL135743 (to D.K.S.), and American Heart Association Grant AHA 15SDG24470170 (to S.C.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.O.A., J.Z., S.C.M., V.L.G., B.K.L., D.K.S., and A.A.U. conceived and designed research; B.O.A., J.Z., R.G., A.L.A., and A.A.U. performed experiments; B.O.A., J.Z., S.C.M., D.K.S., and A.A.U. analyzed data; B.O.A., J.Z., S.C.M., D.K.S., and A.A.U. interpreted results of experiments; B.O.A., J.Z., S.C.M., and A.A.U. prepared figures; B.O.A., J.Z., S.C.M., R.G., A.L.A., V.L.G., B.K.L., D.K.S., and A.A.U. drafted manuscript; B.O.A., J.Z., S.C.M., R.G., A.L.A., V.L.G., B.K.L., D.K.S., and A.A.U. edited and revised manuscript; B.O.A., J.Z., S.C.M., R.G., A.L.A., V.L.G., B.K.L., D.K.S., and A.A.U. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the University of Maryland, Baltimore; Genomic Core Facility; the Translational Genomics Laboratory; the Electron Microscopy Core Imaging Facility; and the Department of Diagnostic Radiology and Nuclear Medicine for expertise.
REFERENCES
- 1.Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest 117: 1271–1278, 2000. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- 2.Kochanek KD, Murphy SL, Xu J, Arias E. Deaths: final data for 2017. Natl Vital Stat Rep 68: 1–77, 2019. [PubMed] [Google Scholar]
- 3.Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Fattori R, Ince H. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 6: 407–416, 2013. doi: 10.1161/circinterventions.113.000463. [DOI] [PubMed] [Google Scholar]
- 4.Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Molecular mechanisms of thoracic aortic dissection. J Surg Res 184: 907–924, 2013. doi: 10.1016/j.jss.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoff E, Eagle T, Pyeritz RE, Ehrlich M, Voehringer M, Bossone E, Hutchison S, Peterson MD, Suzuki T, Greason K, Forteza A, Montgomery DG, Isselbacher EM, Nienaber CA, Eagle KA. Pulse pressure and type A acute aortic dissection in-hospital outcomes (from the International Registry of Acute Aortic Dissection). Am J Cardiol 113: 1255–1259, 2014. doi: 10.1016/j.amjcard.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg 37: 724–732, 2003. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- 7.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 286: 1218–1227, 2001. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 8.Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011 Jul 6;(7):CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piepoli MF, Davos C, Francis DP, Coats AJS; ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 328: 189, 2004. doi: 10.1136/bmj.37938.645220.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott MM. Exercise rehabilitation for peripheral artery disease: a review. J Cardiopulm Rehabil Prev 38: 63–69, 2018. [Erratum in J Cardiopulm Rehabil Prev 38: 347, 2018]doi: 10.1097/HCR.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mas-Stachurska A, Siegert AM, Batlle M, Gorbenko Del Blanco D, Meirelles T, Rubies C, Bonorino F, Serra-Peinado C, Bijnens B, Baudin J, Sitges M, Mont L, Guasch E, Egea G. Cardiovascular benefits of moderate exercise training in Marfan syndrome: insights from an animal model. J Am Heart Assoc 6: e006438, 2017. doi: 10.1161/JAHA.117.006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson C, Nielsen C, Alex R, Cooper K, Farney M, Gaufin D, Cui JZ, van Breemen C, Broderick TL, Vallejo-Elias J, Esfandiarei M. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J Appl Physiol (1985 ) 123: 147–160, 2017. doi: 10.1152/japplphysiol.00132.2017. [DOI] [PubMed] [Google Scholar]
- 13.Myers J, McElrath M, Jaffe A, Smith K, Fonda H, Vu A, Hill B, Dalman R. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc 46: 2–9, 2014. doi: 10.1249/MSS.0b013e3182a088b8. [DOI] [PubMed] [Google Scholar]
- 14.Kothmann E, Batterham AM, Owen SJ, Turley AJ, Cheesman M, Parry A, Danjoux G. Effect of short-term exercise training on aerobic fitness in patients with abdominal aortic aneurysms: a pilot study. Br J Anaesth 103: 505–510, 2009. doi: 10.1093/bja/aep205. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama A, Morita H, Nagayama M, Hoshina K, Uemura Y, Tomoike H, Komuro I. Cardiac rehabilitation protects against the expansion of abdominal aortic aneurysm. J Am Heart Assoc 7: e007959, 2018. doi: 10.1161/JAHA.117.007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corone S, Iliou M-C, Pierre B, Feige J-M, Odjinkem D, Farrokhi T, Bechraoui F, Hardy S, Meurin P; Cardiac Rehabilitation working Group of the French Society of Cardiology. French registry of cases of type I acute aortic dissection admitted to a cardiac rehabilitation center after surgery. Eur J Cardiovasc Prev Rehabil 16: 91–95, 2009. doi: 10.1097/HJR.0b013e32831fd6c8. [DOI] [PubMed] [Google Scholar]
- 17.Fuglsang S, Heiberg J, Hjortdal VE, Laustsen S. Exercise-based cardiac rehabilitation in surgically treated type-A aortic dissection patients. Scand Cardiovasc J 51: 99–105, 2017. doi: 10.1080/14017431.2016.1257149. [DOI] [PubMed] [Google Scholar]
- 18.Chanoki M, Ishii M, Kobayashi H, Fushida H, Yashiro N, Hamada T, Ooshima A. Increased expression of lysyl oxidase in skin with scleroderma. Br J Dermatol 133: 710–715, 1995. doi: 10.1111/j.1365-2133.1995.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilad GM, Kagan HM, Gilad VH. Evidence for increased lysyl oxidase, the extracellular matrix-forming enzyme, in Alzheimer's disease brain. Neurosci Lett 376: 210–214, 2005. doi: 10.1016/j.neulet.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 20.Guo D-C, Regalado ES, Gong L, Duan X, Santos-Cortez RL, Arnaud P, Ren Z, Cai B, Hostetler EM, Moran R, Liang D, Estrera A, Safi HJ, Leal SM, Bamshad MJ, Shendure J, Nickerson DA, Jondeau G, Boileau C, Milewicz DM; University of Washington Center for Mendelian Genomics. LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ Res 118: 928–934, 2016. doi: 10.1161/CIRCRESAHA.115.307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee VS, Halabi CM, Hoffman EP, Carmichael N, Leshchiner I, Lian CG, Bierhals AJ, Vuzman D, Mecham RP, Frank NY, Stitziel NO; Brigham Genomic Medicine. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci USA 113: 8759–8764, 2016. doi: 10.1073/pnas.1601442113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayorca-Guiliani A, Erler JT. The potential for targeting extracellular LOX proteins in human malignancy. Onco Targets Ther 6: 1729–1735, 2013. doi: 10.2147/OTT.S38110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remus EW, O’Donnell RE Jr, Rafferty K, Weiss D, Joseph G, Csiszar K, Fong SFT, Taylor WR. The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol 303: H1067–H1075, 2012. doi: 10.1152/ajpheart.00217.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staiculescu MC, Kim J, Mecham RP, Wagenseil J. Mechanical behavior and matrisome gene expression in aneurysm-prone thoracic aorta of newborn lysyl oxidase knockout mice. Am J Physiol Heart Circ Physiol 313: H446–H456, 2017. doi: 10.1152/ajpheart.00712.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509, 2002. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 26.Bruel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis 140: 135–145, 1998. doi: 10.1016/s0021-9150(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 27.Eberson LS, Sanchez PA, Majeed BA, Tawinwung S, Secomb TW, Larson DF. Effect of lysyl oxidase inhibition on angiotensin II-induced arterial hypertension, remodeling, and stiffness. PLoS One 10: e0124013, 2015. doi: 10.1371/journal.pone.0124013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huffman MD, Curci JA, Moore G, Kerns DB, Starcher BC, Thompson RW. Functional importance of connective tissue repair during the development of experimental abdominal aortic aneurysms. Surgery 128: 429–438, 2000. doi: 10.1067/msy.2000.107379. [DOI] [PubMed] [Google Scholar]
- 29.Jia L-X, Zhang W-M, Zhang H-J, Li T-T, Wang Y-L, Qin Y-W, Gu H, Du J. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J Pathol 236: 373–383, 2015. doi: 10.1002/path.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar D, Trent MB, Boor PJ. Allylamine and β-aminopropionitrile induced aortic medial necrosis: mechanisms of synergism. Toxicology 125: 107–115, 1998. doi: 10.1016/s0300-483x(97)00168-6. [DOI] [PubMed] [Google Scholar]
- 31.Li J-S, Li H-Y, Wang L, Zhang L, Jing Z-P. Comparison of β-aminopropionitrile-induced aortic dissection model in rats by different administration and dosage. Vascular 21: 287–292, 2013. doi: 10.1177/1708538113478741. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Revelles S, Garcia-Redondo AB, Avendano MS, Varona S, Palao T, Orriols M, Roque FR, Fortuno A, Touyz RM, Martinez-Gonzalez J, Salaices M, Rodriguez C, Briones AM. Lysyl oxidase induces vascular oxidative stress and contributes to arterial stiffness and abnormal elastin structure in hypertension: role of p38MAPK. Antioxid Redox Signal 27: 379–397, 2017. doi: 10.1089/ars.2016.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren W, Liu Y, Wang X, Jia L, Piao C, Lan F, Du J. β-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sc Rep 6: 28149, 2016. doi: 10.1038/srep28149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou B, Li W, Zhao G, Yu B, Ma B, Liu Z, Xie N, Fu Y, Gong Z, Dai R, Zhang X, Kong W. Rapamycin prevents thoracic aortic aneurysm and dissection in mice. J Vasc Surg 69: 921–932.e3, 2019. doi: 10.1016/j.jvs.2018.05.246. [DOI] [PubMed] [Google Scholar]
- 35.Jordao MT, Ladd FV, Coppi AA, Chopard RP, Michelini LC. Exercise training restores hypertension-induced changes in the elastic tissue of the thoracic aorta. J Vasc Res 48: 513–524, 2011. doi: 10.1159/000329590. [DOI] [PubMed] [Google Scholar]
- 36.Les AS, Shadden SC, Figueroa CA, Park JM, Tedesco MM, Herfkens RJ, Dalman RL, Taylor CA. Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann Biomed Eng 38: 1288–1313, 2010. doi: 10.1007/s10439-010-9949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N, Okada Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation 126: 3070–3080, 2012. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 38.Poole DC, Copp SW, Colburn TD, Craig JC, Allen DL, Sturek M, O’Leary DS, Zucker IH, Musch TI. Guidelines for animal exercise and training protocols for cardiovascular studies. Am J Physiol Heart Circ Physiol 318: H1100–H1138, 2020. doi: 10.1152/ajpheart.00697.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aicher BO, Mukhopadhyay S, Lu X, Muratoglu SC, Strickland DK, Ucuzian AA. Quantitative micro-CT analysis of aortopathy in a mouse model of β-aminopropionitrile-induced aortic aneurysm and dissection. J Vis Exp, 57589, 2018. doi: 10.3791/57589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE; Subcommittee of Professional and Public Education of the American Heart Association. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45: 299–310, 2005. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- 41.Prado CM, Rossi MA. Circumferential wall tension due to hypertension plays a pivotal role in aorta remodelling. Int J Exp Pathol 87: 425–436, 2006. doi: 10.1111/j.1365-2613.2006.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Lv X, Hu Y, Chen X, Chen X, Chen L, Lin Y, Hou Y. Establishment and effect evaluation of an aortic dissection model induced by different doses of β-aminopropionitrile in rats. Vascular 1708538120984056, 2020. Dec 23 (Epub ahead of print): 1708538120984056. doi: 10.1177/1708538120984056. [DOI] [PubMed] [Google Scholar]
- 44.Nakashima Y, Sueishi K. Alteration of elastic architecture in the lathyritic rat aorta implies the pathogenesis of aortic dissecting aneurysm. Am J Pathol 140: 959–969, 1992. [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y-Y, Li L-Y, Jiao X-L, Jia L-X, Zhang X-P, Wang Y-L, Yang S, Li J, Du J, Wei Y-X, Qin Y-W. Intermittent hypoxia alleviates β-aminopropionitrile monofumarate induced thoracic aortic dissection in C57BL/6 mice. Eur J Vasc Endovasc Surg 59: 1000–1010, 2020. doi: 10.1016/j.ejvs.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Pei Y-F, Wang L, Liao M-F, Lu Q-S, Zhuang Y-F, Zhang S-M, Jing Z-P. Dramatic decrease of aortic longitudinal elastic strength in a rat model of aortic dissection. Ann Vasc Surg 26: 996–1001, 2012. doi: 10.1016/j.avsg.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Bailey TG, Perissiou M, Windsor MT, Schulze K, Nam M, Magee R, Leicht AS, Green DJ, Greaves K, Golledge J, Askew CD. Effects of acute exercise on endothelial function in patients with abdominal aortic aneurysm. Am J Physiol Heart Circ Physiol 314: H19–H30, 2018. doi: 10.1152/ajpheart.00344.2017. [DOI] [PubMed] [Google Scholar]
- 48.Hammond MA, Wallace JM. Exercise prevents β-aminopropionitrile-induced morphological changes to type I collagen in murine bone. Bonekey Rep 4: 645, 2015. doi: 10.1038/bonekey.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNerny EMB, Gardinier JD, Kohn DH. Exercise increases pyridinoline cross-linking and counters the mechanical effects of concurrent lathyrogenic treatment. Bone 81: 327–337, 2015. doi: 10.1016/j.bone.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood LK, Brooks SV. Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. J Orthop Res 34: 346–353, 2016. doi: 10.1002/jor.22824. [DOI] [PubMed] [Google Scholar]
- 51.Maguire EM, Pearce SWA, Xiao R, Oo AY, Xiao Q. Matrix metalloproteinase in abdominal aortic aneurysm and aortic dissection. Pharmaceuticals (Basel) 12: 118, 2019. doi: 10.3390/ph12030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Windsor MT, Bailey TG, Perissiou M, Greaves K, Jha P, Leicht AS, Russell FD, Golledge J, Askew CD. Acute inflammatory responses to exercise in patients with abdominal aortic aneurysm. Med Sci Sports Exerc 50: 649–658, 2018. doi: 10.1249/MSS.0000000000001501. [DOI] [PubMed] [Google Scholar]
- 53.El Hajj EC, El Hajj MC, Ninh VK, Gardner JD. Inhibitor of lysyl oxidase improves cardiac function and the collagen/MMP profile in response to volume overload. Am J Physiol Heart Circ Physiol 315: H463–H473, 2018. doi: 10.1152/ajpheart.00086.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, Yamamoto T, Ito K, Yan X, Shirakawa K, Shimizu-Hirota R, Yamada Y, Ueha S, Shinmura K, Okada Y, Fukuda K, Sano M. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res 116: 612–623, 2015. doi: 10.1161/CIRCRESAHA.116.304918. [DOI] [PubMed] [Google Scholar]
- 55.Onoda M, Yoshimura K, Aoki H, Ikeda Y, Morikage N, Furutani A, Matsuzaki M, Hamano K. Lysyl oxidase resolves inflammation by reducing monocyte chemoattractant protein-1 in abdominal aortic aneurysm. Atherosclerosis 208: 366–369, 2010. doi: 10.1016/j.atherosclerosis.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 56.Huang G, Wang A, Li X, Long M, Du Z, Hu C, Luo C, Wu Z, Tang L. Change in high-sensitive C-reactive protein during abdominal aortic aneurysm formation. J Hypertens 27: 1829–1837, 2009. doi: 10.1097/HJH.0b013e32832db36b. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Yin P, Chen Y-H, Yu Y-S, Ye W-X, Huang H-Y, Ji Z-C, Shen Z-Y. A functional variant of SMAD4 enhances macrophage recruitment and inflammatory response via TGF-β signal activation in Thoracic aortic aneurysm and dissection. Aging (Albany NY) 10: 3683–3701, 2018. [Erratum in Aging (Albany NY) 11: 836, 2019]doi: 10.18632/aging.101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King DE, Carek P, Mainous AG 3rd, Pearson WS. Inflammatory markers and exercise: differences related to exercise type. Med Sci Sports Exerc 35: 575–581, 2003. doi: 10.1249/01.mss.0000058440.28108.cc. [DOI] [PubMed] [Google Scholar]
- 59.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301: H1025–H1032, 2011. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C, Hancock MA, Sehgal P, Zhou S, Reinhardt DP, Philip A. Soluble CD109 binds TGF-β and antagonizes TGF-β signalling and responses. Biochem J 473: 537–547, 2016. doi: 10.1042/BJ20141488. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho RL, Itoh F, Goumans MJ, Lebrin F, Kato M, Takahashi S, Ema M, Itoh S, van Rooijen M, Bertolino P, Ten Dijke P, Mummery CL. Compensatory signalling induced in the yolk sac vasculature by deletion of TGFβ receptors in mice. J Cell Sci 120: 4269–4277, 2007. doi: 10.1242/jcs.013169. [DOI] [PubMed] [Google Scholar]
- 62.Frutkin AD, Shi H, Otsuka G, Levéen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-Cre, not SMMHC-Cre. J Mol Cell Cardiol 41: 724–731, 2006. doi: 10.1016/j.yjmcc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 63.Jaffe M, Sesti C, Washington IM, Du L, Dronadula N, Chin MT, Stolz DB, Davis EC, Dichek DA. Transforming growth factor-β signaling in myogenic cells regulates vascular morphogenesis, differentiation, and matrix synthesis. Arterioscler Thromb Vasc Biol 32: e1–e11, 2012. doi: 10.1161/ATVBAHA.111.238410. [DOI] [PubMed] [Google Scholar]
- 64.Langlois D, Hneino M, Bouazza L, Parlakian A, Sasaki T, Bricca G, Li JY. Conditional inactivation of TGF-β type II receptor in smooth muscle cells and epicardium causes lethal aortic and cardiac defects. Transgenic Res 19: 1069–1082, 2010. doi: 10.1007/s11248-010-9379-4. [DOI] [PubMed] [Google Scholar]
- 65.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest 124: 755–767, 2014. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang P, Hou S, Chen J, Zhang J, Lin F, Ju R, Cheng X, Ma X, Song Y, Zhang Y, Zhu M, Du J, Lan Y, Yang X. Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ Res 118: 388–399, 2016. doi: 10.1161/CIRCRESAHA.115.308040. [DOI] [PubMed] [Google Scholar]
- 67.Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, van Erp C, Gerber EE, Parker SJ, Sauls K, Judge DP, Cooke SK, Lindsay ME, Rouf R, Myers L, Ap Rhys CM, Kent KC, Norris RA, Huso DL, Dietz HC. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest 124: 448–460, 2014. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121, 2006. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332: 358–361, 2011. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 71.Duan XY, Guo DC, Regalado ES, Shen H, Coselli JS, Estrera AL, Safi HJ, Bamshad MJ, Nickerson DA, LeMaire SA, De Backer J, Milewicz DM; University of Washington Center for Mendelian Genomics. SMAD4 rare variants in individuals and families with thoracic aortic aneurysms and dissections. Eur J Hum Genet 27: 1054–1060, 2019. doi: 10.1038/s41431-019-0357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Huang H-Y, Bian G-L, Yu Y-S, Ye W-X, Hua F, Chen Y-H, Shen Z-Y. A functional variant of SMAD4 enhances thoracic aortic aneurysm and dissection risk through promoting smooth muscle cell apoptosis and proteoglycan degradation. EBioMedicine 21: 197–205, 2017. doi: 10.1016/j.ebiom.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honnesh S, Goosen L, Adams N, Forni L, Lavies N, Venn R. Effect of short-term exercise training on aerobic fitness in patients with abdominal aortic aneurysms. Br J Anaesth 104: 265–266, 2010. doi: 10.1093/bja/aep388. [DOI] [PubMed] [Google Scholar]