Abstract
Background
We have investigated the use of nebulized surfactant as a potential therapeutic option for the patients with coronavirus disease 2019 (COVID-19)-associated acute respiratory distress syndrome (ARDS) undergoing non-invasive ventilation.
Methods
The patients were divided into 2 groups: surfactant (n = 33) and control (n = 32). The subjects in the surfactant group received the inhaled surfactant at daily dose of 150–300 mg. The oxygenation parameters and several clinical outcomes were analyzed.
Results
On the 5 day of therapy, PaO2/FiO2 improved significantly in the surfactant group compared to the control group (184 (155–212) mmHg vs 150 (91–173) mmHg, p = 0.02). The inhaled surfactant significantly reduced the need for transfer of patients to intensive care units (24.2% vs 46.9%, p = 0.05) and invasive mechanical ventilation (18.2% vs 40.6%, p = 0.04). Even more, the nebulized surfactant shortened the length of non-invasive ventilation (7 (3–13) days vs 11 (5–22) days, p = 0.02) and time spent in hospital (18 (16–27) days vs 26 (21–31) days, p = 0.003) in patients suffering from COVID-19-linked ARDS.
Conclusions
Our preliminary data provided indications that inhaled surfactant therapy may represent a promising option for patients with COVID-19-associated ARDS. However, larger clinical trials are crucially needed.
Keywords: Inhalation, Surfactant, COVID-19, Acute respiratory distress syndrome
1. Introduction
The patients suffering from coronavirus disease 2019 (COVID-19) may develop acute respiratory distress syndrome (ARDS). COVID-19 appears due to the infection with the novel severe acute respiratory syndrome coronavirus (SARS-CoV-2). This virus preferentially molests the alveolar type II cells and subsequently causes apoptosis, cellular damage, and altered production of the pulmonary surfactant [1,2]. Dysregulated function of the surfactant may result in alveolar collapse and inflammation, augmented capillary permeability, edema, and microvascular thrombosis [2,3]. In the past, the treatment strategy using the exogenous surfactant did not show the full therapeutic efficacy in the context of adult ARDS [4]. Up to date, there are only few studies with a limited number of patients, which describe the potential of the treatment with exogenous surfactant in COVID-19-associated ARDS [[5], [6], [7]]. The surfactant was administered in intubated patients via endotracheal instillation or through the bronchoscope [[5], [6], [7]]. The results were promising and the application of exogenous surfactant provided an improvement in oxygenation, an increase in pulmonary static compliance, and a tendency to reduce the mortality rate [[5], [6], [7]].
In the present study, we hypothesized that early initiation of therapy with exogenous surfactant in patients with COVID-19-associated ARDS, before endotracheal intubation (ETI) and invasive mechanical ventilation (IMV) maybe even more effective. The objective of our study was to evaluate the feasibility, safety and effectiveness of nebulized surfactant therapy in patients with COVID-19-associated ARDS treated with non-invasive ventilation (NIV).
2. Methods
This prospective case-control study was conducted in COVID-19 care units of university-affiliated hospital (Sechenov University) between April 8 and November 12, 2020. We prospectively enrolled patients over 18 years old with laboratory-confirmed SARS-CoV-2 infection (real-time PCR) admitted to the general wards (outside intensive care units (ICU)). The study was approved by the University ethics committee (approval number 16–20), and written informed consent was obtained from all patients. Inclusion criteria were ARDS defined according to Berlin definition [8], arterial oxygen tension to inspired oxygen fraction ratio (PaO2/FiO2)<200 mmHg and treatment with mask NIV. The exclusion criteria were as follows: need for immediate ETI and unstable hemodynamics.
The primary NIV mode was continuous positive airway pressure (CPAP) that was administered with NIV ventilators. CPAP was initially set at 10cmH2O and then adjusted according to oxygen saturation (SpO2) and clinical tolerance. FiO2 was adjusted to maintain SpO2 during NIV. We used non-vented oronasal masks and the expiratory limb of the circuit was equipped with an antimicrobial filter.
All patients received the standard therapy including hydroxychloroquine (400 mg daily), azithromycin (500 mg daily), dexamethasone (6–12 mg daily), prophylactic enoxaparin (40 mg daily), and tocilizumab (8 mg/kg). Patients in the surfactant group were treated with standard therapy and inhaled surfactant. Surfactant (Surfactant-BL, Biosurf LLC, Russia) was administered at a daily dose of 75–150 mg b.i.d., for at least 5 days, by means of vibrating mesh nebulizer (Aerogen Solo, Aerogen Ltd, Ireland) positioned in the breathing circuit between the leak port and the mask.
All control patients had the same enrollment criteria described for the surfactant group, and the measured parameters were collected prospectively on the same data chart, according to a standardized treatment procedure. A matching control patient was selected for each patient treated with surfactant, according to the following criteria: age (within ±5 years); PaO2/FiO2 ratio on admission (within ±20 mmHg); and National Early Warning Score (NEWS)2 score on admission (within ±1 points).
The data were recorded at admission, and at days 3 and 5. We also analyzed the length of hospitalization and outcome of the disease, such as transfer to ICU, need for ETI and IMV, and 28-day mortality. Criteria for ETI and IMV were worsening respiratory failure, SpO2<88% without response to NIV, respiratory acidosis, hemodynamic instability, and exhaustion.
Data are presented as absolute values (%), median (interquartile range) or mean ± SEM. For statistical analyses, we used the following tests: Student's t-test or Mann–Whitney U test, Fisher's exact test or 2-way ANOVA with Sidak's multiple comparisons test. P < 0.05 was considered statistically significant.
3. Results
The baseline characteristics, such as age, gender, body mass index, length of the disease before inclusion in the study, and clinical status data did not differ significantly between the groups (Table 1 ).
Table 1.
Main demographic and clinical characteristics of the patients at baseline and at days 3 and 5 following the treatment with inhaled surfactant.
| Parameters | Surfactant group (n = 33) | Control group (n = 32) | р |
|---|---|---|---|
| Age, years | 59.0 (53.0–69.0) | 63.5 (50.5–68.5) | 0.88 |
| Males, % | 54 | 72 | 0.15 |
| BMI, kg/m2 | 29.2 (24.7–33.2) | 31.3 (27.1–33.8) | 0.24 |
| Duration of the disease before entering the study, days | 10.5 (8.8–15.0) | 12.0 (9.0–15.0) | 0.31 |
| Comorbidities, n (%): | |||
| COPD | 1 (3.0%) | 2 (6.3%) | 0.55 |
| Asthma | 0 | 1 (3.1%) | 0.31 |
| Cardiovascular disease | 19 (57.6%) | 15 (46.9%) | 0.60 |
| Diabetes | 9 (27.3%) | 8 (25%) | 0.78 |
| Malignancy | 1 (3.0%) | 0 | 0.31 |
| At baseline | |||
| SOFA score | 3 (3–4) | 3 (3–3) | 0.45 |
| NEWS scale | 8 (6–10) | 7 (4–8) | 0.09 |
| Body temperature, 0С | 37.7 (37.3–38.0) | 37.5 (36.6–38.0) | 0.14 |
| Dyspnea (Borg scale) | 6 (4–9) | 6 (2–8) | 0.56 |
| Respiratory rate, min−1 | 26 (21–32) | 24 (21–26) | 0.08 |
| Leukocytes, 109/L | 6.0 (5.1–8.3) | 6.8 (4.4–9.4) | 0.99 |
| Lymphocytes, 109/L | 0.9 (0.6–1.3) | 0.7 (0.7–1.2) | 0.98 |
| СRP, mg/L | 121 (56–160) | 119 (52–170) | 0.83 |
| D-dimer, μg/mL | 2.7 (1.5–5.6) | 1.7 (0.7–4.6) | 0.17 |
| Day 3 | |||
| SOFA score | 3 (3–3) | 3 (3–4) | 0.08 |
| NEWS scale | 5 (4–7) | 5 (4–8) | 0.76 |
| Body temperature, 0С | 37.0 (36.5–37.4) | 36.9 (36.6–37.2) | 0.59 |
| Dyspnea (Borg scale) | 5 (3–8) | 5 (2–10) | 0.87 |
| Respiratory rate, min−1 | 22 (20–26) | 22 (19–25) | 0.46 |
| Day 5 | |||
| SOFA score | 2 (2–3) | 3 (2–5) | 0.04 |
| NEWS scale | 3 (2–4) | 4 (2–7) | 0.66 |
| Body temperature, 0С | 37.0 (36.6–37.2) | 36.8 (36.6–37.2) | 0.51 |
| Dyspnea (Borg scale) | 2 (2–3) | 3 (2–6) | 0.35 |
| Respiratory rate, min−1 | 20 (19–23) | 21 (20–24) | 0.35 |
| Leukocytes, 109/L | 7.4 (5.8–10.2) | 9.2 (8.2–12.5) | 0.02 |
| Lymphocytes, 109/L | 1.2 (0.9–1.6) | 1.4 (0.6–2.5) | 0.80 |
| СRP, mg/L | 13 (5–94) | 40 (14–114) | 0.22 |
| D-dimer, μg/mL | 1.3 (0.9–1.8) | 2.3 (0.9–9.6) | 0.24 |
| Outcomes | |||
| Transfer to ICU, n (%) | 8 (24.2) | 15 (46.9) | 0.05 |
| Intubation and invasive mechanical ventilation, n (%) | 6 (18.2) | 13 (40.6) | 0.04 |
| Deaths, n (%) | 5 (15.2) | 9 (28.1) | 0.17 |
| Duration of NIV, days | 7 (3–13) | 11 (5–22) | 0.02 |
| Length of hospitalization, days | 18 (16–27) | 26 (21–31) | 0.003 |
Table 1. Results are presented as absolute values (%) or median (interquartile range). Abbreviations. BMI, body mass index; COPD, chronic obstructive pulmonary disease; SOFA, Sequential Organ Failure Assessment; NEWS, National Early Warning Score; CRP, C-reactive protein; ICU, intensive care unit; NIV, non-invasive ventilation.
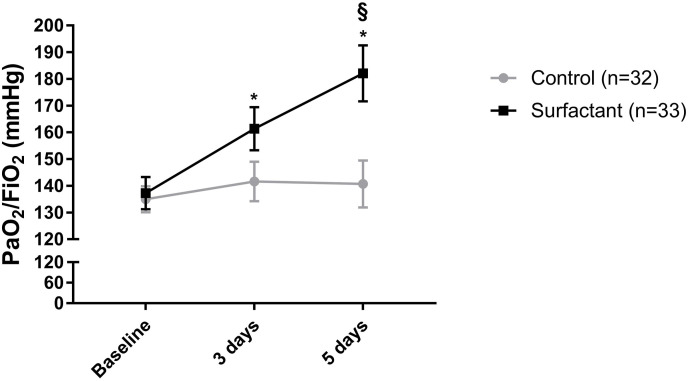
However, the oxygenation index (PaO2/FiO2) significantly increased over time in the surfactant group (Fig. 1 ). In addition, there was a significantly higher value of this parameter in the surfactant group as compared to the control at day 5 upon the treatment (Fig. 1).
Fig. 1.
Effects of inhaled surfactant on oxygenation parameters in patients with COVID-19-associated acute respiratory distress syndrome. Data are shown as mean ± SEM (n = 32–33). PaO2/FiO2: arterial oxygen tension to inspired oxygen fraction ratio. 2-way ANOVA with Sidak's multiple comparisons test was used for statistical analysis. *,§p < 0.05. *baseline versus 3 days and 3 days versus 5 days; §Control versus Surfactant.
Moreover, significantly fewer patients in the surfactant group were transferred to ICU and switched to mechanical ventilation, in comparison to the control group. The duration of NIV and the total length of hospitalization were shorter in the surfactant group compared to the control group. Mortality did not differ significantly between the groups (Table 1).
4. Discussion
Overall, our study demonstrates that the use of nebulized surfactant is feasible in patients with COVID-19-associated ARDS undergoing non-invasive ventilation, and its application results in favorable outcomes, such as improved oxygenation, reduction of the need for IMV and transfer to ICU, and the shorter length of NIV and hospital stay, but did not decrease mortality.
The main function of pulmonary surfactant is to reduce surface tension in alveoli and thus to prevent lung collapse and gas exchange [2,4]. In addition, pulmonary surfactant is involved in the barrier and protective function of the lungs, affecting innate and adaptive local immunity [4]. The exogenous surfactant exhibits anti-inflammatory properties and reduces the production of tumor necrosis factor-alpha, interleukin (IL)-1, IL-6, and, thus, it may efficiently contribute toward the repair of damaged alveoli in SARS-CoV-2-associated ARDS [3].
Recently, few studies reported the promising therapeutic effects of the treatment with exogenous surfactant in COVID-19-associated ARDS [[5], [6], [7]]. In contrast to our study (nebulization), the surfactant was applied in intubated patients via endotracheal instillation or through the bronchoscope [[5], [6], [7]]. Our patients were not intubated and they received non-invasive respiratory support. In addition, we have enrolled a noticeably higher number of patients, as compared to the previously published studies [[5], [6], [7]].
Piva et al. reported a 30-day mortality reduction in the surfactant group, but this reduction was not statistically significant, probably due to the small sample size [7]. The mortality in our study did not differ significantly between the groups, but the number of dead patients was higher in the control group compared to the surfactant group (9 vs 5). Busani et al. showed a progressive improvement in PaO2/FiO2 6–48 h after endotracheal instillation of surfactant, but this case series included five patients only that precluded evaluating mortality [6].
This study has several limitations. The case–control design cannot exclude a bias in the analysis of outcomes, and statistical analysis and interpretation of our study results are further limited by the small sample size.
Therefore, our preliminary data provided indications in favor of conducting further studies with inhaled surfactant therapy for COVID-19 in the near future, including randomized controlled clinical trials. Larger trials of inhaled surfactant therapy for COVID-19-associated ARDS (NCT04362059, NCT04568018) are underway [9,10].
Funding
None.
Disclosures
The authors have nothing to disclose with regard to this study.
CRediT authorship contribution statement
Sergey N. Avdeev: analyzed the data and interpreted the results, study design. Natalia V. Trushenko: analyzed the data and interpreted the results. Svetlana Yu Chikina: performed the measurements and collected the data, analyzed the data and interpreted the results. Natalia A. Tsareva: performed the measurements and collected the data, analyzed the data and interpreted the results. Zamira M. Merzhoeva: performed the measurements and collected the data, analyzed the data and interpreted the results. Andrey I. Yaroshetskiy: performed the measurements and collected the data, analyzed the data and interpreted the results. Violetta I. Sopova: performed the measurements and collected the data, analyzed the data and interpreted the results. Margarita I. Sopova: performed the measurements and collected the data, analyzed the data and interpreted the results. Oleg A. Rosenberg: analyzed the data and interpreted the results. Ralph Theo Schermuly: contributed with significant intellectual content. All authors were involved in writing the manuscript. All authors have read and approved the manuscript. Djuro Kosanovic: analyzed the data and interpreted the results, study design.
Declaration of competing interest
Oleg A. Rosenberg is involved in the patent with regard to the surfactant used in this study. Other authors do not have any conflict of interest.
Acknowledgments
None.
References
- 1.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schousboe P., Wiese L., Heiring C., Verder H., Poorisrisak P., Verder P., Nielsen H.B. Assessment of pulmonary surfactant in COVID-19 patients. Crit. Care. 2020;24:552. doi: 10.1186/s13054-020-03268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghati A., Dam P., Tasdemir D., Kati A., Sellami H., Sezgin G.C., Ildiz N., Franco O.L., Mandal A.K., Ocsoy I. Exogenous pulmonary surfactant: a review focused on adjunctive therapy for severe acute respiratory syndrome coronavirus 2 including SP-A and SP-D as added clinical marker. Curr. Opin. Colloid Interface Sci. 2021;51:101413. doi: 10.1016/j.cocis.2020.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson W.J., Dorscheid D., Spragg R., Schulzer M., Mak E., Ayas N.T. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit. Care. 2006;10:R41. doi: 10.1186/cc4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heching M., Lev S., Shitenberg D., Dicker D., Kramer M.R. Surfactant for treatment of ARDS in COVID-19 patient. Chest. 2021 Jan 22 doi: 10.1016/j.chest.2021.01.028. S0012-3692(21)00100-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busani S., Dall'Ara L., Tonelli R., Clini E., Munari E., Venturelli S., Meschiari M., Guaraldi G., Cossarizza A., Ranieri V.M., Girardis M. Surfactant replacement might help recovery of low-compliance lung in severe COVID-19 pneumonia. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620951043. 1753466620951043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piva S., DiBlasi R.M., Slee A.E., Jobe A.H., Roccaro A.M., Filippini M., Latronico N., Bertoni M., Marshall J.C., Portman M.A. Surfactant therapy for COVID-19 related ARDS: a retrospective case-control pilot study. Respir. Res. 2021;22:20. doi: 10.1186/s12931-020-01603-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson N.D., Fan E., Camporota L., Antonelli M., Anzueto A., Beale R., Brochard L., Brower R., Esteban A., Gattinoni L., Rhodes A., Slutsky A.S., Vincent J.L., Rubenfeld G.D., Thompson B.T., Ranieri V.M. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 9.Dabbagh A., Rajaei S., Ghahremani M., Fathi M., Massoudi N., Tavana S., Fani K., Nooraee N., Malekpour Alamdari N., Besharat S., Najafi Abrandabadi A., Pirsalehi A., Khabiri Khatiri M.A. The effect of surfactant on clinical outcome of patients with COVID-19 under mechanical ventilation: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:919. doi: 10.1186/s13063-020-04815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avdeev S. Open-label Trial to Assess the Efficacy and Safety of Inhalation Use of the Approved Drug Surfactant-BL as a Part of Complex Therapy of Acute Respiratory Distress Syndrome (ARDS) in Patients with SARS-CoV-2 Coronavirus Infection (COVID-19). ClinicalTrials.gov Identifier: NCT04568018..