Abstract
Bacteria and fungi secrete many natural products that inhibit each other’s growth and development. The dynamic changes in secreted metabolites that occur during interactions between bacteria and fungi are complicated. Pyochelin is a siderophore produced by many Pseudomonas and Burkholderia species that induces systemic resistance in plants and has been identified as an antifungal agent. Through imaging mass spectrometry and metabolomics analysis, we found that Phellinus noxius, a plant pathogen, can modify pyochelin and ent-pyochelin to an esterification product, resulting in reduced iron-chelation and loss of antifungal activity. We also observed that dehydroergosterol peroxide, the fungal metabolite, is only accumulated in the presence of pyochelin produced through bacteria–fungi interactions. For the first time, we show the fungal transformation of pyochelin in the microbial interaction. Our findings highlight the importance of understanding the dynamic changes of metabolites in microbial interactions and their influences on microbial communities.
Subject terms: Microbial ecology, Metabolomics
Microorganisms use various strategies to establish themselves within an ecological niche while facing keen competition in the environment. Natural products such as antibiotics, quorum sensing molecules, and siderophores are crucial in microbial interactions [1–3]. Certain microorganisms are equipped with uptake systems that enable them to acquire siderophores, even by those that may not produce them [4]. For example, pyochelin is a siderophore produced by many Pseudomonas and Burkholderia strains. Such bacterial strains are commonly found in soils, as endophytes, and from the rhizosphere where they may inhibit plant pathogens [5, 6].
Burkholderia cenocepacia 869T2 was isolated as an endophyte and showed beneficial abilities to control banana Fusarium wilt [7]. It harbors many biosynthetic gene clusters of secondary metabolites, such as pyochelin, pyrrolnitrin, and pyrroloquinoline quinone [8]. Recently, we found that this strain could temporarily inhibit the growth of P. noxius, a fungal pathogen of brown root rot disease, which is prevalent in tropical and subtropical regions and has a wide host range covering over 200 plant species [9]. However, in the competition between fungi and bacteria, P. noxius can resist this inhibition and overwhelm bacterial colonies after 1–2 weeks under dual-culture conditions (Fig. S1). These results imply that fungi might have resistance responses and undergo metabolic changes in bacteria–fungi interactions [10]. Here we unveiled metabolic changes in the competitive interaction between B. cenocepacia 869T2 and P. noxius 2252 using the matrix-assisted laser desorption ionization-time of flight imaging mass spectrometry (MALDI-TOF IMS) [11, 12].
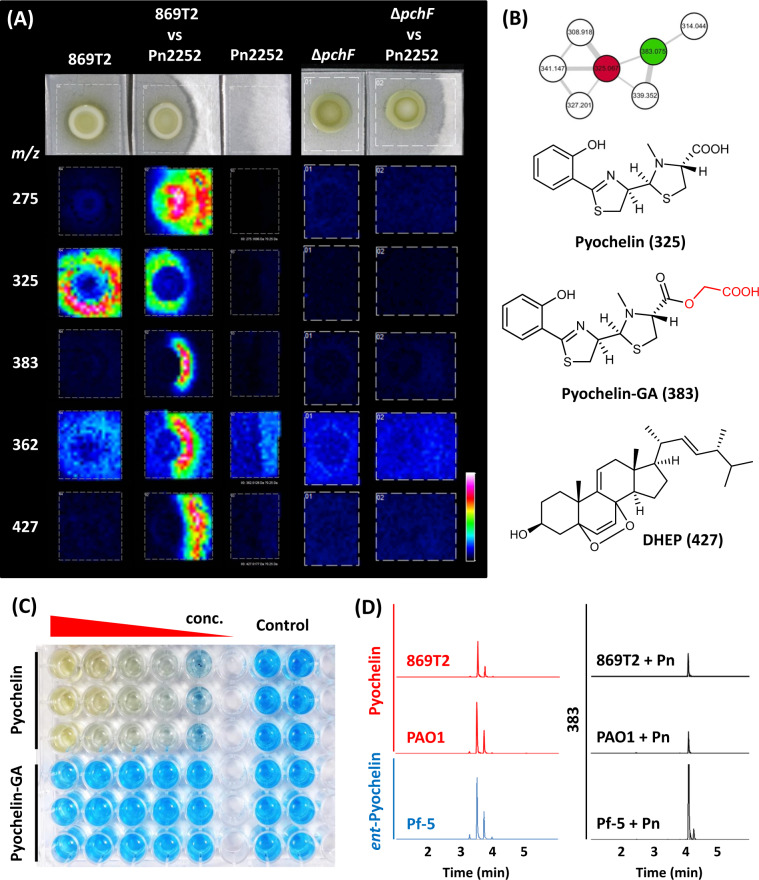
We specifically monitored the metabolites in the inhibition region of B. cenocepacia 869T2 and P. noxius 2252 dual-culture using MALDI-TOF IMS. Several induced or enzymatically modified metabolites were detected, including m/z 275, 362, 383, and 427 (Fig. 1A). In particular, pyochelin (m/z 325), surrounding the B. cenocepacia 869T2 colony, showed asymmetric distribution in dual-culture samples. Near the P. noxius 2252 mycelia, a new metabolite with m/z 383 was detected with a complementary distribution to pyochelin (Fig. 1A). In LC-MS/MS-based molecular networking analysis [13], we found that this new metabolite structure is an esterification product of pyochelin and glycolic acid, which we named pyochelin-GA (Fig. 1B). We then constructed a pchF-null mutant strain, ΔpchF, which cannot produce pyochelin, and then dual cultured it with P. noxius. Pyochelin and pyochelin-GA were not observed in the MALDI-TOF IMS and LC-MS analysis of dual-culture samples (Fig. 1A and Fig. S2). We further inoculated P. noxius 2252 with pyochelin-GA-free extract harvested from B. cenocepacia 869T2 single culture, and the complementary distribution of pyochelin and pyochelin-GA was observed by MALDI-TOF IMS again (Fig. S3). These results demonstrated that pyochelin-GA was transformed from pyochelin by P. noxius 2252, rather than produced by B. cenocepacia 869T2 under dual-culture conditions.
Fig. 1. Metabolic changes in the bacteria–fungi interaction.
A Spatial distribution of selected mass signals (m/z) in MALDI-TOF IMS analysis of Phellinus noxius 2252 (Pn2252) dual-cultured with Burkholderia cenocepacia 869T2 (869T2) and a pchF-null mutant strain (Δ pchF). B Molecular networking analysis of pyochelin and analogs from the dual-culture sample. The red node is pyochelin, and the green node is pyochelin-GA. The structures of pyochelin, pyochelin-GA, and dehydroergosterol peroxide (DHEP), together with their mass signals in MALDI-TOF IMS, are shown. C Iron-chelating abilities of pyochelin and pyochelin-GA were evaluated by Chrome Azurol S liquid assay using different concentrations (2.5, 1.25, 0.63, 0.31, and 0.16 mM, n = 3). Proportions of siderophore units are shown in Fig. S14. D Fungal transformation of pyochelin and ent-pyochelin by treating P. noxius 2252 with ethyl acetate crude extracts of B. cenocepacia 869T2, Pseudomonas aeruginosa PAO1, and P. protegens Pf-5 for 8 h. LC-MS was used to monitor the signals of pyochelin (red), ent-pyochelin (blue), and transformation product 383 (black).
The chemical structure of pyochelin-GA was further confirmed via total synthesis, NMR, and LC-MS/MS analysis (Supplementary Material and Methods, and Figs. S4–7). The purified pyochelin and pyochelin-GA were also evaluated for their iron-chelating ability. Chrome Azurol S assay indicated that pyochelin had the dose-dependent iron-chelating ability, but pyochelin-GA had lower iron-binding efficiency (Fig. 1C, Fig. S8). Pyochelin chelates iron in the extracellular medium and transports it into cells via the specific outer membrane transporter FptA. The X-ray structure of FptA-pyochelin-Fe indicated that the terminal carboxylic acid of pyochelin plays an essential role in the iron uptake ability [14, 15]. Our docking analysis suggested that the glycolic ester moiety of pyochelin-GA would affect the binding pocket shape of FptA and result in different binding properties compared to FptA-pyochelin (Fig. S9).
Pyochelin and ent-pyochelin are produced independently by different biosynthetic gene clusters in Pseudomonas species [16]. To determine whether P. noxius 2252 can transform both enantiomers via this esterification process, we treated P. noxius 2252 with the extracts of pyochelin producers (P. aeruginosa PAO1 and B. cenocepacia 869T2) and an ent-pyochelin producer (P. protegens Pf-5). After 8 h of treatment, both pyochelin and ent-pyochelin were converted to pyochelin-GA (or ent-pyochelin-GA) (Fig. 1D), demonstrating this is a non-stereospecific transformation.
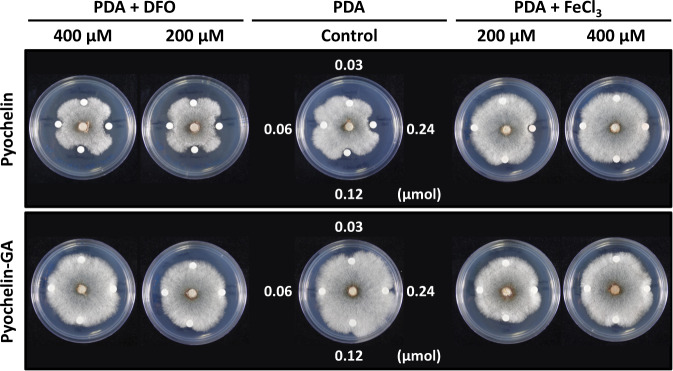
To better understand the iron-chelating ability of pyochelin, we used pyochelin and pyochelin-GA to treat P. noxius 2252 under iron-deficiency conditions, by adding the iron chelator deferoxamine, and iron-rich conditions by adding FeCl3 (Fig. 2). Pyochelin-GA did not affect the growth of P. noxius 2252 under all conditions. However, P. noxius 2252 was more sensitive to pyochelin in iron-deficient conditions and more resistant to pyochelin in iron-rich conditions, demonstrating that iron availability directly affected the tolerance of P. noxius 2252 to pyochelin. A similar phenomenon was reported previously for Aspergillus fumigatus [17].
Fig. 2. Pyochelin inhibition of mycelial growth of Phellinus noxius 2252 is inversely associated with iron concentration.
Pyochelin-GA did not have an inhibition effect on P. noxius 2252. Potato dextrose agar (PDA) with deferoxamine (DFO; 200 and 400 µM) was used to mimic iron-deficiency conditions. Iron-rich conditions was prepared by adding FeCl3 (200 and 400 µM) in PDA. P. noxius 2252 was treated with 0.03, 0.06, 0.12, and 0.24 µmol of pyochelin or pyochelin-GA at 30 °C for 24 h. The antifungal assay was performed in two biological replicates.
Using MALDI-TOF IMS analysis of the dual-culture of B. cenocepacia 869T2 and P. noxius 2252, we observed that several metabolites (e.g., m/z 275, 362, and 427) were only observed in the boundary of fungal mycelia (Fig. 1A). Although those metabolites were not detected in the dual-culture of ΔpchF and P. noxius 2252 (Fig. 1A), they were present when we treated P. noxius 2252 with pyochelin (Fig. S10). We identified the metabolite associated with m/z 427 as dehydroergosterol peroxide (DHEP) (Fig. S11), which was initially oxidized from ergosterol and dehydroergosterol [18]. Pyochelin can enhance intercellular reactive oxygen species (ROS) and ultimately disrupts membrane integrity, leading to cell death [17, 19, 20]. To clarify whether ROS induced the accumulation of DHEP, we treated P. noxius 2252 with pyochelin, pyochelin-GA, and 2,2′-bipyridyl (an iron chelator). Pyochelin and 2,2′-bipyridyl showed antifungal effects on P. noxius 2252 and induced ROS production (Fig. S12). However, the accumulation of DHEP in P. noxius 2252 was only associated with pyochelin treatment (Fig. S13). The induction of ROS in P. noxius 2252 by pyochelin and pyochelin-GA was not significantly different (Fig. S14). Therefore, we predict that pyochelin-induced accumulation of DHEP in P. noxius 2252 is independent of ROS production and iron-deficiency.
Overall, we demonstrate that pyochelin transformation by fungi, in the interaction between pyochelin-producing bacteria and the plant pathogen P. noxius transforms pyochelin and ent-pyochelin into pyochelin-GA (and ent-pyochelin-GA). This product no longer functions as an iron chelator and no longer shows antifungal activity. The production of a fungal metabolite, dehydroergosterol peroxide, was induced explicitly by pyochelin through an unknown mechanism. These results highlight the importance of monitoring dynamic changes of metabolites in situ to better understand the functions and influences of metabolites on microbial community interactions.
Supplementary information
Acknowledgements
This research was supported by the Ministry of Science and Technology of Taiwan (MOST 104-2321-B-001-060-MY3). LC-MS data were collected in the Metabolomics Core Facility of the Instrument Center, Academia Sinica. NMR data were collected in the High Field Nuclear Magnetic Resonance Center, Academia Sinica. Burkholderia cenocepacia 869T2 was generously provided by Prof. Chieh-Chen Huang’s Lab (National Chung Hsing University, Taiwan) and Phellinus noxius strain 2252 by Prof. Chia-Lin Chung’s Lab (National Taiwan University, Taiwan). 9.11-Dehydroergosterol peroxide and ergosterol peroxide were kindly provided by Dr. Ming-Ren Cheng (Bioresource Collection and Research Center, Taiwan). The molecular docking analysis was supported by Dr. Ying-Ta Wu, Genomics Research Center, Academia Sinica.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41396-020-00871-0) contains supplementary material, which is available to authorized users.
References
- 1.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver VB, Kolter R. Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J Bacteriol. 2004;186:2376–84.. doi: 10.1128/JB.186.8.2376-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linares JF, Gustafsson I, Baquero F, Martinez J. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci USA. 2006;103:19484–89. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiessl KT, Janssen EM-L, Kraemer SM, McNeill K, Ackermann M. Magnitude and mechanism of siderophore-mediated competition at low iron solubility in the Pseudomonas aeruginosa pyochelin system. Front Microbiol. 2017;8:1964. doi: 10.3389/fmicb.2017.01964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weller DM. Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology. 2007;97:250–56.. doi: 10.1094/PHYTO-97-2-0250. [DOI] [PubMed] [Google Scholar]
- 6.Ganeshan G, Manoj Kumar A. Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J Plant Interact. 2005;1:123–34.. doi: 10.1080/17429140600907043. [DOI] [Google Scholar]
- 7.Ho Y-N, Chiang H-M, Chao C-P, Su C-C, Hsu H-F, Guo C-T, et al. In planta biocontrol of soilborne Fusarium wilt of banana through a plant endophytic bacterium, Burkholderia cenocepacia 869T2. Plant Soil. 2015;387:295–306. doi: 10.1007/s11104-014-2297-0. [DOI] [Google Scholar]
- 8.Ho Y-N, Huang C-C. Draft genome sequence of Burkholderia cenocepacia strain 869T2, a plant-beneficial endophytic bacterium. Genome Announc. 2015;3:e01327–15.. doi: 10.1128/genomeA.01327-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahashi N, Akiba M, Ishihara M, Ota Y, Kanzaki N. Brown root rot of trees caused by Phellinus noxius in the Ryukyu Islands, subtropical areas of Japan. Pathol. 2012;42:353–61.. doi: 10.1111/j.1439-0329.2012.00767.x. [DOI] [Google Scholar]
- 10.Hoefler BC, Gorzelnik KV, Yang JY, Hendricks N, Dorrestein PC, Straight PD. Enzymatic resistance to the lipopeptide surfactin as identified through imaging mass spectrometry of bacterial competition. Proc Natl Acad Sci USA. 2012;109:13082–87.. doi: 10.1073/pnas.1205586109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y-L, Xu Y, Straight P, Dorrestein PC. Translating metabolic exchange with imaging mass spectrometry. Nat Chem Biol. 2009;5:885–87.. doi: 10.1038/nchembio.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho YN, Shu LJ, Yang YL. Imaging mass spectrometry for metabolites: technical progress, multimodal imaging, and biological interactions. WIRES Syst Biol Med. 2017;9:e1387. doi: 10.1002/wsbm.1387. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotech. 2016;34:828–37.. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobessi D, Celia H, Pattus F. Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J Mol Biol. 2005;352:893–904. doi: 10.1016/j.jmb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Mislin GLA, Hoegy F, Cobessi D, Poole K, Rognan D, Schalk IJ. Binding related properties of pyochelin and structurally molecules to FptA of Pseudomonas aeruginosa. J Mol Biol. 2006;357:1437–48.. doi: 10.1016/j.jmb.2006.01.080. [DOI] [PubMed] [Google Scholar]
- 16.Youard ZA, Wenner N, Reimmann C. Iron acquisition with the natural siderophore enantiomers pyochelin and enantio-pyochelin in Pseudomonas species. Biometals. 2011;24:513–22.. doi: 10.1007/s10534-010-9399-9. [DOI] [PubMed] [Google Scholar]
- 17.Briard B, Mislin GL, Latgé J-P, Beauvais A. Interactions between Aspergillus fumigatus and pulmonary bacteria: current state of the field, new data, and future perspective. J Fungi. 2019;5:48. doi: 10.3390/jof5020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponce MA, Ramirez JA, Galagovsky LR, Gros EG, Erra-Balsells R. A new look into the reaction between ergosterol and singlet oxygen in vitro. Photoch Photobio Sci. 2002;1:749–56.. doi: 10.1039/b204452h. [DOI] [PubMed] [Google Scholar]
- 19.Ong KS, Cheow YL, Lee SM. The role of reactive oxygen species in the antimicrobial activity of pyochelin. J Adv Res. 2017;8:393–98. doi: 10.1016/j.jare.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho Y-N, Lee H-J, Hsieh C-T, Peng C-C, Yang Y-L. Chemistry and biology of salicyl-capped siderophores. Stud Nat Prod Chem. 2018;59:431–90. doi: 10.1016/B978-0-444-64179-3.00013-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.