Abstract
Allergic Rhinitis is one of the most commonly recognised rhinitis globally. Though its not a life threatening entity but it is associated with severe impairment of quality of life along with substantial financial burden on patient. There has been a substantial rise in number of patients of allergic rhinitis over years and researchers worldwide have also found low levels of vitamin D in patients of allergic rhinitis. It is a randomised control trial with 87 subjects divided into two groups. Pre-treatment total nasal symptom score (TNSS) were recorded for these patients. The Group A was given intranasal steroidal spray while Group B was given vitamin D supplementation along with intranasal steroidal spray. Post treatment TNSS scores and rhinitis control assessment test scores were calculated and analysed. 38 patients had severe Vit D deficiency with average TNSS score as 11.3 while 49 patients had insufficient Vit D levels with average TNSS as 8.6. The pre-treatment TNSS score in Group A was 12.5 ± 2.68 while post-treatment score was 8.98 ± 1.009 with difference in both scores of Group A as 3.52. The pre-treatment TNSS score in Group B (fluticasone spay with Vit D) was 11.64 ± 3.09 while post-treatment score was 6.3 ± 1.45 with difference in both scores of Group A as 5.34. The post treatment RCAT in Group A and Group B was 19.72 ± 2.84 and 28.2 ± 1.53 respectively with difference between two groups as 8.48. Though Intranasal steroidal sprays are the first line of management of allergic rhinitis however vitamin D supplementation can have a role in better relief of symptoms when used in conjunction. More multi-institutional studies are encouraged to confirm the validity of results before it can be incorporated in standard treatment guidelines.
Keywords: Vitamin D levels, Vitamin D supplementation, Allergic rhinitis, TNSS score, RCAT score, Diagnosis, Treatment, Roleof
Introduction
Allergic rhinitis is the most common type of chronic rhinitis, affecting 10–20% of the population, and evidence suggests that the prevalence of the disorder is increasing [1, 2]. Severe allergic rhinitis has been associated with significant impairments in quality of life, sleep and work performance. Despite the recent epidemiologic evidence that the incidence of allergic rhinitis is increasing worldwide, less than 12% of allergy sufferers seek medical recommendations from a physician, resulting in allergic rhinitis being frequently under recognized, misdiagnosed, and ineffectively treated [3, 4].
Allergic rhinitis is typically manifested by patients having nasal congestion, rhinorrhea, sneezing, and nasal itching as well as ocular redness, tearing, and itching. Nearly 50% of allergic rhinitis patients will experience symptoms for at least 4 months of the year. Allergic rhinitis, as a non-life threatening disease, has not been regarded as a serious health problem, despite the evidence suggesting it has important short-term and long-term health consequences [5]. The economic burden is further enhanced as allergic rhinitis and asthma are often comorbid conditions. Approximately 85% of asthmatic patients have allergic rhinitis, whereas up to 40% of allergic rhinitis patients have or will develop asthma [6, 7].
Treatment options consist of drugs like anti-histamines, intranasal corticosteroids, decongestants, and more recently immunotherapy. In recent years, there has been worldwide increase in allergic diseases more so in developed countries and this has been associated with low vitamin D. Current lifestyle has led to people spending more times indoors, leading to less sun exposure and less cutaneous vitamin D production. Several studies have investigated the role of vitamin D in the treatment of allergic diseases and asthma, however the results are still controversial [8]. In present study efficacy of vit d supplementation will evaluated in patients suffering from moderate to severe allergic rhinitis in both seasonal and perennial cases.
Methods and Materials
Present study is randomised controlled trial conducted in out-patient department of ENT of a tertiary care centre from April 2017 to September 2019. Total of 87 out of 139 patients of allergic rhinitis were selected and enrolled after thorough consent and assent of subjects. The study complied the principles of the declaration of Helsinki and every effort was made to maintain patient confidentiality at all points of study. This was a non-funded trial and CONSORT (CONsolidated Standards of Reporting Trials) reporting guidelines was used to report the study.
The Primary Objective was to see for any symptom improvement in patients with Vitamin D supplementation and secondary objective was to see the levels of Vitamin D in patients with Allergic Rhinitis.
A thorough history and physical examination of all patients attending ENT OPD with complaints of sneezing and watery discharge from the nose. An evaluation of the patient’s home and work/school environments was also done to determine potential triggers of rhinitis. Environmental history focussed on common and potentially relevant allergens including pollens, furred animals, textile upholstery, tobacco smoke, humidity levels at home, as well as other potential noxious substances that the patient may be exposed to at work or at home.
Patients were also asked about recent medication or drug use, history of atopic disease and the presence of comorbidities. After through history, detailed nasal examination with nasal endoscopy was done. Patients were diagnosed as cases of Allergic rhinitis as per the history and physical examination criteria adapted from Small et al. [1] (Table 1). The patients who were diagnosed as cases of Allergic rhinitis were subjected to serum Vit D levels.
Table 1.
Components of a complete history and physical examination for suspected rhinitis
| History | Physical examination |
|---|---|
|
Personal Congestion Nasal itch Rhinorrhea Sneezing Eye involvement Seasonality Triggers Family Allergy Asthma Environmental Pollens Animals Flooring/upholstery Mould Humidity Tobacco exposure Medication/drug use Beta-blockers SO NSAIDs ACE inhibitors Hormone therapy Recreational cocaine use Quality of life Rhinitis-specific questionnaire Comorbidities Asthma Mouth breathing Snoring ± apnea Impaired smell or taste Sinus involvement Otitis media Nasal polyps Conjunctivitis Response to previous interventions Avoidance measures Saline nasal rinses Second-generation oral antihistamines Intranasal corticosteroids |
Outward signs Mouth breathing Rubbing the nose/transverse nasal crease Frequent sniffling and/or throat clearing Allergic shiners (dark circles under eyes) Nose Mucosal swelling, bleeding Pale, thin secretions Polyps or other structural abnormalities Ears Generally normal Pneumatic otoscopy to assess for Eustachian tube dysfunction Valsalva’s maneuver to assess for fluid behind the ear drum Sinuses Palpation of sinuses for signs of tenderness Maxillary tooth sensitivity Posterior oropharynx Postnasal drip Lymphoid hyperplasia (“cobblestoning”) Tonsillar hypertrophy Chest and skin Atopic disease |
The following were the inclusion and exclusion criteria for the patients to be enrolled into the study
Inclusion Criteria
Allergic rhinitis patients with serum 25 (OH)-Vitamin-D3 levels less than 20 ng/ml.
Age Group 16 years to 60 years.
Exclusion Criteria
Non-Allergic Rhinitis.
Associated Nasal co-morbidities like Nasal Polyposis, Sinusitis, Deviated Nasal Septum, Autoimmune disease of nose.
Associated Systemic co-morbidities affecting vitamin D levels like Juvenile Idiopathic Arthritis, Rickets, Cystic Fibrosis, Ulcerative Colitis, Crohn’s disease, Celiac disease, Thyroid dysfunction.
Individuals who had received medications including corticosteroids, barbiturates, omega-3 and vitamin D.
Total of 139 patients were diagnosed with Allergic Rhinitis but only 87 patients had deficient/insufficient Vit D levels and were enrolled in the study as per the inclusion and exclusion criteria. The patients were randomised into two groups. Total Nasal Symptom Score (TNSS) were calculated and recorded for these patients. TNSS is the sum of scores for each of nasal congestion sneezing, nasal itching, and rhinorrhea at each time point, using a four point scale (0–3) (Table 2). TNSS is calculated by adding the score for each of the symptoms to a total out of 12 [9].
Table 2.
Total nasal symptom scores (TNSS) each symptom (sneezing, congestion, itching, and rhinorrhea) is graded from 0 to 3 by the participants during the screening and NAC visits
| Score symptoms | Severity of symptoms reported by patient |
|---|---|
|
0 = None 1 = Mild 2 = Moderate 3 = Severe |
No symptoms evident Symptom present but easily tolerated Definite awareness of symptom; bothersome but tolerable Symptom hard to tolerate; interferes with daily activity |
The treatment guidelines were based on ARIA 2017 recommendations [10]. Group A patients were put on Fluticasone 50 mcg nasal spray 2 puffs BD for 4 weeks and Group B patients were put on Fluticasone 50 mcg nasal spray 2 puffs BD and Oral Vit D3 Cholecalciferol 60000 IU once a week for 4 weeks. Serum Vit D levels in study population was recorded and tabulated. Post-treatment TNSS score was recorded in both groups.
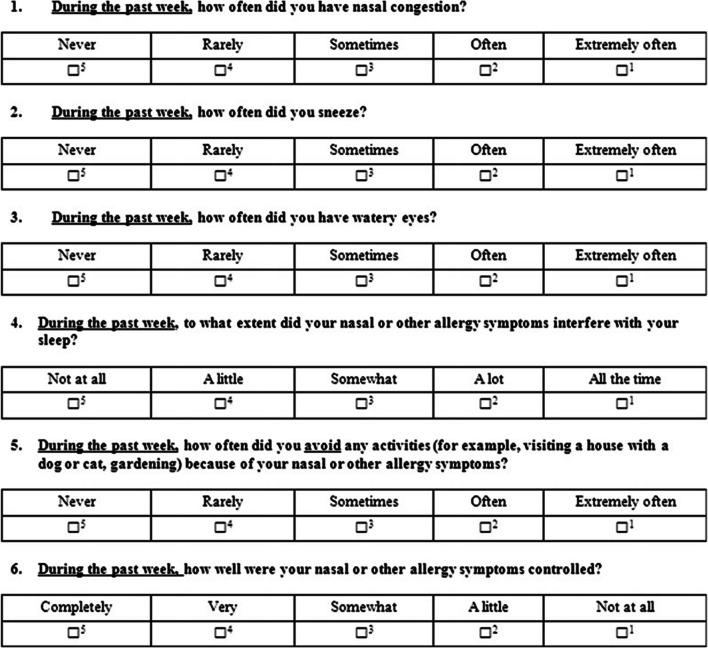
Post-treatment Rhinitis Control Assessment Test score (RCAT) [11] was also recorded in both the groups for all patients. RCAT is a patient-based tool that measures rhinitis symptom control. The RCAT was developed to identify patients whose nasal symptoms, ocular symptoms, or both might warrant a change in management strategy, referral to an allergy specialist, or both. The RCAT has 6 items that include nasal congestion, sneezing, watery eyes, sleep problems caused by rhinitis, activity avoidance, and rhinitis symptom control. Responses are measured on 5-point Likert-type scales. RCAT scores range from 6 to 30, with higher scores indicating better rhinitis control (Fig. 1).
Fig. 1.
RCAT
The proformas were managed by an ENT colleague who was not aware of the treatment protocol of the groups as well as group of the patient. The Data so obtained was statistically analysed using SPSSR software (version 17.0; SPSS, USA). Paired and Unpaired student t test were used as statistical tests.
Results
In Present study, out of 87 patients enrolled male to female ratio was 0.77 (38 males vs. 49 females). Vit D levels were however studied in all 139 patients who were diagnosed with allergic rhinitis. Out of 139 patients 87 had Vitamin D deficiency. Out of these 87; 38 patients had severe Vit D deficiency with average TNSS score as 11.3 while 49 patients had insufficient Vit D levels with average TNSS as 8.6. The percentage of Vit D deficiency in Allergic Rhinitis was 62.5 (Table 3). The pre-treatment TNSS score in Group A (only fluticasone spay) was 12.5 ± 2.68 while post-treatment score was 8.98 ± 1.009 with difference in both scores of Group A as 3.52. t score was 117.387 and p < 0.0001 (Table 4). The pre-treatment TNSS score in Group B (fluticasone spay with Vit D) was 11.64 ± 3.09 while post-treatment score was 6.3 ± 1.45 with difference in both scores of Group A as 5.34. t score was 131.1403 and p < 0.0001 (Table 4). The post treatment RCAT in Group A and Group B was 19.72 ± 2.84 and 28.2 ± 1.53 respectively with difference between two groups as 8.48 and t score as 135.27 and p < 0.05 (Table 5).
Table 3.
Levels of vitamin D in patients with allergic rhinitis and its correlation with TNSS
| Number of patients | Vitamin D levels | Average TNSS |
|---|---|---|
| 52 | 30–100 ng/ml (sufficient) | 5.7 |
| 49 | 20–29 ng/ml (insufficient) | 8.6 |
| 38 | <20 ng/ml (deficient) | 11.3 |
Out of 139 patients diagnosed with allergic rhinitis only 87 patients had decreased levels of serum vitamin D levels which is around 62.5%
Table 4.
The statistical analysis showing the mean difference in TNSS score in both groups
| Group | Mean pre-treatment score | Mean post-treatment score | Difference | t-score/p value |
|---|---|---|---|---|
| A | 12.5 ± 2.68 | 8.98 ± 1.009 | 3.52 |
t-score = 117.387 p < .00001 |
| B | 11.64 ± 3.09 | 6.3 ± 1.45 | 5.34 |
t-score = 131.1403 p < .00001. |
Though both the groups showed statistically significant reduction in TNSS scores but the difference between pre and post treatment scores was more in Group B than Group A
Table 5.
Comparison of RCAT score in both groups
| Group | Mean RCAT score | Difference | t-score | p value |
|---|---|---|---|---|
| A | 19.72 ± 2.84 | 8.48 | 135.27 | p < 0.05 |
| B | 28.2 ± 1.53 |
The symptoms were relieved more in Group B (with vitamin D supplementation) as compared to the Group A (without vitamin D supplementation) and the difference in relief of symptoms in both groups was statistically significant
Discussion
Our trial efficacy of vitamin D supplementation in Allergic Rhinitis studied the effect of adding Vitamin D in management of moderate and severe allergic patients. It was found that adding Vitamin D to nasal steroid spray showed statistically significant improvement in post-treatment RACT score than by not adding Vitamin D (Table 5). Though Post-treatment total nasal score was reduced in both the groups, however Group B (with Vitamin D supplementation) showed better outcome in patients in terms of difference in reduction of total nasal symptom score and average post treatment score than in Group A (Table 4). Another variable which was studied though it was not a primary outcome variable was to study for role of Vit D deficiency and its correlation with TNSS. It was found that more severe the Vit D deficiency more severe was the degree of allergic rhinitis (Table 3). Although technique of randomisation, data collection, careful selection of homogenous allergic rhinitis patients, study of multiple parameters like TNSS and RACT score for evaluation of efficacy of treatment and correlation of severity of Vit D deficiency with severity of symptoms of allergic rhinitis, well accepted dose of Vit D and fluticasone, safety monitoring and appropriate management of non-responders were major strengths of trial, there were many limitations of current trial like small cohort, short follow up, selection of patients only with Vit D deficiency and not all allergic patients, concerns about internal validity, external validity and generalizability of results. Though two parameters were used to study the efficacy of treatment but both these parameters are patient based questionnaires subjective to the attitude and understanding of patients which can be the cause of some bias in treatment outcome.
In our study it was found that pre-treatment TNSS and post-treatment TNSS of both groups with Fluticasone only (Group A) and Fluticasone with Vitamin D (Group B) showed statistically significant improvement in both groups. Mean pre-treatment and post treatment TNSS score in Group A was 12.5 ± 2.68 and 8.98 ± 1.009 respectively. Pre-treatment and post-treatment TNSS Group B was 11.64 ± 3.09 and 6.3 ± 1.45 respectively. The difference of pre-treatment and post-treatment score improvement was more in Group B (5.34) as compared to Group A (3.52) though the difference was not statistically significant (Table 4).
Mean Post-treatment RACT score in Group A and Group B was 28.2 ± 1.53 and 19.72 ± 2.84 respectively and this difference was statistically significant in favour of Group B (Table 5).
Another important observation was the association of vit d deficiency with severity of allergic rhinitis patient. It was observed that 38 patients had severe Vit D deficiency (i.e. 20 ng/ml) and 33/38 patients also had TNSS > 10 which was on higher side taking the average TNSS in this group to 11.3 as compared to patients with moderate Vit D deficiency (i.e. 20–30 ng/ml); with average TNSS coming up to 8.6 (Table 3).
The role of INS in the treatment of AR is well established. They are proven to be efficacious and are recommended as first-line therapy for individuals with persistent moderate/severe rhinitis. Therapeutic efficacy of fluticasone in AR has been proven by several double-blind, placebo-controlled, clinical trials that can be differentiated according to drug dosage, duration of treatment, age of patients, type of rhinitis, and end-points. Martin et al. [12] all concluded that with use of fluticasone nasal spray there is definite reduction in TNSS in their respective studies where they used TNSS as end point of their study these findings also correlated with our present study [13–16].
In a randomized, placebo-controlled, double-blind study in patients with SAR conducted by F.M. Baroody and J. Lane, it was concluded that the addition of Vit D to an Intra nasal spray provide significant additional therapeutic benefit in SAR, this finding also correlated well with our current trial whereby there was not only improvement of TNSS but also significant improvement of RACT score by addition of Vitamin D to fluticasone nasal spray. Another study conducted by Upadhaya et al. in paediatric population also concluded a significant reduction of symptoms of allergic rhinitis when oral Vit D was added to standard treatment of allergic rhinitis trial [17, 18].
Vitamin D has long been known to be an essential nutrient for the human body, particularly with regard to the absorption of dietary calcium and phosphate. Technically, vitamin D is not a true vitamin; it belongs to the family of steroid hormones. Its nuclear hormone receptor, vitamin D receptor (VDR), is expressed in at least seventeen tissues or cells [19, 20].
The prevalence of Vitamin D deficiency is reported worldwide, both in sunshine deficient and sunshine sufficient countries. Still, it is the most underdiagnosed and undertreated nutritional deficiency in the world. However, various studies showed poor Vitamin D status irrespective of age, sex, and geography. As there is no standard guideline which is followed all over the world for classifying the Vitamin D status, these studies had different cut off values for the deficiency. The vast majority of these studies used serum 25 (OH) D level of < 20 ng/ml as Vitamin D deficiency [21, 22].
The community-based Indian studies of the past decade done on apparently healthy controls reported a prevalence ranging from 50 to 94%. These studies which included various age groups reflect the magnitude of the problem. High prevalence was seen throughout the country [23, 24].
It is generally agreed that a shift from a Th1 to Th2 phenotype in the proliferation of CD4 + T cells contributes to the pathogenesis of AR; however, the exact mechanism is still under investigation. Recent studies indicate that Th17 and Treg cells are important in the disease course of AR. Vitamin D inhibits the proliferation of T cells; induces a switch from Th1 to Th2 by enhancing the development of Th2 cells; facilitates the induction of Foxp3 + Treg cells; and suppresses the differentiation, maintenance, bioactivity, and transcription of Th17 cells. Additionally, 1,25 (OH)2D3 inhibits the proliferation and induces apoptosis of activated B cells, and it inhibits plasma cell differentiation and immunoglobulin secretion, including IgE secretion [25, 26]. Yenigun et al., Vatankhah et al., Sudiro et al., in their respective studies showed that vitamin D deficiency was correlated with allergic rhinitis classification and a significant proportion of allergic rhinitis patients showed a severe vitamin D deficiency [27, 28]. Several other researchers in their studies concluded that supplementation of vitamin D in allergic rhinitis patients alters natural course of AR toward significant clinical improvement [29–37].
Conclusion
As chronic AR is a decade old problem, management of which is a difficult task for most of clinicians including physicians and otolaryngologists in the current scenario, supplementation of vitamin D to alter the allergic course has emerged as ray of hope but more extensive multi institutional work is needed to come to conclusion.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest whatsoever.
Ethical Statement
Before starting the study ethical clearance was taken from institutional ethical committee as per Declaration of Helsinki.
Consent for Publication
Informed consent was taken by all the patients before surgery and enrolment into the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bhanu Bhardwaj, Email: entwithdrbhanu@gmail.com.
Jaskaran Singh, Email: jassigill001@gmail.com.
References
- 1.Small P, Frenkiel S, Becker A, Boisvert P, Bouchard J, Carr S, et al. The Canadian Rhinitis Working Group. Rhinitis: a practical and comprehensive approach to assessment and therapy. J Otolaryngol. 2007;36(Suppl 1):S5–S27. doi: 10.2310/7070.2006.X002. [DOI] [Google Scholar]
- 2.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103–S115. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 3.Berger WE. Overview of allergic rhinitis. Ann Allergy Asthma Immunol. 2003;90(Suppl. 3):7–12. doi: 10.1016/S1081-1206(10)61653-5. [DOI] [PubMed] [Google Scholar]
- 4.Malone DC, Lawson KA, Smith DH, et al. A cost of illness study of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99:22–27. doi: 10.1016/s0091-6749(97)70296-3. [DOI] [PubMed] [Google Scholar]
- 5.Foresi A. A comparison of the clinical efficacy and safety of intranasal fluticasone propionate and antihistamines in the treatment of rhinitis. Allergy. 2000;62:12–14. doi: 10.1034/j.1398-9995.2000.055suppl62012.x. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Van Cauwenberge P, Khaltaev N, ARIA Workshop Group. World Health Organization Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(Suppl. 5):S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 7.Global strategy for asthma management and prevention. Global initiative for asthma (GINA). http://www.ginasthma.com/Guidelineitem.asp?11=2&12=1&intId=60. Accessed 16 Sept 2008]
- 8.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9(3):202–207. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A. Vitamin D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6(2):729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer EO, Schatz M, Nathan R, et al. Reliability, validity, and responsiveness of the rhinitis control assessment test in patients with rhinitis. J Allergy Clin Immunol. 2013;131:379–386. doi: 10.1016/j.jaci.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Martin BG, Ratner PH, Hampel FC, et al. Optimal dose selection of fluticasone furoate nasal spray for the treatment of seasonal allergic rhinitis in adults and adolescents. Allergy Asthma Proc. 2007;28(2):216–225. doi: 10.2500/aap.2007.28.2983. [DOI] [PubMed] [Google Scholar]
- 13.Stanford R, Philpot E, Faris M, et al. Fluticasone furoate nasal spray once-daily improves quality of life in subjects with seasonal allergic rhinitis (SAR) Ann Allergy Asthma Immunol. 2007;98:A90. [Google Scholar]
- 14.Nathan R, Berger W, Yang W, et al. Once daily fluticasone furoate nasal spray (FFNS), a novel enhanced affinity steroid, provides 24-hour relief for the nasal symptoms of perennial allergic rhinitis (PAR) J Allergy Clin Immunol. 2007;119:S65. doi: 10.1016/j.jaci.2006.11.278. [DOI] [Google Scholar]
- 15.Fokkens WJ, Jogi R, Reinartz S, et al. Once daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollen. Allergy. 2007;62:1078–1084. doi: 10.1111/j.1398-9995.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser HB, Naclerio RM, Given J, et al. Fluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;119:1430–1437. doi: 10.1016/j.jaci.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Baroody FM, Lane J, Watanabe S, DeTineo M, Pinto J, Naclerio RM. The addition of vitamin D (VitD) to an intranasal steroid (INS) improves control of symptoms in seasonal allergic rhinitis (SAR) J Allergy Clin Immunol. 2012;129(2):AB134. doi: 10.1016/j.jaci.2011.12.449. [DOI] [Google Scholar]
- 18.Upadhyay P, Jain R. The effect of vitamin d supplementation on children with allergic rhinitis. Pediatric Oncall J. 2017;14(3):56–59. [Google Scholar]
- 19.Akbar NA, Zacharek MA. Vitamin D: immunomodulation of asthma, allergic rhinitis, and chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2011;19:224–228. doi: 10.1097/MOO.0b013e3283465687. [DOI] [PubMed] [Google Scholar]
- 20.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25:671–680. doi: 10.1016/j.beem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 24.Suryanarayana P, Arlappa N, Sai Santhosh V, Balakrishna N, Lakshmi Rajkumar P, Prasad U, et al. Prevalence of vitamin D deficiency and its associated factors among the urban elderly population in Hyderabad metropolitan city, South India. Ann Hum Biol. 2018;45:133–139. doi: 10.1080/03014460.2018.1425479. [DOI] [PubMed] [Google Scholar]
- 25.Kapil U, Pandey RM, Goswami R, Sharma B, Sharma N, Ramakrishnan L, et al. Prevalence of vitamin D deficiency and associated risk factors among children residing at high altitude in Shimla district, Himachal Pradesh, India. Indian J Endocrinol Metab. 2017;21:178–183. doi: 10.4103/2230-8210.196031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhury R, Taneja S, Bhandari N, Sinha B, Upadhyay RP, Bhan MK, et al. Vitamin-D deficiency predicts infections in young North Indian children: a secondary data analysis. PLoS ONE. 2017;12:e0170509. doi: 10.1371/journal.pone.0170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srimani S, Saha I, Chaudhuri D. Prevalence and association of metabolic syndrome and vitamin D deficiency amongpostmenopausal women in a rural block of West Bengal, India. PLoS One. 2017;12:e0188331. doi: 10.1371/journal.pone.0188331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra P, Srivastava R, Misra A, Kant S, Kardam P, Vikram NK, et al. Vitamin D status of adult females residing in Ballabgarh health and demographic surveillance system: a community-based study. Indian J Public Health. 2017;61:194–196. doi: 10.4103/ijph.IJPH_176_16. [DOI] [PubMed] [Google Scholar]
- 29.Osguthorpe JD. Pathophysiology of and potential new therapies for allergic rhinitis. Int Forum Allergy Rhinol. 2013;3:384–392. doi: 10.1002/alr.21120. [DOI] [PubMed] [Google Scholar]
- 30.Heine G, Anton K, Henz BM, Worm M. 1alpha,25-dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol. 2002;32:3395–3404. doi: 10.1002/1521-4141(200212)32:12<3395::AID-IMMU3395>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Yenigun A, Dadaci Z, Oncel M. Plasma vitamin D levels of patients with allergic rhino-conjunctivitis with positive skin prick test. Am J Rhinol Allergy. 2015;29:46–49. doi: 10.2500/ajra.2015.29.4164. [DOI] [PubMed] [Google Scholar]
- 32.Vatankhah V, Lotfizadeh M, Iranpoor H, Jafari F, Khazraei H. Comparison vitamin D serum levels in allergic rhinitis patients with normal population. Revue Française d’Allergologie. 2016;56:539–543. doi: 10.1016/j.reval.2016.10.009. [DOI] [Google Scholar]
- 33.Sudiro M, Lestari B, Madiadipoera T, Setiabudiawan B, Boesoirie T. Vitamin D deficiency is correlated with severity of allergic rhinitis. Open Access Libr J. 2017;4:1–9. doi: 10.4236/oalib.1103813. [DOI] [Google Scholar]
- 34.Arshi S, Ghalehbaghi B, Kamrava SK, Aminlou M. Vitamin D serum levels in allergic rhinitis: any difference from normal population? Asia Pac Allergy. 2012;2:45–48. doi: 10.5415/apallergy.2012.2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moradzadeh K, Larijan B, Keshtkar AA, Hossein-Nezhad A, Rajabian R, Nabipour I, et al. Normative values of vitamin D among Iranian population: a population based study. Int J Osteoporos Metab Disord. 2008;1:8–15. doi: 10.3923/ijom.2008.8.15. [DOI] [Google Scholar]
- 36.Wjst M, Hyppönen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–1086. doi: 10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 37.Modh D, Katarkar A, Thakkar B, Jain A, Shah P, Joshi K. Role of vitamin D supplementation in allergic rhinitis. Indian J Allergy Asthma Immunol. 2014;28:35–39. doi: 10.4103/0972-6691.134223. [DOI] [Google Scholar]