Abstract
Introduction
Certolizumab pegol (CZP), an Fc-free, PEGylated anti-tumour necrosis factor biologic, dosed at 400 mg every 2 weeks (Q2W) and 200 mg Q2W over 16 weeks, resulted in improvements in Japanese patients with moderate to severe plaque psoriasis (PSO); no new safety signals were identified. We present 52-week efficacy and safety results.
Methods
Patients ≥ 20 years with PSO ≥ 6 months [Psoriasis Area and Severity Index (PASI) ≥ 12, body surface area ≥ 10%, Physician’s Global Assessment (PGA) ≥ 3] were randomised 1:2:2 to placebo Q2W, CZP 400 mg Q2W and CZP 200 mg Q2W (400 mg weeks 0/2/4) for 16 weeks. Week 16 PASI 50 responders continued through week 52; CZP 200 mg Q2W-randomised patients were re-randomised 1:1 to CZP 200 mg Q2W or CZP 400 mg Q4W; patients initially randomised to other treatment groups continued in the same group. Outcomes included PASI 75/90/100, PGA 0/1, Dermatology Life Quality Index (DLQI) 0/1, Itch Numeric Rating Scale (INRS) 0, modified Nail Psoriasis Severity Index (mNAPSI), durability of response for week 16 PASI 75/90 responders, and safety.
Results
Of 26/53/48 patients randomised to placebo, CZP 400 mg Q2W and CZP 200 mg Q2W, 2/47/39 completed week 52, respectively. PASI 75/90 responses were generally maintained from weeks 16 to 52 for all CZP doses. Most week 16 PASI 75/90 achievers maintained their response through week 52. PASI 75/90/100 responses at week 52 in the CZP 400 mg Q2W and CZP 200 mg Q2W groups were 83.0/81.1/41.5% and 72.9/60.4/18.8%, respectively; DLQI/INRS remission rates were 64.2/50.9% in CZP 400 mg Q2W and 58.3/27.1% in CZP 200 mg Q2W-treated patients. Reductions in mNAPSI observed for CZP-treated groups were maintained through week 52. No new safety signals were identified.
Conclusion
CZP treatment resulted in improvements in signs and symptoms of PSO, which were maintained through week 52. The 400 mg Q2W dose could provide additional clinical benefit.
Trial Registration
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00520-0.
Keywords: Anti-tumour necrosis factor, Certolizumab pegol, Japanese patients, Maintenance therapy, Plaque psoriasis
Key Summary Points
| Why carry out this study? |
| Certolizumab pegol (CZP) is an Fc-free, PEGylated anti-tumour necrosis factor biologic. |
| In a phase 2/3 trial, CZP dosed at 400 mg every 2 weeks (Q2W) and 200 mg Q2W over 16 weeks showed clinically meaningful improvements in Japanese patients with moderate to severe plaque psoriasis (PSO). |
| Due to the chronic nature of PSO, patients are required to remain on treatment for many years. Therefore, it is desirable that treatments remain durable over the longer term. |
| Here we report the 52-week efficacy and safety of CZP in Japanese patients with PSO. |
| What was learned from the study? |
| Treatment with CZP was associated with improvements in signs and symptoms of PSO, which were maintained through week 52. |
| In general, numerically higher response rates were observed with the CZP 400 mg Q2W dose through weeks 0–52. The 400 mg Q2W dose could provide additional clinical benefit. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.14262083.
Introduction
Psoriasis manifests as raised, red, demarcated papules and plaques on the skin, often covered by white or silver scales [1]. Histologically, it is characterised by epidermal hyperplasia, vascular hyperplasia and recruitment of leukocyte subsets [2, 3]. Psoriatic arthritis may also develop in patients with psoriasis, which may result in pain, joint deformation and disability [1], leading to impairment in the quality of life (QoL). Nail deformity may also sometimes co-exist in psoriatic patients [4]. Psoriasis has been considered as a systemic disease [5], and conditions associated with chronic systemic inflammation, such as obesity, are known risk factors [6–8]. Itching, pain, a negative body image due to skin lesions, nail deformities, anxiety and depression impair the QoL in patients with psoriasis [4, 9, 10]. Therefore, the management of psoriasis involves the control of skin symptoms as well as the management of other comorbidities and impaired QoL.
Tumour necrosis factor-alpha (TNF-α), a cytokine mainly produced by dendritic cells, plays a critical role in the production of self-amplifying inflammatory responses in keratinocytes [3, 11]. Consequently, TNF-α inhibition is an effective strategy to manage psoriasis [12, 13].
Certolizumab pegol (CZP) is a unique Fc-free, PEGylated, anti-TNF biologic. PEGylation increases the half-life of CZP to 14 days [14]. Unlike other anti-TNF agents, CZP lacks the IgG Fc region that binds the neonatal Fc receptor for IgG (FcRn). Indeed, recent prospective studies have shown minimal to no placental transfer of CZP from mothers to infants [15]. CZP was first approved in the USA and EU in 2008 and 2009, respectively, for the treatment of rheumatoid arthritis (RA). Currently, it is additionally approved for the treatment of moderate to severe plaque psoriasis (PSO), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) [comprising both ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA)] [16, 17]. In the USA and Switzerland, it is also approved for the treatment of Crohn’s disease [16, 18]. In Japan, CZP is currently approved for the treatment of RA, PSO, PsA, generalised pustular psoriasis (GPP) and erythrodermic psoriasis (EP) [19].
In a 2014 review of all phase 3 studies of biologic therapies (adalimumab, etanercept, infliximab and ustekinumab) used in the treatment of psoriasis, approximately a third of patients receiving biologics tended to lose their PASI 75 (at least 75% reduction from baseline in Psoriasis Area and Severity Index) response during 0.8–3.9 years of follow-up [20], indicating the need for treatments that have long-lasting efficacy profiles to address the chronic nature of the disease.
In a phase 2/3, multi-centre, randomised, double-blind, placebo-controlled Japanese clinical trial, which evaluated the safety and efficacy of CZP for the treatment of moderate to severe PSO in Japanese patients (NCT03051217), CZP dosed at 400 mg every 2 weeks (Q2W) and 200 mg Q2W over 16 weeks showed clinically meaningful improvements in moderate to severe PSO, with no new safety signals identified compared with previously reported data [21]. Here, we present 52-week outcomes from the same trial.
Methods
Study Design
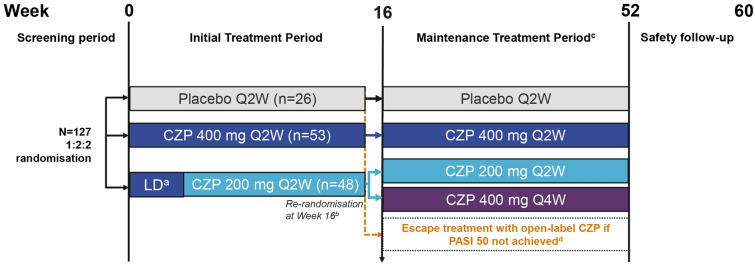
Patients were enrolled across 33 medical institutions in Japan, and were randomised 1:2:2 to placebo Q2W, CZP 400 mg Q2W and CZP 200 mg Q2W (with a loading dose of CZP 400 mg Q2W at weeks 0, 2 and 4), for 16 weeks of treatment (Fig. 1). Patients who achieved PASI 50 (at least 50% reduction from baseline PASI) response at week 16 continued through to week 52 as follows: patients initially randomised to CZP 400 mg Q2W continued to receive CZP 400 mg Q2W, patients initially randomised to CZP 200 mg Q2W were re-randomised (1:1) to receive either CZP 200 mg Q2W or CZP 400 mg every 4 weeks (Q4W) (with placebo administered on alternate dosing weeks to maintain the blind) and patients initially randomised to placebo continued to receive placebo. Patients who did not achieve a PASI 50 response at week 16 entered the escape arm and received open-label CZP 200 mg Q2W (CZP 400 mg Q2W loading dose for the first three visits). Patients who received open-label CZP 200 mg Q2W in the escape arm and did not achieve a PASI 50 response could have their dose increased to CZP 400 mg Q2W, at the discretion of the investigator. All double-blind CZP and placebo treatments were administered subcutaneously by trained institutional personnel not involved in any other study procedures.
Fig. 1.
Study design. aPatients received a loading dose of CZP 400 mg at weeks 0, 2 and 4; bpatients initially randomised to CZP 200 mg Q2W were re-randomised (1:1) to receive either CZP 200 mg Q2W or CZP 400 mg Q4W (with placebo administered on alternate dosing weeks to maintain the blind); cpatients who achieved PASI 50 continued therapy to week 52; dpatients who did not achieve PASI 50 entered an open-label escape arm and received CZP 200 mg Q2W (CZP 400 mg Q2W loading dose for the first three visits). CZP certolizumab pegol, LD loading dose, PASI 50 ≥ 50% reduction from baseline in Psoriasis Area and Severity Index, Q2W every 2 weeks, Q4W every 4 weeks
This study protocol was reviewed by the institutional review board (IRB) of each institution prior to implementation. Written informed consent was obtained from all patients. The study was carried out in accordance with the applicable regulatory and International Council for Harmonization Good Clinical Practice requirements and the Helsinki Declaration of 1964, 739 and its later amendments.
Study Participants
The trial enrolled patients ≥ 20 years of age with moderate to severe PSO of disease duration ≥ 6 months with baseline PASI ≥ 12 and affected body surface area (BSA) of ≥ 10% and Physician’s Global Assessment (PGA) ≥ 3 on a 5-point scale. All patients were candidates for systemic psoriasis therapy, phototherapy or chemophototherapy. Patients were excluded if they had a history of primary failure to any biologic (i.e. no response within the first 12 weeks of treatment) or secondary failure to > 2 biologics (i.e. patient initially responded then discontinued treatment owing to loss of response after 12 weeks of treatment); a diagnosis of any inflammatory arthritis other than psoriatic arthritis; or guttate, drug-induced, erythrodermic or pustular psoriasis. Full inclusion and exclusion criteria have been published previously [21].
Efficacy Evaluations
The primary efficacy endpoint of the study and other secondary outcomes (efficacy and safety) over 16 weeks of CZP treatment were previously reported [21]. Outcomes reported here were other outcomes of the study.
The blinded maintenance treatment groups summarised patients on the basis of the treatment group assigned at week 16. Efficacy outcomes through weeks 16–52 were presented according to the blinded maintenance treatment groups. All other outcomes were presented according to the randomised treatment groups, which summarised patients on the basis of original assignment at baseline, and consisted of placebo, CZP 400 mg Q2W and CZP 200 mg Q2W (includes patients who received CZP 200 mg Q2W and CZP 400 mg Q4W during the maintenance period) groups.
Clinical efficacy during the maintenance treated period was assessed as the proportions of patients achieving PASI 75, PASI 90 (at least 90% reduction from baseline PASI), PASI 100 (100% reduction from baseline PASI) and PGA 0/1 [PGA score of 0 or 1 (‘clear’ or ‘almost clear’) with ≥ 2-point improvement from baseline]. Durability of response was assessed as the proportion of patients achieving PASI 75 and PASI 90 from weeks 16 to 52, in patients who achieved PASI 75 or PASI 90 at week 16. The proportion of patients achieving PASI 75/90/100, PGA 0/1, absolute PASI scores of < 1, < 2, < 3 and < 5, and absolute PASI scores for individual patients from weeks 0 to 52 were also reported.
Patient-reported outcomes (PRO) were assessed by Dermatology Life Quality Index (DLQI) and Itch Numeric Rating Scale (INRS). The proportion of patients who achieved DLQI and INRS remission (i.e. DLQI 0/1 and INRS 0) from weeks 0 to 52 was reported. A DLQI absolute score of ≤ 1 indicates DLQI remission (i.e. no or small impact of the disease on health-related quality of life) [22]. The INRS is a simple, single-item instrument to assess the patient-reported severity of itch at its highest intensity during the past 24-h period. A score of 0 indicates itch remission [23]. The modified Nail Psoriasis Severity Index (mNAPSI) was assessed in patients with psoriatic nail disease at baseline. The most-affected nail at baseline was selected as the target nail and was the only one assessed through the study.
Safety Evaluations
Safety data for all patients who received ≥ 1 dose of CZP through weeks 0–52, under double-blind or open-label escape-arm treatment conditions, were reported. Adverse events (AEs) were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) v.18.1. Serious AEs were defined as AEs that met one or more of the following criteria: death; life threatening; significant or persistent disability or incapacity; congenital anomaly or birth defect (including that occurring in a foetus); an important medical event that may jeopardise the patient and may require surgical intervention; or initial inpatient hospitalisation or prolongation of hospitalisation. Treatment-emergent AEs (TEAEs) were defined as events with onset at the time of or after the first dose of study medication and up to 70 days after the final dose of study medication. The pre-defined AEs of interest (AEOI) were serious infections, including opportunistic infections; malignancies, including lymphoma; congestive heart failure; demyelinating-like disorders; aplastic anaemia, pancytopenia, thrombocytopenia, neutropenia and leukopenia; serious bleeding events; lupus and lupus-like illness; serous skin reactions such as Stevens–Johnson Syndrome, toxic epidermal necrosis and erythema multiforme; and potential Hy’s law case, defined as ≥ 3 × the upper limit of normal (ULN) in alanine transaminase (ALT) or aspartate transaminase (AST) with co-existing ≥ 2 × ULN in total bilirubin, in the absence of ≥ 2 × ULN in alkaline phosphatase (ALP), with no alternative explanation for the biochemical abnormality. Incidence rates (IR) and associated confidence intervals (CI) were calculated as incidence of new cases per 100 patient-years (PY).
Statistical Analyses
The following data were generated post hoc: PASI 75/90/100, PGA 0/1, absolute PASI thresholds, DLQI 0/1 and INRS 0 responder rates, over weeks 0–52. Other analyses were pre-specified in the protocol.
For PASI 75/90/100, PGA 0/1, absolute PASI < 1, < 2, < 3 and < 5, DLQI 0/1 and INRS 0 responder rates, missing data were imputed using non-responder imputation (NRI); for mNAPSI, last observation carried forward (LOCF) was used. Patients who entered the escape arm were imputed as non-responders at the point of escape. Those who discontinued were imputed as non-responders from the point of premature withdrawal through week 52.
Safety data were analysed on the safety set, for patients who received CZP from weeks 0–52 under double-blind and open-label escape treatment conditions.
Data were summarised using descriptive statistics only. Simple proportions were calculated to estimate responder rates. No statistical tests were performed.
Results
Patient Disposition and Baseline Disease Characteristics
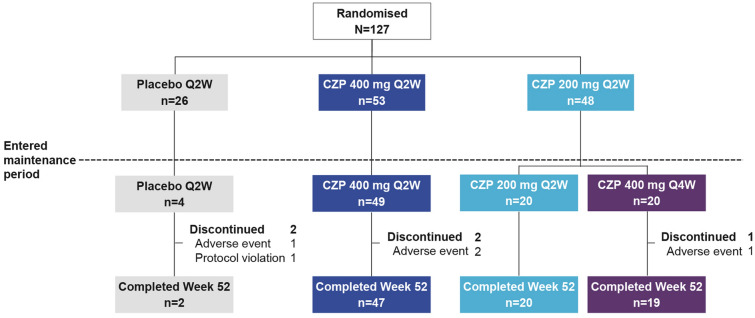
Of the 168 participants screened, 127 patients were randomised to placebo (n = 26), CZP 400 mg Q2W (n = 53) and CZP 200 mg Q2W (n = 48) (Fig. 2). Of the randomised patients, 2/26 placebo-treated patients, 47/53 CZP 400 mg Q2W-treated patients and 39/48 CZP 200 mg Q2W-treated patients completed week 52 (Fig. 2). Demographics and baseline disease characteristics were balanced across treatment groups (Table 1).
Fig. 2.
Patient disposition. Patient disposition during the initial treatment period (weeks 0–16) was previously reported [21]. CZP certolizumab pegol, Q2W every 2 weeks, Q4W every 4 weeks
Table 1.
Demographics and baseline disease characteristics
| Characteristic | Placebo Q2W n = 26 |
CZP 400 mg Q2W n = 53 |
CZP 200 mg Q2W n = 48 |
|---|---|---|---|
| Demographics | |||
| Age, years, mean ± SD | 47.9 ± 11.4 | 52.4 ± 11.6 | 48.4 ± 13.5 |
| Male, n (%) | 21 (80.8) | 42 (79.2) | 36 (75.0) |
| Weight, kg, mean ± SD | 75.1 ± 15.8 | 71.6 ± 14.3 | 72.6 ± 14.3 |
| Height, cm, mean ± SD | 165.7 ± 7.1 | 165.6 ± 7.5 | 167.5 ± 7.5 |
| BMI, kg/m2, mean ± SD | 27.3 ± 5.1 | 26.0 ± 4.3 | 26.0 ± 5.5 |
| Baseline disease characteristics | |||
| Duration of disease, years, mean ± SD | 12.7 ± 8.6 | 13.2 ± 9.3 | 12.7 ± 10.1 |
| Concurrent PsA, n (%) | 5 (19.2) | 10 (18.9) | 6 (12.5) |
| PASI, mean ± SD | 24.5 ± 10.4 | 26.5 ± 11.0 | 24.5 ± 12.6 |
| DLQI, mean ± SD | 10.5 ± 7.2 | 9.2 ± 7.4 | 10.5 ± 6.6 |
| INRS, mean ± SD | 6.1 ± 2.5 | 5.7 ± 2.6 | 5.6 ± 2.4 |
| BSA affected, %, mean ± SD | 38.3 ± 17.2 | 39.7 ± 21.4 | 38.7 ± 21.9 |
| PGA score, n (%) | |||
| 3: moderate | 17 (65.4) | 31 (58.5) | 31 (64.6) |
| 4: severe | 9 (34.6) | 22 (41.5) | 17 (35.4) |
| Any systematic PSO treatment, n (%) | 20 (76.9) | 37 (69.8) | 39 (81.3) |
| Prior biologic use, n (%) | |||
| One therapy | 8 (30.8) | 16 (30.2) | 14 (29.2) |
| Two therapies | 0 (0) | 3 (5.7) | 0 (0) |
| Anti-TNF | 1 (3.8) | 3 (5.7) | 3 (6.3) |
Full analysis set. BMI body mass index, BSA body surface area, CZP certolizumab pegol, DLQI Dermatology Life Quality Index, INRS Itch Numeric Rating Scale, PASI Psoriasis Area and Severity Index, PGA Physician’s Global Assessment, PsA psoriatic arthritis, PSO plaque psoriasis, Q2W every 2 weeks, SD standard deviation, TNF tumour necrosis factor
Efficacy During the Maintenance Treatment Period
The efficacy of CZP during weeks 0–16 has been previously shown [21]. Among patients who were PASI 50 responders at week 16 and who received CZP treatment during the maintenance treatment period, PASI 75 responder rates were generally maintained from weeks 16 to 52 for all treatment groups, with ≥ 85.0% PASI 75 responders in each group at week 52 (Table 2). Similar trends were observed for PASI 90, PASI 100 and PGA 0/1. For patients who were initially treated with CZP 200 mg Q2W and re-randomised at week 16 to either CZP 200 mg Q2W or CZP 400 mg Q4W, similar responder rates were observed between the treatment groups during the maintenance treatment period.
Table 2.
Efficacy outcomes during maintenance treatment period
| CZP 400 mg Q2W/CZP 400 mg Q2W (n = 49) | CZP 200 mg Q2W/CZP 200 mg Q2W (n = 20) | CZP 200 mg Q2W/CZP 400 mg Q4W (n = 20) | CZP 200 mg Q2W combined (n = 40) | |
|---|---|---|---|---|
| PASI 75, n (%) | ||||
| Week 16 | 46 (93.9) | 17 (85.0) | 17 (85.0) | 34 (85.0) |
| Week 52 | 44 (89.8) | 18 (90.0) | 17 (85.0) | 35 (87.5) |
| PASI 90, n (%) | ||||
| Week 16 | 40 (81.6) | 12 (60.0) | 13 (65.0) | 25 (62.5) |
| Week 52 | 43 (87.8) | 15 (75.0) | 14 (70.0) | 29 (72.5) |
| PASI 100, n (%) | ||||
| Week 16 | 9 (18.4) | 6 (30.0) | 5 (25.0) | 11 (27.5) |
| Week 52 | 22 (44.9) | 4 (20.0) | 5 (25.0) | 9 (22.5) |
| PGA 0/1, n (%) | ||||
| Week 16 | 35 (71.4) | 11 (55.0) | 13 (65.0) | 24 (60.0) |
| Week 52 | 38 (77.6) | 15 (75.0) | 15 (75.0) | 30 (75.0) |
Responder rates are from the BM Full Analysis Set (IA definition) and summarised according to blinded maintenance treatment group. Missing values were imputed using NRI. Only two patients continued placebo treatment to week 52; therefore, placebo data are not shown. BM blinded maintenance, IA interim analysis, NRI non-responder imputation, PASI 75/90/100 at least 75%/90%/100% improvement from baseline in Psoriasis Area and Severity Index, PGA Physician’s Global Assessment, Q2W every 2 weeks, Q4W every 4 weeks
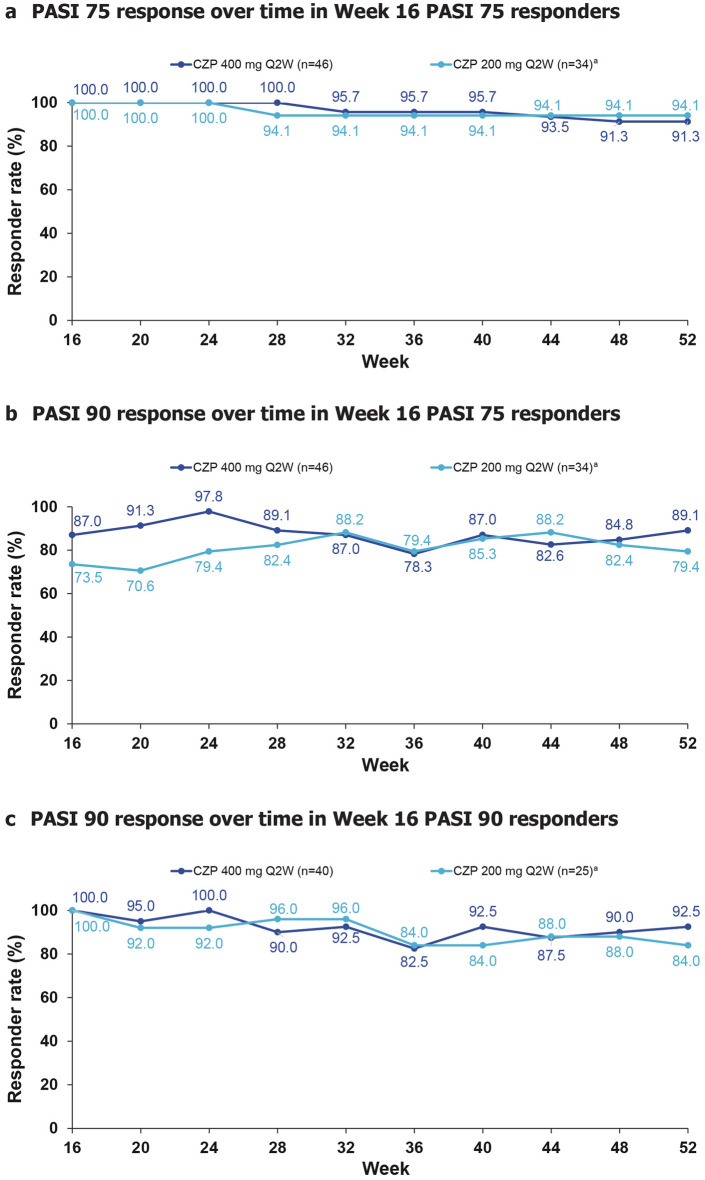
Durability of Week 16 PASI 75 and PASI 90 Response during the Maintenance Treatment Period
In patients who achieved PASI 75 at week 16, 91.3% of CZP 400 mg Q2W- and 94.1% of CZP 200 mg Q2W-treated (includes patients receiving CZP 200 mg Q2W and CZP 400 mg Q4W) patients maintained their response to week 52 (Fig. 3A) Of the patients who achieved PASI 75 at week 16, 87.0% of CZP 400 mg Q2W- and 73.5% of CZP 200 mg Q2W-treated patients also achieved PASI 90; 89.1% of CZP 400 mg Q2W- and 79.4% of CZP 200 mg Q2W-treated patients maintained the PASI 90 response to week 52 (Fig. 3B). In patients who achieved PASI 90 at week 16, 92.5% of CZP 400 mg Q2W- and 84.0% of CZP 200 mg Q2W-treated patients maintained their PASI 90 response to week 52 (Fig. 3C).
Fig. 3.
Durability of response. Responder rates are summarised according to patients’ blinded maintenance treatment group. Missing values were imputed using NRI. Placebo data are not shown as there were only two patients in the placebo group who were week 16 PASI 75 responders and no patients in the placebo group were week 16 PASI 90 responders. aIncludes patients who received CZP 200 mg Q2W and CZP 400 mg Q4W during maintenance. CZP certolizumab pegol, NRI non-responder imputation, PASI 75/90 at least 75%/90% improvement from baseline in Psoriasis Area and Severity Index, Q2W every 2 weeks, Q4W every 4 weeks
Efficacy Outcomes over 52 Weeks
A higher proportion of patients achieved PASI 75 in both CZP groups versus placebo at week 16 (placebo: 7.7%; CZP 400 mg Q2W: 86.8%; CZP 200 mg Q2W: 70.8%) (Fig. 4A); the proportion of responders in the CZP groups were maintained through to week 52. Similar trends were observed with PASI 90, PASI 100 and PGA 0/1 (Fig. 4B–D). PASI 75 response in patients receiving CZP 400 mg Q2W was numerically greater compared with CZP 200 mg Q2W from week 8 onwards (Fig. 4A). At week 52, 81.1% CZP 400 mg Q2W patients achieved PASI 90 (Fig. 4B), and 41.5% achieved total skin clearance (PASI 100) (Fig. 4C); 84.9% CZP 400 mg Q2W-treated patients and 75.0% CZP 200 mg Q2W-treated patients achieved PASI < 3 at week 52 (Fig. S1 in Supplementary Material). Absolute PASI thresholds were reached by a higher proportion of patients in the CZP 400 mg Q2W group than in the CZP 200 mg Q2W group. The absolute PASI for each patient in both CZP groups decreased over weeks 0–52 (Figs. S2 and S3 in Supplementary Material). The mean absolute PASI of the CZP 400 mg Q2W group was lower than that of CZP 200 mg Q2W group at week 52 (1.5 versus 2.6, respectively) (Fig. S2B in Supplementary Material).
Fig. 4.
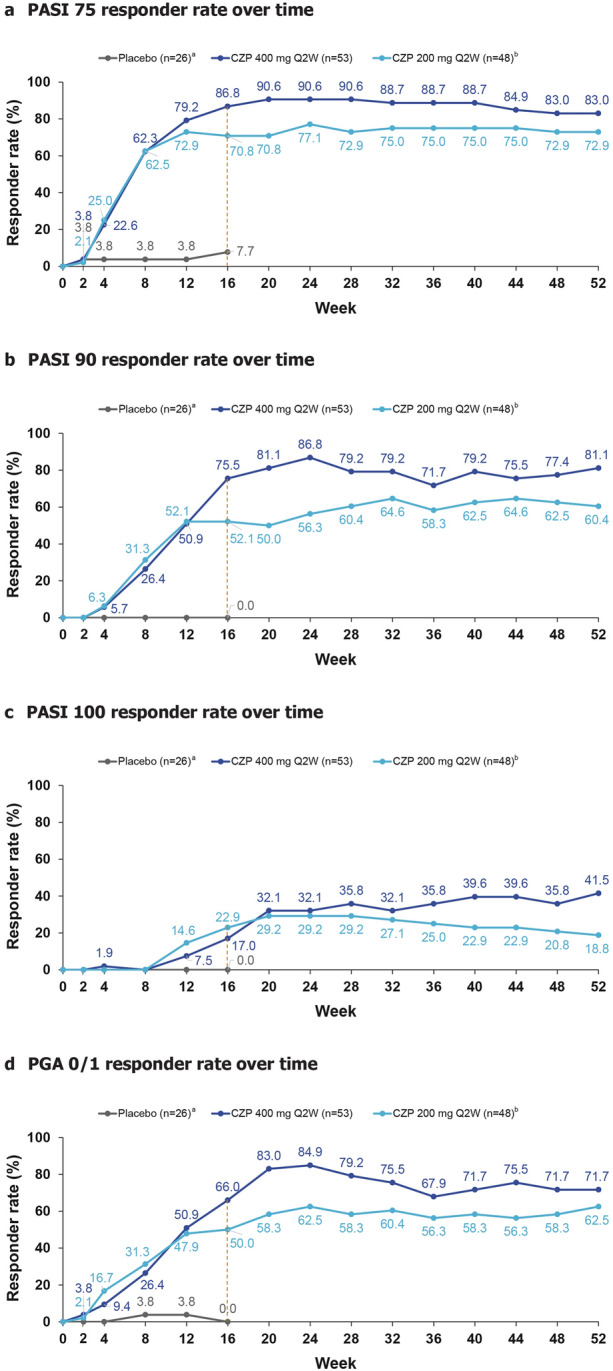
Efficacy outcomes to week 52. Responder rates are summarised according to patients’ originally randomised treatment group. Missing values were imputed using NRI. aOnly four patients continued placebo treatment in maintenance; therefore, placebo data are only shown to week 16; bincludes patients who received CZP 200 mg Q2W and CZP 400 mg Q4W during maintenance. CZP certolizumab pegol, NRI non-responder imputation, PASI 75/90 at least 75%/90% improvement from baseline in Psoriasis Area and Severity Index, PGA 0/1 Physician's Global Assessment (‘clear’ or ‘almost clear’ with ≥ 2-point improvement from baseline), Q2W every 2 weeks, Q4W every 4 weeks
Other Efficacy Outcomes
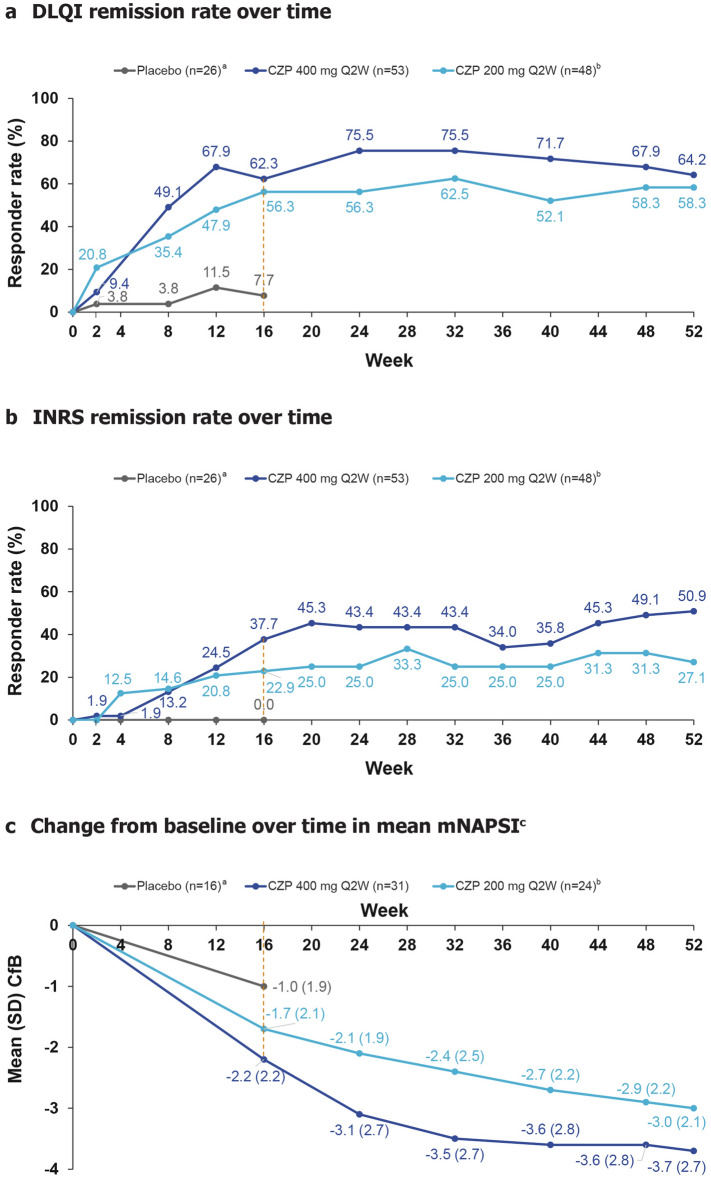
Higher DLQI remission rates were observed for both CZP groups versus placebo at week 16 (placebo: 7.7%; CZP 400 mg Q2W: 62.3%; CZP 200 mg Q2W: 56.3%) (Fig. 5A). The response to CZP was maintained to week 52 for both CZP doses. At week 52, 64.2% of CZP 400 mg Q2W- and 58.3% of CZP 200 mg Q2W-treated patients achieved DLQI remission. The DLQI 0/1 response rates were higher in patients receiving CZP 400 mg Q2W compared with CZP 200 mg Q2W from weeks 8 to 52. Similar trends were observed for INRS remission. At week 52, 50.9% of CZP 400 mg Q2W- and 27.1% of CZP 200 mg Q2W-treated patients achieved INRS remission (Fig. 5B). Reductions in mNAPSI score were observed for both CZP-treated groups from baseline to week 16, and these were maintained through to week 52. At week 52, there was a mean (standard deviation; SD) decrease of −3.7 (2.7) and −3.0 (2.1) in the CZP 400 mg Q2W and CZP 200 mg Q2W groups, respectively (Fig. 5C).
Fig. 5.
Other efficacy outcomes to week 52. Responder rates and mean change from baseline are summarised according to patients’ originally randomised treatment group. Missing values were imputed using NRI (for DLQI and INRS) and LOCF for mNAPSI. aOnly four patients continued placebo treatment in maintenance; therefore, placebo data are only shown to week 16; bincludes patients who received CZP 200 mg Q2W and CZP 400 mg Q4W during maintenance; cmNAPSI was assessed only in patients with psoriatic nail disease at baseline. CfB change from baseline, CZP certolizumab pegol, DLQI Dermatology Life Quality Index, INRS Itch Numeric Rating Scale, mNAPSI modified Nail Psoriasis Severity Index, NRI non-responder imputation, Q2W every 2 weeks, Q4W every 4 weeks, SD standard deviation
Safety Assessments
The safety of CZP versus placebo up to week 16 has been reported previously [21]. After 52 weeks of treatment, the patient exposures were 62.9, 63.5 and 126.5 patient-years in the CZP 400 mg Q2W, CZP 200 mg Q2W and All CZP groups, respectively (Table 3). The IRs (95% CI) for any TEAE were 261.0 (196.1–340.5) for the CZP 400 mg Q2W and 352.7 (271.0–451.3) for the CZP 200 mg Q2W treatment group. The most common TEAE was nasopharyngitis, with an IR of 56.7 (42.5–74.2) in the All CZP group. In total, nine serious TEAEs were reported in eight CZP-treated patients (6.6%, IR 6.6, 95% CI 2.8–12.9): six were considered drug-related by the investigator (thrombocytopenia, herpes zoster, neutropenia, sarcoidosis and two latent tuberculosis). No other AEOIs were reported from weeks 0 to 52 in that there were no opportunistic infections (including active tuberculosis), malignancies (including lymphoma), TEAEs related to congestive heart failure, serious cardiovascular events, serious skin disorders (such as Stevens–Johnson syndrome or lupus erythematosus) or deaths. One case of increased anti-nuclear antibody and two cases of increased cell marker (KL-6) were reported.
Table 3.
Summary of TEAEs from baseline to week 52
| Number of patients (% of patients) [events] IR (95% CI) |
CZP 400 mg Q2W (n = 64) Patient exposure: 62.9 PY |
CZP 200 mg Q2Wa (n = 72) Patient exposure: 63.5 PY |
All CZP (N = 122) Patient exposure: 126.5 PY |
|---|---|---|---|
| Any TEAE |
54 (84.4) [187] 261.0 (196.1–340.5) |
63 (87.5) [230] 352.7 (271.0–451.3) |
111 (91.0) [417] 307.5 (252.9–370.3) |
| Most commonly reported TEAE (PT ≥ 10% in All CZP group) | |||
| Nasopharyngitis |
28 (43.8) [48] 64.1 (42.6–92.6) |
25 (34.7) [30] 49.1 (31.8–72.5) |
53 (43.4) [78] 56.7 (42.5–74.2) |
| Psoriasis |
5 (7.8) [5] 8.1 (2.6–18.9) |
8 (11.1) [8] 13.1 (5.7–25.9) |
13 (10.7) [13] 10.6 (5.7–18.2) |
| Serious TEAEs |
6 (9.4) [7] 10.1 (3.7–22.1) |
2 (2.8) [2] 3.2 (0.4–11.5) |
8 (6.6) [9] 6.6 (2.8–12.9) |
| Blood and lymphatic system disorders |
1 (1.6) [1] 1.6 (0.0–8.9) |
1 (1.4) [1] 1.6 (0.0–8.9) |
2 (1.6) [2] 1.6 (0.2–5.8) |
| Neutropenia | 0 |
1 (1.4) [1] 1.6 (0.0–8.9) |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Thrombocytopenia |
1 (1.6) [1] 1.6 (0.0–8.9) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Eye disorders |
1 (1.6) [1] 1.6 (0.0–8.9) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Eyelid ptosis |
1 (1.6) [1] 1.6 (0.0–8.9) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Gastrointestinal disorders |
1 (1.6) [1] 1.6 (0.0–9.0) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Large intestine polyp |
1 (1.6) [1] 1.6 (0.0–9.0) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Immune system disorders | 0 |
1 (1.4) [1] 1.6 (0.0–8.8) |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Sarcoidosis | 0 |
1 (1.4) [1] 1.6 (0.0–8.8) |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Infections and infestations |
2 (3.1) [3] 3.3 (0.4–11.8) |
0 |
2 (1.6) [3] 1.6 (0.2–5.8) |
| Herpes zoster |
1 (1.6) [1] 1.6 (0.0–9.0) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Latent tuberculosis |
2 (3.1) [2] 3.3 (0.4–11.7) |
0 |
2 (1.6) [2] 1.6 (0.2–5.8) |
| Skin and subcutaneous tissue disorders |
1 (1.6) [1] 1.6 (0.0–8.9) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Henoch–Schonlein purpura |
1 (1.6) [1] 1.6 (0.0–8.9) |
0 |
1 (0.8) [1] 0.8 (0.0–4.4) |
| Discontinuation due to TEAEs | 4 (6.3) [4] | 5 (6.9) [5] | 9 (7.4) [9] |
| Drug-related TEAEs | 18 (28.1) [34] | 21 (29.2) [43] | 39 (32.0) [77] |
| Severe TEAEs | 4 (6.3) [4] | 3 (4.2) [3] | 7 (5.7) [7] |
| Deaths | 0 | 0 | 0 |
Safety set. TEAEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) v.18.1. Only two patients continued placebo treatment to week 52; therefore, placebo data are not shown. Patients who received both CZP 200 mg Q2W and CZP 400 mg Q2W are included once in the population count for the All CZP group. aPatients may receive either CZP 200 mg Q2W or CZP 400 mg Q4W during the maintenance treatment period
CI confidence interval, CZP certolizumab pegol, IR incidence rates, PT preferred term, PY patient year, Q2W every 2 weeks, Q4W every 4 weeks, TEAE treatment-emergent adverse events
Discussion
In this Japanese phase 2/3 trial, treatment with CZP dosed at either 400 mg Q2W or 200 mg Q2W resulted in improvements in signs and symptoms of PSO compared with placebo to week 16 [21], and these improvements with CZP were sustained through week 52. Responder rates were generally sustained at each visit from week 16 to 52 for all CZP-treated groups and there were no meaningful differences in efficacy in patients who were re-randomised to either CZP 200 mg Q2W or CZP 400 mg Q4W. In general, greater response rates through weeks 0–52 were observed with the CZP 400 mg Q2W dose; week 52 PASI 90 and PASI 100 responses in this group were comparable to other biologics evaluated in Japanese patients with PSO [24–26]. In this Japanese study, week 52 PASI 75 response was achieved by 83% of patients in the CZP 400 mg Q2W arm. Based on current literature of other anti-TNF agents, PASI 75 was achieved by 67% of Japanese patients with PSO on adalimumab [27]. Compared with other classes of biologics, the week 52 PASI 90 response observed with CZP 400 mg Q2W in this trial was comparable to the response of brodalumab (anti-IL-17A) [24], guselkumab (anti-IL-23) [25], ixekizumab (anti-IL-17A) [26], risankizumab (anti-IL-23) [28] and secukinumab (anti-IL-17A) [29] in Japanese patients with PSO (41–93%), and similar trends were also observed for PASI 100 (39–56%) [24–26, 28].
The results of this Japanese study were comparable to the two larger global phase 3 studies examining the efficacy of CZP in PSO patients (CIMPASI-1 and CIMPASI-2). Three-year efficacy data pooled from the CIMPASI-1 and CIMPASI-2 phase 3 trials demonstrated sustained and durable response to CZP dosed at either 400 mg or 200 mg Q2W; additional clinical benefits may be conferred with the higher dose [30].
In this study, we found that the majority of the initial week 16 responders were able to maintain their response through to week 52. Due to the chronic nature of psoriasis, patients are required to remain on treatment for many years. Therefore, it is desirable that treatments remain durable over the longer term. Evidence of loss of response over time has been observed with some biologics used in the treatment of PSO; over 33–52 weeks, 24% of patients receiving adalimumab in a phase 3 clinical trial were reported to lose their PASI 75 response [20], and in a real world study, 19% of patients receiving secukinumab reported loss of efficacy at around 24–32 weeks of therapy [31].
Increasing expert opinion recommends the use of absolute PASI for the assessment of treatment efficacy; and absolute PASI ≤ 3 has been suggested to be a suitable treatment target for PSO [32, 33]. In this study, the absolute PASI in both CZP groups decreased over weeks 0–52, with 85% of CZP 400 mg Q2W- and 75% of CZP 200 mg Q2W-treated patients achieving PASI < 3 at week 52.
DLQI and INRS are important PRO tools to confirm that any documented clinical improvements in PSO are meaningful to the patient [9, 23]. The notable improvements in QoL from CZP treatment at week 16 as demonstrated by the DLQI and INRS remission, was maintained through to Week 52 in this study. Over 50% of patients receiving CZP reported DLQI remission at week 52, indicating that disease was no longer impacting their QoL. In a survey on patient perspectives in the management of PSO, 43% of patients indicated itching to be among the most bothersome signs or symptoms of PSO [34], highlighting the need for suitable treatments that will improve the patients’ QoL. In this study, the proportion of patients receiving CZP who were itch-free increased over time through week 52, with half of the CZP 400 mg Q2W-treated patients being itch-free at week 52.
No new safety signals were identified in this study as compared with previously reported data for CZP in other indications, and to other anti-TNF medications approved for PSO [35–39], and the incidence rates of TEAEs did not increase with longer exposure [21].
One of the limitations of this trial was the lack of an active comparator. The trial also did not include patients with a history of primary failure to biologic therapy. As all clinical trials have strict inclusion and exclusion criteria which may affect the generalisability of these results to clinical practice, there is a need to use real-world data in registries to monitor efficacy and safety of CZP over the longer term. Additionally, this trial had a small sample size. However, both safety and efficacy results were comparable to those from the larger global studies of CZP in PSO patients.
Conclusion
Overall, these data show that CZP is an effective treatment in Japanese patients with moderate to severe PSO over 52 weeks of treatment, with no new safety signals identified as compared with previously reported data. After initial improvements observed at week 16, PASI 75 and PASI 90 responses remained high through to week 52. CZP could provide a suitable treatment option for patients with PSO in Japan.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients, the investigators and their teams who took part in this study.
Funding
This study was sponsored by UCB Pharma. This article was based on the original study PS0017 (NCT03051217) sponsored by UCB Pharma. The journal's Rapid Service Fee was funded by UCB Pharma.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Substantial contributions to study conception and design: YU, AA, SI, YT, SS, AM, SS, NH, NT, HN; substantial contributions to data acquisition, analysis and interpretation: YU, AA, SI, YT, SS, AM, SS, NH, NT, HN; drafting the article or revising it critically for important intellectual content: YU, AA, SI, YT, SS, AM, SS, NH, NT, HN; final approval of the version of the article to be published: YU, AA, SI, YT, SS, AM, SS, NH, NT, HN.
Medical writing, editorial, and other assistance
The authors also acknowledge Sadayoshi Onodera, PhD, UCB Pharma, Japan, for publication coordination and Yi Ling Teo, PhD, from Costello Medical, Singapore, for medical writing and editorial assistance based on the authors’ input and direction. This assistance was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
List of Investigators
The authors would like to thank the PS0017 Study Group: Masatoshi Abe of Kojinkai Sapporo Skin Clinic, Satoru Arai of St Luka’s International Hospital, Akihiko Asahina of The Jikei University Hospital, Yoshihide Asano of The University of Tokyo Hospital, Koki Endo of Iwate Medical University Hospital, Takafumi Etoh of Tokyo Teishin Hospital, Susumu Fujiwara of Kobe University Hospital, Mari Higashiyama of Nippon Life Saiseikai Public Interest Foundation and Nippon Life Hospital, Masaru Homma of Asahikawa Medical University Hospital, Atsuyuki Igarashi of NTT Medical Center Tokyo, Shinichi Imafuku of Fukuoka University Hospital, Norito Katoh of University Hospital Kyoto Prefectural University, Akira Kawada of Kindai University Hospital, Mayumi Komine of Jichi Medical University Hospital, Atsuko Matsuo of Kumamoto Shinto General Hospital, Hiroshi Mitsui of The Fraternity Memorial Hospital, Akimichi Morita of Nagoya City University Hospital, Osamu Nemoto of Kojinkai Sapporo Skin Clinic, Eisaku Ogawa of Shinshu University Hospital, Chika Ohata of Kurume University Hospital, Yukari Okubo of Tokyo Medical University Hospital, Shigetoshi Sano of Kochi Medical School Hospital, Mariko Seishima of Gifu University Hospital, Fumiaki Shirasaki of Shirasaki Dermatology Clinic, Yayoi Tada of Tokyo Teikyo University Hospital, Shunsuke Takahagi of Hiroshima University Hospital, Hidetoshi Takahashi of Takagi Dermatological Clinic, Chiharu Tateishi of Osaka City University Hospital, Tadahis Terui of Nihon University Itabashi Hospital, Yoshiki Tokura of Hamamatsu University Hospital, Hideshi Torii of Tokyo Yamate Medical Center, Noriko Umegaki of Keio University Hospital, Toshiyuki Yamamoto of Fukushima Medical University Hospital, Keiichi Yamanaka of Mie University Hospital, Kenshi Yamasaki of Tohoku University Hospital, and Masahiro Amano of University of Miyazaki Hospital.
Prior presentation
This manuscript is based on work that has been previously presented at the 2019 Japan Dermatological Association Tokyo/East Congress (Tokyo, Japan, 16th November 2019).
Disclosures
Yoshinori Umezawa received consulting and/or speaker fees from AbbVie GK, Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co., Ltd., Maruho Co., Ltd. and UCB Pharma. Akihiko Asahina received consulting and/or speaker fees from AbbVie GK, Eisai Co., Ltd., Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Kyowa Hakko Kirin Co., Ltd., LEO Pharma Japan, Maruho Co., Ltd., Mitsubishi Tanabe Pharma, TAIHO Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd. and UCB Pharma. Shinichi Imafuku received consulting and/or speaker fees from Celgene, Kyowa Hakko Kirin, LEO Pharma, Maruho, Novartis, and UCB Pharma. Yayoi Tada received grants for research from AbbVie, Celgene, Eisai, Eli Lilly, Kyowa Hakko Kirin, LEO Pharma, Maruho, Taiho Pharmaceutical and UCB Pharma, and honoraria for lectures from AbbVie, Eisai, Eli Lilly, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Taiho Pharmaceutical and Torii Pharmaceutical. Shigetoshi Sano received consulting fees and/or honoraria from AbbVie, Celgene, Eisai, Eli Lilly & Co., Janssen Pharmaceuticals, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Taiho Pharmaceutical, Torii Pharmaceutical, UCB Japan and Yakuhin. Akimichi Morita received research grants, consulting fees, and/or speaker’s fees from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi-Iko, Nippon Kayaku, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical and Torii Pharmaceutical, Ushio. Shinya Sakurai, Naoki Hoshii and Nicola Tilt are employees of UCB Pharma. Hidemi Nakagawa received consulting fees, honoraria and/or speaker fees from AbbVie GK, Bristol-Myers Squibb, Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Japan Tobacco Inc., Kyowa Hakko Kirin Co., Ltd., LEO Pharma, Maruho Co., Ltd., Mitsubishi-Tanabe Pharma, Novartis Pharma K.K., Torii Pharmaceutical Co., Ltd. and UCB Pharma.
Compliance with Ethics Guidelines
This study protocol was reviewed by the institutional review board (IRB) of each institution prior to implementation. Written informed consent was obtained from all patients. The study was carried out in accordance with the applicable regulatory and International Council for Harmonization-Good Clinical Practice requirements, and the Helsinki Declaration of 1964, and its later amendments.
Data Availability
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient-level data and redacted trial documents which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password protected portal.
References
- 1.World Health Organization. Global report on Psoriasis. 2016. https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_eng.pdf?sequence=1&isAllowed=y. Accessed 10 Dec 2020.
- 2.Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(Suppl 2):ii30–ii36. doi: 10.1136/ard.2004.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–653. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassell SE, Bieber JD, Rich P, et al. The modified Nail Psoriasis Severity Index: validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J Rheumatol. 2007;34(1):123–129. [PubMed] [Google Scholar]
- 5.Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. 2018;9:579. doi: 10.3389/fimmu.2018.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi H, Tsuji H, Takahashi I, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Prevalence of obesity/adiposity in Japanese psoriasis patients: adiposity is correlated with the severity of psoriasis. J Dermatol Sci. 2009;55(1):74–76. doi: 10.1016/j.jdermsci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Budu-Aggrey A, Brumpton B, Tyrrell J, et al. Evidence of a causal relationship between body mass index and psoriasis: a Mendelian randomization study. PLoS Med. 2019;16(1):e1002739. doi: 10.1371/journal.pmed.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borska L, Kremlacek J, Andrys C, et al. Systemic inflammation, oxidative damage to nucleic acids, and metabolic syndrome in the pathogenesis of psoriasis. Int J Mol Sci. 2017;18(11):2238. doi: 10.3390/ijms18112238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi T, Yamaoka H, Kojima T, Ikoma N, Akasaka E, Ozawa A. Psoriasis affects patient’s quality of life more seriously in female than in male in Japan. Tokai J Exp Clin Med. 2012;37(3):84–88. [PubMed] [Google Scholar]
- 10.Griffiths CEM, Jo SJ, Naldi L, et al. A multidimensional assessment of the burden of psoriasis: results from a multinational dermatologist and patient survey. Br J Dermatol. 2018;179(1):173–181. doi: 10.1111/bjd.16332. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264–272. doi: 10.1111/1346-8138.14139. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842–1847. doi: 10.1016/S0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- 13.Bissonnette R, Bolduc C, Poulin Y, Guenther L, Lynde CW, Maari C. Efficacy and safety of adalimumab in patients with plaque psoriasis who have shown an unsatisfactory response to etanercept. J Am Acad Dermatol. 2010;63(2):228–234. doi: 10.1016/j.jaad.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Weir N, Athwal D, Brown D, et al. A new generation of high-affinity humanized PEGylated Fab' fragment anti-tumor necrosis factor-α monoclonal antibodies. Therapy. 2006;3(4):535–546. [Google Scholar]
- 15.Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77(2):228–233. doi: 10.1136/annrheumdis-2017-212196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Certolizumab Pegol prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125160s237lbl.pdf. Accessed 15 Nov 2019.
- 17.European Medicines Agency. Certolizumab Pegol summary of product characteristics. 2014. https://www.ema.europa.eu/en/medicines/human/EPAR/cimzia-0#product-information-section. Accessed 15 Nov 2019.
- 18.Swissmedic. Cimzia®/-AutoClicks® Injektionslösung product information. 2020. https://www.swissmedicinfo.ch/?Lang=EN. Accessed 10 Dec 2020.
- 19.Pharmaceuticals and Medical Devices Agency. Cimzia® 200mg Syringe for S.C. Injection. Cimzia® 200mg AutoClicks® for S.C. Injection. 2020. https://www.info.pmda.go.jp/go/interview/1/820110_3999437G1022_1_014_1F.pdf. Accessed 10 Dec 2020.
- 20.Levin EC, Gupta R, Brown G, Malakouti M, Koo J. Biologic fatigue in psoriasis. J Dermatol Treat. 2014;25(1):78–82. doi: 10.3109/09546634.2013.826341. [DOI] [PubMed] [Google Scholar]
- 21.Umezawa Y, Sakurai S, Hoshii N, Nakagawa H. Certolizumab pegol for the treatment of moderate to severe plaque psoriasis: results from 16 weeks of a Phase 2/3 multicenter, randomised, double-blind, placebo-controlled study in Japan. Dermatol Ther. 2021. 10.1007/s13555-021-00494-z. [DOI] [PMC free article] [PubMed]
- 22.Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27–33. doi: 10.1159/000365390. [DOI] [PubMed] [Google Scholar]
- 23.Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(1):157–162. doi: 10.1111/bjd.14464. [DOI] [PubMed] [Google Scholar]
- 24.Umezawa Y, Nakagawa H, Niiro H, Ootaki K. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30(11):1957–1960. doi: 10.1111/jdv.13785. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–1062. doi: 10.1111/1346-8138.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J) J Dermatol. 2017;44(4):355–362. doi: 10.1111/1346-8138.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahina A, Ohtsuki M, Etoh T, et al. Adalimumab treatment optimization for psoriasis: results of a long-term phase 2/3 Japanese .study. J Dermatol. 2015;42(11):1042–1052. doi: 10.1111/1346-8138.13001. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–694. doi: 10.1111/1346-8138.14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41(12):1039–1046. doi: 10.1111/1346-8138.12668. [DOI] [PubMed] [Google Scholar]
- 30.Gordon K, Warren RB, Gottlieb AB, et al. Long-term efficacy of certolizumab pegol for the treatment of plaque psoriasis: three-year results from two randomised Phase 3 trials (CIMPASI-1 and CIMPASI-2) Br J Dermatol. 2020 doi: 10.1111/bjd.19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang YYM, Ruth JS, Hsu S. Loss of efficacy of secukinumab for psoriasis at 24 to 32 weeks. J Am Acad Dermatol. 2016;75(4):e169. doi: 10.1016/j.jaad.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 32.Mahil SK, Wilson N, Dand N, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR) Br J Dermatol. 2020;182(5):1158–1166. doi: 10.1111/bjd.18333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carretero G, Puig L, Carrascosa JM, et al. Redefining the therapeutic objective in psoriatic patients candidates for biological therapy. J Dermatol Treat. 2018;29(4):334–346. doi: 10.1080/09546634.2017.1395794. [DOI] [PubMed] [Google Scholar]
- 34.Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871.e1–30–81.e1–30. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Curtis JR, Mariette X, Gaujoux-Viala C, et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn's disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open. 2019;5(1):e000942. doi: 10.1136/rmdopen-2019-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon K, Papp K, Poulin Y, Gu Y, Rozzo S, Sasso EH. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. J Am Acad Dermatol. 2012;66(2):241–251. doi: 10.1016/j.jaad.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 38.Tyring S, Gordon KB, Poulin Y, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Arch Dermatol. 2007;143(6):719–726. doi: 10.1001/archderm.143.6.719. [DOI] [PubMed] [Google Scholar]
- 39.Blauvelt A, Paul C, van de Kerkhof P, et al. Long-term safety of certolizumab pegol in plaque psoriasis: pooled analysis over 3 years from three phase 3, randomised, placebo-controlled studies. Br J Dermatol. 2020 doi: 10.1111/bjd.19314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient-level data and redacted trial documents which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password protected portal.