Abstract
Atopic dermatitis (AD) is a highly prevalent chronic inflammatory skin disease that is characterized by intense pruritus, seriously affecting patients’ quality of life. Its pathophysiology, which involves both the adaptive and innate immune responses as well as skin barrier defects, is still poorly understood. We recently identified a microRNA, miR-335, as a key driver of keratinocyte differentiation and cornification, which is essential for the establishment of a healthy skin barrier. However, expression of miR-335 is lost in AD, leading to barrier defect. We further demonstrated how belinostat, a histone deacetylase inhibitor, can effectively restore miR-335 and resolve the barrier defect in a dry skin model. Here, in this commentary, we highlight the role of belinostat in the treatment of AD and discuss the need for more research into crosstalk between epigenetic and non-coding RNA-based regulation, as well as possible therapeutic strategies targeting the epigenome.
Keywords: Atopic dermatitis, Barrier defect, Pruritis, Inflammation, microRNA
Key Summary Points
| Atopic dermatitis (AD), a pro-inflammatory skin disorder, is characterized by recurrent severe episodes of scaling and uncontrolled pruritis that are exacerbated by genetic and environmental factors. |
| Two recent key findings in AD have been the recent identification of miR-335 as a driver of keratinocyte differentiation and cornification and that miR-335 expression is lost in AD, leading to barrier defect. |
| Belinostat, a histone deacetylase (HDAC) inhibitor, can restore miR-335 expression and repair the defective barrier. |
| The toxicity issue with HDAC inhibitors which can be addressed using analogues of belinostat with chemical modifications; thiese may be less toxic and could serve as a therapeutic alternatives for the alleviation of AD. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14333519.
Commentary
Atopic dermatitis (AD) is a chronic inflammatory skin disease. Also known as eczema, AD most commonly manifests as repeated episodes of skin inflammation, scaling and intense itching in young children [1]. Although the precise aetiology underlying AD remains poorly defined, its pathogenesis involves both immune hypersensitization and a skin barrier defect in which epidermal maturation is disrupted, resulting in loss of the skin’s normal watertight properties. This end result is excessive water loss leading to dryness and itching, while environmental allergen penetration encourages immune hypersensitization, inflammation and atopy. Here, we discuss our study on how dysfunctional epigenetic regulation in AD leads to the loss of a crucial microRNA (miRNA) involved in skin barrier maturation, and why these findings are important to the therapeutic management of AD.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Extensive evidence points strongly towards a synergy of genetic and environmental factors in the manifestation of AD and suggests that pathogenesis may be highly context-dependent and specific to each individual [2, 3]. The disease has a known heritable component [4], and risk factors include family history of atopy and loss-of-function mutations in the gene encoding filaggrin (FLG). However, not all AD patients carry FLG null mutations, and not all carriers of FLG null alleles develop AD. Furthermore, the sudden increase in AD prevalence in the latter half of the 20th century happened too quickly to be attributable to changes in allele frequencies [5], and disease manifestation appears to correlate with latitude and birth season [6]. Various environmental factors, such as lack of childhood exposure to pathogens (the “Hygiene Hypothesis”) [7, 8] and UV light intensity [5], have been put forward to explain these phenomena. While the exact contribution of each factor is unclear, the environment is likely to have an overall impact on epigenetic regulation over gene expression in the skin [9, 10]. These epigenetic effects, in combination with the endogenous genotype, ultimately lead to skin barrier defect and allergic sensitization associated with AD.
In a recently published study [11], we describe how the loss of a crucial miRNA affects epigenetic regulation of skin maturation. The epidermis is a stratified structure in which cell division occurs in the basal layer, above which keratinocytes progressively differentiate as they move suprabasally. The skin’s barrier function is largely regulated by the outermost layer (the stratum corneum), which consists of cornified keratinocytes tightly bonded together in an insoluble lipid matrix [12]. SOX6, a transcription factor which suppresses keratinocyte differentiation, is normally restricted to the basal layer [11] where it recruits components of the SMARCA (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A) complex, which epigenetically silence critical differentiation-related genes.
A pro-differentiation miRNA, miR-335, is expressed in the suprabasal layers of the healthy epidermis, where it drives keratinocyte differentiation and cornification by directly suppressing SOX6 [11] (Fig. 1). In AD lesional skin, miR-335 is lost, leading to aberrant expression of SOX6 throughout the epidermis. This in turn prevents normal expression of differentiation-related proteins, such as involucrin, small proline rich proteins and transglutaminase-1 and impairs skin barrier development, leading to barrier defect [11]. Crucially, miR-335 itself is epigenetically regulated via histone acetylation, and its expression may be altered by using histone deacetylase inhibitors (HDACis) [11]. Sodium butyrate (NaB), a broad-spectrum HDACi, significantly increases the expression of miR-335 in keratinocytes. This miR-335 upregulation occurs in tandem with increased abundance of differentiation markers, indicating that NaB treatment induces pro-differentiation effects by regulating the expression of miR-335 [11]. Although the observation that HDACis can alter miRNA expression is not new [13, 14], and past literature indicates that both HDACis [15, 16] and miRNAs [15, 17, 18] stimulate keratinocyte differentiation, in our study we report a mechanistic link between HDAC-mediated miRNA regulation and keratinocyte differentiation. These findings prompted us to investigate the possibility of targeting HDACs to address the skin barrier defect in AD.
Fig. 1.
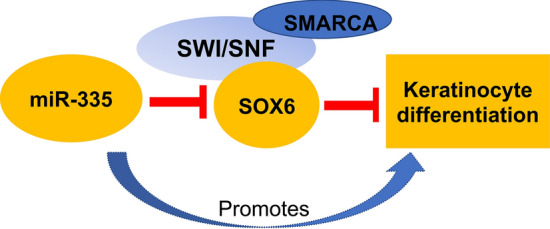
Schematic representation of the microRNA miR-335 in action
The epigenome plays a significant role in skin barrier phenotype and represents an ideal target for therapeutic intervention in skin disease. Crucially, epigenetic changes are largely reversible through the administration of suitable compounds, such as HDACis. We therefore screened a library of HDACis and identified five potential candidates which can effectively control miR-335 expression and associated differentiation-related genes in keratinocytes. Of these compounds, belinostat most consistently induced effective keratinocyte differentiation, with minimal toxicity. Belinostat, also known as beleodaq or PXD101, is a hydroxamate-type inhibitor that targets class I and II HDACs [19]. Discovered in 2003, it was originally proposed as an anti-tumour agent [20] and has been used for treating peripheral T-cell lymphoma with a good safety profile with respect to haematological toxicity [21]. When evaluating the efficacy of belinostat, we were able to induce miR-335 expression and upregulate differentiation markers in an ex vivo human skin organ culture system in which we used a dry-skin model to mimic the AD phenotype. This raised the possibility of repurposing belinostat as topical therapeutic to alleviate the skin barrier defect in AD.
While the concept of using HDACis to treat epithelial disease is currently met with resistance, it is not without precedent. HDACi-mediated reversal of detrimental epigenetic modifications has been proposed for the treatment of skin cancer [22]. Their use as prophylactics in cancer prevention has also been discussed [23], but questions remain regarding long-term safety. A different HDACi, JNJ-2648158, was also successfully used in the short-term to improve integrity in the nasal epithelium [24]; no unwanted side effects were reported. This latter study was carried out in the context of allergic rhinitis and presents an interesting parallel to AD and skin barrier integrity.
However, the use of belinostat or any other HDACi as a potential therapeutic to treat AD should be done with caution. This risk could be mitigated in several ways. For example, chemical modification of belinostat to destabilize it on contact with the bloodstream would enable a temporal effect, confined only to the skin. Another HDACi, remetinostat, was modified for topical use in basal cell carcinoma and cutaneous T-cell lymphoma. While the effect of remetinostat on AD lesions is unknown, similar principles can be adopted to design analogues of this compound, such as the incorporation of labile groups, and modify belinostat for use in AD. Restricting belinostat use to address acute flare-ups will avoid the side effects related to long-term use; this adoption of drug ‘breaks’ is already in use for other topical prescription drugs used to manage AD [25].
In addition to addressing safety concerns, it would be prudent to consider criteria for adopting belinostat as an AD therapeutic. Given the heterogeneous nature of AD pathogenesis, the development of a standardized treatment regime has remained elusive to date. Current clinical management strategies, such as the use of emollients, are aimed at restricting epidermal water loss and soothing itch; other therapeutic regimes include phototherapy to stimulate lipid synthesis and maturation in the skin, as well as therapeutics targeted at the immune component. Many of these strategies also come with associated risks: emollients are not often tested on individuals with skin barrier defects, while the benefits of phototherapy must be weighed against photosensitivity and an increased risk of skin cancer. Drugs targeting the immune component include immunosuppressants, such as mycophenolate mofetil, the prescription of which necessarily involves careful consideration of risk versus reward. While we strongly propose that restoring the skin barrier will resolve a host of downstream complications, including allergen sensitization and pruritus resulting from water loss, we recognize that the use of belinostat in AD will likely be subject to the same cost–benefit considerations. There may never be a universal therapeutic of choice for AD, but belinostat has the potential to be beneficial as part of a tailored therapeutic regime designed to alleviate AD and alleviate further atopic progression.
Our study also opens up new avenues for future research into AD pathogenesis. Histone deacetylation causes the loss of miR-335 from the epidermis, but the ultimate cause of this epigenetic alteration is still unknown. Investigating the relationship between known environmental risk factors for AD and FLG mutation status in donor skin may prove informative in this context. The observation that miR-335 is epigenetically regulated raises the possibility that other dysregulated miRNAs may be regulated in a similar fashion. Epigenetic modification is particularly critical in the immune component of AD, and it will be of interest to find out if a cohort of miRNAs including miR-155, which is strongly upregulated in infiltrating immune cells [17], are regulated epigenetically in AD. miR-155 is implicated in inflammation [17, 26] and is known to be epigenetically regulated in other contexts [27].
Overall, AD is a highly heterogenous disease whose pathogenesis, manifestation, and association with other atopic conditions vary significantly between individuals. It is our hope that continued investment into AD research leads to a deeper understanding of the disease, and the potential for tailored therapeutics.
Acknowledgements
Funding
This work and the journal’s rapid service fee was supported by the Biomedical Research Council of Singapore and an Agency for Science, Technology, and Skin Innovation Grant (SIG18006).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Shan Quah, Gowtham Subramanian and Prabha Sampath all contributed to manuscript writing.
Disclosures
Shan Quah, Gowtham Subramanian and Prabha Sampath have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Eichenfield LF, Tom WL, Chamlinet SL, et al. Guidelines of care for the management of atopic dermatitis. J Am Acad Dermatol. 2014;70:338–51. [DOI] [PMC free article] [PubMed]
- 2.Cabanillas B, Brehler AC, Novak N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol. 2017;17:309–315. doi: 10.1097/ACI.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPherson T. Current understanding in pathogenesis of atopic dermatitis. Indian J Dermatol. 2016;61:649–655. doi: 10.4103/0019-5154.193674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Regan GM, Sandilands A, McLean WHI, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Thyssen JP, Zirwas MJ, Elias PM. Potential role of reduced environmental UV exposure as a driver of the current epidemic of atopic dermatitis. J Allergy Clin Immunol. 2015;136:1163–1169. doi: 10.1016/j.jaci.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 6.Weiland SK. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609–615. doi: 10.1136/oem.2002.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true? Br J Dermatol. 2005;152:202–216. doi: 10.1111/j.1365-2133.2004.06436.x. [DOI] [PubMed] [Google Scholar]
- 8.Flohr C, Yeo L. Atopic dermatitis and the hygiene hypothesis revisited. In: Shiohara T, editor. Pathogenesis and management of atopic dermatitis. Current problems in dermatology, vol. 41. Basel: Karger; 2011. p. 1–34. 10.1159/000323290. [DOI] [PubMed]
- 9.Shen Y, Stanislauskas M, Li G, Zheng D, Liu L. Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci Rep. 2017;7:42646. doi: 10.1038/srep42646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Si J, Lee S, Park JM, Sung J, Ko G. Genetic associations and shared environmental effects on the skin microbiome of Korean twins. BMC Genom. 2015;16:992. doi: 10.1186/s12864-015-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liew WC, Sundaram GM, Quah S, et al. Belinostat resolves skin barrier defects in atopic dermatitis by targeting the dysregulated miR-335:SOX6 axis. J Allergy Clin Immunol. 2020; 146(3):606–20.e12. 10.1016/j.jaci.2020.02.007. [DOI] [PubMed]
- 12.Elias PM. Structure and function of the stratum corneum extracellular matrix. J Invest Dermatol. 2012;132:2131–2133. doi: 10.1038/jid.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JH, Shih KS, Wu YW, Wang AW, Yang CR. Histone deacetylase inhibitors increase microRNA-146a expression and enhance negative regulation of interleukin-1β signaling in osteoarthritis fibroblast-like synoviocytes. Osteoarthr Cartil. 2013;21:1987–1996. doi: 10.1016/j.joca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Bourassa MW, Ratan RR. The interplay between microRNAs and histone deacetylases in neurological diseases. Neurochem Int. 2014;77:33–39. doi: 10.1016/j.neuint.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer L, Kalinina P, Köcher M, et al. Mir-155 contributes to normal keratinocyte differentiation and is upregulated in the epidermis of psoriatic skin lesions. Int J Mol Sci. 2020;21:1–16. [DOI] [PMC free article] [PubMed]
- 16.Lemper M, Snykers S, Vanhaecke T, De Paepe K, Rogiers V. Current status of healthy human skin models: can histone deacetylase inhibitors potentially improve the present replacement models. Skin Pharmacol Physiol. 2013;27:36–46. doi: 10.1159/000351363. [DOI] [PubMed] [Google Scholar]
- 17.Sonkoly E, Janson P, Majuri, M-L, et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol. 2010;126:581-589.e20. [DOI] [PubMed]
- 18.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature. 2008;452:225–229. doi: 10.1038/nature06642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramalingam SS, et al. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol. 2009;4:97–101. doi: 10.1097/JTO.0b013e318191520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plumb JA, Finn PW, William RJ, et al. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2003;2:721–8. [PubMed]
- 21.Allen PB, Lechowicz MJ. Hematologic toxicity is rare in relapsed patients treated with belinostat: a systematic review of belinostat toxicity and safety in peripheral T-cell lymphomas. Cancer Manag Res. 2018;10:6731–6742. doi: 10.2147/CMAR.S149241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders N, Dicker A, Popa C, Jones S, Dahler A. Histone deacetylase inhibitors as potential anti-skin cancer agents. Cancer Res. 1999;59:399–404. [PubMed] [Google Scholar]
- 23.Katiyar SK, Singh T, Prasad R, Sun Q, Vaid M. Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: Interaction of bioactive dietary components on epigenetic targets. Photochem Photobiol. 2012;88:1066–1074. doi: 10.1111/j.1751-1097.2011.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steelant B, Wawrzyniak P, Martens K, et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144:1242-1253.e7. [DOI] [PubMed]
- 25.Sidbury R, Kodama S. Atopic dermatitis guidelines: diagnosis, systemic therapy, and adjunctive care. Clin Dermatol. 2018;36:648–652. doi: 10.1016/j.clindermatol.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Ma L, Xue HB, Wang F, Shu CM, Zhang JH. MicroRNA-155 may be involved in the pathogenesis of atopic dermatitis by modulating the differentiation and function of T helper type 17 (Th17) cells. Clin Exp Immunol. 2015;181:142–149. doi: 10.1111/cei.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krzeminski P, Sarasquete ME, Misiewicz-Krzeminska I, et al. Insights into epigenetic regulation of microRNA-155 expression in multiple myeloma. Biochim Biophys Acta Gene Regul Mech. 2015;1849:353–66. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


