Abstract
Introduction
The clinical benefits of biologic and oral treatments for moderate-to-severe plaque psoriasis are well-established, but efficacy outcomes can vary across therapies. Comparative efficacy analysis can be highly informative in clinical settings with multiple therapeutic options. This study assessed the short-term and long-term comparative efficacy of biologic and oral treatments for moderate-to-severe psoriasis.
Methods
A systematic literature review identified phase 2/3/4 randomized controlled trials (RCTs) through to 1 July 2020 for Food and Drug Administration- or European Medicines Agency-licensed treatments for moderate-to-severe psoriasis. Psoriasis Area and Severity Index (PASI) 75/90/100 response rates at the end of the primary response (short-term: 10–16 weeks from baseline) and maintenance periods (long-term: 48–52 weeks from baseline) were estimated using Bayesian network meta-analysis. Surfaces under the cumulative ranking curves (SUCRA) were estimated to present the relative ranking of treatments.
Results
In the short term (N = 71 RCTs), the PASI 90 response rates were highest for ixekizumab (72.9%, SUCRA 0.951), risankizumab (72.5%, 0.940), and brodalumab (72.0%, 0.930), which were significantly higher than those for guselkumab (65.0%, 0.795), secukinumab (65.0%, 0.794), infliximab (56.8%, 0.702), certolizumab (400 mg: 49.6%, 0.607; 200 mg: 42.2%, 0.389), ustekinumab (90 mg: 47.9%, 0.568; weight-based: 45.7%, 0.505; 45 mg: 44.6%, 0.460), adalimumab (43.0%, 0.410), tildrakizumab (200 mg: 39.7%, 0.327; 100 mg: 37.2%, 0.268), etanercept (18.0%, 0.171), apremilast (12.4%, 0.090), and dimethyl fumarate (12.2%, 0.092). The PASI 100 response rates were highest for ixekizumab (41.4%), risankizumab (40.8%), and brodalumab (40.3%). In the long term (N = 11 RCTs), the PASI 90 rate was highest for risankizumab (85.3%, SUCRA: 0.998), which were significantly higher than those for brodalumab (78.8%, 0.786), guselkumab (78.1%, 0.760), ixekizumab (72.1%, 0.577), secukinumab (67.0%, 0.450), ustekinumab (weight-based: 55.0%, 0.252), adalimumab (51.6%, 0.176), and etanercept (37.9%, 0.001). Risankizumab had the highest PASI 100 response rate (65.4%), followed by brodalumab (55.7%) and guselkumab (54.8%).
Conclusions
Ixekizumab, risankizumab, and brodalumab had the highest short-term efficacy, and risankizumab had the highest long-term efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00511-1.
Keywords: Biologic therapies, Network meta-analysis, Plaque psoriasis
Key Summary Points
| Why carry out this study? |
| A comparative efficacy analysis of the clinical benefits of biologic and oral treatments for moderate-to-severe plaque psoriasis can help inform treatment decisions when multiple therapeutic options are available. |
| This study assessed the short-term and long-term comparative efficacy of biologic and oral treatments for moderate-to-severe plaque psoriasis licensed by the US Food and Drug Administration or European Medicines Agency using data from their phase 2, 3, or 4 randomized controlled trials. |
| Psoriasis Area and Severity Index (PASI) 75/90/100 response rates at the end of the primary response period (short-term: 10–16 weeks from baseline) and the maintenance period (long-term: 48–52 weeks from baseline) were estimated using Bayesian network meta-analysis. |
| What was learned from the study? |
| In the short term (N = 71 trials), the PASI response rates were highest for ixekizumab, risankizumab, and brodalumab; in the long term (N = 11 trials), the PASI response rates were highest for risankizumab. |
| Ixekizumab, risankizumab, and brodalumab had the highest short-term efficacy, and risankizumab had the highest long-term efficacy. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14102903.
Introduction
Novel biologic therapies have shifted the treatment paradigm for moderate-to-severe plaque psoriasis [1–3]. Anti-tumor necrosis factor (TNF) agents (e.g., etanercept, infliximab, and adalimumab) and an anti-interleukin (IL) 12/23 agent (ustekinumab) were among the earliest biologic treatments licensed by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for psoriasis. In recent years, biologic drugs targeting IL-17 (ixekizumab, brodalumab, and secukinumab) and IL-23 (risankizumab, guselkumab, and tildrakizumab) have become available and expanded the therapeutic options for psoriasis.
To facilitate the selection of appropriate treatment regimens among the available candidates, it is important to evaluate multiple aspects of each treatment, including the efficacy, safety, treatment adherence, and health-related quality of life (HRQoL), and to consider the contraindications of the target patient population [4]. Specifically, the comparative efficacy data, particularly a high level of skin clearance as an outcome (i.e., a 90 or 100% reduction in Psoriasis Area and Severity Index [PASI 90, PASI 100, respectively]), are of key interest. These efficacy outcomes have been associated with improvement in HRQoL, psoriasis symptoms, and work productivity. For example, Elewski et al. [5] reported that patients who achieved PASI 90–100 at week 12 in the ERASURE and FIXTURE trials were more likely to achieve a sustained response in HRQoL measured by the Dermatology Life Quality Index. Viswanathan et al. [6] showed that patients who achieved PASI 100 response at week 12 in a clinical trial had significantly lower psoriasis symptom severity [6]. Feldman et al. [7] showed that employed patients in the CLEAR trial who achieved at least PASI 90 had significantly lower work productivity loss and reduced annual indirect costs. Outcomes assessing a high level of skin clearance have also become increasingly used in clinical development programs [8–11], making it possible to conduct an indirect comparison of such outcomes associated with various treatments.
While several recent network meta-analyses (NMAs) have been conducted to compare the relative efficacy of treatments for moderate-to-severe plaque psoriasis, knowledge gaps still exist [12–19]. First, PASI 100 results were not available in some of the recent NMAs [13, 15]. Second, such NMAs may lack sufficient statistical power to detect potential differences between treatments with the highest PASI 90 and 100 response rates. Third, comparative evidence assessing the long-term efficacy of treatments is limited due to the dearth of head-to-head trials that did not implement crossover or re-randomization in the study design [14]. Expert opinions have stressed the importance of maintaining long-term skin clearance, even if short-term skin clearance is achieved [20]. Compared with the short-term PASI response, the long-term PASI response can additionally reflect the variation in response over time, accounting for the gradual loss of response among some patients.
Recently, several large head-to-head trials have been published that compare various novel treatments for psoriasis, including IXORA-R comparing ixekizumab with guselkumab [8], ECLIPSE comparing guselkumab with secukinumab [21], CLARITY comparing secukinumab with ustekinumab [22], and IMMerge comparing risankizumab with secukinumab [9]. Incorporating these studies in an indirect comparison of the PASI response rates may not only improve the statistical power to detect differences in both short- and long-term PASI response rates, but also enhance and inform the evidence network for comparisons of long-term treatment efficacy. To this end, this study updated the NMA conducted by Armstrong et al. [14] by incorporating recently published clinical trial data to provide a comprehensive assessment of the short-term and long-term PASI response rates, including PASI 90 and 100 as outcomes, associated with licensed treatments for moderate-to-severe plaque psoriasis.
Methods
Data Source
A systematic literature review (SLR) was conducted to identify randomized controlled clinical trials of treatments for moderate-to-severe psoriasis through to 1 July 2020, which was an update of the SLR by Armstrong et al. [14]. The search strategy is detailed in the Methods section of the electronic supplementary material. Eligible trials were required to (1) be a phase 2, 3, or 4 randomized clinical trial (RCT) on treatments for moderate-to-severe plaque psoriasis among adults who were eligible for systemic therapies or phototherapy; (2) include treatments and dosages licensed by the US FDA or the EMA; and (3) report at least one of the efficacy outcomes of interest (PASI 75, 90, and 100; indicating the proportions of patients who achieved at least a 75, 90, or 100% reduction in PASI) by the end of the primary response period (short-term: 10–16 weeks from baseline) or the end of the maintenance period (long-term: 48–52 weeks from baseline). For the long-term NMA, trials were excluded if (1) patients switched to a different treatment during the post-induction period compared with the induction period; (2) patients received a different dosage during the post-induction period compared with the induction period, such that the entire treatment regimen considering both the induction period and the post-induction period was not licensed; or (3) patients were re-randomized based on certain efficacy criteria, such as PASI 75, during the post-induction period.
As this is a post-hoc NMA of previously published results of clinical trial data, no institutional board review was required. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Comparators
The comparators in this study included anti-IL-23 agents (guselkumab 100 mg at weeks 0 and 4, then every 8 weeks; risankizumab 150 mg at weeks 0 and 4, then every 12 weeks; tildrakizumab 100 mg and 200 mg at weeks 0 and 4, then every 12 weeks), anti-IL-17 agents (brodalumab 210 mg at weeks 0, 1, and 2, then every 2 weeks; ixekizumab 160 mg at week 0, 80 mg every 2 weeks until week 12, then 80 mg every 4 weeks; secukinumab 300 mg at weeks 0, 1, 2, 3, and 4, then every 4 weeks), anti-TNF agents (adalimumab 80 mg at week 0, then 40 mg every 2 weeks starting at week 1; certolizumab 400 mg at weeks 0, 2, and 4, then 200 mg or 400 mg every 2 weeks; etanercept 25 mg twice-weekly/50 mg weekly; infliximab 5 mg/kg at weeks 0, 2 and 6, then every 8 weeks), an anti-phosphodiesterase type 4 inhibitor (PDE4) agent (apremilast 30 mg twice daily after the initial titration schedule), an anti-IL-12/23 agent (ustekinumab 45 mg, 90 mg, or with a weight-based dosage [45 mg ≤ 100 kg, 90 mg > 100 kg] at weeks 0 and 4, then every 12 weeks), as well as dimethyl fumarate uptitrated to a maximum daily dose of 720 mg for the treatment of moderate-to-severe plaque psoriasis.
Outcomes
The outcomes were the proportions of patients who achieved PASI 75, 90, and 100 response by the end of the pre-specified primary assessment period for the short-term NMA (weeks 10–16 after baseline) and the end of the pre-specified maintenance period for the long-term NMA (weeks 48–52 after baseline). The number needed to treat (NNT) for each treatment relative to placebo by the end of the primary assessment period was also calculated. These pre-specified periods for the primary assessment period and maintenance period were chosen because the studies for the various medications were designed and a priori powered for those time periods. Additionally, these time points often corresponded to the primary or secondary endpoints of the clinical trials.
Statistical Analyses
NMA Models
Bayesian probit NMAs [23] were implemented to jointly model the PASI 75, 90, and 100 response rates. Due to the rich set of clinical trials in the short-term network, a reference-arm adjustment was implemented to account for potential cross-trial heterogeneities in treatment effects associated with the placebo response rate of each trial [19, 24]. Additionally, a random-effects model was applied to the short-term NMA to account for the potential heterogeneities that cannot be explained by placebo response rates. For the long-term NMA, a fixed-effects NMA model was fit due to the relative sparsity of the network.
For each treatment, the posterior distributions of the PASI 75, 90, and 100 response rates in the short term (10–16 weeks after baseline) and the long term (48–52 weeks after baseline) were summarized using posterior medians and 95% credible intervals (CrI). As the response rates were correlated, an overlap in two CrIs did not rule out the possibility of a statistically significance difference between two treatments. Therefore, odds ratios (ORs) were used to formally compare the PASI response rates between each pair of treatments and were summarized using posterior medians and 95% CrIs. Additionally, the treatments were ranked using the surface under the cumulative ranking curve (SUCRA) and mean rank with 95% CrI [25].
For the short-term NMA, the NNT for each treatment relative to the placebo was calculated as the reciprocal of difference of a treatment in PASI 75, 90, and 100 response rates versus placebo.
Additionally, the heterogeneity in treatment contrasts for the short-term NMA was summarized using (1) the posterior distribution measuring the effect of placebo response on treatment contrasts and (2) the posterior distribution measuring the residual cross-trial variance in treatment contrasts.
Computation
The posterior samples of the Bayesian NMA models were drawn using the Markov Chain Monte Carlo technique. Three parallel chains, each with 5000 adaptation iterations, 50,000 burn-in iterations, 50,000 posterior simulations, and a thinning factor of 10, were implemented. Vague prior distributions were applied for all parameters, such that the posterior distributions were driven primarily by the observed data. All analyses were conducted in R statistical software (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria) and JAGS 4.3.0 (Free Software Foundation, Inc., Boston, MA, USA)
Results
Literature Search
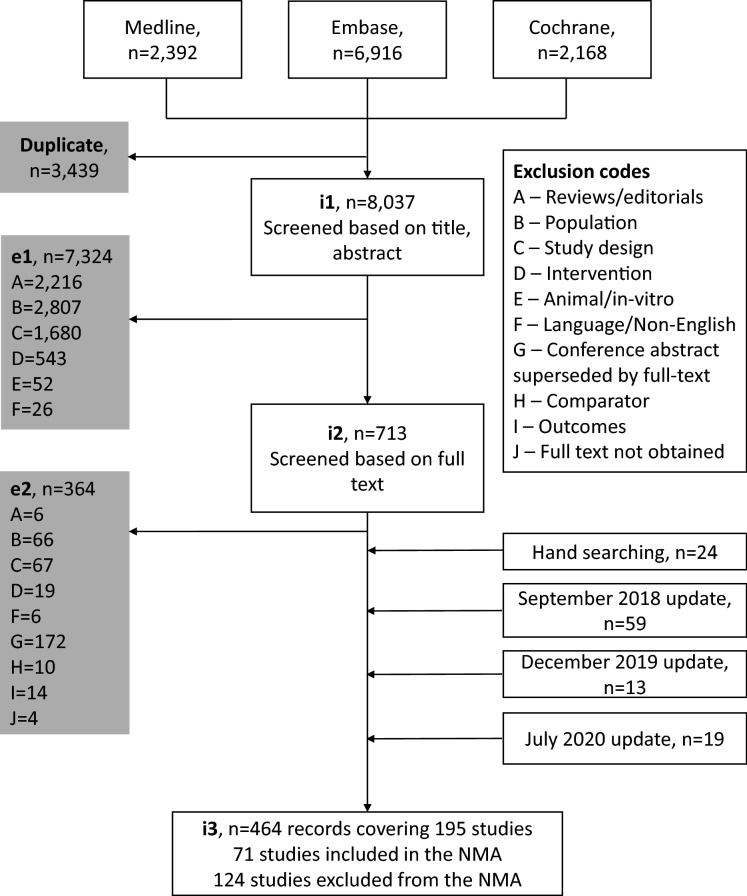
A total of 464 publications covering 195 studies were identified in the SLR through to 1 July 2020, with 71 studies included in the NMAs and 124 studies excluded from the NMAs (Fig. 1). All 71 studies were included in the short-term NMA, and 11 of the 71 studies were included in the long-term NMA. Compared with the SLR conducted by Armstrong et al. [14], 52 additional records were identified, with 11 additional studies qualifying for the short-term NMA and four additional studies qualifying for the long-term NMA.
Fig. 1.
Study screening and selection flow. e1 exclusion 1, e2 exclusion 2, i1 inclusion 1, i2 inclusion 2, i3 inclusion 3, NMA network meta-analysis
Short-term Efficacy
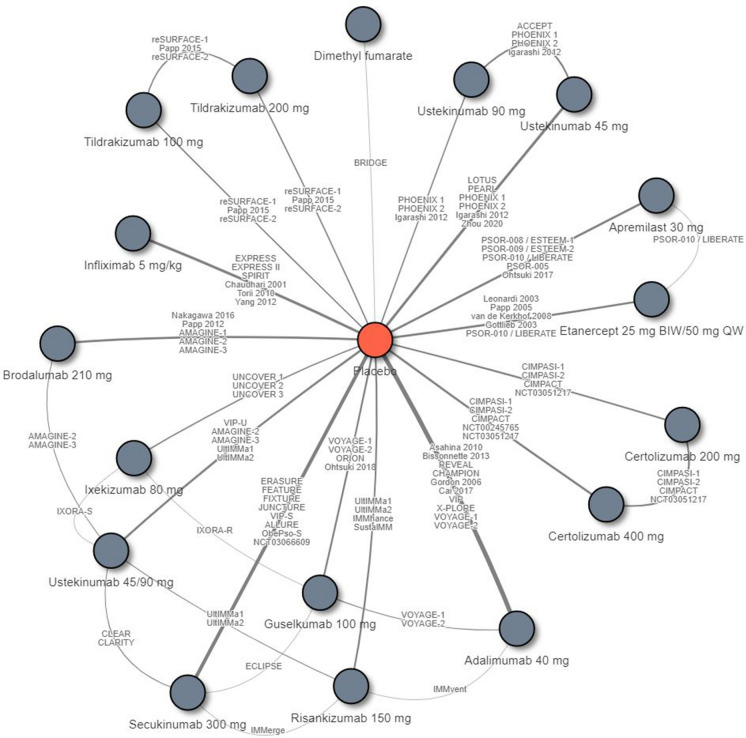
A total of 71 eligible RCTs connecting 18 treatment regimens were included in the NMA of short-term PASI response rates (10–16 weeks after baseline) (Fig. 2). A list of the trials included in the short-term NMA is included in Table S1 in the electronic supplementary material.
Fig. 2.
Evidence network for the network meta-analysis (NMA) of the Psoriasis Area and Severity Index (PASI) response by the end of the primary response period (short-term; 10–16 weeks after baseline). The included trials were: Asahina et al. [27], Bissonnette et al. [28], REVEAL [29], CHAMPION [30], Gordon et al. [31], Cai et al. [32], VIP [33], Leonardi et al. [34], Papp et al. [35], van de Kerkhof et al. [36], Gottlieb et al. [37], EXPRESS [38], EXPRESS II [39], SPIRIT [40], Chaudhari et al. [41], Torii et al. [42], Yang et al. [43], UNCOVER 1 [44], UNCOVER 2 [45], UNCOVER 3 [45], IXORA-S [10], IXORA-R [8], ERASURE [46], FEATURE [47], FIXTURE [46], JUNCTURE [48], CLEAR [49], CLARITY [11], VIP-S [50], ALLURE [51], ObePso-S [52], NCT03066609 [53], ACCEPT [54], LOTUS [55], PEARL [56], PHOENIX 1 [57], PHOENIX 2 [58], Igarashi et al. [59], VIP-U [60], Zhou et al. [61], X-PLORE [62], VOYAGE-1 [63], VOYAGE-2 [64], ORION [65], Ohtsuki et al. [66], ECLIPSE [21], Nakagawa et al. [67], Papp et al. [68], AMAGINE-1 [69], AMAGINE-2 [70], AMAGINE-3 [70], CIMPASI-1 [71], CIMPASI-2 [71], CIMPACT [72], NCT00245765 [73], NCT03051217 [74], reSURFACE-1 [75], Papp et al. [76], reSURFACE-2 [75], UltIMMa1 [77], IMMvent [78], IMMhance [79], SustalMM [80], IMMerge [9], BRIDGE [81], PSOR-008/ESTEEM-1 [82], PSOR-009/ESTEEM-2 [83], PSOR-010/LIBERATE [84], PSOR-005 [85], and Ohtsuki et al. [86]. BIW Twice weekly, QW once weekly
The posterior medians of the PASI 90 response rates were highest for ixekizumab (median 72.9% [95% CrI 68.3%, 77.1%]), risankizumab (72.5% [68.1%, 76.7%]), and brodalumab (72.0% [67.3%, 76.7%]), followed by guselkumab (65.0% [60.3%, 69.7%]), secukinumab (65.0% [61.0%, 68.7%]), infliximab (56.8% [50.4%, 62.9%]), certolizumab 400 mg (49.6% [43.0%, 56.3%]), ustekinumab 90 mg (47.9% [41.4%, 54.2%]), ustekinumab weight-based dosage (45.7% [41.2%, 50.3%]), ustekinumab 45 mg (44.6% [39.2%, 49.8%]), adalimumab (43.0% [38.7%, 47.4%]), certolizumab 200 mg (42.2% [35.3%, 49.4%]), tildrakizumab 200 mg (39.7% [33.2%, 46.8%]), tildrakizumab 100 mg (37.2% [30.8%, 44.1%]), etanercept (18.0% [14.5%, 22.2%]), apremilast (12.4% [9.7%, 15.9%]), and dimethyl fumarate (12.2% [7.2%, 20.2%]) (Table 1). Similarly, the posterior medians of the PASI 100 response rates were highest for ixekizumab (median 41.4% [95% CrI 36.3%, 46.6%]), risankizumab (40.8% [36.1%, 46.0%]), and brodalumab (40.3% [35.2%, 46.1%]). SUCRA and mean rank suggested a similar ranking of treatments as the median PASI response rates. Ixekizumab, risankizumab, and brodalumab were associated with the highest SUCRA (0.951, 0.940, and 0.930, respectively) and mean rank (1.8, 2.0, and 2.2, respectively) (Table 1; and ESM Fig. S1).
Table 1.
Estimated response rates, SUCRA, and mean rank from the NMA of short-term PASI response
| Treatment | Posterior median, % (95% CrI) | SUCRAa | Mean rank (95% CrI) | ||
|---|---|---|---|---|---|
| PASI 75 | PASI 90 | PASI 100 | |||
| Ixekizumab 160 mg at week 0, then 80 mg Q2W |
89.9 (87.3, 92.0) |
72.9 (68.3, 77.1) |
41.4 (36.3, 46.6) |
0.951 |
1.8 (1.0, 3.0) |
| Risankizumab 150 mg at weeks 0, and 4, then Q12W |
89.6 (87.2, 91.8) |
72.5 (68.1, 76.7) |
40.8 (36.1, 46.0) |
0.940 |
2.0 (1.0, 3.0) |
| Brodalumab 210 mg at weeks 0, 1, and 2, then Q2W |
89.4 (86.7, 91.9) |
72.0 (67.3, 76.7) |
40.3 (35.2, 46.1) |
0.930 |
2.2 (1.0, 3.0) |
| Guselkumab 100 mg at weeks 0, and 4, then Q8W |
85.3 (82.3, 88.1) |
65.0 (60.3, 69.7) |
32.9 (28.5, 37.7) |
0.795 |
4.5 (4.0, 5.0) |
| Secukinumab 300 mg at weeks 0, 1, 2, 3, and 4, then Q4W |
85.3 (82.7, 87.6) |
65.0 (61.0, 68.7) |
32.9 (29.2, 36.7) |
0.794 |
4.5 (4.0, 5.0) |
| Infliximab 5 mg/kg at weeks 0, 2, and 6, then Q8W |
79.8 (75.0, 84.0) |
56.8 (50.4, 62.9) |
25.6 (20.7, 31.0) |
0.702 |
6.1 (5.0, 7.0) |
| Certolizumab 400 mg Q2W |
74.4 (68.7, 79.5) |
49.6 (43.0, 56.3) |
20.1 (15.7, 25.2) |
0.607 |
7.7 (6.0, 11.0) |
| Ustekinumab 90 mg at weeks 0, and 4, then Q12W |
73.0 (67.3, 77.9) |
47.9 (41.4, 54.2) |
18.9 (14.8, 23.5) |
0.568 |
8.3 (7.0, 12.0) |
| Ustekinumab 45 mg ≤ 100 kg, 90 mg > 100 kg at weeks 0, and 4, then Q12W |
71.1 (67.1, 74.9) |
45.7 (41.2, 50.3) |
17.4 (14.7, 20.6) |
0.505 |
9.4 (7.0, 12.0) |
| Ustekinumab 45 mg at weeks 0, and 4, then Q12W |
70.1 (65.3, 74.5) |
44.6 (39.2, 49.8) |
16.7 (13.5, 20.3) |
0.460 |
10.2 (8.0, 13.0) |
| Adalimumab 80 mg at week 0, then 40 mg Q2W |
68.7 (64.7, 72.5) |
43.0 (38.7, 47.4) |
15.7 (13.2, 18.6) |
0.410 |
11.0 (8.0, 14.0) |
| Certolizumab 400 mg at weeks 0, 2, and 4, then 200 mg Q2W |
68.0 (61.3, 74.2) |
42.2 (35.3, 49.4) |
15.3 (11.4, 20.0) |
0.389 |
11.4 (8.0, 14.0) |
| Tildrakizumab 200 mg at weeks 0, and 4, then Q12W |
65.7 (59.1, 72.1) |
39.7 (33.2, 46.8) |
13.8 (10.3, 18.2) |
0.327 |
12.4 (9.0, 14.0) |
| Tildrakizumab 100 mg at weeks 0, and 4, then Q12W |
63.3 (56.5, 69.7) |
37.2 (30.8, 44.1) |
12.4 (9.2, 16.5) |
0.268 |
13.4 (11.0, 14.0) |
| Etanercept 25 mg BIW/50 mg QW |
40.2 (34.7, 45.9) |
18.0 (14.5, 22.2) |
4.1 (2.9, 5.5) |
0.171 |
15.1 (15.0, 16.0) |
| Apremilast 30 mg BID after initial titration schedule |
31.3 (26.4, 36.8) |
12.4 (9.7, 15.9) |
2.4 (1.7, 3.4) |
0.090 |
16.5 (16.0, 17.0) |
| Dimethyl fumarate (LAS 41008) |
30.9 (21.4, 43.3) |
12.2 (7.2, 20.2) |
2.3 (1.1, 4.8) |
0.092 |
16.4 (15.0, 17.0) |
| Placebo |
5.3 (4.8, 5.8) |
1.1 (1.0, 1.3) |
0.1 (0.1, 0.1) |
0.000 |
18.0 (18.0, 18.0) |
BID twice daily, BIW twice weekly, CrI credible interval, NMA network meta-analysis, PASI Psoriasis Area and Severity Index, PASI 75, 90, 100 75, 90, or 100% decrease from baseline PASI, respectively, QW once weekly, Q2W once every 2 weeks, Q4W once every 4 weeks, Q8W once every 8 weeks, Q12W once every 12 weeks
aSUCRA (surfaces under the cumulative ranking curves) measures the probability of a treatment being in the top ranks
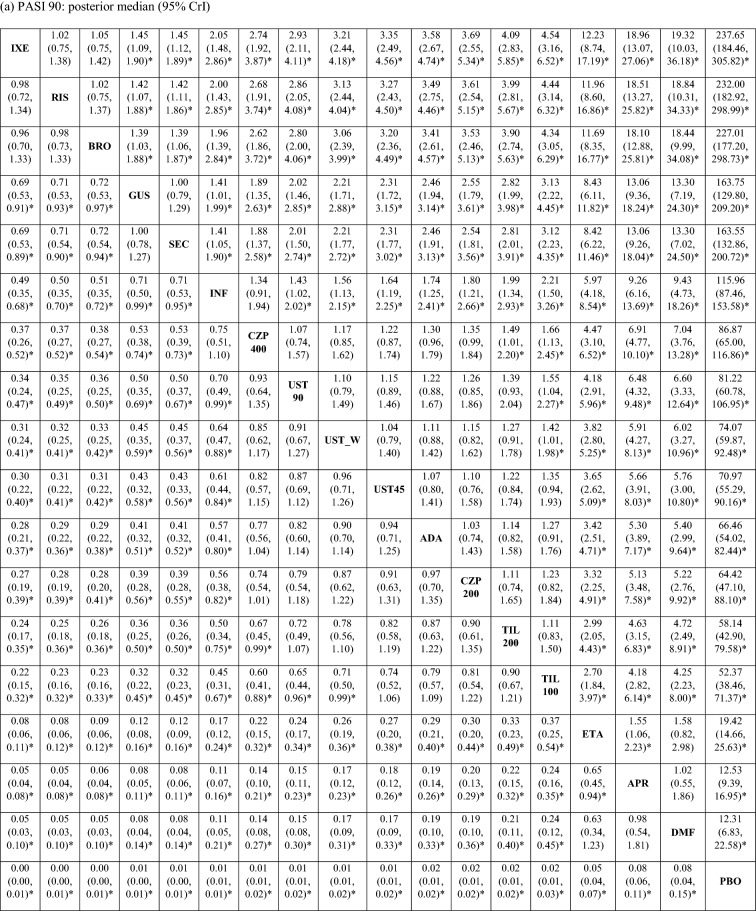
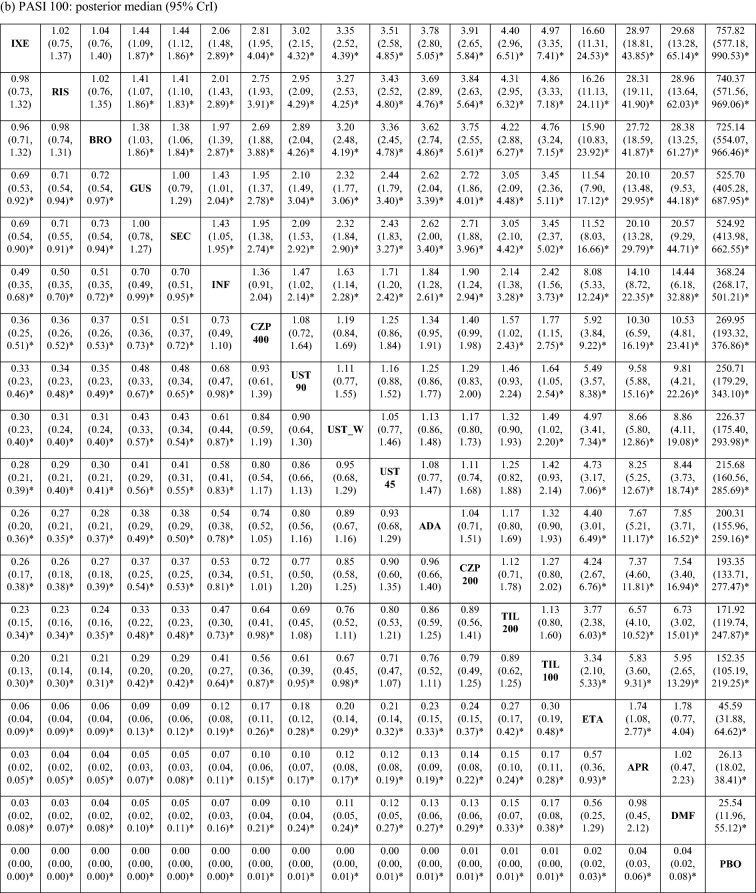
The posterior distributions of the pairwise ORs suggested that the PASI response rates were comparable between ixekizumab, risankizumab, and brodalumab, which were significantly higher than those of all other treatments, including guselkumab and secukinumab, with 95% probability (Table 2 [PASI 90 and 100]; ESM Table S2 [PASI 75]). Guselkumab and secukinumab were associated with significantly higher PASI response rates compared with infliximab, certolizumab (200 and 400 mg), ustekinumab (45 mg, 90 mg, and weight-based), adalimumab, tildrakizumab (100 and 200 mg), etanercept, apremilast, and dimethyl fumarate, with 95% probability.
Table 2.
Pairwise odds ratio of achieving PASI 90 and 100 response in the short term
Values in table are presented as the pairwise odds ratio with the 95% credible interval in parenthesis. An odds ratio > 1 indicates that the treatment in that row has a higher probability of achieving PASI response compared with the treatment in that column. An odds ratio < 1 indicates that the treatment in that row has a lower probability of achieving PASI response compared with the treatment in that column
ADA adalimumab, APR apremilast, BRO brodalumab, CZP 200 certolizumab 200 mg, CZP 400 certolizumab 400 mg, DMF dimethyl fumarate, ETA etanercept, GUS guselkumab, INF infliximab, IXE ixekizumab, PBO placebo, RIS risankizumab, SEC secukinumab, TIL 100 tildrakizumab 100 mg, TIL 200 tildrakizumab 200 mg, UST_W ustekinumab weight based, UST 45 ustekinumab 45 mg, UST 90 ustekinumab 90 mg
aDenotes that 95% CrI excludes 1
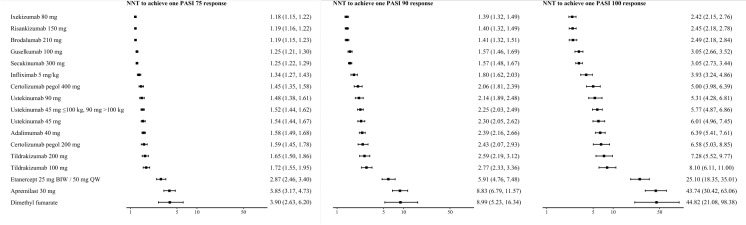
The NNTs to achieve PASI 75, 90, or 100 for each treatment relative to placebo are presented in Fig. 3. The NNTs to achieve one additional PASI 90 response were 1.39 (95% CrI 1.32, 1.49) for ixekizumab, 1.40 (1.32, 1.49) for risankizumab, and 1.41 (1.32, 1.51) for brodalumab. The NNTs to achieve one additional PASI 100 response were 2.42 (95% CrI 2.15, 2.76) for ixekizumab, 2.45 (2.18, 2.78) for risankizumab, and 2.49 (2.18, 2.84) for brodalumab.
Fig. 3.
Estimated numbers needed to treat (NNTs) relative to placebo for short-term PASI response. Values are presented with the 95% credible interval (Crl) in parenthesis. BIW Twice weekly, PASI 75, 90, 100 75, 90, or 100% decrease from baseline PASI, respectively, QW once weekly
The random-effects model with reference-arm adjustment revealed cross-trial heterogeneities in treatment contrasts. On the probit scale, each unit increase in the placebo PASI response rate was associated with a statistically significant decrease of 0.750 (95% CrI 0.534, 1.008) in the difference between the active treatment and placebo. The residual cross-trial variance of treatment contrasts on the probit scale was 0.014 (95% CrI 0.006, 0.028), which differs from zero with 95% probability.
Long-Term Efficacy
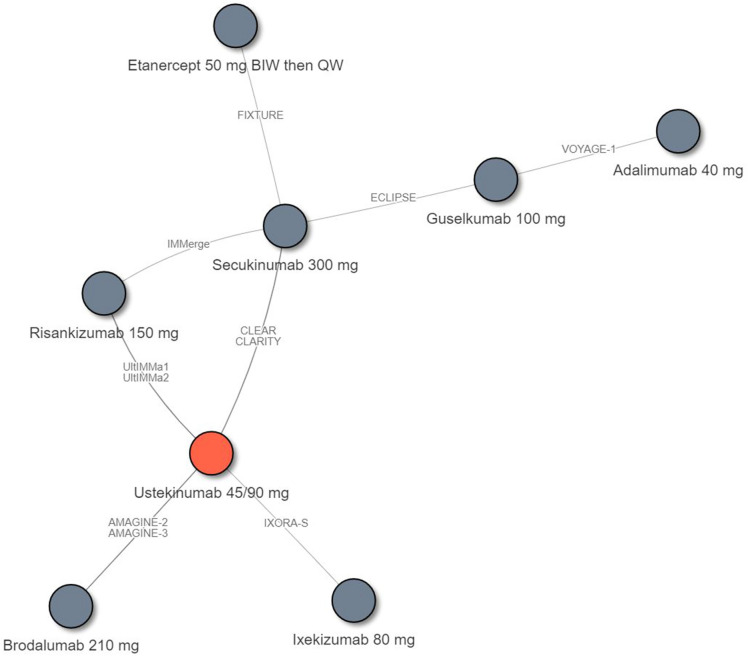
A total of 11 eligible RCTs connecting eight treatment regimens were included in the NMA of long-term PASI response rates (48–52 weeks after baseline) (Fig. 4). A list of the trials included in the long-term NMA is shown in ESM Table S3.
Fig. 4.
Evidence network for the NMA of PASI response by the end of the maintenance period (long-term; 48–52 weeks after baseline). The included trials were: AMAGINE-2 [70], ECLIPSE [21], VOYAGE-1 [63], CLEAR [87], FIXTURE [46], CLARITY [22], IXORA-S [88], UltIMMa1 [77], and IMMerge [9]
The posterior PASI 90 response rates were highest for risankizumab (median 85.3% [95% CrI 81.4%, 88.7%]), followed by brodalumab (78.8% [74.0%, 83.0%]), guselkumab (78.1% [72.5%, 83.0%]), ixekizumab (72.1% [62.7%, 80.1%]), secukinumab (67.0% [62.8%, 71.0%]), ustekinumab (weight-based 55.0% [52.7%, 57.3%]), adalimumab (51.6% [41.8%, 61.3%]), and etanercept (37.9% [30.4%, 45.8%]) (Table 2). Similarly, the posterior PASI 100 response rates were highest for risankizumab (median: 65.4% [95% CrI 59.3%, 71.1%]), followed by brodalumab (78.1% [72.5%, 83.0%]) and guselkumab (54.8% [47.6%, 61.9%]). SUCRA and mean rank suggested a consistent ranking of treatments as the PASI response rates, with risankizumab associated with a SUCRA of 0.998 and a mean rank of 1.0, followed by brodalumab and guselkumab (Table 3; ESM Fig. S2).
Table 3.
Estimated response rates, SUCRA, and mean rank from the NMA of long-term PASI response
| Treatment | Posterior median, % (95% CrI) | SUCRAa | Mean rank (95% Crl) | ||
|---|---|---|---|---|---|
| PASI 75 | PASI 90 | PASI 100 | |||
| Risankizumab 150 mg at weeks 0, and 4, then Q12W |
93.6 (91.2, 95.4) |
85.3 (81.4, 88.7) |
65.4 (59.3, 71.1) |
0.998 |
1.0 (1.0, 1.0) |
| Brodalumab 210 mg at weeks 0, 1, and 2, then Q2W |
89.7 (86.6, 92.3) |
78.8 (74.0, 83.0) |
55.7 (49.4, 61.8) |
0.786 |
2.5 (2.0, 4.0) |
| Guselkumab 100 mg at weeks 0, and 4, then Q8W |
89.3 (85.6, 92.3) |
78.1 (72.5, 83.0) |
54.8 (47.6, 61.9) |
0.760 |
2.7 (2.0, 4.0) |
| Ixekizumab 160 mg at week 0, 80 mg Q2W until week 12, then 80 mg Q4W |
85.4 (78.5, 90.6) |
72.1 (62.7, 80.1) |
47.2 (37.0, 57.6) |
0.577 |
4.0 (2.0, 5.0) |
| Secukinumab 300 mg at weeks 0, 1, 2, 3, and 4, then Q4W |
81.8 (78.5, 84.7) |
67.0 (62.8, 71.0) |
41.5 (37.0, 46.1) |
0.450 |
4.9 (4.0, 5.0) |
| Ustekinumab 45 mg ≤ 100 kg, 90 mg > 100 kg at weeks 0, and 4, then Q12W |
72.4 (70.2, 74.4) |
55.0 (52.7, 57.3) |
29.8 (27.6, 32.1) |
0.252 |
6.2 (6.0, 7.0) |
| Adalimumab 80 mg at week 0, then 40 mg Q2W |
69.4 (60.2, 77.5) |
51.6 (41.8, 61.3) |
26.9 (19.3, 35.7) |
0.176 |
6.8 (6.0, 7.0) |
| Etanercept 50 mg BIW until week 12, then QW |
56.3 (48.1, 64.2) |
37.9 (30.4, 45.8) |
16.7 (12.1, 22.4) |
0.001 |
8.0 (8.0, 8.0) |
aSUCRA measures the probability of a treatment being in the top ranks
The posterior distributions of the pairwise odds ratios suggested that the PASI response rates associated with risankizumab were significantly higher than all other treatments, including brodalumab, guselkumab, ixekizumab, and secukinumab with 95% probability (Table 4 [PASI 90 and 100] and Table S4 [PASI 75] in the electronic supplementary material). Brodalumab and guselkumab were associated with significantly higher PASI response rates than ustekinumab, adalimumab, and etanercept with 95% probability.
Table 4.
Pairwise odds ratio of achieving PASI 90 and 100 response in the long term
| PASI 90 response: posterior median (95% CrI) | |||||||
|---|---|---|---|---|---|---|---|
| RIS | 1.57 (1.08, 2.28)a | 1.63 (1.12, 2.37)a | 2.25 (1.35, 3.73)a | 2.86 (2.16, 3.82)a | 4.76 (3.64, 6.29)a | 5.48 (3.49, 8.61)a | 9.56 (6.46, 14.28)a |
| 0.64 (0.44, 0.92)a | BRO | 1.04 (0.71, 1.53) | 1.44 (0.87, 2.34) | 1.82 (1.36, 2.46)a | 3.03 (2.37, 3.92)a | 3.49 (2.21, 5.52)a | 6.09 (4.07, 9.16)a |
| 0.61 (0.42, 0.89)a | 0.96 (0.66, 1.42) | GUS | 1.38 (0.82, 2.31) | 1.76 (1.39, 2.25)a | 2.92 (2.19, 3.94)a | 3.36 (2.56, 4.42)a | 5.88 (4.08, 8.50)a |
| 0.44 (0.27, 0.74)a | 0.70 (0.43, 1.15) | 0.72 (0.43, 1.22) | IXE | 1.27 (0.81, 2.02) | 2.11 (1.39, 3.26)a | 2.43 (1.37, 4.33)a | 4.24 (2.51, 7.26)a |
| 0.35 (0.26, 0.46)a | 0.55 (0.41, 0.74)a | 0.57 (0.45, 0.72)a | 0.79 (0.50, 1.23) | SEC | 1.66 (1.42, 1.95)a | 1.91 (1.35, 2.71)a | 3.34 (2.54, 4.41)a |
| 0.21 (0.16, 0.28)a | 0.33 (0.26, 0.42)a | 0.34 (0.25, 0.46)a | 0.47 (0.31, 0.72)a | 0.60 (0.51, 0.71)a | UST_W | 1.15 (0.78, 1.69) | 2.01 (1.47, 2.77)a |
| 0.18 (0.12, 0.29)a | 0.29 (0.18, 0.45)a | 0.30 (0.23, 0.39)a | 0.41 (0.23, 0.73)a | 0.52 (0.37, 0.74)a | 0.87 (0.59, 1.28) | ADA | 1.75 (1.12, 2.73)a |
| 0.10 (0.07, 0.15)a | 0.16 (0.11, 0.25)a | 0.17 (0.12, 0.25)a | 0.24 (0.14, 0.40)a | 0.30 (0.23, 0.39)a | 0.50 (0.36, 0.68)a | 0.57 (0.37, 0.89)a | ETA |
| PASI 100 response: posterior median (95% CrI) | |||||||
|---|---|---|---|---|---|---|---|
| RIS | 1.50 (1.08, 2.10)a | 1.56 (1.11, 2.19)a | 2.11 (1.32, 3.40)a | 2.66 (2.06, 3.45)a | 4.44 (3.49, 5.68)a | 5.14 (3.29, 8.13)a | 9.40 (6.30, 14.23)a |
| 0.67 (0.48, 0.93)a | BRO | 1.03 (0.72, 1.48) | 1.41 (0.88, 2.24) | 1.77 (1.34, 2.34)a | 2.96 (2.35, 3.73)a | 3.42 (2.16, 5.49)a | 6.26 (4.12, 9.60)a |
| 0.64 (0.46, 0.90)a | 0.97 (0.68, 1.38) | GUS | 1.36 (0.83, 2.22) | 1.71 (1.37, 2.14)a | 2.86 (2.18, 3.75)a | 3.31 (2.50, 4.42)a | 6.05 (4.15, 8.89)a |
| 0.47 (0.29, 0.76)a | 0.71 (0.45, 1.14) | 0.74 (0.45, 1.20) | IXE | 1.26 (0.81, 1.95) | 2.10 (1.40, 3.16)a | 2.43 (1.37, 4.34)a | 4.45 (2.60, 7.66)a |
| 0.38 (0.29, 0.49)a | 0.56 (0.43, 0.75)a | 0.58 (0.47, 0.73)a | 0.79 (0.51, 1.23) | SEC | 1.67 (1.43, 1.95)a | 1.93 (1.34, 2.81)a | 3.53 (2.62, 4.84)a |
| 0.23 (0.18, 0.29)a | 0.34 (0.27, 0.43)a | 0.35 (0.27, 0.46)a | 0.48 (0.32, 0.71)a | 0.60 (0.51, 0.70)a | UST_W | 1.16 (0.78, 1.74) | 2.11 (1.50, 3.04)a |
| 0.19 (0.12, 0.30)a | 0.29 (0.18, 0.46)a | 0.30 (0.23, 0.40)a | 0.41 (0.23, 0.73)a | 0.52 (0.36, 0.74)a | 0.86 (0.57, 1.29) | ADA | 1.83 (1.13, 2.95)a |
| 0.11 (0.07, 0.16)a | 0.16 (0.10, 0.24)a | 0.17 (0.11, 0.24)a | 0.22 (0.13, 0.38)a | 0.28 (0.21, 0.38)a | 0.47 (0.33, 0.67)a | 0.55 (0.34, 0.88)a | ETA |
Values in table are presented as the pairwise odds ratio with the 95% CrI. An odds ratio > 1 indicates that the treatment in that row has a higher probability of achieving PASI response compared with the treatment in that column. An odds ratio < 1 indicates that the treatment in that row has a lower probability of achieving PASI response compared with the treatment in that column
ADA adalimumab, BRO brodalumab, ETA etanercept, GUS guselkumab, IXE ixekizumab, RIS risankizumab, SEC secukinumab, UST_W ustekinumab weight based
aDenoted that 95% CrI excludes 1
Discussion
The results suggest that ixekizumab, risankizumab, and brodalumab were associated with significantly higher PASI response rates than the other licensed treatments in the short term, and that risankizumab was associated with significantly higher PASI response rates than the other licensed treatments in the long term. The results established the benefits of these treatments towards achieving a high level of skin clearance (PASI 100 and PASI 90).
Compared with the prior NMA by Armstrong et al. [14], this study identified 11 additional trials for the short-term PASI NMA and four additional trials for the long-term PASI NMA. These additions included several large head-to-head trials between active treatments reporting short-term PASI results, such as IXORA-R (n = 1027) and ECLIPSE (n = 1048) [8, 21]. The increase in statistical power made it possible to detect previously unidentified significant differences in PASI response rates between the treatments. Specifically, the present study found statistically significantly higher short-term PASI response rates associated with ixekizumab, risankizumab, and brodalumab compared with guselkumab following the addition of the 11 recent trials. Furthermore, the four newly added trials with long-term PASI results extended the long-term NMA conducted by Armstrong et al. [14] and further connected guselkumab and adalimumab to facilitate an indirect comparison of eight active treatments. The large sample sizes of the newly included head-to-head trials, such as CLARITY (n = 1102) and ECLIPSE (n = 1048) [21, 22], also made it possible for the present long-term NMA to detect statistical differences between treatments.
In the short-term NMAs of this study, two anti-IL-17 agents (ixekizumab and brodalumab) and an anti-IL-23 agent (risankizumab) were associated with the most favorable efficacy outcomes, including achievement of a high level of skin clearance. This result is consistent with the findings of other recent NMAs for moderate-to-severe plaque psoriasis. For example, Sbidian et al. [15] suggested that anti-IL-17 agents (ixekizumab, secukinumab, bimekizumab, and brodalumab), anti-IL-23 agents (risankizumab and guselkumab), and infliximab were significantly more effective, in terms of PASI 90 response rates, than ustekinumab and other anti-TNF agents (adalimumab, certolizumab, and etanercept). Mahil et al. [13] showed that in terms of achieving clear/nearly clear skin status, ixekizumab was associated with the highest SUCRA value followed by risankizumab. Sawyer et al. [12] reported that the anti-IL-17 agents guselkumab and risankizumab were more efficacious than tildrakizumab, ustekinumab, anti-TNF agents, and non-biologic systemic treatments. Tada et al. [17] similarly reported that brodalumab, ixekizumab, and risankizumab were associated with the highest rates of PASI 90 and PASI 100 response and the highest SUCRA values.
The long-term NMA suggested that PASI 90 and 100 response rates were highest for risankizumab by the end of the maintenance period (PASI 90: 85.3%; PASI 100: 65.4%), followed by brodalumab and guselkumab. To date, few studies have attempted an NMA comparing the relative efficacy of treatments in the long term. Yasmeen et al. [16] found that risankizumab, brodalumab, and guselkumab were associated with a higher probability of achieving a PASI response than other biologics, but did not detect statistically significant differences between the three treatments.
This study benefits from the following strengths. First, the inclusion of newly published clinical trials permitted the addition of guselkumab and adalimumab into the long-term NMA and increased the sample sizes and statistical power for detection of differences in PASI response rates between treatments in both the short-term and long-term NMAs. Second, the use of the random-effects model adjusted for reference-arm response for the short-term NMAs addressed the potential heterogeneities in treatment contrasts across trials, and insured the validity for the statistical inference for the short-term NMAs under a rich network.
Limitations
This study is subject to the following limitations. First, NMAs rely on the transitivity assumption, requiring that the study conduct and patient populations be comparable across trials. There may be observed or unobserved factors, such as differences in study design, patient characteristics, and concomitant treatments, that may modify the treatment efficacy and influence the comparability of the clinical trials in the NMAs. The assessment time points also varied across trials. However, as the included clinical trials were designed a priori for the assessments at these time points, these pre-specified time points were chosen for this analysis. Second, the potential differences between patients with moderate-to-severe plaque psoriasis in the clinical trials and the real world, such as patient characteristics, adherence, and persistence to treatments, may limit the generalizability of the study results [26]. Third, due to the relative dearth of available data, the long-term network is still sparse. Fourth, while the long-term NMA was able to assess the relative efficacy of treatments by 48–52 weeks after baseline, direct evidence comparing the PASI response rates beyond 1 year is lacking. Lastly, PASI may only represent one of the many aspects measuring the efficacy of treatments for moderate-to-severe plaque psoriasis. With additional data, future studies may consider comparisons of other measures of treatment efficacy, such as the resolution of itch, HRQoL, and treatment adherence.
Conclusions
This study provides an up-to-date, comprehensive indirect comparison of the relative efficacy of licensed treatments for moderate-to-severe plaque psoriasis. Ixekizumab, risankizumab, and brodalumab were associated with the highest PASI response rates by the end of the primary response period. Risankizumab was associated with the highest PASI response rates by the end of the maintenance period. Future research can take an integrative approach to assess multiple aspects of each treatment, including efficacy, safety, treatment adherence, and HRQoL, and identify subgroups of patients (using clinical, laboratory, and genomic data) who can benefit the most from each biologic and oral treatment for moderate-to-severe plaque psoriasis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Sponsorship for this study and the Rapid Service Fee were funded by AbbVie Inc.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical writing and editorial assistance
Editorial assistance in the preparation of this article was provided by Dr. Shelley Batts of Analysis Group, Inc. Support for this assistance was funded by AbbVie Inc.
Prior presentation
Portions of this research are planned to be presented at the 2021 American Academy of Dermatology (AAD) Virtual Meeting Experience (VMX).
Disclosures
April W. Armstrong serves as investigator and/or scientific advisor to AbbVie, BMS, Incyte, Leo, UCB, Janssen, Lilly, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed. Ahmed M. Soliman is an employee of AbbVie and owns AbbVie stock. Luis Puig has served as investigator and/or consultant or paid speaker to AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Janssen, Leo, Lilly, Merck, Novartis, Pfizer, and UCB. Keith A. Betts, Yan Wang, and Yawen Gao are employed by Analysis Group, Inc., which received payment from AbbVie Inc. for participation in this research. Matthias Augustin has served as consultant to or paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli-Lilly, GSK, Janssen-Cilag, Leo, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. No honoraria or payments were made for authorship.
Compliance with ethics guidelines
As this is a post-hoc NMA of previously published results of clinical trial data, no institutional board review was required. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
The datasets analyzed during the current study are in the electronic supplementary materials of the published article.
References
- 1.Ighani A, Partridge ACR, Shear NH, Lynde C, Gulliver WP, Sibbald C, et al. Comparison of management guidelines for moderate-to-severe plaque psoriasis: a review of phototherapy, systemic therapies, and biologic agents. J Cutan Med Surg. 2019;23(2):204–221. doi: 10.1177/1203475418814234. [DOI] [PubMed] [Google Scholar]
- 2.Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63(4):278–285. [PMC free article] [PubMed] [Google Scholar]
- 3.Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–848. doi: 10.1111/bjd.18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 5.Elewski BE, Puig L, Mordin M, Gilloteau I, Sherif B, Fox T, et al. Psoriasis patients with psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75-89 response: results from two phase 3 studies of secukinumab. J Dermatol Treat. 2017;28(6):492–499. doi: 10.1080/09546634.2017.1294727. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan HN, Chau D, Milmont CE, Yang W, Erondu N, Revicki DA, et al. Total skin clearance results in improvements in health-related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatol Treat. 2015;26(3):235–239. doi: 10.3109/09546634.2014.943687. [DOI] [PubMed] [Google Scholar]
- 7.Feldman SR, Zhao Y, Gilloteau I, Graham CN, Miles L, McBride D, et al. Higher psoriasis skin clearance is associated with lower annual indirect costs in the United States: a post hoc analysis from the CLEAR study. J Manag Care Spec Pharm. 2018;24(7):617–622. doi: 10.18553/jmcp.2018.24.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blauvelt A, Papp K, Gottlieb A, Jarell A, Reich K, Maari C, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–1358. doi: 10.1111/bjd.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren RB, Blauvelt A, Poulin Y, Beeck S, Kelly M, Wu T, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase 3, randomised, open-label, efficacy assessor-blinded clinical trial. Br J Dermatol. 2020;184:50–59. doi: 10.1111/bjd.19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reich K, Pinter A, Lacour JP, Ferrandiz C, Micali G, French LE, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–1023. doi: 10.1111/bjd.15666. [DOI] [PubMed] [Google Scholar]
- 11.Bagel J, Nia J, Hashim PW, Patekar M, de Vera A, Hugot S, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week CLARITY results) Dermatol Ther (Heidelb) 2018;8(4):571–579. doi: 10.1007/s13555-018-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawyer LM, Malottki K, Sabry-Grant C, Yasmeen N, Wright E, Sohrt A, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS ONE. 2019;14(8):e0220868. doi: 10.1371/journal.pone.0220868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahil SK, Ezejimofor MC, Exton LS, Manounah L, Burden AD, Coates LC, et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol. 2020;183:638–649. doi: 10.1111/bjd.19325. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong AW, Puig L, Joshi A, Skup M, Williams D, Li J, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269. doi: 10.1001/jamadermatol.2019.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sbidian E, Chaimani A, Afach S, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasmeen N, Sawyer LM, Malottki K, Levin L, Didriksen Apol E, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2020;1–15. 10.1080/09546634.2020.1743811. [DOI] [PubMed]
- 17.Tada Y, Watanabe R, Noma H, Kanai Y, Nomura T, Kaneko K. Short-term effectiveness of biologics in patients with moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. J Dermatol Sci. 2020;99(1):53–61. doi: 10.1016/j.jdermsci.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong AW, Betts KA, Signorovitch JE, Sundaram M, Li J, Ganguli AX, et al. Number needed to treat and costs per responder among biologic treatments for moderate-to-severe psoriasis: a network meta-analysis. Curr Med Res Opin. 2018;34(7):1325–1333. doi: 10.1080/03007995.2018.1457516. [DOI] [PubMed] [Google Scholar]
- 19.Signorovitch JE, Betts KA, Yan YS, LeReun C, Sundaram M, Wu EQ, et al. Comparative efficacy of biological treatments for moderate-to-severe psoriasis: a network meta-analysis adjusting for cross-trial differences in reference arm response. Br J Dermatol. 2015;172(2):504–512. doi: 10.1111/bjd.13437. [DOI] [PubMed] [Google Scholar]
- 20.Strober BE, van der Walt JM, Armstrong AW, Bourcier M, Carvalho AVE, Chouela E, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb) 2019;9(1):5–18. doi: 10.1007/s13555-018-0279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839. doi: 10.1016/S0140-6736(19)31773-8. [DOI] [PubMed] [Google Scholar]
- 22.Bagel J, Blauvelt A, Nia J, Hashim P, Patekar M, de Vera A, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY) J Eur Acad Dermatol Venereol. 2020;35:135–142. doi: 10.1111/jdv.16558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Mak. 2013;33(5):607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity-subgroups, meta-regression, bias, and bias-adjustment. Med Decis Mak. 2013;33(5):618–640. doi: 10.1177/0272989X13485157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damiani G, Conic RRZ, Pigatto PDM, Bragazzi NL, Pacifico A, Malagoli P, et al. From randomized clinical trials to real life data. An Italian clinical experience with ixekizumab and its management. Dermatol Ther. 2019;32(3):e12886. doi: 10.1111/dth.12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310. doi: 10.1111/j.1346-8138.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 28.Bissonnette R, Tardif JC, Harel F, Pressacco J, Bolduc C, Guertin MC. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6(1):83–90. doi: 10.1161/CIRCIMAGING.112.975730. [DOI] [PubMed] [Google Scholar]
- 29.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158(3):558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 31.Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Cai L, Gu J, Zheng J, Zheng M, Wang G, Xi LY, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89–95. doi: 10.1111/jdv.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11(6):e007394. doi: 10.1161/CIRCIMAGING.117.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 35.Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- 36.van de Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159(5):1177–1185. doi: 10.1111/j.1365-2133.2008.08771.x. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139(12):1627–1632. doi: 10.1001/archderm.139.12.1627. [DOI] [PubMed] [Google Scholar]
- 38.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 39.Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51(4):534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842–1847. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- 42.Torii H, Nakagawa H. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59(1):40–49. doi: 10.1016/j.jdermsci.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX, Xu JH, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl) 2012;125(11):1845–1851. [PubMed] [Google Scholar]
- 44.Gordon K, Blauvelt A, Langley R, editors. Ixekizumab for treatment of moderate-to-severe plaque psoriasis: 60-week results from a double-blind phase 3 induction and randomized withdrawal study (UNCOVER-1). In: 73rd Annual Meeting of the American Academy of Dermatology; March 20–24, 2015; San Francisco.
- 45.Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 46.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 47.Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE) Br J Dermatol. 2015;172(2):484–493. doi: 10.1111/bjd.13348. [DOI] [PubMed] [Google Scholar]
- 48.Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE) J Eur Acad Dermatol Venereol. 2015;29(6):1082–1090. doi: 10.1111/jdv.12751. [DOI] [PubMed] [Google Scholar]
- 49.Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S) J Investig Dermatol. 2020;140(9):1784–1793. doi: 10.1016/j.jid.2020.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ClinicalTrials.gov. Study of secukinumab with 2 mL pre-filled syringes (ALLURE). https://clinicaltrials.gov/ct2/show/NCT02748863. Accessed 13 Mar 2020.
- 52.ClinicalTrials.gov. Study to explore the effect of secukinumab, compared to placebo, on fat tissue and skin in plaque psoriasis patients (ObePso-S). https://clinicaltrials.gov/ct2/show/NCT03055494Accessed 13 Mar 2020.
- 53.ClinicalTrials.gov. Study of efficacy and safety of secukinumab in subjects with moderate to severe chronic plaque-type psoriasis. https://clinicaltrials.gov/ct2/show/study/NCT03066609. Accessed 24 Sept 2020.
- 54.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 55.Zhu X, Zheng M, Song M, Shen YK, Chan D, Szapary PO, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS) J Drugs Dermatol. 2013;12(2):166–174. [PubMed] [Google Scholar]
- 56.Tsai TF, Ho JC, Song M, Szapary P, Guzzo C, Shen YK, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL) J Dermatol Sci. 2011;63(3):154–163. doi: 10.1016/j.jdermsci.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 58.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 59.Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–252. doi: 10.1111/j.1346-8138.2011.01347.x. [DOI] [PubMed] [Google Scholar]
- 60.Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U trial) J Investig Dermatol. 2020;140(1):85–93. doi: 10.1016/j.jid.2019.07.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J, Shen JY, Liu LF, Chen JS, Dou TT, Zheng M, et al. Indirect regulation and equilibrium of p35 and p40 subunits of interleukin (IL)-12/23 by ustekinumab in psoriasis treatment. Med Sci Monit. 2020;26:e920371. doi: 10.12659/MSM.920371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373(2):136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- 63.Blauvelt A, Papp KA, Griffiths CE, Randazzo B, Wasfi Y, Shen YK, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 64.Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 65.Ferris LK, Ott E, Jiang J, Hong HC, Li S, Han C, et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (One-Press), for moderate-to-severe psoriasis: results from the phase 3 ORION study. J Dermatol Treat. 2020;31(2):152–159. doi: 10.1080/09546634.2019.1587145. [DOI] [PubMed] [Google Scholar]
- 66.Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–1062. doi: 10.1111/1346-8138.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44–52. doi: 10.1016/j.jdermsci.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 69.Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- 70.Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 71.Gottlieb AB, Blauvelt A, Thaçi D, Leonardi CL, Poulin Y, Drew J, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2) J Am Acad Dermatol. 2018;79(2):302–314. doi: 10.1016/j.jaad.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Lebwohl M, Blauvelt A, Paul C, Sofen H, Węgłowska J, Piguet V, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT) J Am Acad Dermatol. 2018;79(2):266–276. doi: 10.1016/j.jaad.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 73.Reich K, Ortonne JP, Gottlieb AB, Terpstra IJ, Coteur G, Tasset C, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab' certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167(1):180–190. doi: 10.1111/j.1365-2133.2012.10941.x. [DOI] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov. A study to test the efficacy and safety of certolizumab pegol in Japanese subjects with moderate to severe chronic psoriasis. https://clinicaltrials.gov/ct2/show/NCT03051217Accessed 13 Mar 2020.
- 75.Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288. doi: 10.1016/S0140-6736(17)31279-5. [DOI] [PubMed] [Google Scholar]
- 76.Papp K, Thaçi D, Reich K, Riedl E, Langley RG, Krueger JG, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930–939. doi: 10.1111/bjd.13932. [DOI] [PubMed] [Google Scholar]
- 77.Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661. doi: 10.1016/S0140-6736(18)31713-6. [DOI] [PubMed] [Google Scholar]
- 78.Reich K, Gooderham M, Thaçi D, Crowley JJ, Ryan C, Krueger JG, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–586. doi: 10.1016/S0140-6736(19)30952-3. [DOI] [PubMed] [Google Scholar]
- 79.Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: A phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):1–11. doi: 10.1001/jamadermatol.2020.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohtsuki M, Fujita H, Watanabe M, Suzaki K, Flack M, Huang X, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–694. doi: 10.1111/1346-8138.14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mrowietz U, Szepietowski JC, Loewe R, van de Kerkhof P, Lamarca R, Ocker WG, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm®—and placebo-controlled trial (BRIDGE) Br J Dermatol. 2017;176(3):615–623. doi: 10.1111/bjd.14947. [DOI] [PubMed] [Google Scholar]
- 82.Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 83.Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 84.Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE) J Eur Acad Dermatol Venereol. 2017;31(3):507–517. doi: 10.1111/jdv.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–746. doi: 10.1016/S0140-6736(12)60642-4. [DOI] [PubMed] [Google Scholar]
- 86.Ohtsuki M, Okubo Y, Komine M, Imafuku S, Day RM, Chen P, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: Efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol. 2017;44(8):873–884. doi: 10.1111/1346-8138.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76(1):60–69. doi: 10.1016/j.jaad.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Paul C, Griffiths CEM, van de Kerkhof PCM, Puig L, Dutronc Y, Henneges C, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: Results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80(1):70–79. doi: 10.1016/j.jaad.2018.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are in the electronic supplementary materials of the published article.