Abstract
Introduction
Cryotherapy is an efficient method to treat various cutaneous lesions. In the current clinical evaluation, the efficacy of the Pixie® Skin Tag cryogenic pen as a home treatment for benign skin tags was evaluated against a marketed comparator device. In addition, the safety, tolerability, and expected visual effects of the treatment were assessed.
Methods
Fifty-six healthy volunteers presenting with skin tags were included and randomized in a prospective, single-blinded, parallel, single-center, comparative trial and subjected to treatment with either Pixie® Skin Tag or a comparator device, Wortie® skin tag remover. Selected skin tags located on the neck, breast, and under the armpits were topically treated according to device prescriptions for maximally three times with a 15-day interval between treatments.
Results
Of the skin tags treated with Pixie® Skin Tag, 64.3% completely disappeared during the study, of which half of the skin tags were cleared after one treatment, compared with 7.1% of the study population treated with Wortie® skin tag remover (p < 0.001). Both medical devices were safe to use, painless, and very well tolerated by 64.3% in the Pixie® Skin Tag and 96.4% in the Wortie® skin tag remover group. In addition, 72% of the subjects using Pixie® Skin Tag were satisfied with the results, and two-thirds of this study group would buy and use the device for the treatment of other skin tags. For the comparator device, only 11.0% were satisfied and 7.0% would buy the device.
Conclusion
Treatment of skin tags with Pixie® Skin Tag showed superior clinical performance when compared to Wortie® skin tag remover. Both treatments were safe and well tolerated, with the majority of skin response serving as a predictor for clinical performance in the Pixie® Skin Tag treated group.
Trial Registration Number
ANSL Registration: 2018-A01804-51.
Keywords: Cryotherapy, Efficacy, Home treatment, Nitrous oxide, Pixie® Skin Tag, Prescription-free, Skin tags, Tolerability
Summary Slide
| Why carry out this study? |
| Despite their harmless nature, skin tags can be bothersome to the patient for aesthetic reasons or by causing irritation or wounds through friction against clothes or jewelry. |
| The current clinical trial investigated the efficacy, safety, tolerability, and visual aspects related to treatment of skin tags with a cryogenic medical device indicated for home use. |
| What was learned from the study? |
| The Pixie® Skin Tag cryogenic pen demonstrated a superior efficacy of skin tag treatment compared with a similar comparator device, as illustrated by a 57.1% increase in successful and complete skin tag disappearance. |
| Pixie® Skin Tag is safe, painless, and well tolerated for the treatment of skin tags, while most observed skin responses are predictive for the device’s efficacy. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14381387.
Introduction
Skin tags, also known as acrochordons or fibroepithelial polyps, are very common benign skin outgrowths. They consist of soft, loose epidermal tissue often connected to the underlying skin via a thin stalk [1]. Skin tags generally have a size ranging from 2 to 5 mm in diameter, tend to grow progressively, and do not disappear on their own [1, 2]. It is estimated that about half of the adult population develops at least one of these harmless cutaneous excrescences. People suffering from diabetes, obesity, and metabolic syndrome or with a family history of skin tags tend to be more prone to the development of such skin lesions [3]. Skin tags are also significantly correlated with age and might even be a marker of increased risk for atherosclerosis and cardiovascular disease [4]. Normally, skin tags are skin-colored or brown and most frequently observed in skin folds such as the neck, axillae, and groin, where skin irritations might be a causative factor [2]. Although skin tags are painless in themselves, they often represent as an aesthetic problem and might be troublesome when friction with clothes or jewelry induces itching or bleeding, subsequently causing pain or even an infection.
Skin tags can be treated by several medical procedures, such as surgical removal, cauterization, laser therapy, or cryosurgery [3]. In addition, many over-the-counter treatments are also available that do not require specialist support and can be applied at home. Such therapies include oils, cremes, patches, or banding of the skin tag creating a lack of blood flow and resulting in its removal [5]. However, efficacy of these home treatments has not been clinically demonstrated. This in contradiction to cryotherapy, an established technique based on the well-aimed destruction of tissue through usage of extreme cold temperatures ceasing epidermal cell growth and proliferation [6, 7]. As such, irreversible damage is inflicted to the cell membrane by controlled frostbite and formation of ice crystals in the affected skin. After thawing, vascular stasis is induced, causing oxygen supply shortage and necrosis, ultimately leading to disappearance of the skin tag. This procedure produces excellent cosmetic results in various types of cutaneous lesions, is well tolerated, and is considered safe since adverse events are limited and serious reactions rarely reported [8–10]. In addition, many advantages related to cryotherapy are observed, including a short preparation time, no need for expensive specialized material or support, minimal wound care, low risk of infection, fast results starting from the first treatment round, and induction of the release of inflammatory mediators important in the immunologic response associated with cryotherapy [8]. Despite the proven benefits of cryotherapy in the treatment of skin tags used by health care professionals, the home-used marketed medical devices serving similar purposes do not achieve equal efficacy. This is likely due to limited freezing temperatures [11, 12]. It is after all demonstrated that various skin cell populations express different cold temperature tolerance and that the speed of cell freezing is directly correlated with cell destruction efficacy [13, 14]. To assure efficient cell and tissue destruction, freezing temperatures as low as − 50 to − 60 °C are recommended [9, 15].
On that note, a cryogenic pen has been developed, Pixie® Skin Tag, designed to reach freezing temperatures of − 80 °C for topical treatment of skin tags at home. This extreme low temperature is obtained through evaporation of pressured liquid nitric oxide. In addition, the device contains a flexible foam nib at the application side that has a flat surface, which allows optimal contact between the cold surface and the often convex, unleveled skin tags. Pixie® Skin Tag is demonstrated to effectively treat warts by transferring generated cold to the warts and destroying affected tissue [16].
The main objective of the current study was to clinically evaluate the efficacy of Pixie® Skin Tag for the treatment of skin tags versus a comparator product. In addition, the number of treatments needed for complete disappearance of skin tags, device satisfaction assessed by the subjects themselves, safety, and tolerability of the treatment were analyzed. Finally, the global effect of the device on skin tags was evaluated.
Methods
Clinical Study
This study was designed as an investigator-blinded, prospective, randomized, parallel, single-center, comparative clinical investigation and performed by Dermscan (Villeurbanne, France). The investigational design was unblinded for the study participants and laboratory technician applying the treatment, since the investigational products were distinctly different and therefore did not allow blinding. Clinical evaluation of the treatment was performed by a blinded investigator.
The clinical trial was conducted in accordance with the ethical principles initially outlined in the Declaration of Helsinki (1964) and according to EN ISO standard 14155:2012 and their updates. The study was approved by the local Ethical Committee, Comité de Protection des Personnes (Reference number 18.063), and is registered at ANSL: 2018-A01804-51.
Study Participants and Sample Size
The sample size of the study population was based on the goal to establish non-inferiority of the test product with an anticipated response rate of 95% over the comparator product, of which a response rate of 80% was anticipated. Accounting for a power of 80% at a 5% significance level, 24.25 subjects would be needed per treatment group. Rounding up and adding a 10% drop-out rate bring the total number of subjects to 28 per treatment group.
Study participants included male and female healthy volunteers, aged between 18 and 65 years, with skin types I–III according to Fitzpatrick’s scale and presenting with at least one skin tag that could be treated by the test devices on the neck, breast, or under the armpits with a diameter of 2–5 mm. All subjects who participated in the current clinical investigation were informed verbally and in writing about the study, the medical device under investigation, and related procedures. Informed consent forms were obtained from study participants prior to study initiation.
Medical Device Under Investigation
The Pixie® Skin Tag medical device is pen-shaped and has a gas dispenser containing pressurized liquified nitric oxide gas. The nitric oxide induces temperatures up to − 80 °C for at least 20 s at the applicator side. The application side consists of a reticulated foam nib which is flatand flexible and has pores enlarging the surface area thereby creating a denser section which works as a cold capacitor to allow optimal surface contact with the skin tags. The nib is a disposable part of the medical device and should be replaced after use. Before using Pixie® Skin Tag, the area surrounding the skin tag containing healthy skin cells is protected with a temperature-resistant plaster, approved according to ISO 10993-5 and 10993-10.
The medical device under investigation was compared to a dimethylether-based product, Wortie® skin tag remover, which has a comparable mechanism of action to Pixie® Skin Tag. Wortie® skin tag remover is a cryogenic pen marketed for home treatment of warts.
Study Set-up and Treatment Regimen
Fifty-six study participants were randomly allocated by the study staff to either the group treated with Pixie® Skin Tag or the group treated with Wortie® skin tag remover. Per patient, one skin tag was selected for treatment by the health care professional and was located on the neck, breast, or under the armpits. On Day 0, treatment of the selected skin tags was carried out according to the devices’ instructions by study professionals. In brief, the Pixie® Skin Tag cryogenic pen was topically applied for 20 s on the selected skin tag, starting on top of the skin tag. For subjects treated with the Wortie® skin tag remover, the cryogenic pen was topically applied for 40 s starting on top of the skin tag. Both treatments were repeated on Day 15 and 30 if considered necessary in the opinion of the investigator, in other words, if the skin tag was still present. In case of a successful treatment on Day 15 or Day 30, these corresponding time points were considered as the closing visit and subsequent scheduled visits were cancelled. Treatments were thus maximally repeated for three times with a 15-day interval. Final follow-up of the subjects and the last possible closing visit was scheduled at Day 45 of the study. Throughout the investigational period, study participants kept a daily diary for self-assessment purposes of the skin tags.
Study Outcomes
Efficacy Evaluation
The efficacy of Pixie® Skin Tag and the comparator device were evaluated by a blinded investigator and by subjects’ self-assessment. At baseline (Day 0), the blinded investigator measured the skin tag in terms of width and length in millimeters. Subsequently, efficacy was defined by a clinical evaluation of the selected skin tag at each time point (Day 15, 30, and 45) during the study rating the evolution of healing according to a predefined score (0 = no change, 1 = improvement, and 2 = disappearance of the skin tag). If the skin tag disappeared in between two visits, the subjects were asked to note the exact date in their daily log. The number of treatments needed to reach clinical success were also collected. The subjects in their turn assessed the efficacy and interest in future use by completing a questionnaire at the end of the investigation (Day 45). Macrophotography of the treatment-selected skin tag was performed on Day 0, 15, 30, and 45 before treatment, if applicable, with a digital camera (Nikon D90).
Safety Evaluation
For the test product, all required quality, safety, and efficacy issues were addressed and evaluated based on the documentation essential to fulfill the requirements of the European Council Directive 93/42 EEC. According to the Risk Management Procedure for the medical device in compliance with ISO 14971, no unacceptable risks were identified. In addition, a previous clinical evaluation of Pixie® Skin Tag as a treatment of warts only revealed transient, non-serious adverse events (AEs) related to cryotherapy [16].
AEs related and unrelated to the medical device under investigation were recorded throughout the study at each time point.
Tolerability Evaluation
Tolerability of both treatments was assessed by the blinded investigator and the unblinded subjects. As such, the blinded investigator clinically evaluated skin conditions at each intermittent investigational time point after study initiation (Day 3, 15, and 30) before treatment and at the end of the study on Day 45. Both the skin tag and the surrounding skin were subjected to signs of erythema, hypopigmentation, hyperpigmentation, scab, wound, blister, and frostbite and rated trough a 5-point score (0 = none, 1 = slight, 2 = moderate, 3 = severe, 4 = very severe). During this dermatological evaluation, subjects were asked about the sensations they experienced with regard to stinging, tightness, itching, and warm, burning sensation using a 5-point scoring system (0 = none, 1 = slight, 2 = moderate, 3 = severe, 4 = very severe). According to the signs observed at each time point, the overall local tolerance of both devices was defined during the last examination (0 = bad tolerance, 1 = moderate tolerance, 2 = good tolerance, 3 = very good tolerance).
Subjects evaluated the degree of pain on a visual analog scale ranging from 0, equaling no pain, to 10, corresponding to the worst imaginable pain. In addition, every study participant scored the skin tag under treatment every day in their diary for pain (none, slight, moderate, or severe) and redness, appearance of black color, a blister, wound, and scab (none, only on the skin tag, only on the skin surrounding the skin tag, or on the skin tag and the surrounding skin) to evaluate the healing process and also indicated any other intolerance reaction, if it occurred.
Data Analysis and Statistics
Results are regrouped and analyzed for both treatment groups in three categories according to the number of treatments realized (1, 2, or 3). Comparison of the proportion of successfully cured subjects was determined through a chi-squared test for independence. All statistical tests were assessed at a 5% level of significance in a bilateral approach.
Results
Baseline Data
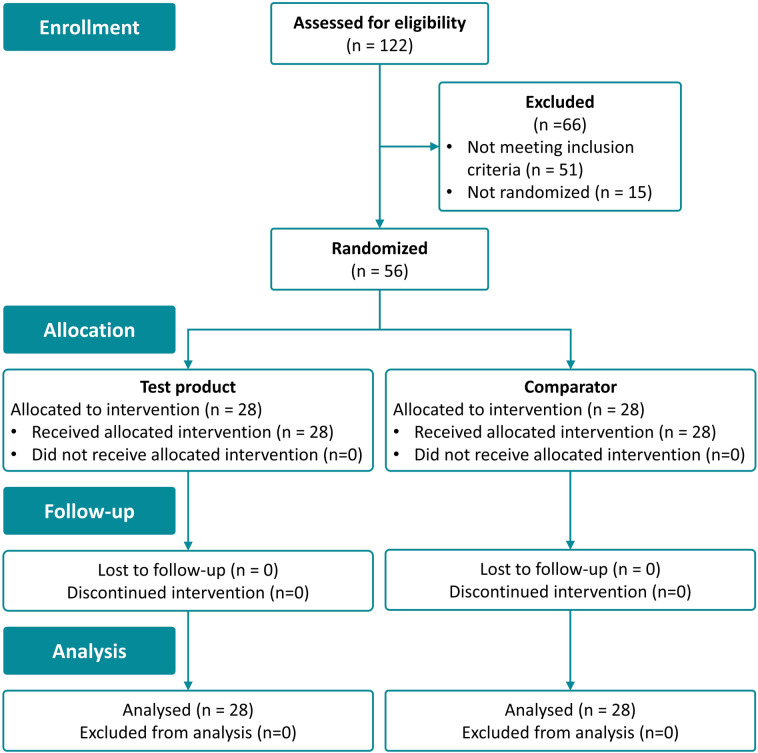
The clinical investigation was carried out between October 2018 and January 2019. One hundred twenty-two subjects were screened for eligibility of the study. Seventy-one healthy volunteers fulfilled the inclusion criteria, of which 56 were randomized. Fifteen subjects were not randomized. All included study participants completed the clinical investigation according to the study protocol. A CONSORT flow chart providing an overview of the clinical investigation process is shown in Fig. 1. The average age of the included population was 48.6 years. Forty-three women and 13 men with skin phototype I, II, or III were included. The average skin tag diameter size was balanced between the two study groups with an average size of 3.0 mm, ranging from 2 to 5 mm in diameter. Skin tags were located on the breast, neck, and under the armpits in both treatment groups. Subject demographic and baseline characteristics are summarized in Table 1.
Fig. 1.
CONSORT flow chart
Table 1.
Subject demographics and baseline characteristics
| Subject demographics and baseline characteristics | Pixie® Skin Tag | Wortie® skin tag remover | Total |
|---|---|---|---|
| Number of subjects | 28 | 28 | 56 |
| Age (years) (mean) | 47.8 | 49.4 | 48.6 |
| Sex | |||
| Male | 4 | 9 | 13 |
| Female | 24 | 19 | 43 |
| Skin type (Fitzpatrick) | |||
| I | 1 | 0 | 1 |
| II | 16 | 18 | 34 |
| III | 11 | 10 | 21 |
| Race | |||
| Caucasian | 28 | 28 | 56 |
| Skin tag size | |||
| Diameter (mm) | 3.1 | 3.0 | 3.1 |
| Height (mm) | 3.7 | 3.7 | 3.7 |
| Skin tag localization | |||
| Breast | 5 | 3 | 8 |
| Armpit | 18 | 17 | 35 |
| Neck | 5 | 8 | 13 |
Medical Device Efficacy
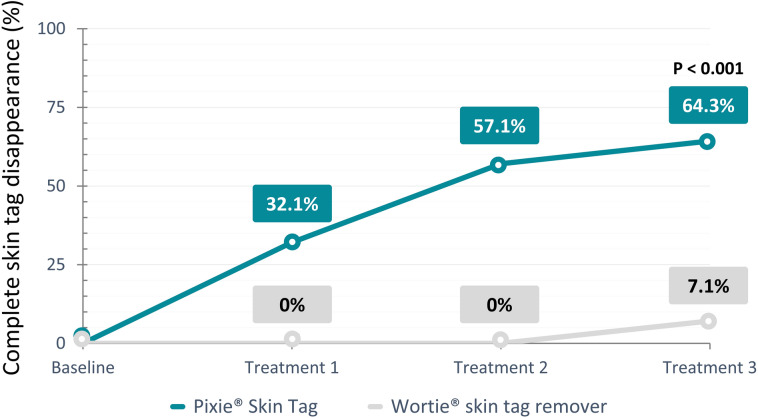
After a maximum of three treatments, skin tags completely disappeared in 64.3% of the subjects treated with Pixie® Skin Tag in comparison with only 7.1% of the Wortie® skin tag remover treated group. These results demonstrated clear superiority of Pixie® Skin Tag over Wortie® skin tag remover with a successful treatment proportion of 57.1% (p < 0.001) higher in the Pixie® Skin Tag group compared to its comparator device; 28.6% of subjects in the investigational device group presented with an improvement of the condition of their skin tag, and 7.1% did not notice any change in skin tag condition after a maximum of three treatments. For the subjects in the Wortie® skin tag remover group, 14.3% noted improvement of the skin tag condition, whereas 78.6% of the population did not demonstrate any change in the selected skin tags after treatment completion. Figure 2 provides an overview of the number of treatments needed for complete healing of skin tags after treatment with Pixie® Skin Tag and the comparator device, Wortie® skin tag remover. Notably, none of the skin tags were cured after one or two treatment rounds with Wortie® skin tag remover, whereas in the study population receiving treatment with Pixie® Skin Tag, 32.1% and 25.0% of the skin tags completely disappeared after one and two treatments, respectively. Details on the treatment efficacy per treatment round can be found in Table 2.
Fig. 2.
Graph demonstrating the percentage of complete healing of skin tags per treatment for the investigational device (Pixie® Skin Tag) and the comparator device (Wortie® skin tag remover)
Table 2.
Number of treatments needed for complete healing of skin tags
| Treatment status | Treatment 1 (Day 0) | Treatment 2 (Day 15) | Treatment 3 (Day 30) | Final status after three treatments |
|---|---|---|---|---|
| Pixie® Skin Tag | ||||
| Dissapeared | 32.1% (9/28) | 36.8% (7/19) | 16.7% (2/12) | 64.3% (18/28) |
| Improved | 17.9% (S/28) | 36.8% (7/19) | 58.3% (7/12) | 28.6% (8/28) |
| No change | 50.0% (14/28) | 26.3% (5/19) | 33.3% (4/12) | 7.1% (2/28) |
| Wortie® skin tag remover | ||||
| Dissapeared | 0.0% (0/28) | 0.0% (0/28) | 7.1% (2/28) | 7.1% (2/28) |
| Improved | 3.6% (1/28) | 17.8% (5/28) | 14.3% (4/28) | 14.3% (4/28) |
| No change | 96.4% (27/28) | 82.1% (23/28) | 78.6% (22/28) | 78.6% (22/28) |
The subjects’ self-assessments of the devices’ efficacy were compared as well. Overall, the Pixie® Skin Tag cryopen was considered efficient by 75% of the treated subjects, 82% of the subjects considered the device a fast treatment for skin tags, and over half of the study population thought the complete treatment until skin tag disappearance was fast as well. Based on these results, it can be stated that Pixie® Skin Tag's efficacy was very well appreciated by the study subjects. When looking at the results for Wortie® skin tag remover, the overall rating was less satisfying compared with the investigational device. As such, Wortie® skin tag remover was only considered efficient by 11% of the subjects. A treatment session with the device was perceived to be fast by most of the subjects; however, only 7% agreed that disappearance of the skin tag was accomplished quickly. Details on the efficacy self-assessment evaluation are presented in Table 3.
Table 3.
Subject evaluation of the medical devices
| Statement | Pixie® Skin Tag (n = 28) (% agreement) | Wortie® skin tag remover (n = 28) (% agreement) |
|---|---|---|
| The device is efficient | 75 | 11 |
| I am satisfied with the treatment | 72 | 11 |
| A treatment session is fast | 82 | 64 |
| The complete treatment until skin tag disappearance is fast | 57 | 7 |
| The adhesive foam plaster effectively protects the healthy skin surrounding the skin tag | 68 | 82 |
| The treatment is painless | 57 | 100 |
| I would like to use the device to treat other skin tags | 64 | 21 |
| At the end of the test, I would buy the device (regardless of its price) | 61 | 7 |
Safety Assessment
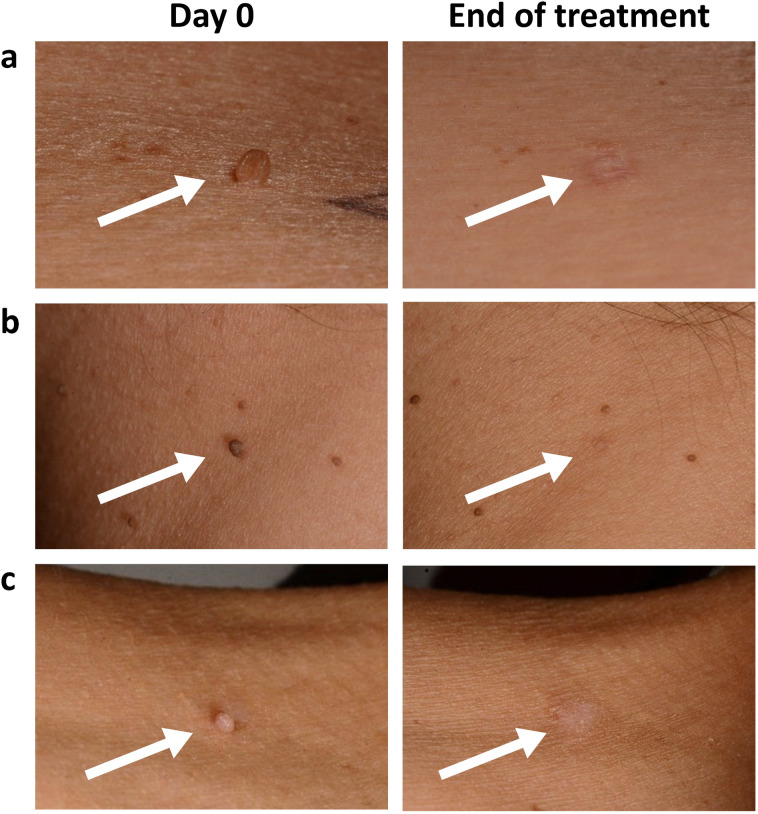
During the study, 48 AEs were observed in the subjects treated with Pixie® Skin Tag, of which 35 were related to the medical device, including erythema (17), hypopigmentation (7), hyperpigmentation (5), scab formation on the skin tag (2), scar on the lesion (2), skin thickening on and around the lesion area (1), and occurrence of pus after removal of the skin tag (1). The adverse effects lasted on average 15 days, and the majority were of mild intensity. Seven of the reported AEs were still present after the end of the study but were expected to disappear within a few weeks and required no additional follow-up. In the subjects treated with Wortie® skin tag remover, 16 AEs were reported with three device-related AEs, including erythema (2), which lasted for 5 days, and hyperpigmentation (1). One serious AE occurred during the study between screening and randomization and therefore had no relationship with the study procedure (Fig. 3).
Fig. 3.
Skin tags at Day 0 before treatment and after completion of Pixie® Skin Tag treatment at the end of the study in subject a, b, and c. Arrows indicate the treated skin tag
Tolerability Assessment
Forty-three percent of the study participants treated with the Pixie® Skin Tag cryogenic pen experienced a very limited amount of pain, represented by a mean scoring rate of 2.2 at the first and second treatment round and 1.3 at the third round as assessed by the pain scale. All patients in the comparative device group found the Wortie® skin tag remover treatment painless, as illustrated by a mean pain scoring of 0, 0.4, and 0.3 at first, second, and third treatment, respectively. The clinical evaluation of local tolerance resulted in similar findings. As such, fewer incidences of erythema, hypopigmentation, hyperpigmentation, wound occurrence, and blister and scab formation were observed in the Wortie® skin tag remover group throughout the study compared with the Pixie® Skin Tag treated group as observed by the reported AEs. However, all dermatological observations were minor and transient, and no bad local tolerance was noted in either of the treatment groups. Moreover, both erythema and hyperpigmentation are expected signs after cryotherapy, with the latter often indicating treatment success. Overall, the blinded investigator concluded a very good tolerance based on the clinical signs in 64.3% of the subjects in the Pixie® Skin Tag treated group and in 96.4% of the Wortie® skin tag remover treated subjects.
Product Performance
The Pixie® Skin Tag device was well appreciated for its efficacy by the majority of the treated subjects as 72% of the participants expressed their satisfaction about the device. Over two-thirds of the study population treated with the investigational medical device would like to use the device to treat other skin tags and would buy it in the future. This is different from the Wortie® skin tag remover device, where only 11% of the study population was satisfied with the device, barely one in five patients would like to use the device again, and even fewer subjects would buy the device. Details regarding the product performance as evaluated by the unblinded subjects are presented in Table 3.
Discussion
Cold has been a treatment method for several centuries and for numerous conditions, ranging from inflammation therapy and pain control to cancer and a wide variety of skin conditions [17]. Extensive research demonstrates that cryotherapy is safe for the treatment of various benign cutaneous lesions and selected premalignant and malignant skin tumors with high cure rates and a favorable cosmetic outcome [18, 19]. Only a few adverse effects are expected, such as temporary and local pain during and shortly after cryotherapy, bulla formation, and local edema. Major long-term AEs observed after cryotherapy treatment include lesional hypopigmentation and peripheral hyperpigmentation [9]. Given the high efficacy and optimal treatment results, the benefits of cryotherapy for skin lesions clearly outweigh potential risks and limited side effects.
The medical device under investigation, Pixie® Skin Tag, is a cryogenic pen developed for the treatment of benign skin tags in the neck, breast, or under armpits. The device consists of a dispenser filled with liquid nitric oxygen under high pressure that, upon activation, evaporates on a flexible foam nib lowering its temperature to about − 80 °C. The temperature reached with Pixie® Skin Tag is therefore significantly lower compared with the commercially available similar medical devices for home use of approximately − 55 °C. It has been previously demonstrated that efficacy of treatment with cryotherapy is correlated with lower freezing temperatures. Reduced temperatures result in higher clearance rates of skin lesions due to increased cell necrosis [20]. Also, rapid cooling leads to more intracellular and extracellular ice crystal formation, contributing to cell membrane disruption [21].
The results of the current clinical investigation reflect the beneficial effect of efficiently and rapidly applying ultra-low freezing temperatures on skin tags. As such, use of Pixie® Skin Tag resulted in complete disappearance of skin tags in 64.3% of the patient population after a maximum of three treatments, of which half of the skin tags were successfully treated after one treatment round. These results clearly demonstrate superiority over its comparator device, Wortie® skin tag remover, where only 7.1% of all subjects were successfully treated. In addition, Pixie® Skin Tag efficacy was very much appreciated by the vast majority of study participants in contradiction to 11.0% of the Wortie® skin tag remover treated patients. Differences in treatment efficacy are probably due to differences in applied freezing temperatures. The freezing temperature reached with Pixie® Skin Tag is after all significantly lower than the dimethyl ether-based comparator device and is a result of the use of different cooling agents.
However, lower temperatures and a higher treatment efficacy are commonly associated with more pronounced side effects, as is also the case in the current investigation. Although use of Pixie® Skin Tag did not induce significant pain or unexpected AEs, they were more common than with Wortie® skin tag remover treatment. Notably, the majority of skin response in the Pixie® Skin Tag group was predictive for the clinical performance. Overall, the local tolerance and safety for both treatments were evaluated to be very good in the majority of the study population.
Importantly, the study subjects—and potential end-users of the marketed test product—were very satisfied with the Pixie® Skin Tag cryogenic device in general, and over two-thirds would like to buy the device and use it for other skin tags, regardless of the price. Satisfaction about the Wortie® skin tag remover was limited to 11.0% of the study population, where only 7.0% would buy and use the device again.
A potential limitation of the current study is that the treatments in this study were applied at the investigational site by professional laboratory technicians. Treatment efficacy might be lower when the device is used at home by the consumers. However, this is true for all home-used over-the-counter cryogenic treatments.
Conclusion
The current clinical investigation demonstrates that cryotherapy with Pixie® Skin Tag removes skin tags safely, efficiently, and quickly. The high observed efficacy results from effective transfer of extremely low temperatures obtained from liquid nitric oxide to the skin tag. Throughout the study, most observed skin responses were predictive for the clinical performance of Pixie® Skin Tag. Nearly all subjects were satisfied with the results of the device, and two-thirds would purchase it for personal use. These results indicate that the Pixie® Skin Tag cryogenic pen can be used at home to effectively remove skin tags without posing risks to the overall health of end-users.
Acknowledgements
The authors thank all subjects for participating in the clinical trial.
Funding
Sponsorship for this study and article processing charges were funded by Oystershell Laboratories, which is also the manufacturer of the investigational product. The sponsor was involved in the study planning as well as in the decision to publish, but was not involved in data collection, data analysis, and data interpretation. All authors had full access to all data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Other Assistance
Oystershell Laboratories engaged and funded Dermscan to independently design and perform the clinical investigation. Writing of the manuscript and development of the figures were performed by an independent, external consultant, Leni Vandekerckhove (MediGraphX, Retie, Belgium, email: info@medigraphx.be). Statistical analyses were performed by an independent, external consultant, Els Adriaens (Adriaens Consulting, Bellegem, Belgium). Support for writing and statistical analysis assistance was funded by Oystershell Laboratories.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work, and have given final approval for the version to be published.
Authorship Contributions
André Antunes and Bart Rossel were involved in the study concept, design, planning, and data interpretation, as well as critical revision of the manuscript and providing final approval of the manuscript to be published. Els Adriaens performed the statistical analysis, data interpretation and revision, and approves the manuscript to be published.
Disclosures
André Antunes and Bart Rossel are employees of the company Oystershell Laboratories, who is the manufacturer of the test product and funded this study. Els Adriaens have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013, and according to EN ISO standard 14155:2012. The study was approved by the local Ethics Committee, Comité de Protection des Personnes (Reference number 18.063), and is registered at ANSL: 2018-A01804-51. Informed consent was obtained from all patients for being included in the study.
Data Availability
Datasets generated during and/or analysed during the current study are not publicly available because all relevant clinical results, necessary to make correct conclusions, are presented in the publication. Data are available from the corresponding author on reasonable request.
References
- 1.Platsidaki E, Vasalou V, Gerodimou M, Markantoni V, Kouris A, Vryzaki E, et al. The association of various metabolic parameters with multiple skin tags. J Clin Aesthet Dermatol. 2018;11:40–43. [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins JC, Maher MH, Douglas MS. Diagnosing Common Benign Skin Tumors. Naval Hospital Jacksonville, Jacksonville, Florida.
- 3.Pandey A, Sonthalia S. Skin Tags. [Updated 2021 Mar 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547724/.
- 4.Sari R, Akman A, Alpsoy E, Balci MK. The metabolic profile in patients with skin tags. Clin Exp Med. 2010;10:193–197. doi: 10.1007/s10238-009-0086-5. [DOI] [PubMed] [Google Scholar]
- 5.Fredriksson CH, Ilias M, Anderson CD. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9–9. [PubMed] [Google Scholar]
- 6.Taylor JE, Osmun WE. Just a pinch: technique for skin tag removal in sensitive areas. Can Fam Phys. 2016;62:998–999. [PMC free article] [PubMed] [Google Scholar]
- 7.Afsar FS, Erkan CD, Karaca S. Clinical practice trends in cryosurgery: a retrospective study of cutaneous lesions. Postep Dermatologii i Alergol. 2015;32:88–93. doi: 10.5114/pdia.2015.48048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews Mark D. Cryosurgery for common skin conditions. Am Fam Physician. 2004;69(10):2365–2372. [PubMed] [Google Scholar]
- 9.Zouboulis CC. Principles of cutaneous cryosurgery: an update. Dermatology. 1999;198:111–117. doi: 10.1159/000018084. [DOI] [PubMed] [Google Scholar]
- 10.Kuflik EG. Cryosurgery updated. J Am Acad Dermatol. 1994;31:925–944. doi: 10.1016/S0190-9622(94)70261-6. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart CG, Pchalek I, Adler M, Burkhart CN. An in vitro study comparing temperatures of over-the-counter wart preparations with liquid nitrogen. J Am Acad Dermatol. 2007;57:1019–1020. doi: 10.1016/j.jaad.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Wetmore SJ. Cryosurgery for common skin lesions: treatment in family physicians’ offices. Can Fam Phys. 1999;45:964–974. [PMC free article] [PubMed] [Google Scholar]
- 13.Gage AA. Experimental cryogenic injury of the palate: observations pertinent to cryosurgical destruction of tumors. Cryobiology. 1978;15:415–425. doi: 10.1016/0011-2240(78)90060-3. [DOI] [PubMed] [Google Scholar]
- 14.Gage AA, Meenaghan MA, Natiella JR, Greene GW. Sensitivity of pigmented mucosa and skin to freezing injury. Cryobiology. 1979;16:348–361. doi: 10.1016/0011-2240(79)90048-8. [DOI] [PubMed] [Google Scholar]
- 15.Torre D. Depth dose in cryosurgery. J Dermatol Surg Oncol. 1983;9:219–225. doi: 10.1111/j.1524-4725.1983.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 16.Walczuk I, Eertmans F, Rossel B, Cegielska A, Stockfleth E, Antunes A, et al. Efficacy and safety of three cryotherapy devices for wart treatment: a randomized, controlled, investigator-blinded, comparative study. Dermatol Ther. 2018;8:203–216. doi: 10.1007/s13555-017-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouganim N, Freiman, A. History of cryotherapy. Dermatol Online J. (2005);11(2). Retrieved from https://escholarship.org/uc/item/4f62h9vt. [PubMed]
- 18.Graham GF. Cryosurgery. Clin Plast Surg. 1993;20:131–147. doi: 10.1016/S0094-1298(20)30779-3. [DOI] [PubMed] [Google Scholar]
- 19.Graham GF. Cryosurgery in the management of cutaneous malignancies. Clin Dermatol. 2001;19:321–327. doi: 10.1016/S0738-081X(01)00171-7. [DOI] [PubMed] [Google Scholar]
- 20.Sterling JC, Handfield-Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144:4–11. doi: 10.1046/j.1365-2133.2001.04066.x. [DOI] [PubMed] [Google Scholar]
- 21.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1985;247(3 Pt 1):C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analysed during the current study are not publicly available because all relevant clinical results, necessary to make correct conclusions, are presented in the publication. Data are available from the corresponding author on reasonable request.