Abstract
Objective
Human immunodeficiency virus (HIV) infection and antiretroviral therapy (ART) have been associated with reduced bone mineral density (BMD) in persons with HIV (PWH). BMD provides information only about bone mineral quantity. Trabecular bone score (TBS) is a noninvasive tool that estimates bone microarchitecture. The aim of this study is to measure BMD and TBS of Chinese PWH after one-year ART.
Methods
We designed a retrospective study of adult Chinese PWH. Patients with a dual-energy X-ray absorptiometry (DXA) scan prior to ART initiation, and again 48 weeks later were included. Information regarding demographic and clinical history, HIV treatment history, BMD and TBS were collected. We analyzed differences in BMD and TBS over 48 weeks and associations between key risk factors and changes in BMD and TBS.
Results
Our study included 233 PWH (mean age = 36.6 ± 11.1 years). Before ART initiation, 19.3% of PWH had normal BMD but abnormal TBS. Both BMD and TBS decreased after one-year ART. TDF and LPV/r-containing regimens were associated with greater declines in BMD at different site. Traditional risk factors such as old age, low BMI and female sex were associated with lower baseline TBS. Greater change in TBS over one year was associated with lower BMI and lower baseline CD4+ cell count, but unlike BMD measures, it was not correlated with treatment with TDF and LPV/r in our study population.
Conclusions
We present the first longitudinal analysis of change in TBS over 48 weeks compared with BMD among Asian PWH receiving ART. Before ART initiation, approximately 20% of PWH with impaired bone microarchitecture would not have been identified if DXA were used alone to assess for bone damage. Both BMD and TBS decreased after one-year ART. Change in TBS was not associated with different antiretroviral agents.
The translational potential of this article
The trabecular microarchitecture measured indirectly by TBS may provide clinicians additional information about bone damage in PWH.
Keywords: Human immunodeficiency virus (HIV), Antiretroviral therapy (ART), Bone mineral density (BMD), Trabecular bone score (TBS), Tenofovir disoproxil fumarate (TDF)
1. Introduction
Although the use of antiretroviral therapy (ART) has resulted in a significant reduction in human immunodeficiency virus (HIV)-related morbidity and mortality [1,2], studies have shown that chronic comorbidities including decreased bone density and fragility fracture occur more frequently in persons with HIV (PWH) than in the general population [3]. It has been reported that in PWH the prevalence of osteoporosis is three times higher [4] and risk for fracture increases 20%–60% compared to uninfected individuals [5,6].
Dual-energy X-ray absorptiometry (DXA) is the gold standard used for the diagnosis of osteoporosis [7]. However, bone mineral density (BMD) accounts for only 60%–70% of the variation in bone strength, which has many determinants including bone quantity and bone quality [8]. A more recent tool that has been used to measure bone quality is trabecular bone score (TBS). This noninvasive, indirect tool produces a score, which has been shown to be related to 3D bone microarchitecture parameters such as trabecular number, trabecular separation and connectivity density [9]. TBS has also been shown to improve fracture prediction independent of BMD [10,11]. Previous studies have shown that TBS in HIV-infected individuals are lower compared to TBS in individuals without HIV [12].
In China, there were an estimated 1,250,000 PWH by the end of 2018. The incidence of newly diagnosed cases continues to rise [2]. Tenofovir disoproxil fumarate (TDF) + lamivudine (3 TC) + efavirenz (EFV) was designated the first-line ART regimen in China's 2011 national guidelines for HIV/AIDS treatment. Lopinavir/ritonavir (LPV/r), which is a protease inhibitor (PI), has been used as the most common alternative agent for EFV since 2011 [13]. Due to the increasing availability of ART in China, life expectancy among Chinese PWH has also increased. With the acceleration of aging, the problem of osteoporosis and fractures has become an issue of increasing concern [14]. To date, few studies have been conducted in China regarding bone health of PWH after ART [15,16], and no studies have measured changes of TBS.
In order to address this gap, we retrospectively analyzed medical records data from patients receiving HIV care at a large Chinese tertiary-care hospital and evaluated changes in BMD and TBS after initiation of ART from baseline to 48 weeks. We also systematically investigated potential clinical and HIV-associated risk factors associated with change in BMD and TBS during this time.
2. Materials and methods
2.1. Study design and population
We conducted a retrospective chart review of adult PWH receiving care in the outpatient HIV clinic of the Infectious Diseases Department of Peking Union Medical College Hospital (PUMCH) between April 2007 and April 2019. Patients were eligible for inclusion if they had a diagnosis of HIV, which was confirmed by Western blot, and had obtained a DXA scan prior to ART initiation, and again at 48 ± 4 weeks post-ART initiation. Patients were excluded from the study if they had any of the following conditions: (1) significant renal, hepatic, thyroid or parathyroid dysfunction; (2) use of systemic glucocorticoids; (3) use of bone active drugs; (4) pregnant or nursing women; (5) patients who were younger than 20 years old, because the reference Asia DXA database is not available for this population; (6) patients with BMI below 15 kg/m2 or over 37 kg/m2, because TBS values are not recommended for use in these groups [9]; and (8) poor-quality DXA images. The study was reviewed and exempt by the Peking Union Medical College Hospital Institutional Review Board (No. S–K1367).
2.2. Measures
For each patient, we obtained information regarding general demographic and clinical history, HIV diagnosis and treatment history, BMD and TBS information.
BMD of the lumbar spine (LS), femoral neck (FN), and total hip (TH) were measured on the same GE Lunar Prodigy Advance DXA scanner (GE Healthcare, Madison, WI), using the same software (enCORE version 10.50.086) for both scan acquisition and analysis. The precision of DXA measurements was 0.94% for lumbar spine, 1.75% for femoral neck, and 0.74% for total hip respectively, which is notably lower than the minimal acceptable precision advised by the International Society for Clinical Densitometry (ISCD) [LS: 1.9%; FN: 2.5%; TH: 1.8%] [17].
According to WHO criteria, for postmenopausal women and men aged 50 and older, a T-score of ≥ −1.0 denotes normal BMD; a T-score between −1.0 and −2.5 denotes low bone density (or osteopenia), and a T-score of ≤ −2.5 denotes osteoporosis [18]. Due to the retrospective nature of this study, we were unable to collect details regarding menopausal status, therefore 50 years was also used as the age threshold for postmenopausal status, based upon data showing the average age for menopause in China is 45–50 years [19]. For patients younger than 50 years of age, a Z-score of −2.0 or lower is defined as “below the expected range for age” and a Z-score above −2.0 is “within the expected range for age” according to the ISCD [17].
TBS was measured using iNsight v2.1 software (Med-Imaps, Merignac, France) applied to lumbar spine DXA images. TBS value above 1.310 is considered to be “normal”. A value between 1.230 and 1.310 is considered “partially degraded bone microarchitecture”, and a value below 1.230 is categorized as “degraded microarchitecture” [20]. We performed TBS precision analyses by measuring lumbar spine DXA in 30 patients 2 times, repositioning the patient after each scan at first. Then these lumbar spine DXA images were used to calculate TBS. Finally, the precision of TBS was calculated using ISCD precision calculating tool (https://iscd.org/learn/resources/calculators/). The precision for TBS was 1.14% in our study.
For all patients, peripheral lymphocyte subpopulation analysis, HIV RNA and routine laboratory tests were performed. Peripheral lymphocyte subpopulation analysis was performed using BD FACS canto (BD Bioscience, USA) and whole blood samples to determine the counts of CD4+T cells. Roche COBAS TaqMan (Roche, CA, USA) was used to detect HIV plasma viral load.
2.3. Statistical analysis
Mean, standard deviations (SD) and frequencies were calculated to describe baseline and follow-up variables. We used Paired-Samples T test to compare continuous variables and χ2 tests or Fisher's exact tests to compare categorical variables between baseline and follow-up data.
Univariate linear regression analyses were performed to examine the associations between the change in BMD and TBS over 48 weeks with key covariates including age (per 10-year increment), sex, BMI, hepatitis B virus (HBV) co-infection, route of HIV transmission, baseline CD4+ T-cell count, baseline HIV viral load, TDF exposure, LPV/r exposure. Variables with p < 0.20 in the univariate analysis were entered into the multivariable model. We also added age and BMI into every multivariable model regardless of its p-value in univariate analysis considering its established influence on bone health.
All statistical analyses were performed using the software package SPSS 22.0 (SPSS Inc., Chicago, IL, USA). P-values of <0.05 were considered statistically significant and all p-values were 2-sided.
3. Results
3.1. Baseline characteristics
A total of 233 patients had received a DXA examination both prior to ART initiation and 48 ± 4 weeks post-initiation, and met other eligibility criteria for inclusion in this analysis. The baseline characteristics of the patients are shown in Table 1. The mean age of the overall sample was 36.6 ± 11.1 years, including 212 men (91.0%) and 21 women (9.0%). The mean BMI was 22.9 ± 3.7 kg/m2. The major route of transmission among study population was sexual transmission (190 patients, 81.5%), the second most common was blood-borne transmission (8 patients, 3.4%). The mean baseline CD4+ T cell count was 256 ± 178 cells/μL, and the mean baseline plasma HIV RNA load was 4.8 ± 0.7 lg copies/mL. One hundred fifty patients in the sample had been screened for HBV infection with a HBsAg test, of which 12 patients (8%) were positive. No patients were hepatitis C virus (HCV) antibody positive among the 145 patients who had a HCV testing documented in the medical record.
Table 1.
Demographic and clinical characteristics at baseline.
| Characteristics | |
|---|---|
| N | 233 |
| Age (years) | 36.6 ± 11.1 |
| <50 years, n (%) | 199 (85.4%) |
| ≥50 years, n (%) | 34 (14.6%) |
| Sex, n (%) | |
| Male | 212 (91.0%) |
| Female | 21 (9.0%) |
| BMI (kg/m2) | 22.9 ± 3.7 |
| CD4 + T cell count (cells/μL) | 256 ± 178 |
| HIV viral load (lg copies/mL) | 4.8 ± 0.7 |
| Route of HIV transmission, n (%) | |
| Sexual transmission | 190 (81.5%) |
| Homosexual | 155 (66.5%) |
| Heterosexual | 29 (12.4%) |
| Bisexual | 6 (2.6%) |
| Blood-borne transmission | 8 (3.4%) |
| Unknown | 35 (15.0%) |
Abbreviations: BMI, body mass index.
3.2. BMD and TBS at baseline and week 48 in overall study population
At baseline, the mean BMD of the overall sample was 1.171 ± 0.142 kg/cm2, 0.989 ± 0.138 kg/cm2, and 1.020 ± 0.132 kg/cm2 at the LS, FN, and TH, respectively (Table 2). One hundred ninety-nine patients were under 50 years of age. Among them, 189/199 patients (95.0%) had BMD results within the expected range for age (Z-score > −2.0) for at least one site, and 10/199 patients (5.0%) had BMD results below the expected range for age (Z-score ≤ −2.0) for at least one site. Thirty-four patients were aged 50 years and older. Among them, 7/34 patients (20.6%) had low bone density (−2.5<T-score < −1.0) as measured by DXA for at least one site, and no patients met criteria for osteoporosis (T-score ≤ −2.5).
Table 2.
Lumbar spine, femoral neck, total hip BMD and TBS at baseline and week 48.
| Characteristics | Baseline | Week 48 | p-value |
|---|---|---|---|
| BMD (g/cm2) | |||
| Lumbar Spine | 1.171 ± 0.142 | 1.136 ± 0.145 | 0.018 |
| Femoral Neck | 0.989 ± 0.138 | 0.954 ± 0.137 | <0.001 |
| Total Hip | 1.020 ± 0.132 | 0.988 ± 0.135 | <0.001 |
| TBS | 1.409 ± 0.087 | 1.384 ± 0.093 | <0.001 |
| TBS category, n (%) | 0.111 | ||
| Normal (≥1.310) | 201 (86.3%) | 189 (81.1%) | |
| Partly Degraded (1.230–1.310) | 26 (11.2%) | 29 (12.5%) | |
| Degraded (≤1.230) | 6 (2.5%) | 15 (6.4%) | |
Abbreviations: BMD, bone mineral density; TBS, trabecular bone score.
BMD of the overall study population at each of the sites decreased significantly during the first year of ART: LS BMD decreased to 1.136 ± 0.145 g/cm2 (p = 0.118, percent change: −3.0% ± 4.0%); FN BMD to 0.954 ± 0.137 g/cm2 (p < 0.001, percent change: −3.5% ± 3.9%); and TH BMD to 0.988 ± 0.135 g/cm2 (p < 0.001, percent change: −3.2% ± 3.2%) (Table 2).
The mean baseline TBS of the overall population was 1.409 ± 0.087. While 201 patients (86.3%) had normal TBS results, 26 patients (11.2%) and 6 patients (2.5%) had TBS results categorized as partially degraded and degraded, respectively. We observed a significant decrease in the average TBS to 1.384 ± 0.093 (−1.6% ± 5.7%, p < 0.001) in the first 48 weeks. No significant changes in distribution across TBS categories were observed between baseline and week 48 (p = 0.111) (Table 2).
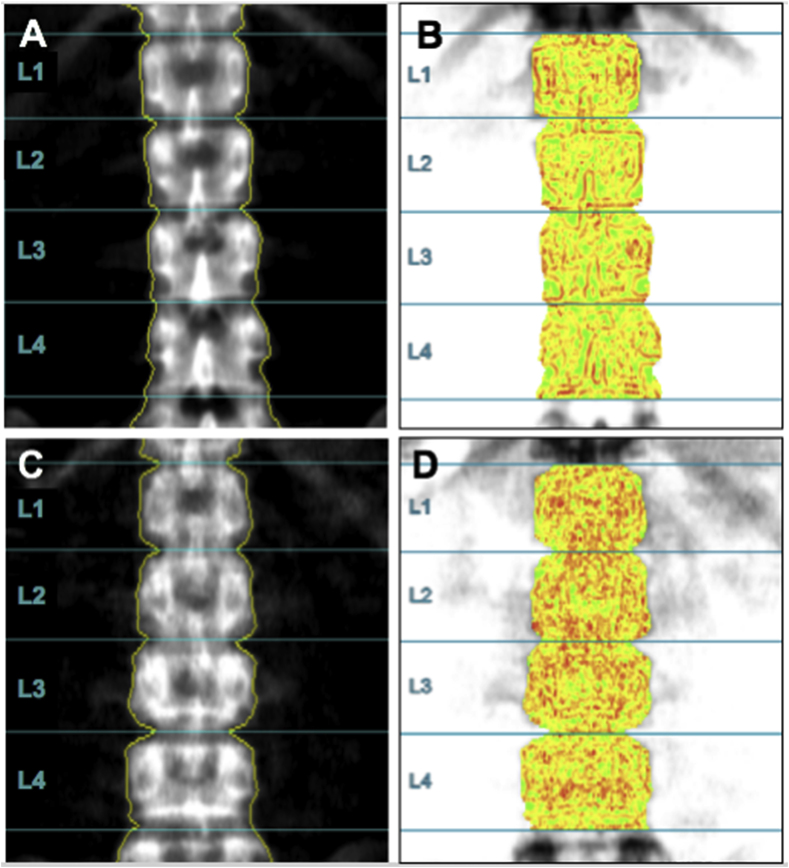
Forty-five patients (19.3%) had a normal BMD but partially degraded TBS (42 patients, 18.0%) or degraded TBS (3 patients, 1.3%). Fig. 1-A is the lumbar spine DXA image and Fig. 1-B is the TBS image of a 26.1-year-old male, whose lumbar spine BMD is within the expected range for age (BMD is 1.074 g/cm2, Z-score is −0.2) and TBS is normal (1.431). Fig. 1-C shows the lumbar spine DXA image and Fig. 1-D is the TBS image of a 26.5-year-old male, his lumbar spine BMD is within the expected range for age (BMD is 1.232 g/cm2, Z-score is −0.8) but his TBS is 1.189, which shows the degraded bone microarchitecture.
Figure 1.
Fig. 1-A is the lumbar spine DXA image (BMD: 1.074 g/cm2, Z-score: −0.2) and Fig. 1-B is the TBS image (TBS: 1.431) of a 26.1-year-old male, whose BMD is within the expected range for age and TBS is normal; Fig. 1-C is the lumbar spine DXA image (BMD: 1.232 g/cm2, Z-score: −0.8) and Fig. 1-D is the TBS image (TBS: 1.189) of a 26.5-year-old male, his BMD is within the expected range for age but his TBS shows the degraded bone microarchitecture.
3.3. Factors associated with percent change in lumbar spine, femoral neck, and total hip BMD as well as TBS
In the univariate analysis, we found that higher baseline HIV viral load [β coefficient (95%CI): LS BMD: −1.578 (−2.437, −0.718), p < 0.001; FN BMD: −1.903 (−2.765, −1.041), p < 0.001; TH BMD: −1.332 (−2.047, −0.617), p < 0.001], TDF exposure [β coefficient (95%CI): LS BMD: −1.423 (−2.258, −0.589), p = 0.001; FN BMD: −1.138 (−1.958, −0.318), p = 0.001; TH BMD: −1.572 (−2.226, −0.919), p < 0.001] and LPV/r exposure [β coefficient (95%CI): LS BMD: −1.364 (−2.289, −0.439), p = 0.004; FN BMD: −1.255 (−2.159, −0.351), p = 0.007; TH BMD: −1.708 (−2.430, −0.986), p < 0.001] were risk factors significantly associated with larger BMD decrease at each site during the first 48 weeks. Additionally, older baseline age (per 10-year increment) was significantly associated with decreased LS BMD [β coefficient (95%CI): −0.213 (−0.373, −0.053), p = 0.009]. Lower baseline BMI [β coefficient (95%CI): 0.100 (−0.035, 0.235), p = 0.146] was risk factor significantly associated with larger FH BMD decrease. There was a slight association between lower baseline CD4+ cell count and larger TBS decrease [β coefficient (95%CI): 0.005 (0.001, 0.009), p = 0.061].
In the multivariable model including baseline age, BMI, HIV viral load, TDF exposure and LPV/r exposure, higher baseline HIV viral load and TDF exposure were significantly associated with larger BMD decrease at each site during the first 48 weeks. Additionally, the use of LPV/r was significantly associated with decreased TH BMD [β coefficient (95% CI): −1.367 (−2.260, −0.474), p = 0.003]. In the lumbar spine, there was a significant association between lower baseline BMI and greater BMD loss [β coefficient (95% CI): 0.205 (0.045, 0.365), p = 0.012] (Table 3).
Table 3.
Factors associated with percent change in lumbar spine, femoral neck, total hip BMD and TBS over the first 48 weeks in the multivariable linear regression analysis (N = 233).
| Variable | Percent Change in Lumbar Spine BMD |
Percent Change in Femoral Neck BMD |
Percent Change in Total Hip BMD |
Percent Change in TBS |
||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Age (per 10-year increment) | −0.379 (−0.879, 0.121) | 0.136 | 0.008 (−0.499, 0.515) | 0.975 | −0.170 (−0.569, 0.230) | 0.403 | −0.472 (−1.207, 0.263) | 0.207 |
| BMI | 0.205 (0.045, 0.365) | 0.012 | 0.111 (−0.051, 0.274) | 0.177 | 0.060 (−0.067, 0.188) | 0.352 | 0.237 (−0.005, 0.478) | 0.045 |
| CD4 + T cell count | NA | NA | NA | NA | NA | NA | 0.005 (0.0005, 0.0104) | 0.032 |
| HIV viral load | −1.174 (−2.049, −0.298) | 0.009 | −1.716 (−2.605, −0.827) | <0.001 | −1.240 (−1.939, −0.541) | 0.001 | NA | NA |
| TDF exposure | −1.392 (−2.334, −0.451) | 0.004 | −1.096 (−2.052, −0.140) | 0.025 | −1.409 (−2.162, −0.657) | <0.001 | −0.516 (−1.984, 0.952) | 0.489 |
| LPV/r exposure | −0.635 (−1.753, 0.483) | 0.264 | −1.084 (−2.220, 0.051) | 0.061 | −1.367 (−2.260, −0.474) | 0.003 | −0.048 (−1.733, 1.677) | 0.956 |
Abbreviations: CI, confidence interval; NA, not applicable (denotes p ≥ 0.20 in the univariate analysis); BMD, bone mineral density; TBS, trabecular bone score; BMI, body mass index; TDF, tenofovir disoproxil fumarate; LPV/r, lopinavir/ritonavir.
In the multivariable model including baseline age, BMI, HIV viral load and combination usage of TDF + LPV/r, more significant associations were observed between combination usage of TDF + LPV/r and larger declines in BMD [β coefficient (95% CI): LS BMD: −3.177 (−5.641, −0.714), p = 0.012, FN BMD: −2.873 (−5.380, −0.365), p = 0.025, and TH BMD: −4.337 (−6.346, −2.328), p < 0.001].
Factors associated with lower baseline TBS included older age (per 10-year increment) [β coefficient (95% CI): −0.013 (−0.023, −0.004), p = 0.007], lower baseline BMI [β coefficient (95% CI): 0.004 (0.001, 0.007), p = 0.018] and female sex [β coefficient (95% CI): 0.043 (0.004, 0.081), p = 0.029]. During the observation period, in the multivariable model including baseline age, BMI, CD4+ T cell count, TDF exposure and LPV/r exposure, both lower baseline BMI [β coefficient (95% CI): 0.237 (−0.005, 0.478), p = 0.045] and lower baseline CD4+ cell count [β coefficient (95% CI): 0.005 (0.0005, 0.0104), p = 0.032] were significantly associated with larger decreases in TBS. No associations were found between TBS changes and TDF or LPV/r exposure in our study (Table 3). Even after adjusting for age, BMI and baseline CD4+ cell count, there was no association remained between combination usage of TDF + LPV/r and percent change in TBS (p = 0.384).
4. Discussion
This is the first longitudinal study to evaluate TBS and report change in TBS as compared with change in BMD after initiation of ART in Asia PWH. Both BMD and TBS decreased in the year following ART initiation. We found that traditional risk factors such as old age, low BMI and female sex were associated with low baseline TBS. Greater change in TBS over one year was associated with lower baseline BMI and lower baseline CD4+ cell count, but unlike BMD measures, it did not correlate with treatment with different antiretroviral agents in our study population.
Our group has previously published two smaller studies reporting longitudinal BMD changes among Chinese PWH. One small early study evaluating data from 40 PWH (prior to inclusion of TDF in as part of first-line regimens in the Chinese national free AIDS treatment program) demonstrated that prior to ART, Chinese PWH had lower lumbar spine BMD compared with healthy controls. During the first 48 weeks after ART initiation, BMD of lumbar spine (2.10%), femoral neck (3.28%), and total hip (1.78%) decreased significantly [15]. In a recent study focused on understanding the impact of TDF on BMD among Chinese PWH, we found greater declines in total hip BMD among 136 Chinese PWH during the initial 96 weeks of TDF-containing ART compared with patients treated with non-TDF-containing ART [16]. Our present study adds to this body of knowledge by specifically evaluating changes in TBS and evaluating the association of two ART agents (TDF and LPV/r) that are staples of ART in China and commonly used in other low- and middle-income countries for treatment of HIV.
Currently, American and European guidelines exist for the evaluation and management of bone disease in PWH [21,22]. However, in Asia, including China, locally appropriate guidelines for screening for osteoporosis and fragility fracture are still not available due to the paucity of primary data in these populations on the epidemiology, mechanisms, and prevention strategies for these outcomes. In our clinic, DXA availability and integration into clinical care occurred originally as a means of screening for lipodystrophy when stavudine was used as first-line therapy prior to 2012. However, there is a wide resource variability across China. To this end, our team has also been collaborating with a national network of HIV research centers in China to address the gaps in knowledge with regards to aging-related comorbidities among PWH including bone disease [2], with the goal of ultimately developing evidence-based guidelines appropriate for HIV-care settings in China.
Compared to the large number of studies evaluating BMD among PWH, only a limited number of studies have focused on TBS in this population. While BMD provides information regarding the bone mass, bone strength and risk for fracture are also influenced by other structural skeletal determinants, such as bone microarchitecture and geometry. Modalities such as quantitative computed tomography (QCT) [23], microindentation [24], high resolution peripheral quantitative computed tomography (HRpQCT) [25] and quantitative ultrasound [26] provide detailed in vivo information regarding microarchitecture. For example, QCT is an alternative method to evaluate 3-dimensional volumetric density measurements and allows for measures of cortical and trabecular bone separately. The 3-dimensional volumetric density measured by QCT is closely related to vertebral fragility fracture, so QCT is an effective technique to accurately discriminate vertebral fragility fracture. Compared with DXA, the measurement of QCT is less influenced by calcifications and degenerative changes in the spine. Thus, use of QCT may provide additional information in PWH [23,27]. However, these tools are not routinely available in clinical practice, even in high resource settings. By contrast, TBS indirectly quantifies trabecular microarchitecture by performing a gray-scale textural analysis of lumbar spine DXA images [9]. A number of studies have shown that TBS correlates with direct measures of trabecular microarchitecture such as HRpQCT and bone biopsy [28,29], and predicts fracture risk independent of DXA-derived BMD measures [20,28]. Another advantage of TBS is that even though osteoarthritic changes can confound lumbar spine DXA measurements, its severity has little effect on TBS [30]. Our study reports that before ART initation, 19.3% of PWH had normal BMD but abnormal TBS, which means approximately 20% of PWH with impaired bone microarchitecture would not have been identified if DXA were used alone to assess for bone damage.
Only two prior studies have examined longitudinal change in TBS among PWH on ART [12,23]. One study, by Güerri-Fernández and colleagues [24], reported a decrease in TBS of 2.5% among 40 Spanish PWH (mean age: 38 ± 9 years, 33% males) after one year of ART (elvitegravir/cobicistat + TDF + FTC), in contrast to the decrease in TBS of 1.6% observed in our study population. Potential explanations for this discrepancy include differences in composition of the study population (greater proportion of younger males in our study), ART regimens and race. However, the decline in TBS among PWH in our study is higher than that reported in the general population [[31], [32], [33]]. In the literature, a decrease in TBS by 0.2%–0.5% per year has been reported in populations aged 30–90 years in Hong Kong [31], Japan [32] and Thailand [33], providing a basis of comparison. Therefore, our data suggest that the decline in TBS reflects skeletal microstructure damage beyond that from aging alone, but also due to other factors relevant to PWH.
We systematically investigated clinical and HIV-associated risk factors associated with change in BMD and TBS during the first 48 weeks. The linear regression analyses examining the associations between the change in BMD and TBS over 48 weeks with TDF, LPV/r, and combination of TDF and LPV/r showed that the combination of TDF and LPV/r was associated with greater BMD loss compared with either agent alone. With regards to ART exposure, we did not find any significant association between TBS over 48 weeks and different treatment groups. This was in contrast to changes observed in BMD, underscoring that skeletal parameters may be differentially impacted by ART. McGinty et al. [10] previously reported that exposure to PIs [β coefficient (95% CI): −0.051 (−0.087, −0.015), p = 0.006], but not TDF [β coefficient (95% CI): −0.023 (−0.065, 0.019), p = 0.27] was independently associated with lower TBS among 201 PWH (median age: 39 years, 59.2% males, median ART duration: 2.7 years).
Prior studies have demonstrated a more pronounced loss of BMD after exposure to TDF-containing ART compared to regimens without TDF [34,35], a nucleotide reverse-transcriptase inhibitor, which is hypothesized to affect bone metabolism and BMD through renal proximal tubule dysfunction, leading to abnormal phosphaturia and osteomalacia [36], and potentially secondary hyperparathyroidism mediated through aberrations in vitamin D metabolism [37]. In our study, use of LPV/r, a PI, was also associated with BMD loss. PIs, in particular LPV/r, have been associated with lower BMD and higher rates of fracture [38,39]. However, the mechanisms underlying PI-associated bone loss still remain to be elucidated [40].
This study has some limitations that warrant mention. First, our study represents data from a single tertiary care hospital in China, which limits the generalizability of our findings. Additionally, the relatively small sample size limited our ability to perform subgroup analyses. However, given the dearth of longitudinal studies evaluating TBS among PWH on ART, our findings are an important addition to the literature. Moreover, because our study was a retrospective chart review, we were unable to systematically collect information about bone turnover markers, data regarding osteoporosis and fracture risk factors such as smoking and alcohol status, and personal or parental risk of fractures, which could affect bone health. Finally, our study only explored the influence of exposure to TDF and LPV/r, however we selected two agents according to China national guidelines for HIV/AIDS treatment and compelling reasons from the perspective of bone health.
5. Conclusions
We present the first longitudinal analysis of change in TBS over 48 weeks compared with BMD among Chinese PWH receiving ART. Before ART initiation, approximately 20% of PWH with impaired bone microarchitecture would not have been identified if DXA were used alone to assess for bone damage. Both BMD and TBS decreased after one-year ART. Lower baseline BMI and lower baseline CD4+ cell count may be associated with greater declines in TBS. More research is needed to confirm these findings, and better understand if there is a potential role for TBS in evaluation and monitoring of bone health in ART-treated PWH. Finally, future multi-center prospective studies with larger samples and longer duration of follow up should to explore whether TBS and BMD differentially contribute to fracture risk prediction among PWH.
Declaration of competing interest
None.
Acknowledgements
We thank all the patients and their families for their participation during this study. This study was funded by the National Key Technologies Research & Development Program for the 13th Five-year Plan (2017ZX10202101), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2017–12M-1–014), and the Yale Center for Clinical Investigation K Supplement Award (NIH/National Center for Advancing Translational Sciences) (UL1TR001863). Dr. Guan is supported by the 2019 Peking Union Medical College Doctoral Student Short-term Visiting Fund. Dr. Hsieh is supported by NIH/Fogarty International Center K01TW009995.
Contributor Information
Wei Yu, Email: weiyu5508@yahoo.com.
Tai-Sheng Li, Email: litsh@263.net.
References
- 1.Palella F.J.J., Delaney K.M., Moorman A.C., Loveless M.O., Fuhrer J., Satten G.A. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/nejm199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Cao W., Hsieh E., Li T. Optimizing treatment for adults with HIV/AIDS in China: successes over two decades and remaining challenges. Curr HIV AIDS Rep. 2020;17(1):26–34. doi: 10.1007/s11904-019-00478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran C.A., Weitzmann M.N., Ofotokun I. Bone loss in HIV infection. Curr Treat Options Infect Dis. 2017;9(1):52–67. doi: 10.1007/s40506-017-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown T.T., Qaqish R.B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/qad.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 5.Shiau S., Broun E.C., Arpadi S.M., Yin M.T. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS. 2013;27(12):1949–1957. doi: 10.1097/qad.0b013e328361d241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin F., Duval X., Le Moing V., Piroth L., Al Kaied F., Massip P. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23(8):1021–1024. doi: 10.1097/qad.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanis J.A., McCloskey E.V., Johansson H., Oden A., Melton L.J., 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Dalle Carbonare L., Giannini S. Bone microarchitecture as an important determinant of bone strength. J Endocrinol Invest. 2004;27(1):99–105. doi: 10.1007/bf03350919. [DOI] [PubMed] [Google Scholar]
- 9.Martineau P., Leslie W.D. Trabecular bone score (TBS): method and applications. Bone. 2017;(104):66–72. doi: 10.1016/j.bone.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 10.McGinty T., Cotter A.G., Sabin C.A., Macken A., Kavanagh E., Compston J. Assessment of trabecular bone score, an index of bone microarchitecture, in HIV positive and HIV negative persons within the HIV UPBEAT cohort. PloS One. 2019;14(3) doi: 10.1371/journal.pone.0213440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciullini L., Pennica A., Argento G., Novarini D., Teti E., Pugliese G. Trabecular bone score (TBS) is associated with sub-clinical vertebral fractures in HIV-infected patients. J Bone Miner Metabol. 2018;36(1):111–118. doi: 10.1007/s00774-017-0819-6. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A., Ma Y., Tien P.C., Scherzer R., Anastos K., Cohen M.H. HIV infection is associated with abnormal bone microarchitecture: measurement of trabecular bone score in the women's interagency HIV study. J Acquir Immune Defic Syndr. 2018;78(4):441–449. doi: 10.1097/qai.0000000000001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AIDS Professional Group Society of infectious diseases Chinese medical association: guidelines for diagnosis and treatment of HIV/AIDS in China. Chin J Infect Dis. 2011;29(10):629–649. doi: 10.3760/cma.j.issn.1674-2397.2011.06.001. [DOI] [Google Scholar]
- 14.National Health Commission of the People's Republic of China. Regular press conference: Progress in the prevention and treatment of AIDS in China. http://ncaidschinacdccn/xxgx/yqxx/201811/t20181123_197488.htm. (Accessed 23 February 2020).
- 15.Zhang L., Su Y., Hsieh E., Xia W., Xie J., Han Y. Bone turnover and bone mineral density in HIV-1 infected Chinese taking highly active antiretroviral therapy -a prospective observational study. BMC Muscoskel Disord. 2013;14:224. doi: 10.1186/1471-2474-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo F., Song X., Li Y., Guan W., Pan W., Yu W. Longitudinal change in bone mineral density among Chinese individuals with HIV after initiation of antiretroviral therapy. Osteoporos Int. 2021;32(2):321–332. doi: 10.1007/s00198-020-05584-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adult official positions of the international society for clinical Densitometry. 2020. http://www.iscd.org/offcial positions/offcial.ISCD position-adult (English) [DOI] [PubMed] [Google Scholar]
- 18.Kanis J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–381. doi: 10.1007/bf01622200. [DOI] [PubMed] [Google Scholar]
- 19.Chinese Medical Association Menopause Professional Group Guidelines for diagnosis of menopause and treatment of menopausal hormone therapy in China (2018) Chin J Obstet Gynecol. 2018;53(11):729–739. doi: 10.3969/j.issn.1674-9081.2018.06.007. [DOI] [Google Scholar]
- 20.McCloskey E.V., Oden A., Harvey N.C., Leslie W.D., Hans D., Johansson H. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31(5):940–948. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- 21.Brown T.T., Hoy J., Borderi M., Guaraldi G., Renjifo B., Vescini F. Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis. 2015;60(8):1242–1251. doi: 10.1093/cid/civ010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biver E., Calmy A., Aubry-Rozier B., Birkhäuser M., Bischoff-Ferrari H.A., Ferrari S. Diagnosis, prevention, and treatment of bone fragility in people living with HIV: a position statement from the Swiss Association against Osteoporosis. Osteoporos Int. 2019;30(5):1125–1135. doi: 10.1007/s00198-018-4794-0. [DOI] [PubMed] [Google Scholar]
- 23.Thomsen M.T., Wiegandt Y.L., Gelpi M., Knudsen A.D., Fuchs A., Sigvardsen P.E. Prevalence of and risk factors for low bone mineral density assessed by quantitative computed tomography in people living with HIV and uninfected controls. J Acquir Immune Defic Syndr. 2020;83(2):165–172. doi: 10.1097/qai.0000000000002245. [DOI] [PubMed] [Google Scholar]
- 24.Güerri-Fernández R., Lerma-Chippirraz E., Fernandez Marron A., García-Giralt N., Villar-García J., Soldado-Folgado J. Bone density, microarchitecture, and tissue quality after 1 year of treatment with tenofovir disoproxil fumarate. AIDS. 2018;32(7):913–920. doi: 10.1097/qad.0000000000001780. [DOI] [PubMed] [Google Scholar]
- 25.Foreman S.C., Wu P.H., Kuang R., John M.D., Tien P.C., Link T.M. Factors associated with bone microstructural alterations assessed by HR-pQCT in long-term HIV-infected individuals. Bone. 2020;133:115210. doi: 10.1016/j.bone.2019.115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang L., Xia C., Xu H., Ge Q., Shi Z., Kong L. Defining disease progression in Chinese mainland people: association between bone mineral density and knee osteoarthritis. J Orthop Translat. 2020;26:39–44. doi: 10.1016/j.jot.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao Y.-F., Zhang Y., Li K., Wang L., Ma Y.-M., Xiao W.-L. Discrimination of vertebral fragility fracture with lumbar spine bone mineral density measured by quantitative computed tomography. J Orthop Translat. 2019;16:33–39. doi: 10.1016/j.jot.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hans D., Stenova E., Lamy O. The trabecular bone score (TBS) complements DXA and the FRAX as a fracture risk assessment tool in routine clinical practice. Curr Osteoporos Rep. 2017;15(6):521–531. doi: 10.1007/s11914-017-0410-z. [DOI] [PubMed] [Google Scholar]
- 29.Hans D., Barthe N., Boutroy S., Pothuaud L., Winzenrieth R., Krieg M.A. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14(3):302–312. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Silva B.C., Leslie W.D., Resch H. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29(3):518–530. doi: 10.1002/jbmr.2176. [DOI] [PubMed] [Google Scholar]
- 31.Su Y., Leung J., Hans D., Aubry-Rozier B., Kwok T. Added clinical use of trabecular bone score to BMD for major osteoporotic fracture prediction in older Chinese people: the Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporos Int. 2017;28(1):151–160. doi: 10.1007/s00198-016-3785-2. [DOI] [PubMed] [Google Scholar]
- 32.Iki M., Tamaki J., Sato Y., Winzenrieth R., Kagamimori S., Kagawa Y. Age-related normative values of trabecular bone score (TBS) for Japanese women: the Japanese Population-based Osteoporosis (JPOS) study. Osteoporos Int. 2015;26(1):245–252. doi: 10.1007/s00198-014-2856-5. [DOI] [PubMed] [Google Scholar]
- 33.Sritara C., Thakkinstian A., Ongphiphadhanakul B., Amnuaywattakorn S., Utamakul C., Akrawichien T. Age-adjusted dual X-ray absorptiometry-derived trabecular bone score curve for the lumbar spine in Thai females and males. J Clin Densitom. 2016;19(4):494–501. doi: 10.1016/j.jocd.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 34.McComsey G.A., Kitch D., Daar E.S., Tierney C., Jahed N.C., Tebas P. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stellbrink H.J., Orkin C., Arribas J.R., Compston J., Gerstoft J., Van Wijngaerden E. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51(8):963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 36.Casado J.L., Santiuste C., Vazquez M., Bañón S., Rosillo M., Gomez A. Bone mineral density decline according to renal tubular dysfunction and phosphaturia in tenofovir-exposed HIV-infected patients. AIDS. 2016;30(9):1423–1431. doi: 10.1097/qad.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh E., Fraenkel L., Han Y., Xia W., Insogna K.L., Yin M.T. Longitudinal increase in vitamin D binding protein levels after initiation of tenofovir/lamivudine/efavirenz among individuals with HIV. AIDS. 2016;30(12):1935–1942. doi: 10.1097/qad.0000000000001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briot K., Kolta S., Flandre P., Boué F., Ngo Van P., Cohen-Codar I. Prospective one-year bone loss in treatment-naïve HIV+ men and women on single or multiple drug HIV therapies. Bone. 2011;48(5):1133–1139. doi: 10.1016/j.bone.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Brown T.T., McComsey G.A., King M.S., Qaqish R.B., Bernstein B.M., da Silva B.A. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51(5):554–561. doi: 10.1097/qai.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 40.Moran C.A., Weitzmann M.N., Ofotokun I. The protease inhibitors and HIV-associated bone loss. Curr Opin HIV AIDS. 2016;11(3):333–342. doi: 10.1097/coh.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]