Abstract
Chordoma is a rare and aggressive intracranial bone tumor that is difficult to diagnose and resect with a peak incident between the ages of 20–40 years old and high recurrence rate when not completely resected. We present the case of clival chordoma in an 11-year-old female patient, who reported with a chronic right-sided headache, progressive loss of vision, hoarseness of voice, and slurred speech. Fluid-fluid level on fluid-attenuated inversion recovery magnetic resonance imaging sequence can be an atypical radiological sign for clival chordoma. Thumbing of the pons as well as extension of the chordoma to the sinonasal, intracranial, vertebral, intraspinal, and orbital regions were observed. The patient underwent partial resection of the tumor and discharge home after by the end of the third week after the surgery. Histopathology report confirmed the diagnosis of chordoma.
Keywords: Brain tumor, Chordoma, Fluid-fluid level, Clival lesion, Bone tumor
Introduction
Chordoma is a rare and aggressive bone tumor with an incidence of 0.08 per 1,000,000. It accounts for 0.5% of all central nervous system tumors. It emerges from the residual notochord tissue as part of the axial skeleton, which usually develops in the sacrococcygeal, clival, and vertebral bodies [1], [2], [3]. The incidence of clival chordomas peaks between the ages of 20–40 years old. Meanwhile, sacrococcygeal most commonly develops during the fourth and fifth decades of life [3]. The 10-year survival of chordoma patients is reportedly 40% [4]. The presentation of clival chordomas depends on their size and location within the clivus and the mass effect they exert on the surrounding structures, particularly the brain stem, which causes cranial neuropathies and headache [5,6]. Due to its location, it is difficult to access and completely resect [7]. Chordomas can present with atypical radiological features, making them difficult to diagnose.
Case presentation
An 11-year-old previously healthy girl presented with a chronic right-sided headache for one year, which was partially relieved by painkillers. The headache was associated with unintentional weight loss for the past year. Two months prior to reporting at our hospital, she had developed progressive visual impairment in the right eye, which progressed to complete vision loss. The visual acuity of her left eye began deteriorating as well one month prior. She also exhibited slurred speech, hoarseness of voice, and dysphagia to solids.
Imaging findings
Computed tomography (CT) scan of the head (Fig. 1) revealed a large destructive skull base soft tissue mass, measuring approximately 6.5 × 5 × 6 cm (anteroposterior, transverse, and craniocaudal dimensions). The mass involved and penetrated the clivus, petrous apices, jugular foramina, and occipital condyles, and extended intracranially into the sellar and right parasellar regions. Posteriorly, it extended to the interpeduncular, pre-pontine, and right cerebellopontine angle cisterns. Inferiorly, the mass extended into the upper cervical vertebrae, encasing the odontoid process and C1 anterior arch, damaging the left side of the body and lateral mass of C2, extending intraspinally through the C1-2 neural foramen. Anteriorly, the mass involved the nasopharynx, sphenoid sinus, posterior ethmoid cells, right nasal cavity posterior nostril, right pterygoid plate, and right pterygopalatine fossa, reaching the posterior wall of the right maxillary sinus. The planum sphenoidale was destroyed, and the mass extended intracranially into the floor of the anterior cranial fossa. Additionally, it extended intra-orbitally into the right orbital apex, with the destruction of the posterior lamina papyracea. The optic canal and superior and inferior orbital fissures were also involved. Significant obliteration of the nasopharynx and right side of the oropharyngeal airway was noted.
Fig. 1.
Unenhanced computed tomography (CT) of the head at the level of the mass showed a large destructive skull base hyperdense soft tissue mass with sinonasal, intracranial, vertebral, intraspinal, and right orbital involvement; as detailed. A) Axial. B) Coronal. C) Sagittal.
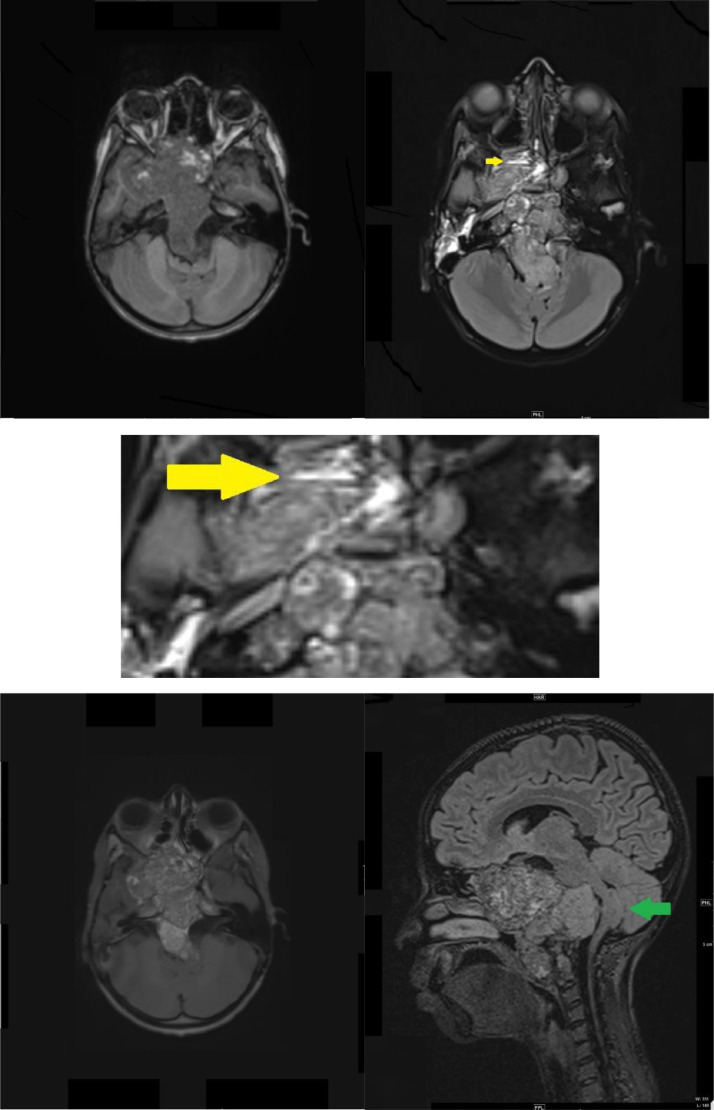
Brain magnetic resonance imaging (MRI) performed with intravenous (IV) gadolinium administration (Fig. 2) demonstrated a large destructive lesion at the base of the skull, centered within the clivus. It exhibited a heterogenous low signal intensity on T1-weighted imaging, with intervening linear hyperintense signal intensity. Additionally, few fluid levels were observed in the tumor in the right ethmoid sinus on fluid-attenuated inversion recovery (FLAIR) imaging, and multiple foci of diffusion restriction were seen. The lesion had intermediate heterogeneous signal intensity on T2/FLAIR images and heterogenous enhancement on post-contrast images. The intensity of the posterior component of the lesion was more enhanced than the internal component. There was disfiguring, deformity, and mass effect on the brainstem, with a U-shaped appearance. This simulated the thumb sign or thumbing of the pons. The peri-pontine cistern and the fourth ventricle were effaced. Inferiorly, the mass abutted the distal vertebral arteries bilaterally, with a destructive lesion at the C1 and C2 levels, causing encasement of the distal left vertebral artery.
Fig. 2.
Magnetic resonance imaging (MRI) of the brain axial T1 (A), axial FLARE (B), axial flare magnified (C), axial T1 post-contrast (D), sagittal axial fluid-attenuated inversion recovery (FLAIR) (E), demonstrated a large mass arising from clivus. (A) A heterogeneous low signal intensity with multiple foci of high signal intensity indicating intralesional hemorrhage was seen. (B, C) Intermediate signal intensities with fluid levels were noted (Yellow arrow) in the FLAIR images. (D) Heterogenous enhancement were observed in the post-contrast images. The posterior component of the lesion was enhanced compared to the internal component mass effect upon the brainstem with a U-shaped appearance. (E) Simulating the thumb sign or thumbing of the pons (Green arrow).
The patient underwent surgical resection and debulking and the diagnosis of chordoma was confirmed histopathologically where it states multiple brown tan tissue fragments with multiple calcifications grossly and chondroid matrix with diffuse growth pattern microscopically. The symptoms reduced and her deteriorating vision stabilized following the surgery. The patient was discharged from the hospital on day 22 post-op with 80% of the chordoma resected. Her Glasgow Coma Score was 15/15 with residual motor, bulbar, and visual deficits.
Discussion
Clival chordoma can present with atypical radiological features. On CT, it appears as a well-defined soft tissue lesion, destroying the surrounding bone, with heterogeneous enhancement and calcification. On MRI, it exhibits low signal intensity in T1 with intralesional hyperintense signal intensity representing hemorrhage. In T2, it exhibits high signal intensity, and with IV gadolinium, it demonstrates a heterogeneous enhancement [8]. Thumbing of the pons is a specific sign for clival chordoma [1]. However, chordomas are difficult to diagnose based on radiological findings alone. A histopathological study is required to reach the definitive diagnosis. Fluid-fluid level has not been observed in chordomas with its various locations and subtypes in the literature. Multiple studies have Investigated bone lesions that present radiographically with fluid-fluid level, none of which were chordomas. Instead, aneurysmal bone lesions as well as giant cell tumor were the most common while chondrosarcoma found to be uncommon [9], [10], [11].
Conclusion
Clival chordomas are rare intracranial tumors that can be challenging to diagnose, especially when they present with atypical radiological features such as fluid-fluid level.
Data availability
The data are archived in the database of the radiology department of King Khalid university hospital
Author Contribution
All the authors participated to the completion of this work. The final version of the manuscript was read and edited by all authors.
Patient consent
The consent to publish the radiological findings was obtained verbally from the patient's father. All patient's identifying information's was not included in the manuscript.
Footnotes
Competing Interests: None
References
- 1.Frezza AM, Botta L, Trama A, Dei Tos AP, Stacchiotti S. Chordoma: update on disease, epidemiology, biology and medical therapies. Curr Opin Onc. 2019;31(2):114–120. doi: 10.1097/CCO.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 2.Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–e76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 3.Das P, Soni P, Jones J, Habboub G, Barnholtz-Sloan JS, Recinos PF, Kshettry VR. Descriptive epidemiology of chordomas in the United States. J Neuro Onc. 2020;148(1):173–178. doi: 10.1007/s11060-020-03511-x. [DOI] [PubMed] [Google Scholar]
- 4.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. CCC. 2001;12(1):1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 5.Koutourousiou M, Snyderman CH, Fernandez-Miranda J, Gardner PA. Skull base chordomas. Otolaryngol Clin North Am. 2011;44(5):1155–1171. doi: 10.1016/j.otc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Gay E, Sekhar LN, Rubinstein E, Wright DC, Sen C, Janecka IP, Snyderman CH. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. J Neurosurg. 1995;36(5):887–896. doi: 10.1227/00006123-199505000-00001. discussion 896-7. [DOI] [PubMed] [Google Scholar]
- 7.Jägersberg M, El Rahal A, Dammann P, Merkler D, Weber DC, Schaller K. Clival chordoma: a single-centre outcome analysis. Acta neurochirurgica. 2017;159(10):1815–1823. doi: 10.1007/s00701-017-3163-7. [DOI] [PubMed] [Google Scholar]
- 8.Doucet V, Peretti-Viton P, Figarella-Branger D, Manera L, Salamon G. MRI of intracranial chordomas. Extent of tumour and contrast enhancement: criteria for differential diagnosis. J Neuroradiol. 1997;39(8):571–576. doi: 10.1007/s002340050469. [DOI] [PubMed] [Google Scholar]
- 9.Van Dyck P, Vanhoenacker FM, Vogel J, Venstermans C, Kroon HM, Gielen J, Parizel PM, Bloem JL, De Schepper AM. Prevalence, extension and characteristics of fluid-fluid levels in bone and soft tissue tumors. Eur Radiol. 2006;16(12):2644–2651. doi: 10.1007/s00330-006-0250-1. [DOI] [PubMed] [Google Scholar]
- 10.Sone M, Ehara S, Sasaki M, Nakasato T, Tamakawa Y, Shiraishi H, Abe M. [Fluid-fluid levels in bone and soft tissue tumors demonstrated by MR imaging]. Nihon Igaku Hoshasen Gakkai zasshi. Nippon acta radiologica. 1992;52(8):1110–1115. [PubMed] [Google Scholar]
- 11.Tsai JC, Dalinka MK, Fallon MD, Zlatkin MB, Kressel HY. Fluid-fluid level: a nonspecific finding in tumors of bone and soft tissue. J Radiol. 1990;175(3):779–782. doi: 10.1148/radiology.175.3.2160676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are archived in the database of the radiology department of King Khalid university hospital