Abstract
MxB/Mx2 is an interferon-induced dynamin-like GTPase, which restricts a number of life-threatening viruses. Because of its N-terminal region, predicted to be intrinsically disordered, and its propensity to self-oligomerize, purification of the full-length protein has not been successful in conventional E. coli expression systems. Here, we describe an expression and purification procedure to obtain pure full-length wild-type MxB from suspension-adapted mammalian cells. We further describe how to characterize its GTPase activity and oligomerization function.
Keywords: Dynamin; Mx protein; GTPase, Mammalian expression; Purification; MBP-fusion; CryoEM, Helical assembly; Negative staining EM
1. Introduction
MxB/Mx2 is an interferon-stimulated mx2 gene product, found in eukaryotes, that restricts viral pathogens such as human immunodeficiency virus (HIV) (Goujon, Moncorge et al. 2013, Kane, Yadav et al. 2013, Liu, Pan et al. 2013), herpesvirus (Schilling, Bulli et al. 2018), murine cytomegalovirus (MCMV) (Jaguva Vasudevan, Bahr et al. 2018), equine infectious anemia virus (EIAV) (Meier, Jaguva Vasudevan et al. 2018), and porcine reproductive and respiratory syndrome virus (PRRSV) (Wang, Bai et al. 2016), among others. Although the mechanistic details are still unclear, it is thought that MxB acts during early post-entry stages of the infection by blocking viral genome replication.
MxB belongs to the dynamin family of large GTPases, and thus shares the core architecture of its members (Fribourgh, Nguyen et al. 2014, Haller, Staeheli et al. 2015, Alvarez, He et al. 2017). The core is composed of a GTPase (G) domain, which catalyzes the hydrolysis of GTP; a stalk domain, which contains the major interfaces for homo-oligomerization; and a bundle-signaling element (BSE) domain, which connects the GTPase and stalk domains, thus transmitting conformational changes between the two domains. Unique to MxB is its N-terminal region (NTR), which contains a nuclear localization signal. For the anti-HIV activity of human MxB, its NTR and oligomerization properties are critical determinants, but the GTPase activity is dispensable. For equine MxB, a fragment of the NTR (1–25 aa) is not necessary, while the GTPase activity is important for restriction of EIAV (Meier, Jaguva Vasudevan et al. 2018). In the case of herpesvirus restriction, both the NTR and the GTPase activity of MxB are important for its antiviral activity. These observations suggest a versatility of MxB in exerting different modes of inhibition for viral targets.
Structural analyses of human MxB have been hampered by the difficulty in purifying soluble full-length protein for in vitro studies (Xu, Kong et al. 2015). The crystal structure of MxB was solved by removing its NTR and introducing stalk mutations preventing higher-order oligomerization (Fribourgh, Nguyen et al. 2014). Clearly, study of the full length protein with intact GTPase function and self-assembly properties is important to understanding its mode of action. Furthermore, modifications introduced into the crystallization constructs of MxB alters the MxB structure, particularly in regions involved the oligomeration interfaces, as compared to the cryoEM structure of full-length wild-type MxB (Alvarez, He et al. 2017). In our laboratory, we exhausted various strategies of expressing and purifying the full-length wild type protein in E. coli. We used a comprehensive list of expression constructs containing fusions and affinity tags and expressed them in various strains at different expression conditions, but none resulted in soluble protein, in amounts necessary for biochemical and biophysical studies. We then switched to baculovirus expression in insect cells and observed very good expression of MxB during our preliminary experiments. However, throughput is slow when testing different expression constructs and fusion tags for optimal protein production, because it usually takes a few weeks for the generation and characterization of the viruses.
Here, we present expression, purification and characterization of recombinant full-length wild type MxB in suspension-adapted mammalian cells.
2. Materials
Prepare all solutions using deionized water and analytical grade reagents. Prepare and store all purification reagents at 4°C temperature (unless indicated otherwise). No sodium azide was added to reagents.
2.1. Transfection
Plasmid DNA
Expifectamine 293 Expression Kit (Thermo Fisher Scientific)
Expi293F cells (A14527, Thermo Fisher Scientific)
ExpiFectamine 293 Transfection Kit contains the ExpiFectamine™ 293 Reagent, Transfection Enhancer 1 and Transfection Enhancer 2 (A14524 Thermo Fisher Scientific)
Expi293 Expression Medium (A14351, Thermo Fisher Scientific)
Opti-MEM I Reduced Serum Medium (31985-062, Thermo Fisher Scientific)
Sterile Vent-cap Erlenmeyer Flasks with Baffled Bottom
Required infrastructure: basic cell culture room with Class II laminar flow hood and incubator with temperature and carbon dioxide control
Equipment and reagents for cell counting and viability (light microscope, hematocytometer with trypan blue).
2.2. Purification
Phosphate-buffered saline (PBS)
HNG (50 mM HEPES-KOH pH 8, 250mM NaCl, 5% glycerol)
Amylose resin (New England Biolabs)
Type B pestle Dounce homogenizer (Kimble Chase)
Empty chromatography column (Bio-rad)
Sephacryl S500-HR (Sigma-Aldrich)
Concentrator with molecular weight cut-off (MWCO) 50 kDa (Millipore)
Refrigerated table-top centrifuge (Eppendorf)
2.3. GTPase Assay
Spectrophotometer
quartz microcuvette
GTPase reaction buffer (50 mM Hepes-KOH (pH 7), 150 mM NaCl, 10 mM MgCl2, 2 mM dithiothreitol (DTT), 4 mM phosphenolpyruvate (PEP), 0.35 mM NADH, 25 Units of pyruvate kinase/lactate dehydrogenase, and 1mM GTP)
2.4. Tube assembly and immune-gold labeling
-
1.
400 mesh carbon coated electron microscopy grid (Ted Pella)
-
2.
anti-MBP (Abcam)
-
3.
5nm gold-conjugated secondary antibody (Ted Pella)
-
4.
bovine serum albumin (BSA)
-
5.
2% uranyl formate
-
4.
Purified HRV-3C protease or PreScission protease
3. Methods
3.1. Construction and purification of plasmid DNA
We used pcDNA3.1(+) (Invitrogen), which is a mammalian expression vector under the control of the cytomegalovirus (CMV) promoter and uses ampicillin for antibiotic selection in E. coli. We made the construct by Gibson assembly to insert the expression construct (Figure 1A) within the multiple cloning site (MCS) of the vector. The construct begins with a Kozak consensus sequence to initiate translation in eukaryotic cells, followed by the maltose-binding protein (MBP) tag, a linker containing the human rhinovirus (HRV) 3C protease (e.g. PreScission protease) cleavage site, the mxb gene, a hexa-histidine tag and stop codon. We designed tags on both ends to allow for multi-step affinity purification, if necessary, and for monitoring the expression of a full, intact protein using antibodies against both tags. (Note 4.1)
Figure 1 |.

A, Expression construct containing the gene for full-length (1–715) wild type human MxB, N-terminal maltose binding protein (MBP) fusion tag and C-terminal hexa-histidine tag. The schematic of the domain structure of MxB is shown: N-terminal region (NTR), BSE domain (B), GTPase domain and Stalk domain. B, Time-course, small scale expression of MBP-MxB-H6 (black arrow) starting from day of transfection (Day 1). A western blot using antibody against MBP is used to detect total expression of MBP-MxB-H6, which is increasing in amount during the course of expression. C, Solubility of expressed protein is assayed by lysis of a small aliquot of cells followed by centrifugation to separate supernatant (S) and pellet (P), representing the soluble and insoluble fractions, respectively. The amount of MBP-MxB-H6 in the pellet increases during the course of expression.
The quality of plasmid DNA is critical to the success of transfection. We maintain the plasmid in E. coli DH5ɑ cells and grow up enough culture for a maxiprep purification (the volume will depend on the kit or procedure used). We tested different DNA purification kits and various protocols based on Cesium Chloride (CsCl2) density-gradient purification. We found that using the NucleoBond Maxiprep kit (Takara) was the most efficient method that yielded high-quality plasmid DNA, wherein purification is finished in a few hours and transfection can be performed on the same day. (Note 4.2)
3.2. Maintenance of cells
Frozen Expi293F cells are thawed at 37℃ in a water bath and added to pre-warmed expression media in a disposable sterile vent-cap erlenmeyer flask with baffled bottom to achieve a concentration of 3–5 × 106 cells/ml (see more details from manufacturer’s manual). The minimum volume should be 20% of the volume of the baffled flask to minimize foaming and maximum of 40% of the flask volume to allow for proper aeration of the cell suspension. The cells are monitored for growth and viability, ensuring that cells are not aggregated by visualization under the light microscope and at least 90–95% of cells are alive using trypan blue as indicator of dead cells. After a few passages when cells are consistently at >95% viability, the cells can be used for transfection.
Because the cells are not maintained in the presence of antibiotics or antifungal reagents, aseptic technique must be practiced at every step to prevent contamination of the cells. Sterile single use polycarbonate flasks also help in preventing contamination.
If an incubator with shaker, carbon dioxide and humidity controls is not available, one can fit an orbital shaker, preferably with digital control for consistent agitation, in a standard cell culture incubator. Place a tray of distilled water at the bottom level to keep the incubator humidified. (Note 4.3)
3.3. Transfection
We essentially followed the manufacturer’s instructions for using the transfection kit. Ensure that the cells will be at ~5 × 106 cells/ml on the day of transfection by splitting the cells in new media the day before (Note: doubling time is 24 hours). On the day of transfection, pre-warm all the media at 37℃. Decontaminate all containers/equipment and perform all the steps inside a Class II laminar flow hood. Observe aseptic technique. For a small-scale culture:
Add 30 μg of plasmid DNA to 1.5 ml in Opti-MEM I Reduced Serum. Incubate for 5 minutes.
Add 80 μl of ExpiFectamine reagent 1.5 ml in Opti-MEM I Reduced Serum. Incubate for 5 minutes.
Mix tubes containing DNA and ExpiFectamine reagent and incubate for 25 minutes.
Add the DNA/ExpiFectamine mixture to 25.5 ml of 2.9 × 106 cells/ml of Expi293F cells in a 125-ml erlenmeyer flask. Final volume will be ~28.5 ml of ~2.5 × 106 cells/ml (25.5 ml cells + 3 ml of DNA/ExpiFectamine mixture).
Incubate at 37°C and 125-rpm agitation with 8% CO2 in air and in humidified environment.
After 18 hrs of incubation, add 150 μl transfection enhancer 1 and 1.5 ml of enhancer 2 into the suspension.
Scale up the culture size following the DNA/transfection reagent/culture size above. Perform an expression and solubility test for new constructs to determine the optimum duration of transient expression. For MxB, we found that most of the expressed protein is soluble 24 hrs after addition of transfection enhancers (Figure 1B&C).
3.4. Purification
Twenty-four hours after the addition of the enhancers, harvest cells by centrifugation at low-speed (100 × g). Resuspend the cell pellet gently with cold phosphate-buffered saline to wash the cells. Spin the cells again at low speed, flash-freeze the cell pellet and store the pellet at −80°C for later use.
3.4.1. Lysis and collection of soluble fraction
-
1.
Prepare the purification buffer (HNG): 50 mM Hepes-KOH (pH 8), 250 mM NaCl, and 5% glycerol. (Note 4.4)
-
2.
Thaw and resuspend the frozen cell pellet in HNG buffer supplemented with the following: 1% Tween, 0.3 % NP-40, 5mM MgCl2, protease inhibitors (1 tablet Roche per 50ml), 50 μg/ml DNAse, and 2 mM DTT. (Note 4.5)
-
3.
Incubate the cells in lysis buffer with rotation at 4°C for 1 hour. Then, homogenize the lysate by 15 strokes in an ice-cold, tight-fitting Dounce homogenizer (Pestle type B).
-
5.
Centrifuge the homogenate at 21,000g at 4°C for 30 min using a refrigerated table top centrifuge.
3.4.2. Affinity chromatography
After centrifugation, collect the supernatant and mix with amylose agarose resin (1 ml per 50 ml of cell suspension) pre-equilibrated with buffer HNG + 2 mM DTT.
Transfer the supernatant/resin mixture into a disposable column. Let flow through.
Wash unbound protein from the resin with at least 50x the volume of the resin. For some MxB mutants, a high salt (750mM NaCl) wash is added as a step before completing the wash in 250mM NaCl.
To elute the protein, plug the end of the column to stop flow and add 3x resin volume buffer A + 100 mM maltose. Gently mix the resin + elution buffer using a clean plastic inoculation loop. Keep the column at 4°C for 15 mins before collecting the elution. At this point, ~90% of the protein is already eluted off the resin but the elution step may be repeated to possibly elute more protein.
Combine and concentrate the elution volumes using concentrator MWCO50 up to 5mg/ml, if necessary. Protein concentration is determined by measuring absorbance at 280 nm and using the calculated molecular weight and extinction coefficients, 129421.8 g/mol and 116660 M−1cm−1, respectively.
3.4.3. Size-exclusion chromatography
Single step affinity purification yields ~1 mg total protein per liter of culture media of fairly pure MxB. To further polish the purity, mainly to remove the excess MBP tag, we injected the protein into a Sephacryl S500-HR (Sigma-Aldrich), which allows separation of a wide range of molecular weight proteins (40–20,000 kDa) (Note 4.6). MxB eluted into a single peak but examination of the fractions under the peak showed that MxB exists in various oligomeric conformations (Figure 2), which indicated its propensity to form helical tubes akin to the behavior of members within the dynamin superfamily (Ramachandran and Schmid 2018).
Figure 2 |. Full-length, wild-type MxB purified as oligomers.

A, Purification of MBP-MxB-H6 from Expi293 cells by amylose affinity chromatography, followed by gel filtration through Sephacryl S500-HR column. The fractions indicated by arrowheads were visualized by negative-stain EM. Inset, Coomassie-stained SDS-PAGE gel of untransfected cells (1), transfected cells (2) and elution from amylose resin (3). Molecular weight (MW) markers are shown in kDa. B–E, Representative micrographs by negative-stain EM of the indicated fractions in (A). F, 2D class averages of the negative-stained MxB sample from the fraction “C”. Scale bars, 200 nm. Reproduced from Alvarez et al, 2017 with permission from Sci. Adv. (Alvarez, He et al. 2017).
3.5. Characterization
3.5.1. GTPase activity
The ability of MxB to hydrolyze GTP was assessed using a continuous NADH (reduced form of nicotinamide adenine dinucleotide)–coupled assay (Note 4.7) in a microcuvette format.
Set the spectrophotometer to read continuously for 30 minutes at 37°C at 340nm. Blank the machine with a quartz microcuvette containing deionized distilled water.
Prepare a 100 μl-reaction mixture by mixing the reagents in a microcentrifuge tube to achieve the following final concentrations in the assay solution: 50 mM Hepes-KOH (pH 7), 150 mM NaCl, 10 mM MgCl2, 2 mM dithiothreitol (DTT), 4 mM phosphenolpyruvate (PEP), 0.35 mM NADH, 25 Units of pyruvate kinase/lactate dehydrogenase, and 1mM GTP. Warm the mixture at 37°C in a heat block for a few minutes. (Note 4.8)
Add 1.5 μM MxB into the reaction mixture and quickly place 90 μl of the mixture into the microcuvette for measurement. For negative control, record measurements using the purification buffer only, in place of the protein. (Note 4.9)
Record the decrease in the NADH absorbance at 340 nm over time. Subtract the reading from the negative control to correct for background. The rate of NADH oxidation is taken as the slope of the linear portion of the resulting plot and is directly proportional to the amount of GTP hydrolyzed. (Figure 3A)
Figure 3 |. MxB GTPase activity and tubular assembly.
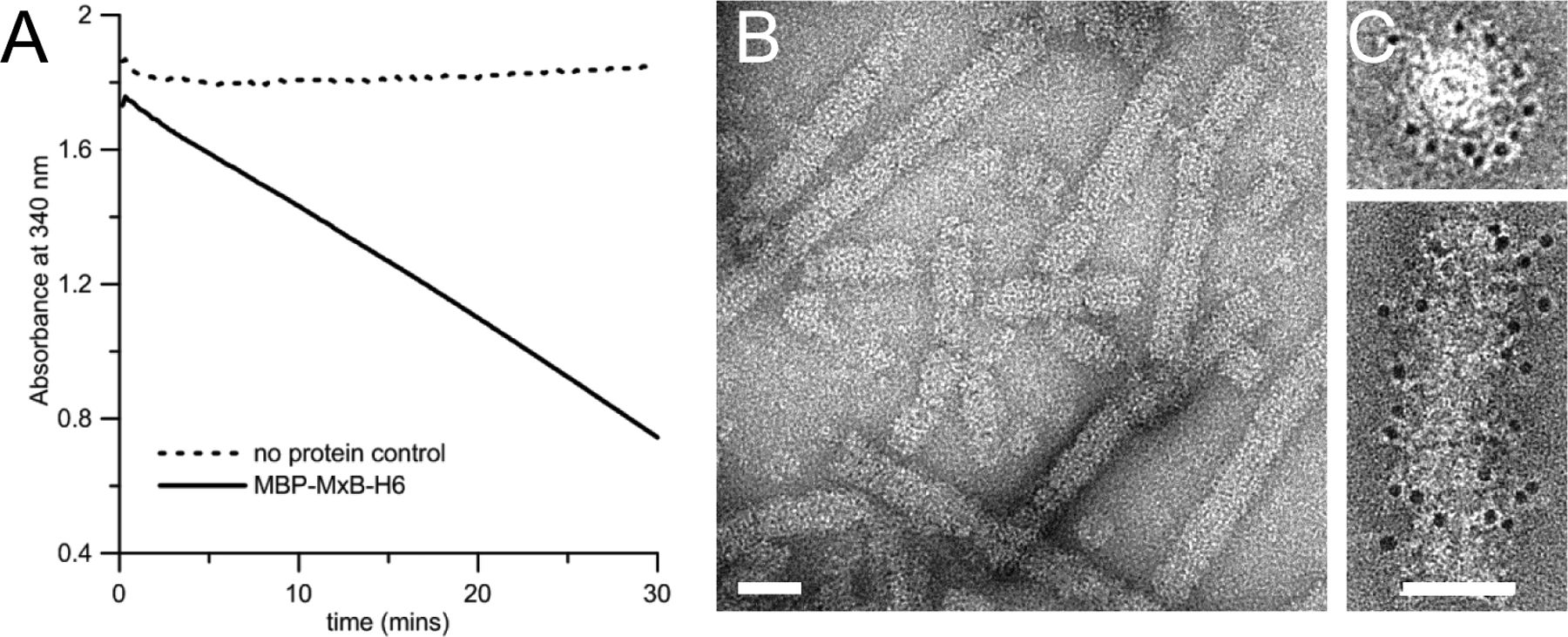
A, The decrease in the NADH absorbance at 340 nm over time. B, MBP-MxB self-assembled into tubular structures at 150 mM NaCl. C, Gold-labeling of MBP shows that MBP is located on the surface of assembled MBP-MxB tubes. End on view (top) and side view (bottom) are shown. Scale bars, 50 nm in B&C. Reproduced from Alvarez et al, 2017 with permission from Sci. Adv. (Alvarez, He et al. 2017).
3.5.2. Tubular assembly
The assembly of MxB into tubes is initiated by diluting the salt in the sample buffer to 150 mM NaCl or less, for at least an hour at room temperature. MxB tubes grow longer with extended incubation (overnight to few days). (Figure 3B)
To determine the position of the NTR of MxB in its helical form, we performed on-grid immuno-gold labeling of MBP visualized by negative stain electron microscopy (EM) as indirect localization of the NTR. We observed decoration of gold particles on the surface of the tubes indicating that the NTR of MxB is oriented on the surface of the tubes. (Figure 3C)
Prepare MxB tubes as described above. Apply 3.5 μl of the sample on a 400 mesh carbon-coated EM grid. Incubate for 1 minute and blot the rest of the liquid using a Whatman #1 filter paper.
- Float the grid on the following solutions at 4°C. (Note 4.10)
- blocking buffer [bovine serum albumin (BSA;10 mg/ml) in oligomerization buffer] for 5 min, twice
- blocking buffer containing primary antibody against MBP tag (Abcam) (1:250 dilution) for 1 hour
- blocking buffer for 5 min, twice
- blocking buffer containing a 5-mm gold-labeled secondary antibody (Ted Pella) (1:250 dilution) for 1 hour.
- blocking buffer for 5 min
- oligomerization buffer for 1 min, twice
- 2 % uranyl formate for 1 min (Note 4.11)
Blot the grid with filter paper and let the grid completely dry before imaging on the electron microscope.
3.5.4. Cleavage of MBP-tag
The MBP tag is cleaved from MxB by adding purified HRV-3C (also known as PreScission protease) in 1:10 (protease to MxB molar ratio) to MxB in the purification buffer at 4°C for 1 hour. Monitor the completeness of the reaction by running an SDS-PAGE, followed by western blots using antibodies against MxB or MBP (Figure 4A). We found that cleavage of MBP from MxB induced tube formation of MxB even at 250 mM NaCl, but those tubes are bundled together (Figure 4B&C).
Figure 4 |. MxB assembles into helical tubes with and without MBP-tag.

A, Coomassie-stained SDS-PAGE gel and the corresponding western blots, with the indicated primary antibody or probe, of MBP-MxB-H6 without or with HRV-3C at 150mM NaCl. Protein bands are indicated by arrows on the right. Dashed arrow points to a minor species of MxB with both MBP and the linker between MBP and MxB removed. B&C, Negative stain EM images of samples in (A) without the protease (B) and with the protease (C). Removal of MBP-tag resulted in ordered MxB tubes, but largely aggregated. Scale bar, 50 nm. Reproduced from Alvarez et al, 2017 with permission from Sci. Adv. (Alvarez, He et al. 2017).
4. Notes
-
4.1
We made a similar construct using glutathione S-transferase (GST) tag in place of MBP but the protein did not express well, which highlights the importance of testing different tags and constructs for optimum expression.
-
4.2
Ensure that plasmid DNA is fully dried and free of ethanol prior to resuspension in sterile double distilled water.
-
4.3
We attempted using a glass spinner culture bottle with a magnetic stir plate to maintain the cells, but in our hands, the cell viability was consistently low. Reagents used for cleaning the glass or the heat generated by the spinning of the magnetic stirrer may account for low cell viability.
-
4.4
We performed small scale purification using higher concentrations of NaCl in the purification buffer, but the amount of protein bound to resin also decreased.
-
4.5
Avoid concentrating the cells more than five times (i.e. for 30 ml cells worth of cell pellet, re-suspend with no less than 6 ml of lysis buffer) to prevent aggregation or higher-order oligomerization.
-
4.6
Affinity-purified MxB elutes in the void volume of a Superdex 200 (GE Healthcare) column.
-
4.7
The recipe used in this study is modified for cuvette measurements based on a plate assay by Ingerman E, et al 2005 (Ingerman and Nunnari 2005).
-
4.8
A 5x concentrated stock of the reaction mixture can be made ahead of time, frozen in −20°C and kept away from light (NADH is light-sensitive). The source of potassium needed in the assay is in the form of KOH used to adjust the pH of HEPES. If HEPES solution is prepared another way, supplement the reaction mixture with KCl to a final concentration of 7.5 mM.
-
4.9
The volume of the MxB added into the reaction mixture should be kept small enough to minimize the effect of NaCl carried from the purification buffer to assay conditions.
-
4.10
For negative control, prepare another grid without applying MxB tubes.
-
4.11
Uranyl formate is classified as a radioactive material and appropriate handling must be observed.
5. References
- Alvarez FJD, He S, Perilla JR, Jang S, Schulten K, Engelman AN, Scheres SHW and Zhang P (2017). “CryoEM structure of MxB reveals a novel oligomerization interface critical for HIV restriction.” Sci Adv 3(9): e1701264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourgh JL, Nguyen HC, Matreyek KA, Alvarez FJD, Summers BJ, Dewdney TG, Aiken C, Zhang P, Engelman A and Xiong Y (2014). “Structural insight into HIV-1 restriction by MxB.” Cell Host Microbe 16(5): 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, Hue S, Barclay WS, Schulz R and Malim MH (2013). “Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection.” Nature 502(7472): 559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Staeheli P, Schwemmle M and Kochs G (2015). “Mx GTPases: dynamin-like antiviral machines of innate immunity.” Trends Microbiol 23(3): 154–163. [DOI] [PubMed] [Google Scholar]
- Ingerman E and Nunnari J (2005). “A continuous, regenerative coupled GTPase assay for dynamin-related proteins.” Methods Enzymol 404: 611–619. [DOI] [PubMed] [Google Scholar]
- Jaguva Vasudevan AA, Bahr A, Grothmann R, Singer A, Haussinger D, Zimmermann A and Munk C (2018). “MXB inhibits murine cytomegalovirus.” Virology 522: 158–167. [DOI] [PubMed] [Google Scholar]
- Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, Schoggins JW, Rice CM, Yamashita M, Hatziioannou T and Bieniasz PD (2013). “MX2 is an interferon-induced inhibitor of HIV-1 infection.” Nature 502(7472): 563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Pan Q, Ding S, Qian J, Xu F, Zhou J, Cen S, Guo F and Liang C (2013). “The interferon-inducible MxB protein inhibits HIV-1 infection.” Cell Host Microbe 14(4): 398–410. [DOI] [PubMed] [Google Scholar]
- Meier K, Jaguva Vasudevan AA, Zhang Z, Bahr A, Kochs G, Haussinger D and Munk C (2018). “Equine MX2 is a restriction factor of equine infectious anemia virus (EIAV).” Virology 523: 52–63. [DOI] [PubMed] [Google Scholar]
- Ramachandran R and Schmid SL (2018). “The dynamin superfamily.” Curr Biol 28(8): R411–R416. [DOI] [PubMed] [Google Scholar]
- Schilling M, Bulli L, Weigang S, Graf L, Naumann S, Patzina C, Wagner V, Bauersfeld L, Goujon C, Hengel H, Halenius A, Ruzsics Z, Schaller T and Kochs G (2018). “Human MxB Protein Is a Pan-herpesvirus Restriction Factor.” J Virol 92(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bai J, Fan B, Li Y, Zhang Q and Jiang P (2016). “The Interferon-Induced Mx2 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication.” J Interferon Cytokine Res 36(2): 129–139. [DOI] [PubMed] [Google Scholar]
- Xu B, Kong J, Wang X, Wei W, Xie W and Yu XF (2015). “Structural insight into the assembly of human anti-HIV dynamin-like protein MxB/Mx2.” Biochemical and Biophysical Research Communications 456(1): 197–201. [DOI] [PubMed] [Google Scholar]


