Abstract
Background
Anemia and chronic kidney disease (CKD) are common in patients with heart failure with preserved left ventricular fraction (HFpEF). However, it is entirely unknown about the impact of anemia on prognosis in HFpEF patients with CKD. In this study, we investigated the impact of anemia on prognosis and the optimal hemoglobin (Hb) levels to predict prognosis in HFpEF patients with CKD.
Methods and Results
We prospectively examined 523 consecutive HFpEF patients enrolled in Japanese heart failure syndrome with preserved ejection fraction registry. CKD was defined as an estimated glomerular filtration rate (eGFR) of <60 mL /min/1.73 m2. The prevalence rate of anemia was 78% in HFpEF patients with CKD by using the World Health Organization criteria. Kaplan-Meier analysis for all-cause mortality and heart failure rehospitalization demonstrated that anemic patients had poor prognosis compared with non-anemic patients in HFpEF patients with CKD, but not those without CKD. According to the degree of CKD, anemia affected prognosis in HFpEF patients with mild CKD (45 ≤ eGFR < 60), but not those with moderate to severe CKD (15 ≤ eGFR < 45). Additionally, multivariate analysis revealed that anemia and Hb levels were independent predictors of composite outcomes in HFpEF patients with mild CKD, but not those with moderate to severe CKD. Finally, survival classification and regression tree analysis showed that the optimal Hb levels to predict composite outcomes were 10.7 g/dL in those with mild CKD.
Conclusions
Anemia has an impact on prognosis in HFpEF patients, especially among those with mild CKD.
Keywords: Anemia, Chronic kidney disease, Hemoglobin, Heart failure with preserved left ventricular ejection fraction
1. Introduction
Anemia is common and is associated with worse outcomes in patients with heart failure (HF) [1], [2]. In addition, chronic kidney disease (CKD) is a frequent comorbidity and is associated with poor prognosis in patients with HF [3]. The interaction among HF, CKD, and anemia is called as cardio-renal anemia syndrome [4].
Compared with HF with reduced left ventricular ejection fraction (HFrEF), the prevalence of anemia is reported to be higher in HF patients with preserved left ventricular ejection fraction (HFpEF) [5], [6], while the prevalence of CKD is not significantly different between HFpEF and HFrEF patients [7]. In fact, a previous report has shown that hemoglobin (Hb) levels are lower in HFpEF patients than in HFrEF patients, whereas eGFR is not significantly different between HFpEF and HFrEF patients [8]. Thus, anemia has emerged as an important factor in HFpEF patients with CKD.
Regarding with the prognosis, both anemia and CKD are associated with poor prognosis in HFpEF patients [9], [10], [11]. However, it has been entirely unknown about the impact of anemia on prognosis in HFpEF patients, by the degree of CKD. In addition, the optimal Hb levels to predict prognosis in HFpEF patients with CKD remain largely unknown. Because renal insufficiency causes anemia, the value of anemia as a contributing factor of prognosis in HFpEF may be overlap by the value of CKD. Therefore, in this study, we investigated the impact of anemia on prognosis and the optimal Hb levels to predict prognosis in HFpEF patients with CKD enrolled in Japanese heart failure syndrome with preserved ejection fraction (JASPER) registry [12].
2. Methods
2.1. Study protocol
We studied 535 individuals with HFpEF enrolled in JASPER registry. The JASPER registry is a multicenter, observational, prospective cohort that includes patients aged 20 and above requiring hospitalization with a diagnosis of acute HF according to the Framingham criteria [13] by at least two experienced cardiologists, between November 2012 and March 2015 at 15 sites in Japan. Patients in the registry had preserved left ventricular systolic function, defined as left ventricular ejection fraction ≥ 50% with the modified Simpson method, or left ventricular fractional shortening ≥ 25% on echocardiography. Patients with acute coronary syndrome, receiving hemodialysis, or with a history of heart transplantation were excluded. In addition, we excluded 7 participants who died during the first hospitalization and 5 participants whose clinical data until discharge was unknown, which left a final analytical cohort of 523 participants. The patients’ demographic data including co-morbid conditions, clinical signs, laboratory and echocardiographic data, in-hospital treatment including oral and intravenous medication, and length of hospital stay were obtained. Anemia was defined according to the World Health Organization (WHO) criteria (Hb levels < 13 g/dL in male, Hb levels < 12 g/dL in female) [14].
Serum creatinine levels (Cr) were measured in all patients. Estimated glomerular filtration rate (eGFR) was calculated using the modified modification of diet in renal disease (MDRD) formula in the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × Cr −1.094 × Age −0.287 (Male), 0.739 × 194 × Cr −1.094 × Age −0.287 (Female) [15]. CKD was defined as an eGFR of < 60 mL/min/1.73 m2, which is defined CKD stage 3 as suggested by the National Kidney Foundation classification. Then, HFpEF patients were divided into 2 groups: patients with CKD, or those without CKD defined as an eGFR ≥ 60 mL/min/1.73 m2. For the purposes of this study, we compared the impacts of anemia on prognosis between HFpEF patients with and without CKD.
Follow-up was performed at discharge, 12 months after discharge, and 24 months after discharge by direct contact with patients or their physicians at the hospital or outpatient clinic, telephone interview of patients or, if deceased, of family members, and mail, by dedicated coordinators and investigators. In this study, because patient information was anonymized and de-identified prior to analyses, written informed consent was not obtained from each patient. However, we publicized the study by posting a summary of the protocol (with an easily understood description) on the website of the National Cerebral and Cardiovascular Center; the notice clearly informed patients of their right to refuse enrollment. These procedures for informed consent and enrolment were in accordance with the detailed regulations regarding informed consent described in the guidelines, and this study, including the procedure for enrolment, has been approved by the Institutional Review Board of each site, and registered under the Japanese UMIN Clinical Trials Registration (UMIN000010601).
2.2. Statistical analysis
Continuous variables were presented as mean ± standard deviation when normally distributed, and as median and interquartile range (IQR) when non-normally distributed. Continuous variables were compared using an unpaired t test. Categorical variables were made by chi-squared test or Fisher’s exact test for dichotomous variables, when appropriate. The correlations of Hb levels with eGFR were assessed by Pearson correlation analysis. The cumulative incidence of the composite of all-cause mortality and HF rehospitalization was estimated using Kaplan-Meier analysis. The associations of parameters with all-cause mortality and HF rehospitalization were assessed by Cox proportional-hazards regression analysis. Multivariate analysis was performed using covariates which were related to all-cause mortality and HF rehospitalization. To investigate the impacts of cardio-renal anemia syndrome on prognosis, covariates which were closely related to cardio-renal anemia syndrome (age, male, body mass index, atrial fibrillation, anemia, systolic blood pressure, serum albumin, Hb levels, beta-blockers use, and loop diuretics use) were chosen in this study. In additon, to evaluate the optimal Hb levels, we used a survival classification and regression tree (CART) analysis. All tests were two tailed, and a value of p < 0.05 was considered statistically significant. All analyses were performed with R version 3.5.2.
3. Results
3.1. Patients characteristics
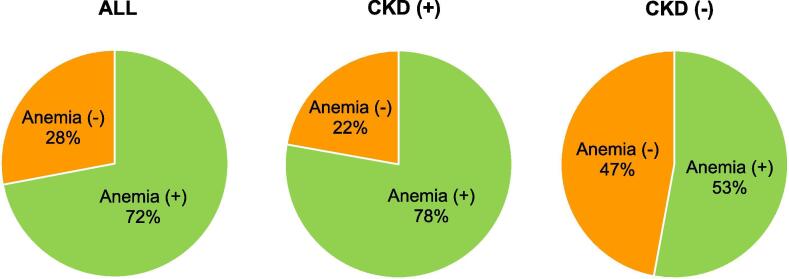
About 70% of HFpEF patients were shown anemia in this study. The proportion was higher in HFpEF patients with than those without CKD (78% and 53%, respectively) (Fig. 1).
Fig. 1.
Prevalence rate of anemia in HFpEF patients with CKD. Prevalence rate of anemia in (A) all HFpEF patients (n = 523), (B) HFpEF patients with CKD (n = 402), and (C) HFpEF patients without CKD (n = 121).
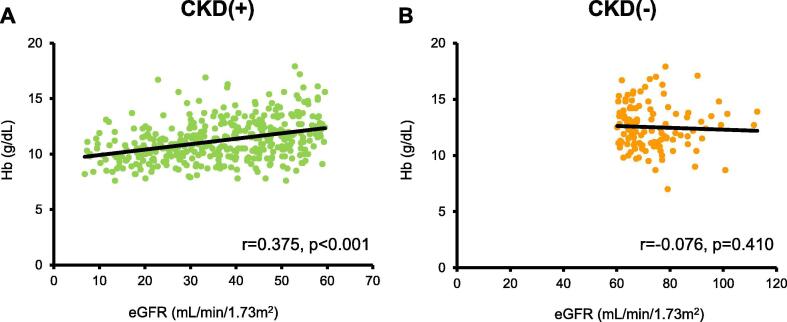
The patient characteristics are described in Table 1. 77% of HFpEF patients had CKD (n = 402/523). HFpEF patients with CKD were older (80 versus 73 years, p < 0.001), lower systolic blood pressure (116 versus 112 mmHg, p = 0.037), and lower heart rate (82 versus 92 beats/min, p < 0.001) than those without CKD. History of past hospitalization for HF was more frequently in HFpEF patients with than those without CKD (44% versus 18%, p < 0.001). The prevalence of hypertension, diabetes mellitus, and cerebrovascular disease were higher in HFpEF patients with than those without CKD. There were no differences in gender, body mass index, and diastolic blood pressure between the two groups. Serum albumin and Hb levels were lower in HFpEF patients with than those without CKD. HFpEF patients with CKD were more frequently treated with calcium blockers and loop diuretics compared with those without CKD. As shown in Fig. 2, Hb levels were positively correlated with eGFR in HFpEF patients with CKD, but not those without CKD.
Table 1.
Patient characteristics.
| Characteristics | CKD (+) (n = 402) | CKD (−) (n = 121) | P value |
|---|---|---|---|
| Age (years) | 80 ± 9 | 73 ± 14 | <0.001 |
| Male, n (%) | 198 (49) | 62 (51) | 0.756 |
| BMI (kg/m2) | 22 ± 4.1 | 23 ± 5.0 | 0.312 |
| SBP (mmHg) | 116 ± 17 | 112 ± 14 | 0.037 |
| DBP (mmHg) | 61 ± 11 | 63 ± 11 | 0.067 |
| HR (bpm) | 82 ± 27 | 92 ± 30 | <0.001 |
| Past HF, n (%) | 170 (44) | 21 (18) | <0.001 |
| AF, n (%) | 256 (64) | 66 (56) | 0.080 |
| OMI, n (%) | 55 (14) | 10 (8.5) | 0.155 |
| HT, n (%) | 325 (81) | 81 (67) | 0.001 |
| DLp, n (%) | 175 (44) | 47 (39) | 0.328 |
| DM, n (%) | 168 (42) | 34 (28) | 0.006 |
| CVD, n (%) | 108 (28) | 13 (11) | <0.001 |
| Alb (g/dL) | 3.6 ± 0.5 | 3.7 ± 0.5 | 0.032 |
| Hb (g/dL) | 11.2 ± 1.8 | 12.5 ± 2.0 | <0.001 |
| eGFR (mL/min/1.73 m2) | 37 ± 14 | 73 ± 11 | <0.001 |
| Median BNP (pg/mL) (IQR) | 171 (86–327) | 102 (53–187) | 0.017 |
| β blockers, n (%) | 263 (65) | 75 (62) | 0.488 |
| ACE-Is, n (%) | 129 (32) | 32 (26) | 0.238 |
| ARBs, n (%) | 178 (44) | 44 (36) | 0.123 |
| Ca blockers, n (%) | 225 (56) | 51 (42) | 0.008 |
| Loop diuretics, n (%) | 314 (78) | 73 (60) | <0.001 |
| Warfarin, n (%) | 196 (49) | 53 (44) | 0.339 |
| DOACs, n (%) | 53 (13) | 16 (13) | 0.999 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; Past HF, past hospitalization for heart failure; AF, atrial fibrillation; OMI, old myocardial infarction; HT, hypertension; DLp, dyslipidemia; DM, diabetes mellitus; CVD, cerebrovascular disease; Alb, albumin; Hb, hemoglobin; eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide; IQR, interquartile range; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; DOAC, direct oral anticoagulant.
Fig. 2.
Correlation between hemoglobin levels and estimated glomerular filtration rate in HFpEF patients with and without CKD. Correlation between hemoglobin levels and estimated glomerular filtration rate in HFpEF patients (A) with CKD (n = 402) and (B) without CKD (n = 121). Hb, hemoglobin levels; eGFR, estimated glomerular filtration rate.
3.2. Prognosis
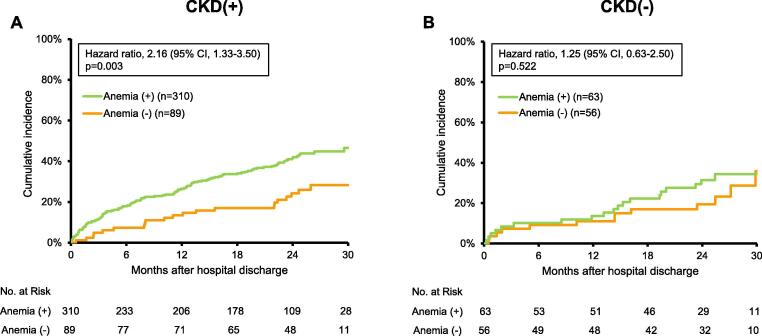
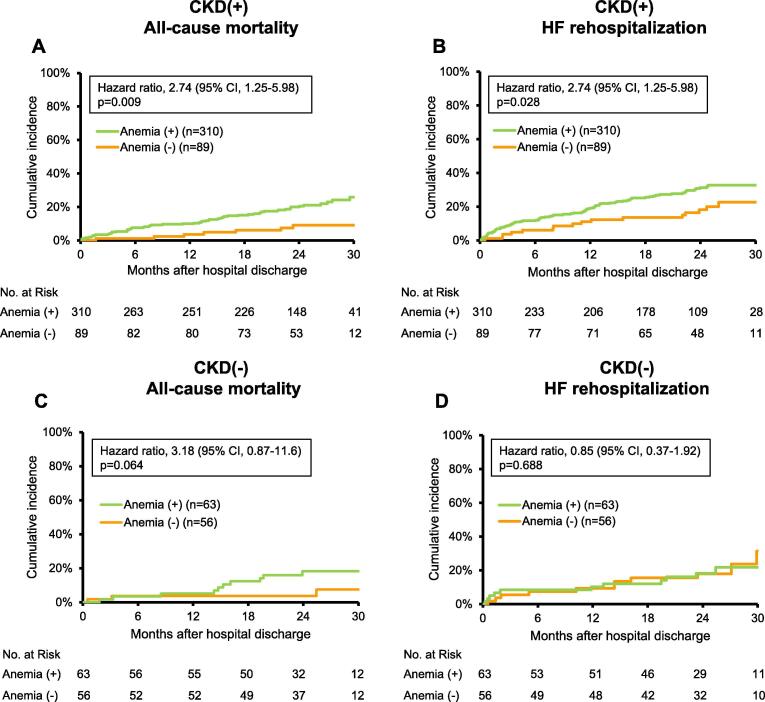
During a median follow-up of 23.3 months, composite outcomes (all-cause mortality and HF rehospitalization) occurred in 145 HFpEF patients with CKD and 33 those without CKD. We then assessed the relation between anemia and prognosis in HFpEF patients with and without CKD. Kaplan-Meier analyses for all-cause mortality and HF rehospitalization showed that anemic patients had significantly poorer prognosis compared with non-anemic patients among HFpEF patients with CKD (47% versus 28%, p = 0.003; Fig. 3A). On the other hand, there was no significant different prognosis between anemic and non-anemic patients among HFpEF patients without CKD (34% versus 36%, p = 0.522; Fig. 3B). Among HFpEF patients with CKD, both all-cause mortality and HF rehospitalization rates were higher in anemic patients compared with non-anemic patients (26% versus 9%, p = 0.009 and 33% versus 23%, p = 0.028, respectively; Fig. 4A, B). However, no statistically significant different prognosis was observed in anemic or non-anemic patients among HFpEF patients without CKD (18% versus 8%, p = 0.064 for all-cause mortality; Fig. 4C, 31% versus 22%, p = 0.688 for HF rehospitalization; Fig. 4D).
Fig. 3.
Survival curves for all-cause mortality and heart failure rehospitalization by anemia status in HFpEF patients with and without CKD. Kaplan-Meier analysis for all-cause mortality and heart failure rehospitalization in anemic and non-anemic HFpEF patients (A) with and (B) without CKD. CI, confidence interval.
Fig. 4.
Survival curves for all-cause mortality or heart failure rehospitalization by anemia status in HFpEF patients with and without CKD. Kaplan-Meier analysis for (A) all-cause mortality and (B) heart failure (HF) rehospitalization in HFpEF patients with CKD. Kaplan-Meier analysis for (C) all-cause mortality and (D) HF rehospitalization in HFpEF patients without CKD. CI, confidence interval.
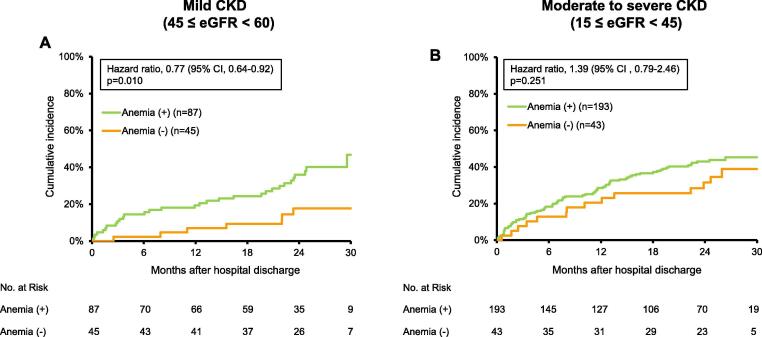
Then, we investigated the prognostic impact of anemia on all-cause mortality and HF rehospitalization in HFpEF patients with CKD, by the degree of CKD. Anemic patients increased the cumulative proportion of composite outcomes compared with non-anemic patients among HFpEF patients with mild CKD (45 ≤ eGFR < 60) (47% vs. 18%, p = 0.01; Fig. 5A), while anemia did not affect the cumulative proportion of composite outcomes in those with moderate to severe CKD (15 ≤ eGFR < 45) (Fig. 5B). To further investigate the prognostic impact of anemia on these composite outcomes after discharge, we performed univariate and multivariate Cox regression analyses for all-cause mortality and HF rehospitalization in these patients. Multivariate analysis showed that anemia and Hb levels at discharge were independent predictors of all-cause mortality and HF rehospitalization in HFpEF patients with CKD (Table 2). According to the degree of CKD, anemia and Hb levels at discharge were found to be independent predictors of all-cause mortality and HF rehospitalization in HFpEF patients with mild CKD, but not in those with moderate to severe CKD (Table 2).
Fig. 5.
Survival curves for all-cause mortality and heart failure rehospitalization by anemia status in HFpEF patients with mild and moderate to severe CKD. Kaplan-Meier analysis for all-cause mortality and heart failure rehospitalization in HFpEF patients with (A) mild CKD (45 ≤ eGFR < 60) and (B) moderate to severe CKD (15 ≤ eGFR < 45). CI, confidence interval.
Table 2.
Univariate and multivariate Cox regression analysis for all-cause mortality and heart failure rehospitalization.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| HFpEF patients with CKD (eGFR < 60) | ||||
| Anemia | 2.02 (1.27–3.20) | 0.003 | 1.92 (1.06–3.46) | 0.031 |
| Hb (1 g/dL increase) | 0.81 (0.74–0.90) | <0.001 | 0.83 (0.73–0.94) | 0.004 |
| HFpEF patients with mild CKD (45 ≤ eGFR < 60) | ||||
| Anemia | 2.79 (1.23–6.34) | 0.014 | 3.05 (1.02–9.15) | 0.046 |
| Hb (1 g/dL increase) | 0.77 (0.64–0.92) | 0.004 | 0.74 (0.59–0.94) | 0.012 |
| HFpEF patients with moderate to severe CKD (15 ≤ eGFR < 45) | ||||
| Anemia | 1.39 (0.79–2.46) | 0.253 | 1.28 (0.62–2.61) | 0.503 |
| Hb (1 g/dL increase) | 0.87 (0.76–0.99) | 0.043 | 0.89 (0.75–1.05) | 0.168 |
HR, hazard ratio; CI, confidence interval.
Therefore, we finally assessed the optimal Hb levels to predict composite outcomes in HFpEF patients with mild CKD. Survival CART analysis revealed that the optimal Hb levels to predict composite outcomes were 10.7 g/dL in HFpEF patients with mild CKD.
4. Discussion
This study demonstrated that both all-cause mortality and HF rehospitalization rates were higher in anemic patients compared with non-anemic patients among HFpEF patients with CKD. According to the degree of CKD, anemia has an impact on prognosis in HFpEF patients, especially among those with mild CKD. The optimal Hb levels to predict prognosis were 10.7 g/dL in those with mild CKD.
Anemia and CKD are common in HFpEF patients [9], [16]. In the present study, the prevalence rate of anemia was 72% in all HFpEF patients by the WHO criteria. On the other hand, the prevalence rate of CKD was 77% in this study. HFpEF is a heterogeneous syndrome, and various factors are associated with the pathological condition of HFpEF. Although anemia and CKD are associated with the pathophysiology of HFpEF, it is completely unknown whether anemia and CKD are causally related to prognosis in HFpEF patients. In this study, we assessed the impact of anemia on prognosis in HFpEF patients with and without CKD. Of note, anemic patients had worse prognosis on both all-cause mortality and HF rehospitalization than non-anemic patients among HFpEF patients with CKD, while there was no statistically significant different prognosis between anemic and non-anemic patients among HFpEF patients without CKD. These results indicate that anemia is mainly associated with worse prognosis in HFpEF patients with CKD.
In the present study, HFpEF patients with CKD were older and had higher prevalence of past hospitalization for HF, hypertension, diabetes mellitus, and cerebrovascular disease than those without CKD. In addition, HFpEF patients with CKD were more frequently treated with loop diuretics compared with those without CKD. These characteristics indicate that HFpEF patients with CKD are in more advanced stage in HF compared with those without CKD. Therefore, the differences of prognosis impact by anemic status may occur between HFpEF patients with and without CKD. Among HFpEF patients without CKD, all-cause mortality was tended to be higher in anemic patients compared with non-anemic patients, but the difference was not statistically significant (p = 0.064). More continuous models analyzing the impact of Hb across the spectrum of eGFR would provide additional insight.
Several factors are associated with anemia in HF patients. Renal insufficiency is one of the important factors of anemia in HF patients, which is called as cardio-renal anemia syndrome [4]. In the present study, Hb levels were lower in HFpEF patients with CKD than in those without CKD, indicating an association between renal insufficiency and anemia in HF. In fact, eGFR was positively correlated with Hb levels in HFpEF patients with CKD, but not those without CKD.
Because the prognostic factors have yet to be known in HFpEF patients, we don't have effective strategy to improve their prognosis. Anemia is one of the possible factors which can be intervened. In this study, anemia did not impact the cumulative proportion of composite outcomes in HFpEF patients with moderate to severe CKD. Since renal insufficiency has been already established in patients with moderate and severe CKD, intervention of anemia may not affect prognosis in these patients. On the other hand, the cumulative proportion of composite outcomes was increased in anemic patients compared with non-anemic patients among HFpEF patients with mild CKD. In addition, anemia and Hb levels at discharge were independent predictors of composite outcomes in these patients. Therefore, anemia may be one of the factors which can be intervened in HFpEF patients with mild CKD.
By using survival CART analysis, the optimal Hb levels to predict the composite outcomes were found to be 10.7 g/dL in HFpEF patients with mild CKD. It has been completely unknown about the optimal Hb levels to predict prognosis in HFpEF patients with CKD. To the best of our knowledge, this study showed for the first time the optimal Hb levels to predict the composite outcomes in HFpEF patients with mild CKD. Because HFpEF patients have few proven therapeutic options, treatment of anemia may lead to improve prognosis in HFpEF patients, especially in those with mild CKD. Further studies are needed to assess this point.
Our study has limitations. This study was an observational cohort study and was not designed to assess the mechanism of anemia. Most unfortunately, we have no data on serum iron, ferritin levels, mean corpuscular volume, and history of occult gastrointestinal bleeding or cancer, etc. Thus, all cases of anemia are included and there is no apparent attempt to ascertain or describe the etiology of anemia other than assuming that this is due to cardio-renal anemia syndrome.
5. Conclusions
Anemia has an impact on prognosis in HFpEF patients, especially among those with mild CKD. The optimal Hb levels to predict prognosis were 10.7 g/dL in those with mild CKD.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
We deeply thank the contributions of all the investigators, clinical research coordinators, data managers, and laboratory technicians in the JASPER registry. This work was supported by a grant from the Japan Cardiovascular Research Foundation (T.A., 24-4-2).
References
- 1.Groenveld H.F., Januzzi J.L., Damman K. Anemia and mortality in heart failure patients: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Okuno K., Naito Y., Asakura M. Effective blood hemoglobin level to predict prognosis in heart failure with preserved left ventricular ejection fraction: Results of the Japanese heart failure syndrome with preserved ejection fraction registry. Heart Vessels. 2019;34:1168–1177. doi: 10.1007/s00380-019-01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Hillege H.L., Nitsch D., Pfeffer M.A. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg D., Wexler D., Blum M., Wollman Y., Iaina A. The cardio-renal anemia syndrome: does it exist? Nephrol. Dial. Transplant. 2003;18(Suppl 8) doi: 10.1093/ndt/gfg1084. viii:7–12. [DOI] [PubMed] [Google Scholar]
- 5.Lee D.S., Gona P., Vasan R.S. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaku H., Ozasa N., Morimoto T. Demographics, Management, and In-Hospital Outcome of Hospitalized Acute Heart Failure Syndrome Patients in Contemporary Real Clinical Practice in Japan - Observations From the Prospective, Multicenter Kyoto Congestive Heart Failure (KCHF) Registry. Circ. J. 2018;82:2811–2819. doi: 10.1253/circj.CJ-17-1386. [DOI] [PubMed] [Google Scholar]
- 7.Nayor M., Larson M.G., Wang N. The association of chronic kidney disease and microalbuminuria with heart failure with preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 2017;19:615–623. doi: 10.1002/ejhf.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tribouilloy C., Rusinaru D., Mahjoub H. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur. Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 9.Latado A.L., Passos L.C., Darzé E.S., Lopes A.A. Comparison of the effect of anemia on in-hospital mortality in patients with versus without preserved left ventricular ejection fraction. Am. J. Cardiol. 2006;98:1631–1634. doi: 10.1016/j.amjcard.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Damman K., Valente M.A., Voors A.A. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur. Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed A., Rich M.W., Sanders P.W. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am. J. Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai T., Yoshikawa T., Saito Y. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction: A report from the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) registry. Circ. J. 2018;82:1534–1545. doi: 10.1253/circj.CJ-18-0073. [DOI] [PubMed] [Google Scholar]
- 13.McKee P.A., Castelli W.P., McNamara P.M., Kannel W.B. The natural history of congestive heart failure: the Framingham study. N. Engl. J. Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Nutritional anaemias, Report of a WHO Scientific Group, WHO Techn. Rep. Ser. 405 (1968) 9–10. [PubMed]
- 15.Matsuo S., Imai E., Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Felker G.M., Shaw L.K., Stough W.G., O'Connor C.M. Anemia in patients with heart failure and preserved systolic function. Am. Heart J. 2006;151:457–462. doi: 10.1016/j.ahj.2005.03.056. [DOI] [PubMed] [Google Scholar]