Abstract
Background
Hyalinization is a process of conversion of stromal connective tissue into a homogeneous, acellular translucent material. Nevertheless, hyalinization could provide insights into the biologic behaviour and prognosis of pathological lesions. Few studies with limited sample size have intended to assess the correlation of hyalinization and biologic behaviour in oral lesions.
Aim
The current review aims to comprehensively appraise the mechanism of hyalinization in pathological oral hyalinizing lesions (OHL) and its clinical implications with emphasis on differential stains employed.
Methods
An electronic search was performed in the PubMed database (from year 2000–2020) using the keywords “special stains in oral hyalinizing lesions”, “significance of hyalinization in oral lesions” and “hyalinization and biologic behaviour”. Original research articles analyzing the effect of hyalinization on biologic characteristics of the lesion were evaluated in this review. Narrative review articles that provided insights into the mechanism of hyalinization and maturity of collagen fibers were also considered for analysis.
Conclusion
The presence of hyalinization does seem to have a significant effect on the biologic behaviour of pathological lesions. There is substantial scope to further investigate the process of hyalinization on larger samples and its correlation with the aggressive behaviour of OHLs. Special stains and advanced investigations such as immunohistochemistry for stromal markers would define the nature of hyalinized material and validate the correlation.
Clinical significance
The prediction of the biologic behaviour of a lesion established through assessment of hyalinization would prevent unwanted over or under treatment leading to a better prognosis.
Keywords: Alcian blue, Hyalinization, Odontogenic tumors, Oral potentially malignant disorders, Oral cancer, Periodic acid schiff, Picrosirius red, Safranin O, Salivary gland tumors
1. Introduction
Hyaline is a pale, glassy, structureless, acellular, and usually proteinaceous material that stains eosinophilic. The entomology of hyaline relates to a colourless, transparent substance and literally, the term means “glassy” derived from the Greek word “hyalinos.“1
The term hyaline change is applied to a tissue that has accumulated hyaline resulting is a glassy pink appearance, usually associated with the accumulation of a protein. Hyaline degeneration concerns the deposition of eosinophilic plaques, circular masses, or bands in the extra-cellular tissue spaces.2 Hyalinization is the process of deposition of hyaline-like homogeneous material that stains intensely with acid stains. It is recognized as a product of cellular and acellular matrix interaction process.3
The hyalinized stroma present in pathological lesions is modified biochemically and thus influences the biologic behaviour of neoplasms.4 It is often observed in long-standing lesions as well, indicating a degenerative process. However, a hyalinized stroma in several benign and malignant tumors could indicate a pathological phenomenon in progress that mandates evaluation. While in numerous lesions of odontogenic origin, it is the result of epithelial-mesenchymal interactions (EMI).5 It is believed to be an aberrant deposition of basement membrane material produced by tumor cells. It is postulated that GAG's, hyaluronic acid, and proteoglycans are chiefly responsible for the content of hyalinized stroma.6 These materials when present in excess are accountable for the aggressive biologic behaviour of lesions. Only a few studies have aimed to correlate the presence of hyalinized stroma with the aggressive biologic behaviour of oral lesions. The presence of hyalinization in oral lesions is a scarcely researched area in pathology. This review aims to appraise the nomenclature, hypothesis, differential stains and markers, clinical significance, and prognostic implications of hyalinization related to oral pathological lesions.
2. The hypothesis of hyalinization in oral hyalinizing lesions
We assume that the hyalinization process differs for each group of oral pathological lesions. Before the process of hyalinization, there is an alteration within the cells particularly in the levels of the polysaccharide hyaluronan. The concentration of hyaluronan is postulated to define the intensity of hyalinization, however, this still requires further investigation in tissue samples. Hyalinized tissue is rich in type IV collagen, laminin, proteoglycans, GAG's, and hyaluronic acid.7,8
The nature of fibrous tissue differs in oral lesions varying from cellular, to thick fibrous, fibro-myxoid, and purely myxoid. The stromal elements and the ground substance seem to impact the epithelial components of OHLs through EMI and influence the biologic behaviour of these neoplasms.9,10 However, this has not been investigated thoroughly. Whether hyalinization density and stromal composition are indicative of biologic behaviour in OHLs is yet to be established. Whether a correlation exists between intensity and pattern of staining of hyalinized tissue with the clinical parameters of OHLs remains inconclusive.
The influence of the odontogenic stroma on the biologic behaviour in odontogenic tumors is a strong indication of the existence of EMI in odontogenic tumors.11 To conclude, whether the content of type IV collagen, laminin, proteoglycans, GAG's and hyaluronic acid in hyalinized tissue influence the biologic behaviour of OHL's remains an unsolved domain in research.
Hyalinization is a characteristic histologic feature that is often associated with low-grade sarcomas. Perivascular hyalinization is often noted around the blood vessels in sarcomatous lesions. Smith et al. proposed two possible theories to explain its formation. First, tumor infiltration causes the release of vasoactive amines from mast cells which leads to vascular permeability. Second, as a result of vascular invasion by tumor cells, there is endothelial damage and leakage of plasma contents. These materials get organized and result in perivascular hyaline deposits. The continued deposition of hyaline leads to progressive obliteration and vascular thrombosis resulting in hypoxia and necrosis. The cascade of tumorigenesis proceeds in this above-mentioned manner regulated by the process of hyalinization.12
3. Methodology
A web-based search was performed via the PubMed database (from year 2000–2020) with the keywords “special stains in oral hyalinizing lesions”, “significance of hyalinization in oral lesions” and “hyalinization and biologic behaviour”. Original research studies describing special stains and their role in oral hyalinizing lesions and studies explaining the significance of hyalinization in oral lesions were considered. Reviews, case reports, and short communications published in the English language were included to assess numerous topics. The findings are elaborated in Table 1.
Table 1.
Correlation of Hyalinization and biologic behaviour of OHL's.
| Sl No | Group | Hyalinization Hypothesis | Differential Stains and Markers | Correlation with Biological Behaviour |
|---|---|---|---|---|
| 1. |
Odontogenic Lesions
|
The influence exerted by the odontogenic cells on the stroma The immune response against the tumor or secondary stromal change |
PAS Alcian Blue Picrosirius Red |
Hyalinization affects tumor growth and prevents stromal-tumor cell interaction. There is inhibition of heparanase function and angiogenesis. Eventually, the tumor cells adjacent to hyalinized areas undergo programmed cell death (Sathi et al., 2008).13 However, this needs to be verified on larger sample size, as the above-mentioned study considered only 6 cases |
|
Deposition of basement membrane material Contribution via hyaluronic acid and mucopolysaccharides |
PAS Alcian Blue Picrosirius Red Antibody to Type IV Collagen Antibody to Laminin-5 PAS Alcian Blue |
Subepithelial hyalinization of the underlying connective tissue capsule (P = 0.006) is significantly more common in OKC that recurred (Cottom et al., 2011).14 The correlation of hyalinization and recurrence needs to be validated on OKC samples via special staining and IHC |
|
| Myxomas rich in hyaluronic acid tend to be more aggressive and locally invasive (Sarkar, 2013).15 Overexpression of matrix metalloproteinases 2 and 9 in odontogenic myxomas lend invasive characters (Miyagi et al., 2012).16 | ||||
| 2. |
Potentially Malignant Disorders and Oral Cancer
|
TGF-β downregulates collagenolytic activity & promotes collagen deposition Downregulation of the inhibitory effect of SMAD6 and SMAD7 |
PAS Alcian Blue Picrosirius Red Antibody to Type I & III Collagen |
Perpendicular type III fibers in zones of hyalinization increase with advancement in the histopathological grade of OSMF (Nishat and Kumar, 2019).17 Fibrosis is due to the upregulation of SMAD 3 and downregulation of SMAD 7 through the TGF-β1 pathway. Long-standing fibrosis leads to hyalinization (Kamath et al., 2014).18 Procollagen genes increase collagen production while TIMP and PAI genes inhibit collagenase and thereby decrease collagen degradation, which results in increased insoluble collagen (Auluck et al., 2008).19 |
|
Desmoplasia plays a protective role in tumor invasion | PAS Alcian Blue Picrosirius Red Antibody to Type I, III & IV Collagen |
Tumour desmoplasia occurs in highly developed invasive tumors of OSCC. The collagen and extracellular matrix proteins secreted by stromal cells initiate the desmoplastic process to facilitate invasion (Kawashiri et al., 2009).20 Stromal desmoplasia is a forerunner of hyalinization. Stromal desmoplasia is a strong prognostic indicator in grades of OSCC (Zainab et al., 2019).21 The polarizing birefringence shifts from reddish-orange to yellowish-green from well-differentiated OSCC to poorly differentiated OSCC indicating a shift from mature to immature collagen fibers as the tumor progresses (Arun Gopinathan 2015).22 |
|
| 3. | Salivary Gland Tumors | Basement membrane material secreted by the tumor cells or degeneration of the connective tissue | PAS Alcian Blue Picrosirius Red Antibody to Laminin-5 |
There are no studies to relate the amount of hyalinized areas with the behaviour of ACC (Naik et al., 2013).23 Prominent zones of hyalinization have been associated with a greater likelihood of malignant transformation (Zarbo, 2002).24 |
| 4. | Benign Connective Tissue Tumors | Degenerative change of connective tissue commonly encountered in long-standing lesions | PAS Alcian Blue |
Degenerative hyaline changes in long-standing neurilemmomas are referred to as ancient schwannomas (Muruganandhan et al., 2013).25 Degenerative changes such as hyalinization are often found in leiomyomas (Billings et al., 2001).26 |
| 5. | Malignant Connective Tissue Tumors | Organization of endothelial damage and exudation of plasma contents | PAS Reticulin Stain |
Perivascular hyalinization promotes hypoxia and VEGF production in low-grade sarcomas (Groisman et al., 2000).12 Hyalinization/fibrosis was a significant independent favourable predictor for RFS (hazard ratio 0.49, P =.007) and OS (hazard ratio 0.36, P = 0.02) (Schaefer et al., 2017).27 The correlation of hyalinization and prognosis in sarcomas needs to be determined on a larger sample size via special staining and IHC to arrive at a definitive consensus |
4. Differential stains to assess hyalinization
The use of differential stains help to establish a link between hyalinization and proteoglycan content which in turn correlates with the biological behaviour of OHLs to predict aggressiveness. The popular stains to assess hyalinization are as follows:
-
•
Periodic Acid Schiff (PAS)
PAS is an inexpensive special stain that was first used by Mc Mannus to demonstrate mucin. Periodic acid oxidizes the amino or alkyl amino derivatives to form dialdehydes. Schiff reagent of PAS stain reacts with these dialdehydes and gives an insoluble magenta color. It is used to detect polysaccharides such as glycogen, and mucosubstances such as glycoproteins, glycolipids, and mucins in tissues.28,29
PAS stains collagen, precisely it stains carbohydrates and carbohydrate-rich macromolecules a deep red colour (magenta). The intensity of staining is directly proportional to the degree of hyalinization seen. This property of PAS can be utilized to evaluate the degree of hyalinization in oral pathological lesions. The same can be correlated to observe a link with biological behaviour and intensity of PAS staining.
Song et al. conducted a study on 62 patients with primary oral mucosal melanomas (OMM) and reported a correlation between the presence of PAS-positive patterns/networks and poor prognosis with metastasis in OMM. A completely closed vessel that stained with PAS was termed as a PAS-positive loop and three back to back loops were termed as PAS-positive network. The presence of a loop or a network was considered a PAS-positive pattern. The study concluded that using a PAS stain to detect these patterns is a cost-efficient and time effective method to assess the prognosis of OMM.30
Another significant application of PAS is its ability to determine glycogen and mucin in salivary gland tumors through the use of diastase enzymes.31, 32, 33 Most mucins are diastase resistant whereas glycogen is diastase sensitive. This property enables the diagnosis of tumors based on mucin content. The reactivity of the glycoproteins in the basement membrane makes PAS valuable in identifying the hyaline material in cases of cylindroma.34 The ability of PAS to stain the glycogen in the basement membrane is utilized in detecting potential early invasive carcinomas that indicate a breach in the basement membrane. Minor breaks in the basement membrane would otherwise be undetectable in routine hematoxylin and eosin staining.
-
•
Alcian Blue
Alcian blue is a large phthalocyanine molecule that has a net positive charge. The positive dye molecules are attracted by negatively charged mucin sites. This staining gives a blue colour to mucin rich sites, and PAS will stain neutral mucins rose red.35 Additionally, the presence of increased amounts of acid mucin in oral epithelial dysplasia and oral squamous cell carcinoma (OSCC) has been demonstrated by alcian blue stain.36
Satpathy et al. analyzed 26 specimens of pleomorphic adenomas of minor salivary glands and 5 control minor salivary glands of the hard palate to compare the stromal patterns and expression of mucin by employing the histochemical method of alcian blue staining. The stained slides revealed varying heterogeneity of mucin expression of normal and neoplastic minor salivary glands. The study concluded that the neoplastic myoepithelial cells differentiate to stellate cells and produce the myxoid matrix. The alcian blue staining is a reliable indicator of mucin.37
Alcian blue stains GAGs and hyaluronic acid in the stroma of hyalinized lesions. It is postulated that GAGs and hyaluronic acid are abundant in hyalinized tissue and are responsible for the aggressive biologic behaviour of such lesions.38,39 Hence, alcian blue can be utilized to study hyalinizing oral lesions.
-
•
Picrosirius Red
Picrosirius red method was adopted after Puchtler et al. and Junqueira et al. Picrosirius red is a strong cationic dye, by reacting with sulphonic acid and basic groups in the collagen molecules they impart a yellowish-green color on thin fibers and yellowish-orange color to thick fibers. Picrosirius red displays the nature of collagen fibers due to its ability to stain thick as well as thin fibers.40
Mature, immature, and pathologic fibers can also be differentiated with the stain. Picrosirius red is a useful tool to appraise collagen networks in normal and pathological tissues. Studies have well-utilized picrosirius red polarizing microscopy to evaluate the nature of collagen in hyalinizing lesions. The reddish color indicates closely packed thick fibers whereas greenish-yellow indicates loosely packed fibers in turn implicating a transition from mature to immature collagen.
A comparative study by Segnani et al. was undertaken to study collagen fibers revealed that picrosirius red is superior to van Gieson staining and the Sirius Red/Fast Green method is the most sensitive, in terms of both qualitative and quantitative evaluation of collagen fibers. Collagen fibers could be appreciated, even in their thinner networks.41
With the gradual change in polarizing colors correlating well with the advancing grade of the tumor from yellowish orange to greenish-yellow picrosirius red polarizing microscopy can be employed as an effective tool to correlate hyalinized collagen and aggressive biologic behaviour of OHLs.
Kulkarni et al. conducted a study on 56 odontogenic lesions which included odontogenic follicle, dentigerous cyst, unicystic ameloblastoma, keratocystic odontogenic tumor, multicystic/solid ameloblastoma, and ameloblastic carcinoma. The lesions were stained with picrosirius red and evaluated under a polarizing microscope. Collagen fibers of odontogenic follicles and dentigerous cyst showed orange-red birefringence, unicystic ameloblastoma and keratocystic odontogenic tumor fibers showed both orange-red and greenish-yellow birefringence, greenish-yellow birefringence was seen in multicystic/solid ameloblastoma, in ameloblastic carcinoma complete greenish birefringence was seen. The lesions with increased immature collagen fibers were found to be biologically aggressive. This study suggested that collagen fibers play a vital role in predicting the biological behaviour of odontogenic lesions and picrosirius red is a reliable stain.42
-
•
Safranin O
Safranin O is a basic dye with a positive charge and binds electrostatically to the proteoglycans, staining it with varying shades of red depending on proteoglycan content in the tissue. The fast-green solution, a protein stain, stains the non-collagen sites which provide a clear contrast to the safranin O staining.43 Proteoglycans are proteins to which GAGs are attached. GAGs comprise of repeating disaccharide units which give it a net negative charge.44 The intensity of safranin O staining is proportional to the proteoglycan content in the tissue.
No study has been performed to date correlating the intensity of safranin O staining in hyalinized tissue with aggressive biologic behaviour of pathological lesions. Thus, it's an exciting prospect to evaluate proteoglycans in hyalinized tissue and to establish their correlation with the aggressive biologic behaviour of OHLs.
5. Immunohistochemistry (IHC) to assess oral hyalinizing lesions
Immunohistochemically areas of hyalinization show strong diffuse reactivity to an antibody to type IV collagen and laminin. Type IV collagen and laminin are basal lamina products,45 these markers can be utilized in lesions where hyalinization is the result of basement membrane secretions.
A study by Agarwal & Ballabh, evaluated the expression of type IV collagen in the histological grades of OSCC and concluded that the type IV collagen staining intensity was more enhanced in well-differentiated OSCC than poorly-differentiated OSCC. This indicates a direct relationship between the presence of type IV collagen and the differentiation degree of SCC cells. The study stated that SCC cells lose their capability to form the basement membrane as they become less differentiated.46 Type IV collagen quantification has also been used as a predictor for invasion and metastasis in a study by Hirota et al. who stated that the amount of type IV collagen decreased in advanced T stage (T3 and T4) tumors. Basement membrane deposition significantly correlated with lymph node metastasis. The authors concluded that immunohistochemical examinations of basement membrane deposition on biopsied specimens seem beneficial for cancer treatment.47 A recent study by Kumari et al. conducted to assess the nature of collagen in grades of OSCC revealed that type of collagen fibers transit from type I to type III from well-differentiated to poorly differentiated OSCC. The authors concluded that the determination of the type of collagen in different grades of OSCC can facilitate the therapeutic targeting of molecules responsible for the invasion and progression of oral cancer.48
Laminin is a significant basement membrane glycoprotein. A study by Yellapurkar et al., revealed that laminin staining significantly correlated with the grade of OSCC. A weak/absent linear staining of laminin around the tumor-host interface was observed in poorly differentiated tumors. The authors concluded that laminin can be adopted as a useful marker in evaluating the histological differentiation and aggressiveness of oral carcinoma. This study also proposed the interesting concept of the role of laminin in inducing cell migration and metastasis by regulating the formation of lamellipodia as well as its interactions with integrins and proteases.49
Other oral lesions where hyalinization is not directly linked to basement membrane components like type IV collagen and laminin have adjunctive contributory components like proteoglycans, GAG's, and hyaluronic acid.
6. Working classification of oral hyalinizing lesions
(According to the tissue of origin and biologic behaviour).
6.1. Odontogenic lesions
-
•Solid Multicystic Ameloblastoma
-
➢Follicular
-
➢Plexiform
-
➢Acanthomatous
-
➢
-
•
Odontogenic Myxoma
-
•
Adenomatoid Odontogenic Tumor
-
•
Ameloblastic Fibroma
-
•
Clear cell hyalinizing odontogenic carcinoma
-
•
Unicystic Ameloblastoma
-
•
Odontogenic Keratocyst
6.2. Potentially malignant and malignant oral lesions
-
•
Oral submucous fibrosis
-
•
Basaloid Oral Squamous cell carcinoma
-
•
Metastatic carcinoma to the jaws
6.3. Salivary gland tumors
-
•
Pleomorphic Adenoma
-
•
Mucoepidermoid Carcinoma
-
•
Adenoid Cystic Carcinoma
-
•
Carcinoma ex-pleomorphic adenoma
6.4. Benign connective tissue tumors
-
•
Fibroma
-
•
Peripheral ossifying fibroma
-
•
Neurilemmoma
-
•
Neurofibroma
-
•
Leiomyoma
-
•
Nodular Fasciitis
6.5. Malignant connective tissue tumors
-
•
Neurofibrosarcoma
-
•
Leiomyosarcoma
6.6. Bone tumors
-
•
Fibrous Dysplasia
-
•Juvenile aggressive ossifying fibroma
-
➢Psammomatoid Type
-
➢Trabecular type
-
➢
6.7. Reactive lesions
-
•
Irritational Fibroma
-
•
Traumatic Neuroma
7. Discussion
Hyalinization and its debated origin pertaining to significant oral lesions have been discussed below.
-
•
Odontogenic Lesions
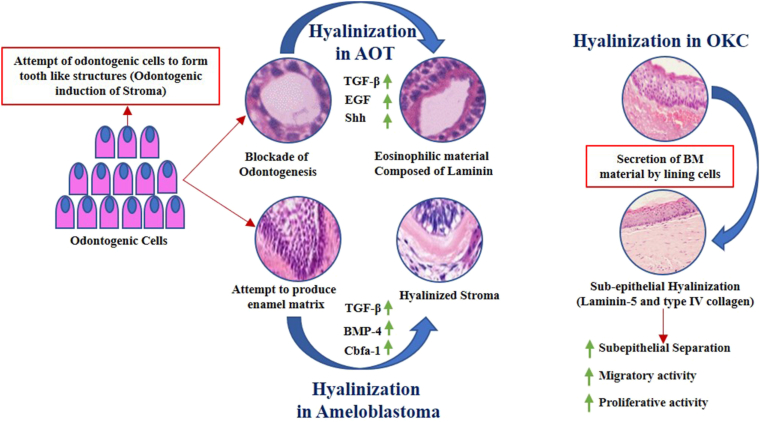
In odontogenic tumors, the epithelial cells significantly contribute to hyalinization ∖1. 1. The influence exerted by the tumor cells on the stroma is an attempt to complete the process of odontogenesis. However, there is a failure of formation of tooth-like structures, instead, a matrix is secreted.50 The evidence for the same can be found in adenomatoid odontogenic tumor (AOT) where an eosinophilic rim of varying thickness is observed around the lumina of microcysts. At the epithelial-connective tissue interface a fine fibrillar eosinophilic material has also been noted in AOT's which is usually immunopositive for basement membrane material laminin.51 Solid multicystic ameloblastomas often demonstrate an intensely hyalinized stroma. The odontogenic cells exert an inductive effect on the surrounding stroma which is noted as hyalinized areas around the odontogenic follicles. Even though the ameloblast cells attempt to induce hard tissue formation in the adjacent stroma by secretion of an enamel matrix, the fibroblasts are unable to differentiate into odontoblasts to complete the process of odontogenesis. The hyalinized zones represent a blockade of this process.52,53
∖1. 1.
Mechanism of hyalinization in Odontogenic Lesions.
A pathognomonic feature of calcifying epithelial odontogenic tumor is the presence of a homogenous eosinophilic substance that has been interpreted as amyloid, basement membrane material, glycoprotein, keratin, or enamel matrix. The material that is present in large quantities or as microparticles appears to be formed intracellularly and then extruded into the extracellular environment following exocytosis or degeneration.50 The eosinophilic material stains metachromatically with crystal violet. It exhibits an apple-green birefringence under polarized light after staining with Congo red. It fluoresces with thioflavin T under ultraviolet light similar to amyloid. Most ameloblastic fibromas present with a paucity of blood vessels and juxta-epithelial hyalinization. The thick hyalinized areas that often resemble dysplastic dentin in ameloblastic fibromas have postulated to be basement membrane material following electron microscopic studies.54
Ultrastructural studies by White and associates have revealed the presence of a pale secretory cell that is believed to secrete non-sulphated mucopolysaccharides. This myxoid material is secreted into the intercellular spaces through a transport mechanism.55 Hudson and Prout have suggested that OM's contain two types of acid mucopolysaccharides namely large quantities of hyaluronic acid and lesser amounts of chondroitin sulphate. They stated that the quantity of hyaluronic acid dictates the aggressive behaviour of the neoplasm.55
In odontogenic keratocyst (OKC) the most common theory postulated for subepithelial hyalinization is the deposition of basement membrane material by basal cells that include laminin V and type IV collagen.56 The modification of the basement membrane has been directly linked with separation of the lining epithelium resulting in recurrence of OKC.57
Excess of laminin V surges the migratory activity of the lining epithelial cells by interaction of laminin V with cell surface integrins and membrane-type matrix metalloproteinases. It is believed that significant hyalinization in OKC with an excess of laminin V induces epithelial proliferation. The same has been demonstrated in cancers with hyalinized stroma.58 Clear cell odontogenic carcinomas often show stromal hyalinization which has been inadequately researched.
The significance of hyalinization in odontogenic lesions seem to indicate the correlation of hyalinization with aggressive biologic behaviour. The implication of hyalinization on the biologic behaviour in unicystic ameloblastomas remains to be an interesting prospect to investigate. Execution of original research studies on odontogenic lesions with hyalinization and correlation with their biologic behaviour would provide an insight into improved understanding of this concept. Expression of IHC markers of hyalinization in odontogenic lesions and their association with clinical behaviour is another unaddressed area of research.
-
•
Potentially Malignant Lesions and Oral Cancer
Several researchers have endeavoured to evaluate the connective tissue stroma of oral potentially malignant disorders (OPMDs) and oral cancer. The stromal components play a regulatory role in the host defense mechanism against oral cancer. Considerable emphasis has been placed upon the type of collagen fibers, the orientation of fibers, and the severity of hyalinization.
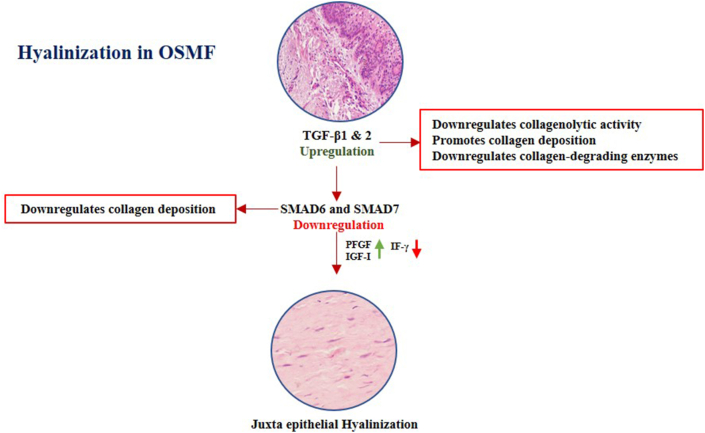
In OSMF, TGF-β plays an indispensable role in downregulating the collagenolytic activity and promotes collagen deposition. It impedes the transcription of collagen-degrading enzymes. The diminished degradation of collagen along with downregulation of the inhibitory effect of SMAD6 and SMAD7 proteins could be an initial step in fibrosis subsequently leading to hyalinization ∖1. 2. Whether special stains can indicate areas of hyalinization due to dysregulated TGF-β activity requires further exploration.59
∖1. 2.
Mechanism of hyalinization in OSMF.
A study conducted by Nishat and Kumar stated that OSMF stained with picrosirius red and observed under a polarizing microscope showed a majority of parallel type I fibers in early stages and perpendicular type III fibers increased with the advancement in the histopathological grade. In the early stage of OSMF, out of 15 cases, only 2 cases showed an overall greenish-yellow polarization color. Whereas, in the moderately advanced stage, out of 51 cases no significant birefringent color association was found with the stage of OSMF.17
A study conducted by Savita and Nail analyzed 40 cases of OSMF and concluded that there was a shift in the color of collagen fibers from greenish-yellow to an orangish-red and red color as the severity of the disease increased.60 A study by Ashalata et al. concluded that as the histopathological grade of the disease increased there was a shift in the polarizing color from yellow-green to orange-red.61
A picrosirius red polarizing study conducted to assess the nature of collagen in grades of OSCC revealed that collagen fibers show a change in birefringence ranging from reddish-orange to greenish-yellow in well- to poorly-differentiated OSCC indicating a transition from mature to immature. This indicates that studying collagen fibers with differential stains for stromal changes around tumor islands along with routine staining may help in predicting the prognosis of tumors.62
Homogenous condensation of collagen defines hyalinization in oral cancer. This is viewed as an adaptive mechanism of the body to wall-off the tumor cells from invasion. Stromal desmoplasia is the result of increased stromal proteins produced as a reaction to the inductive tumor stimuli. The consequences of tumor desmoplasia are debatable. It was assumed that tumor desmoplasia and hyalinization are protective mechanisms that prevent infiltration of tumor cells.63 Recent evidence suggests that hyalinization favors tumor cross-talk between malignant cells and stromal fibroblasts with subsequent cancerous changes in the stroma. The modified stroma influences the proliferative capacity and invasive potential of the tumor cells.21,64
A study by Kawashiri et al. confirmed the reciprocal relationship between tumor cells and stromal cells called molecular crosstalk that regulates the invasive potential of OSCC. The authors proposed that the mechanism of molecular crosstalk should be considered as a potential therapeutic target.65 A study by Jung et al. has demonstrated that upregulation of the chemokine (C–C motif) ligand 7 (CCL7) in carcinoma-associated fibroblasts favors migration and invasion of OSCC, the findings of this study confirm the presence of molecular tumor-stromal crosstalk.66 Evaluating this tumor - stroma cross talk on a large sample size could unravel potential therapeutic targets for OSCC. The phenomenon of hyalinization and its significance in OPMDs and oral cancer needs further exploration.
-
•
Salivary Gland Tumors
Tumors of the major salivary glands frequently exhibit hyalinization. When prominent zones of hyalinization are found in large benign salivary gland tumors especially in elderly individuals, they seem to have a higher predisposition for malignant transformation.67 A study by Ito et al. stated that hyalinization in pleomorphic adenoma is often present in small quantities, however, the authors reported 22 cases where hyalinization prevailed over myxoid or chondroid tissue.68 Hyalinization is considered as a histomorphological risk marker in pleomorphic adenomas, when a pleomorphic adenoma is extensively hyalinized a complete sampling of the tumor must be done to exclude malignancy.24
Low-grade mucoepidermoid carcinoma (MEC) is characterized by increased cyst formation, low cytologic atypia, a smaller number of epidermoid cells, more of mucous cells, and relatively less hyalinized areas. High-grade MECs show marked cytologic atypia, mitosis, necrosis, stromal invasion, and markedly more zones of hyalinization.69
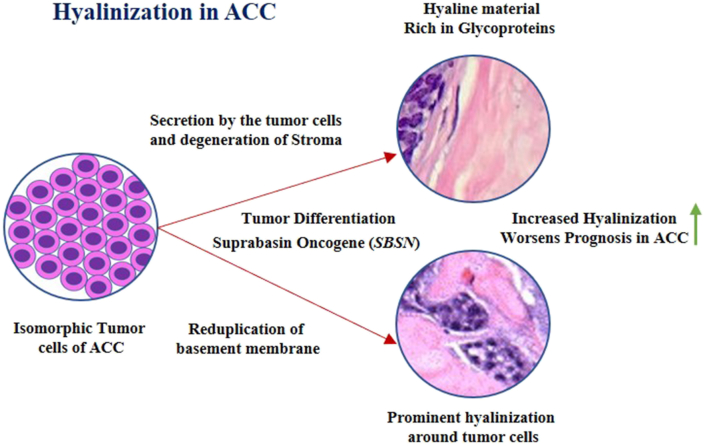
The hyaline basement membrane material characterizes the adenoid cystic carcinoma (ACC), it can be detected by employing PAS stain.70 The origin of this basement membrane material is theorized to be secreted by the tumor cells or as a result of the degeneration of connective tissue ∖1. 3. The basaloid islands and nests are bound by a hyalinized reduplicated basement membrane and have coalescent membrane droplets. The glycoproteins present in the basement membrane react with PAS stain making it pivotal in the diagnosis of cylindroma.
∖1. 3.
Mechanism of hyalinization in Adenoid Cystic Carcinoma.
In clear cell carcinomas of the salivary glands (CCCSG), PAS stains the granular cytoplasm with diastase sensitivity, PAS also stains the predominantly present hyalinized tissue in CCCSG. Despite the hyalinized areas being similar to amyloid Congo red is always negative. The hyalinized tissue is positive for fibronectin and type I collagen, but negative for type IV collagen and laminin indicating the presence of non-basement membrane material.71,72
It is evident from the above discussion that a hyalinized stroma in SGTs favours aggressive behaviour or malignant transformation of benign salivary gland tumors. Analysis of SGTs with a correlation of hyalinization characteristics with clinical behaviour will enable us to validate this hypothesis. The use of IHC to grade the degree of hyalinization in SGTs and to analyze its link with the clinical behaviour of SGTs is future scope for work in this field.
8. Clinical significance and conclusion
Predictive information on the behaviour of the lesion preoperatively can greatly influence treatment outcomes. Predicting biologic behaviour histologically can help clinicians to decide effective treatment plans.
The correlation of aggressive biologic behaviour with differential staining characteristics of a lesion is a novel idea to implement. Differential stains are inexpensive, can be easily prepared in the laboratory, and generate highly reproducible results. Once standardized, they can accurately stain and reveal stromal compositions through the intensity of staining, pattern, and type of collagen fibers. The need of the hour is to perform IHC studies on a large sample of OHLs to validate the findings of the correlation between biologic behaviour and differential staining characteristics.73
Several vital questions remain unanswered and further research is required to deduce the biochemical changes in hyalinized tissue such as: Is there any abnormal proteoglycan and GAGs in the hyalinized zones? Does the total proteoglycan and GAGs content influence hyalinization? Do GAGs modulate the growth and differentiation of fibroblasts leading to hyalinization? Do proteoglycans and GAGs influence stromal microenvironment and molecular crosstalk?74,75
The assessment of the biological behaviour of OHLs will resolve the dilemma in decision making while managing the lesion along with close follow up. The findings will navigate the surgeons in the treatment strategies of individual lesions.
Source of funding
None Declared.
Declaration of conflicting interest
Absent.
Acknowledgments
Nil.
References
- 1.Hamamura M., Oshikata T., Katoku K., Tsuchitani M., Yamaguchi R. Two types of deposits, hyaline droplets and eosinophilic bodies, associated with α2u-globulin accumulation in the rat kidney. J Toxicol Pathol. 2017;30(4):275–282. doi: 10.1293/tox.2017-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller M.A., Zachary J.F. Mechanisms and morphology of cellular injury, adaptation, and death. Pathol Basis Vet Dis. 2017;2–43:e19. doi: 10.1016/B978-0-323-35775-3.00001-1. [DOI] [Google Scholar]
- 3.Saluja T., Iyer J. Unmasking the grey zone of hyalinization with a proposed classification of oral hyalinizing lesions. J Interdiscipl Histopathol. 2017;5(1):18–21. [Google Scholar]
- 4.Siwach P., Joy T., Tupkari J., Thakur A. Controversies in odontogenic tumours: review. Sultan Qaboos Univ Med J. 2017;17(3):e268–e276. doi: 10.18295/squmj.2017.17.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puthiyaveetil J.S., Kota K., Chakkarayan R., Chakkarayan J., Thodiyil A.K. Epithelial - mesenchymal interactions in tooth development and the significant role of growth factors and genes with emphasis on mesenchyme - a review. J Clin Diagn Res. 2016;10(9):ZE05–ZE09. doi: 10.7860/JCDR/2016/21719.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elgundi Z., Papanicolaou M., Major G. Cancer metastasis: the role of the extracellular matrix and the heparan sulfate proteoglycan perlecan. Front Oncol. 2020;9:1482. doi: 10.3389/fonc.2019.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouw J.K., Ou G., Weaver V.M. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15(12):771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith M.M., Melrose J. Proteoglycans in normal and healing skin. Adv Wound Care. 2015;4(3):152–173. doi: 10.1089/wound.2013.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babu R.S.A., Reddy B.V.R., H A C. Histogenetic concepts, terminology and categorization of biphasic tumours of the oral and maxillofacial region. J Clin Diagn Res. 2014;8(2):266–270. doi: 10.7860/JCDR/2014/7506.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santosh A.B., Jones T.J. The epithelial-mesenchymal interactions: insights into physiological and pathological aspects of oral tissues. Onco Rev. 2014;8(1):239. doi: 10.4081/oncol.2014.239. Published2014 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali A.N., Elshamy I.S., Essa A.A. Influence of stromal architecture on the aggressiveness of ameloblastomas and keratocystic odontogenic tumors. Tanta Dent J. 2018;15:247–254. [Google Scholar]
- 12.Groisman G.M., Bejar J., Amar M., Ben-Izhak O. Pleomorphic hyalinizing angiectactic tumor of soft parts. Immuno-histochemical study including the expression of vascular endothelial growth factor. Arch Pathol Lab Med. 2000;124:423–426. doi: 10.5858/2000-124-0423-PHATOS. [DOI] [PubMed] [Google Scholar]
- 13.Sathi G.S., Fujii M., Tamamura R. Juxta-epithelial hyalinization inhibits tumor growth and invasion in ameloblastoma. J Hard Tissue Biol. 2008;17(2):63–68. [Google Scholar]
- 14.Cottom H.E., Bshena F.I., Speight P.M., Craig G.T., Jones A.V. Histopathological features that predict the recurrence of odontogenic keratocysts. J Oral Pathol Med. 2012 May;41(5):408–414. doi: 10.1111/j.1600-0714.2011.01113.x. Epub 2011 Nov 16. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar R.R. Oncologic profile of maxillary odontogenic myxoma: a rare case. Contemp Clin Dent. 2013;4(3):374–377. doi: 10.4103/0976-237X.118351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyagi S.P., Maranduba C.M., Silva Fde S., Marques M.M. Dental pulp stem cells express proteins involved in the local invasiveness of odontogenic myxoma. Braz Oral Res. 2012;26:139–144. doi: 10.1590/s1806-83242012000200009. [DOI] [PubMed] [Google Scholar]
- 17.Nishat R., Kumar H. Collagen fibers in oral submucous fibrosis - a polarizing microscopy study using two special stains. Indian J Pathol Microbiol. 2019;62:537–543. doi: 10.4103/IJPM.IJPM_324_19. [DOI] [PubMed] [Google Scholar]
- 18.Kamath V.V., Satelur K.P., Rajkumar K., Krishnamurthy S. Transforming growth factor beta 1 in oral submucous fibrosis: an immunohistochemical study - understanding the pathogenesis. J Dent Res Rev. 2014;1:75–80. [Google Scholar]
- 19.Auluck A., Rosin M.P., Zhang L., Sumanth K.N. Oral submucous fibrosis, a clinically benign but potentially malignant disease: report of 3 cases and review of the literature. J Can Dent Assoc. 2008;74:735–740. [PubMed] [Google Scholar]
- 20.Kawashiri S., Tanaka A., Noguchi N. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31:1346–1353. doi: 10.1002/hed.21097. [DOI] [PubMed] [Google Scholar]
- 21.Zainab H., Sultana A., Shaimaa Stromal desmoplasia as a possible prognostic indicator in different grades of oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2019;23(3):338–343. doi: 10.4103/jomfp.JOMFP_136_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arun Gopinathan P., Kokila G., Jyothi M., Ananjan C., Pradeep L., Humaira Nazir S. Study of collagen birefringence in different grades of oral squamous cell carcinoma using picrosirius red and polarized light microscopy. Sci Tech Rep. 2015;2015:802980. doi: 10.1155/2015/802980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naik K.L., Shetty P., Hegde P. Adenoid cystic carcinoma of buccal mucosa with extensive hyalinization: a unique case report. Ann Trop Med Publ Health. 2013;6:571–574. [Google Scholar]
- 24.Zarbo R. Salivary gland neoplasia: a review for the practicing pathologist. Mod Pathol. 2002;15:298–323. doi: 10.1038/modpathol.3880525. [DOI] [PubMed] [Google Scholar]
- 25.Muruganandhan J., Prasad T.S., Selvakumar T., Kumar S.N. Ancient neurilemmoma: a rare oral tumor. J Oral Maxillofac Pathol. 2013;17(3):447–450. doi: 10.4103/0973-029X.125218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billings S.D., Folpe A.L., Weiss S.W. Do leiomyomas of deep soft tissue exist? An analysis of highly differentiated smooth muscle tumors of deep soft tissue supporting two distinct subtypes. Am J Surg Pathol. 2001;25(9):1134–1142. doi: 10.1097/00000478-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer I.M., Hornick J.L., Barysauskas C.M. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: assessment of the European organization for research and treatment of cancer-soft tissue and bone sarcoma group response score. Int J Radiat Oncol Biol Phys. 2017;98(2):375–383. doi: 10.1016/j.ijrobp.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 28.Tabatabaei Shafiei M., Carvajal Gonczi C.M., Rahman M.S., East A., François J., Darlington P.J. Detecting glycogen in peripheral blood mononuclear cells with periodic acid schiff staining. JoVE. 2014;94:52199. doi: 10.3791/52199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shedge S., Roy P., Shedge A., Doshi M.A. Periodic acid schiff (PAS) staining: a useful technique for demonstration of carbohydrates. Med Leg Update. 2020;20(2):353–357. [Google Scholar]
- 30.Song H., Jing G., Wang L., Guo W., Ren G. Periodic acid–Schiff-positive loops and networks as a prognostic factor in oral mucosal melanoma. Melanoma Res. 2016;26(2):145–152. doi: 10.1097/CMR.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 31.Pujar A., Pereira T., Tamgadge A., Bhalerao S., Tamgadge S. Comparing the efficacy of hematoxylin and eosin, periodic acid schiff and fluorescent periodic acid schiff-acriflavine techniques for demonstration of basement membrane in oral lichen planus: a histochemical study. Indian J Dermatol. 2015;60(5):450–456. doi: 10.4103/0019-5154.159626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bobati S.S., Patil B.V., Dombale V.D. Histopathological study of salivary gland tumors. J Oral Maxillofac Pathol. 2017;21:46–50. doi: 10.4103/0973-029X.203762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latti B.R., Birajdar S.B., Latti R.G. Periodic Acid Schiff-Diastase as a key in Exfoliative cytology in diabetics: a pilot study. J Oral Maxillofac Pathol. 2015;19(2):188–191. doi: 10.4103/0973-029X.164531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoj M.G., Shelly D. Periodic acid Schiff's: a definitive stain in histopathological diagnosis of Cylindroma. Med J Armed Forces India. 2016;72(4):404–406. doi: 10.1016/j.mjafi.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahni A., Rehani S., Kardam P., Sethi S., Kumari R., Mathias Y. Analysis of stromal mucin in oral epithelial dysplasia & oral squamous cell carcinoma- A histochemical study. J Oral Biol Craniofac Res. 2019;9(1):40–46. doi: 10.1016/j.jobcr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George J., Narang R.S., Rao N.N. Stromal response in different histological grades of oral squamous cell carcinoma: a histochemical study. Indian J Dent Res. 2012;23:842. doi: 10.4103/0970-9290.111291. [DOI] [PubMed] [Google Scholar]
- 37.Satpathy Y., Spadigam A.E., Dhupar A., Syed S. Epithelial and stromal patterns of pleomorphic adenoma of minor salivary glands: a histopathological and histochemical study. J Oral Maxillofac Pathol. 2014;18(3):379–385. doi: 10.4103/0973-029X.151319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casale J., Crane J.S. StatPearls Publishing; Treasure Island (FL): 2020. Biochemistry, Glycosaminoglycans. In: StatPearls. [PubMed] [Google Scholar]
- 39.Kubaski F., Osago H., Mason R.W. Glycosaminoglycans detection methods: applications of mass spectrometry. Mol Genet Metabol. 2017;120(1-2):67–77. doi: 10.1016/j.ymgme.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Street J.M., Souza A.C., Alvarez-Prats A. Automated quantification of renal fibrosis with Sirius Red and polarization contrast microscopy. Phys Rep. 2014;2(7) doi: 10.14814/phy2.12088. Published 2014 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segnani C., Ippolito C., Antonioli L. Histochemical detection of collagen fibers by Sirius red/fast green is more sensitive than van Gieson or Sirius red alone in normal and inflamed rat colon. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0144630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulkarni P.G., Kumari M.A., Jahagirdar A., Nandan S., Reddy D.S.P., Keerthi M. Collagen and its role in predicting the biological behaviour of odontogenic lesions. J Contemp Dent Pract. 2017;18(2):137–141. doi: 10.5005/jp-journals-10024-2004. Published 2017 Feb 1. [DOI] [PubMed] [Google Scholar]
- 43.Hyllested J.L., Veje K., Ostergaard K. Histochemical studies of the extracellular matrix of human articular cartilage—a review. Osteoarthritis Cartilage. 2002;10(5):333–343. doi: 10.1053/joca.2002.0519. [DOI] [PubMed] [Google Scholar]
- 44.Miller T., Goude M.C., McDevitt T.C., Temenoff J.S. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014;10(4):1705–1719. doi: 10.1016/j.actbio.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hewitt R.E., Powe D.G., Morrell K. Laminin and collagen IV subunit distribution in normal and neoplastic tissues of colorectum and breast. Br J Canc. 1997;75(2):221–229. doi: 10.1038/bjc.1997.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal P., Ballabh R. Expression of type IV collagen in different histological grades of oral squamous cell carcinoma: an immunohistochemical study. J Canc Res Therapeut. 2013;9(2):272–275. doi: 10.4103/0973-1482.113382. [DOI] [PubMed] [Google Scholar]
- 47.Hirota J., Yoneda K., Osaki T. Basement-membrane type-IV collagen in oral squamous-cell carcinoma. Head Neck. 1990;12:400–405. doi: 10.1002/hed.2880120505. [DOI] [PubMed] [Google Scholar]
- 48.Kumari K., Ghosh S., Patil S., Augustine D., Samudrala Venkatesiah S., Rao R.S. Expression of type III collagen correlates with poor prognosis in oral squamous cell carcinoma. J Invest Clin Dent. 2017 Nov;8(4) doi: 10.1111/jicd.12253. [DOI] [PubMed] [Google Scholar]
- 49.Yellapurkar S., Natarajan S., Boaz K. Expression of laminin in oral squamous cell carcinomas. Asian Pac J Cancer Prev APJCP. 2018 Feb 26;19(2):407–413. doi: 10.22034/APJCP.2018.19.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shafer A.W., Hine M.K., Levy B.M. Elsevier, a division of Reed Elsevier India Private Limited; 2015. Shafer's Textbook of Oral Pathology. [Google Scholar]
- 51.Kurra S., Gunupati S., Prasad P.R., Raju Y.S., Reddy B.V. An adenomatoid odontogenic cyst (AOC) with an assorted histoarchitecture: a unique entity. J Clin Diagn Res. 2013;(6):1232–1235. doi: 10.7860/JCDR/2013/5771.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masthan K.M., Anitha N., Krupaa J., Manikkam S. Ameloblastoma. J Pharm BioAllied Sci. 2015;(Suppl 1):S167–S170. doi: 10.4103/0975-7406.155891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kessler H.P., Schwartz-Dabney C., Ellis E., III. Recurrent left mandibular enlargement. J Contemp Dent Pract. 2003;3(4):127–137. [PubMed] [Google Scholar]
- 54.Biancalana M., Koide S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 2010;1804(7):1405–1412. doi: 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tasnime S., Saxena C., Bansal V., Wadhwan V. Peripheral odontogenic myxoma. Indian J Dent Res. 2016;27:437–440. doi: 10.4103/0970-9290.191896. [DOI] [PubMed] [Google Scholar]
- 56.Kato N., Takeda J., Fukase M., Motoyama T. Hyalinized stroma in clear cell carcinoma of the ovary: how is it formed? Hum Pathol. 2012;43(11):2041–2046. doi: 10.1016/j.humpath.2012.02.012. Epub 2012 May 18. [DOI] [PubMed] [Google Scholar]
- 57.Philipsen H.P., Fejerskov O., Donatsky O., Hjorting-Hansen E. Ultrastructure of epithelial lining of keratocysts in nevoid basal cell carcinoma syndrome. Int J Oral Surg. 1976;5:71–81. doi: 10.1016/s0300-9785(76)80051-8. [DOI] [PubMed] [Google Scholar]
- 58.Kato N., Takeda J., Fukase M., Motoyama T. Alternate mucoid and hyalinized stroma in clear cell carcinoma of the ovary: manifestation of serial stromal remodelling. Mod Pathol. 2010;23:881–888. doi: 10.1038/modpathol.2010.75. [DOI] [PubMed] [Google Scholar]
- 59.Shih Y.H., Wang T.H., Shieh T.M., Tseng Y.H. Oral submucous fibrosis: a review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci. 2019;20(12):2940. doi: 10.3390/ijms20122940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thakkannavar S.S., Naik V.V. Histochemical and immunohistochemical analysis of collagen fibers and microvascular density in various grades of oral submucous fibrosis. Iran J Pathol. 2019;14(2):127–134. doi: 10.30699/IJP.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashalata G., Baghirath P.V., Krishna A.B., Kumar P.U., Tom A. Quantitative and qualitative analysis of collagen in oral submucous fibrosis. J NTR Univ Health Sci. 2012;1:99–105. [Google Scholar]
- 62.Manjunatha B.S., Agrawal A., Shah V. Histopathological evaluation of collagen fibers using picrosirius red stain and polarizing microscopy in oral squamous cell carcinoma. J Canc Res Therapeut. 2015;11:272–276. doi: 10.4103/0973-1482.154061. [DOI] [PubMed] [Google Scholar]
- 63.Sudhakara M., Reshma V., Khan N., Amulya S.R. Uncommon features in conventional oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2016;20(2):316–319. doi: 10.4103/0973-029X.185905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valkenburg K.C., de Groot A.E., Pienta K.J. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–381. doi: 10.1038/s41571-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kawashiri S., Tanaka A., Noguchi N. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31(10):1346–1353. doi: 10.1002/hed.21097. [DOI] [PubMed] [Google Scholar]
- 66.Jung D.W., Che Z.M., Kim J. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Canc. 2010;127(2):332–344. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 67.Auclair P.L., Ellis G.L. Atypical features in salivary gland mixed tumors: their relationship to malignant transformation. Mod Pathol. 1996;9:652–657. [PubMed] [Google Scholar]
- 68.Ito F.A., Jorge J., Vargas P.A., Lopes M.A. Histopathological findings of pleomorphic adenomas of the salivary glands. Med Oral Patol Oral Cir Bucal. 2009 Jan 1;14(2):E57–61. [PubMed] [Google Scholar]
- 69.Weinreb I. Hyalinizing clear cell carcinoma of salivary gland: a review and update. Head Neck Pathol. 2013;7(Suppl 1):S20–S29. doi: 10.1007/s12105-013-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manoj M.G., Shelly D. Periodic acid Schiff's: a definitive stain in histopathological diagnosis of Cylindroma. Med J Armed Forces India. 2016;72(4):404–406. doi: 10.1016/j.mjafi.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanjai K., Shivalingaiah D., Sharath R., Pandey B. Clear cell carcinoma of palatine salivary gland: a diagnostic challenge. J Oral Maxillofac Pathol. 2018;22:128–131. doi: 10.4103/jomfp.JOMFP_236_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Félix A., Rosa J.C., Nunes J.F., Fonseca I., Cidadão A., Soares J. Hyalinizing clear cell carcinoma of salivary glands: a study of extracellular matrix. Oral Oncol. 2002;38(4):364–368. doi: 10.1016/s1368-8375(01)00072-0. [DOI] [PubMed] [Google Scholar]
- 73.Khan W., Augustine D., Rao R.S., Sowmya S.V., Haragannavar V.C., Nambiar S. Stem cell markers SOX-2 and OCT-4 enable to resolve the diagnostic dilemma between ameloblastic carcinoma and aggressive solid multicystic ameloblastoma. Adv Biomed Res. 2018;7:149. doi: 10.4103/abr.abr_135_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker C., Mojares E., Del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19(10):3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghatak S., Maytin E.V., Mack J.A. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol. 2015;2015 doi: 10.1155/2015/834893. [DOI] [PMC free article] [PubMed] [Google Scholar]