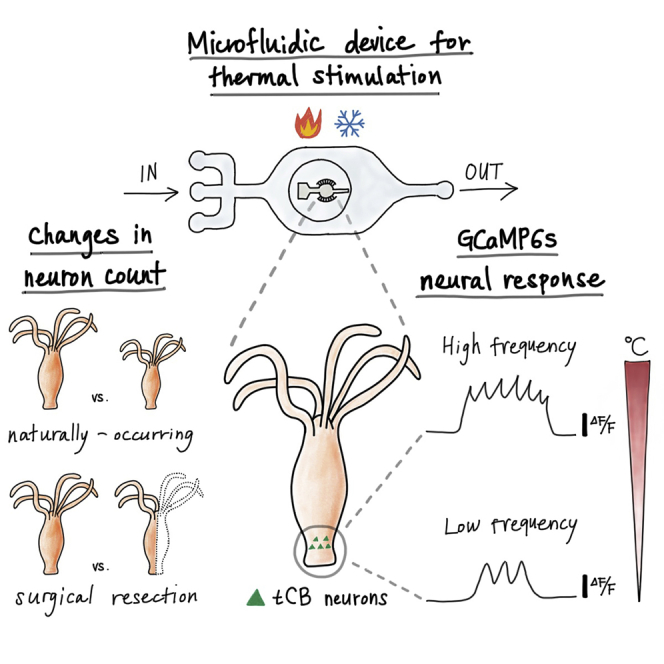
Summary
Many animals that lose neural tissue to injury or disease can maintain behavioral repertoires by regenerating new neurons or reorganizing existing neural circuits. However, most neuroscience small model organisms lack this high degree of neural plasticity. We show that Hydra vulgaris can maintain stable sensory-motor behaviors despite 2-fold changes in neuron count, due to naturally occurring size variation or surgical resection. Specifically, we find that both behavioral and neural responses to rapid temperature changes are maintained following these perturbations. We further describe possible mechanisms for the observed neural activity and argue that Hydra's radial symmetry may allow it to maintain stable behaviors when changes in the numbers of neurons do not selectively eliminate any specific neuronal cell type. These results suggest that Hydra provides a powerful model for studying how animals maintain stable sensory-motor responses within dynamic neural circuits and may lead to the development of general principles for injury-tolerant neural architectures.
Subject areas: Behavioral neuroscience, Biological sciences, Developmental neuroscience, Neuroscience
Graphical abstract
Highlights
-
•
Thermal stimulation drives temperature-dependent firing rate in specific Hydra neurons
-
•
Hydra show stable neural responses to temperature despite 2× decrease in neuron count
-
•
Injury tolerance of Hydra offers model for stable neural architecture
Behavioral neuroscience; Biological sciences; Developmental neuroscience; Neuroscience;
Introduction
One remarkable feature of the nervous system is plasticity, the ability to alter or reorganize neural circuits to gain or restore function, particularly in response to injury. In mammals, individual neurons can alter their excitability based on presynaptic transmission frequencies to maintain stable activity levels (Turrigiano, 2008). Deficits in this process of homeostatic plasticity have been associated with neural disorders (Tatavarty et al., 2020). Synaptic plasticity at the circuit level allows collections of neurons to remap connections. This produces behavioral compensation after broad injuries such as strokes and uses molecular mechanisms similar to those involved in neural development (Murphy and Corbett, 2009). Non-mammalian animals, such as salamanders and crustaceans, can regenerate limbs and their associated peripheral nerves (Alwes et al., 2016; Joven et al., 2019), whereas the cnidarian polyp Hydra vulgaris can regrow its entire body and complete nervous system from a fragment as small as ~300 cells (Shimizu et al., 1993). A major challenge in understanding how neural plasticity supports and maintains animal behavior during nervous system regeneration is the difficulty in observing complete neural activity during reorganization.
Millimeter-sized invertebrates are ideal model systems for studying neural plasticity and recovery from injury because they have relatively small nervous systems amenable to live imaging of neural circuit reorganization. However, most small invertebrate model organisms either show modest neural regeneration or lack well-developed transgenic techniques. For instance, neurons in the central nervous system of Drosophila do not regenerate following axotomies in either whole-brain cultures (Ayaz et al., 2008) or larvae (Song et al., 2012). In C. elegans, axons of select neurons can regenerate after being severed (Yanik et al., 2004); however, growth trajectories are error prone (Wu et al., 2007) even when identical neurons across animals are subjected to similar injury protocols (Hammarlund and Jin, 2014). Exceptional neural plasticity can be found in planarians, which regenerate entire organisms (Massobrio et al., 2015) from small fragments of tissue, but these animals lack a suite of transgenic tools similar to that of Drosophila and C. elegans (Scimone et al., 2017).
Hydra vulgaris is unique among invertebrate model organisms in that it is amenable to a variety of transgenic techniques and displays remarkable regenerative capabilities. These regenerative abilities include complete regeneration of the entire organism from fragments of only a few hundred cells (Shimizu et al., 1993; Technau et al., 2000), and its entire nervous system can be rebuilt from a single interstitial stem cell (David and Murphy, 1977). The robustness of Hydra's nervous system arises from its constitutively active multipotent interstitial stem cells, which give rise to several cell types, including neurons. In an uninjured, homeostatic animal, all differentiated cell types, including neurons, are continually shed and replaced every 20 days by stem cells (Campbell, 1967). This results in the continual differentiation of neurons in an adult animal, and thus the continual remodeling of existing neural circuits to account for the loss and gain of neurons. Likewise, Hydra's radial symmetry offers opportunities to experimentally modulate the number of neurons while preserving the presence of cell types. For example, when cut along the oral-aboral axis, we expect that the remaining tissue will have cells from all neuronal cell types. Thus, when the animal reseals its body column, its radially symmetric neural structure can be preserved while containing fewer total neurons. These properties of the Hydra nervous system make it an excellent model to study how new neurons integrate into existing circuits and how neuronal circuits are rebuilt following injury.
The small size and genetic tractability of Hydra provide additional advantages. As a millimeter-scale invertebrate, an entire Hydra can be imaged at a single-cell resolution (Dupre and Yuste, 2017). Furthermore, the ability to generate transgenic lines by embryo injection (Wittlieb et al., 2006; Juliano et al., 2014) has enabled functional calcium imaging in neurons (Dupre and Yuste, 2017) and epitheliomuscular cells (Szymanski and Yuste, 2019). In addition, cell-type specific promoters have been identified using single-cell transcriptomic analysis of the Hydra nervous system. These data reveal that Hydra has 12 neuronal subtypes, each expressing distinct sets of biomarkers linked to neuronal development and function (Noro et al., 2019; Siebert et al., 2019).
Given Hydra's potential as a model for neural regeneration and plasticity, it is critical to establish behavioral assays that indicate if and when neural circuits have regained their ability to regulate behavior. Although Hydra has been studied for over 300 years, we lack quantitative characterization of behaviors like sensory-motor responses. Studies of behavior dating back to the 1700s (Campbell, 2008) qualitatively describe responses to light (Passano and McCullough, 1963), chemicals (Kepner and Hopkins, 1924), and temperature (Mast, 1903; Schroeder and Callaghan, 1981; Bosch et al., 1988), but these experiments fall short of quantitative evaluation of behavior and neural activity. Recently, machine learning approaches have been used to identify behavioral motifs in freely moving Hydra, but these experiments have not been extended to sensory-motor responses (Han et al., 2018).
Hydra's response to a rapid change in temperature is one sensory-motor behavior that could be used to assess neural plasticity, but this behavior must first be quantified. Although prior experiments suggest that Hydra can sense and react to temperature, more work is needed to precisely demonstrate and quantify the animal's neural and behavioral response patterns to a rapid temperature change. Mast showed that when touched by heated objects, Hydra responds by bending toward the stimulus, but these experiments cannot fully distinguish between mechanosensory and thermosensory behaviors (Mast, 1903). Schroeder and Callaghan reported the highest and lowest temperatures in which Hydra oligactis and Hydra pseudoligactis could survive (Schroeder and Callaghan, 1981). Bosch et al. demonstrated that exposing Hydra to moderately elevated temperatures (30°C for two hours) increases their ability to survive culture at high temperatures (34°C for 4 days) that would otherwise be lethal (Bosch et al., 1988). Yamamoto and Yuste likewise found that extended exposure to elevated temperatures did not affect various behavioral features in Hydra, including long-term contraction burst frequency and detachment events (Yamamoto and Yuste, 2020). These experiments show that Hydra's viability depends on the ambient temperature and that Hydra can adapt to elevated environmental temperatures, but they do not reveal the specific sensory-motor response and neural processing of an acute thermal stimulus.
Here we show the first quantitative description of Hydra's behavioral and neural response to acute thermal stimulation. We demonstrate that these responses are maintained even if the number of neurons in the animal changes by a factor of 2 due to surgery or naturally occurring variation in animal size, assuming that there is no complete loss of any particular neural cell type. To perform this study, we developed a microfluidic device capable of delivering rapid and precise thermal stimuli without the confounding effect of mechanical stimulation. Using this technique, we find that Hydra elongates after the onset of positive thermal stimulation, followed by contraction. Synchronous with body movements, we find temperature-related oscillatory neural activity within a ring of neurons in Hydra's peduncle. The frequency of this oscillation decreases for negative thermal stimulation (i.e., cooling) and increases for positive thermal stimulation (i.e., heating). We show that the frequency of the neural oscillation varies with the absolute temperature of the thermal stimuli, rather than relative changes from culture temperature. Importantly, we find that these oscillation frequencies are nearly unchanged even if the number of neurons in these animals changes by a factor of 2, whether due to surgical resectioning or natural size variation. Overall, these data describe a specific sensory-motor behavior and associated neural activity that is robust to large changes in the number of neurons in this animal.
Results
Microfluidic device enables thermal stimulation and whole-body imaging of Hydra vulgaris
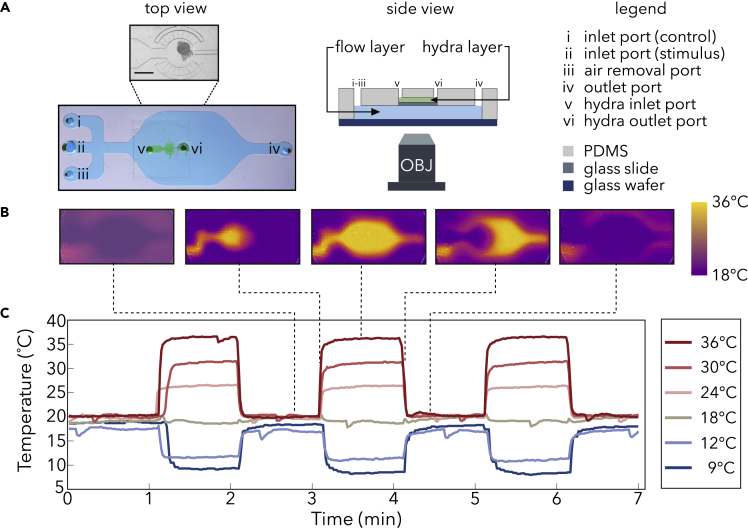
Because Hydra are sensitive to mechanical stimulation (e.g., touch and changes in fluid flow [Rushforth et al., 1963; Passano and McCullough, 1964; Khetan et al., 2018]), we designed a two-layer microfluidic device to deliver thermal stimuli without mechanical confounds: a Hydra immobilization chamber fabricated above a flow layer with multiple inlet ports (Figure 1A, see transparent methods). By connecting the flow layer's inlet ports to in-line heaters at different temperature setpoints, we can provide rapid and precise thermal stimulation by switching fluid flow through the different inlet ports—a process that we automate using microcontrollers (see transparent methods). In our experiments, one inlet (i in Figure 1A) provides fluid that is maintained at Hydra's culture temperature to serve as a control. A second inlet provides fluid that is maintained at a stimulus temperature ranging from 9°C to 36°C. To validate our ability to rapidly and repeatedly modulate the temperature of the Hydra immobilization chamber, we measured the temperature of the device glass with an infrared camera (Figures 1B and 1C). We found that the temperature plateaus in approximately 5 s after the start of the temperature change and that the temperature set points were repeatable to within a standard deviation of 0.5°C across multiple stimulation cycles.
Figure 1.
Characterization and validation of two-layer microfluidic device for thermal stimulation of Hydra vulgaris
(A) (Left) Photograph of microfluidic device with blue dye in flow layer and green dye in Hydra chamber. Scale bar, 1 mm. (Middle) Schematic of cross-section of the device. (Right) Legend for left and middle panels.
(B) Infrared image showing temperature of the flow layer before (leftmost), the start of (second leftmost), during (center), end of (second rightmost), and after (rightmost) thermal stimulation.
(C) Time course of thermal stimulation at temperatures above and below Hydra's baseline culture temperature of 18°C.
Positive thermal stimulation drives sequential elongation and contraction in Hydra vulgaris
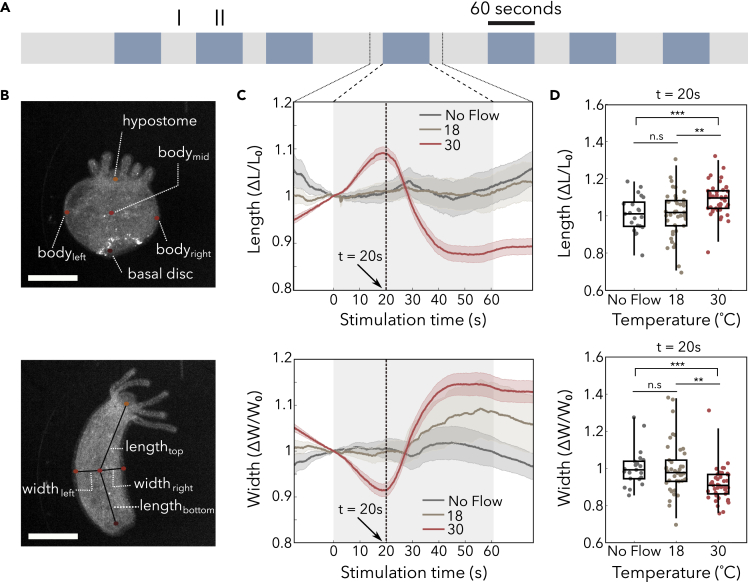
Having fabricated a microfluidic device that allows us to thermally stimulate and simultaneously image Hydra, we supplied fluids of different temperatures to the flow layer to measure how Hydra responds to a rapid change in temperature. To avoid synchronizing thermal stimuli with natural rhythmic behavior (Passano and McCullough, 1964; Rushforth, 1971) or entraining a periodic response, we applied thermal stimuli for a period of 60 s and randomized the time interval between successive stimuli (Figure 2A). We quantified changes in Hydra's posture by using DeepLabCut (Nath et al., 2019) to automate labeling of Hydra's body width and length using hypostome (oral end), basal disc (aboral end), and the leftmost, middle, and rightmost points in the body column region (Figure 2B, see transparent methods). We observed no statistically significant changes to Hydra's body length in experiments when we switched the source of the flow layer between two sources at 18°C compared with experiments with no fluid flow (“18°C” and “No flow” curves in Figure 2C), showing that the developed microfluidic device does not produce mechanical stimulation. However, we found that Hydra responds to a rapid change in temperature by first elongating and then longitudinally contracting. The peak body length occurs approximately 20 s after the stimulus onset (Figures 2C and 2D).
Figure 2.
Hydra's body length initially increases and width initially decreases when stimulated at 30°C
(A) Schematic of a representative thermal stimulation protocol. Gray regions (I) indicate non-stimulus periods at Hydra's culture temperature lasting between 30 and 90 s, and blue-colored regions (II) indicate stimulus periods at designated temperatures for 60 s.
(B) Representative frame annotated from DeepLabCut. Top panel describes which body points were annotated, and the bottom panel shows how the length and width of the animals are determined. The hypostome is the end of the body with Hydra's mouth and tentacles (oral end), whereas the basal disc is used to adhere to substrates (aboral end). Scale bar, 500 μm.
(C) Average length and width of Hydra calculated for stimulation periods and time-aligned to stimuli, with 15 s before and after stimulation periods. Curve corresponds to mean, and shaded error bars correspond to standard error of the mean. N = 3 Hydra for No Flow, N = 6 Hydra for 18°C and 30°C.
(D) The change in length (top panel) and width (bottom panel) at 20 s after onset of stimulation (t = 20 s), for each stimulus period. Box-and-whisker plots indicate Q1, median, and Q3; whisker lengths indicate the 2nd and 98th percentiles. N = 3 Hydra for No Flow, N = 6 Hydra for 18°C and 30°C. (n.s = not significant, ∗∗p < 0.001, ∗∗∗p < 0.0001, unpaired t test).
Subset of peduncle neurons show periodic calcium spikes in response to thermal stimulation
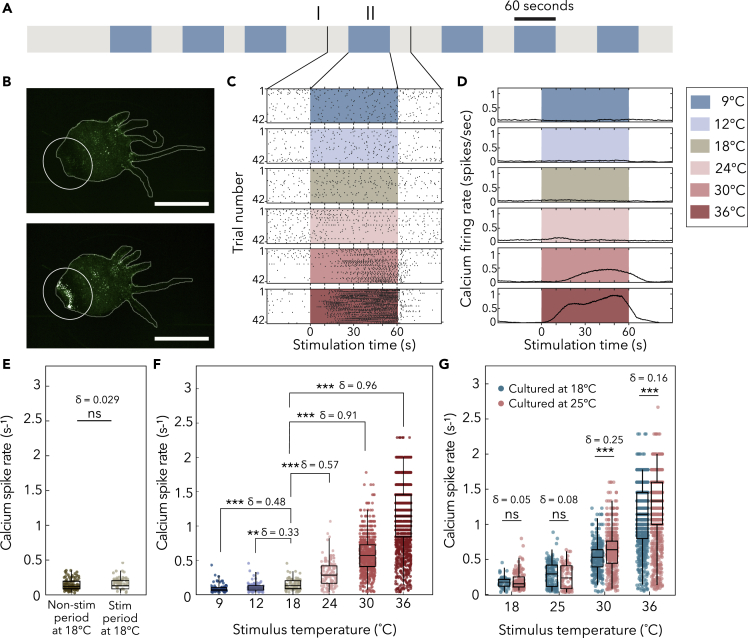
Using fluorescence imaging of transgenic Hydra that express the calcium-sensitive fluorophore GCaMP6s in neurons, we observed bursts of synchronous calcium spikes following thermal stimulation in a group of neurons in the animal's peduncle (Figures 3A and 3B, Video S1, Video S2). We will refer to these neurons as temperature-linked contraction burst (tCB) neurons because their activity is closely linked to body contractions as described previously (Szymanski and Yuste, 2019) and are active following thermal stimulation. To quantify the calcium spike rates in tCB neurons following thermal stimulation, we selected a region of interest (ROI) that encompassed the entire Hydra peduncle (Figure 3B). Although not all neurons in this region are putative tCB neurons, the strong signal produced by the synchronous tCB neuron activity allowed us to measure the collective synchronous calcium spike rate using this ROI (Figures 3C, 3D, and S1; Videos S1 and S2, see transparent methods). We found that Hydra's calcium spike rate did not significantly change when switching between fluid reservoirs at 18°C, suggesting that changing fluids in the flow layer did not produce a mechanical stimulation artifact (Figure 3E, p value > 0.1, Cliff's delta effect size δ = 0.029).
Figure 3.
Thermal stimulation modulates the synchronous neural activity in the peduncle of Hydra vulgaris, see also Figures S1 and S2.
(A) Schematic of a representative thermal stimulation protocol. Gray-colored regions (I) indicate non-stimulus periods at Hydra's culture temperature (either 18°C or 25°C) lasting between 30 and 90 s. The blue-colored regions (II) indicate stimulus periods at designated temperatures for 60 s.
(B) Images of Hydra without synchronous neural firing (top) and during firing (bottom) in the peduncle (white circle). Scale bar, 1 mm.
(C) Raster plot of synchronous firing events in peduncle in (B). One black vertical line indicates one synchronous firing event. Each row represents the raster plot of spikes time-aligned with one stimulation (boxed regions) with 30 s before and after stimulation (white non-boxed region).
(D) Peristimulus time histogram (calcium firing rate) of (C), which was calculated with a 10-s sliding window.
(E) Calcium spike rate comparison between the non-stimulation (gray-colored region I in A) and stimulation (blue-colored region II in A) periods with a stimulus temperature of 18°C. (ns = not significant, Mann-Whitney U test. δ = Effect size using Cliff's delta). Box-and-whisker plots indicate Q1, median, and Q3; whisker lengths indicate the 2nd and 98th percentiles.
(F) Calcium spike rates from Hydra, all cultured at 18°C. N = 6 animals per stimulus temperature. (ns = not significant, ∗∗p < 0.0001, ∗∗∗p < 0.00001, Mann-Whitney U test. δ = Effect size using Cliff's delta). Box-and-whisker plots indicate Q1, median, and Q3; whisker lengths indicate the 2nd and 98th percentiles.
(G) Calcium spike rates from Hydra cultured at 18°C and 25°C. x axis notes the stimulation temperatures. N = 3 per culture temperature at each stimulation temperature. (ns = not significant, ∗∗∗p < 0.00001, Mann-Whitney U test. δ = Effect size using Cliff's delta). Box-and-whisker plots indicate Q1, median, and Q3; whisker lengths indicate the 2nd and 98th percentiles.
To estimate the population-level variability in calcium spike rates against which we could compare the response to thermal stimulation, we calculated the effect size of natural variation (Figures 3C–3G and S2). We subjected three organisms to identical temperature stimulation 48 h apart, finding that Hydra could exhibit significant day-to-day variability. For instance, one animal did not have statistically significant day-to-day variation in calcium spike rates (Figure S2, p > 0.1, δ = 0.04), whereas another exhibited highly statistically significant differences in its neural responses to identical temperature stimulation on different days (Figure S2, p < 0.0001, δ = 0.61). When day-to-day trials between the three organisms are aggregated based on the experimental day (analogous to Figure 3G), the collective difference between experimental days has an effect size of δ = 0.35, providing an indication of day-to-day, random variation that can be expected from data collected across groups of Hydra. As a conservative measure for evaluating our experimental findings, δ = 0.61 will be used for reference to day-to-day variation that is discussed hereafter. Because of Hydra's day-to-day variability, we proceeded to interpret subsequent results based on both p values (for determining whether medians of calcium spike rate distributions are different) and effect size (as key context on the magnitude of experimentally induced changes in Hydra's neural activity).
When we thermally stimulated Hydra across a wide range of temperatures both above and below their culture temperature, we observed that the tCB neurons' calcium spike rate during positive thermal stimulation (i.e., heating) was significantly higher than in control experiments (compare conditions labeled “24°C,” “30°C,” and “36°C” with the control condition of “18°C” in Figures 3C, 3D, and 3F). Likewise, tCB neurons' spike rate decreased during negative thermal stimulation (i.e., cooling; compare conditions labeled “9°C” and “12°C” with the control condition of “18°C” in Figures 3C, 3D, and 3F). We also found that the firing rate of the tCB neurons during thermal stimulation depends primarily on the absolute temperature of the thermal stimulus and not on the relative increase in temperature. When we increased the Hydra culture temperature from 18°C to 25°C, there was no significant change in the calcium spike rate for low thermal stimulus temperatures (i.e., stimulus at 18°C and 25°C in Figure 3G). At high stimulus temperatures (i.e., 30°C and 36°C in Figure 3G), we observed a statistically significant difference based on culture temperature, but the effect size was small compared with Hydra's day-to-day variability (δ = 0.25 at 30°C stimulation, δ = 0.16 at 36°C stimulation, δ of up to 0.61 for identical stimulus on the same organism across different days). Furthermore, the effect size of changing Hydra's culture temperature was small compared with changing the stimulation temperature by a comparable amount (e.g., δ = 0.71 between Hydra stimulated at 30°C or 36°C, but both cultured at 18°C). These results support the conclusion that the calcium spike rate of tCB neurons depends primarily on the absolute temperature of a thermal stimulus, rather than relative changes from a culture temperature baseline.
Hydra vulgaris' neural response to thermal stimulation is robust against naturally-occurring 2-fold changes in neuron count
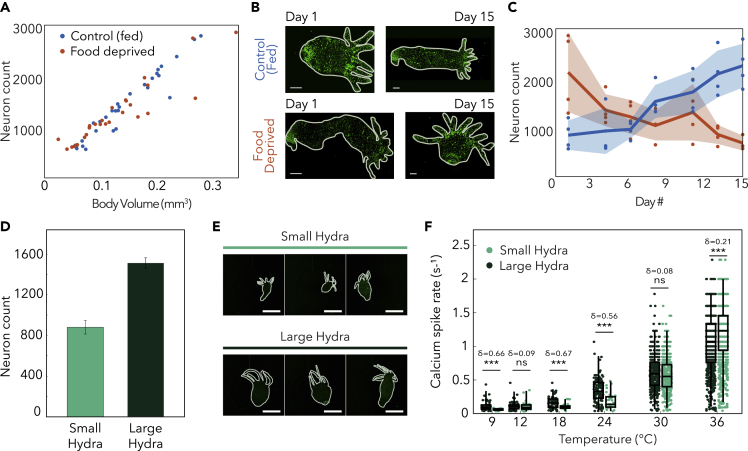
Having identified that the calcium spike rate of tCB neurons correlates with the absolute temperature of a thermal stimulus, we asked if this relationship is maintained even if the animals have significantly different numbers of neurons. To answer this question, we first determined that the number of neurons in Hydra is linearly related to the size of the animal. Using longitudinal fluorescence imaging of Hydra over 2 weeks of starvation or regular feedings (see transparent methods), we found that the number of neurons scaled with Hydra's body volume regardless of how that body volume was reached (e.g., small organism by food deprivation versus small organism due to recent budding), consistent with prior reports of the density of neurons as a function of animal size (Bode et al., 1973; Otto and Campbell, 1977; Siebert et al., 2019) (Figures 4A–4C). Given the gradual change in body size over the time course of days, we expect that neuronal subtypes and structures will be preserved, despite changes in the total number of neurons.
Figure 4.
Calcium spike rate of tCB neurons does not depend on Hydra's body size and number of neurons, see also Figure S3
(A) Number of neurons as a function of animal size (N = 4 per diet condition).
(B) Fluorescence images of Hydra at days 1 and 15 for both control and food-deprived groups (pseudo colored). Scale bar, 200 μm.
(C) Change in the number of neurons over the time course of 15 days. Curve corresponds to the mean, and shaded region corresponds to standard deviation.
(D) Estimated number of neurons from measurements in (A). Data represented as mean ± standard error of the mean.
(E) Three representative Hydra from small and large groups (pseudo colored). Scale bar, 1 mm.
(F) Calcium spike rates from large (N = 3 per size at each temperature) and small (N = 3 per size at each temperature) Hydra, all cultured at 18°C. (ns = not significant, ∗∗∗p < 0.00001, Mann-Whitney U test. δ = Effect size using Cliff's delta). Box-and-whisker plots indicate Q1, median, and Q3; whisker lengths indicate the 2nd and 98th percentiles.
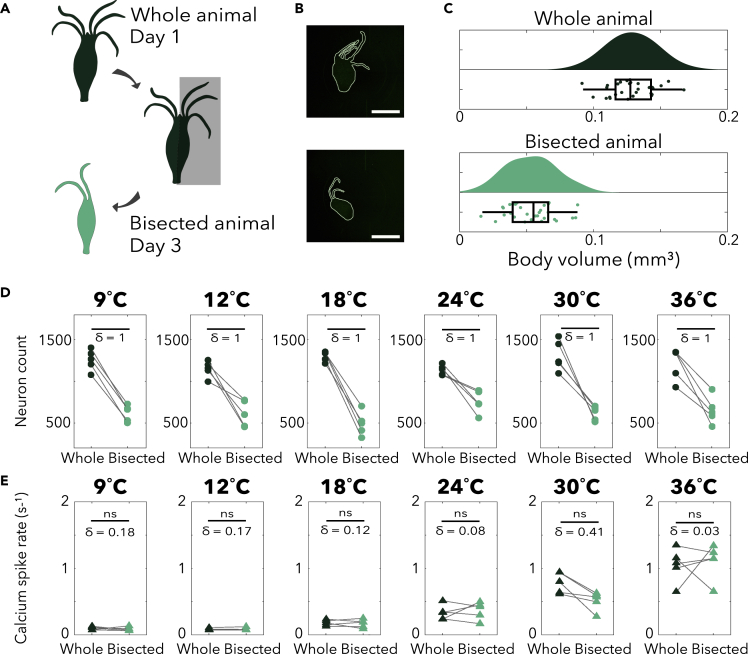
To compare the thermal responses of animals with different sizes, we separated animals into “large” and “small” cohorts according to their body size (area calculated through binarization of Hydra images). Based on the differences in body size and our measured relationship between body size and number of neurons, we estimate that the difference in neuron count between these groups is approximately 2-fold (Figures 4D and 4E). Note that although the small and large populations have a 2-fold difference in the mean, the distributions do overlap due to the naturally occurring inhomogeneity in the small and large populations (Figure S3). Nevertheless, we expect that these groups would show differences in behavior if the thermal response were dependent on the number of neurons in the animal.
When we measured the calcium spike rates during thermal stimulation, we found a nearly identical temperature relationship in both small and large animals (Figure 4F), suggesting that Hydra maintain stable neural responses to thermal stimuli despite significant, 2-fold differences in the number of neurons. Both groups exhibit decreased calcium spike rates during negative thermal stimulation and increased calcium spike rates during positive thermal stimulation. Although some temperatures did show statistically significant differences between the thermal response of the “small” and “large” animals (Figure 4F), the effect size (δ) is small compared with the effect size of changes in stimulus temperature (e.g., δ = 0.96 when stimulating at 36°C when compared with culture temperature baseline; δ = 0.21 between large and small animals when stimulated at 36°C). At 9°C stimulation and control temperature (18°C), the effect size (δ) between the small and large groups was 0.66 and 0.67, respectively, which is comparable to the effect size of when comparing the calcium spike rate from the same animal measured on subsequent days (δ = 0.61, Figure S2).
Surgical removal of half of Hydra's neurons does not significantly affect neural response to thermal stimulation
While animals with naturally occurring large and small nervous systems showed similar responses to thermal stimuli, we also wondered if this behavioral stability would apply to individual animals that had recently suffered the injury of losing neural tissue. To answer this question, we cut the organisms in half along their oral-aboral axis. Thanks to Hydra's radial symmetry and regenerative abilities, these surgically altered animals closed their wounds but did not regenerate to their original size within 48 h (Itayama and Sawada, 1995), yielding an animal with roughly half the number of neurons as the original whole (Figures 5A–5C, see transparent methods). Additionally, the radial symmetry helps to ensure that a resection along the oral-aboral axis does not remove the entirety of any one cell type (Javois et al., 1988; Wang et al., 2020). These resections are different from cuts normal to the oral-aboral axis that are known to produce significant behavioral deficits due to the removal of neuronal subtypes that are organized along the body column (Passano and McCullough, 1964). Given the radial symmetry of its nervous system and accompanying preservation of neuronal subtypes, it is a natural hypothesis that Hydra will maintain a level of stability when bisected along the oral-aboral axis. However, we could not find any reports quantifying the neural stability of bisected Hydra. Therefore, we set out to experimentally evaluate the stability of Hydra's neural activity and behavior following injury.
Figure 5.
Changes in Hydra's number of neurons due to surgical resection do not affect neural response to thermal stimulation, see also Figure S4
(A) Experimental schematic. A whole animal is imaged on Day 1 and then surgically resected, producing the bisected animal that is imaged on Day 3 (48−50 h after being cut).
(B) Representative image of whole Hydra (top panel), representative image of bisected Hydra (bottom panel). Scale bar, 1 mm.
(C) Size distribution of whole and bisected Hydra. (N = 5 per size at each stimulation temperature). Box-and-whisker plots indicate Q1, median, and Q3; whisker lengths indicate the 2nd and 98th percentiles.
(D) Comparison of neuron count between whole (N = 5 per size at each temperature) and bisected (N = 5 per size at each temperature) Hydra. The dark green data points on the left correspond to the number of neurons in the whole Hydra, which are connected to the corresponding bisected Hydra; the light green points on the right. δ, the effect size using Cliff's delta shown is calculated after bootstrapping with 100 iterations.
(E) Mean calcium spike rate comparison between whole and bisected Hydra. (ns = not significant, Wilcoxon signed-rank test. δ = Effect size using Cliff's delta).
By measuring neural responses to thermal stimulation both before and 2 days after this longitudinal resectioning, we could experimentally modulate the number of neurons in an organism and quantify resultant changes in neural activity patterns (Figure 5B-E). Specifically, we generated on average a 2-fold decrease in neuron count between whole and bisected Hydra (Figures 5C and 5D); however, we found that the neural response to thermal stimulation remained stable across all stimulus temperatures from 9°C to 36°C (Figure 5E). Namely, we found no statistically significant changes in the calcium spike rate when comparing Hydra before and after surgical resection of half of the animal's neurons (N = 5 animals per stimulus temperature). To account for naturally occurring variability between animals, we performed bootstrapping on the calcium spike rates within size groups and calculated the corresponding effect size (δ) for comparison (Figure S4). We found that the effect sizes between the calcium spike rates of whole and bisected Hydra are negligible to small (δ < 0.18) for all stimulation temperatures except for 30°C and moderate (δ = 0.41) for 30°C stimulation. These effect sizes are all small compared with the day-to-day variability within an animal (δ = 0.61, Figure S2) and to the change in number of neurons, which has a maximum Cliff's delta of 1 (Figure 5D). Note that the surgical resection we chose, which cuts the animal along the oral-aboral axis, helps to preserve the diversity of cell types as cells differentiate along the oral-aboral axis (Siebert et al., 2019). Thus, these perturbations are expected to maintain all neuronal cell types. We would not necessarily expect that all potential resections (e.g., removing the oral or aboral ends of the animal) would show the same stable behavioral responses as we find in these experiments.
Evaluation of mechanisms of the tCB circuit
After finding that activity in Hydra's tCB neurons changes according to the absolute temperature of an acute stimulus and is nearly invariant with neuron count, we asked what mechanism could link absolute temperature to these phenomena. We considered whether the simple hypothesis of diffusion coefficients' dependence on absolute temperature could explain elevated calcium spike rates (e.g., faster diffusion of ions and signaling molecules). The Stokes-Einstein equation states that diffusion coefficients are proportional to the absolute temperature of a system. The increase in the stimulus temperature from 18°C to 36°C corresponds to a 6.2% increase in absolute temperature and diffusion coefficients. However, we observe a nearly 800% increase in median calcium spike rate (Figure 3F). As observed changes in calcium spike rates are two orders of magnitude larger than changes in diffusion coefficient, the temperature-dependent increase in chemical diffusion is an unlikely mechanism for the observed relationship between Hydra neural activity and the absolute temperature of thermal stimuli.
As a more promising biophysical mechanism, we considered the temperature-dependent activity of the tCB neurons themselves, which can be quantified with the temperature coefficient, Q10, a metric to describe the intrinsic thermal sensitivity of a system. As a point of reference, Q10 values greater than 2 indicate high temperature dependence, as has been described in studies on the effects of temperature on ion channel currents (Ito et al., 2015) and grasshopper sensory receptor neuron firing rates (Roemschied et al., 2014). This value can be calculated as a function of the rates of a process measured at two different temperatures (Equation 1).
| (Equation 1) |
We determine the value of Q10 for the tCB neurons to be 3.3 by measuring the slope of the log-linear relationship between rate ratio R2/R1 and temperature difference T2-T1 from our data of 18°C through 36°C in Figure 3F (see transparent methods). Performing similar calculations on calcium spike rates of Hydra cultured at 18°C or 25°C (Figure 3G) we found that Q10 is similar across different culture temperatures (Q10 = 3.09 for animals cultured at 18°C versus 3.45 for animals cultured at 25°C). These values of Q10 are within the range of values designated as thermosensitive in individual cells (Eisenman and Jackson, 1967; Binda et al., 2002; Ramot et al., 2008; Ito et al., 2015), which supports the hypothesis that the temperature sensitivity of the tCB neural firing rate may be a property of individual cells (which could be sensory or tCB neurons, see below) and that synchrony is achieved due to the Hydra peduncle's strong gap junctional coupling (Wolf et al., 2014).
It is important to note that although the Q10 value of the tCB neurons suggests they are highly temperature sensitive, they are likely not the primary thermosensory neurons. The onset of tCB calcium bursts was approximately 24 s (median value at 30°C stimulus) following thermal stimulation, a large time interval given the hertz-scale frequency of tCB neurons after the start of firing and that the chamber temperature plateaus approximately 5 s after the start of the temperature change (median value at 30°C stimulus) (Figure S1). Instead, the long latency between the thermal stimuli and the neural activity suggests that the tCB neurons may be downstream of primary sensory neurons.
Furthermore, Hym-176A, C, and D-positive neurons that are primarily localized to the peduncle do not express clear homologs to thermosensitive ion channels like the TRPM or TRPA family (Peng et al., 2015; Malafoglia et al., 2016; McLaughlin, 2017; Klimovich et al., 2020). Instead, TRPM-like channels are expressed in pacemaker neurons in Hydra's head region that control the regularity of spontaneous rhythmic contractions (Klimovich et al., 2020), whereas neurons associated with Hydra's nematocytes express homologs for thermosensitive TRPA channels (Beckmann, 2013). These TRPM- and TRPA-expressing neurons are localized to Hydra's hypostome and tentacles, a region known to contain broader types of sensory neurons (Bode et al., 1988; Koizumi et al., 1988), providing evidence that tCB neurons do not sense temperature directly. Qualitative examinations of tCB neurons' morphology (e.g., Videos S1 and S2) also support the idea of tCB neurons not being direct sensory neurons, with tCB neurons more closely resembling the compact shape of ganglion cells than the small narrow cell bodies of sensory neurons (e.g., as described in the basal disk ganglion cells of David [1973] or the multipolar neurons of Epp and Tardent [1978]).
Based on our data, we expect that the most likely explanation for the tCB neurons' temperature sensitivity is that individual cells in the tCB ensemble are downstream of other sensory neurons, supported by (1) extended latencies between thermal stimulus and tCB firing, (2) hypostome and tentacle localization of Hydra homologs to known thermosensitive channel genes, and (3) comparison of tCB neurons' morphology against that of known sensory neurons. The presented data are likewise consistent with a hypothesis that synchrony arises from gap junction coupling between radially symmetric neurons of Hydra's peduncle. As initial support, Hydra's genome contains gap junction proteins enriched in the peduncle (Alexopoulos et al., 2004; Chapman et al., 2010). For example, imaging data have validated the peduncle localization of the gap junction protein innexin-2, which is required for the normal synchronous activity of neurons that coordinate contraction bursts (Wolf et al., 2014). Similar gap junction proteins could likewise be responsible for the coordinated activity of tCB neurons, providing directions for future studies probing the circuit architecture of Hydra's thermosensory response.
Discussion
Unlike many invertebrate model systems like C. elegans and Drosophila that have stereotyped neural architectures with well-defined numbers of neurons, Hydra's highly plastic nervous system, in conjunction with recent optical and genetic advances, facilitates studying how behaviors like sensory-motor responses are maintained as the number of neurons change under shifts like naturally-occurring size variation or oral-aboral resectioning. The two-layer microfluidic device shown here is a well-controlled method to study this process because it allows us to simultaneously image neural activity and behavior while we deliver thermal stimuli without mechanical stimulation artifacts. Using this technology, we found that when stimulated with a rapid temperature change, the animal first elongates and then contracts, with the peak body length occurring approximately 20 s after the stimulus onset. This behavioral response to a thermal stimulus is accompanied by synchronous periodic activity in a group of neurons near the animal's aboral end that we refer to as the tCB neurons. The frequency of tCB neuron activity, which we define as calcium spike rate, varies with the absolute temperature of the thermal stimulus, as opposed to relative changes from the baseline culture temperature. This relationship is maintained even if the number of neurons in the animal changes by a factor of 2 either by natural changes to the animal's size or by surgical resection along the oral-aboral axis.
Interestingly, neurons enriched in the peduncle do not express homologs for canonical thermoreceptors, but their large Q10 values (Q10 > 3) indicate a strong thermal dependence in these cells. These data suggest that either the tCB neurons are downstream of other sensory neurons or they express temperature-sensitive ion channels outside of the TRPA and TRPM family homologs that are found in other regions of the animal.
It bears emphasizing that the number of neurons changes naturally with animal size, so one may view the surgical resection and caloric restriction approaches as complementary methods of experimentally modulating the size of the animal and the number of neurons in the nervous system. Thanks to the gradual adaptation enabled by caloric restriction and orientation of oral-aboral resectioning along Hydra's radial axis of symmetry, we expect that these experimental perturbations allow for modulating neuron counts while not eliminating specific cell types. This feature allows us to independently modulate the number of neurons in the nervous system without significantly altering the overall structure of the nervous system. These features of Hydra are not true of other commonly used neuroscience model organisms (e.g., C. elegans, with its stereotyped number of neurons), underscoring Hydra's value in studies on neural structures.
Although the radial symmetry of Hydra may lead one to expect that the surgical resections along the oral-aboral axis would not completely remove any neural structures, it is not yet known if there are neural specializations that could be removed from this manipulation. For example, the closely related cnidarian Clytia hemisphaerica is also radially symmetric, yet calcium imaging has found that spontaneous neural activity is not always radially symmetric (Weissbourd et al., 2021). Likewise, there are well-known left-right asymmetries in bilaterians including neurons that span both hemispheres that are important for mesoscale synchronization in computational simulations (Crick and Koch, 2005; Wang et al., 2017; Choi and Mihalas, 2019). In our experiments, we found no significant functional deficits following the oral-aboral resections suggesting that the neural circuits coordinating the response to thermal stimulation have no significant radial asymmetries in Hydra.
The ability to quantitatively study neural and behavioral activity as a function of nervous system size is one of the intriguing advantages of Hydra as a model organism. One potential future direction is to exploit this animal's ability to grow and shrink based on its environment, to study how organisms can dynamically regulate and reorganize their nervous systems based on satiety and nutrient availability. Hydra holds promise as a model organism for probing links between environmental conditions, internal signaling patterns, and mechanisms of homeostasis/regeneration. As we demonstrate in our work, the biochemical decision-making process of whether and which tissue to expand or sacrifice occurs while maintaining robust function to environmental stimuli. This provides means to repeatedly perturb Hydra's size while studying the accompanying mechanisms for maintaining neural and behavioral homeostasis. With Hydra's evolutionary similarity as the sister group to bilaterians and examples of other key body patterning mechanisms being conserved across evolution (e.g., Hox genes from Drosophila), we are enthusiastic for long-term efforts focused on uncovering Hydra's intercellular signaling and computation mechanisms for adapting the nervous system to changes in body mass, as well as the mechanisms' applicability to broader species.
Future work is needed to confirm the molecular identity of the tCB neurons and understand the molecular mechanisms of the temperature response. Building on the quantitative input-output relationships established here across neural function and behavior, functional calcium imaging with cell type-specific labeling would help to identify molecular markers of the tCB sub-population and demonstrate relationships with previously defined neuron populations (e.g., CB neurons or clusters from single-cell RNA sequencing [scRNA-seq] datasets). The development of tools for cell type-specific labeling based on molecular identity would not only enable the identification of candidate proteins important in tCB neurons' functional activity (e.g., temperature-sensitive channels, gap junction proteins) but also open tremendous possibilities for perturbing tCB neurons' functionality (e.g., knockdown/knockin experiments).
Further work is also needed to fully elucidate the thermosensory response circuit. For example, modulating thermoreceptors with TRP agonists could help reveal their role in the circuit; computational algorithms for single-neuron tracking could enable us to directly measure the activity of neurons throughout Hydra to see which neurons precede or correlate with tCB activity, pointing toward upstream drivers (e.g., sensory neurons expressing TRP family homologs in Hydra's tentacles). Similarly, blocking innexin-2 gap junction coupling could test if gap junctions indeed mediate the synchrony found in tCB activity.
Another remaining direction is how the tCB neurons maintain their thermal response properties in circuits of different sizes. The simplest explanation may be that the temperature sensitivity is a cellular property of specific neurons, such as other sensory neurons or tCB neurons expressing thermosensitive channels other than TRPA or TRPM family members; in this scenario, tCB neurons' activity could be synchronized by innexin-2 gap junction coupling. Alternatively, it is possible that Hydra have homeostatic mechanisms at the level of circuit connectivity to maintain a neural circuit architecture that has the same thermal response even as neurons are added and removed. For instance, Hydra's radial symmetry ensures that no neuronal subtype is completely removed during surgical resectioning along the oral-aboral axis, potentially contributing to robust neural response patterns following this perturbation in the number of neurons. It is also possible that the thermal response circuit is simply not affected by the overall number of neurons in the circuit, provided there is a critical minimum number of each constituent cell type. In principle, patch-clamp electrophysiology could provide insights into these possibilities. However, several features of Hydra have resulted in no successful patch-clamp experiments to date: in an intact Hydra, attempts to use an extracellular electrode to record activity from Hydra's peduncle neurons were overwhelmed by electrical signals from accompanying muscle contractions (Dupre and Yuste, 2017), whereas Hydra's flexibility and lack of endoskeleton present difficulties for holding a fixed position with intracellular recording electrodes (Taddei-Ferretti and Musio, 1999). And even for dissociated neurons, the fact that Hydra is a freshwater organism means that patch-clamp techniques must use low-ionic-strength media, which causes difficulty with successfully rupturing cell membranes and obtaining large signal-to-noise ratios (Santillo et al., 1997). As alternate approaches, blocking of cell-cell signaling or alteration of properties of neuronal subsets through cell type-specific promoters could help reveal if the stable behavioral response is a cellular-level or circuit-level property of Hydra.
Overall, Hydra's combination of extreme neural plasticity and promise for genetically targeted manipulations could help evaluate the relative contributions of cellular and ensemble-level properties to the tCB circuit and its stability to perturbations in the number of constituent neurons. Additionally, by studying sensory-motor transformations like this thermal response, we may also learn about fundamental properties of neural circuits that are common to many species. For example, the tCB neurons show characteristics of a neural oscillator, which is a common motif across phylogeny, whereas mechanisms for selectively pruning or expanding the nervous system based on body mass (and environmentally driven conditions like satiety) could provide insights for robustly maintaining neural and behavioral function. These types of cross-species comparative studies could point toward conserved principles of neural circuits that appeared early in our evolutionary history. As described here, the ability to study neural and behavioral responses to thermal stimulation in a highly regenerative animal amenable to cellular-resolution fluorescence imaging offers many advantages as a model system for uncovering how neural circuits remodel without compromising their function.
Limitations of the study
One limitation of this study is that we cannot yet provide the precise molecular identity of the tCB neurons. Based on our calcium imaging data and how the location of the tCB neurons compares to in situ hybridization experiments, we are confident that the population is a subset of the CB neurons. However, Hydra still lacks established neuron-specific promoters, which makes it difficult for us to know the exact cell type. Fortunately, recent scRNA-seq studies in Hydra provide promise for CB neuron-specific markers (Siebert et al., 2019; Klimovich et al., 2020) suggesting that more accurate cell type identification will be possible in the near future. These new molecular techniques, combined with computational algorithms for single-neuron tracking, would enable future experiments to identify the role of each cell type in the types of sensory-motor behaviors studied here.
Resource availability
Lead contact
Information and requests for resources should be directed to and will be fulfilled by the lead contact, Jacob T. Robinson (jtrobinson@rice.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
All data and code are available from the lead contact upon request.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
General: We would like to thank Dr. Christophe Dupre and Dr. Rafael Yuste (Columbia University), Dr. Celina Juliano (UC Davis) and Dr. Robert Steele (UC Irvine) for sharing transgenic Hydra lines, and Abby Primack (UC Davis) and Dr. Juliano for comments on the manuscript. Funding: This work was supported in part by the NSF IOS-1829158 and National Institutes of Health in the United States R21AG067034. C.N.T. is funded by a Fannie and John Hertz Foundation Fellowship and by a National Science Foundation Graduate Research Fellowship (1122374). K.N.B. is funded by training fellowships from the Keck Center of the Gulf Coast Consortia on the NSF Integrative Graduate Education and Research Traineeship (IGERT): Neuroengineering from Cells to Systems 1250104.
Author contributions
C.N.T. and S.K. performed and analyzed Hydra thermal stimulation experiments. K.N.B. performed and analyzed longitudinal imaging experiments on Hydra's number of neurons as a function of body size. C.N.T., K.N.B., and B.W.A. designed, prototyped, and fabricated the microfluidic device. C.N.T., S.K., and B.W.A. implemented microcontroller automation of thermal stimulation experiments. C.N.T., J.T.R. supervised the research. C.N.T., S.K., K.N.B., and J.T.R. co-wrote the paper. All authors read and commented on the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102490.
Supplemental information
References
- Alexopoulos H., Böttger A., Fischer S., Levin A., Wolf A., Fujisawa T., Hayakawa S., Gojobori T., Davies J.A., David C.N. Evolution of gap junctions: the missing link? Curr. Biol. 2004;14:R879–R880. doi: 10.1016/j.cub.2004.09.067. [DOI] [PubMed] [Google Scholar]
- Alwes F., Enjolras C., Averof M. Live imaging reveals the progenitors and cell dynamics of limb regeneration. eLife. 2016;5:e19766. doi: 10.7554/eLife.19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz D., Leyssen M., Koch M., Yan J., Srahna M., Sheeba V., Fogle K.J., Holmes T.C., Hassan B.A. Axonal injury and regeneration in the adult brain of Drosophila. J. Neurosci. 2008;28:6010–6021. doi: 10.1523/JNEUROSCI.0101-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann A. Molecular factors of nematocyst morphogenesis and discharge in the freshwater polyp Hydra. 2013. [DOI]
- Binda F., Bossi E., Giovannardi S., Forlani G., Peres A. Temperature effects on the presteady-state and transport-associated currents of GABA cotransporter rGAT1. FEBS Lett. 2002;512:303–307. doi: 10.1016/s0014-5793(02)02271-8. [DOI] [PubMed] [Google Scholar]
- Bode H., Berking S, David C.N., Gierer A., Schaller H., Trenkner E. Quantitative analysis of cell types during growth and morphogenesis in Hydra. Wilhelm Roux Arch. Entwickl. Mech. Org. 1973;171:269–285. doi: 10.1007/BF00577725. [DOI] [PubMed] [Google Scholar]
- Bode H.R., Heimfeld S., Koizumi O., Llttlefield C.L., Yaross M.S. Maintenance and regeneration of the nerve net in Hydra. Am. Zool. 1988;28:1053–1063. [Google Scholar]
- Bosch T.C., Krylow S.M., Bode H.R., Steele R.E. Thermotolerance and synthesis of heat shock proteins: these responses are present in Hydra attenuata but absent in Hydra oligactis. Proc. Natl. Acad. Sci. U S A. 1988;85:7927–7931. doi: 10.1073/pnas.85.21.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R.D. Tissue dynamics of steady state growth in Hydra littoralis. II. Patterns of tissue movement. J. Morphol. 1967;121:19–28. doi: 10.1002/jmor.1051210103. [DOI] [PubMed] [Google Scholar]
- Campbell R.D. Taxonomy of the European Hydra (Cnidaria: Hydrozoa): a re-examination of its history with emphasis on the species H. vulgaris Pallas, H. attenuata Pallas and H. circumcincta Schulze. Zool. J. Linn. Soc. 2008;95:219–244. [Google Scholar]
- Chapman J.A., Kirkness E.F., Simakov O., Hampson S.E., Mitros T., Weinmaier T., Rattei T., Balasubramanian P.G., Borman J., Busam D. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H., Mihalas S. ‘Synchronization dependent on spatial structures of a mesoscopic whole-brain network. PLoS Comput. Biol. 2019;15:e1006978. doi: 10.1371/journal.pcbi.1006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F.C., Koch C. What is the function of the claustrum? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C.N. A quantitative method for maceration of hydra tissue. Wilhelm Roux Arch. Entwickl. Mech. Org. 1973;171:259–268. doi: 10.1007/BF00577724. [DOI] [PubMed] [Google Scholar]
- David C.N., Murphy S. Characterization of interstitial stem cells in hydra by cloning. Dev. Biol. 1977;58:372–383. doi: 10.1016/0012-1606(77)90098-7. [DOI] [PubMed] [Google Scholar]
- Dupre C., Yuste R. Non-overlapping neural networks in Hydra vulgaris. Curr. Biol. 2017;27:1085–1097. doi: 10.1016/j.cub.2017.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman J.S., Jackson D.C. Thermal response patterns of septal and preoptic neurons in cats. Exp. Neurol. 1967;19:33–45. doi: 10.1016/0014-4886(67)90005-2. [DOI] [PubMed] [Google Scholar]
- Epp L., Tardent P. The distribution of nerve cells inHydra attenuata Pall. Wilehm Roux Arch. Dev. Biol. 1978;185:185–193. doi: 10.1007/BF00848677. [DOI] [PubMed] [Google Scholar]
- Hammarlund M., Jin Y. Axon regeneration in C. elegans. Curr. Opin. Neurobiol. 2014;27:199–207. doi: 10.1016/j.conb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Taralova E., Dupre C., Yuste R. Comprehensive machine learning analysis of Hydra behavior reveals a stable basal behavioral repertoire. eLife. 2018;7 doi: 10.7554/eLife.32605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itayama T., Sawada Y. Development of electrical activity in regenerating aggregates of hydra cells. J. Exp. Zool. 1995;273:519–526. doi: 10.1002/jez.1402730608. [DOI] [PubMed] [Google Scholar]
- Ito E., Ikemoto Y., Yoshioka T. Thermodynamic implications of high Q 10 of thermo-TRP channels in living cells. Biophysics (Nagoya-shi) 2015;11:33–38. doi: 10.2142/biophysics.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javois L.C., Bode P.M., Bode H.R. Patterning of the head in hydra as visualized by a monoclonal antibody: II. The initiation and localization of head structures in regenerating pieces of tissue. Dev. Biol. 1988;129:390–399. doi: 10.1016/0012-1606(88)90386-7. [DOI] [PubMed] [Google Scholar]
- Joven A., Elewa A., Simon A. Model systems for regeneration: salamanders. Development. 2019;146:dev167700. doi: 10.1242/dev.167700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C.E., Lin H., Steele R.E. Generation of transgenic Hydra by embryo microinjection. J. Vis. Exp. 2014:e51888. doi: 10.3791/51888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepner W.A., Hopkins D.L. Reactions of Hydra to chloretone. J. Exp. Zool. 1924;38:437–448. [Google Scholar]
- Khetan N., Maheshwari S., Athale C.A. Quantitative detachment mechanics of Hydra from substrates. Proc. Indian Natl. Sci. Acad. 2018;99 [Google Scholar]
- Klimovich A., Giacomello S., Björklund Å., Faure L., Kaucka M., Giez C., Murillo-Rincon A.P., Matt A.S., Willoweit-Ohl D., Crupi G. Prototypical pacemaker neurons interact with the resident microbiota. Proc. Natl. Acad. Sci. U S A. 2020;117:17854–17863. doi: 10.1073/pnas.1920469117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi O., Heimfeld S., Bode H.R. Plasticity in the nervous system of adult hydra. II. Conversion of ganglion cells of the body column into epidermal sensory cells of the hypostome. Dev. Biol. 1988;129:358–371. doi: 10.1016/0012-1606(88)90383-1. [DOI] [PubMed] [Google Scholar]
- Malafoglia V., Traversetti L., Del Grosso F., Scalici M., Lauro F., Russo V., Persichini T., Salvemini D., Mollace V., Fini M. Transient receptor potential melastatin-3 (TRPM3) mediates nociceptive-like responses in Hydra vulgaris. PLoS ONE. 2016;11:1–15. doi: 10.1371/journal.pone.0151386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massobrio P., Tessadori J., Chiappalone M., Ghirardi M.I. In vitro studies of neuronal networks and synaptic plasticity in invertebrates and in mammals using multielectrode arrays. Neural Plast. 2015;2015:1–18. doi: 10.1155/2015/196195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast S.O. Reactions to temperature changes in spirillum, Hydra, and fresh-water planarians. Am. J. Physiol. 1903;10:165–190. [Google Scholar]
- McLaughlin S. Evidence that polycystins are involved in Hydra cnidocyte discharge. Invert. Neurosci. 2017;17:1. doi: 10.1007/s10158-016-0194-3. [DOI] [PubMed] [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Nath T., Mathis A., Chen A.C., Patel A., Bethge M., Mathis M.W. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 2019;14:2152–2176. doi: 10.1038/s41596-019-0176-0. [DOI] [PubMed] [Google Scholar]
- Noro Y., Yum S., Nishimiya-Fujisawa C., Busse C., Shimizu H., Mineta K., Zhang X., Holstein T.W., David C.N., Gojobori T., Fujisawa T. Regionalized nervous system in Hydra and the mechanism of its development. Gene Expr. Patterns. 2019;31:42–59. doi: 10.1016/j.gep.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Otto J.J., Campbell R.D. Tissue economics of hydra. Regulation of cell cycle, animal size and development by controlled feeding rates. J. Cell Sci. 1977;8:117–132. doi: 10.1242/jcs.28.1.117. [DOI] [PubMed] [Google Scholar]
- Passano L.M., McCullough C.B. Pacemaker hierarchies controlling the behaviour of hydras. Nature. 1963;199:1174–1175. doi: 10.1038/1991174a0. [DOI] [PubMed] [Google Scholar]
- Passano L.M., McCullough C.B. Co-ordinating systems and behaviour in Hydra: I. Pacemaker system of the periodic contractions. J. Exp. Biol. 1964;41:643–664. [Google Scholar]
- Peng G., Shi X., Kadowaki T. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 2015;84:145–157. doi: 10.1016/j.ympev.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Ramot D., MacInnis B.L., Goodman M.B. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 2008;11:908–915. doi: 10.1038/nn.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemschied F.A., Eberhard M.J., Schleimer J.H., Ronacher B., Schreiber S. Cell-intrinsic mechanisms of temperature compensation in a grasshopper sensory receptor neuron. In: Calabrese R.L., editor. Vol. 3. eLife Sciences Publications, Ltd; 2014. p. e02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushforth N.B. Behavioral and electrophysiological studies of Hydra. I. Analysis of contraction pulse patterns. Biol. Bull. 1971;140:255–273. doi: 10.2307/1540284. [DOI] [PubMed] [Google Scholar]
- Rushforth N.B., Burnett A.L., Maynard R. Behavior in hydra: contraction responses of hydra pirardi to mechanical and light stimuli. Science. 1963;139:760–761. [Google Scholar]
- Santillo S., Taddei-Ferretti C., Nobile M. ‘Resting potentials recorded in the whole-cell configuration from epithelial cells of Hydra vulgaris’. Ital. J. Zool. 1997;64:7–11. [Google Scholar]
- Schroeder L.A., Callaghan W.M. Thermal tolerance and acclimation of two species of Hydra1. Limnol. Oceanogr. 1981;26:690–696. [Google Scholar]
- Scimone M.L., Cote L.E., Reddien P.W. Orthogonal muscle fibres have different instructive roles in planarian regeneration. Nature. 2017;551:623. doi: 10.1038/nature24660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Sawada Y., Sugiyama T. Minimum tissue size required for Hydra regeneration. Dev. Biol. 1993;155:287–296. doi: 10.1006/dbio.1993.1028. [DOI] [PubMed] [Google Scholar]
- Siebert S., Farrell J.A., Cazet J.F., Abeykoon Y., Primack A.S., Schnitzler C.E., Juliano C.E. Stem cell differentiation trajectories in Hydra resolved at single-cell resolution. Science. 2019;365:eaav9314. doi: 10.1126/science.aav9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Ori-McKenney K.M., Zheng Y., Han C., Jan L.Y., Jan Y.N. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 2012;26:1612–1625. doi: 10.1101/gad.193243.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski J.R., Yuste R. Mapping the whole-body muscle activity of Hydra vulgaris. Curr. Biol. 2019;29:1807–1817.e3. doi: 10.1016/j.cub.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Taddei-Ferretti C., Musio C. Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 1999. The neural net of hydra and the modulation of its periodic activity; pp. 123–137. [Google Scholar]
- Tatavarty V., Torrado Pacheco A., Groves Kuhnle C., Lin H., Koundinya P., Miska N.J., Hengen K.B., Wagner F.F., Van Hooser S.D., Turrigiano G.G. Autism-associated Shank3 is essential for homeostatic compensation in rodent V1. Neuron. 2020;106:769–777.e4. doi: 10.1016/j.neuron.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau U., Cramer von Laue C., Rentzsch F., Luft S., Hobmayer B., Bode H.R., Holstein T.W. Parameters of self-organization in Hydra aggregates. Proc. Natl. Acad. Sci. U S A. 2000;97:12127–12131. doi: 10.1073/pnas.97.22.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G.G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Ng L., Harris J.A., Feng D., Li Y., Royall J.J., Oh S.W., Bernard A., Sunkin S.M., Koch C., Zeng H. Organization of the connections between claustrum and cortex in the mouse. J. Comp. Neurol. 2017;525:1317–1346. doi: 10.1002/cne.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Steele R.E., Collins E.-M.S. Wnt signaling determines body axis polarity in regenerating Hydra tissue fragments. Dev. Biol. 2020;467:88–94. doi: 10.1016/j.ydbio.2020.08.012. [DOI] [PubMed] [Google Scholar]
- Weissbourd B., Momose T., Nair A., Kennedy A., Hunt B., Anderson D. Functional modules within a distributed neural network control feeding in a model medusa. bioRxiv. 2021 [Google Scholar]
- Wittlieb J., Khalturin K., Lohmann J.U., Anton-Erxleben F., Bosch T.C. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc. Natl. Acad. Sci. U S A. 2006;103:6208–6211. doi: 10.1073/pnas.0510163103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A., Hwang J.S., Wolf A., Böttger A., Shimizu H., David C.N., Gojobori T.I. Innexin gap junctions in nerve cells coordinate spontaneous contractile behavior in Hydra polyps. Sci. Rep. 2014;4:1–8. doi: 10.1038/srep03573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Ghosh-Roy A., Yanik M.F., Zhang J.Z., Jin Y., Chisholm A.D. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc. Natl. Acad. Sci. U S A. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto W., Yuste R. Whole-body imaging of neural and muscle activity during behavior in Hydra vulgaris: effect of osmolarity on contraction bursts. eNeuro. 2020;7 doi: 10.1523/ENEURO.0539-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik M.F., Cinar H., Cinar H.N., Chisholm A.D., Jin Y., Ben-Yakar A. Functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code are available from the lead contact upon request.