Abstract
Purpose
Influenza-associated pulmonary aspergillosis (IAPA) is a frequent complication in critically ill influenza patients, associated with significant mortality. We investigated whether antifungal prophylaxis reduces the incidence of IAPA.
Methods
We compared 7 days of intravenous posaconazole (POS) prophylaxis with no prophylaxis (standard-of-care only, SOC) in a randomised, open-label, proof-of-concept trial in patients admitted to an intensive care unit (ICU) with respiratory failure due to influenza (ClinicalTrials.gov, NCT03378479). Adult patients with PCR-confirmed influenza were block randomised (1:1) within 10 days of symptoms onset and 48 h of ICU admission. The primary endpoint was the incidence of IAPA during ICU stay in patients who did not have IAPA within 48 h of ICU admission (modified intention-to-treat (MITT) population).
Results
Eighty-eight critically ill influenza patients were randomly allocated to POS or SOC. IAPA occurred in 21 cases (24%), the majority of which (71%, 15/21) were diagnosed within 48 h of ICU admission, excluding them from the MITT population. The incidence of IAPA was not significantly reduced in the POS arm (5.4%, 2/37) compared with SOC (11.1%, 4/36; between-group difference 5.7%; 95% CI − 10.8 to 21.7; p = 0.32). ICU mortality of early IAPA was high (53%), despite rapid antifungal treatment.
Conclusion
The higher than expected incidence of early IAPA precludes any definite conclusion on POS prophylaxis. High mortality of early IAPA, despite timely antifungal therapy, indicates that alternative management strategies are required. After 48 h, still 11% of patients developed IAPA. As these could benefit from prophylaxis, differentiated strategies are likely needed to manage IAPA in the ICU.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-021-06431-0.
Keywords: Aspergillosis, Influenza, Posaconazole, Critical illness, Prophylaxis
Take-home message
| The higher than expected incidence of IAPA at ICU admission (71% of IAPA cases) and the lower than expected incidence in the remaining MITT population precludes any definite conclusions on posaconazole as prophylacticum. Immediate diagnostic fungal assessment upon ICU admission combined with differentiated strategies are likely needed to manage IAPA in the ICU. |
Introduction
Invasive pulmonary aspergillosis (IPA) is a life-threatening fungal infection, typically occurring in severely immunocompromised patients with prolonged, profound neutropenia. In recent years, IPA has been increasingly described in non-traditional risk groups, such as patients in medical intensive care units (ICUs), particularly in the setting of severe viral pneumonia. Recent cohort studies reported influenza-associated pulmonary aspergillosis (IAPA) as a severe secondary infection with poor outcome [1–6]. In a retrospective analysis of 432 influenza patients admitted to Belgian-Dutch ICU facilities, 19% suffered from IAPA, with higher organ support requirements, longer duration of ICU stay and an almost doubled mortality rate compared to critically ill influenza patients without IAPA (51% vs. 28%) [2]. Furthermore, a mortality rate as high as 90% was reported in influenza patients who presented with invasive Aspergillus tracheobronchitis, which may affect up to 30% of IAPA patients [7]. Early diagnosis is often challenging, and management may be complicated by the presence of azole resistance in Aspergillus fumigatus [8]. Given the high incidence and ICU mortality of IAPA, prevention by administering antifungal prophylaxis might be a feasible management strategy. However, no antifungal agents are currently licensed for prophylaxis in patients admitted to the ICU. Posaconazole (POS) was shown to be effective in neutropenic patients with acute myeloid leukaemia and those with graft-versus-host disease following allogeneic haematopoietic stem cell transplantation, lowering the incidence of IPA to below 2% in these high-risk patient groups as well as attributable mortality [9–11]. The favourable safety profile, availability as an intravenous formulation, accumulation in lung tissue, and good residual activity against azole-resistant A. fumigatus, make this drug a suitable candidate to be evaluated as prophylaxis in the setting of severe influenza in ICU [12–15].
However, at present, several critical parameters remain unknown, which preclude performing a large blinded randomised controlled prophylaxis trial. One factor is the time of onset of IPA in patients who present with influenza in the ICU. Although the retrospective cohort studies consistently indicate that IAPA develops early on, with a median of 3 days after influenza diagnosis [2], this estimate is influenced by the timing of diagnostic procedures. Excluding the presence of IAPA is critical as patients with evidence of IAPA require immediate antifungal therapy. A second factor is the duration of antifungal prophylaxis. Influenza infection is likely to be associated with a temporary risk for IPA. Animal models have shown a time-dependent increased risk of bacterial superinfection in influenza, implying a transitory modulation of host immune responses [16]. The associated increased susceptibility to co-infections likely also applies to fungal superinfection [17–19]. A third knowledge gap is the pharmacokinetic profile of POS in critically ill patients, with limited data favouring the use of the intravenous formulation [20, 21]. To gain insight into the abovementioned factors, a proof-of-concept, randomised, open-label clinical pilot trial on POS prophylaxis in critically ill influenza patients was performed.
Methods
Study design
In this prospective, randomised, open-label, proof-of-concept trial, we compared POS prophylaxis vs. no prophylaxis (standard-of-care only, SOC) as management strategies for IAPA in critically ill influenza patients. The trial was conducted in nine centers participating in the Dutch-Belgian Mycosis Study Group, in collaboration with three centers in France (supplementary Table 1), from December 2017 until March 2020. The study protocol was approved by the ethical committee of all participating centers in Belgium and France, and by the independent ethical committee Arnhem-Nijmegen for all five participating Dutch sites (CMO 2018-4041), and written informed consent from each patient or their legal representative was obtained prior to any study procedures. The authors designed the study, gathered the clinical data, and were responsible for the analysis of the data.
Participants
Patients 18 years of age or older were eligible if admitted to a participating ICU due to respiratory distress, with PCR-confirmed influenza within 7 days before or 48 h after ICU admission. To be eligible, influenza-compatible symptoms had to be present for no more than 10 days before ICU admission. Exclusion criteria were pregnancy, expected survival on ICU admission of less than 48 h, mycological evidence for IAPA at study inclusion, active treatment with antifungal agents for IPA, a history of intolerance of or hypersensitivity to azole antifungal agents, a prolonged QTc interval (≥ 500 ms), liver cirrhosis Child–Pugh class C or participation in another interventional clinical trial. Patients were not excluded if they were receiving medications known to interact adversely with POS, yet continuous monitoring for adverse events and therapeutic drug monitoring (TDM) of calcineurin-inhibitors was advised according to the study protocol.
Randomisation and masking
Patients who met eligibility criteria were randomly assigned in a 1:1 ratio to receive either POS prophylaxis or SOC, within 48 h of ICU admission. Randomisation was done by a centralised online randomisation system (Sealed Envelope Ltd, UK), with variable permuted blocks without stratification. Participants, caregivers and study staff were aware of treatment allocation.
Procedures
At study inclusion all patients underwent comprehensive evaluation for the presence of an invasive fungal infection, consisting of bronchoscopy with broncho-alveolar lavage (BAL, if considered safe as judged by the treating physician) for galactomannan (GM) detection, Aspergillus PCR and culture, and serum GM assessment. Per protocol a 48 h window was defined to complete this diagnostic assessment, with a preference for assessment and randomisation on the first day of ICU admission. Throughout the ICU admission period, presence of invasive fungal infection was assessed in both groups by similar diagnostic procedures.
Patients assigned to the prophylaxis arm, received the first dose of POS prophylaxis within 48 h of ICU admission. Prophylaxis consisted of commercially available POS intravenous formulation (Noxafil, MSD), starting with a loading dose of 300 mg twice daily on the day of randomisation (day 1), followed by a once-daily administration of 300 mg from day 2 onwards for a total treatment duration of 7 days (or less, in case of occurrence of a protocol specified endpoint: evidence of invasive fungal infection requiring treatment and/or an adverse event requiring discontinuation of study medication). Treatment was administered by slow infusion over 90 min through central venous access (except for the first dose, where peripheral venous access was permissible). Oseltamivir treatment was permitted in either group, and was started or continued at the discretion of the treating physician.
Although therapeutic and prophylactic intravenous POS doses employed are identical, this azole is currently not indicated as a first-line treatment for IPA [11]. Therefore, when IAPA was diagnosed based on clinical and mycological evidence with radiological abnormalities, targeted antifungal treatment was started and POS prophylaxis was discontinued. The type and duration of targeted systemic antifungal treatment were determined by the treating physician according to national guidelines on the management of IPA.
Clinical data, routine biochemistry, mycological diagnostic testing and antifungal drug use were recorded in electronic case report forms (eCRFs) using Castor EDC (Castor electronic data capture, Amsterdam, The Netherlands). Patients were followed up for 90 days after randomisation.
An independent data review committee, consisting of a clinician (JM) and a microbiologist (JBB), both not involved in patient care of the included patients, with substantial expertise in invasive fungal infections, were blinded to the treatment allocation and reviewed and classified all cases of fungal infection according to the European Organisation for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) [22], modified AspICU algorithm [2], and ICM2020 case definition [23]. If IAPA was diagnosed based on diagnostic work-up performed within 48 h of ICU admission, this was considered as ‘early IAPA’; if first diagnostic evidence was found from day 3 of ICU admission onwards, it is further referred to in this manuscript as ‘late IAPA’. In accordance with modified AspICU criteria, the presence of a single sputum/bronchial aspirate culture positive for Aspergillus species was considered colonization, and thus not a reason for prophylaxis cessation.
Outcomes
The primary endpoint was the incidence of IAPA during ICU stay, as adjudicated by the independent data review panel based on the modified AspICU criteria [2]. Secondary endpoints included the timing of IAPA diagnosis, length of ICU and hospital stay, ICU and hospital mortality and mortality evaluated at 90 days after ICU admission. If patients were discharged before day 90, the local investigator contacted the patient or relatives by telephone to ensure the status of the patient and record 90-day mortality. Adverse events were recorded from randomisation until 90 days thereafter. These events were classified according to the Common Terminology Criteria for Adverse Events of the National Cancer Institute, version 4.0. Reasons for early discontinuation of study medication were recorded.
Statistical analysis
The primary efficacy analysis was based on a one-sided hypothesis test using a modified intention-to-treat (MITT) approach, excluding those patients in whom IAPA was diagnosed based on the mycological work-up performed at study randomisation because in fact IAPA was already present at the day of inclusion in these patients. Within the POS prophylaxis arm of the study, patients who received at least 1 full dose of POS and did not present with early IAPA were included in the MITT population. Sample size calculation was based on the primary efficacy endpoint. IAPA incidence was estimated at 25% and considering an 80% reduction in incidence to 5% in the POS prophylaxis group, and a power of 80%, 47 patients in each study arm were required. To allow for sufficient patient inclusion in the MITT study population, a study enrolment of 110 patients in total was anticipated to be sufficient.
Categorical variables are reported as numbers and percentages, and continuous variables as means ± standard deviation (SD) or medians and interquartile range (IQR), as appropriate. Differences in categorical variables were assessed using the Chi-Square test or the Fisher’s Exact test and for the analysis of continuous variables the Student’s t test or the Mann–Whitney U test was used, as appropriate. Confidence intervals for comparison of proportions are based on the Newcombe-Wilson method. The time to onset of IAPA in the MITT population was evaluated with the use of the Kaplan–Meier analysis and log-rank test, patient data were censored at 90 days after ICU admission. Hazard ratios and 95% confidence intervals (CI) were calculated with the use of log-rank analysis of the effect of POS prophylaxis in the MITT population in this time-to-event analysis. The comparison of the treatment groups with respect to the length of stay (ICU and hospital) was performed using Gray’s test for competing event data. For this analysis, length of stay was defined as the time to discharge alive, while death was considered as a competing event. Significance was defined as p values < 0.05 (two-sided analyses except for the primary endpoint). Statistical analyses were performed with IBM SPSS Statistics for Windows version 26 (IBM Corp., Armonk, NY, USA) and Prism version 8.4.2 (GraphPad Software, San Diego, CA, USA). This study was registered with ClinicalTrials.gov, number NCT03378479 and the protocol can be found in the supplementary materials.
Results
Study population
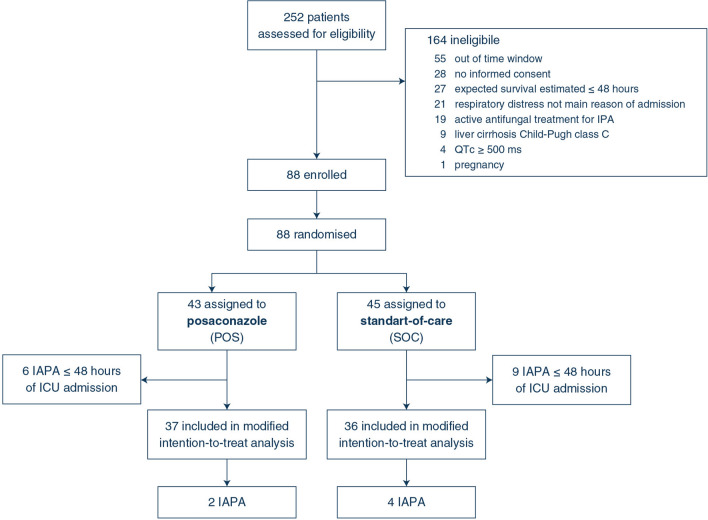
Between December 1, 2017 and March 31, 2020, a total of 252 critically ill influenza patients were screened at 12 intensive care centers in Belgium, The Netherlands and France and 88 patients were found eligible and were randomised (Fig. 1). Baseline diagnostic work-up (serum GM and BAL GM if the bronchoscopic evaluation was feasible) was performed to exclude fungal infection within 48 h of ICU admission. Study medication was initiated before test results were available.
Fig. 1.
Trial profile. ICU intensive care unit, IAPA influenza-associated pulmonary aspergillosis, IPA invasive pulmonary aspergillosis, QTc corrected QT-interval
Based on the results of the baseline diagnostic work-up, 15 patients were diagnosed with IAPA within the first 48 h of ICU admission (early IAPA) and were therefore excluded from the MITT population. Of the 73 patients in the MITT population, 51% (37/73) received POS prophylaxis and 49% (36/73) received SOC (Fig. 1). Both the MITT patient group and the patient group with early IAPA were well balanced in terms of baseline and ICU clinical characteristics (Table 1 and supplementary table 2).
Table 1.
Baseline and ICU characteristics of the modified intention-to-treat population
| Posaconazole prophylaxis (n = 37) |
Standard-of-care (n = 36) |
|
|---|---|---|
| Mean age—years (SD) | 59 (16) | 63 (15) |
| Sex | ||
| Male | 23 (62%) | 18 (50%) |
| Female | 14 (38%) | 18 (50%) |
| BMI > 30 kg/m2 | 14/37 (38%) | 9/35 (26%) |
| Diabetes mellitus | 5 (14%) | 5 (14%) |
| Liver cirrhosis | 0 (0%) | 1 (3%) |
| COPD | 6 (16%) | 8 (22%) |
| EORTC/MSGERC host factor | 6 (16%) | 5 (14%) |
| Haematological malignancy | 3 (8%) | 0 (0%) |
| Solid organ transplant | 0 (0%) | 0 (0%) |
| Solid organ malignancy | 1 (3%) | 4 (11%) |
| Neutropenia | 0 (0%) | 0 (0%) |
| Systemic CS 30 days before ICU admission | 12/36 (33%) | 10/36 (28%) |
| Median dose CS 30 days before ICU admission, mg/kg/day prednisone equivalent (IQR) | 0.07 (0.02–0.18), n = 12 | 0.11 (0.02–0.36), n = 10 |
| Smoking in the past year | 14/31 (45%) | 14/28 (50%) |
| Influenza type | ||
| Influenza A | 27 (73%) | 32 (89%) |
| Influenza B | 10 (27%) | 3 (8%) |
| Influenza A and B | 0 (0%) | 1 (3%) |
| Influenza vaccination status | 5/26 (19%) | 8/23 (35%) |
| Mean APACHE II score on ICU admission (SD) | 20 (8), n = 37 | 19 (7), n = 35 |
| Median days between onset influenza symptoms and ICU admission (IQR) | 3 (3–7), n = 35 | 4 (3–6), n = 33 |
| Median days between hospital and ICU admission (IQR) | 1 (0–3) | 0 (0–2) |
| Ventilatory supporta | 33 (89%) | 34 (94%) |
| Invasive ventilatory support | 21 (57%) | 20 (56%) |
| Median duration of non-invasive ventilation—days (IQR) | 5 (2–8), n = 27 | 3 (1–6), n = 24 |
| Median duration of invasive ventilation—days (IQR) | 14 (8–28), n = 21 | 14 (6–25), n = 20 |
| Nitric oxide inhalation | 5/34 (15%) | 2/36 (6%) |
| Prone ventilation | 8 (22%) | 8 (22%) |
| ECMO | 7 (19%) | 3 (8%) |
| Vasopressor therapy | 25 (68%) | 22 (61%) |
| Renal replacement therapy | 8 (22%) | 4 (11%) |
| Neuraminidase inhibitor treatment | 37 (100%) | 33 (92%) |
| Median duration of NAI treatment—days (IQR) | 7 (5–9), n = 37 | 6 (4–9), n = 32 |
| CS treatment during ICU | 21 (57%) | 20 (56%) |
| Median dose CS during ICU admission, mg/kg/day prednisone equivalent (IQR) | 0.27 (0.08–0.71) n = 21 | 0.42 (0.31–0.87), n = 20 |
Data are n (%) unless otherwise indicated. All p values were > 0.05
APACHE acute physiology and chronic health evaluation, BMI body mass index, BIPAP bilevel positive airway pressure, COPD chronic obstructive pulmonary disease, CPAP continuous positive airway pressure, CS corticosteroids, ECMO extracorporeal membrane oxygenation, EORTC/MSGERC European Organisation for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium, ICU intensive care unit, IQR interquartile range, NAI neuraminidase inhibitor, SD standard deviation
aVentilatory support includes high flow nasal cannula, non-invasive BIPAP/CPAP and invasive mechanical ventilation
Efficacy and safety
Within the MITT population, the incidence of proven and putative IAPA during ICU stay was 5.4% in patients receiving POS prophylaxis (2/37) and 11.1% in patients receiving SOC [4/36, between-group difference 5.7% (95% CI – 10.8 to 21.7); p = 0.32, Table 2]. Although IAPA diagnosis seemed to occur later on during ICU stay in patients receiving POS prophylaxis [10 (IQR 8–12) days vs. 5 (IQR 3–8) days after ICU admission], the hazard ratio was not statistically significant [0.46 (0.09–2.29), p = 0.36] (Fig. 2). Length of ICU and hospital stay were similar in both study arms (Table 2).
Table 2.
Primary and secondary outcome measures of the modified intention-to-treat population
| Posaconazole prophylaxis (n = 37) |
Standard-of-care (n = 36) |
p value | |
|---|---|---|---|
| Primary endpointa | |||
| IAPA incidence during ICU stay | 2 (5.4%) | 4 (11.1%) | 0.32 |
| Between-group difference (95% CI) IAPA incidence | 5.7% (− 10.8 to 21.7) | – | – |
| Secondary endpoints | |||
| Timing IAPA diagnosisb | |||
| Median timing of IAPA diagnosis after ICU admission—days (IQR) | 10 (8–12) | 5 (3–8) | 0.27 |
| Length of stayc | |||
| Median length of ICU stay—days (IQR) | 16 (8–29), n = 30 | 6 (3–12), n = 27 | 0.76 |
| Median length of hospital stay—days (IQR) | 25 (18–45), n = 28 | 12 (9–35), n = 25 | 0.56 |
| Mortalityd | |||
| ICU | 7 (18.9%) | 9 (25.0%) | 0.58 |
| Between group difference (95% CI) ICU mortality | 6.1% (− 14.3 to 26.9) | – | – |
| Hospital | 8 (21.6%) | 10 (27.8%) | 0.60 |
| Between group difference (95% CI) hospital mortality | 6.2% (− 14.8 to 27.6) | – | – |
| 90-day | 9 (24.3%) | 11 (30.6%) | 0.61 |
| Between group difference (95% CI) 90-day mortality | 6.2% (− 15.1 to 8.2) | – | – |
CI confidence interval, IAPA influenza-associated pulmonary aspergillosis, ICU intensive care unit, IQR interquartile range
ap value based on a one-sided Fisher’s Exact test, confidence interval for comparison of proportions based on the Newcombe-Wilson method
bTime to onset of IAPA was evaluated with the use of the Kaplan–Meier analysis and two-sided log-rank test, patient data were censored at 90 days after ICU admission
cLength of stay was defined as the time to discharge alive, while death was considered as a competing event. Median and IQR of days to alive discharge are shown. Two-sided statistical significance testing was performed using Gray’s test for competing event data
dp value based on a two-sided Fisher’s Exact test, confidence interval for comparison of proportions based on the Newcombe-Wilson method
Fig. 2.
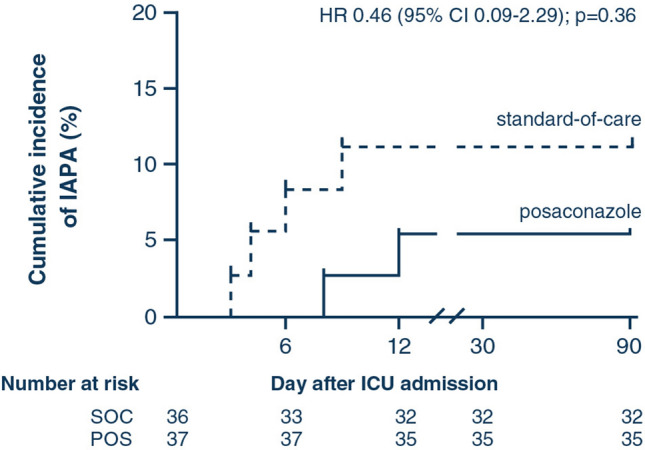
Time to influenza-associated pulmonary aspergillosis. Data derived from modified intention-to-treat population; at 90 days after intensive care admission all patient data were censored. IAPA diagnosis based on modified AspICU criteria. CI confidence interval, HR hazard ratio, IAPA influenza-associated pulmonary aspergillosis, ICU intensive care unit, N° number, POS posaconazole prophylaxis, SOC standard-of-care
In this study, POS prophylaxis was discontinued prematurely in 24% (9/37) of patients, after a mean of 4.6 days of administration (SD 1.2). Reasons for POS prophylaxis discontinuation and adverse events were considered treatment-unrelated and are listed in supplementary table 3. Similar rates of grade 3 liver enzyme elevation [POS prophylaxis 3/37 (8%) vs. SOC 1/36 (3%), p = 0.61] and QTc prolongation [POS prophylaxis 2/37 (5%) vs. SOC 1/36 (3%), p > 0.99] were found in both treatment groups.
Invasive pulmonary aspergillosis
Characteristics of all IAPA cases are summarized in Table 3. Early IAPA was found in 15 cases (71% of all IAPA) and late IAPA occurred in 6 cases (29% of all IAPA). All early IAPA patients required ventilator support, with a median of 12 (IQR 7–22) intubation days and need for ECMO in a third (5/15) of the cases. In early IAPA cases mycological work-up at admission included GM determination in BAL samples more often compared to MITT population (supplementary table 2). BAL GM was positive in 11/14 (79%) early IAPA cases [optical density index (ODI) ≥ 1, median value of positivity 2.9 (IQR 1.4–5.6)] and bronchial aspirate (BA) or BAL culture grew A. fumigatus in 8 early IAPA patients, with 1 patient additionally showing evidence of A. terreus infection. BAL GM was positive in 4/6 (67%) of late IAPA cases and BAL cultures were positive in 2. Positive serum GM was found in 5/13 (38%) early IAPA cases [ODI ≥ 0.5, median value of positivity 0.7 (IQR 0.6–2.3)], none of these positive patients had a host factor as per EORTC/MSGERC criteria (Table 3). Furthermore, IAPA tracheobronchitis, defined as airway plaques in conjunction with hyphae on biopsy or with positive GM in serum or BAL or positive BAL culture, was identified in 4 (27%) early IAPA cases. Antifungal treatment was initiated on average 3 days (SD 2) after ICU admission in early IAPA cases (Table 3). Screening for azole resistance (using broth microdilutation testing with EUCAST methodology and clinical breakpoints [24] or PCR-based detection of TR34/L98H and TR46/Y121F/T289A resistance mechanisms) was performed in nine early IAPA cases, all of which were POS susceptible. Despite early diagnosis and treatment, ICU mortality of patients with early IAPA was 53% (8/15). In the only patient that underwent autopsy, IAPA was confirmed.
Table 3.
Overview of all influenza-associated pulmonary aspergillosis cases
| IAPA; study arm | Age/sex | Underlying risk factor | Serum GM ODI (first; highest) | BAL GM ODI (first; highest) | BAL Aspergillus PCR | Culture (source; isolate) | Azole suscep-tibilitya | Tracheo-bronchitisb | EORTC/MSGERC [22] | Modified AspICU [2] | ICM2020 case definition [23] | Days between ICU admission and diagnosis IAPA | Days between IAPA and start antifungal therapy | Therapy | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Early | 69/F | COPD | 1.13; 1.13 | 2.94; 2.94 | NP | BAL, BA; A. fumigatus, A. terreus | No data | No | NC | IPA | Probable IAPA | 1 | 0 | Vorico (11d) | Died in ICU (12d) |
| 2 | Early | 61/F | COPD | NP; NP | 1.24; 1.24 | Positive | NA | No data | No | NC | IPA | Probable IAPA | 2 | 3 | Vorico (12d) | Died in ICU (18d) |
| 3 | Early | 70/M | / | NP; NP | 6.5; 6.5 | Positive | NA | Susceptible | No | NC | IPA | Probable IAPA | 1 | NA | None | Died in ICU (3d) |
| 4 | Early | 85/M | / | 0.1; 0.1 | 1.3; 1.3 | Negative | NA | No data | No | NC | IPA | Probable IAPA | 1 | 1 | Vorico (43d) | Alive |
| 5 | Early | 50/M | CS before ICU (0.06 mg/kg/day)c | 0; 0.1 | 5.3; 5.6 | Positive | NA | Susceptible | No | NC | IPA | Probable IAPA | 2 | 1 | Vorico and Caspo, Amph B (69d) | Alive |
| 6 | Early | 49/M | / | 0.05; 0.05 | 2.09; 2.09 | Positive | BAL, BA; A. fumigatus | Susceptible | No | NC | IPA | Probable IAPA | 2 | 0 | Vorico (43d) | Alive |
| 7 | Early | 60/F | COPD, CS before ICU (0.22 mg/kg/day)c | 0.7; 0.7 | NP; NP | NP | BAL, BA, sputum; A. fumigatus | Susceptible | No | NC | IPA | Probable IAPA | 1 | 1 | Vorico and Caspo (10d) | Died in ICU (12d) |
| 8 | Early | 51/F | / | 0.3; 0.3 | 2.6; 2.6 | Negative | NA | No data | No | NC | IPA | Probable IAPA | 1 | 0 | POS and Micafun (4d) | Died in ICU (6d) |
| 9 | Early | 63/M | / | 3.5; 3.5 | 8.9; 8.9 | Positive | BAL; A. fumigatus | Susceptible | Yes | NC | IPA | Probable IAPA | 2 | 0 | Vorico and Micafun (2d) | Died in ICU (5d) |
| 10 | Early | 66/F | COPD | 0.6; 0.6 | 0.6; 0.6 | NP | BA, sputum; A. fumigatus | Susceptible | No | NC | IPA | Probable IAPA | 1 | 3 | Vorico and Anidula (10d) | Alive |
| 11 | Early | 60/M | COPD, CS before ICU (dose missing)c | 0.1; 0.2 | 0.2; 0.2 | NP | BAL; A. fumigatus | Susceptible | Yes | NC | IPA | Probable IAPA | 1 | 3 | Vorico and Anidula and nebAmph B (16d) | Alive |
| 12 | Early | 52/M | / | 0.5; 0.5 | 0.1; 0.1 | NP | NA | No data | Yes | NC | IPA | NC | 0 | 2 | Vorico and Anidula, POS and nebAmph B (14d) | Died in hospital (23d) |
| 13 | Early | 60/M | Lung transplant, use of calcineurin inhibitor and CS before ICU (0.14 mg/kg/day)c | 0; 0 | 4.3; 4.3 | Positive | BA; A. fumigatus | Susceptible | Yes | Proven IPA | Proven IPA | Proven IAPA | 2 | 2 | POS (7d) | Died in ICU (12d); autopsy proven |
| 14 | Early | 62/F | / | 0.2; 0.2 | 5.5; 5.5 | NP | NA | No data | No | NC | IPA | Probable IAPA | 0 | 0 | Vorico (97d) | Alive |
| 15 | Early | 39/M | / | 0.04; 0.04 | 1.38; 1.38 | Positive | BAL; A. fumigatus | Susceptible | No | NC | IPA | Probable IAPA | 1 | 4 | Vorico and Caspo (12d) | Died in ICU (17d) |
| 16 | Late POS | 55/F | Undifferentiated autoinflammatory disorder; CS before and during ICU (0.21 and 1.16 mg/kg/day)c | 0.4; 0.4 | 0.4; 1 | Positive | NA | Susceptible | No | NC | IPA | Probable IAPA | 8 | 1 | Vorico, POS (62d) | Alive |
| 17 | Late; POS | 59/M | COPD; CS before and during ICU (0.01 and 0.96 mg/kg/day)c | 0; 0.1 | 0.1; 5.7 | Negative | NA | No data | No | NC | IPA | Probable IAPA | 12 | 1 | Vorico (1d) | Died in ICU (13d) |
| 18 | Late; SOC | 79/M | COPD | 0.32; 0.32 | 0.72; 0.72 | NP | BAL, BA; A. fumigatus | Susceptible | No | NC | IPA | Probable IAPA | 3 | 0 | Vorico (31d) | Died in ICU (37d) |
| 19 | Late; SOC | 51/M | CS during ICU (0.01 mg/kg/day)c | 0.19; 0.19 | 2.14; 2.14 | NP | BAL, sputum; A. fumigatus | Susceptible | No | Proven IPA | Proven IPA | Proven IAPA | 4 | 0 | Vorico, Anidula (28d) | Died in ICU (33d), autopsy proven |
| 20 | Late; SOC | 64/F | Inherited immunodeficiency | 0.1; 0.1 | 0.1; 5.4 | Positive | NA | Susceptible | No | Probable IPA | IPA | Probable IAPA | 6 | 1 | Vorico (23d) | Alive |
| 21 | Late; SOC | 66/M | CS before and during ICU (0.34 and 0.38 mg/kg/day)c | 0.1; 0.1 | 0.2; 0.2 | Positived | NA | Susceptible | No | Proven IPA | Proven IPA | Proven IAPA | 9 | NA | None | Died in ICU (9d), autopsy proven |
All patients presented with at least one clinical sign and radiological criterion according to the modified AspICU algorithm, data not mentioned in table
Amph B amphotericin B, Anidula anidulafungin, BA bronchial aspirate, BAL broncho-alveolar lavage, Caspo caspofungin, COPD chronic obstructive pulmonary disease, CS corticosteroids, d day(s), EORTC/MSGERC European Organisation for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium, F female, GM galactomannan, IAPA influenza-associated pulmonary aspergillosis, IPA invasive pulmonary aspergillosis, M male, Micafun micafungin, NA not applicable, NC not classifiable, nebAmph B nebulized amphotericin B, NP not performed, ODI optical density index, PCR polymerase chain reaction, POS posaconazole, Vorico voriconazole
aAzole susceptibility: based on broth microdilution testing of isolates using EUCAST methodology and clinical breakpoints [24] or on detection of TR34/L98H and TR46/Y121F/T289A resistance mechanisms via PCR
bTracheobronchitis: signs of Aspergillus tracheobronchitis include ulceration(s), nodule(s), pseudomembrane(s), plaque(s) and eschar(s)
cCorticosteroid dose expressed as mean dose in mg/kg/day of prednisone equivalent
dPerformed post-mortem on stored BAL fluid (obtained during ICU admission)
IAPA was diagnosed in two patients who had received POS prophylaxis after completing the full 7-day course, at day 8 and day 12 of ICU admission, respectively. The first case was a patient receiving corticosteroids (at a lower dose than defined as an EORTC/MSGERC host factor criterion) for an auto-inflammatory disorder, who was treated with high dose corticosteroid treatment during ICU stay. The second case had a history of chronic obstructive pulmonary disease (COPD) and received high dose corticosteroid treatment during ICU stay as well (Table 3, case 16 and 17 respectively). Screening for azole resistance was negative. As their ICU stay was 23 and 13 days respectively, these two late IAPA cases did not drive the extended length of stay of the POS prophylaxis arm of the study.
IAPA infection was diagnosed in four patients in the SOC group, one of which was a proven case diagnosed post-mortem. No cases of invasive Aspergillus tracheobronchitis nor GM serum positivity were found in the late IAPA cases (Table 3, supplementary table 2).
In the entire study cohort, IAPA cases had higher ICU mortality than non-IAPA critically ill influenza patients (57% vs. 18%, p = 0.0013; supplementary table 2).
Discussion
We describe the first randomised clinical trial on antifungal prophylaxis in critically ill patients admitted to the ICU with respiratory failure due to influenza. The overall incidence of IAPA in the entire study population was as high as anticipated (24%). However, as 15 of the IAPA cases (15/21, 71%) were diagnosed immediately after ICU admission, they had to be excluded from the MITT population as predefined in the protocol. This resulted in a substantially lower incidence of IAPA in the MITT population and as such underpowered the study. Although the number of IAPA cases in the POS prophylaxis arm was half of that in the SOC arm (5.4% vs. 11.1%), this reduction was not statistically significant. Moreover, POS prophylaxis did not positively impact mortality, the type and median duration of respiratory support or length of stay. In our opinion, the most important and clinically relevant findings of our study were that in 71% of the cases IAPA was present at ICU admission, and that the mortality of early IAPA was 53% despite prompt diagnosis and treatment.
The early IAPA cases demonstrated clues towards a more advanced disease process (positivity of serum GM and presence of tracheobronchitis) compared with the late IAPA cases, whereas the host risk profile was similar in both groups. Although previous cohort studies have indicated IAPA as an early secondary infection after ICU admission [2, 23], our results indicate that the majority of patients may suffer from IAPA at the time of ICU admission, making IAPA a co-infection in the majority of ICU patients with influenza, rather than a secondary infection.
Given the high incidence of IAPA [1, 2, 5, 25], the high proportion of early cases, and high associated mortality, our findings support prompt initiation of empirical antifungal therapy in critically ill influenza patients at ICU admission, and an instant mycological diagnostic work-up within 24–48 h. We realize that this approach is only possible when bronchoscopy is safe and performed on-demand, and in settings where mycological tests are available with short turnaround times. Point-of-care Aspergillus tests, such as lateral flow device assays [26], may facilitate this strategy. If the initial diagnostic work-up is not suggestive for IAPA or when bronchoscopy is not feasible and/or safe, antifungal prophylaxis may be an option as the incidence of late IAPA was still 11%. Further studies are required to determine if such a differentiated approach, e.g. de-escalation for early IAPA and prophylaxis to prevent late IAPA, is successful. However, a very large, pragmatic, randomised clinical trial would be required to demonstrate efficacy, as a 11% incidence in this patient population as observed in our randomised controlled trial would require two groups of 315 patients to have a reasonably powered study, assuming 80% power. Other interventions, such as nebulized antifungal therapy in patients with invasive Aspergillus tracheobronchitis, might also be considered to achieve therapeutic antifungal drug concentrations at the site of infection as soon as possible.
This study has several limitations. Our study was underpowered. However, we decided not to continue patient enrolment in this prophylaxis study for a fourth influenza season because of a number of reasons. The large proportion of patients that were excluded from the study due to early IAPA infection questioned the efficacy of an antifungal prophylaxis strategy initiated at the time of ICU admission. Moreover, it has become clear that the required number of patients to be recruited would be an unfeasible (within current study frame) number of 630 patients, and increasing our sample size would not change the main message of our manuscript.
The limited sample size and absence of correction for multiple testing should be kept in mind in the interpretation of all univariate p values. Additionally, azole susceptibility testing was not part of our study protocol though local resistance rates in part of our study region are high [27, 28]. However, azole resistance testing of A. fumigatus is routinely performed in most centers and posaconazole resistance was not detected in all 14 IAPA cases that were tested. Finally, we diagnosed IAPA using the modified AspICU algorithm as was defined in the study protocol in 2017 [2]. Recently, a novel case definition of IAPA was proposed by a group of experts to facilitate homogeneity in clinical studies and increase the number of classifiable patients [23]. When applying this definition to our patient cohort, all but one putative IAPA case could be classified as probable IAPA. The ICM2020 unclassifiable case showed signs of tracheobronchitis on bronchoscopic evaluation in conjunction with a threshold serum GM-index of 0.5. Treatment for IAPA was administered but the outcome was unfavourable.
In conclusion, the higher than expected incidence of IAPA at ICU admission and the lower than expected incidence in the remaining MITT population of this proof-of-concept trial precludes any definite conclusions on posaconazole as prophylacticum. Given the high incidence and mortality rate of early IAPA, prompt mycological diagnostic work-up of influenza patients who are admitted to the ICU is recommended. The benefit of a differentiated approach, applying immediate antifungal therapy in early IAPA cases and antifungal prophylaxis for the prevention of late IAPA, requires further study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients and their families, and the study teams at all participating centers. We would like to thank the Dutch-Belgian Mycosis Study Group members for their efforts: Bart Rijnders; Paul Verweij; Frank van de Veerdonk; Alexander Schauwvlieghe; Tom Wolfs; Joost Wauters; Katrien Lagrou.
Author contributions
BR, FLvdV, IS, JW, KL, PEV, and RJMB contributed to the concept and the design of the study. All authors participated in data collection. LV, NAFJ, CJ, KL, IS, RVD, FLvdV, PEV, RJMB, and JW analyzed the data. LV and CJ wrote the first draft with input from NAFJ, KL, BR, IS, KT, RVD, PEV, RJMB and JW, and all the authors contributed to and approved the manuscript in its final form.
Funding
MSD (Merck Sharp & Dohme Belgium) sponsored the study medication and provided a restricted grant for study organization. The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. We acknowledge support via IOF (Industrial research fund, KU Leuven) (3M180282) to JW. LV is supported by a Ph.D. fellowship of the Research Foundation—Flanders (FWO; Grant Number 11E9819N) and JW is supported by a FWO Fundamental Clinical Mandate (1833317N). IS is supported by the Clinical Research Fund, University Hospitals Leuven. KT acknowledges a mandate of IOF, KU Leuven (IOFm/05/022). FLvdV was supported by a Vidi grant of the Netherlands Association for Scientific Research, the Europeans Union’s Horizon 2020 research and innovation programme under Grant Agreement No 847507, and the “La Caixa” foundation (ID 100010434).
Data availability
Individual participant data that underlie the results reported in this article are available from the corresponding author upon reasonable request, providing the request meets local ethical and research governance criteria after publication. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants.
Declarations
Conflicts of interest
JBB reports grants from F2G, grants from Gilead Sciences, grants from Thermo Fisher Scientific, outside the submitted work. KL received consultancy fees from MSD, SMB Laboratoires Brussels and Gilead, non-financial support from Pfizer and MSD, received speaker fees from Gilead Sciences, FUJIFILM WAKO and Pfizer and a grant from Thermo Fisher Scientific. VL reports financial support of Pfizer, Fisher Paykel, Gilead Sciences, Alexion and Celgene to her research group, outside the submitted work. JM reports grants, personal fees and non-financial support from Gilead Sciences, MSD and Pfizer; personal fees and non-financial support from Cidara, F2G and Mundipharma; outside the submitted work. AWT reports personal fees and non-financial support from Fisher&Paykel, outside the submitted work. RVD reports non-financial support from Pfizer and Gilead Sciences, outside the submitted work. LV reports non-financial support from Gilead Sciences, outside the submitted work. PEV reports grants from Mundipharma, grants from F2G, grants from Pfizer, grants from Thermofisher, grants from Gilead Sciences, non-financial support from IMMY, grants from Cidara, outside the submitted work. RJMB reports consultancy fees from Mundipharma, Cidara, Amplyx, F2G, Gilead, Pfizer and MSD, speaker fees from Pfizer and Gilead, grants from Pfizer, Gilead and MSD. JW received speakers fee from MSD, Pfizer and Gilead, consultancy fee from Gilead and he obtained investigator-initiated grants from Gilead, Pfizer and MSD.
Ethical approval
All study procedures were performed in compliance with the principles of the Declaration of Helsinki, the principles of good clinical practice and in accordance with all applicable regulatory requirements. The study protocol was approved by the ethical committee of all participating centers in Belgium and France, and by the independent ethical committee Arnhem-Nijmegen for all 5 participating Dutch sites (CMO 2018-4041).
Consent to participate
Written informed consent was obtained from all individual patients or their legal representative.
Footnotes
The members of the Dutch-Belgian Mycosis Study Group are listed in the Acknowledgement Section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lore Vanderbeke and Nico A. F. Janssen contributed equally to the publication. Roger J. M. Brüggemann and Joost Wauters contributed equally to the publication.
Contributor Information
Joost Wauters, Email: joost.wauters@uzleuven.be.
the Dutch-Belgian Mycosis Study Group:
Bart Rijnders, Paul Verweij, Frank van de Veerdonk, Alexander Schauwvlieghe, Tom Wolfs, Joost Wauters, and Katrien Lagrou
References
- 1.van de Veerdonk FL, Kolwijck E, Lestrade PPA, et al. Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017;196:524–527. doi: 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- 2.Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Han X, Li Y, et al. Invasive pulmonary aspergillosis in immunocompetent patients hospitalised with influenza A-related pneumonia: a multicenter retrospective study. BMC Pulm Med. 2020;20:239. doi: 10.1186/s12890-020-01257-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ku Y-H, Chan K-S, Yang C-C, et al. Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J Formos Med Assoc. 2017;116:660–670. doi: 10.1016/J.JFMA.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz IS, Friedman DZP, Zapernick L, et al. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from alberta, canada. Clin Infect Dis. 2020;71:1760–1763. doi: 10.1093/cid/ciaa007. [DOI] [PubMed] [Google Scholar]
- 6.Thevissen K, Jacobs C, Holtappels M, et al. International survey on influenza-associated pulmonary aspergillosis (IAPA) in intensive care units: responses suggest low awareness and potential underdiagnosis outside Europe. Crit Care. 2020;24:84. doi: 10.1186/s13054-020-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyga R, Maizel J, Nseir S, et al. Invasive tracheobronchial aspergillosis in critically ill patients with severe influenza a clinical trial. Am J Respir Crit Care Med. 2020;202:708–716. doi: 10.1164/rccm.201910-1931OC. [DOI] [PubMed] [Google Scholar]
- 8.Verweij PE, Chowdhary A, Melchers WJG, Meis JF. Azole resistance in aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 10.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 11.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl I):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Krekels EHJ, Verweij PE, et al. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs. 2020;80:671–695. doi: 10.1007/s40265-020-01306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seyedmousavi S, Mouton JW, Melchers WJG, Verweij PE. Posaconazole prophylaxis in experimental azole-resistant invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2015;59:1487–1494. doi: 10.1128/AAC.03850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauwvlieghe AFAD, Buil JB, Verweij PE, et al. High-dose posaconazole for azole-resistant aspergillosis and other difficult-to-treat mould infections. Mycoses. 2020;63:122–130. doi: 10.1111/myc.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maertens JA, Rahav G, Lee DG, et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021;397:499–509. doi: 10.1016/S0140-6736(21)00219-1. [DOI] [PubMed] [Google Scholar]
- 16.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 17.Rynda-Apple A, Robinson KM, Alcorn JF. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun. 2015;83:3764–3770. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira LVN, Costa MC, Magalhães TFF, et al. Influenza a virus as a predisposing factor for cryptococcosis. Front Cell Infect Microbiol. 2017;7:419. doi: 10.3389/fcimb.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobin JM, Nickolich KL, Ramanan K, et al. Influenza suppresses neutrophil recruitment to the lung and exacerbates secondary invasive pulmonary aspergillosis. J Immunol. 2020;205:480–488. doi: 10.4049/jimmunol.2000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sime FB, Stuart J, Butler J, et al. Pharmacokinetics of intravenous posaconazole in critically ill patients. Antimicrob Agents Chemother. 2018;62:e00242–e318. doi: 10.1128/AAC.00242-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray J, Campbell L, Rudham S, et al. Posaconazole plasma concentrations in critically ill patients. Ther Drug Monit. 2011;33:387–392. doi: 10.1097/FTD.0b013e31821fb197. [DOI] [PubMed] [Google Scholar]
- 22.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2019;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EUCAST (2018) European Committee on antimicrobial susceptibility testing antifungal agents breakpoint tables for interpretation of MICs version 9.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_9.0_180212.pdf. Accessed 30 Apr 2021
- 25.Rijnders BJA, Schauwvlieghe AFAD, Wauters J. Influenza-associated pulmonary aspergillosis: a local or global lethal combination? Clin Infect Dis. 2020;71:1764–1767. doi: 10.1093/cid/ciaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercier T, Dunbar A, Veldhuizen V, et al. Point of care aspergillus testing in intensive care patients. Crit Care. 2020;24:642. doi: 10.1186/s13054-020-03367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in aspergillus diseases. Antimicrob Agents Chemother. 2015;59:4569–4576. doi: 10.1128/AAC.00233-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lestrade PPA, Buil JB, Van Der Beek MT, et al. Paradoxal trends in azole-resistant Aspergillus fumigatus in a national multicenter surveillance program, the Netherlands, 2013–2018. Emerg Infect Dis. 2020;26:1447–1455. doi: 10.3201/eid2607.200088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article are available from the corresponding author upon reasonable request, providing the request meets local ethical and research governance criteria after publication. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants.