Abstract
Background
The ongoing global pandemic of coronavirus disease 2019 (COVID-19) has resulted in the infection of over 128 million people and has caused over 2.8 million deaths as of April 2021 in more than 220 countries and territories. Currently, there is no effective treatment for COVID-19 to reduce mortality. We investigated the potential anti-coronavirus activities from an oral liquid of traditional medicine, Respiratory Detox Shot (RDS), which contains mostly herbal ingredients traditionally used to manage lung diseases.
Results
Here we report that RDS inhibited the infection of target cells by lenti-SARS-CoV, lenti-SARS-CoV-2, and hybrid alphavirus-SARS-CoV-2 (Ha-CoV-2) pseudoviruses, and by infectious SARS-CoV-2 and derived Ha-CoV-2 variants including B.1.1.7, B.1.351, P.1, B.1.429, B.1.2, B.1.494, B.1.1.207, B.1.258, and B.1.1.298. We further demonstrated that RDS directly inactivates the infectivity of SARS-CoV-2 virus particles. In addition, we found that RDS can also block the infection of target cells by Influenza A virus.
Conclusions
These results suggest that RDS may broadly inhibit the infection of respiratory viruses.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, Antiviral therapy, Respiratory detox shot, Traditional Chinese medicine, SARS-CoV, Influenza A, Ha-CoV-2, SARS-CoV-2 pseudovirus
Background
The ongoing coronavirus disease 2019 (COVID-19) global pandemic has afflicted more than 128 million people in over 220 countries and territories, resulting in more than 2.8 million deaths as of April 2021. Currently, there is no effective treatment for COVID-19 to reduce mortality. The newly emerged viral pathogen causing COVID-19 is the coronavirus SARS-CoV-2 [1], a sister virus of SARS-CoV in the species of Severe acute respiratory syndrome-related coronavirus [2, 3]. Both SARS-CoV and SARS-CoV-2 were first identified in China; SARS-CoV was first identified in Guangdong Province in November 2002 [4–6], and SARS-CoV-2 was first identified in Wuhan in December 2019 [1, 7, 8]. In both coronavirus-caused pandemics, traditional Chinese medicines (TCM) have been widely used in China for the urgent management of coronavirus diseases. For the current COVID-19 pandemic, greater than 85% of SARS-CoV-2 infected patients in China have received TCM treatments of some sort [9, 10]. Whether many of the TCMs used have active anti-coronavirus properties and are clinically effective are important questions that have not been fully answered. Lack of systemic studies, both in vitro and in vivo, have hampered the development and rational use of TCMs as effective therapeutics for the treatment of coronavirus diseases.
To identify potential anti-SARS-CoV-2 activities from traditional herbal medicines, we screened multiple herbal extracts, and discovered anti-SARS-CoV and anti-SARS–CoV-2 activities from an oral liquid, Respiratory Detox Shot (RDS), a commercial food supplement in the United States. RDS is used to manage the general wellness of the human respiratory system, and contains multiple herbal ingredients, such as Panax ginseng and Schizonepeta tenuifolia, that are Chinese herbal medicines traditionally used to manage inflammation and lung diseases [11–13]. Here we report that RDS inhibited the infection of target cells by SARS-CoV, lenti- and hybrid alphavirus-SARS-CoV-2 pseudoviruses, and by infectious wild-type SARS-CoV-2. We further demonstrate that RDS inhibits viral infection by direct inactivation of virions. In addition, we found that RDS potently blocks the infection of Influenza A virus. These results suggest that RDS may broadly inhibit the infection of respiratory viruses.
Results
To discover potential anti-SARS-CoV-2 activities from traditional herbal medicines, we screened extracts from approximately 40 medicinal herbs, using a SARS-CoV-2 S protein pseudotyped lentivirus [14, 15] and human lung A549(ACE2) target cells, in which the human ACE2 gene is over-expressed through lentiviral vector-mediated stable transduction. The lenti-pseudoviruses use either the green fluorescent protein (GFP) or luciferase (Luc) as the reporter, and were validated with a broad-spectrum antiviral entry inhibitor, Arbidol [16], and human antiserum against SARS-CoV-2 (Fig. 1A, C). We were able to detect inhibition of SARS-CoV-2 pseudovirus by Arbidol and the antiserum, while we did not find inhibition from any of the 40 herbal extracts tested even in the presence of high toxicity from some of them (Fig. 1A–C). Nevertheless, given that the lenti-pseudovirus only measures SARS-CoV-2 viral entry, we cannot exclude the possibility that these herbal extracts may inhibit SARS-CoV-2 at post-entry steps.
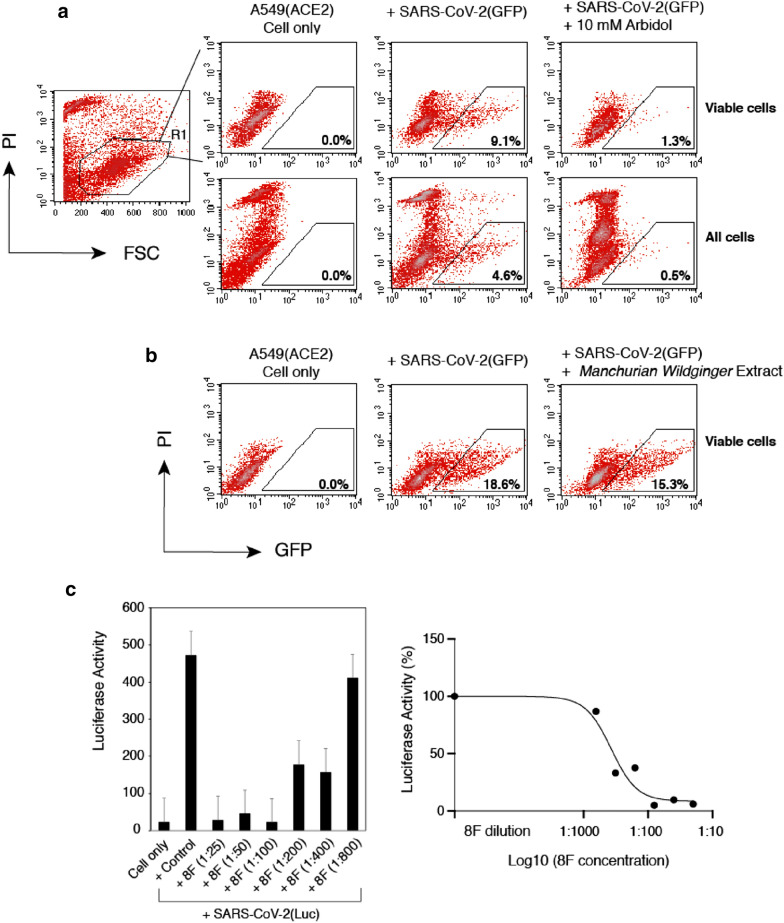
Fig. 1.
Validation of SARS-CoV-2 S protein pseudotyped reporter viruses for the screening and quantification of antiviral drugs and neutralization antibodies. A A lentiviral particle, SARS-CoV-2(GFP) that was pseudotyped with the SARS-CoV-2 S protein, was used to infect A549(ACE2) target cells. GFP was used as the reporter to quantify viral infection, and was measured at 48 h post infection with flow cytometry. Propidium iodide (PI) was added during flow cytometry to stain for dying and dead cells. Arbidol (10 mM) was tested in the system for blocking viral infection. GFP + cells were quantified only in the viable cell population or in the whole cell population. B An example of using SARS-CoV-2(GFP) to screen for TCMs. Extract from Manchurian Wildginger (2 mg/ml) was used to pretreat cells which were infected in the presence of M. Wildginger. After infection, cells were cultured in the absence of M. Wildginger for 48 h. GFP expression was quantified. C A lentiviral particle, SARS-CoV-2(Luc) pseudotyped with the SARS-CoV-2 S protein, was used to infect A549(ACE2) target cells. Luciferease (Luc) was used as the reporter to quantify viral infection. Luc was measured at 72 h post infection. The SARS-CoV-2 neutralizing antiserum 8F was serially diluted and incubated with viral particles for 1 h. The complex was added to infect cells. Luc expression was quantified at 72 h post infection
We further screened possible anti-SARS-CoV-2 activity from an oral liquid of a traditional medicine, Respiratory Detox Shot (RDS), which contains nine ingredients (Lonicera japonica, Forsythia suspensa, Panax ginseng, Schizonepeta tenuifolia, Scrophularia ningpoensis, Prunus armeniaca, Polistes mandarinus saussure, Gleditsia sinensis, Glycyrrhiza uralensis) traditionally used in China to manage lung diseases [11–13]. Lonicera japonica contains methyl caffeate, 3,4-di-O-caffeoylquinic acid, methyl 3,4-di-O-caffeoylquinate, protocatechuic acid, methyl chlorogenic acid, and luteolin; its flow buds also contains loniceracetalides A and B and 10 known iridoid glycosides [17]; the plant also contains saponins loniceroside A and B, and the anti-inflammatory loniceroside C [18, 19]. Forsythia suspensa contains the lignans Pinoresinol and phillyrin [20]. Panax ginseng contains steroid saponins known as ginsenosides that are unique phytochemicals of the Panax species [21, 22]. The main bioactive constituents in Schizonepeta tenuifolia are four monoterpenes, (−)-menthone, (+)-pulegone, (−)-limonene and (+)-menthofuran; the plant also contains other compounds such as 1-octen-3-ol, 3-octanone, β-myrcene, and β-caryophyllene [23]. Scrophularia ningpoensis contains over 162 compounds including iridoids and iridoid glycosides, phenylpropanoid glycosides, organic acids, terpenoids, saccharides, flavonoids, sterols, and saponins [24]. Prunus armeniaca contains phenolic and cyanogenic compounds and pectin polysaccharides [25]. Gleditsia sinensis contains saponins and lupane acid [26, 27], and Glycyrrhiza uralensis contains a major active component, Glycyrrhizin [28].
To test the anti-SARS-CoV-2 activity of RDS, A549(ACE2) cells were pretreated with serially diluted RDS, and then infected in the presence of RDS for 4–6 h. Following infection, cells were cultured in the absence of RDS, and then quantified for the inhibition of viral infection by flow cytometry at 48 and 72 h. To control for cytotoxicity, propidium iodide (PI) was used to stain for dying and dead cells, and GFP + cells were analyzed only in the viable cell population. As shown in Fig. 2, we observed RDS dosage-dependent inhibition of the SARS-CoV-2(GFP) pseudovirus. To confirm these results, we repeated the infection using Vero E6 cells that endogenously express ACE2; Vero E6 supports productive SARS-CoV and SARS-CoV-2 infection and is commonly used in studies of coronaviruses [7]. Given the low infectivity of pseudoviruses for Vero E6 in the absence of ACE2 overexpression [15, 29, 30], we also used a Luc reporter pseudovirus, in which the reporter expression is driven by HIV-1 LTR and Tat for higher reporter sensitivity and signal to noise ratio. As shown in Fig. 3A, using the Luc reporter pseudovirus and Vero E6, we observed RDS dosage-dependent inhibition of infection, and the IC50 (50% inhibition dosage) was determined to be at 1: 230 RDS dilution (Fig. 3B). We also quantified effects of RDS on Vero E6 cell viability, and the LC50 (50% cell death dosage) was determined to be at 1: 11.8 RDS dilution (Fig. 3C). To further validate the results obtained from using pseudoviruses, we tested the ability of RDS to block the infection of infectious SARS-CoV-2. As shown in Fig. 3D, RDS also blocked the infection of Vero E6 cells by SARS-CoV-2. RDS greatly diminished the formation of viral plaques at dosages above 1:40 dilution. Together, the results from SARS-CoV-2 pseudoviruses and infectious virus demonstrated that RDS contains active ingredients inhibting SARS-CoV-2 infection, likely by directly inactivating virons or by blocking viral early infection steps.
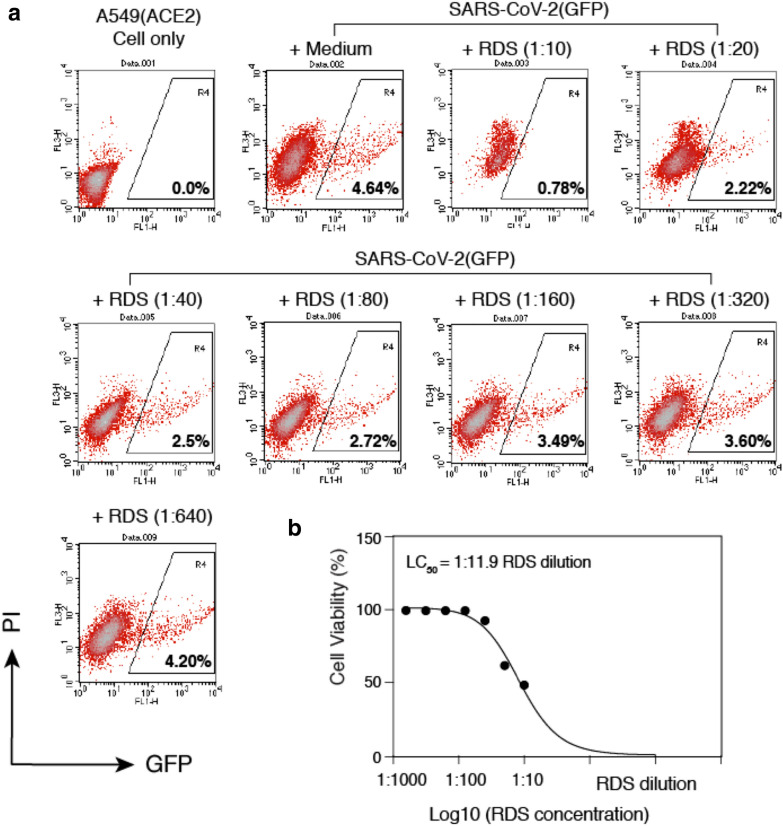
Fig. 2.
RDS inhibits SARS-CoV-2(GFP) pseudovirus infection of A549(ACE2) cells. A A549(ACE2) cells were treated with serially diluted RDS for 30 min, and then infected with SARS-CoV-2(GFP) pseudovirus. Cells were washed to remove the virus and RDS, and cultured in the absence of RDS. Inhibition of viral infection was quantified by flow cytometry. Uninfected cell and SARS-CoV-2(GFP)-infected but RDS-untreated cells were used as controls. The percentages of GFP + cells are shown. PI, propidium iodide. B Quantification of the cytotoxicity of RDS. A549(ACE2) cells were treated with serially diluted RDS for 4 h, washed to remove RDS, and cultured in the absence of RDS for 48 h. Cells were stained with propidium iodide to identify dying and dead cells, and analyzed with flow cytometry. The dose–response cytotoxicity curve was plotted, and the LC50 of RDS was calculated to be at 1:11.9 dilution
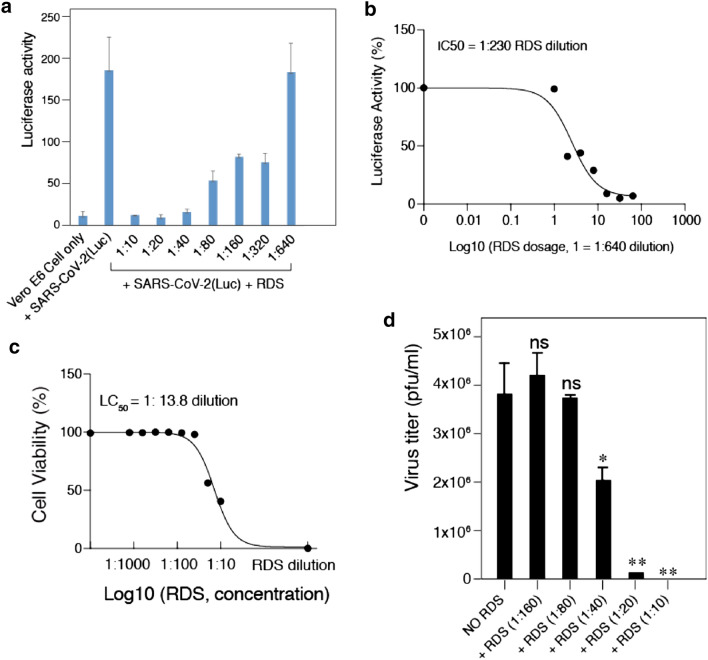
Fig. 3.
RDS dosage-dependent inhibition of SARS-CoV-2(Luc) pseudovirus and wild-type SARS-CoV-2. A and B Vero E6 cells were pretreated with serially diluted RDS, and infected with SARS-CoV-2(Luc) pseudovirus. Cells were washed to remove the virus and RDS, and cultured in the absence of RDS. Inhibition of viral infection was quantified at 72 h post infection by luciferase assay. Uninfected cell and SARS-CoV-2-Luc-infected but RDS-untreated cells were used as controls. The assay was performed in triplicate. The dose–response curve was plotted, and the IC50 of RDS was quantified to be at 1:230 dilution. C The cytotoxicity of RDS on Vero E6 cells was also quantified using propidium iodide staining and flow cytometry. Cells were treated with serially diluted RDS for 4 h, washed to remove RDS, and cultured in the absence of RDS for 72 h. The dose–response cytotoxicity curve was plotted, and the LC50 of RDS was calculated to be at 1:13.8 dilution. D RDS inhibits infectious SARS-CoV-2 infection. Vero E6 cells were pretreated with serially diluted RDS, and infected with SARS-CoV-2 in the presence of RDS. Inhibition of viral replication was quantified by plaque assays of the virus released at 48 h post infection. Inhibition assays were performed in triplicate, and statistical significance was determined using One-Way ANOVA with Dunnett’s Post Test in Prism 7 (Graph Pad). Significance values are indicated using asterisks as follows; *p < 0.02, **p < 0.01. The designation “ns” stands for “not significant”
To further investigate possible mechanisms, we pre-incubated infectious SARS-CoV-2 virus particles with serially diluted RDS for 1 h at 37 °C. Subsequently, the mixture were further serially diluted (10–1 to 10–4), and added to Vero cells for plaque assays to determine reduction of virus infectivity. As shown in Fig. 4A, we observed RDS dosage-dependent reduction of the infectious titer of SARS-CoV-2 with this brief one hour exposure of virus particles to RDS. This result confirmed that RDS can directly inactivate SARS-CoV-2 particle infectivity.
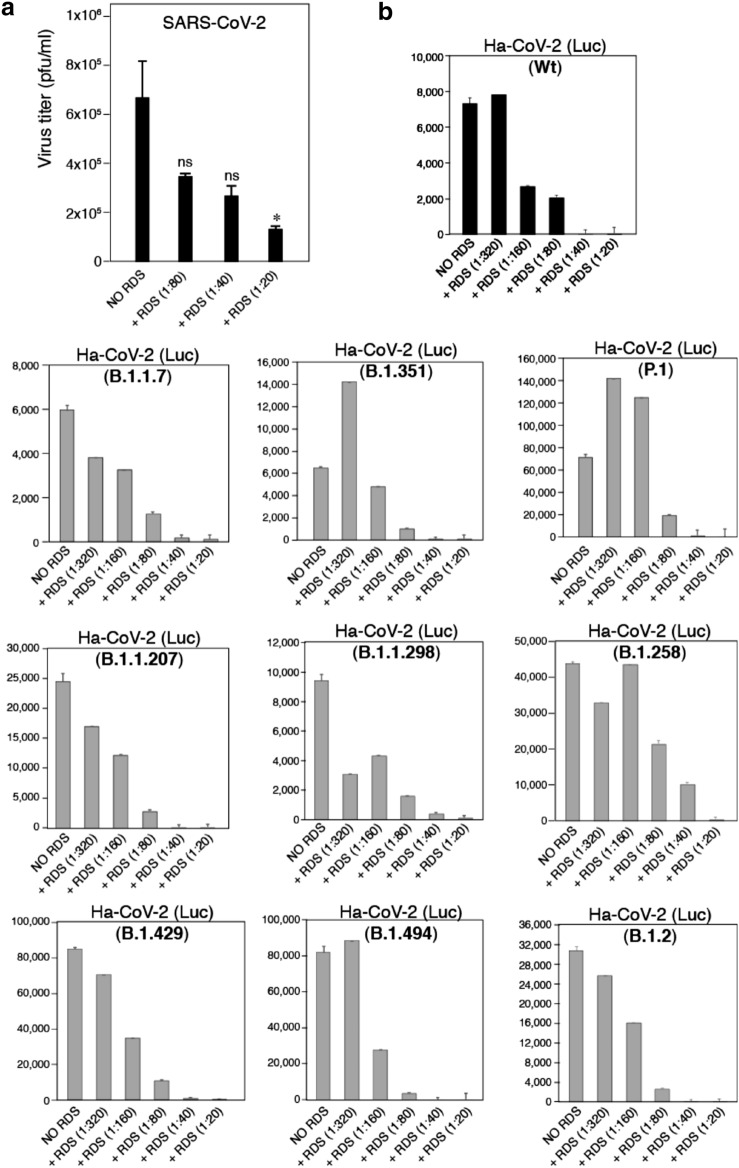
Fig. 4.
RDS dosage-dependent inactivation of the infectivity of SARS-CoV-2 and derived Ha-CoV-2 variants. A SARS-CoV-2 particles were incubated with serially diluted RDS at 37 °C for 1 h. Subsequently, the mixture were further serially diluted and added to Vero cells for plaque assays to determine reduction of virus infectivity. Inhibition assays were performed in triplicate, and statistical significance was determined using One-Way ANOVA with Dunnett’s Post Test in Prism 7 (Graph Pad). Significance values are indicated using asterisks as follows; *p < 0.02. The designation "ns" stands for "not significant". B Ha-CoV-2(Luc) and related S protein variants were incubated with serially diluted RDS at 37 °C for 1 h. Subsequently, the mixture were used to infect HEK293T (ACE2/TMPRESS2) target cells. Inhibition of viral infection was quantified at 12 h post infection by luciferase assay. The IC50 values of RDS were calculated to be at dilutions of 1:177 (wt), 1:828 (B.1.1.7), 1:124 (B.1.351), 1:88 (P.1), 1:134 (B.1.1.207), 1:2601 (B.1.1.298), 1:70 (B.1.258), 1:362 (B.1.429), 1:163 (B.1.494), 1:137 (B.1.2)
We further tested whether RDS can also inhibit the infection of SARS-CoV-2 variants. For this purpose, we took advantage of a recently developed hybrid alphavirus-SARS-CoV-2 pseudovirus (Ha-CoV-2) [31] for the assembly of a series of S protein variants, including the UK variant (B.1.1.7), the South Africa variant (B.1.351), the Brazil variant (P.1), the California variant (B.1.429), and several other emerging variants (B.1.2, B.1.494, B.1.1.207, B.1.258, and B.1.1.298). Ha-CoV-2(Luc) and the related S protein variants were incubated with serially diluted RDS for 1 h at 37 °C. Subsequently, the mixture were used to infect HEK293T (ACE2/TMPRESS2) target cells. Inhibition of viral infection was quantified at 12 h post infection by luciferase assay. As shown in Fig. 4B, we also observed RDS dosage-dependent inhibition of Ha-CoV-2(Luc) and all the S protein variants.
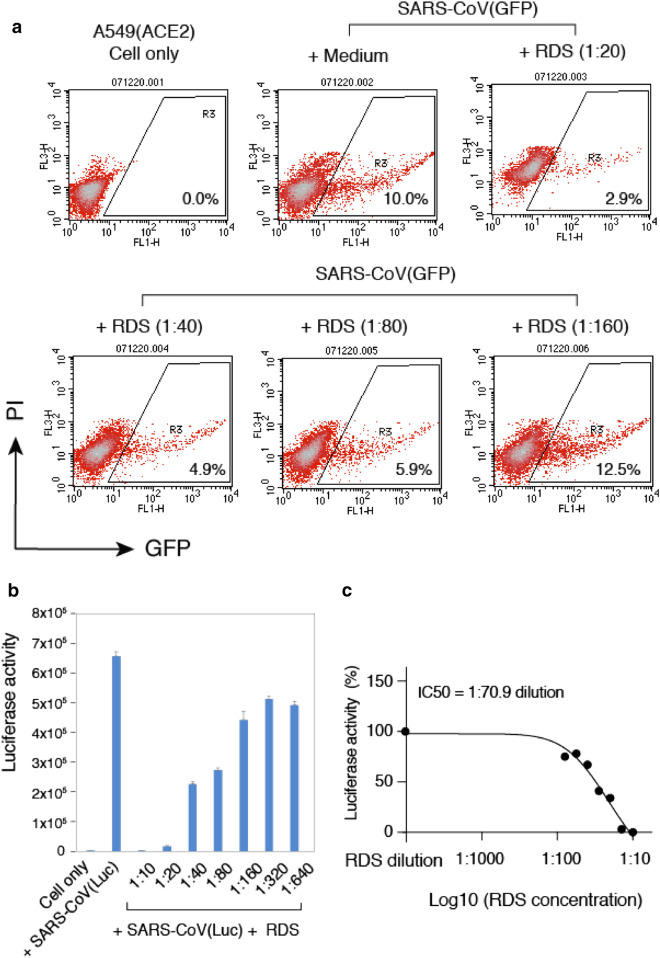
We also tested the ability of RDS to block the infection of SARS-CoV, using a GFP reporter lentivirus pseudotyped with the SARS-CoV spike protein [15]. Human A549(ACE2) cells was used as the target cells, which were pretreated with serially diluted RDS, and then infected with SARS-CoV(GFP) reporter pseudovirus for 4–6 h. Following infection, cells were cultured in the absence of RDS, and then quantified for the inhibition of viral infection by flow cytometry. Similarly, propidium iodide was used to exclude dying and dead cells, and GFP + cells were analyzed only in the viable cell population. As shown in Fig. 5A, we observed RDS dosage-dependent inhibition of SARS-CoV(GFP) pseudovirus. We further confirmed these results and quantified the RDS-mediated inhibition with a Luc reporter SARS-CoV pseudovirus, SARS-CoV(Luc). We observed dosage-dependent RDS inhibition of SARS-CoV(Luc), and the IC50 was determined to be at 1:70.88 RDS dilution (Fig. 5B, C).
Fig. 5.
RDS inhibits SARS-CoV pseudovirus infection of A549(ACE2) cells. A and B Cells were pretreated with serially diluted RDS, and infected with SARS-CoV(GFP) (A) or SARS-CoV(Luc) B pseudovirus. Cells were washed to remove the virus and RDS, and cultured in the absence of RDS. Inhibition of viral infection was quantified at 48 h or 72 h post infection by flow cytometry or luciferase assay. The assay was performed in triplicate. The dose–response curve was plotted, and the IC50 of RDS was determined to be at 1:70.9 dilution (C)
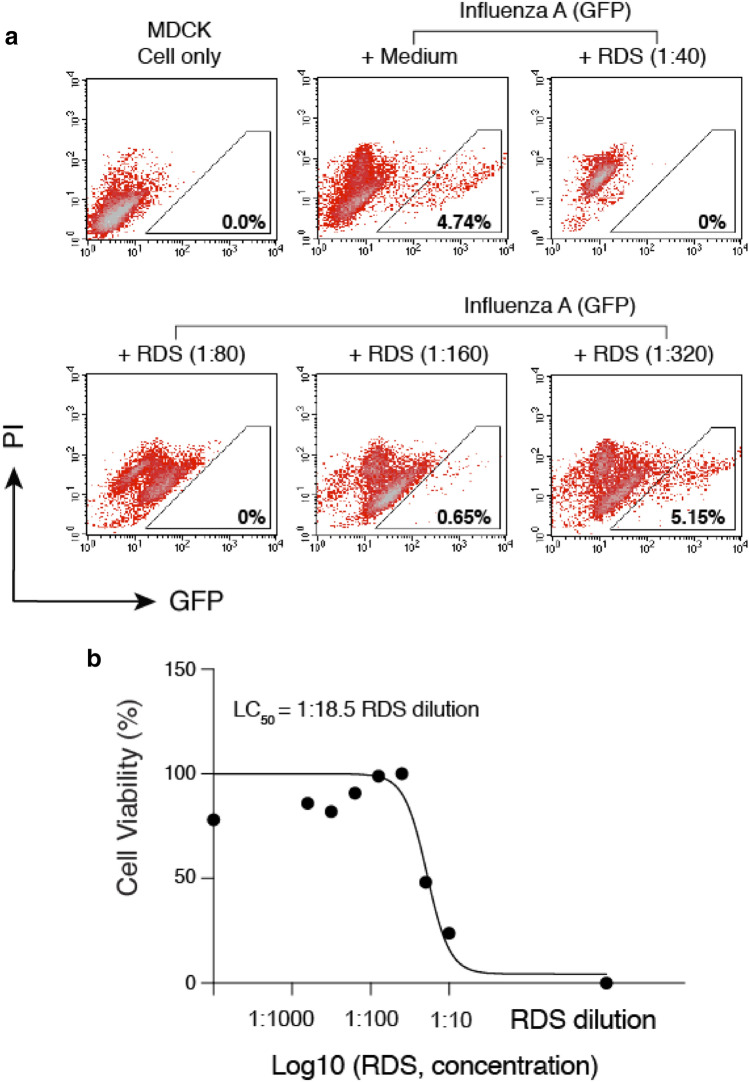
Given that both SARS-CoV and SARS-CoV-2 use ACE2 to infect target cells, we also tested whether the anti-viral activity of RDS is specific to coronaviruses interacting with ACE2. For this purpose, we tested an unrelated, negative-sense RNA virus, Influenza A, which uses viral hemagglutinin (HA) and cellular α-sialic acid to infect target cells. To assemble influenza A virus, eight vectors expressing each of the segments of the influenza A/WSN/33 (H1N1) genome plus a GFP-reporter vector were cotransfected into HEK293T cells. Viral particles were harvested and used to infect target MDCK cells in the presence of RDS. As shown in Fig. 6A, we observed dosage-dependent inhibition of Influenza A virus by RDS. RDS completely blocked viral infection at dilutions of 1:40 and 1:80, and partially inhibited Influenza A at 1:160. The cytotoxicity LC50 of RDS on MDCK cells was determined to be at 1:18.5 dilution (Fig. 6B). These results suggest that the anti-viral activities of RDS are not specific to particular viruses, and may broadly inhibit multiple respiratory viruses such as coronaviruses and Influenza A.
Fig. 6.
RDS inhibits influenza A virus infection of MDCK cells. A MDCK cells were pre-treated with serially diluted RDS for 30 min, and then infected with FluA(GFP) virus. Following infection, cells were cultured in the presence of RDS. Inhibition of viral infection was quantified at 36 h post infection with flow cytometry. Uninfected cell and FluA(GFP)-infected but RDS-untreated cells were used as controls. The percentages of GFP + cells are shown. PI, propidium iodide. B The cytotoxicity of RDS on MDCK cells was also quantified using MTT assay. The dose–response cytotoxicity curve was plotted, and the LC50 of RDS was calculated to be at 1:18.5 dilution
Discussion
In this report, we demonstrate that an oral liquid of a traditional medicine, Respiratory Detox Shot (RDS), contains broad-spectrum antiviral activity, blocking the infection of SARS-CoV, SARS-CoV-2, and Influenza A viruses. While RDS is capable of inhibiting multiple viruses, its antiviral activities vary with virus types and strains. For example, the IC50 for inhibiting lenti-SARS-CoV pseudovirus is at 1:70.9 dilution, and the IC50 for inhibiting lenti-SARS-CoV-2 pseudovirus is at 1:230 dilution. For infectious wild-type viruses, the IC50 of RDS for inhibiting SARS-CoV-2 is 1:40 dilution, and the IC50 for inhibiting influenza A is approximately 1:250 dilution. RDS also inhibited Ha-CoV-2 and its variants differently, with IC50 values varied from 1:70 to 1:2601 dilutions (Fig. 4B).
We further demonstrated that RDS inhibits the early infection steps of coronaviruses. Although the detailed anti-viral mechanisms were not studied, RDS may block viral infection by directly inactivating virions or by blocking viral entry or early post-entry steps. Anti-SARS-CoV and SARS-CoV-2 activities have also been identified in several other traditional Chinese medicines (TCM). For example, a common TCM herbal medicine liquorice root has been shown to contain glycyrrhizin that inhibits the replication of clinical isolates of SARS virus [32]. In addition, another TCM for respiratory diseases, Shuanhuanglian preparation, has been shown to inhibit SARS-CoV-2 3CL protease (3CLpro) activity in vitro in a dose-dependent manner [33]. Baicalin and baicalein were proposed to be the active ingredients of Shuanhuanglian for blocking 3CLpro [33]. The active anti-viral ingredients of RDS have not been identified. However, RDS is different from baicalin and baicalein, as RDS can block viral infection by direct inactivation of virions (Fig. 4), whereas baicalin and baicalein act at a later stage of the viral life cycle by blocking the activity of viral protease. Nevertheless, the in vitro anti-SARS-CoV-2 activity of RDS needs to be confirmed in future animal studies and human clinical trails. Currently, we are conducting small animal studies to determine potential in vivo efficacy of RDS for blocking SARS-CoV-2 infection.
Conclusions
Our studies suggest that RDS may broadly inhibit the infection of respiratory viruses such as SARS-CoV, SARS-CoV-2, and Influenza A.
Methods
Cells and cell culture
HEK293T (ATCC, Manassas, VA), MDCK (ATCC, Manassas, VA), Vero E6 (ATCC, Manassas, VA), and A549(ACE2) (a gift from Virongy, Manassas, VA), and HEK293T(ACE2/TMPRESS2) (a gift from Virongy, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific) containing 10% heat-inactivated FBS and 1× penicillin–streptomycin (Thermo Fisher Scientific). Puromycin and hygromycin B were added to the HEK293T(ACE2/TMPRESS2) cell media at concentrations of 1 µg/ml and 200 µg/ml, respectively.
Plasmid transfection and virus assembly
Lentiviral particles pseudotyped with the SARS-CoV S protein or the SARS-CoV-2 S protein were provided by Virongy LLC (Manassas, VA), or were assembled as previously described [15]. Briefly, for the production of GFP reporter lentiviral pseudovirus, HEK293T cells were cotransfected with the vector expressing the SARS-CoV S protein or the SARS-CoV-2 S protein, pCMVΔR8.2, and pLKO.1-puro-TurboGFP. For the production of luciferase reporter lentiviral pseudovirus, HEK293T cells were co-transfected with the vector expressing the SARS-CoV S protein or the SARS-CoV-2 S protein, pCMVΔR8.2, and pLTR-Tat-IRES-Luc. Virus supernatants were collected at 48 h post transfection, concentrated with centrifugation, and stored at − 80 °C. Wild-type SARS-CoV-2 virus (Isolate USA-WA1/2020) was provided by BEI Bioresources (Manassas, VA). The pHW-NA-GFP (ΔAT6) Reporter plasmid and the A/WSN/1933 H1N1-derived plasmids pHW2000-PB2, pHW2000-PB1, pHW2000-PA, pHW2000-HA, pHW2000-NP, pHW2000-NA, pHW2000-M, and pHW2000-NS were kindly provided by Dr. Feng Li. For influenza A-GFP reporter particle assembly, HEK293T cells were cotransfected with pHW2000-PB2, pHW2000-PB1, pHW2000-PA, pHW2000-HA, pHW2000-NP, pHW2000-NA, pHW2000-M, pHW2000-NS, and pHW-NA-GFP (ΔAT6). Viral supernatants were harvested at 48 h.
The SARS-CoV-2 S, M, E, or N expression vectors were purchased from Sinobiological. The Ha-CoV-2(Luc) vectors and S protein variant vectors were synthesized by Twist Bioscience. Ha-CoV-2(Luc) and S protein variant particles were assembled as previously described [31].
Virus infection and drug inhibition assays
RDS (Respiratory Detox Shot) (a gift from Dejia Harmony, Leesburg, VA) is a commercial product manufactured by Dr. Ma’s Laboratories (Burnaby, BC, Canada). All herbal ingredients in the RDS formula meets “Yin Pian” standard based on the “Chinese Pharmacopeia 2015 edition” which includes active constituents contents and limit tests of heavy metal and pesticide level. RDS is a co-decoction of herbal medicine and the final product was evaporated under vacuum conditions. SARS-CoV-2 antiserum was kindly provided by Dr. Lance A. Liotta. Arbidol-hydrochloride (Sigma) was resuspended in Dimethyl sulfoxide (Sigma). For pseudovirus infection, A549(ACE2) cells (a gift from Virongy LLC, Manassas, VA) or Vero E6 cells in 12-well plates were pre-treated with RDS for 30 min, infected for 4–6 h at 37 °C, and then washed and cultured in fresh medium for 48–72 h. For the infection of Vero E6 cells, cells were also pretreated with CoV-2 Pseudovirus Infection Enhancer (CoV-2 PIE) (a gift from Virongy LLC, Manassas, VA) for another 30 min at 37 °C following pretreatment with RDS. Cell lysates were analyzed for luciferase activity using GloMax Discover Microplate Reader (Promega). For wild-type SARS-CoV-2 infection, Vero E6 cells were pretreated with RDS for 30 min at 37 °C, and then infected with SARS-CoV-2 (Isolate USA-WA1/2020; BEI Bioresources) at MOI of 0.05 for 1 h inside the BSL-3 containment facility at George Mason University. Cells were washed twice with PBS and cultured for 48 h with medium containing RDS. Virus was harvested from the supernatant and the vial titers were determined by plaque assay in Vero cell monolayers grown in 12-well plates. Briefly, serial tenfold dilutions of each sample were prepared in complete Dulbecco's Modified Eagle Medium (VWR) containing 1X Penicilin-Streptomycin (VWR) and supplemented with 10% FBS (Thermo Fisher Scientific). Two hundred microliters of each dilution were then adsorbed onto triplicate wells of Vero E6 cell monolayers for 1 h. The monolayers were then overlaid with 1 to 2 ml of a mixture of 1 part 0.6% agarose (Invitrogen) and 1 part complete Eagle Minimal Essential Medium (VWR) containing 1X Penicillin–Streptomycin and supplemented with 10% FBS. At 48 h, monolayers were fixed in 10% formaldehyde solution for 1 h, and the overlay agar plugs were removed. To stain for plaques, 1% crystal violet dye solution containing 20% ethanol was added for 5 min, followed by washing with deionized water. For influenza A virus infection of MDCK cells, cells were pre-treated with RDS for 30 min at 37 °C, and then infected with influenza A-GFP reporter virus for 6 h. Cells were washed and cultured for 36 h with medium containing RDS. GFP expression was quantified by flow cytometer (FACSCalibur, BD Biosciences).
For RDS inactivation of SARS-CoV-2 viral particle assay, 100 µl of serially diluted RDS was added to 1 ml of SARS-CoV-2 virus stock (3.65 × 105 PFU/ml) for a final RDS dilution of 1:20, 1:40, or 1:80. A control condition (1 ml virus + 100 µl of media only) was also included. The mixtures were incubated at 37 °C for 1 h. Subsequently, serial dilutions of the mixtures were performed to generate additional 1: 10, 1:100, 1:1,000, and 1:10,000 dilutions, and the serially diluted samples were added to Vero cells in 12-well plates for performing plaque assay analysis. The final RDS dilutions in the plaque assays are 1:200 to 1:200,000; 1:400 to 1:400,000; and 1:800 to 1:800,000 dilutions of RDS.
Ha-CoV-2(Luc) and S protein variant particles were assembled as previously described [31]. For RDS inactivation of Ha-CoV-2(Luc), 5 µl of serially diluted RDS was added to 45 µl of Ha-CoV-2(Luc) or variants for a final RDS dilution of 1:20, 1:40, 1:80, 1:160, or 1:320. The mixtures were incubated at 37 °C for 1 h, and subsequently used to infect HEK293T(ACE2/TMPRESS2) cells for 12 h in the presence of RDS. Cell lysates were analyzed for luciferase activity using GloMax Discover Microplate Reader (Promega).
Cytotoxicity assays
Drug cytotoxicity on A549(ACE2) cells and Vero E6 cells were quantify by propidium iodide staining and flow cytometry as described (34). Drug toxicity on MDCK cells was quantified using Cell Proliferation Kit I (MTT) (Sigma) and the protocol suggested by the manufacturer. Briefly, MDCK cells (ATCC) were seeded into a 12 well plate at 1 × 105 cells per well. Cells were cultured overnight, and then treated with RDS for one day, and then cultured in the medium supplemented with MTT labeling reagent (Sigma). Cells were incubated with the labeling reagent for 4 h, followed by the addition of MTT solubilization solution. The plate was incubated overnight, and then the absorbance was measured using GloMax Discover Microplate Reader (Promega).
Acknowledgements
The authors wish to thank Feng Li for providing influenza viral expression vectors, Lance Liotta for providing antiserum; Ted Ci, He Sun, Zhigang Gao, and Wanying Wu for discussions and advices; Kevin Carter, Mark Mamdar, Rich Keurajian, Karen Freidouni for providing RDS and herbal extracts.
Abbreviations
- SARS-CoV
Severe acute respiratory syndrome-related coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome-related coronavirus-2
- TCM
Traditional Chinese medicine
- RDS
Respiratory detox shot
- Ha-CoV-2
Hybrid alphavirus-SARS-CoV-2 pseudovirus
Authors’ contributions
Experiments were designed by YW, RH and LAH. Manuscript was written by YW and edited by LAH. Experiments were performed by BH, DY, AAO, LDC, SH, DD, GA, and YM. All authors have read and approved the final manuscript.
Funding
This work was funded by George Mason University internal grant 223741 (DeJia Harmony/Anti-SARS-CoV-2) provided by Dejia Harmony.
Availability of data and materials
All data generated or analyzed during this study are included in this article. Reagents are available from Y.W. upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
RMH and YW at the National Center for Biodefense and Infectious Diseases, George Mason University have received research grants from Dejie Harmony, and LAH does consultancy for Dejia Harmony and received honorariums; no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, Ho W, Huang Y, Jin D, Li S, Liu S, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395(10228):949–50. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. Nat Microbiol. 2020;5:536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drosten C, Günther S, Preiser W, van der Werf S, Brodt H-R, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 5.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 6.Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–25. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–9. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–17. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling CQ. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) Journal Integr Medicine. 2020;18(2):87–8. doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan M-Q, Qian Y, Yu S, Guo S-C, Zhang L, Ding A-W, et al. Anti-inflammatory effect of volatile oil from Schizonepeta tenuifolia on carrageenin-induced pleurisy in rats and its application to study of appropriate harvesting time coupled with multi-attribute comprehensive index method. J Ethnopharmacol. 2016;194:580–6. doi: 10.1016/j.jep.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Jung ID, Kim HY, Park JW, Lee CM, Noh KT, Kang HK, et al. RG-II from Panax ginseng C.A. Meyer suppresses asthmatic reaction. BMB Reports. 2012;45(2):79–84. doi: 10.5483/BMBRep.2012.45.2.79. [DOI] [PubMed] [Google Scholar]
- 13.Wu W, Li R, Li X, He J, Jiang S, Liu S, et al. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2015;8(1):6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA. 2009;106(14):5871–6. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Waheed AA, Hetrick B, Dabbagh D, Akhrymuk IV, Kehn-Hall K, et al. PSGL-1 inhibits the incorporation of SARS-CoV and SARS-CoV-2 spike glycoproteins into pseudovirus and impairs pseudovirus attachment and infectivity. Viruses. 2021;13(1):46. doi: 10.3390/v13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15(10):997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 17.Kakuda R, Imai M, Yaoita Y, Machida K, Kikuchi M. Secoiridoid glycosides from the flower buds of Lonicera japonica. Phytochemistry. 2000;55(8):879–81. doi: 10.1016/S0031-9422(00)00279-X. [DOI] [PubMed] [Google Scholar]
- 18.Son KH, Jung KY, Chang HW, Kim HP, Kang SS. Triterpenoid saponins from the aerial parts of Lonicera japonica. Phytochemistry. 1994;35(4):1005–8. doi: 10.1016/S0031-9422(00)90656-3. [DOI] [PubMed] [Google Scholar]
- 19.Kwak WJ, Han CK, Chang HW, Kim HP, Kang SS, Son KH. Loniceroside C, an antiinflammatory saponin from Lonicera japonica. Chem Pharm Bull. 2003;51(3):333–5. doi: 10.1248/cpb.51.333. [DOI] [PubMed] [Google Scholar]
- 20.Davin LB, Bedgar DL, Katayama T, Lewis NG. On the stereoselective synthesis of (+)-pinoresinol in Forsythia suspensa from its achiral precursor, coniferyl alcohol. Phytochemistry. 1992;31(11):3869–74. doi: 10.1016/S0031-9422(00)97544-7. [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Woo JY, Han CK, Chang IM. Safety analysis of panax ginseng in randomized clinical trials: a systematic review. Medicines. 2015;2(2):106–26. doi: 10.3390/medicines2020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–93. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 23.Yu S, Chen Y, Zhang L, Shan M, Tang Y, Ding A. Quantitative comparative analysis of the bio-active and toxic constituents of leaves and spikes of Schizonepeta tenuifolia at different harvesting times. Int J Mol Sci. 2011;12(10):6635–44. doi: 10.3390/ijms12106635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren D, Shen Z-y, Qin L-p, Zhu B. Pharmacology, phytochemistry, and traditional uses of Scrophularia ningpoensis Hemsl. J Ethnopharmacol. 2021;269:113688. doi: 10.1016/j.jep.2020.113688. [DOI] [PubMed] [Google Scholar]
- 25.Sefer F, Misirli A, Gülcan R, editors. A RESEARCH ON PHENOLIC AND CYANOGENIC COMPOUNDS IN SWEET AND BITTER KERNELLED APRICOT VARIETIES. 2006: International Society for Horticultural Science (ISHS), Leuven, Belgium.
- 26.Chong W, Feng XY, Zhen GZ, Dan L, Yue D. Inhibition of mast cell degranulation by saponins from Gleditsia sinensis–structure-activity relationships. Nat Prod Commun. 2009;4(6):777–82. [PubMed] [Google Scholar]
- 27.Li WH, Zhang XM, Tian RR, Zheng YT, Zhao WM, Qiu MH. A new anti-HIV lupane acid from Gleditsia sinensis Lam. J Asian Nat Prod Res. 2007;9(6–8):551–5. doi: 10.1080/10286020600883419. [DOI] [PubMed] [Google Scholar]
- 28.Nazari S, Rameshrad M, Hosseinzadeh H. Toxicological effects of Glycyrrhiza glabra (Licorice): a review. Phytother Res. 2017;31(11):1635–50. doi: 10.1002/ptr.5893. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer B, Dabbagh D, Guo J, Kashanchi F, Tyagi M, Wu Y. Tat controls transcriptional persistence of unintegrated HIV genome in primary human macrophages. Virology. 2018;518:241–52. doi: 10.1016/j.virol.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Tang Z, Zheng Y, Yu D, Spear M, Iyer SR, et al. Development of a nonintegrating Rev-dependent lentiviral vector carrying diphtheria toxin A chain and human TRAF6 to target HIV reservoirs. Gene Ther. 2010;17(9):1063–76. doi: 10.1038/gt.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetrick B, He S, Chilin LD, Dabbagh D, Alem F, Narayanan A, et al. Development of a novel hybrid alphavirus-SARS-CoV-2 particle for rapid in vitro screening and quantification of neutralization antibodies, viral variants, and antiviral drugs. bioRxiv. 2020 doi: 10.1101/2020.12.22.423965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–6. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su H-x, Yao S, Zhao W-f, Li M-j, Liu J, Shang W-j, et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacologica Sinica. 2020;41(9):1167–77. doi: 10.1038/s41401-020-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowley LC, Scott AP, Marfell BJ, Boughaba JA, Chojnowski G, Waterhouse NJ. Measuring cell death by Propidium Iodide uptake and flow cytometry. Cold Spring Harb Protoc. 2016 doi: 10.1101/pdb.prot087163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Reagents are available from Y.W. upon request.