Summary
Here, we describe a protocol to construct, calibrate, and operate a versatile tiling light sheet microscope for imaging cleared tissues. The microscope uses adjustable tiling light sheets to achieve higher spatial resolution and better optical sectioning ability than conventional light sheet microscopes and to image cleared tissues with the cellular to the subcellular spatial resolution. It is compatible with all tissue clearing methods and aligned semiautomatically through the phase modulation of the illumination light.
For complete details on the use and execution of this protocol, please refer to Chen et al. (2020).
Subject areas: Microscopy, Neuroscience
Graphical abstract
Highlights
-
•
Protocol for constructing a tiling light sheet microscope
-
•
Calibration and operation of the microscope for imaging cleared tissues
-
•
Method to analyze images captured with the microscope
Here, we describe a protocol to construct, calibrate, and operate a versatile tiling light sheet microscope for imaging cleared tissues. The microscope uses adjustable tiling light sheets to achieve higher spatial resolution and better optical sectioning ability than conventional light sheet microscopes and to image cleared tissues with the cellular to the subcellular spatial resolution. It is compatible with all tissue clearing methods and aligned semiautomatically through the phase modulation of the illumination light.
Before you begin
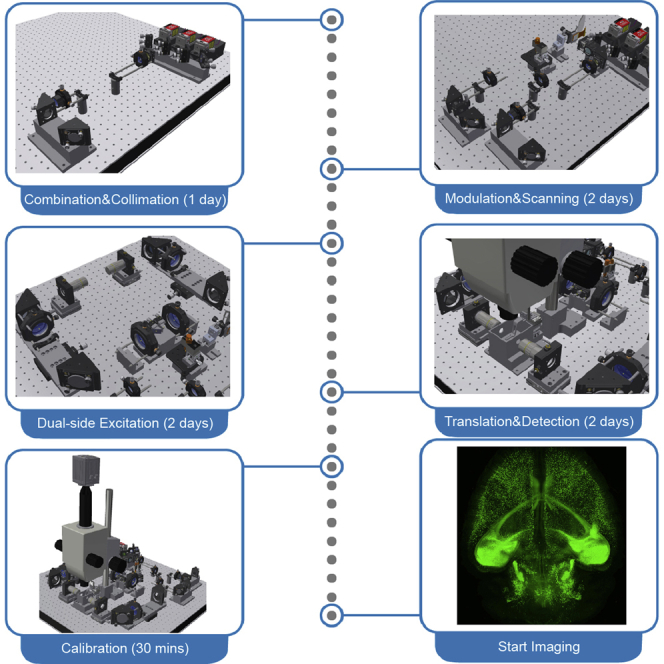
This protocol below describes the construction, calibration, and operation procedures of a tiling light sheet microscope for imaging cleared tissues. A more detailed description of the microscope design principle and working mechanism can be found in the previous publication (Chen et al., 2020). The optical, mechanical, and electrical design of the microscope (Figure 1), the associated technical drawings, and the custom software (Figure 2) are available from Westlake University after the execution of a nondisclosure agreement (NDA). The resources are recommended for general researchers to build the microscope. Researchers with experiences of fluorescence microscope development could construct the microscope and develop similar control software following this protocol and the previous publication without acquiring the resources. The design of the presented tiling light sheet microscope can be modified to satisfy specific requirements without affecting the imaging performance as long as the working mechanism of the microscope remains the same (Chen et al., 2020; Fu et al., 2016; Gao, 2015).
Figure 1.
The design and construction of the presented tiling light sheet microscope
(A) The control system diagram of the microscope.
(B) The optical system diagram of the microscope.
(C) The optomechanical design of the microscope.
(D) The excitation path of the microscope and the associated sub-assemblies.
(E and F) The construction steps of the microscopes. 1, Laser combiner assembly. 2, Laser collimation assembly. 3, PBS assembly. 4, SLM assembly. 5, Optical slit assembly. 6, Galvanometer assembly. 7, Prism mirror assembly. 8, Relay lens assembly. 9, Excitation objective assembly. 10, Imaging chamber assembly. 11, Sample scan assembly. 12, Detection path mounting base.
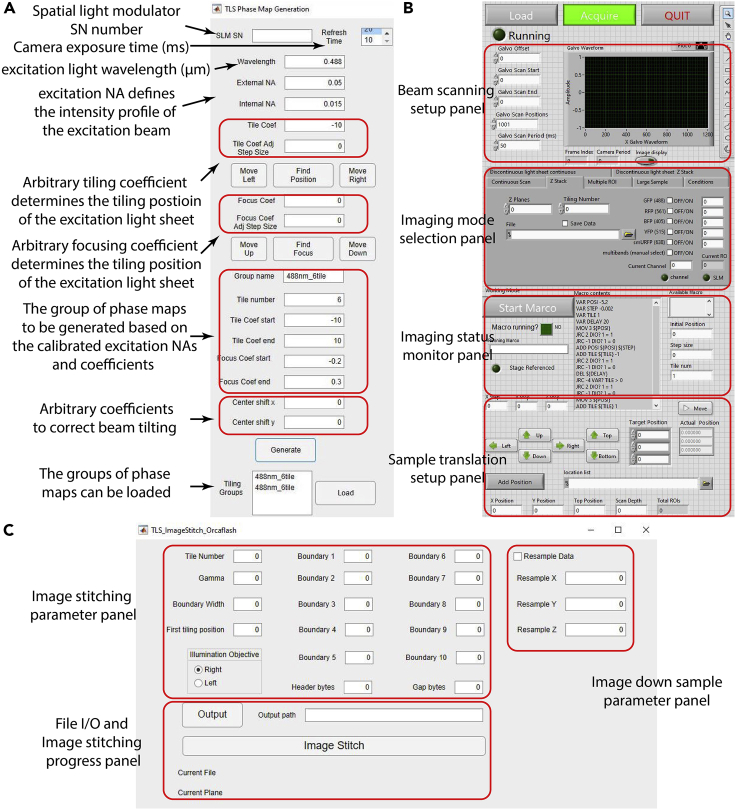
Figure 2.
The custom software
The control panel layout and functions of the custom (A) phase map generation, (B) main control, and (C) image reconstruction software.
Preparation of microscope components
Timing: 1–3 months
-
1.
Acquire all components and software listed in the key resources table.
Preparation of reagents
Timing: 30 mins
-
2.
Prepare 100 mL dye solution of 0.5 μg/ml Alexa Fluor 488 hydrazide sodium salt in water for microscope alignment. The dye solution can be stored at 4°C–25°C and reused as long as the solution is not bleached.
Preparation of cleared tissues
Timing: 2–4 weeks
-
3.
Prepare cleared tissue samples and the corresponding imaging buffers for microscope characterization following previously reported protocols, such as CLARITY, uDISCO, and CUBIC (Chung et al., 2013; Pan et al., 2016; Susaki et al., 2014; Tainaka et al., 2018). Besides the methods mentioned above, the microscope is compatible with all commonly used hydrophobic, hydrophilic, and hydrogel-based tissue clearing methods.
Note: It usually takes 2–4 weeks to prepare a cleared biological tissue sample, such as a cleared whole mouse brain, depending on the type of the tissue and the selected tissue clearing method. Tissues cleared with hydrophobic methods, such as uDISCO, can be easily preserved for several months and reused, so that they are more ideal for the initial characterization of the microscope.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Alexa Fluor 488, Hydrazide, sodium salt | Thermo | A10436 |
| Experimental models: organisms/strains | ||
| Mouse: Thy1-eGFP-M | The Jackson Laboratory | JAX: 007788 |
| Software and algorithms | ||
| LabVIEW 2019 developer suite | National Instruments | https://www.ni.com/en-us/shop/labview.html |
| MATLAB 2019a | MathWorks | https://www.mathworks.com |
| Amira (optional) | Thermo Fisher | https://www.thermofisher.com/us/en/home/industrial/electron-microscopy/electron-microscopy-instruments-workflow-solutions/3d-visualization-analysis-software/amira-life-sciences-biomedical.html |
| Main control software | Westlake University | Lab developed |
| Phase map generation software | Westlake University | Lab developed |
| Image reconstruction software | Westlake University | Lab developed |
| Other | ||
| 405 nm CW Laser (150 mW), qty: 1 | Coherent | OBIS 405 |
| 488 nm CW Laser (150 mW), qty: 1 | Coherent | OBIS 488 |
| 561 nm CW Laser (150 mW), qty: 1 | Coherent | OBIS 561 |
| Spatial light modulator and controller, qty: 1 | Fourth Dimension Displays | SXGA-3DM |
| Galvanometer and controller (6215H, 7 mm, Y axis), qty: 1 | Cambridge Technology | 6SD11490 |
| Linear translational stage and controller, 26 mm distance, qty: 3 | Physik Instruments | Q545.240 |
| ORCA-Fusion Digital CMOS camera and frame grabber, qty: 1 | Hamamatsu | C14440-20UP |
| Filter wheel, qty: 1 | Thorlabs | FW102-C |
| Lens, f=30 mm, Ø=25 mm, qty: 1 | Edmund Optics | 47633 |
| Lens, f=175 mm, Ø =25 mm, qty: 1 | Edmund Optics | 47644 |
| Lens, f=200 mm, Ø =25 mm, qty: 1 | Edmund Optics | 47645 |
| Lens, f=300 mm, Ø =25 mm, qty: 1 | Edmund Optics | 47649 |
| Lens, f=150 mm, Ø =50 mm, qty: 2 | Edmund Optics | 49285 |
| Lens, f=250 mm, Ø =50 mm, qty: 2 | Edmund Optics | 49287 |
| Prism mirror, qty: 2 | Edmund Optics | 87390 |
| Polarizing beam splitter, qty: 1 | Thorlabs | CCM1-PBS251/M |
| 1 inch broadband dielectric mirror, qty: 7 | Thorlabs | BB1-E02 |
| 2 inch broadband dielectric mirror, qty: 4 | Thorlabs | BB2-E02 |
| LaserMUX single-edge laser dichroic beamsplitter, qty: 1 | Semrock | LM01-427-25 |
| LaserMUX single-edge laser dichroic beamsplitter, qty: 1 | Semrock | LM01-503-25 |
| 447/60 nm single-band bandpass filter, qty: 1 | Semrock | FF02-447/60-25 |
| 525/50 nm single-band bandpass filter, qty: 1 | Semrock | FF03-525/50-25 |
| 617/73 nm single-band bandpass filter, qty: 1 | Semrock | FF02-617/73-25 |
| Halfwave plate, qty: 2 | Bolder Vision | AHWP3 |
| Excitation objective, qty: 2 | Mitutoyo | MY5X-802 |
| Detection objective, qty: 1 | Olympus | MVPLAPO 1X |
| Excitation objective (optional), qty: 2 | Mitutoyo | MY10X-803 |
| Detection objective (optional), qty: 1 | Olympus | MVPLAPO 2XC |
| Detection objective (optional), qty: 1 | Olympus | XLSLPLN25XGMP |
| Metric optical table (>0.9 m×0.9 m), qty: 1 | Thorlabs | B9090B |
| Precision kinematic mirror mounts, qty: 3 | Thorlabs | KS1 |
| Threaded precision kinematic mirror mounts, qty: 2 | Thorlabs | KS1T |
| Right-angle kinematic mirror mount 30 mm, qty: 5 | Thorlabs | KCB1/M |
| Right-angle kinematic mirror mount 60 mm, qty: 4 | Thorlabs | KCB2/M |
| Kinematic platform mount, qty: 1 | Thorlabs | KM200B/M |
| Compact kinematic mirror mount, qty: 2 | Thorlabs | KMS/M |
| 1 inch translating lens mount, qty: 4 | Thorlabs | CXY1 |
| 2 inch translating lens mount, qty: 4 | Thorlabs | CXY2 |
| Cage rotation mount, qty: 2 | Thorlabs | CRM1/M |
| Adjustable slit, qty: 1 | Thorlabs | VA100C/M |
| XY dovetail translation stage, qty: 1 | Thorlabs | DT12XY/M |
| XZ dovetail translation stage, qty: 1 | Thorlabs | DT12XZ/M |
| 1 inch translation stage, qty: 2 | Thorlabs | PT1B/M |
| Objective XY translator, qty: 2 | Thorlabs | ST1XY-A/M |
| Complete kinematic base, qty: 1 | Thorlabs | KB25/M |
| 50 mm Pillar post, qty: 6 | Thorlabs | RS50/M |
| 30 mm cage mounting bracket, qty: 6 | Thorlabs | CP02B |
| 60 mm cage mounting bracket, qty: 2 | Thorlabs | LCP01B |
| Thread adapter, qty: 2 | Thorlabs | SM1A27 |
| Lens tube, qty: 2 | Thorlabs | SM1L05 |
| 1 inch cage assembly rod, qty: 4 | Thorlabs | ER1 |
| 2 inch cage assembly rod, qty: 4 | Thorlabs | ER2 |
| 3 inch cage assembly rod, qty: 4 | Thorlabs | ER3 |
| 4 inch cage assembly rod, qty: 8 | Thorlabs | ER4 |
| 6 inch cage assembly rod, qty: 4 | Thorlabs | ER6 |
| 25 mm Pillar POST, qty: 4 | Thorlabs | RS50/M |
| Other mechanical components | Westlake University | Lab developed |
| Control unit, qty: 1 | Olympus | SZX-MDHSW |
| Control unit hand switch, qty: 1 | Olympus | U-ACAD4515 |
| 400 mm pillar, qty: 1 | Olympus | SZH-P400 |
| Drop prevention collar, qty: 1 | Olympus | SZX-R |
| Motorized focus unit, qty: 1 | Olympus | SZX2-FOA |
| MVX10 zoom body, qty: 1 | Olympus | MVX-ZB10 |
| Tube lens unit, qty: 1 | Olympus | MVX-TLU |
| Camera port, qty: 1 | Olympus | MVX-TV 1XC |
| Workstation (Optional configuration: Supermicro X11SPA-TF motherboard, Intel Xeon W-3235 processor, >256 GB memory, >12 TB SSD storage space.) | Customized | |
| PCIe-6323 multifunction I/O card, qty: 1 | National Instruments | 781045-01 |
| SCB-68A connector block, qty: 2 | National Instruments | 782536-01 |
| SHC68-68-EPM shielded cable, qty: 2 | National Instruments | 782536-01 |
Step-by-step method details
Microscope construction and alignment
Timing: 1–4 weeks
This step describes the construction and alignment of the presented tiling light sheet microscope from scratch. All components should be installed according to the microscope design to achieve the designed imaging performance.
Note: The mechanical and electrical design of the microscope is not included in this protocol. It needs to be obtained separately from Westlake University after the execution of an NDA.
CRITICAL: The alignment of the tiling light sheet microscope should comply with Class 3B laser safety regulations for optical system alignment. Follow the local institutional guidelines and acquire the approval prior to the construction of the microscope if applicable.
-
1.Construct the control system of the microscope (Figure 1A). The Windows 10 professional 64-bit operating system is required to be installed on the control workstation.
-
a.Install the NI PCIe-6323 control board and the device driver.
-
b.Install the camera frame grabber and the device driver.
-
c.Install the required commercial software listed in the key resources table, including Labview 2019 developer suite or later version and Matlab 2019a or later version.
-
d.Install the vendor provided control software and device drivers for the optoelectrical components listed in the key resources table, including the laser sources, spatial light modulator (SLM), PI translation stages, filter wheel, and detection camera.
-
e.Confirm all devices work properly using the vendor provided control software.
-
f.Install the custom software listed in the key resources table, including the main control software, the phase map generation software, and the image reconstruction software.
-
g.Connect the control workstation to the SH-68A connector blocks with the SHC68-68-EPM shielded cables.
-
h.Connect the microscope optoelectrical devices to the assigned analog outputs, digital inputs, and digital outputs of the SH-68A connector blocks with BNC cables.
-
a.
The microscope control system construction is completed. Ensure all optoelectrical components can be controlled with the main control software (Figure 2B) and work properly.
-
2.Construct the optomechanical system of the microscope (Figures 1B–1D). Combine and collimate the excitation continuous wave (CW) laser beams.
-
a.Install the laser sources, laser combiner, and the associated components on the optical table according to the microscope design (Figure 1E).
-
b.Combine all CW laser beams into a single coincident beam before the expanding and collimating lenses L1 (f=30 mm) and L2 (f=200 mm). Align the rest of the microscope using the 488 nm CW laser.
-
c.Expand and collimate the combined laser beam using lenses L1 and L2.
-
a.
Note: Ensure the collimation of the 488 nm laser beam with a shear interferometer. Inspect the collimation of other laser beams. It is normal to have a slight divergence or convergence for other laser beams as different laser sources have different beam waist positions and divergences. The divergence and convergence of different lasers are corrected by the spatial light modulator (SLM) in the microscope calibration steps.
A coincident laser beam with ∼10 mm beam diameter (1/e2) is obtained after this step.
-
3.Align the spatial light modulator (SLM) assembly and the Galvanometer assembly (Figure 1F). Troubleshooting 1Note: The SLM, galvo mirror, and rear pupil of the excitation objectives are conjugated with each other through relay lenses in a 4f configuration. The galvo mirror is used as the reference plane of all conjugation planes. The custom phase map generation software (Figure 2A) is required to perform this step and the following steps of the protocol.
-
a.Install the polarizing beamsplitter (PBS) assembly, SLM assembly and the associated components on the optical table according to the design (Figure 1F). Ensure the collimated laser beam from the polarizing beam splitter incidents vertically at the center of the SLM liquid crystal surface.
-
b.Rotate the half wave plate HWP1 to maximize the intensity of transmitted light through the PBS.
-
c.Install the galvanometer assembly on the optical table according to the design.
-
d.Center the galvo rotating axis to the reference mounting hole on the optical table according to the design using the XY translation stage included in the assembly.Note: The conjugation from the SLM to the galvo is aligned reversely as the galvo mirror is used as the reference conjugation plane.
-
e.Install the optical slit assembly.
-
f.Conjugate the galvo mirror to the SLM through relay lenses L4 (f=175 mm) and L3 (f=300 mm).
-
g.The lens L4 needs to be installed and aligned first. Direct the reflected collimated laser beam from the SLM through the lens L4 without the lens L3. Adjust the position the lens L4 until the laser beam is focused on the galvo mirror.
-
h.Install the lens L3. Adjust the lens L3 by evaluating the collimation of the laser beam after the lens L4. The lens L3 is aligned if the collimated laser beam passing through both L3 and L4 remains collimated.
-
i.Apply a phase map with zero tiling phase to the SLM using the provided phase map generation software (Figure 2A). The SLM must stay on to align the rest of the microscope.
-
j.Readjust the SLM position to ensure the SLM liquid crystal surface conjugates with the galvo mirror. The conjugate image of the phase map applied to the SLM on the galvo mirror has the sharpest edges when the SLM liquid crystal surface locates at a conjugation plane of the galvo mirror.
-
k.Adjust the position of the optical slit along the excitation light optical axis so that it locates at the back focal plane of the lens L3.
-
l.Rotate the half wave plate HWP2 to maximize the intensity of the +1 and −1 diffraction orders of the laser beam. Either the +1 or −1 diffraction order can be used for sample illumination.
-
m.Adjust the tilting angle of the SLM to let either the +1 or −1 diffraction order fall on the center of the optical slit. Adjust the width of the optical slit to enable complete transmission of the selected diffraction order while other diffraction orders are blocked. The width of the optical slit determines the maximal tiling distance of the excitation beam.
-
a.
The SLM modulation surface is conjugated to the galvo mirror after this step. A sharp and uniform laser beam cross section profile that is identical to the amplitude mask of the applied phase map can be observed on the galvo mirror when the SLM is turned on after this step.
-
4.Conjugate the galvo mirror to the rear pupil of the excitation objectives (Figure 1G).
-
a.Adjust the rotating axis of the galvo mirror and ensure the excitation laser beam reflected by the galvo mirror is parallel to the optical table.
-
b.Install the prism mirror assembly. Adjust the prism mirrors PM1 and PM2 to ensure the laser beams reflected by both prism mirrors are parallel to the optical table.Note: The optical table surface is used as the reference of the detection focal plane for microscope alignment. Alignment steps 4a and 4b ensure the excitation beam through both excitation objectives stays in focus during beam scanning. PM1 and PM2 can be readjusted if the excitation beam is found to be off focus during beam scanning after the initial alignment, which is very rare.
-
c.Install the relay lens assemblies and the excitation objective assemblies according to the design.
-
d.Conjugate the galvo mirror to the rear pupil of both excitation objectives through two relay lens assemblies respectively.
-
e.Adjust the lateral position of the excitation objectives and ensure the excitation laser beam is centered at the rear pupil of both excitation objectives so that the excitation beams generated by both excitation objectives maintain the same propagation direction as the beam enters each excitation objective.
-
a.
The excitation path of the microscope is roughly aligned after this step. Only fine adjustment is required after this step.
-
5.Install the fluorescence detection assembly (Figure 1H).
-
a.Assemble and install the fluorescence detection assembly according to the design.
-
b.Use a piece of coordinate paper as the alignment target. Place the coordinate pater under the detection objective in parallel to the optical table. Focus the detection camera on the alignment target with ambient light. Adjust the magnification by rotating the magnification knob on the MVX10 microscope body. Ensure the alignment target is in focus within the field of view (FOV) at different magnifications. Perform step 5c if the alignment target is not in focus everywhere.
-
c.(Optional) The purpose of this step is to ensure the detection focal plane is parallel to the surface of the optical table. Place a steel shim with appropriate thickness under the detection assembly mounting base if the alignment target is not in focus everywhere within the FOV. Adjust the position of the steel shim until the alignment target is in focus within the FOV.
-
d.Install the imaging chamber assembly. Fill the imaging chamber with the prepared dye solution. Focus the microscope on the excitation beam without scanning the laser beam.
-
e.Readjust the axial position of both excitation objectives along the excitation light optical axis using the manual translation stages of the excitation objective assemblies to place the excitation beam waist at the center of the detection FOV (Figure 1H).
-
f.Readjust the position of the lenses L6 (f=250 mm) and L8 (f=250 mm) along the excitation light optical axis and ensure the conjugation between the galvo mirror and both excitation objectives.Note: Step 5e and 5f are only required when the RI of the imaging buffer changes significantly, e.g., the imaging buffer is changed from water to BABB, as the excitation beam waist could drift for nearly a centimeter which is larger than the maximal tiling distance of the microscope. Small RI changes of the imaging buffer (<0.1) can be corrected by phase modulation of the illumination light using the SLM directly. Step 4e and 4f are rarely needed after the initial alignment because the RI of biological tissues cleared with commonly used tissue clearing methods is usually between 1.45 and 1.56.
-
g.Scan the excitation beam discretely within the FOV using the galvo mirror. Readjust the prism mirror PM1 and PM2 if necessary and ensure that the excitation beam is in focus at all positions within the FOV (Figures 3A–3D).
-
h.Replace the imaging chamber with another imaging chamber filled with the imaging buffer that matches the RI of the cleared tissue sample to be imaged.
-
i.Repeat step 5e and 5f if the RI of the imaging buffer changes significantly.Note: Imaging chambers are made of stainless steel which is resistive to all imaging buffers in our experience. An imaging chamber must be cleaned completely and dried up before it can be filled with a different kind of imaging buffer. It is suggested to used different imaging chambers for different imaging buffers.
-
a.
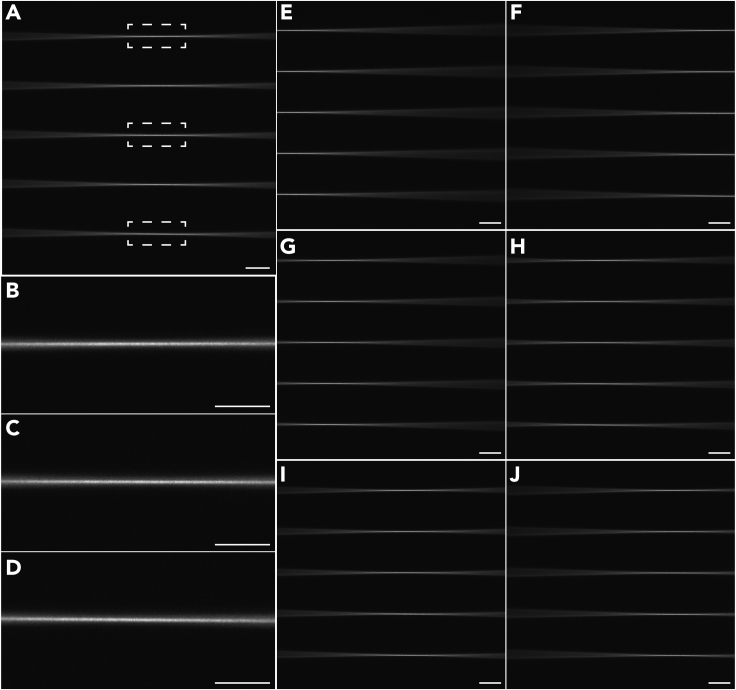
Figure 3.
The calibration of the microscope using dye solution or imaging buffers
(A) The excitation beam stays in focus at all X positions across the detection FOV.
(B–D) Zoom-in views of the excitation beam at the selected positions in (A).
(E and F) The left and right tiling positions defined by the user.
(G–J) The interpolated tiling positions according to the calibrated left and right tiling positions and the desired tiling number.
Scale bars, 200 μm (A, E-J), and 100 μm (B–D).
The microscope is aligned after this step. Hardware adjustment is generally unnecessary in most applications after this step unless otherwise specified.
-
6.Install the sample translation assembly (Figure 1H).
-
f.Install the sample translation assembly on the optical table according to the design.
-
g.Focus the detection camera on the sample holder using ambient light. Move the sample holder in the horizontal directions and ensure the sample translation directions are coincident with the lateral directions of the detection FOV.
-
f.
The microscope is ready for imaging after this step.
Microscope calibration and operation
Timing: 5–30 mins
This step describes the calibration and operation of the microscope for imaging cleared tissues. The custom phase map generation software and main control software are required to calibrate and operate the microscope (Figures 2A and 2B).
Note: This protocol describes the operation procedure using the provided custom software. More detailed descriptions on the microscope imaging modes and phase map generation mechanism can be found in the previous publication (Chen et al., 2020).
-
7.
Microscope calibration (Figures 2 and 3, Methods video S1). Troubleshooting 2–4
-
a.Fill the imaging chamber with the imaging buffer matches the RI of the cleared tissue sample to be imaged. For example, use BABB for tissues cleared with uDISCO.
 CRITICAL: Some imaging buffers, such as BABB, are toxic. Handle the imaging buffer with care.
CRITICAL: Some imaging buffers, such as BABB, are toxic. Handle the imaging buffer with care. -
b.Scan the excitation beam discretely to create a grid pattern in the imaging buffer using one of the excitation objectives. Focus the microscope on the grid pattern.
-
c.Adjust the microscope magnification by adjusting the magnification knob on the MVX 10 microscope body based on the desired lateral spatial resolution.
-
d.Adjust the intensity profile of the excitation beam based on the desired axial resolution and tiling number by changing the illumination numerical apertures (NA) using the phase map generation program (Figure 2A, Methods video S1). The phase map generation mechanism was described in our previous publication (Chen et al., 2020).
-
e.Find the tiling coefficients and focus coefficients to tile the excitation beam on both sides of the detection FOV and keep the excitation beam in focus by adjusting the tiling coefficient and focusing coefficient using the phase map generation software (Figures 2A, 3E, and 3J, Methods video S1).
-
f.Generate the phase maps used to tile the excitation beam within the FOV with the phase map generation software using the calibrated coefficients.
-
g.Confirm the excitation beam is in focus at all tiling positions (Figures 3E–3J, Methods video S1).
-
h.Repeat steps 7e and 7f to calibrate all excitation wavelengths for multicolor imaging and generate the corresponding phase maps using the phase map generation software.
-
i.Repeat steps 7e–7h to calibrate the other excitation objective for the needed excitation wavelengths if both excitation objectives are to be used for imaging.
-
j.Repeat steps 7b–7i when needed after changing the detection objective.Note: Microscope calibration using the phase map generation software should always be performed prior to imaging unless the imaging buffer and imaging conditions are the same. The calibration procedures are the same regardless of the detection objective being used for imaging.
Methods video S1. Microscope calibration, related to step 7Microscope calibration using the provide phase map generation software and the main control software. Associated with Figures 2, 3, and 4. The Movie contains the following calibration steps. 1. Correct the focal position of the scanning excitation beam by changing the focusing coefficient. 2. Change the intensity profile of the scanning excitation beam by changing the illumination NAs (NAOD=0.05, NAID=0.015, NAOD=0.03, NAID=0.01, and NAOD=0.01, NAID=0). 3. Find the tiling coefficients to tile the light sheet at both sides of the FOV. 4. Generate two groups of phase maps to tile the excitation light sheet with the calibrated coefficients at 6 and 3 positions. 5. Load the generated phase maps. 6. Verify the tiling positions of the generated two groups of phase maps. Related to step 7d–7g.Download video file (51.1MB, mp4) -
a.
The microscope is ready for imaging after this step.
-
8.Microscope operation and characterization. Troubleshooting 5
-
a.Mount the sample on the sample holder made of stainless steel and load the sample holder on the sample translation stage.Note: Different sample holders and mounting methods should be used to mount samples of different mechanical properties and shapes. The different sample holder designs, the selection of the appropriate sample holder and mounting method was described in our previous publication (Chen et al., 2020).
-
b.Select the regions of interest to be imaged using the main control program and start imaging. The lateral area of each ROI is the same as the detection camera FOV. The axial dimension of each ROI is determined by the depth of the sample volume to be imaged.
-
c.In order to characterize the performance of the microscope, adjust the excitation light sheet and tiling number using the provide phase map generation software and image the same sample volume with different spatial resolutions and verify the differences. For example, we imaged the same sample volume using the light sheets of different intensity profiles (Methods video S1) and tiling numbers to show the different spatial resolutions of the acquired results (Figure 4).Note: The operation procedure of the microscope for imaging is the same regardless of the sample and the detection objective being used for imaging. A more detailed description of the operation modes of the microscope can be found in the previous publication (Chen et al., 2020).
-
a.
Figure 4.
Comparisons of the different spatial resolutions obtained with different tiling light sheets
(A) Lateral maximum intensity projection (MIP) of a 2.3×2.3×0.5 mm3 sample volume in a Thy1-eGFP mouse brain cleared with uDISCO. (B-D) Axial MIPs of the selected sample volume in (A) imaged using light sheets with different light sheets and tiling numbers.
(B) Excitation NAOD=0.01, NAID=0, 1 tile.
(C) Excitation NAOD=0.03, NAID=0.01, 3 tiles.
(D) Excitation NAOD=0.05, NAID=0.015, 6 tiles.
(E–G) Zoom-in views of the same selected areas in (B–D).
Scale bars, 200 μm (A–D), and 20 μm (E–J).
The sample imaging is completed after this step.
Image reconstruction
Timing: 1–24 hrs
This step describes the procedure to reconstruct the result from the acquired raw images using the custom image reconstruction software (Figure 2C).
Note: The raw image format is dcimg, which is an image format used by Hamamatsu cameras. The reconstruct image format is TIFF that can be opened by most commercial image analysis software. The actual image reconstruction time depends on the data size and the computation power. It typically costs 15 mins to reconstruct 100 GB raw data with the provided image reconstruction software and the suggested computer configuration. The custom image reconstruction software is required to perform the step. A more detailed description of the image reconstruction mechanism can be found in the previous publication (Chen et al., 2020).
-
9.Image reconstruction.
-
a.Input the required parameters, including the tiling number, the boundary positions between each two adjacent tiles, and the size of common areas between each two adjacent tiles. The input parameters are used to calculate the amplitude masks to stitch raw images collected at different tiling positions.
-
b.Select the raw image files to be reconstructed and start reconstructing.
-
c.Register and merge the reconstructed 3D images from adjacent sample volumes with Amira or other software.
-
a.
The image reconstruction is completed after this step.
Expected outcomes
Cleared tissues of centimeter sizes (2.6×2.6×2.6 cm3) can be imaged at variable spatial resolutions using the tiling light sheet microscope described in this protocol (Figures 4 and 5). The spatial resolution can be adjusted by changing the magnification, the intensity profile of the excitation light sheet, and the detection objective following the steps described in this protocol. Air detection objectives should be used for imaging with micron-scale spatial resolution and immersive objectives should be used for imaging with submicron-scale spatial resolution. For example, a Thy1-eGFP mouse brain cleared with CUBIC-L was imaged at 2×2×5 μm3 spatial resolution using a 0.25 NA air objective and 0.6×0.6×1.5 μm3 spatial resolution using a 0.5 NA immerse objective with the presented tiling light sheet microscope (Figure 5, Methods videos S2, S3, S4, S5, and S6). More results collected under different imaging conditions can be found in the previous publication (Chen et al., 2020).
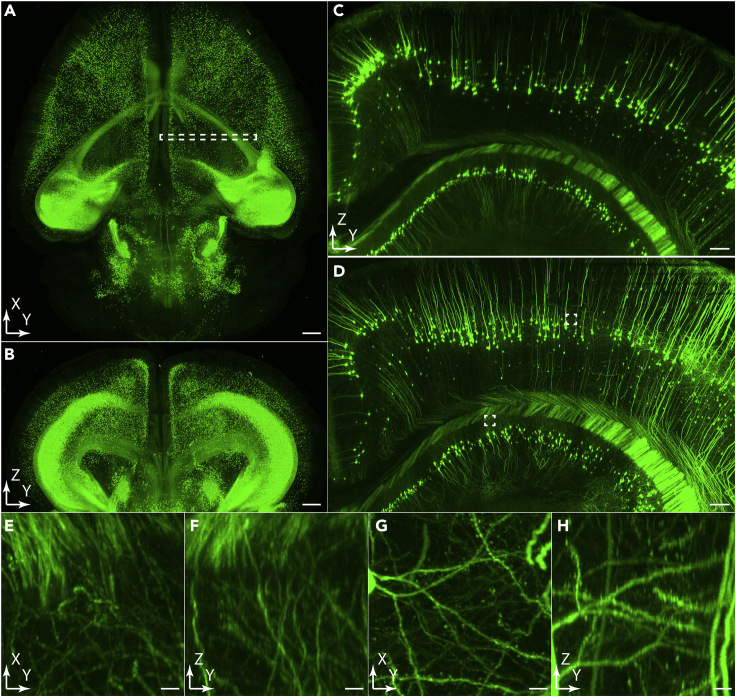
Figure 5.
Cleared tissue imaging with micron-scale to submicron-scale spatial resolution
(A and B) Lateral and axial MIPs of a Thy1-eGFP mouse brain cleared with CUBIC-L imaged at 2×2×5 μm3 spatial resolution using a 0.25 NA air detection objective.
(C) Axial MIP of the selected sample volume in (A).
(D) The same sample volume in (B) imaged at 0.6×0.6×1.5 μm3 spatial resolution using a 0.5 NA immerse detection objective.
(E–H) Lateral and Axial projections of the selected 100×100×100 μm3 regions in (D).
Scale bars, 1 mm (A and B), 200 μm (C and D), and 10 μm (E–H).
A Thy1-eGFP mouse brain cleared with CUBIC-L imaged with 2×2×5 μm3 spatial resolution. Panel 1: 3D volume rendering of the mouse brain. Panel 2: 3D volume rendering of a selected 2.3×2.3×9 mm3 sub volume within the mouse brain. Panel 3: Axial slices of the sub sample volume. Panel 4: Lateral slices of the sub sample volume. Associated with Figure 5.
3D volume rendering of a 2.5×4×2.5 mm3 sub sample volume within the same Thy1-eGFP mouse brain in Movie S2 imaged with 0.6×0.6×1.5 μm3 spatial resolution. Associated with Figure 5.
Lateral slices of the sub sample volume in video S3. Associated with Figure 5.
Axial slices of the sub sample volume in Movie S3. Associated with Figure 5.
Zoom-in view of a 0.6×0.6×2.5 μm3 subsample volume within the sample volume in Movie S3. Panel 1: 3D volume rendering of the sample volume. Panel 2: Axial slices of the sub sample volume. Panel 3: Lateral slices of the sub sample volume. Associated with Figure 5.
Limitations
Tiling light sheet microscopy improves the spatial resolution and optical sectioning ability by tiling thin but short light sheets and collecting additional images. The imaging speed decreases, and the raw image size increases proportionally to the tiling number. Therefore, the tiling number should be determined based on the desired spatial resolution and the imaging throughput.
The maximal sample size is limited by the working distance of the microscope objectives and the travel distance of the sample translation stages, which can be increased by using objectives with longer working distances and translation stages with longer travel ranges. The maximal size of the imaging result is determined by the storage space of the computer. On the other hand, it is important to keep in mind that uniform tissue transparency is essential for acquiring high quality images, which is more challenging to achieve as tissue size increases.
The presented tiling light sheet microscope is more suited for imaging thick tissues and it is not ideal for imaging thin and flat tissues, such as mouse brain slices.
Troubleshooting
Problem 1
A small portion of the illumination light can’t be controlled by the SLM (step 3).
Potential solution
The extinction ratio of the PBS in the SLM assembly is 1000:1. Thus, a small portion of the illumination light that reaches the sample can’t be modulated by the SLM. Despite its existence, it generally doesn’t cause any problem because the ratio of the non-modulated excitation light is very low.
Problem 2
The scanning excitation beam is deformed in the imaging buffer (step 7).
Potential solution
There are two common reasons that cause the problem. 1. The replaceable glass window glued on the imaging chamber that allows the transmission of the excitation light must be attached gently. The deformation of the glass window could introduce aberrations to the excitation light that decrease the quality of the scanning excitation beam. 2. Some imaging buffers are mixtures. It is important to keep the uniformity of the imaging buffer mixture.
Problem 3
Either the spatial resolution or the fluorescence intensity drops quickly as the imaging depth increases (step 8).
Potential solution
It is usually caused by poor tissue transparency or RI mismatch between the imaging buffer and the cleared tissue sample. Properly cleared tissues should be nearly invisible in RI matched imaging buffers. Optimize the tissue clearing and RI matching protocol until good tissue transparency and RI uniformity are obtained.
Problem 4
The microscope loses focus slowly in long term imaging when air detection objectives are used for imaging (step 8).
Potential solution
The microscope uses an open top imaging chamber. The evaporation of the imaging buffer could cause slow drifting of the detection focal plane when air detection objectives are used for imaging. Place a glass window on top of the imaging chamber (included in the microscope design) to prevent focal plane drifting caused by imaging buffer evaporation.
Problem 5
The spatial resolution is not uniform within the FOV (step 8).
Potential solution
The problem could be caused by the excitation light sheet being off focus during beam scanning and tiling. Repeat the microscope calibration steps to ensure the excitation beam stays in focus during beam scanning and tiling.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Liang Gao (gaoliang@westlake.edu.cn)
Materials availability
This study did not generate new material.
Data and code availability
The data of this study are available from the corresponding authors upon reasonable request. The optical, mechanical, and electrical design of the presented tiling light sheet microscope and the associated custom software is available from Westlake University after the execution of a nondisclosure agreement (NDA).
Acknowledgments
This work was supported by the Westlake University, the Westlake Education Foundation, and the Zhejiang Province Natural Science Foundation LR20C070002. We thank advanced biomedical technology core facility and the laboratory animal resource center for the facility support and technical assistance.
Author contributions
L.G. conceived the project and designed the microscope; D.W. and L.G. constructed the microscope. R.F., Y.C., and J.L. prepared the samples, performed the imaging experiments, and analyzed the data; L.G. wrote the paper with the input of all authors.
Declaration of interests
The authors declare no competing interests. A patent regarding the described microscope was filed by Westlake University on behalf of L.G.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100546.
References
- Chen Y., Li X., Zhang D., Wang C., Feng R., Li X., Wen Y., Xu H., Zhang X.S., Yang X. A versatile tiling light sheet microscope for imaging of cleared tissues. Cell Rep. 2020;33:108349. doi: 10.1016/j.celrep.2020.108349. [DOI] [PubMed] [Google Scholar]
- Chung K., Wallace J., Kim S.Y., Kalyanasundaram S., Andalman A.S., Davidson T.J., Mirzabekov J.J., Zalocusky K.A., Mattis J., Denisin A.K. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Martin B.L., Matus D.Q., Gao L. Imaging multicellular specimens with real-time optimized tiling light-sheet selective plane illumination microscopy. Nat. Commun. 2016;7:11088. doi: 10.1038/ncomms11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L. Extend the field of view of selective plan illumination microscopy by tiling the excitation light sheet. Opt. Express. 2015;23:6102–6111. doi: 10.1364/OE.23.006102. [DOI] [PubMed] [Google Scholar]
- Pan C., Cai R., Quacquarelli F.P., Ghasemigharagoz A., Lourbopoulos A., Matryba P., Plesnila N., Dichgans M., Hellal F., Erturk A. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat. Methods. 2016;13:859–867. doi: 10.1038/nmeth.3964. [DOI] [PubMed] [Google Scholar]
- Susaki E.A., Tainaka K., Perrin D., Kishino F., Tawara T., Watanabe T.M., Yokoyama C., Onoe H., Eguchi M., Yamaguchi S. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell. 2014;157:726–739. doi: 10.1016/j.cell.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Tainaka K., Murakami T.C., Susaki E.A., Shimizu C., Saito R., Takahashi K., Hayashi-Takagi A., Sekiya H., Arima Y., Nojima S. Chemical landscape for tissue clearing based on kydrophilic reagents. Cell Rep. 2018;24:2196–2210.e9. doi: 10.1016/j.celrep.2018.07.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microscope calibration using the provide phase map generation software and the main control software. Associated with Figures 2, 3, and 4. The Movie contains the following calibration steps. 1. Correct the focal position of the scanning excitation beam by changing the focusing coefficient. 2. Change the intensity profile of the scanning excitation beam by changing the illumination NAs (NAOD=0.05, NAID=0.015, NAOD=0.03, NAID=0.01, and NAOD=0.01, NAID=0). 3. Find the tiling coefficients to tile the light sheet at both sides of the FOV. 4. Generate two groups of phase maps to tile the excitation light sheet with the calibrated coefficients at 6 and 3 positions. 5. Load the generated phase maps. 6. Verify the tiling positions of the generated two groups of phase maps. Related to step 7d–7g.
A Thy1-eGFP mouse brain cleared with CUBIC-L imaged with 2×2×5 μm3 spatial resolution. Panel 1: 3D volume rendering of the mouse brain. Panel 2: 3D volume rendering of a selected 2.3×2.3×9 mm3 sub volume within the mouse brain. Panel 3: Axial slices of the sub sample volume. Panel 4: Lateral slices of the sub sample volume. Associated with Figure 5.
3D volume rendering of a 2.5×4×2.5 mm3 sub sample volume within the same Thy1-eGFP mouse brain in Movie S2 imaged with 0.6×0.6×1.5 μm3 spatial resolution. Associated with Figure 5.
Lateral slices of the sub sample volume in video S3. Associated with Figure 5.
Axial slices of the sub sample volume in Movie S3. Associated with Figure 5.
Zoom-in view of a 0.6×0.6×2.5 μm3 subsample volume within the sample volume in Movie S3. Panel 1: 3D volume rendering of the sample volume. Panel 2: Axial slices of the sub sample volume. Panel 3: Lateral slices of the sub sample volume. Associated with Figure 5.
Data Availability Statement
The data of this study are available from the corresponding authors upon reasonable request. The optical, mechanical, and electrical design of the presented tiling light sheet microscope and the associated custom software is available from Westlake University after the execution of a nondisclosure agreement (NDA).