Abstract
The precise mechanisms underlying the detrimental effects of early life adversity (ELA) on adult mental health remain still elusive. To date, most studies have exclusively targeted neuronal populations and not considered neuron-glia crosstalk as a crucially important element for the integrity of stress-related brain function. Here, we have investigated the impact of ELA, in the form of a limited bedding and nesting material (LBN) paradigm, on a glial subpopulation with unique properties in brain homeostasis, the NG2+ cells. First, we have established a link between maternal behavior, activation of the offspring's stress response and heterogeneity in the outcome to LBN manipulation. We further showed that LBN targets the hippocampal NG2+ transcriptome with glucocorticoids being an important mediator of the LBN-induced molecular changes. LBN altered the NG2+ transcriptome and these transcriptional effects were correlated with glucocorticoids levels. The functional relevance of one LBN-induced candidate gene, Scn7a, could be confirmed by an increase in the density of voltage-gated sodium (Nav) channel activated currents in hippocampal NG2+ cells. Scn7a remained upregulated until adulthood in LBN animals, which displayed impaired cognitive performance. Considering that Nav channels are important for NG2+ cell-to-neuron communication, our findings provide novel insights into the disruption of this process in LBN mice.
Keywords: Early life stress, NG2+ glia, Transcriptome, Scn7a, Nav-channels, Translational psychiatry
1. Introduction
The brain is particularly sensitive to adversity experienced early in life (Lupien et al., 2009). ELA-such as child neglect and maltreatment-lastingly impacts developmental trajectories of the brain, leading to structural changes (e.g. reduced cortical and hippocampal volumes) but also functional impairment of critical neurocognitive circuits in adulthood (Teicher et al., 2014; Hodel et al., 2015; Kraaijenvanger et al., 2020). In addition, meta-analytical data confirm that exposure to ELA during childhood not only increases the risk for developing neuropsychiatric disorders later in life but also predicts an unfavorable course of illness and treatment outcome in depression (Heim et al., 2008; Nanni et al., 2012; LeMoult et al., 2020). So far, the vast majority of studies into the neurobiology of ELA have exclusively targeted alterations in neuronal function. In recent years, the importance of neuron-glia communication and bidirectional neuron-glia crosstalk is emerging as crucial for proper brain function throughout the lifespan and has been implicated in brain disease-related processes (Chung et al., 2015). There is evidence that, for example impaired brain myelination contributes to the detrimental effects of ELA on brain development across species (Barateiro et al., 2016; Makinodan et al., 2012), but knowledge about the precise molecular mechanisms and glial subpopulations involved is still lacking. Despite its remarkable potential to adapt to different extrinsic stimuli and promote functional recovery (Forbes and Gallo, 2017; Zatorre et al., 2012), myelination can be disturbed if environmental challenges are severe or long-lasting, e.g. under conditions of critical stress, which has been proven by both clinical (Bick et al., 2015) and preclinical studies (Yang et al., 2017; Howell et al., 2013). Myelinating cells -oligodendrocytes within the central nervous system-derive from the differentiation of oligodendrocyte precursor cells (OPCs) (Dimou and Gotz, 2014). Both OPCs and mature oligodendrocytes can be affected by early stressful experiences (Teissier et al., 2020; Tanti et al., 2018). OPCs expressing the type I proteoglycan CSPG4 (NG2) on their cell surface, thus known as NG2+ cells, have been discussed to play additional roles in brain homeostasis, although the exact nature of such roles remains unclear (Nishiyama et al., 2009; Vigano and Dimou, 2016; Dimou and Gallo, 2015; Gallo et al., 2008). In addition to serving as progenitors of myelinating oligodendrocytes (Nishiyama et al., 2009; Trotter et al., 2010), NG2+ cells have unique properties, rendering them particularly interesting in the context of stress and ELA. These properties include the ability to establish synaptic contacts with neurons (Sakry et al., 2011; Bergles et al., 2010; Sun et al., 2016; Sakry and Trotter, 2016), to communicate with microglia (Liu and Aguzzi, 2020) and to modulate glutamatergic signal transduction in adjacent neurons via the shed version of the NG2 ectodomain (Sakry et al., 2014). NG2+ cells express the glucocorticoid receptor (GR) (Matsusue et al., 2014) and their proliferation is regulated by glucocorticoids (Wennstrom et al., 2006; Chetty et al., 2014; Alonso, 2000).
Despite the interesting roles of NG2+ cells and their responsiveness to glucocorticoids (Wennstrom et al., 2006; Chetty et al., 2014) and chronic stress in adulthood (Birey et al., 2015), so far no study has addressed the question as to whether NG2+ cells could be a target population of ELA. Similarly, it has not been addressed whether they could be involved in mediating the long-term negative consequences of ELA on mental health. In rodent models, ELA takes place during the so-called stress hypo-responsive period (SHRP) (Schmidt et al., 2003), which is a period characterized by a markedly reduced responsiveness of the hypothalamus-pituitary adrenal system to moderately challenging conditions. SHRP has been discussed to protect the early postnatal and still developing brain from an excess of glucocorticoid hormones (Sapolsky and Meaney, 1986). Intriguingly SHRP (postnatal day 2–9) largely overlaps with the temporal window during which NG2+ cells reach their peak density (the first postnatal week), and when the majority of newly divided NG2+ cells differentiate into oligodendrocytes (Hill et al., 2014). ELA elicits a significant stress response with increased expression of central corticotropin releasing hormone and enhanced circulating corticosterone (CORT) concentrations, which in turn cause detrimental long-lasting effect on memory formation and hippocampus-dependent cognitive functions (Molet et al., 2016; Singh-Taylor et al., 2018; Maras et al., 2014). However, the individual contribution of an ELA-induced CORT excess and the concomitant activation of central stress neurocircuits on the developing brain remains to be fully dissected.
To this end, we used an established mouse model applying ELA through fragmented maternal care, the LBN paradigm (Rice et al., 2008; van der Kooij et al., 2015; Gallo et al., 2019) and performed cell-type specific transcriptional profiling in hippocampal NG2+ cells directly after stress exposure. To dissect the impact of CORT on modulating LBN-induced negative outcomes, we further correlated the molecular changes with the individual litter's CORT response to LBN manipulation and the extent of maternal care fragmentation. Second, we performed enrichment analyses to test the hypothesis that glucocorticoid responsive genes are overrepresented in our set of LBN- induced candidate genes and to determine whether LBN might shift the molecular identity/profile of NG2+ cells and alter the maturation stage within the lineage. Extending our analyses to the adult stage we identified an impairment of hippocampus-dependent cognitive performance to be accompanied by persistent changes in the NG2+ cell transcriptome profile of adult animals previously exposed to LBN. Finally, the functional relevance of an LBN-induced increase in the expression of Scn7a was confirmed by electrophysiological recordings in hippocampal NG2+ cells.
2. Materials and methods
2.1. Animals
Adult female and male C57BL/6J mice (10 weeks) were obtained from Janvier Labs (France). Mice were single housed with food and water ad libitum in an air-conditioned (temperature = 22 ± 2 °C, relative humidity = 50 ± 5%) housing room with 12 h/12 h light-dark cycle (lights on at 07:00 am). Mice were habituated to the new environment for at least 1 week before the starting of the experiment. The breeding pairs consisted of female and male C57BL/6J. After mating pregnant dams were single housed and put in a cabinet with controlled ventilation, temperature and light cycle until the end of the experiment. From postnatal day (P) 2 to P9 the dams and litter were exposed to LBN procedure or control condition. For electrophysiological experiments C57BL/6N mice were bred to homozygous knock-in NG2 enhanced yellow fluorescent protein (NG2-EYFP) mice, where the expression of the reporter gene is regulated according to the endogenous NG2 promoter, allowing unbiased sampling of this population (Karram et al., 2008), to obtain heterozygous NG2-YFP pups. All experiments were performed in accordance with the European directive 2010/63/EU for animal experiments and were approved by the local authorities (Animal Protection Committee of the State Government, Landesuntersuchungsamt Rheinland-Pfalz, Koblenz, Germany). All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
2.2. Limited bedding and nesting paradigm
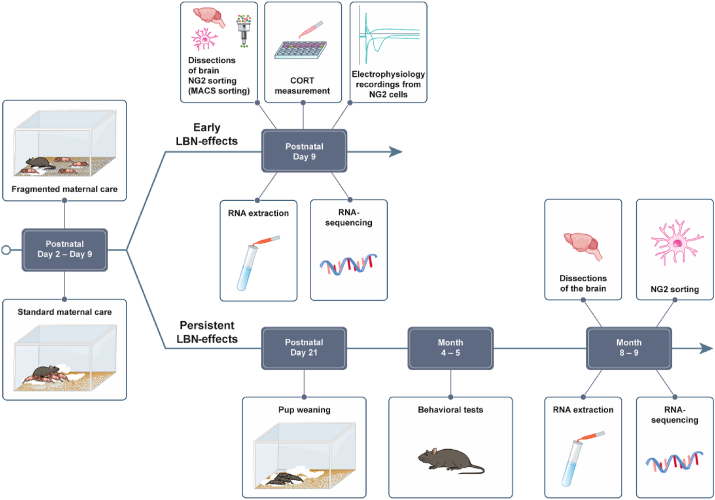
LBN was performed as previously described (Rice et al., 2008; van der Kooij et al., 2015). Briefly, on P2, dams with 5–6 pups (of which at least one female) were randomly subjected to LBN or assigned to the control group from P2 to P9. The dams subjected to LBN were housed in cages with limited bedding and nesting materials (half square of a Nestlet, # 14010 Plexx) on an aluminum mesh platform as for (Rice et al., 2008) (McNichols Co) at least 1.5 cm above the cage floor. Control litters were kept under standard conditions and provided with sufficient bedding and nesting materials (2 Nestlets). All animals were monitored but left undisturbed until the end of the experiment. Maternal behavior was observed three times daily from P2 to P8 in the light phase (9 a.m., 3 p.m.) and dark phase (9 p.m.) during 30-min observation sessions, during which dams were observed every other minute resulting in 15 1 min epochs per session. The maternal behaviors that were scored included: exits of the dam from the nest, time spent outside the nest both for the dams and the pups. For the acute effect of LBN, in the morning of P9 the male pups were weighed and euthanized. For the long-lasting effect of LBN at P9 animals were weighed and were kept and housed again under standard conditions until weaning at P21. After weaning the male offspring were kept group housed until 8 weeks. At week 9–10 the animals were single housed, behavioral phenotype tested at 4–5 months and mice were euthanized at 8 months. For a schematic overview of the different experimental schedules see Fig. 1.
Fig. 1.
Schematic overview of the experimental procedures.
2.3. Measurement of plasma CORT
Concentrations of circulating corticosterone were measured from trunk blood plasma (P9) or tail cut (adult animals) by Corticosterone ELISA Kit (Enzo Life Sciences, #Cat.No. ADI-900-0979) according to the manufacturer's instructions. The blood was collected in an EDTA tube and centrifuged for 10 min at 4 °C and 10 000 rpm.
2.4. Behavioral tests
All behavioral experiments were conducted between 09:00 and 14:00 by experimenters blinded to the treatment groups. All the animals were randomized per condition. The videos of all behavioral tests were scored manually by an experimenter blinded to the animals’ treatment using The Observer XT12 software (Noldus Information Technology). Between each test session, setups and arenas were cleaned with a 5% EtOH solution and dried with tissues. Behavioral testing occurred in custom-made sound-attenuating boxes under constant light conditions (37 lx). Detailed descriptions of the behavioral tests performed (novel object recognition test (NORT), open field (OF) and spatial object location test (SORT) are provided in the Supplementary Information (SI). Data as presented as average of the values from the male animals belonging to the same litter (Jimenez and Zylka, 2021; Lazic and Essioux, 2013).
2.5. Isolation of NG2+ cells by magnetic cell sorting
NG2+ cells were isolated using the NTDK-P Kit (Miltenyi Biotec) according to previously described procedures (Diers-Fenger et al., 2001; Hoch-Kraft et al., 2018), where Magnetic isolation (MACS) was performed with anti-NG2 antibody (Niehaus et al., 1999) conjugated with magnetic beads (Miltenyi Biotec). Cell purity and homogeneity of the sorted population was checked as shown in Supplementary Fig. 1. For the P9 time-point, each sample was a pool of the hippocampi from the male pups of the same litter; n represents litter number (control n = 7, LBN low CORT n = 8, LBN high CORT n = 7, randomized in 3 batches). For the 8-month time-point each sample was a pool of the hippocampi of 3–4 males per sample (control n = 6, LBN n = 7, randomized in 3 batches of experiments).
2.6. RNA extraction and next-generation sequencing (NGS)
After NG2 sorting, cells were pelleted and RNA was extracted using RNeasy Micro Kit (QIAGEN) according to manufacturer's instructions. RNA integrity was assessed by RNA integrity number (RIN) values as shown in Supplementary Table 1. NGS library prep was performed with NuGen Ovation SoLo RNA-Sequencing (RNA-seq) System following NuGen's standard protocol (M01406v2). For the first experiment (at P9) libraries were prepared with a starting amount of 1 ng and amplified in 14 PCR cycles. Libraries were profiled in a High Sensitivity DNA on a 2100 Bioanalyzer (Agilent technologies) and quantified using the ddPCR Library Quantification Kit for Illumina TruSeq in a QX200 Droplet Digital PCR system (BioRad). Details information on the NGS protocol are described in the SI.
2.7. Bioinformatic analysis
Detailed descriptions of the bioinformatic analysis are provided in the SI.
2.8. Electrophysiology
Hippocampal NG2+ cells were electrophysiologically characterized at the age of P9–P10 (n = 11 (control), n = 11 (LBN)) by use of heterozygous knock-in mice expressing the enhanced yellow fluorescent protein EYFP in NG2+ cells (NG2-EYFP ± mice (Karram et al., 2008),). After decapitation the brains were quickly removed and put into ice-cold 95% O2/5% CO2 oxygenated ACSF. Next, the tissue was horizontally cut by use of a vibratome (VT 1200S, LEICA, Germany) to generate hippocampal brain slices with a thickness of 300 μm. The slices were kept in oxygenated ACSF at room temperature for at least 1 h for recovery. Then they were transferred into a submerged-type recording chamber mounted on an upright microscope (Olympus BX-51WI) and perfused with oxygenated 95% O2/5% CO2 HEPES-buffered recording solution at room temperature for 10 min. NG2+ cells located in the stratum radiatum of area CA1 could be visually identified through a 40x objective on the microscope (Olympus) by their EYFP-fluorescence. Whole-cell clamped recordings were performed on these NG2+ cells using glass capillaries filled with a Cesium Gluconate-based internal solution. The resistance of the recording electrode was 3–6 MΩ, and the NG2+ cells were voltage clamped to a holding potential (Vm) of −60 mV. Voltage-gated sodium currents were activated starting from a holding potential of −60 mV followed by application of 20 mV voltage steps in a range from −120 mV to +40 mV with rectangular stimuli lasting 199 ms by use of an AxoPatch 200B amplifier (Molecular Devices, California, USA). Data were acquired with PClamp 11 software (Molecular Devices, California, USA), lowpass Bessel filtered at 1 kHz and sampled at 50 kHz. For Detailed description on the electrophysiology experiments and on data analysis are provided in the SI.
2.9. Statistics
All samples represent biological replicates. Sample sizes are indicated in the figure (Fig) legends. Values are expressed as mean ± SEM. Litter effects were taken in to account by averaging values from pup or offspring from the same litter (Jimenez and Zylka, 2021; Lazic and Essioux, 2013). Data were checked for normal distribution using the Kolmogorov-Smirnov test. Unpaired two-tailed Student's t-test (normally distributed) or Mann–Whitney U test (not normally distributed) were used to compare sets of data obtained from two independent groups of animals. Pearson's or Spearman's (non-parametric) correlation coefficient was used to measure linear correlation between two sets of data. The number of values shown in the correlations is different because for one litter it was not possible to measure the number of dam sorties (Fig. 2D) and for two litters (Fig. 4E) the CORT concentration. Mixed-effects model was used to compare repeated measurements with two variables: stress and time. P values are reported in Fig legends, with P < 0.05 considered statistically significant. All data were analyzed using Prism version 8.3 and 9 (GraphPad Software).
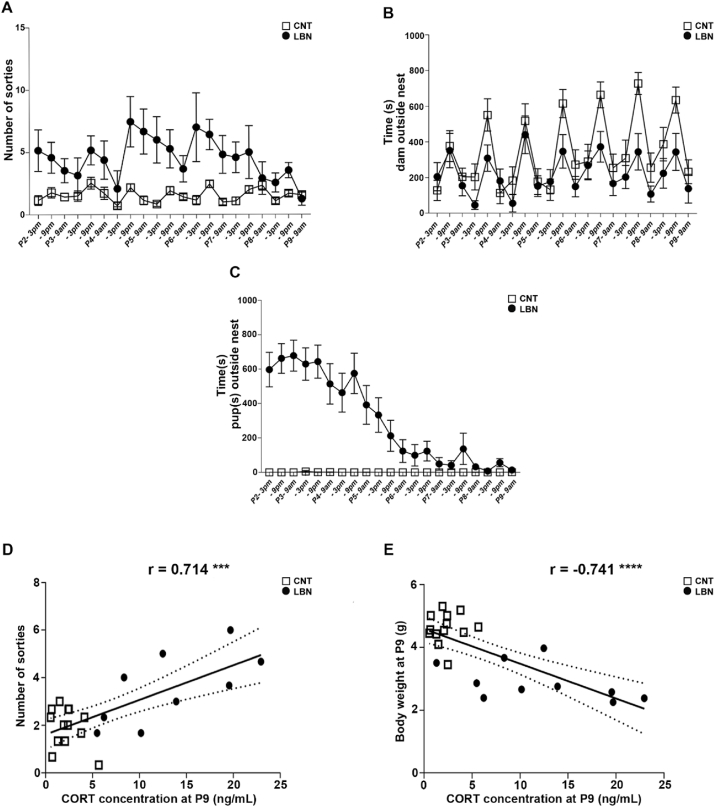
Fig. 2.
Heterogeneity in the LBN enables stratification based on the offspring's CORT response: importance of maternal fragmentation. (A) LBN litters showed an increase in the number of sorties from the nest compared to control (CNT) dams Mixed-effects model, for the stress effect: F(1,26) = 47.17, p < 0.0001, n = 14 CNT and n = 14 LBN litters. (B) LBN dams spend more time inside the nest compared to CNT dams. Mixed-effects model, for the stress effect: F(1,26) = 17.39, p = 0.0003; for time: F(8.712, 193.8) = 7.260, p < 0.0001, n = 14 CNT and n = 14 LBN litters. (C) LBN pups spent more time outside the nest compared to CNT pups. Mixed-effects model, for the stress effect: F(1,26) = 87.42, p < 0.0001; for time: F(6.028, 134.1) = 11.03, p < 0.0001, for interaction time x stress: F(20, 445) = 10.97, p < 0.0001, n = 14 CNT and n = 14 LBN litters. (D) Dam sorties at P3 correlated positively with CORT level of the litter at P9, Pearson r = 0.714, p = 0.0002, n = 22 litters. (E) The decrease in the body weight correlated negatively with the increase in the CORT level at P9, Pearson r = -0.741, p < 0.0001 n = 23 litters. ***p < 0.001 ****p < 0.0001.
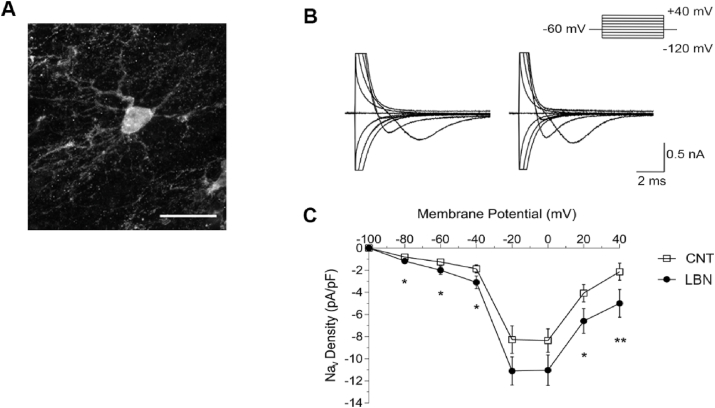
Fig. 4.
LBN exposure alters the current density of Nav channels in hippocampal NG2+ cells. (A) Representative NG2+ cell. Cells were selected by their EYFP expression (NG2-EYFP knock-in mice) and their characteristic morphology. Scale bar 20 μm. (B) Representative voltage traces of voltage sensitive sodium currents in NG2+ cells from control (CNT) (left) and LBN (right) P9 pups at different holding potentials. (C) Summary plot of mean density of Nav activated sodium currents in control and LBN P9 pups. Data are expressed as mean ± SEM. *p < 0.05 vs CNT, **p < 0.01 vs CNT, Mann-Whitney-U test (30–36 cells in 11 animals per group).
3. Results
3.1. Heterogeneity in the LBN enables stratification of the offspring: extend of maternal care fragmentation is linked to the litter's CORT response
We used the LBN paradigm (Rice et al., 2008; Naninck et al., 2015) and investigated the distinct effect of maternal behavior, in particular the extent to which maternal care is fragmented on the stress response of the offspring. Similar to previously published studies (Gallo et al., 2019), we detected fragmentation of maternal care in the LBN litters in terms of the number of sorties from the nest. LBN dams indeed exited the nest more frequently (Fig. 2A) but spent more time in total in the nest (Fig. 2B) compared to the control dams. The pups from LBN dams spent more time outside the nest (Fig. 2C). Interestingly, we identified significant correlations between the magnitude of the CORT response within one LBN litter and the respective mother's number of sorties from the nest (Fig. 2D) and the decrease in body weight of the pups from the same litters (Fig. 2E). Thus, detailed analysis of the heterogeneity in the outcomes after LBN provided evidence for a link between maternal behavior, characteristics of maternal care and the offspring's stress response in this early adversity paradigm.
3.2. Changes of the NG2+ cell transcriptional signatures in response to LBN exposure: impact of glucocorticoids
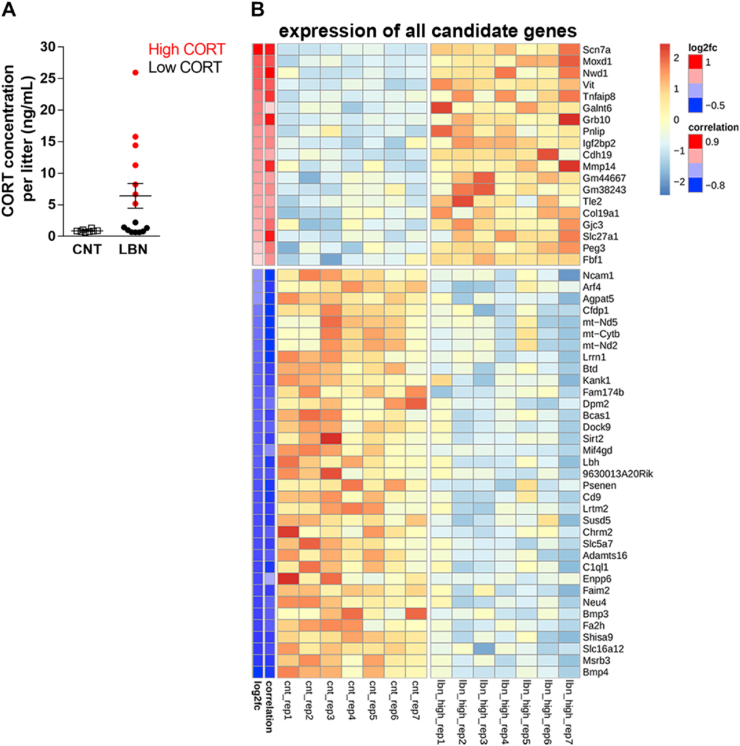
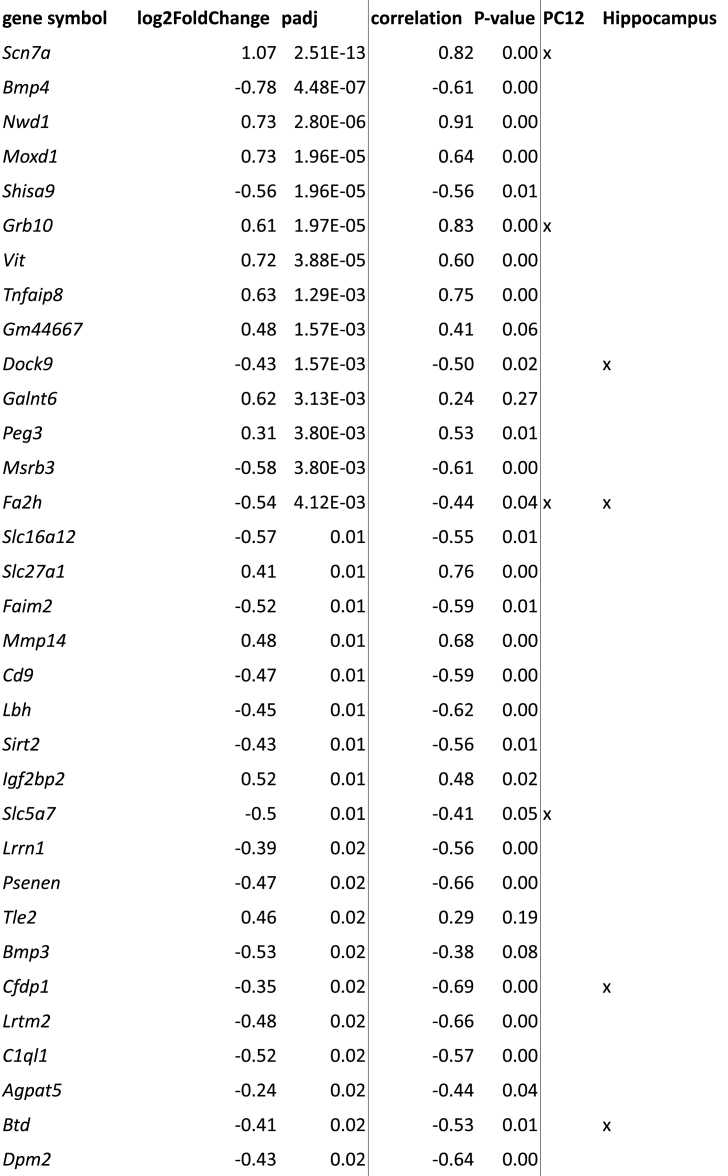
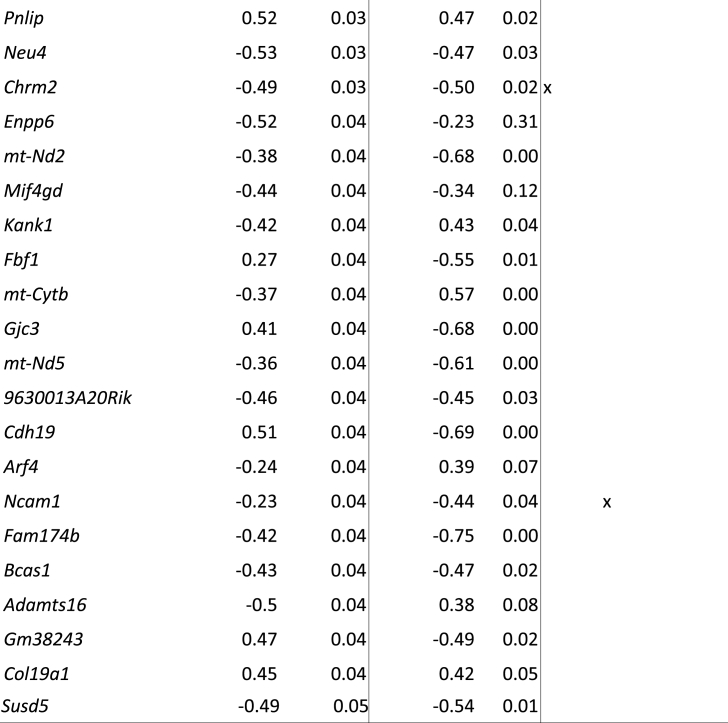
To further investigate whether LBN specifically targets NG2+ cells in brain areas implicated in the behavioral changes seen after LBN exposure, we isolated NG2+ cells from the hippocampus of pups (P9, directly after LBN or respective control pups) and performed bulk RNA-seq followed by differential gene expression (DGE) analysis. When comparing control samples vs all LBN samples, we found only 15 significantly differentially expressed genes (DEGs, 8 upregulated, 7 downregulated, Supplementary Table 2). However, since the impact of LBN in terms of stress-induced increase in plasma CORT concentration varied highly between LBN litters (Fig. 3A), we used the plasma CORT concentration of the control group as reference value and divided the stress group into low-CORT LBN (≤5 ng/mL), high-CORT LBN (≥5 ng/mL) and controls, which always had plasma CORT concentrations below 5 ng/mL (Fig. 3A). Comparison of the RNA-seq data using this stratification revealed 54 DEGs (19 upregulated, 35 downregulated) between controls versus high-CORT LBN samples (Fig. 3B and Table 1). No significant DEGs could be detected for control vs low-CORT LBN and low-CORT LBN vs high-CORT LBN. Further analysis revealed that the expression of some candidate DEGs (e.g Scn7a, Nwd1, Grb10) across samples strongly correlated with the plasma CORT concentrations (values of r ≥ 0.8) (Fig. 3B), whereas some candidates only showed very weak correlation with the stress hormone (r ≤ 0.3) (e.g Agpat5, Fbf1, Ncam1). A significant functional enrichment of our candidate genes in terms of overrepresented molecular pathways could not be found. However, several candidate genes are involved in lipid metabolic processes (Pnlip, Enpp6, Slc27a1, Agpat5, Fa2h, Neu4).
Fig. 3.
Impact of individual increase in circulating plasma CORT concentrations on LBN-induced transcriptional changes in NG2+ cells. (A) Averaging the individual plasma CORT concentrations per litter, this graph illustrates the heterogeneity of the CORT response across litters following LBN with the stratification in high CORT and low CORT concentration. Two-tailed Mann-Whitney Test, U = 30, p = 0.0553, n = 8 control (CNT) litters and n = 15 LBN litters. (B) Heatmap representing DEGs between control and high-CORT litters. In total, 54 genes were significantly upregulated (19) or downregulated (35, |log2fc| > 0.2, padj < 0.05). The heatmap shows scaled expression values for each gene across all relevant samples, whereby higher/lower expression is indicated by red/blue color. The magnitude of change in expression (log2fc) is indicated on the left-hand side of the plot, next to this the Pearson correlation coefficients of gene expression with CORT level in respective samples are shown. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
DE candidate genes (control vs high CORT comparison) sorted by significance.
Since we found strong correlations between the LBN-induced plasma CORT concentrations and some of our DEGs (Fig. 3B and Table 1), we wanted to understand whether the identified DEGs are glucocorticoid responsive genes. We therefore took advantages of already existing data on genomic binding sites of glucocorticoid receptor (GR) in neuronal PC12 cells (Polman et al., 2012a) and hippocampi (Polman et al., 2012b) of rats treated with dexamethasone (DEX), a synthetic glucocorticoid receptor agonist (Bachmann et al., 2003). Overlap analysis of genes binding due to DEX treatment in PC12 cells (918) and our 54 candidate DEGs revealed a significant enrichment represented by 5 genes: Scn7a, Grb10, Fa2h, Slc5a7 and Chrm2 (Fisher enrichment fold 2.79, p-value 0.0416; see Table 1 for overlapping genes). Overlap with the rat hippocampus study showed no significant enrichment represented by 5 genes (5 out of 1828/54 candidates, 1.34 fold/p = 0.431, see Table 1).
3.3. Comparison between LBN associated transcriptional signature and stage-wise gene expression in the oligodendrocyte lineage
Since NG2+ cells can maturate into oligodendrocytes, we wondered whether the LBN induced change in their transcriptional signature could reflect a shift towards a different stage in the oligodendrocyte lineage. We therefore performed a comparative analysis between our 54 candidate DEGs and previously published transcriptional signatures characterizing six maturation stages (13 substages) of the mouse oligodendrocyte lineage (Marques et al., 2016) or three maturational stages (Zhang et al., 2014). In the first analysis we found that DEGs downregulated in the LBN samples tended to be more strongly expressed in early stages of the oligodendrocyte lineage (Supplementary Figs. 2A and B). This tendency was further confirmed by a significant enrichment of 7 out of the 35 LBN downregulated-DEGs and the 50 early-stage indicator genes identified in the single-cell study (Marques et al., 2016) (7 out of 35/50, hypergeometric test p-value = 2.013*10⁻12, as shown in Supplementary Fig. 2C). The expression levels of these seven genes in CNT and LBN samples can be found in Supplementary Fig. 2D. However, we did not find that LBN up-regulated DEGs were increased in late oligodendroglial lineage stages (Supplementary Figs. 2E and F) and the findings of an enrichment of LBN down-regulated DEGs at early stages of the oligodendrocyte lineage failed to be confirmed by the second analysis with data from another widely used data set (Zhang et al., 2014) (See Supplementary Table 3). In this second analysis we identified our strongest up-regulated gene, Scn7a, as overlapping with a more immature stage in the cell lineage.
LBN modulates voltage-sensitive sodium currents in hippocampal NG2+ cells.
Since Scn7a encodes subunits of sodium channels and it has been previously identified in NG2+ cells (Zhang et al., 2014; De Biase et al., 2010; Spitzer et al., 2019; Cahoy et al., 2008), we further tested the hypothesis that LBN exposure could change the electrical properties, thereby modulating the activity, of hippocampal NG2+ cells. To this end, we investigated the impact of LBN on the density of Voltage-Gated sodium (Nav) channels by the use of whole-cell patch-clamp electrophysiological recordings in heterozygous NG2-EYFP knock-in P9 pups immediately after stress (Karram et al., 2008; Spitzer et al., 2019) (Fig. 4A). As expected, and consistent with our data from the C57BL/6J strain, the NG2-EYFP line (±) mouse was responsive to LBN and showed the characteristic LBN-induced increase in plasma CORT concentrations as well as a decrease in body weight (Supplementary Table 4). In addition, electrophysiological recordings from visually identified NG2+ cells (through EYFP-expression) in acute hippocampal slices revealed an increased density of Nav mediated sodium currents in the LBN condition at various membrane holding potentials (Fig. 4B and C).
3.4. Long-lasting effects of LBN on adult hippocampal NG2+ cell transcriptome and cognitive performance: persistent upregulation of Scn7a
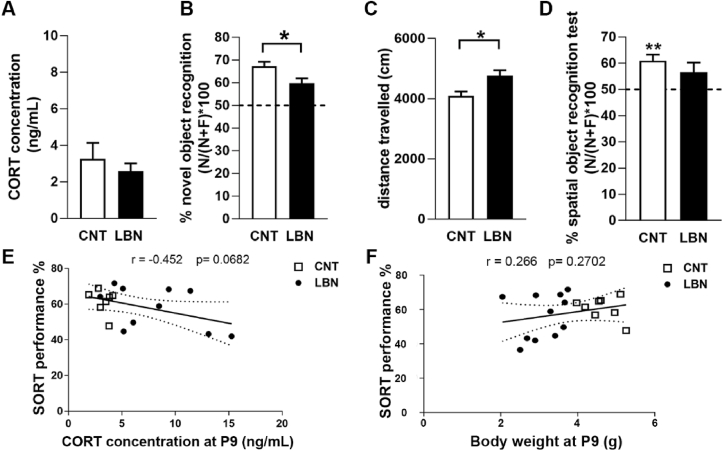
To further evaluate the functional impact of the observed transcriptional changes on cognitive integrity, we examined the behavioral outcome 4–5 months after LBN exposure using three behavioral tests: the novel object recognition test (NORT, assessing cognitive performance), the open field test (OF, investigating locomotor activity) and the spatial location test (SORT, assessing hippocampal-dependent function (Li and Chao, 2008; Mumby et al., 2002). Differently from the pups at P9, LBN offspring did not display any difference in the basal plasma CORT concentration compared to control animals (Fig. 5A), but showed an impaired cognitive performance in the NORT (Fig. 5B), increased locomotor activity in the OF (Fig. 5C) and did not perform above chance level (50%) in the SORT (Fig. 5D). No correlation was found between decreased NORT performance and increased locomotor activity (Supplementary Fig. 3).
Fig. 5.
LBN-induced impairment of cognitive performance in adult animals. (A) Plasma CORT concentrations in adult animals calculated as average per litter. Unpaired Student's t-test t (16) = 0.723, p = 0.4798, n = 8 CNT (control) and 11 LBN litters. (B) The % of novel object recognition of adult animals calculated as average per litter. Unpaired Student's t-test t (18) = 2.416, p = 0.0266, n = 8 CNT and 12 LBN litters. (C) The distance travelled in the OF of adult animals calculated as average per litter. Unpaired Student's t-test t (18) = 2.566, p = 0.0194, n = 8 CNT and 12 LBN litter (D) The % of spatial object recognition of adult animals calculated per litter, control (CNT) mice performed above chance level t (7) = 4.71, p = 0.0022 but not LBN mice t (11) = 1.836, p = 0.0934, n = 8 CNT and 12 LBN litters. (E) SORT performance correlated with the CORT concentration at P9 of the pups belonging to the same litters of the offspring tested in the SORT. Pearson r = −0.452, p = 0.0682, n = 17. (F) SORT performance did not correlate with the body weight at P9 of the pups belonging to the same litters of the offspring tested in the SORT. Pearson r = 0.266, p = 0.2702, n = 19.
Interestingly the decrease in SORT performance correlated with the increase in plasma CORT at P9 in the pups belonging to the same litter of the offspring tested in the SORT (Fig. 5E) but not with the decrease in body weight at P9 (Fig. 5F).
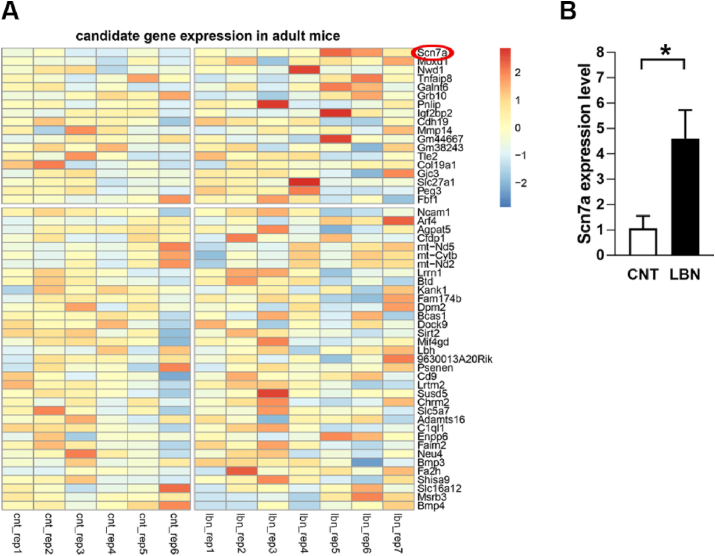
In order to understand whether the LBN-associated alterations in the transcriptome of NG2+ cells isolated from P9 hippocampi are long-lasting and might explain the accelerated cognitive decline, we performed an additional series of NG2+ cell transcriptional profiling on those cognitively impaired, 8-months old animals. At the transcriptome-wide level, we found no DEGs between control and mice previously exposed to LBN, which points to a relative normalization of the NG2+ cell transcriptome profile in the adult stage (Fig. 6A). However, when choosing a candidate-driven approach and performing a targeted t-test of the same DEGs that had been identified in LBN pups, we found that one DEG (Scn7a) was significantly upregulated also in the adult animals (target-t-test p = 0.01969, t (11) = 2.8925, Fig. 6B). The increased expression of Scn7a in NG2+ cells of adult LBN animals was confirmed by RT-qPCR as a second independent method (Supplementary Fig. 4).
Fig. 6.
Persistent upregulation of Scn7a in adult LBN animals.
(A) Heatmap representing the DEGs derived from the P9 analysis (from Fig. 3) re-analyzed at an adult stage. The heatmap shows scaled expression values for each gene across all relevant samples, whereby higher/lower expression is indicated by red/blue color. Only one gene, Vit, was below detection limit ( < 5 reads in all samples) and was therefore omitted from analysis. Only Scn7a remained up-regulated after performing targeted t-test. (B) Graph showing the expression of Scn7a in control (CNT) and LBN animals, target t-test, t (11) = 2.8925, p = 0.0197.
4. Discussion
The present study investigated the impact of LBN manipulation on NG2+ glia transcriptome and function. We employed a mouse model of ELA, the LBN paradigm, to show that LBN distinctly targets the transcriptional signature of hippocampal NG2+ cells, and that changes in the NG2+ cell transcriptome correlated with LBN-induced increase in circulating plasma CORT. The strongest LBN-induced candidate gene, Scn7a, remained upregulated in adult LBN mice, only when performing a targeted t-test of the same DEGs that has been identified in LBN pups. Since Scn7a encodes for a subunit of sodium channels, we finally confirmed the functional relevance of the transcriptional change for one player mediating the hippocampal network activity (Larson et al., 2016) by electrophysiological recordings showing an increase in the density of Nav activated sodium currents after LBN.
In line with previous studies (Rice et al., 2008; Naninck et al., 2015), we confirmed a strong impact of LBN on circulating CORT plasma concentrations in P9 pups. The plasma CORT concentrations showed considerable variability between litters under strictly controlled and similar experimental conditions. Despite the large number of studies using LBN as a translationally relevant animal model, individuality in the LBN procedure outcome is a so far neglected aspect. In several studies, an increase in plasma CORT concentrations is described as a physiological parameter markedly influenced by LBN; still the specific role of CORT in shaping the long-lasting negative effects of LBN on memory and cognitive function remains to be solved (Chen et al., 2016; Wang et al., 2013). It may thus be that the heterogeneity in CORT response across litters, which we identified as correlating with the number of sorties of the dam and with a decrease of the body weight in the pups, as previously reported in Rice et al. (2008), indicates a variance in model efficiency among individuals. Thus, considering LBN subgroups (i.e. dams and their pups resilient or susceptible to the ELA) could be interesting to consider in future studies to enhance the translational value of the paradigm. It would also be relevant to assess how heterogeneity in the stress response across litters early in life, may elicit a different stress response, both in male and female mice (White and Kaffman, 2019), in the face of a second acute challenge later in life, as has been recently proposed in an in vitro study (Provencal et al., 2020).
Our study shows, that the highly dynamic population of NG2+ glia is a specific target of LBN. NG2+ glia have repeatedly been described to quickly respond to different types of challenges or insults to the adult CNS (Kang et al., 2010; Hughes et al., 2013). The fact that the time window of LBN exposure critically hits the period during which NG2+ cells reach their peak density (first postnatal week) provides a strong scientific rationale to investigate the specific role of NG2+ glia in modulating LBN-associated aversive outcomes. LBN-induced changes in the transcriptional signature of NG2+ cells correlated with the plasma concentrations of the stress hormone CORT. This link between glucocorticoids and NG2 transcriptome changes after LBN exposure was further validated by additional bioinformatic analyses: when integrating our transcriptome data with publicly available datasets on genomic binding sites of GR in neuronal PC12 cells treated with DEX (Polman et al., 2012a, 2012b), we identified several overlapping candidates. Thus, LBN-induced activation of the hypothalamus-pituitary-adrenocortical system, which exposes the developing brain to an excess of glucocorticoids seems to play a crucial role major in shifting gene expression, with possible lasting consequences on NG2+ cell function.
The NG2 population is a prototypical cellular target for stress signals (Birey et al., 2015, Birey et al., 2017), as NG2+ cells express both GR and mineralocorticoid receptors (MR) throughout the maturing stages of the lineage, from OPCS to mature oligodendrocytes (Matsusue et al., 2014). Moreover, CORT treatment has been shown to influence oligodendrogliogenesis in the hippocampus (Wennstrom et al., 2006; Chetty et al., 2014; Alonso, 2000), which may in turn affect neurotransmission, alter neuronal function and ultimately behavior (also discussed in (Chetty et al., 2014)). Thus, it is tempting to speculate that ELA, by altering the transcriptome pattern of OPCs may lead to changes in the communication between OPCs and neurons. ELA elicits a stress response, thus putting the various CNS functions that NG2+ cells homeostatically support immediately at risk. Since NG2+ cells are talking back to the neuronal network (Sakry and Trotter, 2016), such changes could alter neuronal function and ultimately lead to long lasting effects on adult emotional behavior and cognitive performance as observed in ELA animals. In support of this hypothesis, Teissier and colleagues found that maternal separation – i.e. different from LBN - caused an increase in mature oligodendrocytes in stressed pups and a consequent decrease in the number of OPCs in adult animals. Comparable results on the OPC/oligodendrocyte ratio were obtained when medial prefrontal cortex neuron excitability was reduced using designed receptors exclusively activated by the designed drug (DREADD) technique during the first 2 postnatal weeks in control pups which mimicked ELA-like behavioral alterations later in adulthood (Teissier et al., 2020). Hence, it may well be that imbalance in the OPC/oligodendrocyte ratio can cause changes in NG2+ cell-neuron-communication and finally lead to negative behavioral outcomes, as with maturation the expression of NG2 is lost concomitant with the loss of synaptic contact between OPCs and neurons (Kukley et al., 2010; Etxeberria et al., 2010).
Considering the remarkable potential of NG2+ cells to homeostatically modulate brain function, LBN-induced upregulation of Scn7a in NG2+ cells is an intriguing finding. This gene encodes for a subunit of sodium channels widely expressed by OPCs throughout their lifespan. The channels are fundamental in the transduction of neuronal input onto OPCs (Spitzer et al., 2019). Given that Scn7a was highly correlated with the concentration of circulating CORT at P9, differentially bound in ChIP-seq studies targeting GR-responsive transcripts and that a targeted analysis revealed its upregulation in adult LBN animals, it is a distinct possibility that Scn7a is involved in modulating LBN-induced behavioral changes observed in our adult mice. So far, evidence for an involvement of Scn7a in psychiatric disease phenotypes is limited to a recent genome-wide association study showing that a single nucleotide polymorphism in the SCN7A gene is associated with response to treatment in depressed patients (Biernacka et al., 2016). Data on the exact role of Nav channels in NG2+ cells both under physiological as well as disease-predisposing conditions such as ELA are still lacking. Patch-clamp recordings have confirmed the expression of functional Nav channels in NG2+ cells (Black and Waxman, 2013; Sontheimer et al., 1989; Karadottir et al., 2008; Chen et al., 2008; Barres et al., 1988). Interestingly, the expression of Nav channels in NG2+ cells follows specific temporal dynamics and changes depend on the developmental stage (Black and Waxman, 2013), with higher Nav channel density in the NG2+ proliferative stage (Spitzer et al., 2019) but decreased density upon maturation (De Biase et al., 2010; Sontheimer et al., 1989; Barres et al., 1988). Under physiological conditions, the expression of Nav channels is relevant for OPC-neuron communication. Nav channel-mediated electrical activity may serve as a signal between unmyelinated axonal sections and OPCs that are ready to differentiate into oligodendrocytes and are in turn capable of myelinating these axonal targets (Black and Waxman, 2013; Karadottir et al., 2008; Paez et al., 2009). Considering that ELA and an excess of glucocorticoid hormones might exert a plethora of cellular and molecular effects it is tempting to speculate that LBN exposure, most likely via an increase in circulating glucocorticoids, enhances Nav density in NG2+ cells. This increase in Nav density may alter the responsiveness of NG2+ cells to neurons with long-lasting negative outcomes on brain function and complex behavioral phenotypes.
There are two major limitations of the present study. We did not take into account sex-differences in the effects of LBN on NG2+ cell transcriptome and function but only focused on male animals. In addition, the finding that LBN caused persistent elevation of Scn7a in NG2+ cells purified from the adult hippocampus should ideally be replicated in an independent cohort of adult animals to elucidate the robustness of our findings.
We are confident that our data will lay the basis for future studies investigating specific effects and impact of stress, early adversity and specifically CORT on the maturation of NG2+ cells, NG2+ cell-neuron-communication and network homeostasis. Specifically, restoration of an LBN-induced dysfunction in NG2+ cell-neuron communication could represent a conceptually novel and promising therapeutic avenue to explore in the future.
CRediT authorship contribution statement
Giulia Treccani: Conceptualization, Methodology, Investigation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. Hatice Yigit: Methodology, Investigation, Formal analysis, Writing – review & editing. Thomas Lingner: Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Vanessa Schleuβner: Methodology, Investigation, Formal analysis, Writing – review & editing. Franziska Mey: Methodology, Investigation, Formal analysis, Writing – review & editing. Michael A. van der Kooij: Methodology, Investigation, Formal analysis, Writing – review & editing. Malin Wennström: Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. David P. Herzog: Methodology, Investigation, Formal analysis, Writing – review & editing. Matthias Linke: Methodology, Investigation, Formal analysis, Writing – review & editing. Markus Fricke: Methodology, Investigation, Formal analysis. Michael J. Schmeisser: Funding acquisition, Writing – review & editing. Gregers Wegener: Funding acquisition, Writing – review & editing. Thomas Mittmann: Funding acquisition, Writing – original draft, Writing – review & editing. Jacqueline Trotter: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. Marianne B. Müller: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of competing interest
GW reported having received lecture/consultancy fees from H. Lundbeck A/S, Servier SA, Astra Zeneca AB, Eli Lilly A/S, Sun Pharma Pty Ltd, Pfizer Inc, Shire A/S, HB Pharma A/S, Arla Foods A.m. b.A., Alkermes Inc, and Mundipharma International Ltd., Janssen AB. All other authors report no biomedical financial interests or potential conflicts of interest. This manuscript has been posted on a preprint server.
Acknowledgment
This work was supported by a DFF Postdoctoral grant from the The Danish Council for Independent Research (DFF-5053-00103), from the Dr.phil. Ragna Rask-Nielsen Grundforskningsfond and by an intramural research funding from the University Medical Center of the Johannes Gutenberg-University Mainz both to GT, and by funding from the German Research Foundation to TM (CRC 1080 TP CO2), to JT and TM (MI 452/6-1) and from the German Foundation to JT (CRC 128, TP B07) and to MJS (CRC 1080, TP B10). MJS was further supported by the Else Kröner Fresenius Foundation (grant 2018_A78), the Volkswagen Foundation and the German Ministry of Education and Research (GeNeRARe, 01GM1519A). The support by the IMB Genomics Core Facility in Mainz, the use of its NextSeq 500 (INST 247/870‐1 FUGG) and the support by the IMB Microscopy Core Facility are gratefully acknowledged. We are very grateful to Dr Inge Sillaber (Genevention) and Dr Filippo Calzolari for critical discussion and to Anna-Lena Schlegelmilch, Annika Hasch, Verena Opitz, Julia Deuster, Jennifer Klüpfel and Dr Konstantin Radyushkin for technical support during the experiments. We thank Roberto Danesi for providing the video editing program. A portion of the work described herein was carried out by Franziska Mey in partial fulfilment of the requirements for a medical doctoral degree at the Johannes Gutenberg University Medical Center Mainz, Germany.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100338.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31(3):219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bachmann C.G., Linthorst A.C., Holsboer F., Reul J.M. Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology. 2003;28(6):1056–1067. doi: 10.1038/sj.npp.1300158. [DOI] [PubMed] [Google Scholar]
- Barateiro A., Brites D., Fernandes A. Oligodendrocyte development and myelination in neurodevelopment: molecular mechanisms in health and disease. Curr. Pharmaceut. Des. 2016;22(6):656–679. doi: 10.2174/1381612822666151204000636. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Chun L.L., Corey D.P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Bergles D.E., Jabs R., Steinhauser C. Neuron-glia synapses in the brain. Brain Res. Rev. 2010;63(1–2):130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Zhu T., Stamoulis C., Fox N.A., Zeanah C., Nelson C.A. Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr. 2015;169(3):211–219. doi: 10.1001/jamapediatrics.2014.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacka J.M., Sangkuhl K., Jenkins G., Whaley R.M., Barman P., Batzler A. The International SSRI Pharmacogenomics Consortium (ISPC): a genome-wide association study of antidepressant treatment response. Transl. Psychiatry. 2016;6(11):e937. doi: 10.1038/tp.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F., Kloc M., Chavali M., Hussein I., Wilson M., Christoffel D.J. Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron. 2015;88(5):941–956. doi: 10.1016/j.neuron.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F., Kokkosis A.G., Aguirre A. Oligodendroglia-lineage cells in brain plasticity, homeostasis and psychiatric disorders. Curr. Opin. Neurobiol. 2017;47:93–103. doi: 10.1016/j.conb.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J.A., Waxman S.G. Noncanonical roles of voltage-gated sodium channels. Neuron. 2013;80(2):280–291. doi: 10.1016/j.neuron.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Cahoy J.D., Emery B., Kaushal A., Foo L.C., Zamanian J.L., Christopherson K.S. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.H., Cai W.Q., Wang L.Y., Deng Q.Y. A morphological and electrophysiological study on the postnatal development of oligodendrocyte precursor cells in the rat brain. Brain Res. 2008;1243:27–37. doi: 10.1016/j.brainres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Chen Y., Molet J., Lauterborn J.C., Trieu B.H., Bolton J.L., Patterson K.P. Converging, synergistic actions of multiple stress hormones mediate enduring memory impairments after acute simultaneous stresses. J. Neurosci. 2016;36(44):11295–11307. doi: 10.1523/JNEUROSCI.2542-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty S., Friedman A.R., Taravosh-Lahn K., Kirby E.D., Mirescu C., Guo F. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol. Psychiatr. 2014;19(12):1275–1283. doi: 10.1038/mp.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.S., Welsh C.A., Barres B.A., Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015;18(11):1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase L.M., Nishiyama A., Bergles D.E. Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 2010;30(10):3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diers-Fenger M., Kirchhoff F., Kettenmann H., Levine J.M., Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001;34(3):213–228. doi: 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- Dimou L., Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63(8):1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L., Gotz M. Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain. Physiol. Rev. 2014;94(3):709–737. doi: 10.1152/physrev.00036.2013. [DOI] [PubMed] [Google Scholar]
- Etxeberria A., Mangin J.M., Aguirre A., Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat. Neurosci. 2010;13(3):287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes T.A., Gallo V. All wrapped up: environmental effects on myelination. Trends Neurosci. 2017;40(9):572–587. doi: 10.1016/j.tins.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Mangin J.M., Kukley M., Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J. Physiol. 2008;586(16):3767–3781. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M., Shleifer D.G., Godoy L.D., Ofray D., Olaniyan A., Campbell T. Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Front. Behav. Neurosci. 2019;13:167. doi: 10.3389/fnbeh.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Mletzko T., Miller A.H., Nemeroff C.B. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hill R.A., Patel K.D., Goncalves C.M., Grutzendler J., Nishiyama A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat. Neurosci. 2014;17(11):1518–1527. doi: 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch-Kraft P., White R., Tenzer S., Kramer-Albers E.M., Trotter J., Gonsior C. Dual role of the RNA helicase DDX5 in post-transcriptional regulation of myelin basic protein in oligodendrocytes. J. Cell Sci. 2018;131(9) doi: 10.1242/jcs.204750. [DOI] [PubMed] [Google Scholar]
- Hodel A.S., Hunt R.H., Cowell R.A., Van Den Heuvel S.E., Gunnar M.R., Thomas K.M. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., McCormack K.M., Grand A.P., Sawyer N.T., Zhang X., Maestripieri D. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biol. Mood Anxiety Disord. 2013;3(1):21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes E.G., Kang S.H., Fukaya M., Bergles D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013;16(6):668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J.A., Zylka M.J. Controlling litter effects to enhance rigor and reproducibility with rodent models of neurodevelopmental disorders. J. Neurodev. Disord. 2021;13(1):2. doi: 10.1186/s11689-020-09353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.H., Fukaya M., Yang J.K., Rothstein J.D., Bergles D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68(4):668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R., Hamilton N.B., Bakiri Y., Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 2008;11(4):450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram K., Goebbels S., Schwab M., Jennissen K., Seifert G., Steinhauser C. NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis. 2008;46(12):743–757. doi: 10.1002/dvg.20440. [DOI] [PubMed] [Google Scholar]
- Kraaijenvanger E.J., Pollok T.M., Monninger M., Kaiser A., Brandeis D., Banaschewski T. Impact of early life adversities on human brain functioning: a coordinate-based meta-analysis. Neurosci. Biobehav. Rev. 2020;113:62–76. doi: 10.1016/j.neubiorev.2020.03.008. [DOI] [PubMed] [Google Scholar]
- Kukley M., Nishiyama A., Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J. Neurosci. 2010;30(24):8320–8331. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson V.A., Zhang Y., Bergles D.E. Electrophysiological properties of NG2(+) cells: matching physiological studies with gene expression profiles. Brain Res. 2016;1638(Pt B):138–160. doi: 10.1016/j.brainres.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazic S.E., Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci. 2013;14:37. doi: 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J., Humphreys K.L., Tracy A., Hoffmeister J.A., Ip E., Gotlib I.H. Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59(7):842–855. doi: 10.1016/j.jaac.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.S., Chao Y.S. Electrolytic lesions of dorsal CA3 impair episodic-like memory in rats. Neurobiol. Learn. Mem. 2008;89(2):192–198. doi: 10.1016/j.nlm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Liu Y., Aguzzi A. NG2 glia are required for maintaining microglia homeostatic state. Glia. 2020;68(2):345–355. doi: 10.1002/glia.23721. [DOI] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Makinodan M., Rosen K.M., Ito S., Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337(6100):1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras P.M., Molet J., Chen Y., Rice C., Ji S.G., Solodkin A. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multi-modal stress. Mol. Psychiatr. 2014;19(7):745. doi: 10.1038/mp.2014.64. [DOI] [PubMed] [Google Scholar]
- Marques S., Zeisel A., Codeluppi S., van Bruggen D., Mendanha Falcao A., Xiao L. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352(6291):1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue Y., Horii-Hayashi N., Kirita T., Nishi M. Distribution of corticosteroid receptors in mature oligodendrocytes and oligodendrocyte progenitors of the adult mouse brain. J. Histochem. Cytochem. 2014;62(3):211–226. doi: 10.1369/0022155413517700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Kinney-Lang E., Harris N.G., Rashid F., Ivy A.S. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016;26(12):1618–1632. doi: 10.1002/hipo.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby D.G., Gaskin S., Glenn M.J., Schramek T.E., Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck E.F., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S.E., Lucassen P.J. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25(3):309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Nanni V., Uher R., Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatr. 2012;169(2):141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Niehaus A., Stegmuller J., Diers-Fenger M., Trotter J. Cell-surface glycoprotein of oligodendrocyte progenitors involved in migration. J. Neurosci. 1999;19(12):4948–4961. doi: 10.1523/JNEUROSCI.19-12-04948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Komitova M., Suzuki R., Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009;10(1):9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Paez P.M., Fulton D., Colwell C.S., Campagnoni A.T. Voltage-operated Ca(2+) and Na(+) channels in the oligodendrocyte lineage. J. Neurosci. Res. 2009;87(15):3259–3266. doi: 10.1002/jnr.21938. [DOI] [PubMed] [Google Scholar]
- Polman J.A., Welten J.E., Bosch D.S., de Jonge R.T., Balog J., van der Maarel S.M. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci. 2012;13:118. doi: 10.1186/1471-2202-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman J.A., Hunter R.G., Speksnijder N., van den Oever J.M., Korobko O.B., McEwen B.S. Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology. 2012;153(9):4317–4327. doi: 10.1210/en.2012-1255. [DOI] [PubMed] [Google Scholar]
- Provencal N., Arloth J., Cattaneo A., Anacker C., Cattane N., Wiechmann T. Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. Proc. Natl. Acad. Sci. U. S. A. 2020;117(38):23280–23285. doi: 10.1073/pnas.1820842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C.J., Sandman C.A., Lenjavi M.R., Baram T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149(10):4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D., Trotter J. The role of the NG2 proteoglycan in OPC and CNS network function. Brain Res. 2016;1638(Pt B):161–166. doi: 10.1016/j.brainres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Sakry D., Karram K., Trotter J. Synapses between NG2 glia and neurons. J. Anat. 2011;219(1):2–7. doi: 10.1111/j.1469-7580.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D., Neitz A., Singh J., Frischknecht R., Marongiu D., Biname F. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 2014;12(11) doi: 10.1371/journal.pbio.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M., Meaney M.J. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396(1):64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V., Enthoven L., van der Mark M., Levine S., de Kloet E.R., Oitzl M.S. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int. J. Dev. Neurosci. 2003;21(3):125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Singh-Taylor A., Molet J., Jiang S., Korosi A., Bolton J.L., Noam Y. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol. Psychiatr. 2018;23(3):648–657. doi: 10.1038/mp.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H., Trotter J., Schachner M., Kettenmann H. Channel expression correlates with differentiation stage during the development of oligodendrocytes from their precursor cells in culture. Neuron. 1989;2(2):1135–1145. doi: 10.1016/0896-6273(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Spitzer S.O., Sitnikov S., Kamen Y., Evans K.A., Kronenberg-Versteeg D., Dietmann S. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron. 2019;101(3):459–471 e5. doi: 10.1016/j.neuron.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Matthews E.A., Nicolas V., Schoch S., Dietrich D. NG2 glial cells integrate synaptic input in global and dendritic calcium signals. Elife. 2016;5 doi: 10.7554/eLife.16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti A., Kim J.J., Wakid M., Davoli M.A., Turecki G., Mechawar N. Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol. Psychiatr. 2018;23(10):2018–2028. doi: 10.1038/mp.2017.231. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Ohashi K., Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol. Psychiatr. 2014;76(4):297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier A., Le Magueresse C., Olusakin J., Andrade da Costa B.L.S., De Stasi A.M., Bacci A. Early-life stress impairs postnatal oligodendrogenesis and adult emotional behaviour through activity-dependent mechanisms. Mol. Psychiatr. 2020;25(6):1159–1174. doi: 10.1038/s41380-019-0493-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J., Karram K., Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res. Rev. 2010;63(1–2):72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij M.A., Grosse J., Zanoletti O., Papilloud A., Sandi C. The effects of stress during early postnatal periods on behavior and hippocampal neuroplasticity markers in adult male mice. Neuroscience. 2015;311:508–518. doi: 10.1016/j.neuroscience.2015.10.058. [DOI] [PubMed] [Google Scholar]
- Vigano F., Dimou L. The heterogeneous nature of NG2-glia. Brain Res. 2016;1638(Pt B):129–137. doi: 10.1016/j.brainres.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Wang X.D., Su Y.A., Wagner K.V., Avrabos C., Scharf S.H., Hartmann J. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat. Neurosci. 2013;16(6):706–713. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- Wennstrom M., Hellsten J., Ekstrand J., Lindgren H., Tingstrom A. Corticosterone-induced inhibition of gliogenesis in rat hippocampus is counteracted by electroconvulsive seizures. Biol. Psychiatr. 2006;59(2):178–186. doi: 10.1016/j.biopsych.2005.08.032. [DOI] [PubMed] [Google Scholar]
- White J.D., Kaffman A. The moderating effects of sex on consequences of childhood maltreatment: from clinical studies to animal models. Front. Neurosci. 2019;13:1082. doi: 10.3389/fnins.2019.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Cheng Z., Tang H., Jiao H., Sun X., Cui Q. Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of wnt signaling. Cerebr. Cortex. 2017;27(5):2871–2884. doi: 10.1093/cercor/bhw121. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Fields R.D., Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O'Keeffe S. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.