Summary
CD8+ T cells are key effector cells in adaptive immune responses against intracellular pathogens and cancer cells. Systemic drug treatments, like chemotherapy, may positively or negatively affect CD8+ T cell function. In this protocol, we describe robust and optimized ex vivo polyclonal activation and cell culture conditions to measure drug treatments' effects on primary human CD8+ T cell activation and cytolytic potential. We provide streamlined methods for measuring effector cytokines and activation markers of CD8+ T cells via flow cytometry.
For complete details on the use and execution of this protocol, please refer to Loo Yau et al. (2021).
Subject areas: Cell isolation, Flow Cytometry/Mass Cytometry, Cell-based Assays, Immunology, Molecular/Chemical Probes
Graphical Abstract

Highlights
-
•
Protocol to measure effects of drug treatments on primary human CD8+ T cell
-
•
Method to measure effector and activation molecules of CD8+ T cells via flow cytometry
-
•
Detailed steps and resources to measure changes in CD8+ T cell cytolytic potential
CD8+ T cells are key effector cells in adaptive immune responses against intracellular pathogens and cancer cells. Systemic drug treatments, like chemotherapy, may positively or negatively affect CD8+ T cell function. In this protocol, we describe robust and optimized ex vivo polyclonal activation and cell culture conditions to measure drug treatments' effects on primary human CD8+ T cell activation and cytolytic potential. We provide streamlined methods for measuring effector cytokines and activation markers of CD8+ T cells via flow cytometry.
Before you begin
Prepare all the necessary buffers in sterile conditions and store them according to the following conditions. Obtain all necessary reagents and materials for CD8+ T cell isolation and downstream functional assessments (See key resources table for a list of suggested reagents).
It is important to note that the lab must obtain permission the institution’s Research Ethics Board approval when working with human biological material; blood donors also need to give and sign consent for blood draws. Additionally, individuals performing this protocol will require safety training on the proper handling and disposal of human samples. We recommend processing the whole blood sample within the same day of collection.
Lymphoprep aliquoting
Lymphoprep is a density gradient medium used for the isolation of mononuclear cells. The density gradient medium ensures the separation of the buffy coat during centrifugation. The buffy coat contains peripheral mononuclear blood cells (PMBCs) in a concentrated suspension (Fuss et al., 2009). Alternative density gradient medium may be used. We suggest aliquoting 12 mL of density gradient medium in 50 mL conical tubes at room temperature (22°C–25°C) prior to starting the protocol.
Drug treatments or compound stock preparation
We recommend aliquoting drug components at 10 mM as one-time use 15 μL stocks; avoid freeze thaw to maintain consistency throughout replicate experiments. The protocol below was followed to activate CD8+ T cells in the presence of DNA demethylating agent, decitabine, as described in Loo Yau et al., However, this protocol is applicable to other drug compounds of interest.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Flow cytometry antibodies/reagents | ||
| Anti-human CD3 | BioLegend | Cat# 317308 (PE), 317306 (FITC), 317342; RRID AB_571913, AB_571907, AB_2563410 |
| Anti-human CD4 | BioLegend | Cat# 317408; RRID AB_571951 |
| Anti-human CD8 | BioLegend | Cat# 344722 (APC), 344704 (FITC), 344714; RRID AB_2075388, AB_1877178, AB_2044006 |
| Anti-human CD69 | BioLegend | Cat# 310914; RRID AB_314849 |
| Anti-human CD25 | BioLegend | Cat# 302606; RRID AB_314276 |
| Anti-human HLA-DR | BioLegend | Cat# 307610; RRID AB_314688 |
| Anti-human/mouse Granzyme B | BioLegend | Cat# 515403; RRID AB_2114575 |
| Anti-human TNF-alpha | BioLegend | Cat# 502908; RRID AB_315260 |
| Anti-human IFN-gamma | BioLegend | Cat# 506510; RRID AB_2623781 |
| FITC Mouse IgG1, κ Isotype Ctrl Antibody | BioLegend | Cat# 400108 |
| PE Mouse IgG1, κ Isotype Ctrl Antibody | BioLegend | Cat# 400112 |
| APC Mouse IgG1, κ Isotype Ctrl Antibody | BioLegend | Cat# 400120 |
| True-Nuclear Transcription Factor Buffer Set | BioLegend | Cat# 424401 |
| SYTOX Blue Viability Dye | Thermo Fisher Scientific | Cat# S34857 |
| LIVE/DEAD Fixable Aqua Dead Cell Dye | Thermo Fisher Scientific | Cat# L-34966 |
| Biological samples | ||
| Human blood from healthy donors | UHN-REB (No. 11-0343-CE) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Lymphoprep | STEMCELL Technologies | Cat# 07851 |
| RPMI 1640 | Wisent | Cat# 350-000-CL |
| HBSS | Wisent | Cat# 311-513-CL |
| Human AB Serum | Sigma | Cat# H4522 |
| Fetal Bovine Albumin | Thermo Fisher Scientific | Cat# 12483020 |
| Bovine Serum Albumin | BioShop | Cat# ALB001 |
| Human IL-2 | Miltenyi Biotec | Cat# 130-097-743 |
| HEPES (1 M) | Thermo Fisher Scientific | Cat# 15630-080 |
| Penicillin-Streptomycin-Glutamine (100×) | Thermo Fisher Scientific | Cat# 10378-016 |
| Ammonium chloride (NH4Cl) | Sigma | Cat# 213330 |
| Potassium bicarbonate (KHCO3) | Sigma | Cat# 237205 |
| EDTA (0.5 M) | Thermo Fisher Scientific | Cat# 15575-020 |
| Phosphate-buffered saline (PBS) | Thermo Fisher Scientific | Cat# 10010049 |
| HBSS | Thermo Fisher Scientific | Cat# 14025092 |
| 5-Aza-2-deoxycytidine | Sigma | Cat# A3656-5MG |
| Critical commercial assays | ||
| CD8+ T cells negative selection kit | Miltenyi Biotec | Cat# 130-096-495 |
| CD8 MicroBeads | Miltenyi Biotec | Cat# 130-045-201 |
| Dynabeads Human T-Activator CD3/CD28 | Thermo Fisher Scientific | Cat# 11131D |
| Cell Stimulation plus Protein secretion inhibitor Cocktail (Cocktail 1 in this protocol) | Thermo Fisher Scientific | Cat# 00-4975-03 |
| Protein secretion inhibitor Cocktail (Cocktail 2 in this protocol) | Thermo Fisher Scientific | Cat# 00-4980-93 |
| QuadroMACS™ Starting Kit (LS) | Miltenyi Biotec | Cat# 130-091-051 |
| Other | ||
| DynaMag-2 Magnet | Thermo Fisher Scientific | Cat# 12321D |
| Suspension, U-bottom, 96-well culture plate | Sarstedt | Cat# 83.3925.500 |
| 96-Well Clear V-Bottom TC-Treated Microplate | VWR | Cat# 29442-068 |
| Bovine Serum Albumin | BioShop | Cat# ALB001 |
| 10 mL Pipette Reservoirs, Sterile | Diamed | Cat# MTCP8010-5S |
| UltraComp eBeads™ Plus Compensation Beads | Thermo Fisher Scientific | Cat# 01-3333-41 |
| ArC™ Amine Reactive Compensation Beads | Thermo Fisher Scientific | Cat# A-10346 |
| Trypan Blue Solution, 0.4% | Thermo Fisher Scientific | Cat# 15250061 |
| Software and algorithms | ||
| FlowJo | BD Biosciences | www.flowjo.com |
Materials and equipment
| Interleukin-2 stock | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| Human IL-2 (powder, research grade) | 5000 IU total | X μg a |
| RPMI 1640 media | n/a | 10 μL |
| Total | n/a | 10 μL |
Store at −80°C for up to 12 months.
This value is calculated by converting 5000 IU to the value in μg based on the Biological Activity in IU/mg collected by the vendor, and may be lot dependent.
Alternatives: Fetal Bovine Serum may be used in place of Human AB Serum. In our experience, Human AB Serum yields consistent results.
Note: IL-2 is often sold in ‘μg’ formats. To maintain consistency throughout experiments, it is recommended to obtain the Biological Activity in IU/mg of the particular stock from the vendor and aliquot as one-time use stocks. For example, 5000 IU of IL-2 are required for 500 mL of media at 10 IU/mL IL-2. We recommend making calculations to resuspend 5000 IU in 10 μL of non-supplemented RPMI 1640 media. Stocks may be stored as 5000 IU per aliquot at −80°C.
| T cell culture medium with IL-2 | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| RPMI 1640 media | n/a | 440 mL |
| L-glutamine, Penicillin streptomycin (100×) | 1× | 5 mL |
| HEPES (1 M) | 10 mM | 5 mL |
| Human AB serum | 10% | 50 mL |
| IL-2 (resuspended stock) | 10 IU/mL | 5000 IU in 10 μL |
| Total | n/a | 500 mL |
Store at 4°C for up to 1 month.
Alternatives: Fetal Bovine Serum may be used in place of Human AB Serum. In our experience, Human AB Serum yields consistent results.
| MACS Buffer | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| HBSS | 1× | 498 mL |
| Bovine Serum Albumin | 0.5% | 2.5 grams |
| EDTA (0.5 M) | 2 mM | 2 mL |
| Total | n/a | 500 mL |
Store at 4°C for up to 1 month.
| FACS buffer | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| HBSS or PBS | 1× | 488 mL |
| Fetal Bovine Albumin | 2% | 10 mL |
| EDTA (0.5 M) | 2 mM | 2 mL |
| Total | n/a | 500 mL |
Store at 4°C for up to 1 month.
| Red blood cell lysis buffer (10×) | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| NH4Cl | 1500 mM | 80.2 g |
| KHCO3 | 100 mM | 10 g |
| EDTA (0.5 M) | 1 mM | 2 mL |
| Sterile Water | n/a | 998 mL |
| Total | n/a | 1000 mL |
Sterile filter and store at 4°C for up to 12 months.
| Decitabine stock (or 5-Aza-2′-deoxycytidine) | ||
|---|---|---|
| Reagent | Final concentration | Amount |
| 5 -Aza-2′-deoxycytidine (powder, 5mg) | 10 mM | 5 mg |
| Sterile Water | n/a | 2.2 mL |
| Total | n/a | 2.2 mL |
Sterile filter, aliquot and store at −80°C for up to 12 months.
Step-by-step method details
Labeling and isolation of primary CD8+ T cells from whole blood
Timing: [2 to 2.5 h]
This section describes how to process human whole blood to obtain the buffy coat. We recommend processing the blood shortly after or within the same day of blood draw. All work in this section needs to be performed in a sterile biological safety cabinet (or tissue culture hood). All equipment (pipettors), plastic ware (serological pipettes, pipette tips, conical tubes) need to be sterile. All work surfaces need to be cleaned with 70% ethyl alcohol before beginning.
-
1.
Aliquot 12 mL Lymphoprep into 50 mL conical tubes.
-
2.Dilute whole blood using non-supplemented RPMI 1640 media.
-
a.In a sterile flask, dilute blood at roughly 1:1 ratio with non-supplemented RPMI 1640 media.
-
b.Mix well by pipetting up and down using a 25 mL serological pipette.
-
a.
Note: In our experience, we obtain 80–100 mL of whole blood collected in 10mL K2-EDTA BD Vacutainer tubes. All tubes are pooled into a sterile flask. Each tube is rinsed with 10 mL of non-supplemented RPMI 1640 and added to the sterile flask. In the end, about 200mL of diluted blood is prepared for layering onto 6 Lymphoprep conical tubes.
CRITICAL: Make sure to set the pipettor to its slowest dispense rate prior to next step. It is critical that the diluted blood does not mix with the Lymphoprep to prevent red blood cell contamination and proper separation of blood components.
-
3.
Carefully layer up to 36 mL of diluted whole blood onto Lymphoprep aliquots.
-
4.Carefully place and balance the conical tubes in the centrifuge.
-
a.Centrifuge for 25 min at 600 g, zero or lowest acceleration and deceleration, room temperature.
-
a.
CRITICAL: The centrifugation settings at zero or lowest acceleration and deceleration are critical to ensure proper separation of blood components (Figure 1).
Figure 1.
Layers of blood components after centrifugation of blood on density gradient medium
Image made with BioRender.
Note: After centrifugation, carefully transport the tubes back to the sterile biological safety cabinet. A clear layering of the different blood components should be seen, with a large white band of cells in the middle of the conical tube.
-
5.
After centrifugation, set the centrifuge back to highest acceleration and deceleration, and 4°C temperature settings.
-
6.
Aspirate and dispose of the plasma component (top layer) from the conical tube.
-
7.
Slowly aspirate the buffy coat and place in clean 50 mL conical tube.
Note: Buffy coats from 2 centrifuged conical tubes may be combined into 1 clean 50 mL conical tube. For example, 6 tubes that were layered with whole blood can be pooled into 3 tubes containing buffy coats.
-
8.
Top up the conical tubes containing pooled buffy coats with non-supplemented RPMI 1640 media.
-
9.
Centrifuge at 300 g for 10 min, at 4°C.
-
10.
Aspirate and discard supernatants.
-
11.
Wash cell pellet by adding 40 mL of non-supplemented RPMI 1640 media and centrifuge at 300 g for 10 min, at 4°C. Aspirate and discard supernatant.
-
12.
Repeat step 11.
Note: Washing steps are critical to remove the Lymphoprep to ensure clean labelling and isolation of CD8+ T cells.
-
13.During centrifugation, prepare 10 mL of 1× Red Blood Cell (RBC) lysis solution from 10× stock.
-
a.Mix 1 part of 10× RBC lysis solution in 9 parts of sterile water.
-
a.
-
14.
Resuspend each cell pellet slowly, with 2 mL 1× RBC lysis solution.
-
15.
Incubate for 2 min at room temperature.
-
16.
Add 5 mL of MACS buffer to each tube and pool all cell suspensions into a singular 50 mL conical tube.
-
17.
Top up to 50 mL with MACS buffer and count the number of cells and the viability with ready-to-use trypan blue (0.4%) solution.
-
18.
Centrifuge at 300 g for 10 min at 4°C and discard supernatant.
-
19.
Proceed to CD8+ T cell magnetic labeling following the manufacturer recommendations.
CRITICAL: There are different cell isolation systems that can be used to purify human CD8+ T cells from a mixture of cells. We recommend the systems from Miltenyi Biotec: “CD8+ T Cell Isolation Kit” or the “CD8 MicroBeads” isolation kit. Catalogue number for both kits is provided in the key resources table. We also recommend setting up the QuadroMACS magnets prior to starting or during centrifugation waiting times.
-
20.
After collecting purified CD8+ T cells, centrifuge at 300 g for 10 min at 4°C and discard MACS buffer supernatant.
-
21.
Resuspend in 2 mL of supplemented T cell culture media with IL-2.
-
22.
Centrifuge at 300 g for 10 min at 4°C and discard supernatant.
-
23.
Resuspend purified CD8+ T cells in 5 mL of supplemented T cell culture media with IL-2.
-
24.
Count the number of cells and viability with ready-to-use trypan blue (0.4%) solution.
Optional: Measure purity of CD8+ T cell populations by staining with 1:100 concentration of CD3, CD4, CD8 antibodies diluted in 100 μL FACS Buffer; incubate for 30 min at room temperature, wash with FACS buffer and proceed for flow cytometry. See Table 1 for a panel to measure CD8+ purity with minimal flow cytometry compensation steps. A recommended minimum of 100,000 cells are needed to measure CD8+ T cell purity.
Table 1.
Flow cytometry antibodies and reagents to measure CD8+ purity after T cell isolation per 100,000 T cells
| Stain | Stain dilution |
|---|---|
| SYTOX Blue Viability Dye | 1 in 1,000 in FACS Buffer |
| CD3 in PE | 1 in 100 in FACS Buffer |
| CD4 in FITC | 1 in 100 in FACS Buffer |
| CD8 in APC | 1 in 100 in FACS Buffer |
Measuring drug response during in vitro activation of human CD8+ T cells
Timing: [1 to 5 days]
This section describes the T cell culture conditions to activate human CD8+ T cells to assess responses to drug treatments of choice.
-
25.
Upon obtaining the total CD8+ T cell count, resuspend the CD8+ T cells at 200,000 cells per mL in supplemented T cell media with IL-2.
-
26.
Transfer resuspended CD8+ T cells into a sterile reagent reservoir.
-
27.
Using a multichannel pipette, transfer 50 μL of the resuspended CD8+ T cells to U-bottom 96-well plates designed to culture suspension cells.
Note: Resuspending CD8+ T cells at 200,000 cells per mL and transferring 50 μL to each well of a U-bottom 96-well plate corresponds to 10,000 cells per well.
-
28.
Transfer sufficient of CD3/CD28 Human T-Activator Dynabeads, at 1 bead to 2 T cells ratio to a sterile 1.5 mL microcentrifuge. This translates to 5,000 Dynabeads for 10,000 T cells in each well.
CRITICAL: The Dynabeads Human T-Activator CD3/CD28 stock is at 40 million beads per mL in phosphate buffered saline (PBS), pH 7.4, with 0.1% human serum albumin. Prior to adding Dynabeads to the seeded T cells, the magnetic beads need to be washed in the T cell culture media. This can be performed either by centrifugation (300 g, 5 min) or using a magnet (e.g., DynaMag2 Magnet).
Example: If 1 million CD8+ T cells resuspended in 5 mL of T cell media were seeded in 100 U-bottom wells, 0.5 million Dynabeads should be resuspended in 5 mL of T cell media. Then, add 50 μL of resuspended Dynabeads for each well.
-
29.
Resuspend CD3/CD28 Human T-Activator Dynabeads at 100,000 beads per mL in supplemented T cell media with IL-2.
-
30.
Transfer resuspended Dynabeads into a sterile reagent reservoir.
-
31.
Using a multichannel pipette, transfer 50 μL of the Dynabeads to the T cells seeded in the U-bottom 96-well plates.
Note: CD3/CD28 Human T-Activator Dynabeads at 100,000 beads per mL and transferring 50 μL to each well of the U-bottom 96-well plate corresponds to 5,000 beads per well. Since 10,000 T cells were seeded prior to bead addition, this ensures 1 bead to 2 T cells in vitro activation conditions.
CRITICAL: Prepare and add the T cells and beads in two separate steps. Combining these two steps together will result in uneven distribution of Dynabeads and T cells as the Dynabeads tend to stick to T cells immediately.
-
32.Prepare working stock of drug of choice.
-
a.Prepare 2× working stock solution of drug treatments.
-
b.Transfer drug of choice to a sterile reagent reservoir.
-
c.Add 100 μL of 2× working stock solution to appropriate wells.
-
d.For the non-treatment wells, add 100 μL of supplemented T cell media with IL-2 with vehicle control.
-
a.
Note: In Loo Yau et al., we expanded T cells with 100 nM and 300 nM decitabine based on previous work published by our group. Thus, 200 nM and 600 nM working stocks were prepared from 10 mM stocks prior to adding to T cells. It is important to note that drug of choice treatments may have apoptotic effects which can impact T cell number at the time of functional read-outs.
Note: Final volume per well, after sequentially adding CD8+ T cells, beads and drug treatments, should be 200 uL.
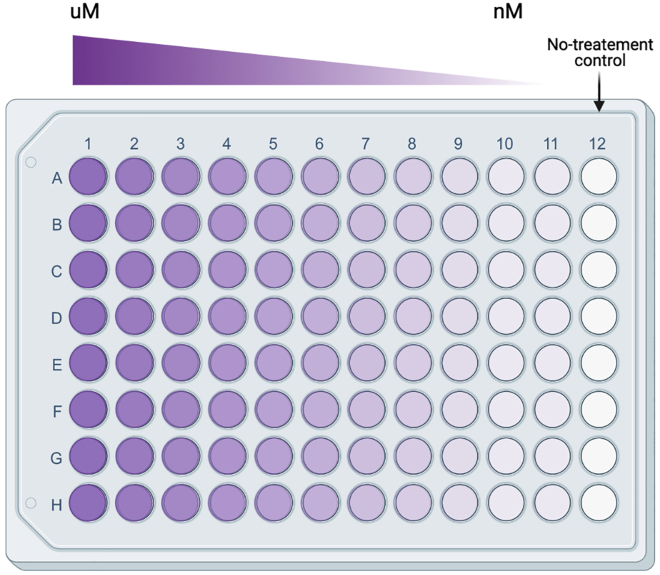
CRITICAL: Initial optimization experiments with these culture conditions should include a dosage titration of concentrations that include nanomolar doses to micromolar doses of drug treatments. It is recommended to test several doses. See Figure 2 for a recommendation on how to set up the culture plate.
Figure 2.
Recommended 96-well set up to test the effects of range of drug treatments on T cell activation and cytolytic potential
Image made with BioRender.
Note: Depending on the half-life of the compound in question, supplemented T cell media with IL-2 containing fresh drug may need to be replenished. See Figure 3 as an example of CD8+ T cell activation in the presence of the pharmacological inhibitor, decitabine.
Figure 3.
Schematic of ex-vivo activation of primary human CD8+ T cells using α-CD3/CD28 beads in the presence or absence of nanomolar concentrations of decitabine (DAC)
Image made with BioRender. Figure reprinted with permission from Loo Yau et al. (2021).
Functional assessment of human CD8+ T cells: Measuring cytokine and activation marker expression
Timing: [8 to 24 h]
This section details how to harvest the CD8+ T cells for functional assessment of cytotoxicity markers in CD8+ T cells via flow cytometry.
Generally, extracellular expression of CD25, CD69, HLA-DR are upregulated upon T cell activation with α-CD3/CD28 antibodies (Legat et al., 2013). The expression of cell surface receptor markers can be assessed immediately after harvesting of the T cells. In addition, intracellular expression of cytokines (e.g., TNF and IFNɣ), as well as cytolytic enzymes (e.g., Granzyme B) are markers to measure cytolytic potential of CD8+ T cells (Seder and Ahmed, 2003). To detect intracellular expression of cytokines however, additional re-stimulation protocols are required.
-
33.
Using a multichannel pipette, harvest the CD8+ T cells by pooling each of the respective treatment conditions into conical tubes.
-
34.
Spin cells at 200 g for 10 min at room temperature. Discard supernatant.
Note: The pellet after centrifugation will contain T cells and magnetics beads, which will need to be removed using the DynaMag2 Magnet.
-
35.
Resuspend pellet in 500 μL of supplemented T cell media with IL-2 and transfer into a 1.5 mL microcentrifuge tube.
-
36.
Place 1.5 mL microcentrifuge tube in the DynaMag2 magnet and leave for 1 min.
Note: The magnetic beads will collect to the side of the microcentrifuge tube, while the T cells will remain in suspension.
-
37.
Transfer the suspension cells into a new 1.5 mL microcentrifuge tube or 15 mL conical tube.
-
38.
Count the number of cells and the viability with ready-to-use trypan blue (0.4%) solution.
-
39.
Resuspend T cells at 1×106 cells per mL in T cell media.
Preparing cells for intracellular staining for flow cytometric analysis
Timing: ~6 h
For intracellular marker staining of functional cytokines TNF and IFNɣ, CD8+ T cells need to be further stimulated with stimulants such as PMA/ionomycin, which are commercially available as optimized cocktail formats. However, intracellular Granzyme B can be detected without additional stimulants.
Note: Fc receptor blocking solution (e.g., anti-CD16/CD32 antibodies) is recommended to block non-antigen specific binding of antibody before specific antibody staining. However, this step is not necessary as the cells used here are purified CD8+ T cells.
-
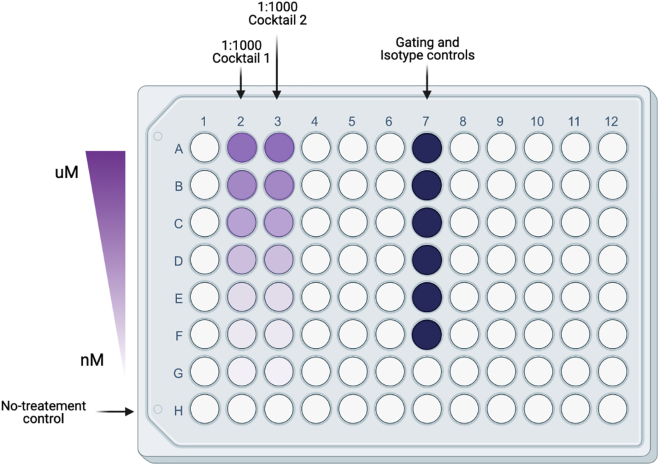
40.Transfer 250 μL of media containing 250,000 T cells for each condition to a V-bottom 96-well plate, including controls (Figure 4).
-
a.Seed 2 wells for each treatment condition.
-
b.One set of cells will receive Cocktail 1: “Cell Stimulation plus Protein secretion inhibitor cocktail” (see “key resources table) for detection of functional cytokines TNF and IFNɣ.
-
c.Another set will receive Cocktail 2: “Protein secretion inhibitor cocktail” (see “key resources table) only to detect the expression of cytolytic enzymes Granzyme B.
-
d.Controls
-
i.Unstained controls.
-
ii.Unstimulated controls for gating controls for TNF and IFNɣ; cells that receive Cocktail 2: ‘Protein secretion inhibitor cocktail’ only.
-
iii.Fluorophore labeled isotype controls for each cocktail condition.
-
i.
-
a.
-
41.
Spin the plate at 200 g for 2 min to pellet the cells.
-
42.Remove 150 μL of media from each well
-
a.Leave behind 100 μL of media containing the 250,000 T cells seeded at step 40.
-
a.
-
43.
Prepare a working 1:500 working stock of the Cocktail 1 and Cocktail 2, respectively..
Note: Add 2 μL of Cocktail 1 or Cocktail 2 in 998 μL of T cell media, respectively. Mix well.
-
44.
Add 100 μL of Cocktail 1 or Cocktail 2 working stock to each corresponding well, bringing the final volume to each well to 200 μL, and the final dilution to 1:1000.
Note: The manufacturer recommends the Cell Stimulation and Protein secretion inhibitor Cocktails at 1:500. However, using these cocktails at 1:1000 final concentrations have yielded consistent results.
-
45.
Mix well components using a multichannel pipette.
-
46.
Incubate at 37°C, 5% CO2 for 4 h.
Note: After the 4 h incubation of T cells with 1:1000 dilution of Cocktail 1 or 1:1000 dilution of Cocktail 2, proceed to the next sections describing steps for flow cytometry staining for analysis. See Table 2 for the recommended flow cytometry staining panel.
Figure 4.
Recommended 96-well set-up for restimulation to detect functional cytokines TNF and IFNɣ
Cocktail 1: Cell Stimulation plus Protein secretion inhibitor cocktail. Cocktail 2: Protein secretion inhibitor cocktail. Image made with BioRender.
Table 2.
Flow cytometry antibodies and reagents to measure intracellular expression of TNF and IFNɣ, and Granzyme B in CD8+ T cells per 250,000 cocktail-stimulated T cells
| Stain | Stain dilution |
|---|---|
| Intracellular Panel 1: Cocktail 1 - Cell Stimulation and Protein secretion inhibitor Cocktail | |
| Viability Dye Fixable Aqua | 1 in 2,000 in PBS |
| CD8 or CD3 in APC-Cy7 | 1 in 100 in FACS Buffer |
| IFNɣ in APC | 1 in 50 in 1× Perm Buffer |
| TNF in PE | 1 in 50 in 1× Perm Buffer |
| Isotype control for Intracellular Panel 1 | |
| Viability Dye Fixable Aqua | 1 in 2,000 in PBS |
| CD8 or CD3 in APC-Cy7 | 1 in 100 in FACS Buffer |
| APC Mouse IgG1 | 1 in 50 in 1× Perm Buffer |
| PE Mouse IgG1 | 1 in 50 in 1× Perm Buffer |
| Gating control for Intracellular Panel 1, treated with Cocktail 2 - Protein secretion inhibitor Cocktail | |
| Viability Dye Fixable Aqua | 1 in 2,000 in PBS |
| CD8 or CD3 in APC-Cy7 | 1 in 100 in FACS Buffer |
| IFNɣ in APC | 1 in 50 in 1× Perm Buffer |
| TNF in PE | 1 in 50 in 1× Perm Buffer |
| Intracellular Panel 2: Cocktail 2 - Protein secretion inhibitor Cocktail | |
| Viability Dye Fixable Aqua | 1 in 2,000 in PBS |
| CD8 or CD3 in APC-Cy7 | 1 in 100 in FACS Buffer |
| Granzyme B in FITC | 1 in 50 in 1× Perm Buffer |
| Isotype control for Intracellular Panel 2 | |
| Viability Dye Fixable Aqua | 1 in 2,000 in PBS |
| CD8 or CD3 in APC-Cy7 | 1 in 100 in FACS Buffer |
| FITC Mouse IgG1 | 1 in 50 in 1× Perm Buffer |
Note: We recommend having a T cell marker in the panel for flow cytometry gating purposes. Some compounds can alter the expression of CD8, whereas CD3 expression is more stable in T cells. In our experience, the flow cytometry panel present here has yielded consistent results and require minimal compensation steps.
-
47.
Spin the plate at 200 g for 5 min to pellet the cells and discard supernatant.
-
48.
Wash cells with PBS, spin the plate at 200 g for 5 min and discard PBS.
-
49.
Prepare 1:2000 dilution of cell viability dye in PBS.
-
50.
Add 100 μL of cell viability dye to each well.
-
51.
Incubate for 30 min at 4°C, in the dark.
-
52.
Add 100 μL of cold FACS buffer to each well, spin the plate at 200 g for 5 min to pellet the cells and discard supernatant.
-
53.Prepare 1× Fix Concentrate.
-
a.Mix 1 part of True-Nuclear Fix Concentrate with 3 parts of the Fix Diluent.
-
a.
-
54.
Add 100 μL of 1× Fix Concentrate to each well.
-
55.Incubate for 30 min at 4°C, in the dark.
 Pause point: After cell fixation, the user may stop here and continue the following steps the next day. In this case:
Pause point: After cell fixation, the user may stop here and continue the following steps the next day. In this case:-
a.Wash each well with 200 μL of cold FACS buffer twice by spinning the plate at 200 g for 5 min to pellet the cells and discard supernatant.
-
b.Resuspend cells with 200 μL of cold FACS buffer and store plate at 4°C in the dark overnight (16–18 h).
-
a.
-
56.Prepare 1× Perm Buffer.
-
a.Mix 1 part of True-Nuclear 10× Perm Buffer with 9 parts of distilled water.
-
a.
-
57.
Add 100 μL of 1× Perm Buffer to each well.
Note: Prepare both the 1× Fix Concentrate and 1× Perm Buffer fresh each time when staining cells.
-
58.
Spin the plate at 200 g for 5 min to pellet the cells, and discard supernatant.
-
59.
Add 200 μL 1× Perm Buffer to wash the cells.
-
60.
Spin the plate at 200 g for 5 min to pellet the cells, and discard supernatant.
-
61.
Prepare antibodies for intracellular markers at 1:50 dilution in 1× Perm Buffer. See Table 2 for antibody dilution.
-
62.
Add 100 μL of antibodies cocktail diluted in 1× Perm Buffer to each well
-
63.
Incubate 4°C for 30 min in the dark
-
64.
Add 100 μL of cold FACS buffer wash to each well, spin the plate at 200 g for 5 min to pellet the cells, and discard supernatant
-
65.
Repeat step 64.
-
66.
Prepare antibodies for CD8 or CD3 surface expression at 1:100 dilution in FACS buffer.
Note: Typically, cell surface marker stain is performed prior to cell fixation (step 53 in this protocol). However, for the particular panel on Table 2, CD3 and CD8 epitopes are not altered after fixation. Thus, we recommend staining for CD3 or CD8 at this step.
Note: For a list of cell surface markers and epitopes that are not altered by fixation, visit https://www.biolegend.com/en-us/fixation
-
67.
Add 100 μL of antibodies cocktail to each well.
-
68.
Incubate for 30 min at 4°C in the dark.
-
69.
Add 100 μL of FACS buffer wash to each well, spin the plate at 200 g for 5 min to pellet the cells, and discard supernatant.
-
70.
Repeat step 69.
-
71.
Resuspend cells in FACS buffer and transfer to flow cytometry appropriate reading vials.
-
72.
Place on cells in the 4°C in the dark and acquire samples on the flow cytometer within 2 h of staining.
CRITICAL: Cells should be analyzed as soon as possible following staining. In our hands, tandem dyes like APC-Cy7 will lose intensity overnight (16–18 h) at 4°C
Preparing cells for cell surface marker staining for flow cytometric analysis
Timing: ~1 h
- 73.
Alternatives: Viability Dye Fixable Aqua can be substituted by SYTOX Blue Viability Dye while keeping same fluorochrome panel.
Note: In our experience, the flow cytometry panel presented here has yielded consistent results and requires minimal compensation steps. Include single color compensation controls using compensation beads, as well as unstained control and Fluorescence minus one (FMO) control. Refer to Tung et al., 2007 and Maecker and Trotter, 2006 for best flow cytometry practices.
-
74.
Spin the plate at 200 g for 5 min to pellet the cells.
-
75.
Wash cells with 200 μL of FACS buffer in each well, spin the plate at 200 g for 5 min to pellet the cells and discard supernatant.
-
76.
Prepare 1:2000 dilution of cell viability dye in PBS.
-
77.
Add 100 μL of diluted cell viability dye to each well.
-
78.
Incubate for 30 min at 4°C in the dark.
-
79.
Add 100 μL of FACS buffer to each well, spin the plate at 200 g for 5 min to pellet the cells and discard supernatant.
-
80.
Prepare antibodies for cell surface markers at 1:100 dilution in FACS buffer.
-
81.
Add 100 μL of antibodies cocktail to each well.
-
82.
Incubate for 30 min at 4°C in the dark.
-
83.
Add 200 μL of FACS buffer wash to each well, spin the plate at 200 g for 5 min to pellet the cells, and discard supernatant.
-
84.
Repeat step 83.
-
85.
Resuspend cells in FACS buffer and transfer to flow cytometry appropriate reading vials.
-
86.
Place on cells in the 4°C, in the dark and acquire samples on the flow cytometer within 2 h of staining.
CRITICAL: Cells should be analyzed as soon as possible following staining. In our hands, tandem dyes like APC-Cy7 will lose intensity overnight (16–18 h) at 4°C.
Table 3.
Flow cytometry antibodies and reagents to measure extracellular expression of activation markers on CD8+ T cells
| Stain | Stain dilution |
|---|---|
| Viability Dye Fixable Aqua | 1 in 2,000 in PBS |
| CD8 or CD3 in FITC | 1 in 100 in FACS Buffer |
| CD25 PE | 1 in 100 in FACS Buffer |
| CD69 APC-Cy7 | 1 in 100 in FACS Buffer |
| HLA-DR APC | 1 in 100 in FACS Buffer |
Table 4.
Fluorescence minus one (FMO) control matrix
| CD3 or CD8 in FITC | CD25 in PE | HLA-DR in APC | CD69 in APC-Cy7 | |
|---|---|---|---|---|
| Unstained | X | X | X | X |
| PE FMO | ✓ | X | ✓ | ✓ |
| APC FMO | ✓ | ✓ | X | ✓ |
| APC-Cy7 FMO | ✓ | ✓ | ✓ | X |
FMO controls are the experimental cells stained with all the fluorophores minus one fluorophore.
Expected outcomes
In this section, we describe, and show expected and possible outcomes when activating human CD8+ T cells with nanomolar doses of the DNA demethylating agent, decitabine. Treatments included: unstimulated (no beads added); stimulated; stimulated plus 100 nM or 300 nM decitabine.
We recommend measuring CD69 expression at 24 h post-activation to ensure the activation protocol was set up properly. CD69 expression should be nearly 100% or at highest in all bead-activated conditions regardless of treatment condition when compared to unstimulated/resting conditions (Figure 5).
Figure 5.
CD69 expression profile 24 h post-stimulation of successful T cell activation culture conditions at 1:2 Dynabead:T cell ratio
Figure reprinted with permission from Loo Yau et al. (2021).
As described in Loo Yau et al., we found that activation of CD8+ T cells in the presence of nanomolar doses of decitabine increases the expression of cell surface activation markers (Figure 6) relative to non-treatment controls as well as functional cytokines and cytolytic enzymes (Figure 7).
Figure 6.
Expression profile of extracellular activation markers in CD8+ T cells during decitabine treatment at day 5 post-stimulation
(A) MFI expression of CD25. Percentage of (B) HLA-DR expression and (C) CD69 expression in CD8+ T cells. The shift of the mean fluorescence intensity to the right represents an increased expression of these various activation markers. Gating to obtain HLA-DR percentage and CD69 percentage levels were gated based on FMO controls. Figure reprinted with permission from Loo Yau et al. (2021).
Figure 7.
Expression profile of cytolytic enzyme (granzyme B) and functional cytokines (IFNɣ, TNF) in CD8+ T cells during decitabine treatment at day 5 post-stimulation
(A–C) (A) MFI expression of Granzyme B. Functional cytokines are represented as frequency for (B) IFNɣ+ and (C) TNF+ expressing T cells. Figure reprinted with permission from Loo Yau et al. (2021).
Limitations
An important consideration to know is that the number of PBMCs and proportion of CD8+ T cells can differ amongst the blood donors. Thus, the number of wells that can be plated for functional assays will need to be adjusted according to the number of CD8+ T cells available to start with. In our experience, we occasionally (but very rarely) have isolated CD8+ T cells from individuals that fail to activate and expand upon Dynabead stimulation.
Though we used the Miltenyi Biotec equipment and cell isolation kits, other commercial isolation kits may also be suitable to isolate purified CD8+ T cell isolation.
We recommend the markers we addressed in the protocol; however, the fluorophore color can be changed.
Troubleshooting
Problem 1
Purity of isolated T cells is low. In our experience, isolation of CD8+ T cells kits should yield 90%+ CD8+ purity (step 19).
Potential solution
Perform additional wash steps on the buffy coat cell pellet prior to cell isolation. Additionally, make sure MACS buffer containing 0.5% BSA is sterile filtered and fresh (less than 1 month old). See Figure 8 for expected CD3 and CD3/CD8 purity after CD8+ T cell isolation.
Figure 8.
Expected purity of CD8+ T cells after isolation from PBMCs
(A). Nearly 100% of the cells isolated should be CD3 positive.
(B). The proportion of CD3+CD8+ T cells should be above at least 90%.
Problem 2
Not enough cells harvested after stimulation (step 38).
Potential solution
Depending of the donor, the expansion rate of CD8+ T cells will differ. Additionally, ~50% of T cells will be lost to activation induced cell death ~24–48 h post Dynabeads activation. T cells numbers should steadily recover by day 5 post-stimulation. We recommend seeding more wells for each condition than anticipated.
Problem 3
Number of live cells after PMA/ionomycin stimulation (1:1000) to detect IFNɣ and TNF may be very low (step 46). In our experience, CD8+ T cells from different donors may respond differently to in vitro activation.
Potential solution
We recommend at minimum, stain 250,000 cells for flow cytometry analysis. The number of cells can be increased up to 750,000 cells per sample with the stimulation conditions described here.
Resource availability
Lead contact
The lead contact Daniel D. De Carvalho (Daniel.DeCarvalho@uhnresearch.ca). Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Daniel D. De Carvalho (Daniel.DeCarvalho@uhnresearch.ca).
Material availability
The study did not generate new unique reagents
Data and code availability
The particular study did not generate any unique datasets or code.
Acknowledgments
This work was funded by Canadian Institute of Health Research (CIHR), New Investigator salary award (201512MSH360794-228629), Helen M Cooke professorship from Princess Margaret Cancer Foundation, Canada Research Chair, CIHR Foundation Grant (FDN 148430), CIHR Project Grant (PJT 165986), NSERC (489073), Terry Fox Research Institute Program Projects Grant (1106), and Ontario Institute for Cancer Research (OICR) with funds from the province of Ontario to D.D.D.C. H.L.Y. is supported by the Vanier Canada Graduate Scholarship (157423) provided by CIHR.
Author contributions
Writing – original draft, H.LY. and D.D.D.C.; revision draft, H.LY. and D.D.D.C.; project administration, H.L.Y. and D.D.D.C.; supervision, D.D.D.C.; funding acquisition, D.D.D.C.
Declaration of interests
D.D.D.C. received research funds from Pfizer and Nektar Therapeutics. D.D.D.C. is a cofounder and shareholder of DNAMx, Inc.
Contributor Information
Helen Loo Yau, Email: helen.loo@uhnresearch.ca.
Daniel D. De Carvalho, Email: daniel.decarvalho@uhnresearch.ca.
References
- Fuss I.J., Kanof M.E., Smith P.D., Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr. Protoc. Immunol. 2009;7:Unit7.1–7.1.8. doi: 10.1002/0471142735.im0701s85. [DOI] [PubMed] [Google Scholar]
- Legat A., Speiser D.E., Pircher H., Zehn D., Fuertes Marraco S.A. Inhibitory receptor expression depends more dominantly on differentiation and activation than “exhaustion” of human CD8 T cells. Front. Immunol. 2013;4:455. doi: 10.3389/fimmu.2013.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo Yau H., Bell E., Ettayebi I., de Almeida F.C., Boukhaled G.M., Shen S.Y., Allard D., Morancho B., Marhon S.A., Ishak C.A. DNA hypomethylating agents increase activation and cytolytic activity of CD8+ T cells. Mol. Cell. 2021 doi: 10.1016/j.molcel.2021.01.038. [DOI] [PubMed] [Google Scholar]
- Maecker H.T., Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- Seder R.A., Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Tung J.W., Heydari K., Tirouvanziam R., Sahaf B., Parks D.R., Herzenberg L.A., Herzenberg L.A. Modern flow cytometry: a practical approach. Clin. Lab. Med. 2007;27:453–468. doi: 10.1016/j.cll.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The particular study did not generate any unique datasets or code.