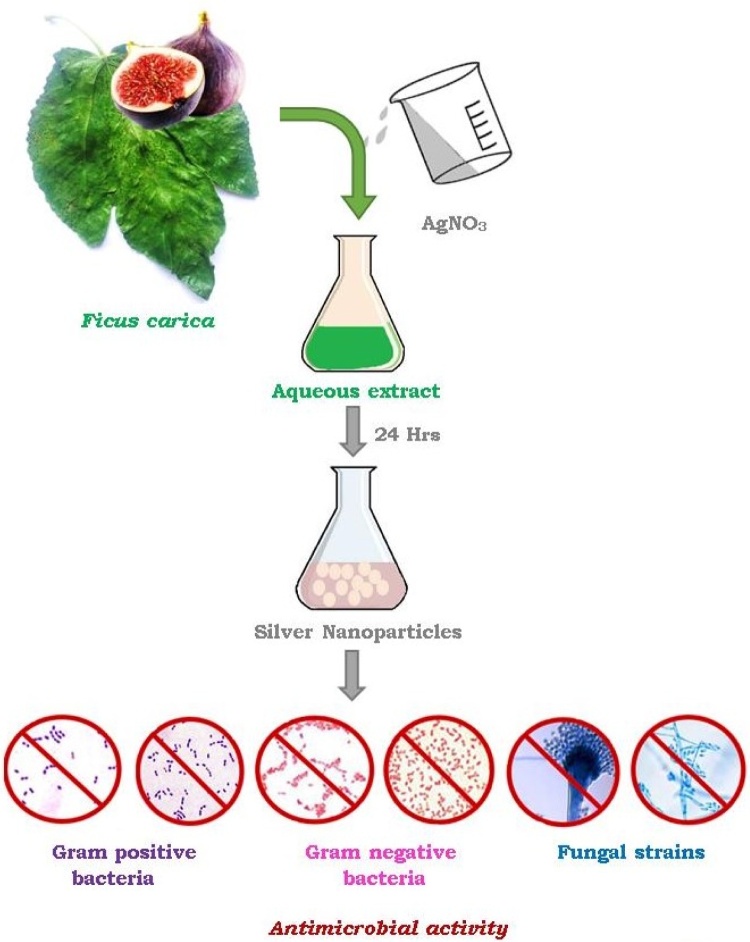
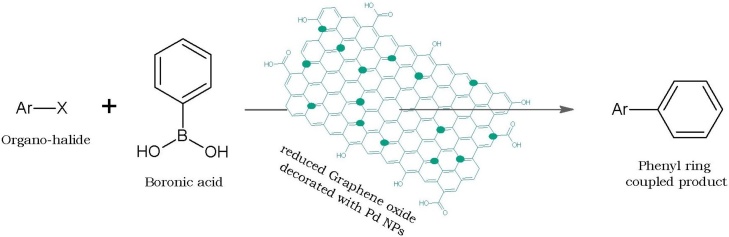
Graphical abstract
Keywords: Nanotechnology, Ficus carica, Silver nanoparticles, Stannic oxide nanoparticles, Palladium nanoparticles
Highlights
-
•
Ficuc carica (Moraceae) has widely been explored for the green synthesis of metallic nanoparticles.
-
•
Silver nanoparticles were found active against several Gram positive, Gram Negative bacteria and some fungal strains.
-
•
Stannic oxide nanoparticles coated glassy carbon electrode determined Hg2+ ions sharply.
-
•
Palladium nanoparticles decorated reduced grapheme oxide could be used in Suzuki coupling reaction.
-
•
Phytochmeicals present in Ficus carica act as both reducing and capping agent for stabilized metallic nanoparticles.
Abstract
In last decade, several attempts were made for the green and economic synthesis of metallic nanoparticle having profound applications in all the arears of science. Ficus carica (Moraceae) is a deciduous plant with edible fruits. It has been widely explored for synthesis of various metallic nanoparticles like silver, gold, stannic oxide, copper oxide, iron oxide, palladium nanoparticles using extracts of Ficus carica leafs or fruits. Phytochemical prospection so far made on Ficus carica leafs or fruits revealed the presence of variety of compounds including organic acids, fatty acids, amino acids, lower terpenes, flavonoids, coumarins etc. Researchers prepared metallic nanoparticles; characterised them by advanced analytical techniques and evaluated for particular application including, antimicrobial activity of silver nanoparticles; improved determination of mercury using stannic oxide nanoparticles coated glassy carbon electrode; carrying of chemical reaction using reduced graphene oxide decorated with palladium nanoparticles as catalyst. On this review, it can be concluded that due to presence of variety of phytocompounds, Ficus caricaplant can be used in preparation of metallic nanoparticles which could be useful in various scientific domains.
1. Introduction
Considering the wide range of applications of metallic nanoparticles, there has been a worldwide increase in investment in nanotechnology based research and development. Traditionally, there are two approaches of nanoparticles synthesis: physical and chemical; which are based on ion sputtering, solvo thermal synthesis, reduction, and sol-gel techniques. Plant-based synthesis of nanoparticles has been considered as green route of achieving nanoparticles because it uses biologically safe solvents and no or low use of harmful chemicals. It is in faster and feasible. Hence, several efforts were made for synthesis of nanoparticles of variety of metals like silver, gold, palladium, zinc, copper, iron, cobalt, nickel etc. using plant extracts. It was reported that, both primary and secondary metabolites present in extracts reduce the metal ions and aggregate them to form nanoparticle. Primary metabolites include carbohydrates (simple sugars and polysaccharides), proteins, and lipids, while secondary metabolites comprise alkaloids, glycosides, terpenes, tannins, flavonoids, acetogenins etc [1]. In last decade, since 2011, several research works have been published on successful utilisation of wide variety of polysaccharides like hydroxypropyl starch [2], curdlan [3], pullulan [4], gum Arabic [5] and pectin [6] in preparation of silver or gold or zinc oxide nanoparticles. In 2016, Abdelgawad et al. [7] synthesised silver nanoparticles by solid state reduction using soy protein isolates.
Applications of metallic nanoparticles explored can be classified into two types. Biological applications includes applications of nanoparticles explored for their anti-bacterial [8], anti-fungal [9], anti-viral [10], anti-inflammatory [11], anti-cancer [12], anti-diabetic [13], anti-oxidant potentials [14]. Non-biological applications include photocatalysis of pollutant dyes like methylene blue, reduction of 4-nitrophenol and its derivatives used in pesticides [15], use in dye-sensitized solar cells (DSSCs) [16]. Using C. gigantea latex, Yttrium nitrate, europium nitrate and sodium chloride, Ramakrishna et al. [17] synthesised Eu3+ doped Y2SiO5 nanophosphors those can be used in light-emitting diodes (LEDs). Recently Wang et al. [18], modified zinc oxide nanoparticles using uniformly dispersed silver nanoparticles and found enhancement in ethanol fumes and H2S gas sensing performance. In 2019, a trial was made to decorate cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide using polymer polymethylol compound (PMC) or functionalized polyethyleneimine compound (FPEI); and it was observed that concomitant presence of these tri-metallic nanoparticles within cotton microstructures imparts long-lasting antibacterial, conductivity and UV protection properties to get ultimately multifunctional cotton fabrics [19]. Another research work suggested two approaches for preparation of poly-(imidazolium vanillyl)-grafted oligochitosan Schiff bases (PIVCSBs) and their silver nano-biocomposites (NBCs). In first in-situ approach, there is use of PIVCSBs as a synergetic reductant and capping agent to afford PIVCSBs/Agin NBCs, while in ex-situ protocol, AgNPs are fabricated first and then capped by PIVCSBs to fabricate PIVCSBs/Agex NBCs. Then, cotton-treated fabrics showed very strong bactericidal activity against E. coli and S. aureus [20].
Several plants have been tried to synthesize metallic or metal oxide nanoparticles and then evaluated for specific application. One of such plant tried for green synthesis of nanoparticles of different metals like silver, gold, stannic oxide, copper oxide, iron oxide and palladium is Ficus carica (Fig.1). In many cases, these metallic nanoparticles have been explored for their applications in any section of applied science. So far, no such review has been made for such a single plant, which has been tried for synthesis of different metal nanoparticles.
Fig. 1.
Ficus carica plant (Moraceae).
Ficus carica, commonly referred as fig, (Fig.1) is the deciduous plant belonging to family Moraceae. It is native to South-west Asian countries and Mediterranean region [21]. It is 15–20 ft tall with many branches, secret milky white latex, containing protein hydrolysing enzyme Ficin. Its leafs are large, bright green in colour, single, alternate, more or less deeply lobed with 1–5 sinuses; rough hairy on the upper surface and soft hairy on the underside. Flowers are like receptacles (saikonium), having axillary origin. Fruits are edible, axillary on leafy branchlets, pear shaped, often get cracked on ripening. The interior portion of fruit is a white, inner ring containing a seed mass bound with sweet jelly-flesh. Seed are numerous in number and vary in size. Bark is smooth, silvery grey or ash-coloured [22].
2. Phytochemical composition
Ficus carica biosynthesizes a variety of plant metabolites (both primary and secondary) in its different organs. Oliveira and his co-workers have reported the presence of diverse phytochemicals from different organs of FC. In 2009, Oliveira et al. presented organic acid profile of fig leaves, composing of: citric, fumaric, malic, oxalic, quinic, and shikimic acids. On HPLC study of FC latex, Oliveira et al. [23] revealed the presence of essential aminoacids (phenylalanine, leucine, tryptophan, histidine and lysine)and eight non-essential amino acids (alanine, asparagine, glutamine, glycine, serine, tyrosine, cysteine, and ornithine); however on GC/MS analysis of FC latex, they claimed the presence of many saturated, monounsaturated and polyunsaturated fatty acids like pentadecylic, myristic, margaric, palmitic, stearic, cis-10-heptadecenoic, linoleic, elaidic, arachidic, oleic, heneicosylic, behenic, lignoceric, andtricosylic acids. Along with these phytocompounds, they also found few volatile principles like a-thujene, a-pinene, b-pinene, limonene, terpinolene, eucalyptol, cis-linalool oxide, linalool and epoxylinalool (monoterpenes) and a-guaiene, a-bourbonene, b-caryophyllene, trans- a-bergamotene, a-caryophyllene, germacrene D, cadinene and a-calacorene (sesquiterpenes). Leaves have been reported to contain flavonoids like quercetin, luteolin, apigenin,kaempferol with their glycosidic forms [24]; coumarins like psoralen, bergapten [25] and triterpenoids like lupeol acetate, and oleanolic acid [26]. Fruits were found to contain phytochemicals of anthocyanin class, namely, glycosides of cyanidin, pelargonidin and peonidin [27] (Fig.2)
Fig. 2.
Phytochemicals present in Ficus carica.
3. Metallic nanoparticles
3.1. Silver nanoparticles (Ag NPs)
First use of FC in green synthesis of metal nanoparticles was made by Logaranjan et al. [28] through successful synthesis of silver nanoparticles by adding 0.5 mL of FC fruit extract to 9.0 mL of 1 mM AgNO3. Further, they evaluated antibacterial activity by agar disc diffusion assay against gram positive bacteria Staphylococcus aureus and gram negative bacteria Escherichia coli.
Then, in 2012 only, another trial was made by Singh and Bhakat for green synthesis of silver nanoparticles (Ag NPs) using FC leaves and bark extract by three methods: Method 1- Solvothermal reduction process (ST), at 15 psi and 1210 °C for 15 min. Method 2- Microwave irradiation (MW), medium cycle (2450 MHz; 700 W) for 5 min. Method 3-Thermal heating (TR), at 800 °C with stirring for 15 min on magnetic stirrer. Ag NPs synthesised by all the three methods were then characterised by advanced techniques like UV, IR, Scanning Tunneling Microscopy, Surface Plasmon resonance.
Next effort of synthesis of silver nanoparticles (Ag NPs) using aqueous extract FC leaf was made by Aldebasi et al. [29]. They characterised as synthesised Ag NPs by UV, FT-IR and TEM analytical tools and evaluated their antimicrobial potential against clinical isolates of gram positive bacteria Staphylococcus aureus, Steptococcus pneumonia; gram negative bacteria Pseudomonas aeruginosa, Proteus vulgaris; and fungus Aspergillus fumigates and Fusarium spp.; by well diffusion method and comparing their zone of inhibition (Fig.3).
Fig. 3.
Green synthesis of silver nanoparticles using aqueous extract of Ficus carica fruit or leaf and their anti-microbial activity.
Another attempt of green synthesis of Ag NPS using aqueous extract of FC fruits was made by Kumar et al. [30]. They determined hydrodynamic particle size distribution and analysed morphology of nanoparticles by using a dynamic light scattering (DLS) and transmission electron microscopy (TEM), X-ray diffraction (XRD).
Last attempt of synthesis of Ag NPs using aqueous extract of FC fruit was made in 2017 by Jacob et al. [31] They characterised Ag NPs by UV, FTIR and SEM; and further they screened anti-cancer potential of Ag NPs against breast cancer cell line MCF7 and also studied acute toxicity in rats.
3.2. Gold nanoparticles (Au NPs)
The rare of attempt of green synthesis of gold nanoparticles was made by Singh and Bhakatin [32]. They used extracts of FC leaves and bark as a reducing agent and capping agent which can rapidly reduce auric ions came from source auric tetra chloride (HAuCl4).
3.3. Stannic oxide nanoparticles (SnO2 NPs)
In 2015, SnO2 NPs were prepared by Hu [33] by mixing aqueous FC leaf extract and hydrated Tin(II) chloride (SnCl2•2H2O)solution with continuous stirring at 80 °C for 24 h. He obtained spherical NPs with average size of 123 nm. Then, SnO2 NPs so prepared were evaluated for their capacity to be used glossy carbon electrode (GCE) for electrochemical determination of Hg2+. The SnO2NPs/GCE exhibited a linear relationship with in the Hg2+ concentration range from 0.001 to 1.5 μM (Fig.4).
Fig. 4.
Determination of Hg + using GCE coated with SnO2 NPs green synthesised using Ficus carica leaf extract.
3.4. Copper oxide nanoparticles (CuO NPs) and their chitosan nanocomposites
The ability of FC aqueous extract to prepare CuO NPs was explored by Syame et al. [34]. They treated 1 mM solution of copper sulphate (CuSO4.5H2O) with FC aqueous extract for 12 h. Then, solution containing CuO NPs was purified by repeated centrifugation at 12,000 rpm for 15 min. They studied absorbance of CuO NPs by UV; morphology and particle size by TEM and SEM; and the crystal structure by X-ray diffractometer. Further, CuO NPs were sonicated with chitosan solution to form CuO NPs- chitosan nanocomposites. Both CuO NPs and their chitosan nanocomposites were then tested for antimicrobial activity against 22 different bacteria, including Methicillin-resistant strain.
3.5. Iron oxide/maghemite nanoparticles (Fe2O3 NPs)
Aqueous extract of dried FC fruits can successfully be used to synthesize Fe2O3 NPs from ferric chloride hexahydrate, Maghemite (FeCl3•6H2O) [35]. These NPs so obtained were characterised and found spherical in shape with average diameter of 9 ± 4 nm.
3.6. Palladium nanoparticles(Pd NPs)
Palladium nanoparticles (Pd NP) have wide applications as a heterogeneous catalyst in C—C bond formation involved in Heck, Suzuki, Stille and Sonogashira reactions which are applied in sections like pharmaceuticals, polymers, herbicides, and fine chemicals. Due to their reactivity, selectivity and stability, heterogeneous Pd NPs are even advantageous over the use of homogenous Pd catalysts [36]. Considering these merits, Pd NPs were successfully green synthesised by Anasdass et al. [37] by treatment of aqueous extract of fresh FC fruits with 1 mL of 1 mM H2PdCl4 for 3 h. Further, Pd NPs –decorated reduced graphene oxide sheets were demonstrated as catalyst in Suzuki cross-coupling reactions where phenyl boronic acid gets coupled with an organo-halide. They optimised the reaction to get 98 % yield using 0.96 Pd NP/rGO (%) and base K2CO3heated at 200 °C (Fig. 5).
Fig. 5.
Suzuki coupling reaction catalysed using reduced graphene oxide decorated with Pd NPs green synthesised using Ficus carica fruit extract.
4. Conclusion
Many researchers have used Ficus carica leaf and/or fruit extracts to synthesize metallic nanoparticles from source compound by green and economic route. Various phytochemicals present in Ficus carica leaf or fruit act as both reducing and capping agent for both synthesizing and stabilizing nanoparticles of selected metal. Most of these metallic nanoparticles synthesised have proved to possess significant application in any scientific domain like antimicrobial activity of silver nanoparticles against Gram positive and negative bacteria and few fungal strains, accurate determination of mercury in water samples using stannic oxide nanoparticles and catalysis of Suzuki reaction by palladium nanoparticles coated reduced graphene oxide. It can be concluded that, new applications of Ficus carica plant extracts assistance synthesised metallic nanoparticles like dye reduction or use in photovoltaic cell can be determined.
5. Future outlook
Based on this review, future scope for the use of Ficus carica extract could include green synthesis of nanoparticles of different metals like nickel, nickel oxide, zinc, zinc oxide, magnesium oxide and their determination of their applications in both similar and different domains (biology, electrochemistry, ecology, synthetic chemistry).
Declaration of Competing Interest
I, an author of this manuscript declare no conflict of interest.
Acknowledgement
Author of this review article is thankful to Dr. (Mrs.) Anagha M. Joshi, Principal, SCES’s Indira College of Pharmacy, Pune for her motivation to write this review and providing library and computer facility at the college premises to access the literature and compiling the data obtained.
References
- 1.Patil S.P., Kumbhar S.T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. Leaves. Biochem. Biophys. Rep. 2017;10:76–81. doi: 10.1016/j.bbrep.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Rafie M.H., El-Naggar M.E., Ramadan M.A., Fouda M.M.G., Al-Deyab S.S., Hebeish A. Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization. Carbohydr. Polym. 2011;86:630–635. doi: 10.1016/j.carbpol.2011.04.088. [DOI] [Google Scholar]
- 3.Hebeis A.A., Shaheen T.I., Fouda M.M.G., El- Naggar M.E. Eco-friendly microwave-assisted green and rapid synthesis of well stabilized gold and core-shell silver-gold nanoparticles. Carbohydr. Polym. 2015 doi: 10.1016/j.carbpol.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Hussein J., El Naggar M.E., Latif Y.A., Medhat D., El-Bana M., Refaat E., Morsy S. Solvent-free and one pot synthesis of silver and zinc nanoparticles: activity toward cell membrane component and insulin signalling pathway in experimental diabetes. Colloids Surf. B Biointerfaces. 2018;170:76–84. doi: 10.1016/j.colsurfb.2018.05.058. [DOI] [PubMed] [Google Scholar]
- 5.Hussein J., Attia M.F., Bana M.E., Daly S.M., Mohamed N., El-Khayat Z., El-Naggar M.E. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 2019;140:1305–1314. doi: 10.1016/j.ijbiomac.2019.08.201. [DOI] [PubMed] [Google Scholar]
- 6.Hussein J., El-Naggar M.E., Fouda M.M.G., Morsy O.M., Ajarem J.S., Almalki A.M., Allam A.A., Mekawi E.M. The efficiency of blackberry loaded AgNPs, AuNPs and Ag@AuNPs mediated pectin in the treatment of cisplatin-induced cardiotoxicity in experimental rats. Int. J. Biol. Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.05.115. [DOI] [PubMed] [Google Scholar]
- 7.Abdelgawad A.M., El-Naggar M.E., Eisa W.H., Rojas O.J. Cleaner and large scale production of silver nanoparticles mediated by soy protein via solid state synthesis. J. Clean. Prod. 2016 doi: 10.1016/j.jclepro.2016.12.122. [DOI] [Google Scholar]
- 8.Antony J.J., Sivalingam P., Siva D., Kamalakkannan S., Anbarasu K., Sukirtha R. Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf. B Biointerfaces. 2011;98:65–72. doi: 10.1016/j.colsurfb.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Logeswari P., Silambarasan S., Abraham J. Synthesis of silver nanoparticles using plant extracts and analysis of their antimicrobial activity. J. Saudi Chem. Soc. 2012;4:23–45. doi: 10.1016/j.jscs.2012.04.007. [DOI] [Google Scholar]
- 10.Suriyakalaa U., Antony J.J., Suganya S., Siva D., Sukirtha R., Kamalakkannan S., Pichiah P.B.T., Achiraman S. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf. B Biointerfaces. 2013;102:189–194. doi: 10.1016/j.colsurfb.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Gurunathan S., Kyung-Jin L., Kalishwaralal K., Sheikpranbabu S., Vaidyanathan R., Eom S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials. 2009;30:6341–6350. doi: 10.1016/j.biomaterials.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Suman T.Y., Rajasree S.R., Kanchana A., Elizabeth S.B. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B Biointerfaces. 2013;106:74–78. doi: 10.1016/j.colsurfb.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Swarnalatha C., Rachela S., Ranjan P., Baradwaj P. Evaluation of invitro antidiabetic activity of Sphaeranthus Amaranthoides silver nanoparticles. Int. J. Nanomat. Biostr. 2012;2:25–29. [Google Scholar]
- 14.Reichelt K.V., Hoffmann-Lu¨cke P., Hartmann B., Weber B., Ley J.P., Krammer G.E., Swanepoel K.M., Engel K.H. Phytochemical characterization of South African bush tea (Athrixia phylicoides DC) S. Afr. J. Bot. 2012;83:1–8. [Google Scholar]
- 15.Gopalakrishnan R., Loganathan B., Dinesh S., Raghu K. Strategic green synthesis, characterization and catalytic application to 4-nitrophenol reduction of palladium nanoparticles. J. Clust. Sci. 2017;28:2123–2131. doi: 10.1007/s10876-017-1207-z. [DOI] [Google Scholar]
- 16.Sharma J.K., Akhtar M.S., Ameen S., Srivastva P., Singh G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys. Compd. 2015;632:321–325. doi: 10.1016/j.jallcom.2015.01.172. [DOI] [Google Scholar]
- 17.Ramakrishna G., Nagabhushana H., Daruka P.D., Vidya Y.S., Sharma S.C., Anantharaju K.S., Prashantha S.C., Choudhary N. Spectroscopic properties of red emitting Eu3+ doped Y2SiO5 nanophosphors for WLED’s on the basis of Judd-Ofelt analysis: calotropis gigantea latex mediated synthesis. J. Lumin. 2016;181:153–163. doi: 10.1016/j.jlumin.2016.08.050. [DOI] [Google Scholar]
- 18.Wang S., Jia F., Wang X., Hu L., Sun Y., Yin G., Zhou T., Feng Z., Kumar P., Liu B. Fabrication of ZnO nanoparticles modified by uniformly dispersed Ag nanoparticles: enhancement of gas sensing performance. ACS Omega. 2020;5:5209–5218. doi: 10.1021/acsomega.9b04243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassabo A.G., El-Naggar M.E., Mohamed A.L., Hebeish A.A. Development of multifunctional modified cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide. Carbohydr. Polym. 2019;210:144–156. doi: 10.1016/j.carbpol.2019.01.066. [DOI] [PubMed] [Google Scholar]
- 20.Elshaarawy R.F.M., Seif G.A., El-Naggar M.E., Mostafa T.B., El-Sawi E.A. In-situ and ex-situ synthesis of poly-(imidazolium vanillyl)-grafted chitosan/ silver nanobiocomposites for safe antibacterial finishing of cotton fabrics. Eur. Polym. J. 2019;116:210–221. doi: 10.1016/j.eurpolymj.2019.04.013. [DOI] [Google Scholar]
- 21.Lodhil F., Bradley M.V., Crane J.C. Auxinsandgibberellin-like substances in parthenocarpic and non-parthenocarpicsyconia of Ficuscarica L. CV. King. Plant Physiol. 1969;44:555–561. doi: 10.1104/pp.44.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badgujar S.B., Patel V.V., Bandivdekar A.H., Mahajan R.T. Traditional uses, phytochemistry and pharmacology of Ficuscarica: a review. Pharm. Biol. 2014;52(11):1487–1503. doi: 10.3109/13880209.2014.892515. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira A.P., Silva L.R., Andrade P.B. Further insight into thelatex metabolite profile of Ficuscarica. J. Agric. Food Chem. 2010;58:10855–10863. doi: 10.1021/jf1031185. [DOI] [PubMed] [Google Scholar]
- 24.Vaya J., Mahmood S. Flavonoid content in leaf extracts of the fig(Ficuscarica L.), carob (Ceratoniasiliqua L.) and pistachio (Pistacialentiscus L.) Biofactors. 2006;28:169–175. doi: 10.1002/biof.5520280303. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira A.P., Valenta˜o P., Pereira J.A. Ficuscarica L.,Metabolic and biological screening. Food ChemToxicol. 2009;47:2841–2846. doi: 10.1016/j.fct.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Saeed M.A., Sabir A.W. Irritant potential of triterpenoids fromFicuscaricaleaves. Fitoterapia. 2002;73:417–420. doi: 10.1016/s0367-326x(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 27.Duen˜as M., Pe´rez-Alonso J.J., Santos-Buelga C., Escribano-Bailo´n T. Anthocyanin composition in fig (Ficuscarica L.) J FoodComp Anal. 2008;21:107–115. [Google Scholar]
- 28.Logaranjan K., Devi S., Pandia K. Biogenic synthesis of silver nanoparticles using fruit extract of ficuscarica and study its antimicrobial activity. Nano Biomed. Eng. 2012;4(4):177–182. doi: 10.5101/nbe.v4i4.p177-182. [DOI] [Google Scholar]
- 29.Aldebasi Y.H., Aly S.M., Khateef R., Khadri H. Noble silver nanoparticles (Ag NPs) synthesis and characterization of fig Ficuscarica(fig) leaf extract and its anti-microbial effect against clinical isolates from corneal ulcer. Afr. J. Biotechnol. 2014;13(45):4275–4281. doi: 10.5897/AJB2014.14133. [DOI] [Google Scholar]
- 30.Kumar B., Kumari S., Cumbal L., Debut A. Ficuscarica (Fig) fruit mediated green synthesis of silver nanoparticles and its antioxidant activity: a comparison of thermal and ultrasonication approach. BioNanoSci. 2016 doi: 10.1007/s12668-016-0193-1. [DOI] [Google Scholar]
- 31.Jacob S.J.P., Siva Prasad V.L., Sivasankar S., Muralidharan P. 2017. Biosynthesis of Silver Nanoparticles Using Dried Fruit Extract of Ficuscarica - Screening for Its Anticancer Activity and Toxicity in Animal Models. [DOI] [PubMed] [Google Scholar]
- 32.Singh P.P., Bhakat C. Green synthesis of gold nanoparticles and silver nanoparticles from leaves and bark of Ficuscarica for nanotechnological applications. Int. J. Sci. Res. Publ. 2012;2(5):1–4. [Google Scholar]
- 33.Hu J. Biosynthesis of SnO2 nanoparticles by fig (Ficuscarica) leaf extract for electrochemically determining Hg(II) in water samples. Int. J. Electrochem. Sci. 2015;10:10668–10676. [Google Scholar]
- 34.Syame S.M., Mohamed W.S., Mahmoud R.K., Omara S.T. Synthesis of copper-chitosan nanocomposites and theirApplications in treatment of local PathogenicIsolates Bacteria. Orient. J. Chem. 2017;33(5):2959–2969. doi: 10.13005/ojc/330632. [DOI] [Google Scholar]
- 35.Demirezen D.A., Yildiz Y.S., Yilmaz S., Yilmaz D.D. Green synthesis and characterization of iron oxide nanoparticles using Ficuscarica (common fig) dried fruit extract. J. Biosci. Bioeng. 2018 doi: 10.1016/j.jbiosc.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Balanta A., Godard C., Claver C. Pd nanoparticles for C±C coupling reactions. Chem. Soc. Rev. 2011;40:4973–4985. doi: 10.1039/c1cs15195a. [DOI] [PubMed] [Google Scholar]
- 37.Anasdass J.R., Kannaiyan P., Raghavachary R., Gopinath S.C.B., Chen Y. Palladium nanoparticle-decorated reduced graphene oxide sheets synthesized using Ficuscarica fruit extract: a catalyst for Suzuki cross-coupling reactions. PLoS One. 2018;13(2):e0193281. doi: 10.1371/journal.pone.0193281. [DOI] [PMC free article] [PubMed] [Google Scholar]