Abstract
Background
Electrocardiographic left ventricular hypertrophy (ECG‐LVH) represents preclinical cardiovascular disease and predicts cardiovascular disease morbidity and mortality. While the newly developed Peguero‐Lo Presti ECG‐LVH criteria have greater sensitivity for LVH than the Cornell voltage and Sokolow–Lyon criteria, its short‐term repeatability is unknown. Therefore, we characterized the short‐term repeatability of Peguero‐Lo Presti ECG‐LVH criteria and evaluate its agreement with Cornell voltage and Sokolow–Lyon ECG‐LVH criteria.
Methods
Participants underwent two resting, standard, 12‐lead ECGs at each of two visits one week apart (n = 63). We defined a Peguero‐Lo Presti index as a sum of the deepest S wave amplitude in any single lead and lead V4 (i.e., SD + SV4) and defined Peguero‐Lo Presti LVH index as ≥ 2,300 µV among women and ≥ 2,800 µV among men. We estimated repeatability as an intraclass correlation coefficient (ICC), agreement as a prevalence‐adjusted bias‐adjusted kappa coefficient (κ), and precision using 95% confidence intervals (CIs).
Results
The Peguero‐Lo Presti index was repeatable: ICC (95% CI) = 0.94 (0.91–0.97). Within‐visit agreement of Peguero‐Lo Presti LVH was high at the first and second visits: κ (95% CI) = 0.97 (0.91–1.00) and 1.00 (1.00–1.00). Between‐visit agreement of the first and second measurements at each visit was comparable: κ (95% CI) = 0.90 (0.80–1.00) and 0.93 (0.85–1.00). Agreement of Peguero‐Lo Presti and Cornell or Sokolow–Lyon LVH on any one of the four ECGs was slightly lower: κ (95% CI) = 0.71 (0.54–0.89).
Conclusion
The Peguero‐Lo Presti index and LVH have excellent repeatability and agreement, which support their use in clinical and epidemiological studies.
Keywords: electrocardiogram, left ventricular hypertrophy, peguero‐lo presti, repeatability, reproducibility
1. INTRODUCTION
Left ventricular hypertrophy (LVH) has been shown to increase the risk of cardiovascular morbidity and mortality as much as a prior myocardial infarction (Koren et al., 1991; Levy et al., 1988; Sullivan et al., 1993). Although LVH is frequently asymptomatic and underdiagnosed, it is associated with hypertension (Casale et al., 1986; Dahlof et al., 1992) and several other cardiac diseases (Levy et al., 1988; McDonagh et al., 1997), including sudden death, end‐stage renal disease (Silberberg et al., 1989; Spirito et al., 2000), and heart failure. Although hypertension detection and control have improved in the United States (Egan et al., 2010; Hajjar & Kotchen, 2003), a large proportion of the population is at risk of developing LVH by virtue of elevated blood pressure. The widespread need to identify and manage left ventricular dysfunction highlights the need for repeatable, sensitive, and specific measures of LVH (McDonagh et al., 1997).
While echocardiographic diagnosis of LVH has shown to be remarkably accurate, echocardiograms are not generally used in the absence of indications that motivate their use (Devereux et al., 1986; Woythaler et al., 1983). Electrocardiograms (ECGs), in contrast, are used routinely to detect and manage conditions at asymptomatic stages or at an early onset (Fortmann et al., 1986). Electrocardiographic and echocardiographic LVH have been shown to be equally predictive of incident heart failure, stroke, and atrial fibrillation, suggesting that the routinely used ECG can be an excellent clinical tool for evaluating potential LVH and predicting subsequent cardiovascular events (Almahmoud et al., 2015; Leigh et al., 2016; O'Neal et al., 2015; Patel et al., 2017). Even so, prior research has shown a wide range of electrocardiographic LVH prevalence estimates (Cuspidi et al., 2012; Levy et al., 1988; Vakili et al., 2001). The variation in these estimates highlights the need for simple and accurate indicators of LVH to ensure similar ECG diagnostic success with this disease compared to other cardiac diseases.
The Peguero‐Lo Presti index, defined as the sum of the deepest S wave amplitude (µV) in any single lead and lead V4, (SD + SV4), was recently developed to diagnose electrocardiographic LVH with a reported sensitivity of 0.62 (95% CI 0.50–0.72) with LVH defined by echocardiograms (Peguero et al., 2017) and sensitivity of 0.47 (95% CI 0.39–0.55) with LVH defined by cardiac magnetic resonance imaging (Guerreiro et al., 2020). The diagnosis of LVH using the most widely used criteria (Cornell voltage) has a specificity of 0.90, but comparatively low sensitivity (0.2–0.4) (Peguero et al., 2017). In addition to its higher sensitivity, Peguero‐Lo Presti LVH has been shown to be as predictive of increased risk of mortality as Cornell voltage LVH (Afify et al., 2018). Because of this, there is evidence that its use in clinical practice would improve detection of LVH and thereby provide opportunities for reducing LVH‐related morbidity and mortality. However, repeatability of Peguero‐Lo Presti has not been reported. Knowledge of the short‐term repeatability of such a measure contributes important information on the reliability of its use in clinical practice, inform study design, and aid in the interpretation of analytic results.
The aim of this study was to estimate the short‐term repeatability of the Peguero‐Lo Presti index and LVH. To aid in the application of these results to study design development and interpretation of results, we estimated changes in the Peguero‐Lo Presti index based on the variance and sample size for one‐ and two‐sample study designs. Additionally, we compared the agreement between Peguero‐Lo Presti LVH and other widely used electrocardiographic LVH criteria.
2. METHODS
2.1. Participants
The analytic data set was drawn from a study conducted in Chapel Hill, North Carolina between July and October 2001, ancillary to the Atherosclerosis Risk in Communities (ARIC) Study. Adults aged 45–64 years were eligible for this study if they were free from diabetes, congestive heart failure, kidney disease, antiarrhythmic medication use, and were not pregnant. Prior to the visit, participants were asked to avoid intense physical activity, smoking, eating, or drinking alcoholic beverages for 10 hr before the visits. Study participants underwent two standardized visits one to two weeks apart (the mean was 10 days between visits, and the range was 7 to 29 days). Participants provided written informed consent, and the study was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
2.2. Electrocardiographic methodology
Details of the ECG methodology for this study have been reported (Schroeder et al., 2004; Vaidean et al., 1989, 2005). Technicians instructed participants to breathe freely and not to talk during ECG recordings. After participants rested for 15 min in the supine position, trained and certified technicians following the standardized protocol used in the ARIC study ("The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators, 1989, 2005) to obtain two 10‐s, standard 12‐lead ECGs at each visit (ECG1 and ECG2 at the initial visit and then ECG3 and ECG4 one to two weeks later). Technicians separated the recordings by 1 to 2 min and did not remove the electrodes between the two recordings. ECGs were recorded using the MAC PC Personal Cardiograph (Marquette Electronics, Inc.,). The Epidemiological Cardiology Research Center (Wake Forest School University of Medicine, Winston Salem, NC) processed all ECGs and used the GE Marquette GE 12‐SL software (GE, Milwaukee, WI) to obtain waveform measurements needed to calculate the Peguero‐Lo Presti index. We defined the index as the sum of the deepest S wave amplitude (µV) in any single lead and lead V4 (SD + SV4) and identified Peguero‐Lo Presti LVH when the index was ≥ 2,300 µV among women and ≥ 2,800 µV among men (Peguero et al., 2017). We also identified Cornell voltage LVH (when SV3 + RaVL was > 2,800 µV among men and > 2,200 µV among women) and Cornell voltage product LVH (when [SV3 + RaVL] ×QRS duration was ≥ 244,000 µV*sec). For both Cornell metrics, we added 600 µV to the voltage sum for women. Additionally, we defined Sokolow–Lyon LVH (when SV1 + [greater of RV5 or RV6] was ≥ 3,500 µV). We excluded participants with complete bundle branch blocks, ventricular pacemakers, Wolff–Parkinson–White Syndrome, and major intraventricular conducting defects (n = 1).
2.3. Statistical analysis
We calculated summary measures for the Peguero‐Lo Presti index and LVH, computing average and absolute differences between pairs of measurements within visits (ECG2 ‐ ECG1 and ECG4 ‐ ECG3) and between visits (ECG3 ‐ ECG1 and ECG4 ‐ ECG2). We used random effects, mixed models to parse the variance of the Peguero‐Lo Presti index into between‐participant (σ2 p), between‐visit (σ2 bv), and within‐visit components (σ2 wv). We calculated the intraclass correlation coefficient (ICC) by dividing the between‐participant variance by the total variance. We calculated the standard error of measurement (SEM) as √(σ2 bv + σ2 wv). To assess within‐ and between‐visit agreement of Peguero‐Lo Presti LVH, we calculated the prevalence‐adjusted, bias‐adjusted kappa coefficient (κ) (Byrt et al., 1993). We also used κ to assess agreement between Peguero‐Lo Presti and Cornell voltage or Sokolow–Lyon LVH on any one of the four ECGs (ECG1, ECG2, ECG3, or ECG4). We estimated precision of the ICC and κ using 95% confidence intervals (CIs). To assess agreement between visits, we created a Bland–Altman plot using the average Peguero‐Lo Presti index for each visit.
To inform study design development and interpretation of the repeatability results, we calculated the minimal detectable change with 95% confidence between two time points for an individual that reflects true change above that of measurement error [MDC95 = SEM×√2 × 1.96]. For a two‐sample study design, we calculated the minimal detectable difference (MDD) between two measurements as MDD = [(√2 × σ2 total)/N] × (tα( df )+tβ( df )). We also calculated the MDD as a percent of the grand mean. The statistical analyses were done using SAS version 9.4 statistical software (SAS Institute, Inc.,).
3. RESULTS
The study population was 49% female and 32% nonwhite (n = 63; Table 1). The prevalence of Peguero‐Lo Presti LVH was 7.9% (n = 5). At baseline, 1 participant had a bundle branch block. Five participants (7.9%) deviated from protocol (ate or smoked within 10 hr of the visit ECGs). The average body mass index (BMI) of participants was 26.9.
TABLE 1.
Participant‐level characteristics of the ECG repeatability study, N = 63
| Variable | Number (percent) or mean (min, max) |
|---|---|
| Female | 31 (49.2) |
| Nonwhite race | 20 (31.8) |
| Age (years) | 52.0 (45.1, 64.6) |
| Body mass index (Kg/m2) | 26.9 (19.4, 42.6) |
| Heart rate (beats/min) on the first ECG 1 | 59.7 (34.0, 92.0) |
| QRS Duration (ms) on the first ECG 1 | 93.3 (74.0, 116.0) |
| Bundle branch block | 1 (1.6) |
| Medication Use | |
| Anticholinergic | 5 (7.9) |
| Beta‐Blocker | 1 (1.6) |
| Selective serotonin reuptake inhibitor | 10 (15.9) |
| Sympathomimetic | 4 (6.4) |
| Deviation from protocol at either visit | 5 (7.9) |
| Peguero‐Lo Presti LVH at any measurement | 5 (7.9) |
n = 62; mean 10 days between visits (range 7–29); participants were advised to not eat, drink or smoke for at least 10 hr before the procedures.
The Peguero‐Lo Presti index had an overall mean value of 1639.9 µV and a range of 2,622.0 µV (Table 2). When evaluating the mean values across the four ECG measurements, the largest difference in the mean of the Peguero‐Lo Presti index was 90.5 µV, showing that there is little variation between visits. The between‐participant variation accounted for 93.6% of the total variation of the Peguero‐Lo Presti index, while the between‐visit variation and within‐visit variation were 5.5% and 0.9%, respectively (Table 3). The index had an ICC of 0.94 (95% CI 0.91–0.97) and a SEM of 133.7 µV (Table 4). The minimal detectible change was 370.7 µV (Table 4). The minimal detectable difference indicated that a population of 1,000 would be needed to detect a difference approximately 5% of the mean (Table 5). The Bland–Altman plot of the agreement between visits 1 and 2 showed an average mean difference of −70.9 µV, and the majority of the differences are within the 95% limits of agreement (Figure 1). The differences are consistent across the range of the measurement.
TABLE 2.
Peguero‐Lo presti index summary statistics, ECG repeatability study (N = 63)
| Measure | Total | ECG 1 | ECG 2 | ECG 3 | ECG 4 |
|---|---|---|---|---|---|
| N | 249 | 62 | 63 | 63 | 61 |
| Mean (µV) | 1639.9 | 1692.1 | 1662.3 | 1603.3 | 1601.6 |
| Median (µV) | 1591.0 | 1627.5 | 1601.0 | 1567.0 | 1,489.0 |
| Standard Deviation (µV) | 525.3 | 497.7 | 528.4 | 535.4 | 545.8 |
TABLE 3.
Sources of Variation in the Peguero‐Lo Presti index. ECG repeatability study (N = 63)
| Source of Variation | Standard Deviation | % Total Variation | Coefficient of Variation (%) |
|---|---|---|---|
| Between‐subject | 512.1 | 93.6 | 31.2 |
| Between‐visit | 123.9 | 5.5 | 7.6 |
| Within‐visit | 50.4 | 0.9 | 3.1 |
| Total | 529.2 | 100.0 | 32.3 |
Peguero‐Lo Presti population mean = 1639.9 µV.
TABLE 4.
Repeatability estimates and minimal detectable change (MDC95) for the Peguero‐Lo Presti index. ECG repeatability study
| Measure | Estimate |
|---|---|
| Standard Error of Measurement (µV) | 133.7 |
| Minimal Detectable Change (µV) | 370.7 |
| Intraclass Correlation Coefficient (95% CI) | 0.94 (0.91–0.97) |
TABLE 5.
Minimal Detectable Difference (MDD) in the Peguero‐Lo Presti index between 2 independent samples, each of size N
| N | Peguero‐Lo Presti index (µV) | |
|---|---|---|
| MDD (µV) | % Mean | |
| 5 | 1,394.26 | 85.0 |
| 10 | 907.65 | 55.3 |
| 50 | 385.81 | 23.5 |
| 100 | 271.28 | 16.5 |
| 500 | 120.79 | 7.4 |
| 1,000 | 85.36 | 5.2 |
| 5,000 | 38.16 | 2.3 |
| 10,000 | 26.98 | 1.6 |
| 50,000 | 12.07 | 0.7 |
| 100,000 | 8.53 | 0.5 |
Peguero‐Lo Presti population mean = 1639.9 µV
FIGURE 1.
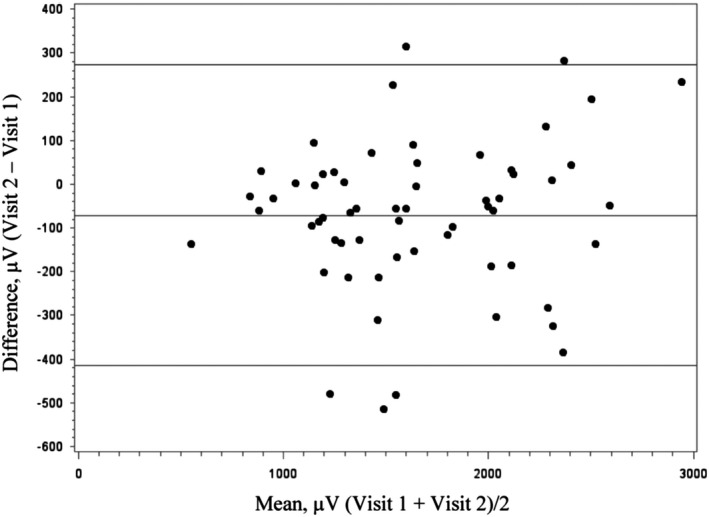
Bland–Altman plot of the agreement between visits 1 and 2 for the Peguero‐Lo Presti index (n = 60). Middle line represents the mean difference, and the two outer lines represent the 95% limits of agreement
Within‐visit κ for Peguero‐Lo Presti LVH was 0.97 (0.91–1.00) at the first visit and 1.00 at the second (Table 6). Between‐visit κ for Peguero‐Lo Presti LVH was 0.90 (0.80–1.00) for the first measurements at each visit and 0.93 (0.85–1.00) for the second measurements (Table 6). Values of κ for Peguero‐Lo Presti and Cornell voltage or Sokolow–Lyon LVH on any one of the four ECGs varied from 0.68 for Sokolow–Lyon LVH to 0.90 for Cornell voltage product LVH (Table 7). The κ was 0.71 (0.54–0.89) for agreement between Peguero‐Lo Presti and any LVH based on Cornell voltage LVH, Cornell voltage product LVH, or Sokolow–Lyon LVH.
TABLE 6.
Between‐Visit and Within‐Visit Prevalence‐Adjusted Bias‐Adjusted Kappa (κ) Coefficient and 95% Confidence Interval Estimates for the Peguero‐Lo Presti LVH
| Visit 1, ECG 1 and 2 | Visit 2, ECG 1 and 2 | First ECG, Visits 1 and 2 | Second ECG, Visits 1 and 2 | |
|---|---|---|---|---|
| κ | 0.97 (0.91–1.00) | 1.00 (1.00–1.00) | 0.90 (0.80–1.00) | 0.93 (0.85–1.00) |
| n (%) of ECG‐LVH+ |
ECG1: 2 (3.2) ECG2: 3 (4.8) |
ECG3: 5 (8.2) ECG4: 5 (8.2) |
ECG1: 2 (3.2) ECG3: 5 (8.1) |
ECG2: 3 (4.9) ECG4: 5 (8.2) |
| N (total) | 62 | 61 | 62 | 61 |
TABLE 7.
Agreement (κ ) between Peguero‐Lo Presti and Cornell voltage or Sokolow–Lyon LVH on any one of the four ECGs (N = 63)
| LVH Measure | Prevalence of ECG‐LVH n (%) | Agreement with Peguero‐Lo Presti LVH (κ, 95% CI) |
|---|---|---|
| Peguero‐Lo Presti | 5 (7.9) | – |
| Cornell voltage | 1 (1.6) | 0.87 (0.75–0.99) |
| Cornell voltage product | 2 (3.2) | 0.90 (0.80–1.00) |
| Sokolow–Lyon | 7 (11.1) | 0.68 (0.50–0.86) |
| Any Cornell or Sokolow–Lyon | 8 (12.7) | 0.71 (0.54–0.89) |
Abbreviations: CI: 95% confidence interval; LVH: left ventricular hypertrophy; κ: prevalence‐adjusted bias‐adjusted kappa.
4. DISCUSSION
To our knowledge, this study is the first to report the repeatability of the Peguero‐Lo Presti index and LVH. Our analysis showed that among healthy adults, the continuous Peguero‐Lo Presti index is highly repeatable, as reflected by its intraclass correlation coefficient of 0.94 (95% CI 0.91–0.97). Furthermore, most of its observed variance originated from differences between individuals (93.6%). As expected, the within‐visit measurements showed greater repeatability than the between‐visit measurements. Indeed, only 5.5% and 0.9% of the variance originated between and within visits. Despite low prevalence of LVH in the study population, we found that the within‐ and between‐visit agreement of Peguero‐Lo Presti LVH was good.
Previous studies of the repeatability of other (e.g., Sokolow–Lyon and Cornell voltage) electrocardiographic LVH indices reported varied repeatability measurements. Within‐ and between‐visit κ in one study were 0.89 and 0.63 and 1 and 0.77 for Sokolow–Lyon and Cornell voltage LVH, respectively (Van Den Hoogen et al., 1992). When dichotomized, the between‐visit agreement of Peguero‐Lo Presti LVH in this study was excellent (0.90 and 0.93) based on κ. When taking into consideration the low prevalence of LVH in our study population, these results are comparable. To quantify the accuracy of Peguero‐Lo Presti LVH, a larger population with a higher prevalence of LVH is needed.
The Cornell voltage is currently one of the most widely used ECG indices of LVH. In the same study population, we previously reported that the ICC for the continuous Cornell voltage index was 0.97 (95% CI: 0.96–0.98) (Meyer et al., 2020). When comparing to the Peguero‐Lo Presti index, the Cornell voltage index showed similar repeatability (determined by the overlapping 95% confidence intervals). Our repeatability and agreement results suggest that the measurement properties of the continuous version of this index support its use in clinical settings, given the previously reported high sensitivity and specificity of the Peguero‐Lo Presti index in detecting LVH. The index is easy to derive from the standard 12‐lead ECG and therefore can be easily used in general practice.
The estimates of the SEM, MDC95, and MDD for the Peguero‐Lo Presti index can aid in estimating sample sizes and evaluating whether differences in the Peguero‐Lo Presti index are meaningful within‐participant or between‐participant groups. The MDC95 for the Peguero‐Lo Presti index was ≥ 133.7 µV, suggesting that a change of at least this magnitude may be necessary in order to determine whether a difference in the Peguero‐Lo Presti index exceeds measurement error and intraindividual variability. It is unknown whether a 133.7 µV change in the Peguero‐Lo Presti index is clinically relevant. However, The Health 2000 Survey, a population‐based study in Finland, showed that a 100 μV increase in the Peguero‐Lo Presti index was associated with a 1.03‐fold (95% CI 1.01–1.05) risk of sudden cardiac death (Porthan et al., 2019).
The prevalence of electrocardiographic LVH varies by criterion, which has been observed in prior studies evaluating differences in prognostic values (Afify et al., 2018; Porthan et al., 2019). In this study, the agreement between Peguero‐Lo Presti and Cornell voltage LVH was higher than with Sokolow–Lyon LVH. Agreement of electrocardiographic LVH metrics also varies among them, with greater overlap between Peguero‐Lo Presti and Cornell voltage LVH (Porthan et al., 2019). However, agreement between electrocardiographic LVH criteria was not formally assessed in the previously cited, population‐based study. The differences in electrocardiographic LVH criteria could be due to the wide range in their sensitivity for LVH (Pewsner et al., 2007), anatomic differences, and conduction alterations (Bacharova et al., 2017). Understanding the underlying differences in electrocardiographic LVH criteria would inform strategies to identify LVH.
As a limitation of this study, we point to the small number of individuals with electrocardiographic LVH in our study population, which constrained the precision of our estimates. However, we used the prevalence‐adjusted and bias‐adjusted κ to account for its low prevalence. Further, the study was adequately powered for the continuous measurements (Donner & Eliasziw, 1987). As this was a study of volunteers, those who participated were likely healthier than patient populations with a higher frequency of LVH. Despite the training of the study personnel and their adherence to a standardized study protocol, measurement variability may have been introduced by the repeated manual placement of ECG leads. Finally, participants in this study were instructed not to eat, drink, or smoke during the 10 hr prior to the ECG procedure. As presented in Table 1, 8% of the study population reported violating one or more of these instructions. We opted for keeping these observations in the analyses as it will more accurately simulate a real clinical setting. However, the ICC estimates were similar after excluding participants with a protocol violation, measurements of lower quality, and records with a PR interval > 200 ms indicative of an AV block.
Based on our results and the unselected nature of our study population, we submit that assessment of the repeatability and agreement of the Peguero‐Lo Presti LVH criterion in patient populations would be useful. Additionally, varying the study conditions, such as lengthening follow‐up time, may facilitate understanding of measurement variability over greater lengths of time or with varying LVH severity.
The short‐term repeatability of the Peguero‐Lo Presti index and LVH criterion was excellent and comparable to Cornell voltage in this study population of mostly healthy volunteers, suggesting that this novel electrocardiographic ECG criterion can be used to characterize left ventricular hypertrophy. Further studies of the repeatability of Peguero‐Lo Presti LVH in larger populations with higher LVH prevalence are needed.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors read and approved the final version of the manuscript and its submission to the journal. Contributed to the concept and design of the study and acquired the data: DD, MLM, EZS, GH, and EAW. Contributed to the analysis and interpretation of the data, drafting of the manuscript, and critically reviewing the manuscript: DD, MLM, EZS, DD, AA, ZZ, and GH.
ETHICAL APPROVAL
The study was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
CONSENT TO PARTICIPATE
Participants provided written informed consent.
Drager D, Soliman EZ, Meyer ML, et al. Short‐term repeatability of the peguero‐lo presti electrocardiographic left ventricular hypertrophy criteria. Ann Noninvasive Electrocardiol. 2021;26:e12829. 10.1111/anec.12829
Funding information
This work was supported by grant (RR00046) from the General Clinical Research Centers program of the Division of Research Resources, National Institutes of Health. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). AA was supported by K24HL148521.
REFERENCES
- Afify, H. M. A. , Waits, G. S. , Ghoneum, A. D. , Cao, X. , Li, Y. , & Soliman, E. Z. (2018). Peguero electrocardiographic left ventricular hypertrophy criteria and risk of mortality. Frontiers in Cardiovascular Medicine, 5, 75. 10.3389/fcvm.2018.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahmoud, M. F. , O'Neal, W. T. , Qureshi, W. , & Soliman, E. Z. (2015). Electrocardiographic versus echocardiographic left ventricular hypertrophy in prediction of congestive heart failure in the elderly. Clinical Cardiology, 38(6), 365–370. 10.1002/clc.22402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharova, L. , Estes, H. E. , Schocken, D. D. , Ugander, M. , Soliman, E. Z. , Hill, J. A. , Bang, L. E. , & Schlegel, T. T. (2017). The 4th report of the working group on ecg diagnosis of left ventricular hypertrophy. Journal of Electrocardiology, 50(1), 11–15. 10.1016/j.jelectrocard.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Byrt, T. , Bishop, J. , & Carlin, J. B. (1993). Bias, prevalence and kappa. Journal of Clinical Epidemiology, 46(5), 423–429. 10.1016/0895-4356(93)90018-v [DOI] [PubMed] [Google Scholar]
- Casale, P. N. , Devereux, R. B. , Milner, M. , Zullo, G. , Harshfield, G. A. , Pickering, T. G. , & Laragh, J. H. (1986). Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Annals of Internal Medicine, 105(2), 173–178. 10.7326/0003-4819-105-2-173 [DOI] [PubMed] [Google Scholar]
- Cuspidi, C. , Sala, C. , Negri, F. , Mancia, G. , & Morganti, A. (2012). Prevalence of left‐ventricular hypertrophy in hypertension: An updated review of echocardiographic studies. Journal of Human Hypertension, 26(6), 343–349. 10.1038/jhh.2011.104 [DOI] [PubMed] [Google Scholar]
- Dahlof, B. , Pennert, K. , & Hansson, L. (1992). Reversal of left ventricular hypertrophy in hypertensive patients. A metaanalysis of 109 treatment studies. American Journal of Hypertension, 5(2), 95–110. 10.1093/ajh/5.2.95 [DOI] [PubMed] [Google Scholar]
- Devereux, R. B. , Alonso, D. R. , Lutas, E. M. , Gottlieb, G. J. , Campo, E. , Sachs, I. , & Reichek, N. (1986). Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. American Journal of Cardiology, 57(6), 450–458. 10.1016/0002-9149(86)90771-x [DOI] [PubMed] [Google Scholar]
- Donner, A. , & Eliasziw, M. (1987). Sample size requirements for reliability studies. Statistics in Medicine, 6(4), 441–448. 10.1002/sim.4780060404 [DOI] [PubMed] [Google Scholar]
- Egan, B. M. , Zhao, Y. , & Axon, R. N. (2010). US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA, 303(20), 2043–2050. 10.1001/jama.2010.650 [DOI] [PubMed] [Google Scholar]
- Fortmann, S. P. , Haskell, W. L. , Williams, P. T. , Varady, A. N. , Hulley, S. B. , & Farquhar, J. W. (1986). Community surveillance of cardiovascular diseases in the Stanford Five‐City Project. Methods and initial experience. American Journal of Epidemiology, 123(4), 656–669. 10.1093/oxfordjournals.aje.a114285 [DOI] [PubMed] [Google Scholar]
- Guerreiro, C. , Azevedo, P. , Ladeiras‐Lopes, R. , Ferreira, N. , Barbosa, A. R. , Faria, R. , Almeida, J. , Primo, J. , Melica, B. , & Braga, P. (2020). Peguero‐Lo Presti criteria for diagnosis of left ventricular hypertrophy: A cardiac magnetic resonance validation study. Journla of Cardiovascular Medicine, 21(6), 437–443. 10.2459/jcm.0000000000000964 [DOI] [PubMed] [Google Scholar]
- Hajjar, I. , & Kotchen, T. A. (2003). Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA, 290(2), 199–206. 10.1001/jama.290.2.199 [DOI] [PubMed] [Google Scholar]
- Koren, M. J. , Devereux, R. B. , Casale, P. N. , Savage, D. D. , & Laragh, J. H. (1991). Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Annals of Internal Medicine, 114(5), 345–352. 10.7326/0003-4819-114-5-345 [DOI] [PubMed] [Google Scholar]
- Leigh, J. A. , O'Neal, W. T. , & Soliman, E. Z. (2016). Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged >/=65 years. American Journal of Cardiology, 117(11), 1831–1835. 10.1016/j.amjcard.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, D. , Anderson, K. M. , Savage, D. D. , Kannel, W. B. , Christiansen, J. C. , & Castelli, W. P. (1988). Echocardiographically detected left ventricular hypertrophy: Prevalence and risk factors. The Framingham Heart Study. Annals of Internal Medicine, 108(1), 7–13. 10.7326/0003-4819-108-1-7 [DOI] [PubMed] [Google Scholar]
- McDonagh, T. A. , Morrison, C. E. , Lawrence, A. , Ford, I. , Tunstall‐Pedoe, H. , McMurray, J. J. , & Dargie, H. J. (1997). Symptomatic and asymptomatic left‐ventricular systolic dysfunction in an urban population. Lancet, 350(9081), 829–833. 10.1016/s0140-6736(97)03033-x [DOI] [PubMed] [Google Scholar]
- Meyer, M. L. , Soliman, E. Z. , Drager, D. , & Heiss, G. (2020). Short‐term repeatability of electrocardiographic criteria of left ventricular hypertrophy. Annals of Noninvasive Electrocardiology, 25(2), e12688. 10.1111/anec.12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal, W. T. , Almahmoud, M. F. , Qureshi, W. T. , & Soliman, E. Z. (2015). Electrocardiographic and Echocardiographic Left Ventricular Hypertrophy in the Prediction of Stroke in the Elderly. Journal of Stroke and Cerebrovascular Diseases, 24(9), 1991–1997. 10.1016/j.jstrokecerebrovasdis.2015.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, N. , O'Neal, W. T. , Whalen, S. P. , & Soliman, E. Z. (2017). Electrocardiographic left ventricular hypertrophy predicts atrial fibrillation independent of left ventricular mass. Annals of Noninvasive Electrocardiology, 22(3), 1–5. 10.1111/anec.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peguero, J. G. , Lo Presti, S. , Perez, J. , Issa, O. , Brenes, J. C. , & Tolentino, A. (2017). Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. Journal of the American College of Cardiology, 69(13), 1694–1703. 10.1016/j.jacc.2017.01.037 [DOI] [PubMed] [Google Scholar]
- Pewsner, D. , Juni, P. , Egger, M. , Battaglia, M. , Sundstrom, J. , & Bachmann, L. M. (2007). Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: Systematic review. BMJ, 335(7622), 711. 10.1136/bmj.39276.636354.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porthan, K. , Kenttä, T. , Niiranen, T. J. , Nieminen, M. S. , Oikarinen, L. , Viitasalo, M. , Hernesniemi, J. , Jula, A. M. , Salomaa, V. , Huikuri, H. V. , Albert, C. M. , & Tikkanen, J. T. (2019). ECG left ventricular hypertrophy as a risk predictor of sudden cardiac death. International Journal of Cardiology, 276, 125–129. 10.1016/j.ijcard.2018.09.104 [DOI] [PubMed] [Google Scholar]
- Schroeder, E. B. , Whitsel, E. A. , Evans, G. W. , Prineas, R. J. , Chambless, L. E. , & Heiss, G. (2004). Repeatability of heart rate variability measures. Journal of Electrocardiology, 37(3), 163–172. 10.1016/j.jelectrocard.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Silberberg, J. S. , Barre, P. E. , Prichard, S. S. , & Sniderman, A. D. (1989). Impact of left ventricular hypertrophy on survival in end‐stage renal disease. Kidney International, 36(2), 286–290. 10.1038/ki.1989.192 [DOI] [PubMed] [Google Scholar]
- Spirito, P. , Bellone, P. , Harris, K. M. , Bernabo, P. , Bruzzi, P. , & Maron, B. J. (2000). Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. New England Journal of Medicine, 342(24), 1778–1785. 10.1056/nejm200006153422403 [DOI] [PubMed] [Google Scholar]
- Sullivan, J. M. , Zwaag, R. V. , El‐Zeky, F. , Ramanathan, K. B. , & Mirvis, D. M. (1993). Left ventricular hypertrophy: Effect on survival. Journal of the American College of Cardiology, 22(2), 508–513. 10.1016/0735-1097(93)90057-8 [DOI] [PubMed] [Google Scholar]
- The ARIC investigators (1989). The atherosclerosis risk in communities (ARIC) study: Design and objectives. American Journal of Epidemiology, 129(4), 687–702. [PubMed] [Google Scholar]
- Vaidean, G. D. , Schroeder, E. B. , Whitsel, E. A. , Prineas, R. J. , Chambless, L. E. , Perhac, J. S. , Heiss, G. , & Rautaharju, P. M. (2005). Short‐term repeatability of electrocardiographic spatial T‐wave axis and QT interval. Journal of Electrocardiology, 38(2), 139–147. 10.1016/j.jelectrocard.2004.09.020 [DOI] [PubMed] [Google Scholar]
- Vakili, B. A. , Okin, P. M. , & Devereux, R. B. (2001). Prognostic implications of left ventricular hypertrophy. American Heart Journal, 141(3), 334–341. 10.1067/mhj.2001.113218 [DOI] [PubMed] [Google Scholar]
- Van Den Hoogen, J. P. , Mol, W. H. , Kowsoleea, A. , Van Ree, J. W. , Thien, T. , & Van Weel, C. (1992). Reproducibility of electrocardiographic criteria for left ventricular hypertrophy in hypertensive patients in general practice. European Heart Journal, 13(12), 1606–1610. 10.1093/oxfordjournals.eurheartj.a060112 [DOI] [PubMed] [Google Scholar]
- Woythaler, J. N. , Singer, S. L. , Kwan, O. L. , Meltzer, R. S. , Reubner, B. , Bommer, W. , & DeMaria, A. (1983). Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: Comparison with postmortem mass measurements. Journal of the American College of Cardiology, 2(2), 305–311. 10.1016/s0735-1097(83)80167-3 [DOI] [PubMed] [Google Scholar]


