Abstract
Background
We investigated whether T‐wave heterogeneity (TWH) can identify patients who are at risk for near‐term cardiac mortality.
Methods
A nested case–control analysis was performed in the 888 patients admitted to the Emergency Department (ED) of our medical center in July through September 2018 who had ≥2 serial troponin measurement tests within 6 hr for acute coronary syndrome evaluation to rule‐in or rule‐out the presence of acute myocardial infarction. Patients who died from cardiac causes during 90 days after ED admission were considered cases (n = 20; 10 women) and were matched 1:4 on sex and age with patients who survived during this period (n = 80, 40 women). TWH, that is, interlead splay of T waves, was automatically assessed from precordial leads by second central moment analysis.
Results
TWHV4‐6 was significantly elevated at ED admission in 12‐lead resting ECGs of female patients who died of cardiac causes during the following 90 days compared to female survivors (100 ± 14.9 vs. 40 ± 3.6 µV, p < .0001). TWHV4‐6 generated areas under the receiver‐operating characteristic (ROC) curve (AUC) of 0.933 in women (p < .0001) and 0.573 in men (p = .4). In women, the ROC‐guided 48‐µV TWHV4‐6 cut point for near‐term cardiac mortality produced an adjusted odds ratio of 121.37 (95% CI: 2.89–6,699.84; p = .02) with 100% sensitivity and 82.5% specificity. In Kaplan–Meier survival analysis, TWHV4‐6 ≥ 48 µV predicted cardiac mortality in women during 90‐day follow‐up with a hazard ratio of 27.84 (95% CI: 7.29–106.36, p < .0001).
Conclusion
Elevated TWHV4‐6 is associated with near‐term cardiac mortality among women evaluated for acute coronary syndrome.
Keywords: cardiac mortality, electrocardiogram, emergency department, repolarization, T‐wave heterogeneity
1. INTRODUCTION
Coronary heart disease represents a major public health problem, which claimed >840,000 deaths in 2016 in the United States, surpassing cancer and chronic lung disease combined (Virani et al., 2020). Huikuri et al. (2003) reported that premature death in the early period after myocardial infarction remains high and that this issue has not been adequately addressed. Solomon et al. (2005) have emphasized the pressing need for improved monitoring in their evaluation of >14,000 patients enrolled in the VALIANT trial, in which it was shown that the risk for SCD was highest in the first few months after MI among patients with or without significant heart failure. There is limited information about ECG parameters to track risk in the early post‐MI phase.
Recently, we reported that T‐wave heterogeneity (TWH), a quantitative measure of the interlead splay of T waves among adjacent leads, correlates with malignant ventricular arrhythmias in preclinical studies (Bonatti et al., 2014; Nearing & Verrier, 2003) and strongly predicts cardiac mortality in clinical studies (Araujo Silva et al., 2020; Bortolotto et al., 2020; Kenttä et al., 2016; Silva et al., 2020; Tan et al., 2017; Verrier & Huikuri, 2017). In a 5600‐subject Health Survey 2000 study, TWH predicted all‐cause mortality and sudden cardiac death in a cross section of the entire Finnish population (Kenttä et al., 2016). This measurement also predicted survival and arrhythmia‐free survival in patients with ischemic and nonischemic cardiomyopathy (Tan et al., 2017; Verrier & Huikuri, 2017). Recently, this laboratory reported that TWH can detect the presence of acute myocardial ischemia verified by single‐photon emission computed tomography during myocardial perfusion imaging and diagnostic coronary angiography (Araujo Silva et al., 2020). TWH was able to detect clinically significant coronary stenosis during pharmacologic stress and exercise tolerance testing, while ST‐segment changes did not distinguish between cases and controls (Araujo Silva et al., 2020; Silva et al., 2020). Finally, preimplantation TWH was found superior to QRS complex duration in predicting mechanical super‐response in patients with non‐left bundle branch block who received cardiac resynchronization therapy (Bortolotto et al., 2020).
The main goal of the current study was to investigate whether repolarization heterogeneity could identify patients who are at risk for near‐term cardiac mortality following discharge from the ED following evaluation for acute coronary syndrome.
2. METHODS
2.1. T‐Wave Heterogeneity Assessment
The 12‐lead ECGs were recorded at emergency department (ED) admission while the subject was at rest with the GE Healthcare CASE 8,000 (Milwaukee WI, USA). The files were downloaded from the MUSE system at 500 samples per second for each channel. Interlead heterogeneity of repolarization (TWH) morphology was assessed from the 10‐s 12‐lead ECG recordings by an investigator blinded to clinical outcomes using automated second central moment analysis with a program written in MatLab. The software program removed noise, baseline wander, and arrhythmias prior to the automated estimation of TWH. After this filter, the software generated mean waveforms separately for the QRS complex and T waves to include the J point and entire T wave of adjoining precordial leads. In our previous studies, we focused on TWH in leads V4–V6 because T waves in this lead set have a lower intrinsic variability due to lead placement or body habitus (Kania et al., 2014). In the current study, we expanded the analysis to include TWH in leads V1‐3 (TWHv1‐3) also including leads I and II (TWHv1‐3LILII, TWHv4‐6LILII) to determine whether incorporating these fields of view offers additional advantages. The mean interlead morphology constitutes the first moment or central axis in the terminology of Newtonian physics. The second central moment, or mean‐square deviation, was then determined to quantify the variability or splay about the mean morphology (Nearing & Verrier, 2003). Finally, the maximum square root of the second central moment was calculated to obtain the TWH values in microvolts (µV). Maximum heterogeneity levels for each patient were reported across the entire JT interval and used to identify associations with mortality. ECG heterogeneity is not unduly weighted by low‐amplitude protracted termination or inflections in the waveforms, which can limit accurate dispersion measurements by conventional analyses.
2.2. ECG analysis
QTc‐interval and ST‐segment data were taken from the clinical record. The cut points adopted for QTc prolongation were ≥470 ms for women and ≥450 ms for men (Straus et al., 2006). For women, ST‐segment elevation was considered positive if there was new ST‐segment elevation at the J point in two or more contiguous leads with the cut point of ≥1.5 mm in V2 or V3 or ≥1 mm in all other leads regardless of age. For men ≥40 years, ST‐segment elevation was considered positive if there was new ST elevation at the J point in two or more contiguous leads with the cut point of ≥2.0 mm in V2 or V3 or ≥1 mm in all other leads (Thygesen et al., 2018).
2.3. Study population
This investigation conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was carried out under a protocol approved by the Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC) (Boston, MA). The inclusion criteria for this retrospective medical records study were admission to BIDMC’s ED between July and September 2018 and two serial troponin measurements within 6 hr for acute coronary syndrome determination following ED admission (n = 888). To identify patients who died within 90 days of ED admission or survived, we used hospital medical records and published obituaries matched for name, birthdate, and geographical location within Massachusetts. Discrimination of the cause of death was based on review of BIDMC’s medical records. Deaths were classified as cardiac if caused by myocardial infarction, acute decompensated heart failure, cardiogenic shock, Takutsubo and other cardiomyopathies, myocarditis, endocarditis, pericarditis and pericardial tamponade or sudden cardiac death.
Patients were not excluded from the study based on clinical characteristics. Among the 888 ED admissions, 42 patients who died within 90 days after the ED admission from a non‐cardiac cause of death and 7 patients who lacked clear causes of death were excluded from the study along with 29 patients whose survival could not be determined from available records (“lost to follow‐up”). If a patient was readmitted to the ED during this period (n = 9), only the data from the first admission were included. Patients whose ECGs were of insufficient quality for TWH analysis (n = 8) were also excluded. The main bases for excluding ECGs were noise due to poor electrode contact (n = 1), 60‐cycle interference (n = 3), and arrhythmias that could not be removed by the software, such as atrial fibrillation with a rapid ventricular response (n = 4).
Of the remaining 793 patients, 20 (10 women) had a cardiac cause of death within 90 days after ED admission, were classified as cases, and were matched 1:4 on age and sex with survivors (controls) by an investigator blinded to TWH and troponin results. Mortality analysis was performed on these 100 patients, with males and females analyzed separately.
2.4. Troponin determination
Troponin T was measured by BIDMC’s hospital laboratory using standard immunoassay methodology (Collinson et al., 2001).
2.5. Statistics
Statistical analyses were performed using XLSTAT (Addinsoft, Inc., New York, NY). Data are reported as means ± standard error of the mean (SEM). TWH in both cases and controls for all patients and separately by sex was not normally distributed according to Shapiro–Wilk test. Statistical differences between cases and controls were calculated using Mann–Whitney test for non‐parametric variables and two‐tailed unpaired Student's t test or Welch's t test, chosen according to the application of the F test for establishing the equality of variances, for parametric variables. Differences in categorical clinical variables were analyzed using Fisher's exact test. Multivariate analysis was used to correct results for confounding effects of factors that differed significantly among controls and cases. ROC curves were plotted for the ECG parameters, and AUCs were calculated. The cut points for TWH were determined for the optimum point on the ROC curve, given by the value with the highest sum of sensitivity and specificity. Kaplan–Meier curves for mortality were determined and statistically evaluated using the log‐rank test. Two‐tailed p‐values ≤.05 were considered significant.
3. RESULTS
3.1. Patient characteristics
Patient characteristics comparing male (n = 10) and female cases (n = 10) and age‐matched male (n = 40) and female controls (n = 40) are provided in Tables 1 and 2 according to sex. Women were somewhat younger than men (69 ± 2.2 vs. 75 ± 1.9 years, p = .01). The frequency of troponin levels >0.1 with │Δ│ >20% differed (p < .02) between cases and controls for both women and men but not comparing women to men (p = .2).
TABLE 1.
Female patients’ baseline characteristics
| Female patients | Controls (40 patients) | Cases (10 patients) | p value |
|---|---|---|---|
| Risk factors—n (%) | |||
| Age (years) | 69 ± 3.3 | 69 ± 4.9 | >.99 |
| Race (black/African American) | 9 (22.5%) | 1 (10.0%) | .3 |
| Obese (BMI ≥ 30 kg/m2) | 18 (45.0%) | 2 (20.0%) | .1 |
| BMI (kg/m2) | 31.5 ± 1.82 | 27.3 ± 2.17 | .3 |
| Hypertension | 29 (72.5%) | 9 (90.0%) | .2 |
| Diabetes type II | 12 (30.0%) | 3 (30.0%) | .3 |
| Hyperlipidemia/Hypercholesterolemia | 33 (82.5%) | 8 (80.0%) | .3 |
| Current smoker | 5 (12.5%) | 2 (20.0%) | .3 |
| History of smoking | 11 (27.5%) | 2 (20.0%) | .3 |
| History—n (%) | |||
| Documented CAD | 20 (50.0%) | 5 (50.0%) | .3 |
| MI Hx | 8 (20.0%) | 1 (10.0%) | .3 |
| AF Hx | 6 (15.0%) | 3 (30.0%) | .2 |
| OSA | 6 (15.0%) | 1 (10.0%) | .4 |
| HFpEF (>40%) | 9 (22.5%) | 1 (10.0%) | .3 |
| HFrEF (≤40%) | 4 (10.0%) | 6 (60.0%) | .002 |
| Chronic kidney disease | 19 (47.5%) | 7 (70.0%) | .1 |
| Interventions—n (%) | |||
| Previous PCI | 3 (7.5%) | 0 (0.0%) | .5 |
| Previous CABG | 3 (7.5%) | 2 (20.0%) | .2 |
| Pacemaker | 0 (0.0%) | 0 (0.0%) | >.99 |
| Medications on admission—n (%) | |||
| Betablocker | 20 (50.0%) | 6 (60.0%) | .2 |
| Calcium antagonist | 6 (15.0%) | 2 (20.0%) | .3 |
| ACE‐I or ARB | 19 (47.5%) | 5 (50.0%) | .3 |
| Diuretics | 14 (35.0%) | 5 (50.0%) | .2 |
| Digitalis | 0 (0.0%) | 1 (10.0%) | .2 |
| Statin | 26 (65.0%) | 4 (40.0%) | .1 |
| Nitrate | 7 (17.5%) | 0 (0.0%) | .2 |
| Biguanides (metformin) | 7 (17.5%) | 1 (10.0%) | .3 |
| Aspirin | 16 (40.0%) | 3 (30.0%) | .2 |
| ECG findings | |||
| QRS (ms) (mean ± SEM) | 100.2 ± 2.59 | 123.7 ± 9.68 | .03 |
| QRS >120 ms – n (%) | 6 (15.0%) | 6 (60%) | .007 |
| QTc (ms) (mean ± SEM) | 427.2 ± 4.14 | 456.6 ± 15.47 | .04 |
| QTc > 470 ms – n (%) | 2 (5.0%) | 1 (10.0%) | .4 |
| TWH (µV) (mean ± SEM) | 40 ± 3.6 | 100 ± 14.9 | .0001 |
| TWH ≥48 µV – n (%) | 7 (17.5%) | 10 (100%) | <.0001 |
| ECG diagnoses—n (%) | |||
| RBBB (complete) | 3 (7.5%) | 3 (30.0%) | .07 |
| LBBB (complete) | 1 (2.5%) | 2 (20.0%) | .09 |
| Left anterior fascicular block | 2 (5.0%) | 1 (10.0%) | .4 |
| Left ventricular hypertrophy | 0 (0.0%) | 1 (10.0%) | .2 |
| ECG changes indicative of ischemia—n (%) | |||
| ST‐segment depression | 3 (7.5%) | 3 (30.0%) | .07 |
| T‐wave inversion | 11 (27.5%) | 6 (60.0%) | .05 |
| ST‐segment elevation | 0 (0.0%) | 2 (20.0%) | .04 |
| Troponin | |||
| 1st or 2nd troponin >0.1 with│Δ│>20% | 1 (2.5%) | 3 (30.0%) | .02 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; MI Hx, myocardial infarction history; AF Hx, atrial fibrillation history; OSA, obstructive sleep apnea; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; ACE‐I, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin receptor blocker; RBBB, right bundle branch block; LBBB, left bundle branch block.
Boldface type indicates statistically significant results (p < .05).
TABLE 2.
Male patients’ baseline characteristics
| Male patients | Controls (40 patients) | Cases (10 patients) | p value |
|---|---|---|---|
| Risk factors—n (%) | |||
| Age (years) | 75 ± 2.1 | 75 ± 2.1 | >.99 |
| Race (black/African American) | 6 (15.0%) | 0 (0.0%) | .2 |
| Obese (BMI ≥ 30 kg/m2) | 12 (30.0%) | 3 (30.0%) | .3 |
| BMI (kg/m2) | 28.0 ± 0.80 | 26.7 ± 2.18 | .5 |
| Hypertension | 35 (87.5%) | 9 (90.0%) | .4 |
| Diabetes type II | 15 (37.5%) | 3 (30.0%) | .2 |
| Hyperlipidemia/Hypercholesterolemia | 33 (82.5%) | 10 (100.0%) | .2 |
| Current smoker | 2 (5.0%) | 2 (20.0%) | .2 |
| History of smoking | 12 (30.0%) | 2 (20.0%) | .3 |
| History‐ n (%) | |||
| Documented CAD | 26 (65.0%) | 8 (80.0%) | .2 |
| MI Hx | 14 (35.0%) | 3 (30.0%) | .3 |
| AF Hx | 11 (27.5%) | 5 (50.0%) | .1 |
| OSA | 3 (7.5%) | 3 (30.0%) | .07 |
| HFpEF (LVEF >40%) | 7 (17.5%) | 2 (20.0%) | .3 |
| HFrEF (LVEF ≤40%) | 9 (22.5%) | 4 (40.0%) | .2 |
| Chronic kidney disease | 21 (52.5%) | 5 (50.0%) | .3 |
| Interventions—n (%) | |||
| Previous PCI | 8 (20.0%) | 2 (20.0%) | .3 |
| Previous CABG | 7 (17.5%) | 2 (20.0%) | |
| Pacemaker | 2 (5.0%) | 3 (30.0%) | .04 |
| Medications on admission—n (%) | |||
| Betablocker | 25 (62.5%) | 5 (50.0%) | .2 |
| Calcium antagonist | 7 (17.5%) | 1 (10.0%) | .3 |
| ACE‐I or ARB | 19 (47.5%) | 4 (40.0%) | .3 |
| Diuretics | 14 (35.0%) | 7 (70.0%) | .04 |
| Digitalis | 3 (7.5%) | 2 (20.0%) | .2 |
| Statin | 28 (70.0%) | 7 (70.0%) | .3 |
| Nitrate | 4 (10.0%) | 1 (10.0%) | .4 |
| Biguanides (metformin) | 5 (12.5%) | 1 (10.0%) | .4 |
| Aspirin | 26 (65.0%) | 5 (50.0%) | .2 |
| ECG findings | |||
| QRS (ms) | 111.4 ± 4.46 | 122.9 ± 9.28 | .3 |
| QRS >120 ms – n (%) | 11 (27.5%) | 5 (50.0%) | .1 |
| QTc (ms) | 442.2 ± 6.50 | 441.8 ± 12.92 | .9 |
| QTc > 450 – n (%) | 13 (32.5%) | 4 (40.0%) | .3 |
| TWH (µV) | 96 ± 11.4 | 87 ± 10.1 | .3 |
| TWH ≥ 56 µV – n (%) | 22 (55.0%) | 9 (90.0%) | .04 |
| ECG diagnoses—n (%) | |||
| RBBB (complete) | 4 (10.0%) | 1 (10.0%) | .4 |
| LBBB (complete) | 7 (17.5%) | 4 (40.0%) | .1 |
| Left anterior fascicular block | 4 (10.0%) | 1 (10.0%) | .4 |
| Left ventricular hypertrophy | 3 (7.5%) | 0 (0.0%) | .5 |
| ECG changes indicative of ischemia—n (%) | |||
| ST‐segment depression | 9 (22.5%) | 2 (20.0%) | .3 |
| T‐wave inversion | 14 (35.0%) | 7 (70.0%) | .04 |
| ST‐segment elevation | 5 (12.5%) | 2 (20.0%) | .3 |
| Troponin | |||
| 1st or 2nd troponin > 0.1 with│Δ│>20% | 3 (7.5%) | 4 (40.0%) | .02 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; MI Hx, myocardial infarction history; AF Hx, atrial fibrillation history; OSA, obstructive sleep apnea; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; ACE‐I, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin receptor blocker; RBBB, right bundle branch block; LBBB, left bundle branch block.
Boldface type indicates statistically significant results (p < .05).
At admission to the Emergency Department, 53 patients (53%) presented with a chief complaint of chest pain; 13 patients (13%) presented with dyspnea / shortness of breath; and 34 patients (34%) presented with other symptoms such as abdominal pain, weakness, syncope, or altered mental status. Diagnostic angiography was indicated in 17 patients (17%). Guided by the cardiac catheterization findings, 14 (82%) were treated medically with beta‐blockers, statins, and aspirin, while 3 (18%) were treated invasively with percutaneous coronary intervention during the index admission. Medical therapy for men and women differed only for aspirin at ED admission and did not differ at discharge (Table 3).
TABLE 3.
Medical therapy at emergency department admission and discharge
| Women (50 patients) | Men (50 patients) | p value | |
|---|---|---|---|
| Medications on admission – n (%) | |||
| Betablocker | 26 (52.0%) | 30 (60.0%) | .1 |
| Calcium Antagonist | 8 (16.0%) | 8 (16.0%) | .2 |
| ACE‐I or ARB | 24 (48.0%) | 23 (46.0%) | .2 |
| Diuretics | 19 (38.0%) | 21 (42.0%) | .1 |
| Digitalis | 1 (2.0%) | 5 (10.0%) | .09 |
| Statin | 30 (60.0%) | 35 (70.0%) | .1 |
| Nitrate | 7 (14.0%) | 5 (10.0%) | .2 |
| Biguanides (metformin) | 8 (16.0%) | 6 (12.0%) | .2 |
| Aspirin | 19 (38.0%) | 31 (62.0%) | .009 |
| New medications/dosage increase at ED discharge: n (%) | |||
| Betablocker | 3 (6.0%) | 8 (16.0%) | .07 |
| Calcium antagonist | 0 (0.0%) | 2 (4.0%) | .2 |
| ACE‐I or ARB | 1 (2.0%) | 4 (8.0%) | .2 |
| Diuretics | 3 (6.0%) | 8 (16.0%) | .07 |
| Digitalis | 0 (0.0%) | 0 (0.0%) | >.99 |
| Statin | 6 (12.0%) | 7 (14.0%) | .2 |
| Nitrate | 5 (10.0%) | 2 (4.0%) | .2 |
| Biguanides | 0 (0.0%) | 0 (0.0%) | >.99 |
| Aspirin | 6 (12.0%) | 5 (10.0%) | .2 |
Abbreviations: ACE‐I, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin receptor blocker; ED, emergency department.
3.2. TWH results
Digitized ECG tracings illustrating T‐wave heterogeneity (TWH) from a representative female control and case are shown in Figure 1. Mean TWHV4‐6 levels differed significantly between the cases and controls for the entire cohort (94 ± 9.1 vs. 68 ± 6.8 µV, p = .0002). The ROC curve showed a close linkage with near‐term cardiac mortality, as TWHV4‐6 generated an AUC of 0.760 (p < .0001) and an optimized cut point of 56 µV, which resulted in an adjusted odds ratio of 16.8 (95% CI: 2.73–103.59, p = .002) for the entire study population. Sensitivity was 90%, and specificity was 67.5% (Table 4). Considering the differences in cardiovascular anatomy and electrophysiology, as well as the different incidences of cardiovascular disease among men and women, a sex‐based analysis was performed. TWHV4‐6 performed better among women than men. Among females, TWHV4‐6 levels differed significantly between cases and controls (100 ± 14.9 vs. 40 ± 3.6 µV, p < .0001) (Table 1, Figure 2 upper panel), while among males, TWHV4‐6 did not differ significantly between the two groups (87 ± 10.1 vs. 96 ± 11.4 µV, p = .3) (Table 2, Figure 2 lower panel). It is noteworthy that the mean TWHV4‐6 levels among control subjects were lower for women than for men (Figure 2).
FIGURE 1.
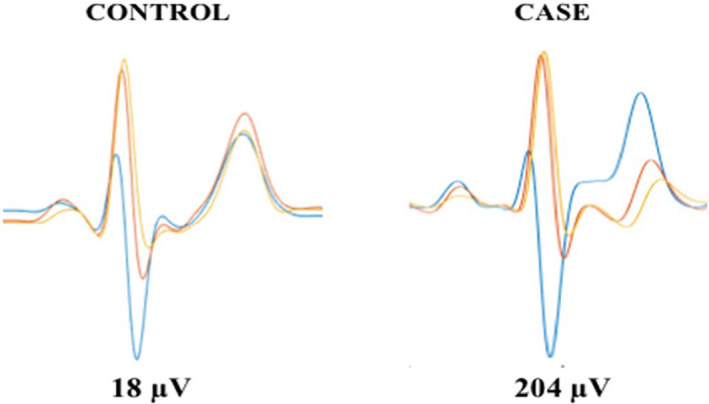
Simultaneous EKGs from leads V4, V5, and V6 are superimposed in a representative female control subject (left panel) and a representative female case (right panel) illustrating T‐wave heterogeneity (TWH) as interlead splay in repolarization morphology. In the case, TWH was markedly elevated in the case to 204 µV compared to 18 µV in the control subject.
TABLE 4.
Optimized cut points for the entire cohort and according to sex
| Cut point | Unadjusted odds ratio | 95% CI | p value | Adjusted odds ratio | 95% CI | p value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 100) | 56 µV | 18.69 | 4.03–86.67 | .0002 | 16.83 a | 2.73–103.59 | .002 | 90% | 67.5% |
| Women (n = 50) | 48 µV | 93.80 | 4.93–1,784.10 | .002 | 121.37 b | 2.20–6,699.84 | .02 | 100% | 82.5% |
| Men (n = 50) | 56 µV | 7.36 | 0.85–63.72 | .06 | 2.89 c | 0.51–16.5 | .1 | 90% | 45.0% |
Results adjusted for seven confounders: HFrEF; Diuretics use; Pacemaker; LBBB; T‐wave inversion; QRS complex; Troponin (1st or 2nd troponin >0.1 with│Δ│>20%).
Results adjusted for six confounders: HFrEF; ST‐segment elevation; T‐wave inversion; QRS complex; QTc interval; Troponin (1st or 2nd troponin >0.1 with│Δ│>20%).
Results adjusted for four confounders: Diuretic use; Pacemaker; T‐wave inversion; Troponin (1st or 2nd troponin >0.1 with│Δ│>20%).
FIGURE 2.
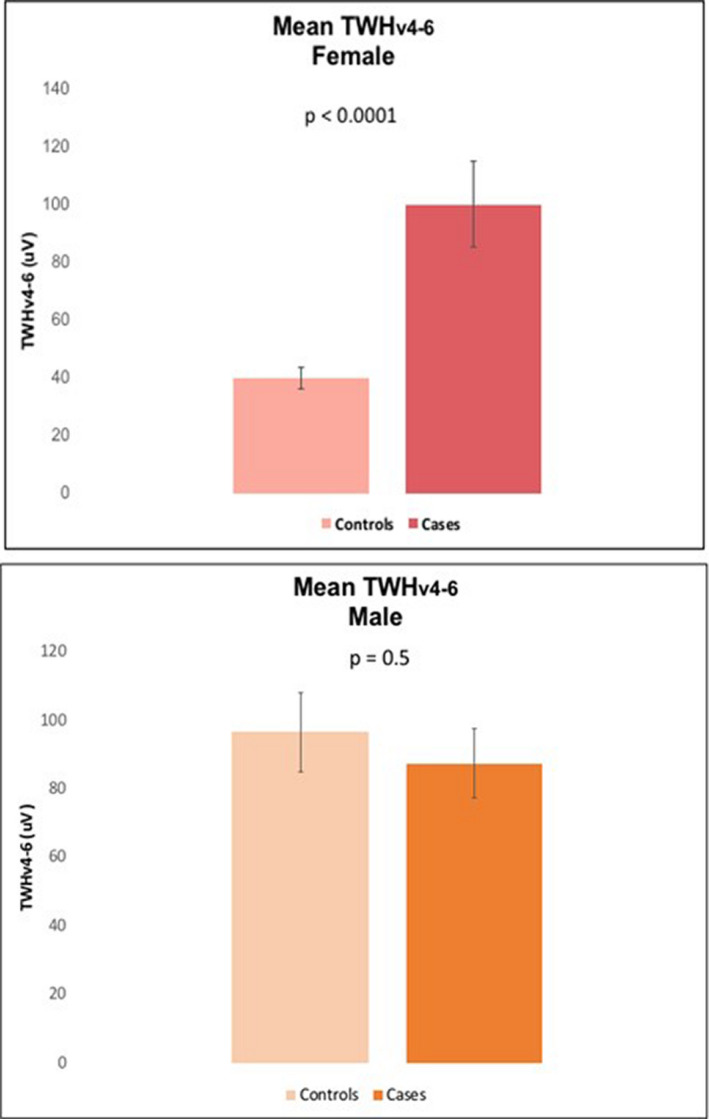
Comparison of mean TWHV4‐6 levels between female controls and cases (upper panel) and male controls and cases (lower panel). The differences between the cases and controls were more marked among females, who showed higher levels of TWH among those who died within the 90‐day post‐admission period.
The ROC for TWHV4‐6 in women was optimum, generating an AUC of 0.933 (p < .0001) (Figure 3) and an optimized cut point of 48 µV, which yielded an adjusted odds ratio of 121.37 (95% CI: 2.20–6,699.84; p = .02) with sensitivity of 100% and specificity of 82.5% (Table 4). In men, the ROC for TWHV4‐6 was also optimum, generating a nonsignificant AUC of 0.573 (p = .4) (Figure 4) with a nonsignificant adjusted odds ratio of 2.89 (95% CI: 0.51–16.5; p = .2) (Table 4).
FIGURE 3.
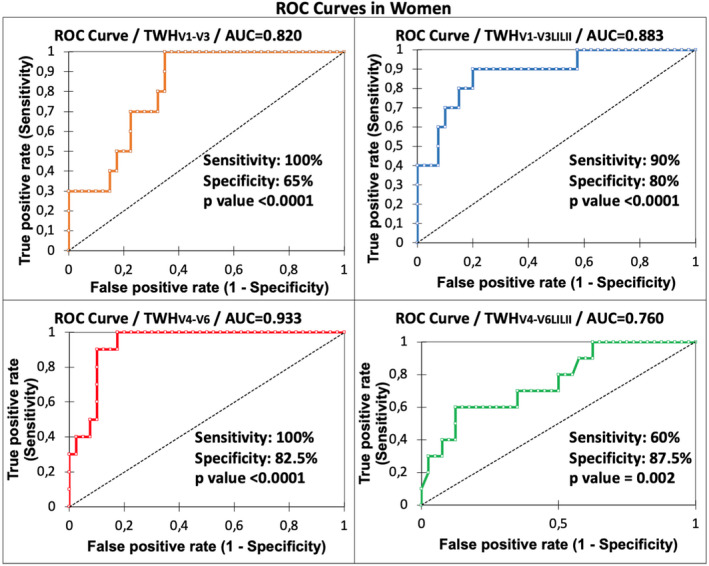
ROC curves for women with AUC for TWH in four lead sets as an indicator of ≤90‐day mortality. Statistically significant AUCs were obtained based on all lead configurations, with the maximum level of 0.933 obtained in leads V4‐V6 (p < .0001).
FIGURE 4.
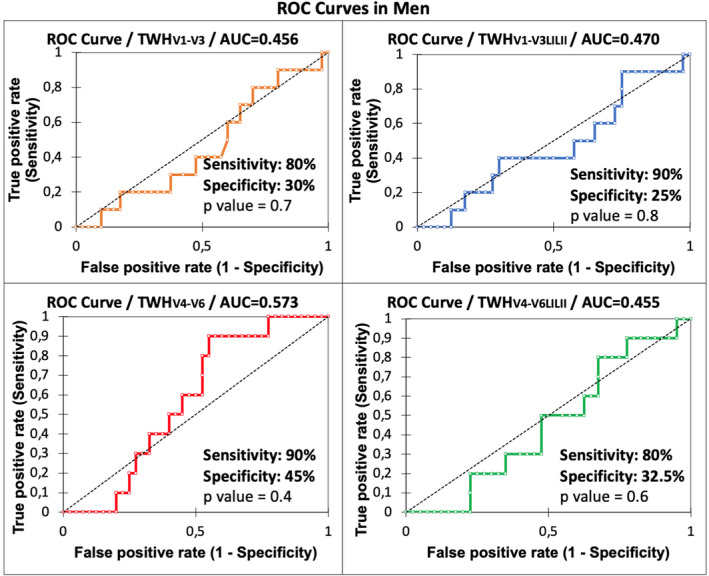
ROC curves for males with AUC for TWH in four lead sets as an indicator of ≤90‐day mortality. In contrast to female patients, none of the lead configurations yielded statistically significant AUCs in male patients.
During the index hospital admission, five male patients underwent cardiac interventions (three percutaneous coronary interventions, two implantable cardiac defibrillator placements). After discharge but during the 90‐day follow‐up period, one male patient underwent balloon valvuloplasty. The ROC for men based on TWHV4‐6 measurements after these interventions generated a nonsignificant AUC of 0.585 (p = .3) with 90% sensitivity and 45% specificity. None of the women underwent cardiac interventions within 90 days following their index ED admission.
3.3. Kaplan–Meier analyses
Kaplan–Meier analysis revealed that 90‐day cardiac mortality was significantly increased in the entire cohort at a TWHV4‐6 cut point of ≥56 μV (p < .0001), which generated a hazard ratio of 13.3 (95% CI: 5.44–32.51, p < .0001). In women, the ROC‐based TWHV4‐6 cut point of ≥48 μV (p < .0001) generated a hazard ratio of 27.84 (95% CI: 7.29–106.37, p < .0001) (Figure 5 upper panel), and in men, the ROC‐based TWHV4‐6 cut point of ≥56 μV (p = .06) generated a hazard ratio of 5.6 (95% CI: 1.57–20.12, p = .06) (Figure 5 lower panel).
FIGURE 5.
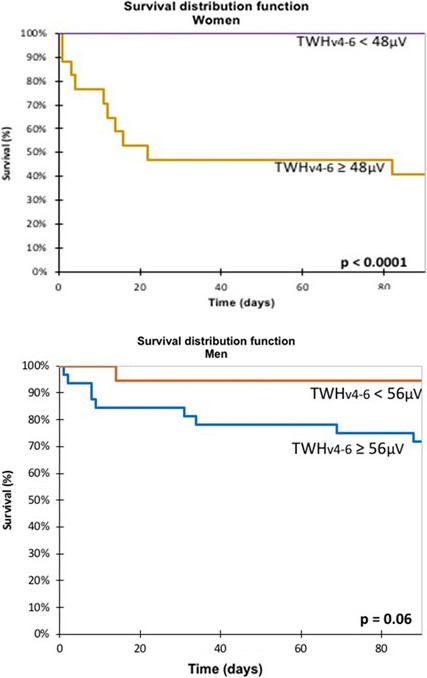
Kaplan–Meier survival curves obtained in women (upper panel) and men (lower panel). When the 48‐µV optimized cut point for TWHV4‐V6 was exceeded, mortality was significantly increased (p < .0001) in females. By contrast, at the optimized cut point of 56 µV for TWHV4‐V6 in men, separation between survivors and nonsurvivors did not achieve statistical significance (p < .06).
4. DISCUSSION
4.1. Main findings
In this retrospective medical records study, increased spatial heterogeneity of repolarization (TWH), measured with the second central moment technique, was associated with cardiac mortality in women during 90 days following ED admission (Table 4, Figure 5). This association remained significant even after adjustment for the patients’ clinical characteristics. Our findings support the use of TWH in noninvasive ECG‐based risk stratification in women admitted to the ED. Elevated TWHV4‐6 levels could constitute an indicator that female patients are at risk for near‐term cardiac‐related death and could prompt early interventions such as adjustments in medications or revascularization. These findings are consistent with those of Solomon et al. (2005) who reported that risk for premature cardiac death is highest early after a coronary event and merits careful monitoring.
4.2. Prior studies and current investigation
The central question addressed in the current study is whether TWH in standard resting 12‐lead ECGs recorded during ED admission can help to stratify risk for cardiovascular mortality during a 90‐day follow‐up period. Selection of TWH was based on the results of clinical investigations that showed its capacity to predict all‐cause mortality and sudden cardiac death in a representative sample of the entire Finnish population (Kenttä et al., 2016). In the current study, we found that this ECG heterogeneity marker was significantly increased in female but not male patients who died of cardiac causes during the 90‐day period. Some factors could account for this sex difference. The lower baseline values in women may relate to their generally smaller heart size, with correspondingly lower T‐wave amplitude, as well as to hormonal and other factors that can influence repolarization.
These findings are important in light of the challenges in ECG‐based evaluation of risk for coronary artery disease‐related events in women (Stoletniy & Pai, 1997). Recently, Haukilahti et al. (2019) reported that 22% of women have normal ECGs prior to sudden cardiac death, suggesting that standard ECG markers may not perform well in women. Moreover, ETT‐induced ST‐segment depression is often a normal variant in women, possibly correlated with estrogen levels, and has lower sensitivity and specificity in identifying coronary artery disease than it does in males (Veseli et al., 2020). ST‐segment changes are also more labile in women, with a greater degree of non‐specific deviation than in men (Zegre‐Hemsey, 2020). These observations point to inherent challenges in ECG‐based risk stratification for cardiac mortality among women.
There are also sex‐based differences in use of medical therapies and interventions and counseling, in part due to the underrepresentation of women in major randomized clinical trials, which can compromise awareness of clinical manifestations and treatment strategies for this population (Davis et al., 2017). Specifically, underuse of medical therapies and interventions in women (Ayanian & Epstein, 1991; Jneid et al., 2008; Steingart et al., 1991; Tobin et al., 1987) could represent important factors in ongoing risk since medication or revascularization would be expected to reduce the patient's repolarization heterogeneity levels compared to the ED admission ECG. The more widespread interventions among men could be confounding factors that could negate risk stratification for near‐term cardiovascular mortality by TWHV4‐6.
In a retrospective study of patients who were discharged from nonfederal acute care hospitals after being hospitalized for coronary artery disease, Ayanian and Epstein (1991) reported that men were more likely than women to undergo diagnostic and revascularization procedures. Steingart et al. (1991) found that while women were more likely to report disability due to ischemic symptoms after myocardial infarction, male patients underwent revascularization >1.8 times as often as female patients. These sex‐based differences in approach persisted despite abnormal results of nuclear testing (1987). These investigators also reported that examining cardiologists were twice as likely to attribute women's symptoms to somatic, psychiatric, or other non‐cardiac causes when compared to men's, even though men and women had approximately the same distribution of the different types of angina and female patients were more symptomatic when compared to male patients.
Prescription of medications was also dissimilar comparing men and women (Jneid et al., 2008). In a sub‐population of patients from the “Get with the Guidelines‐Coronary Artery Disease” (GWTG‐CAD) analysis who experienced an ST‐elevation myocardial infarction, it was found that women were less likely than men to receive aspirin and beta‐blockers in the first 24 hours of hospitalization. In this dataset, sex‐based differences in referrals for catheterization and cardiac reperfusion were also reported.
Thus, sex bias in management of cardiac events cannot be discarded. A further possibility is that women were less compliant with prescribed medications, particularly beta‐blockers (Rasmussen et al., 2007). In this Ontario, Canada, study population, women were 28% less likely to achieve high adherence to this agent, which is known to offer survival benefit.
Another factor that may account in part for differences in care is women's more limited access to primary and secondary cardiovascular counseling (Hayes et al., 2003). A comparative study enrolling 500 physicians documented that women were classified with a lower cardiovascular risk compared to men even though they had identical risk profiles (Mosca et al., 2005) and therefore received fewer recommendations for lifestyle changes and preventive medical therapy.
4.3. Limitations
The results of the current medical records study were obtained in a single tertiary‐care center and were based on routine clinical practice and protocols. The age difference between male and female subjects may limit conclusions. Some patients (n = 29, 3.3%) were excluded because their survival or death could not be documented from BIDMC or public records. Although the number of cases is relatively small, this problem is inherent in studying low‐incidence events. However, by using the nested case–control design, with matching on sex and age and using a robust analytical method for TWH analysis, we found highly a significant ROC relationship with near‐term cardiac mortality. A larger study should expand the evaluation of the prediction of cardiac mortality by ECG heterogeneity in female and male patients.
4.4. Conclusions
Elevated TWHV4‐6 is associated with near‐term cardiac mortality among women admitted to the ED and may identify women whose risk for cardiac events is increased. Female patients with TWHV4‐6 levels ≥48 µV on resting 12‐lead ECG are more likely to experience a cardiac‐related death within 90 days of ED admission than those with lower TWH levels. This marker could provide a useful tool to identify women who may benefit from earlier and more aggressive clinical interventions, filling a gap not addressed in the management of cardiovascular events among women.
5. Ethics approval
This investigation conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was carried out under a protocol approved by the Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC), Boston, MA.
6. Patient consent
As this is a medical records study, no patient consent was required.
CONFLICT OF INTEREST
None of the authors has a conflict of interest in this study.
Monteiro FR, Rabelo Evangelista AB, Nearing BD, et al. T‐wave heterogeneity in standard resting 12‐lead ECGs is associated with 90‐day cardiac mortality in women following emergency department admission: A nested case–control study. Ann Noninvasive Electrocardiol. 2021;26:e12826. 10.1111/anec.12826
DATA AVAILABILITY STATEMENT
Anonymized data not published within this article will be made available by request from any qualified investigator.
REFERENCES
- Araujo Silva, B. , Hauser, T. H. , Nearing, B. D. , Bortolotto, A. L. , Marum, A. A. , Tessarolo Silva, F. , Medeiros, S. A. , Pedreira, G. C. , Gervino, E. V. , & Verrier, R. L. (2020). Regadenoson‐induced T‐wave heterogeneity complements coronary stenosis detection by myocardial perfusion imaging in men and women. European Heart Journal ‐ Cardiovascular Imaging, 10.1093/ehjci/jeaa128 [DOI] [PubMed] [Google Scholar]
- Ayanian, J. Z. , & Epstein, A. M. (1991). Differences in the use of procedures between women and men hospitalized for coronary heart disease. New England Journal of Medicine, 325, 221–225. 10.1056/NEJM199107253250401 [DOI] [PubMed] [Google Scholar]
- Bonatti, R. , Silva, A. F. G. , Batatinha, J. A. P. , Sobrado, L. F. , Machado, A. D. , Varone, B. B. , Nearing, B. D. , Belardinelli, L. , & Verrier, R. L. (2014). Selective late sodium current blockade with GS‐458967 markedly reduces ischemia‐induced atrial and ventricular repolarization alternans and ECG heterogeneity. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 11, 1827–1835. 10.1016/j.hrthm.2014.06.017 [DOI] [PubMed] [Google Scholar]
- Bortolotto, A. L. , Verrier, R. L. , Nearing, B. D. , Marum, A. A. , Araujo Silva, B. , Pedreira, G. C. , Tessarolo Silva, F. , Medeiros, S. A. , Sroubek, J. , Zimetbaum, P. J. , & Chang, J. D. (2020). Pre‐implantation interlead ECG heterogeneity is superior to QRS complex duration in predicting mechanical super‐response in patients with non‐left bundle branch block receiving cardiac resynchronization therapy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 17, 1887–1896. 10.1016/j.hrthm.2020.05.036 [DOI] [PubMed] [Google Scholar]
- Collinson, P. O. , Boa, F. G. , & Gaze, D. C. (2001). Measurement of cardiac troponin. Annals of Clinical Biochemistry, 38, 423–449. 10.1177/000456320103800501 [DOI] [PubMed] [Google Scholar]
- Davis, E. , Gorog, D. A. , Rihal, C. , Prasad, A. , & Srinivasan, M. (2017). "Mind the gap" acute coronary syndrome in women: A contemporary review of current clinical evidence. International Journal of Cardiology, 227, 840–849. 10.1016/j.ijcard.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Haukilahti, M. A. E. , Holmström, L. , Vähätalo, J. , Kenttä, T. , Tikkanen, J. , Pakanen, L. , Kortelainen, M.‐L. , Perkiömäki, J. , Huikuri, H. , Myerburg, R. J. , & Junttila, M. J. (2019). Sudden cardiac death in women. Circulation, 139(8), 1012–1021. 10.1161/CIRCULATIONAHA.118.037702 [DOI] [PubMed] [Google Scholar]
- Hayes, S. N. , Weisman, C. S. , & Clark, A. (2003). The Jacobs Institute of Women's Health report on the prevention of heart disease in women: Findings and recommendations from the "Women and Heart Disease: Putting Prevention into Primary Care" conference. Womens Health Issues, 13, 115–121. 10.1016/s1049-3867(03)00055-0 [DOI] [PubMed] [Google Scholar]
- Huikuri, H. V. , Tapanainen, J. M. , Lindgren, K. , Paatikainen, P. , Makikallio, T. H. , Airaksinen, K. E. J. , & Myerburg, R. J. (2003). Prediction of sudden cardiac death after myocardial infarction in the beta‐blocking era. Journal of the American College of Cardiology, 42, 652–658. 10.1016/s0735-1097(03)00783-6 [DOI] [PubMed] [Google Scholar]
- Jneid, H. , Fonarow, G. C. , Cannon, C. P. , Hernandez, A. F. , Palacios, I. F. , Maree, A. O. , Wells, Q. , Bozkurt, B. , LaBresh, K. A. , Liang, L. I. , Hong, Y. , Newby, L. K. , Fletcher, G. , Peterson, E. , & Wexler, L. (2008). Get With the Guidelines Steering Committee and Investigators. Sex differences in medical care and early death after acute myocardial infarction. Circulation, 16(118), 2803–2810. 10.1161/CIRCULATIONAHA.108.789800 [DOI] [PubMed] [Google Scholar]
- Kania, M. , Rix, H. , Fereniec, M. , Zavala‐Fernandez, H. , Janusek, D. , Mroczka, T. , Stix, G. , & Maniewski, R. (2014) The effect of precordial lead displacement on EKG morphology. Medical & Biological Engineering & Computing, 52, 109–119. 10.1007/s11517-013-1115-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenttä, T. V. , Nearing, B. D. , Porthan, K. , Tikkanen, J. T. , Viitasalo, M. , Nieminen, M. S. , Salomaa, V. , Oikarinen, L. , Huikuri, H. V. , & Verrier, R. L. (2016). Prediction of sudden cardiac death with automated high throughput analysis of heterogeneity in standard resting 12‐lead electrocardiogram. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 13, 713–720. 10.1016/j.hrthm.2015.11.035 [DOI] [PubMed] [Google Scholar]
- Mosca, L. , Linfante, A. H. , Benjamin, E. J. , Berra, K. , Hayes, S. N. , Walsh, B. W. , Fabunmi, R. P. , Kwan, J. , Mills, T. , & Simpson, S. L. (2005). National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation, 111, 499–510. 10.1161/01.CIR.0000154568.43333.82 [DOI] [PubMed] [Google Scholar]
- Nearing, B. D. , & Verrier, R. L. (2003). Tracking heightened cardiac electrical instability by computing interlead heterogeneity of T‐wave morphology. Journal of Applied Physiology, 95, 2265–2272. 10.1152/japplphysiol.00623.2003 [DOI] [PubMed] [Google Scholar]
- Rasmussen, J. N. , Chong, A. , & Alter, D. A. (2007). Relationship between adherence to evidence‐based pharmacotherapy and long‐term mortality after acute myocardial infarction. JAMA, 297, 177–186. 10.1001/jama.297.2.177 [DOI] [PubMed] [Google Scholar]
- Silva, A. C. , de Antonio, V. Z. , Sroubek, J. , Gervino, E. , Ho, K. , Medeiros, S. A. , Silva, F. T. , Pedreira, G. C. , Stocco, F. G. , Nearing, B. D. , & Verrier, R. L. (2020). Exercise and pharmacologic stress‐induced interlead T‐wave heterogeneity analysis to detect clinically significant coronary artery stenosis. International Journal of Cardiology, 298, 32–38. 10.1016/j.ijcard.2019.07.066 [DOI] [PubMed] [Google Scholar]
- Solomon, S. D. , Zelenkofske, S. , McMurray, J. J. V. , Finn, P. V. , Velazquez, E. , Ertl, G. , Harsanyi, A. , Rouleau, J. L. , Maggioni, A. , Kober, L. , White, H. , Van de Werf, F. , Pieper, K. , Califf, R. M. , & Pfeffer, M. A. ; for the Valsartan in Acute Myocardial Infarction Trial (VALIANT) investigators. (2005). Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. New England Journal of Medicine, 352, 2581–2588. 10.1056/NEJMoa043938 [DOI] [PubMed] [Google Scholar]
- Steingart, R. M. , Packer, M. , Hamm, P. , Coglianese, M. E. , Gersh, B. , Geltman, E. M. , Sollano, J. , Katz, S. , Moyé, L. , Basta, L. L. , Lewis, S. J. , Gottlieb, S. S. , Bernstein, V. , McEwan, P. , Jacobson, K. , Brown, E. J. , Kukin, M. L. , Kantrowitz, N. E. , & Pfeffer, M. A. (1991). Sex differences in the management of coronary artery disease. New England Journal of Medicine, 325, 226–230. 10.1056/NEJM199107253250402 [DOI] [PubMed] [Google Scholar]
- Stoletniy, L. N. , & Pai, R. G. (1997). Value of QT dispersion in the interpretation of exercise stress test in women. Circulation, 96, 904–910. 10.1161/01.cir.96.3.904 [DOI] [PubMed] [Google Scholar]
- Straus, S. M. J. M. , Kors, J. A. , De Bruin, M. L. , van der Hooft, C. S. , Hofman, A. , Heeringa, J. , Deckers, J. W. , Kingma, J. H. , Sturkenboom, M. C. J. M. , Stricker, B. H. C. , & Witteman, J. C. M. (2006). Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. Journal of the American College of Cardiology, 47, 362–367. 10.1016/j.jacc.2005.08.067 [DOI] [PubMed] [Google Scholar]
- Tan, A. Y. , Nearing, B. D. , Rosenberg, M. , Nezafat, R. , Josephson, M. E. , & Verrier, R. L. (2017). Interlead heterogeneity of R‐ and T‐wave morphology in standard 12‐lead ECGs predicts sustained ventricular tachycardia/fibrillation and arrhythmic death in patients with cardiomyopathy. Journal of Cardiovascular Electrophysiology, 28, 1324–1333. 10.1111/jce.13288 [DOI] [PubMed] [Google Scholar]
- Thygesen, K. , Alpert, J. S. , & Jaffe, A. S. (2018). Fourth universal definition of myocardial infarction. Circulation, 138, e618–e651. 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- Tobin, J. N. , Wassertheil‐Smoller, S. , Wexler, J. P. , Steingart, R. M. , Budner, N. , Lense, L. , & Wachspress, J. (1987). Sex bias in considering coronary bypass surgery. Annals of Internal Medicine, 107, 19–25. 10.7326/0003-4819-107-1-19 [DOI] [PubMed] [Google Scholar]
- Verrier, R. L. , & Huikuri, H. V. (2017). Tracking interlead heterogeneity of R‐ and T‐wave morphology to disclose latent risk for sudden cardiac death. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 14, 1466–1475. 10.1016/j.hrthm.2017.06.017 [DOI] [PubMed] [Google Scholar]
- Veseli, G. , Jacobson, J. T. , Iwai, S. , & Kadish, A. H. (2020). Sex‐specific definitions of electrocardiographic abnormalities. In Malik M. (Ed.), Sex and cardiac electrophysiology (pp. 153–162). Elsevier. [Google Scholar]
- Virani, S. S. , Alonso, A. , Benjamin, E. J. , Bittencourt, M. S. , Callaway, C. W. , Carson, A. P. , Chamberlain, A. M. , Chang, A. R. , Cheng, S. , Delling, F. N. , Djousse, L. , Elkind, M. S. V. , Ferguson, J. F. , Fornage, M. , Khan, S. S. , Kissela, B. M. , Knutson, K. L. , Kwan, T. W. , Lackland, D. T. , … Tsao, C. W. (2020). Update: A report from the American Heart Association. Circulation, 2020(141), e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Zegre‐Hemsey, J. K. (2020). Electrocardiographic manifestation of suspected acute coronary syndrome. In Malik M. (Ed.), Sex and Cardiac Electrophysiology (pp. 551–559). Elsevier. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.


