Key Points
Question
Are there differences in seroprevalence and antibody levels for SARS-CoV-2 between patients with cancer and health care workers (HCWs) during the COVID-19 pandemic in Japan?
Findings
In this cross-sectional study including 500 patients with cancer and 1190 HCWs, the seroprevalence was 1.0% in patients and 0.67% in HCWs. However, the levels of IgG antibodies against nucleocapsid and spike protein were significantly lower in patients than in HCWs.
Meaning
These findings indicate that seroprevalence was not different in patients with cancer compared with HCWs, but the immune response to SARS-CoV-2 may differ between patients with cancer and HCWs.
This cross-sectional study evaluates whether there are differences in SARS-CoV-2 seroprevalence and antibody levels in patients with cancer compared with health care workers in Japan.
Abstract
Importance
Patients with cancer and health care workers (HCWs) are at high risk of SARS-CoV-2 infection. Assessing the antibody status of patients with cancer and HCWs can help understand the spread of COVID-19 in cancer care.
Objective
To evaluate serum SARS-CoV-2 antibody status in patients with cancer and HCWs during the COVID-19 pandemic in Japan.
Design, Setting, and Participants
Participants were enrolled for this prospective cross-sectional study between August 3 and October 30, 2020, from 2 comprehensive cancer centers in the epidemic area around Tokyo, Japan. Patients with cancer aged 16 years or older and employees were enrolled. Participants with suspected COVID-19 infection at the time of enrollment were excluded.
Exposures
Cancer of any type and cancer treatment, including chemotherapy, surgery, immune checkpoint inhibitors, radiotherapy, and targeted molecular therapy.
Main Outcomes and Measures
Seroprevalence and antibody levels in patients with cancer and HCWs. Seropositivity was defined as positivity to nucleocapsid IgG (N-IgG) and/or spike IgG (S-IgG). Serum levels of SARS-CoV-2 IgM and IgG antibodies against the nucleocapsid and spike proteins were measured by chemiluminescent enzyme immunoassay.
Results
A total of 500 patients with cancer (median age, 62.5 years [range, 21-88 years]; 265 men [55.4%]) and 1190 HCWs (median age, 40 years [range, 20-70 years]; 382 men [25.4%]) were enrolled. In patients with cancer, 489 (97.8%) had solid tumors, and 355 (71.0%) had received anticancer treatment within 1 month. Among HCWs, 385 (32.3%) were nurses or assistant nurses, 266 (22.4%) were administrative officers, 197 (16.6%) were researchers, 179 (15.0%) were physicians, 113 (9.5%) were technicians, and 50 (4.2%) were pharmacists. The seroprevalence was 1.0% (95% CI, 0.33%-2.32%) in patients and 0.67% (95% CI, 0.29%-1.32%) in HCWs (P = .48). However, the N-IgG and S-IgG antibody levels were significantly lower in patients than in HCWs (N-IgG: β, −0.38; 95% CI, −0.55 to −0.21; P < .001; and S-IgG: β, −0.39; 95% CI, −0.54 to −0.23; P < .001). Additionally, among patients, N-IgG levels were significantly lower in those who received chemotherapy than in those who did not (median N-IgG levels, 0.1 [interquartile range (IQR), 0-0.3] vs 0.1 [IQR, 0-0.4], P = .04). In contrast, N-IgG and S-IgG levels were significantly higher in patients who received immune checkpoint inhibitors than in those who did not (median N-IgG levels: 0.2 [IQR, 0.1-0.5] vs 0.1 [IQR, 0-0.3], P = .02; S-IgG levels: 0.15 [IQR, 0-0.3] vs 0.1[IQR, 0-0.2], P = .02).
Conclusions and Relevance
In this cross-sectional study of Japanese patients with cancer and HCWs, the seroprevalence of SARS-CoV-2 antibodies did not differ between the 2 groups; however, findings suggest that comorbid cancer and treatment with systemic therapy, including chemotherapy and immune checkpoint inhibitors, may influence the immune response to SARS-CoV-2.
Introduction
An outbreak of pneumonia of unknown etiology was reported in Wuhan City, Hubei Province, China, in December 2019. It was identified as pneumonia caused by novel coronavirus SARS-CoV-2, and the disease was named COVID-19 by the World Health Organization. COVID-19 has spread globally, and the number of new cases reported is continuously rising. As of February 1, 2021, there have been more than 102 million confirmed cases with 2.22 million deaths worldwide and 389 000 cases with 5722 deaths in Japan.1
Several diagnostic methods have been developed to date aiming to manage and control the infection. The standard diagnostic test for current SARS-CoV-2 infection is a performed by reverse-transcription polymerase chain reaction (RT-PCR) to detect SARS-CoV-2 RNA from the upper respiratory tract.2 However, PCR testing carries the risk of exposure to health care workers (HCWs) at the time of specimen collection and of false-negative results owing specimen type and quality. Meanwhile, a serological test to detect antibodies to SARS-CoV-2 is better suited to measure the extent of the disease by detecting previously infected individuals, including those who were not diagnosed by RT-PCR.3 The serological test also identifies individuals who may have acquired immunity to infection.
Patients with cancer are more susceptible to infections owing to tumor cachexia, malnutrition, and immunosuppression from the cancer itself and anticancer treatment. Although the incidence of COVID-19 among patients with cancer varies across reports,4,5,6,7,8,9 it is higher than in the general population.10 Additionally, patients with cancer diagnosed with COVID-19 have a higher risk of severe illness and death compared with the overall population.11,12,13
Similarly, HCWs work in contact with patients with suspected or confirmed COVID-19 and may be at high risk of COVID-19 infection.14 However, several reports have shown that the seroprevalence of SARS-CoV-2 antibodies in HCWs was similar to that in general populations.15,16 There have been no studies evaluating the seroprevalence of SARS-CoV-2 in asymptomatic patients with cancer and HCWs to evaluate the extent of COVID-19 spread in the community. We aimed to assess the seroprevalence of SARS-CoV-2 infection and antibody levels in patients with cancer and HCWs, considered as controls, to help understand the spread and risk of COVID-19 in patients with cancer, which has significant implications for cancer care.
Methods
Study Design
This prospective cross-sectional study was conducted at the National Cancer Center (NCC), Japan, in collaboration with Sysmex Co. The NCC has 2 comprehensive cancer centers, the 538-bed NCC Hospital located in Tsukiji, Tokyo, and the 425-bed NCC Hospital East, located in Kashiwa, Chiba. The Tokyo and Chiba prefectures have respectively reported the highest and the sixth-highest cumulative numbers of SARS-CoV-2 infections in Japan. The study included 2 cohorts: (1) patients with cancer at the NCC Hospital and (2) HCWs at the NCC Hospital and NCC Hospital East. Participants with suspected current SARS-CoV-2 infection, such as those with fever and/or respiratory symptoms at the time of enrollment, were excluded.
Institutional review board and ethics committee approval was obtained from our institutions. The study was conducted in accordance with the Declaration of Helsinki.17 All participants provided written informed consent before the study-related procedures. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patient Cohort
All inpatients and outpatients with cancer aged 16 years or older were considered eligible. The registration period was from August 3 to 31, 2020. We collected clinical and treatment data of all patients from medical records.
Health Care Worker Cohort
All HCWs at the NCC Hospital and NCC Hospital East were invited by posters to participate in this study. Participants without suspected symptoms of COVID-19 were eligible. The registration period was from September 1 to October 30, 2020. Health care workers who consented to the study were given a self-administered questionnaire. The information collected in the questionnaire is described in eMethods 2 in the Supplement. Only the NCC Hospital had secured an inpatient ward for patients with COVID-19 but without cancer from April 15, 2020.
SARS-CoV-2 Antibody Test
SARS-CoV-2 IgM and IgG antibodies against the nucleocapsid (N) and spike (S) proteins were measured using a highly quantitative and reproducible assay, as previously reported.18 This assay uses a fully automated immunochemistry analyzer based on the chemiluminescence enzyme immunoassay methodology, namely the high-sensitivity chemiluminescence enzyme immunoassay platform (Sysmex Co.) (details in eMethods 1 in the Supplement). The sensitivity and the specificity for N-IgG were 100% and 99.8%, respectively, and those for S-IgG were 98.3% and 99.6% (eFigure 1 in the Supplement). Details of cutoff value setting are provided in eMethods 1 in the Supplement. The seropositivity in this study was defined as a positive result for either the N-IgG or the S-IgG antibody test. All tests were performed at Tsukiji Laboratory, Riken Genesis Co Ltd.
Statistical Analysis
The primary end point was SARS-CoV-2 antibody level and seroprevalence in patients with cancer and HCWs. The SARS-CoV-2 antibody levels and seroprevalence were evaluated in the overall population (intention-to-treat [ITT] population) and in the population without a history of COVID-19 (analysis population). The association between clinical factors and SARS-CoV-2 antibody status was examined. Continuous variables were reported as median (range and interquartile range [IQR]) and compared using the Mann-Whitney U test. Categorical variables were reported as numbers and percentages and compared by the χ2 test. Multivariable regression analysis was performed to analyze the association between SARS-CoV-2 antibody levels and variables. The 2-sided 95% CI of the seroprevalence was calculated. All tests were 2-tailed, and the significance level was set at α = .05. Statistical analyses were performed using StatFlex version 7.0 software (Artech Co. Ltd), STATA version 15.1 (StataCorp), and GraphPad Prism version 8.0 (GraphPad Software).
Results
Baseline Characteristics of the Study Participants
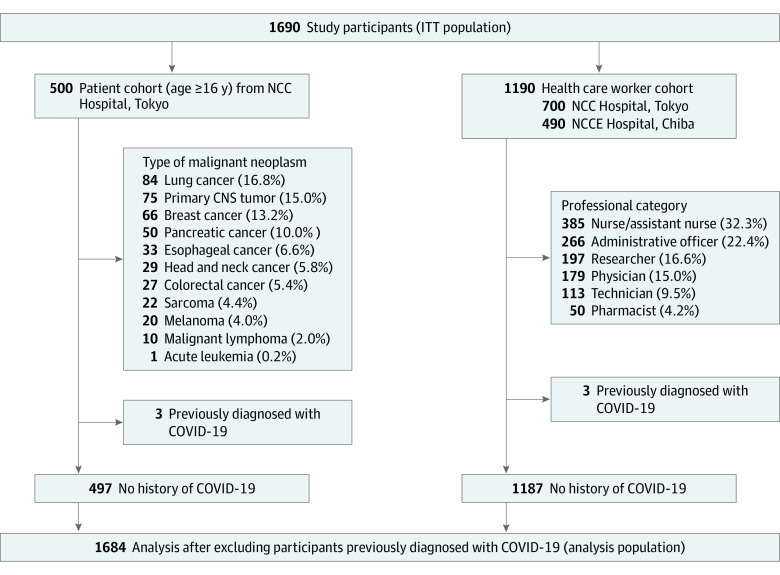
A total of 1690 participants were enrolled in this study: 500 patients with cancer (median age, 62.5 years [range, 21-88 years]; 265 men [55.4%] and 235 women [44.6%]) and 1190 HCWs (median age, 40 years [range, 20-70 years]; 382 men [25.4%] and 888 women [74.6%]). Most patients with cancer had solid tumors (489 of 500 [97.8%]), whereas 11 (2.2%) had hematological malignancies. The most common cancer type was lung cancer (84 of 500 [16.8%]), followed by primary central nervous system cancer (75 of 500 [15.0%]) and breast cancer (66 of 500 [13.2%]). Among HCWs, approximately one-third (385 of 1190 [32.3%]) were nurses or assistant nurses, 22.4% (266) were administrative officers, 16.6% (197) were researchers, 15.0% (179) were physicians, 9.5% (113) were technicians, and 4.2% (50) were pharmacists (Figure 1).
Figure 1. Study Flow Diagram.
ITT indicates intention-to-treat; NCC, National Cancer Center; NCCE, National Cancer Center East; CNS, central nervous system.
The baseline characteristics of the study participants are shown in Table 1. Patients with cancer, compared with HCWs, were more frequently male (265 [53.0%] vs 382 [25.4%]), older (≥65 years, 223 [44.6%] vs 13 [1.1%]), and past or current smokers (265 [53.0%] vs 166 [14.0%]) compared with HCWs. There were also more participants with hypertension (130 [26.0%] vs 65 [3.8%]), diabetes (68 [13.6%] vs 8 [0.7%]), and chronic kidney disease among patients with cancer. Three participants in each cohort (patients, 0.6%; HCWs, 0.25%) had a history of SARS-CoV-2 infection diagnosed by RT-PCR test. In the patient cohort, 287 (57.4%) had advanced disease. Three hundred fifty-five (71.0%) patients had received any anticancer treatment within 1 month of study enrollment, including 35 (7.0%) having received surgery, 24 (4.8%) radiotherapy, 204 (40.8%) cytotoxic chemotherapy, 44 (8.8%) immune checkpoint inhibitors (ICIs), and 91 (18.4%) molecular targeted therapy (Table 2).
Table 1. Baseline Characteristics of 1690 Patients With Cancer and Health Care Workers (HCWs).
| Characteristic | No. (%) | |
|---|---|---|
| Patients (n = 500) | HCWs (n = 1190) | |
| Age, median (range), y | 62.5 (21.0-88.0) | 40.0 (20.0-70.0) |
| <65 | 277 (55.4) | 1177 (98.9) |
| ≥65 | 223 (44.6) | 13 (1.1) |
| Sex | ||
| Male | 265 (53.0) | 382 (25.4) |
| Female | 235 (47.0) | 888 (74.6) |
| Comorbidities | ||
| Hypertension | 130 (26.0) | 65 (3.8) |
| Diabetes | 68 (13.6) | 8 (0.7) |
| COPD | 1 (0.2) | 0 |
| Coronary artery disease | 1 (0.2) | 0 |
| Chronic kidney disease | 9 (1.8) | 0 |
| Malignancy | NA | 8 (1.1) |
| Smoking | ||
| Current | 43 (8.6) | 33 (2.8) |
| Past | 222 (44.4) | 133 (11.2) |
| Never | 222 (44.4) | 1024 (86.1) |
| Unknown | 13 (2.6) | 0 |
| Residence | ||
| Tokyo | 264 (52.8) | 598 (50.3) |
| Non-Tokyo | 236 (47.2) | 592 (49.7) |
| Stage | ||
| Early | 213 (42.6) | NA |
| Advanced | 287 (57.4) | NA |
Abbreviations: COPD, chronic obstructive pulmonary disease; NA, not applicable.
Table 2. Details of Cancer Treatment in 500 Patients With Cancer.
| Details of cancer treatment | No. (%) |
|---|---|
| Cancer treatment within 1 mo of participation | 355 (71.0) |
| Type of treatment | |
| Surgery | 35 (7.0) |
| Radiotherapy | 24 (4.8) |
| Cytotoxic chemotherapy | 204 (40.8) |
| Immune checkpoint inhibitors | 44 (8.8) |
| Molecular targeted therapy | 92 (18.4) |
Serum SARS-CoV-2 Antibody Status in Patients With Cancer and HCWs
Regarding the seroprevalence of SARS-CoV-2 antibodies, 13 of 1690 participants (0.77%; 95% CI. 0.41%-1.31%) were seropositive for either N-IgG or S-IgG, including 7 participants who tested positive for both. Five of the 500 patients with cancer (1.0%; 95% CI, 0.33%-2.32%) were seropositive. Eight of the 1190 HCWs were seropositive (0.67%; 95% CI, 0.29%-1.32%). The difference in the seroprevalence of SARS-CoV-2 antibodies between patients with cancer and HCWs was not significant (P = .48) in the ITT population. In the analysis population, after excluding the 6 participants with a previous diagnosis, there was also no difference in the seroprevalence of SARS-CoV-2 antibodies between patients with cancer and HCWs (0.4% [2 of 497] vs 0.42% [5 of 1187]; P = .96). The seroprevalence was higher in participants who had had COVID-19–related symptoms among both patients with cancer and HCWs (COVID-19–related symptoms vs no symptom; 7.14% [1 of 14] vs 0.21% [1 of 483] in patients with cancer [P < .001]; 2.94% [2 of 68] vs 0.27% [3 of 1119] in HCWs [P < .001]).
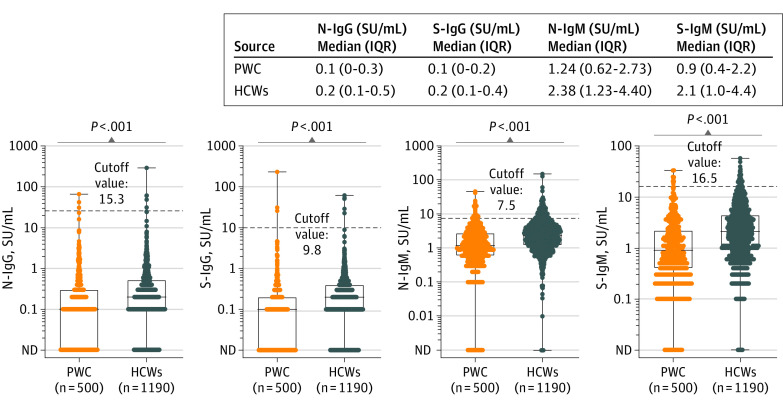
Furthermore, serum SARS-CoV-2 antibody levels were significantly lower in patients with cancer than in HCWs in the ITT population. The median N-IgG and S-IgG levels were 0.1 SU/mL (IQR, 0-0.3 SU/mL) and 0.1 SU/mL (IQR, 0.1-0.2 SU/mL), respectively, in patients with cancer vs 0.2 SU/mL (IQR, 0.1-0.5 SU/mL) and 0.2 SU/mL (IQR, 0.1-0.4 SU/mL), respectively, in HCWs (both P < .001). The median N-IgM and S-IgM levels were 1.24 SU/mL (IQR, 0.62-2.73 SU/mL) and 0.9 SU/mL (IQR, 0.4-2.2 SU/mL), respectively, in patients with cancer vs 2.38 SU/mL (IQR, 1.23-4.4 SU/mL) and 2.1 SU/mL (IQR, 1.0-3.3 SU/mL), respectively, in HCWs (both P < .001) (Figure 2). Antibody levels were also significantly lower in the analysis population. The median N-IgG and S-IgG levels were 0.1 SU/mL (IQR, 0-0.3 SU/mL) and 0.1 SU/mL (IQR, 0-0.2 SU/mL), respectively, in patients with cancer vs 0.2 SU/mL (IQR, 0.1-0.5 SU/mL) and 0.2 SU/mL (0.1-0.4 U/mL), respectively, in HCWs (both P < .001). The median N-IgM and S-IgM levels were 1.24 SU/mL (IQR, 0.62-2.68 SU/mL) and 0.9 SU/mL (IQR, 0.4-2.2 SU/mL), respectively, in patients with cancer vs 2.37 SU/mL (IQR, 1.23-4.4 SU/mL) and 2.1 SU/mL (IQR, 1.0-4.4 SU/mL) respectively, in HCWs (both P < .001) (eFigure 2 in the Supplement). In the multivariable regression analysis, including age, sex, comorbidities, and smoking history as covariates, there was also a significant association between patients with cancer (compared with HCWs) and N-IgG and S-IgG levels (N-IgG: β, −0.38; 95% CI, −0.55 to −0.21; P < .001; S-IgG: β, −0.39; 95% CI, −0.54 to −0.23; P < .001) (eTable 1 in the Supplement).
Figure 2. SARS-CoV-2 Antibody Levels in Patients With Cancer (PWC) and Health Care Workers (HCWs).
Nucleocapsid IgG (N-IgG), spike IgG (S-IgG), nucleocapsid IgM (N-IgM), and spike IgM (S-IgM) antibody levels were compared between PWC and HCWs in the intention-to-treat population. The dots depict antibody levels. The boxes represents the first quartile, median, and third quartile; whiskers represent minimum and maximum values. ND indicates not detected.
Association Between Clinical Factors and SARS-CoV-2 Antibody Status in Patients With Cancer
eFigure 3 in the Supplement shows the seroprevalence of SARS-CoV-2 antibodies according to selected subgroups in the ITT population. There were no significant differences in the seroprevalence of SARS-CoV-2 antibodies between subgroups. Of note, 5 seropositive patients had received systemic treatment of any kind, such as ICIs, chemotherapy, or molecular targeted therapy, within 1 month.
eTable 2 in the Supplement shows the characteristics of seropositive patients with cancer. In 2 patients without a previous diagnosis of COVID-19, only the N-IgG antibody was positive. In 3 patients with a history of COVID-19, high N-IgG and S-IgG antibody levels persisted even though more than 120 days had passed since the diagnosis.
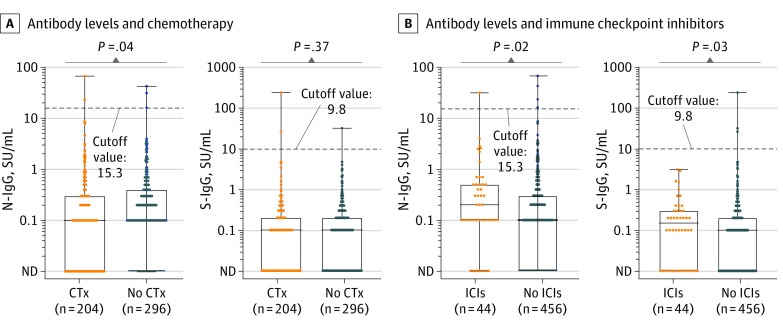
We evaluated whether having received cancer treatment within 1 month and the treatment method received was associated with SARS-CoV-2 antibodies (N-IgG and S-IgG). There were no significant differences in SARS-CoV-2 antibody levels between patients who had received or not received anticancer treatment (eFigure 4 in the Supplement). However, in patients who had received cytotoxic chemotherapy within 1 month, N-IgG antibody levels were significantly lower than in those who did not receive it (median N-IgG levels, 0.1 SU/mL [IQR, 0-0.3 SU/mL] vs 0.1 SU/mL [IQR, 0-0.4 SU/mL]; P = .04) (Figure 3A). In contrast, in patients who had received ICIs within 1 month, both N-IgG and S-IgG antibody levels were significantly higher than in those who did not receive ICIs (median N-IgG levels: 0.2 SU/mL [IQR, 0.1-0.5 SU/mL] vs 0.1 SU/mL [IQR, 0-0.3 SU/mL], P = .02; median S-IgG levels: 0.15 SU/mL [IQR, 0-0.3 SU/mL] vs 0.1 SU/mL [IQR, 0-0.2 SU/mL], P = .03) (Figure 3B). In the multivariable regression analysis including age, sex, comorbidities, and smoking history as covariates, chemotherapy was not significantly associated with N-IgG and S-IgG antibody levels (N-IgG: β, −0.14; 95% CI, −0.37 to 0.09; P = .24; S-IgG: β, −0.00043; 95% CI, −0.21 to 0.31; P = .99) (eTable 3 in the Supplement). However, ICIs were significantly associated with N-IgG and S-IgG antibody levels (N-IgG: β, 0.56; 95% CI, 0.15-0.97; P = .008, S-IgG: β, 0.42, 95% CI, 0.052-0.79; P = .03) (eTable 4 in the Supplement). There was no difference in antibody levels according to sex, age, smoking status, or cancer stage or according to surgery, radiotherapy, or molecular targeted therapy received within 1 month (eFigure 5 in the Supplement).
Figure 3. Association Between Cancer Treatment and SARS-CoV-2 Antibody Levels in Patients With Cancer.
A, Antibody levels in patients having received or not received chemotherapy; B, antibody levels in patients having received or not received immune checkpoint inhibitors. The dots depict the antibody levels. The boxes represents the first quartile, median, and third quartile; the whiskers represent minimum and maximum values. ICI indicates immune checkpoint inhibitor; CTx, chemotherapy; ND, not detected; N-IgG, nucleocapsid IgG; and S-IgG, spike IgG.
Discussion
This is, to our knowledge, the largest prospective study evaluating the seroprevalence of SARS-CoV-2 antibodies among patients with cancer. No significant difference in seroprevalence was observed between patients with cancer and HCWs (1.0% vs 0.67%) in the ITT population, nor after excluding individuals previously diagnosed with COVID-19 (0.4% vs 0.4%).
Patients with cancer have been reported to have a higher risk of COVID-19 infection, according to a case-control study from the United States.10 The mortality rates of COVID-19 infection in patients with cancer, especially those with hematological cancers, were also reported to be higher than those in an unselected population. A recent meta-analysis showed that in patients with cancer, active cancer treatment, including chemotherapy, was associated with mortality for COVID-19, but immunotherapy, surgery, and molecular targeted therapy were not.19 The reasons why comorbid cancer and cancer treatment increase infection risk and mortality are not fully understood.
Serological testing to detect anti–SARS-CoV-2-specific antibodies is an important approach to understand the extent of COVID-19 spread in the community, although the standard diagnostic test for COVID-19 is an RT-PCR test to detect SARS-CoV-2 RNA from the upper respiratory tract. There have been no studies evaluating the seroprevalence of SARS-CoV-2 in patients with cancer without symptomatic COVID-19 infections. We evaluated the seroprevalence of SARS-CoV-2, defined as the presence of IgG antibodies, and compared it between patients with cancer and HCWs. The detection rate of SARS-CoV-2 was quite low, and there was no significant difference in seroprevalence between the 2 groups. However, IgG and IgM antibody levels in patients with cancer were significantly lower than those in HCWs, although the levels were under the respective seropositivity cutoffs. Solodky et al20 reported the detection of SARS-CoV-2 antibodies after symptomatic COVID-19, and the seroconversion rate 15 days after COVID-19 diagnosis was lower in patients with cancer than in HCWs. These results might suggest that the immune response to the virus in patients with cancer differs from that of healthy individuals. Indeed, the prospective observational study showed an inverse correlation between antibody levels and subsequent SARS-CoV-2 infection, even under the positive cutoff level.21 Low IgG antibody levels in patients with cancer could be associated with their higher risk of SARS-CoV-2 infection compared with that of the general population. Additional studies will be needed to confirm whether there is a difference in the immune response to the virus between people with and without cancer.
We also evaluated whether cancer treatment affected SARS-CoV-2 antibody (N-IgG and S-IgG) levels. Of note, the N-IgG antibody levels were lower in patients who had received chemotherapy within 1 month than in those without chemotherapy, although this difference was not significant in the multivariate analysis. Both N-IgG and S-IgG antibody levels were higher in patients having received ICIs than in those without ICIs. It remains unclear how these data affect the risk and mortality of COVID-19. Some reports have investigated the immune response in patients undergoing cytotoxic chemotherapy and ICIs. Verma et al22 reported that in 88 patients with breast cancer who received adjuvant chemotherapy, titers of antipneumococcal and antitetanus antibodies were both significantly reduced after chemotherapy and did not recover during the study period of 9 months. In addition, the VACANCE trial, a prospective open-label study that evaluated the immunogenicity of AS03A-adjuvanted H1N1v vaccine in patients with cancer receiving cytotoxic and/or molecular targeted therapy, showed that the seroconversion rate by seasonal influenza vaccine in patients with cancer undergoing chemotherapy was lower than that in those treated with molecular targeted therapy.23,24 The study suggested that chemotherapy, which induces immunosuppression, but not molecular targeted therapy, reduced the immune response to the vaccine.
Regarding the effect of ICIs on the immune response, Läubli et al25 reported no significant differences in influenza vaccine-induced antibody titers between patients with lung cancer receiving ICIs and healthy controls. However, the seroconversion rate at day 30 was significantly higher in patients receiving ICIs. PD-1/PD-L1 inhibition enhanced T-cell responses to various viral infections in a mouse model,26 suggesting that patients with cancer receiving ICIs may have enhanced immune responses to viral antigens.
The mRNA-1273 SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccines have recently been developed for protection against COVID-19.27,28 Whether vaccination reduces the incidence and mortality of COVID-19 in patients with cancer, or whether cancer treatment affects the efficacy of the vaccines, remains to be seen. Further investigation on how vaccination affects immune response in patients with cancer is warranted.
Limitations
Our study has several limitations. First, there could be a sampling bias in the patient population, because this study included various cancer types, ages, and stages from only 1 hospital. Second, differences in baseline characteristics, such as age, sex, comorbidities, and smoking status, which are associated with the severity of COVID-19 infection, were observed between patients with cancer and HCWs. However, multiple regression analysis revealed that patients with cancer were independently associated with lower IgG antibodies than were HCWs. There were some differences in the potential risk of COVID-19 infection between the 2 groups. Patient care may influence antibody levels in HCWs. In contrast, patients with cancer, especially those treated with chemotherapy, may be more careful of COVID-19 infection and more likely stay at home compared with those who were not treated with chemotherapy. Our results do not fully demonstrate that cancer treatment is associated with antibody production capacity. To evaluate whether cancer treatment is associated with immune response to SARS-CoV-2, we are currently conducting a prospective study to examine the immune response to SARS-CoV-2 vaccination in patients who received various treatments for cancer. Third, our results may not be generalizable, because the seroprevalence in our cohort was much lower than that in previous studies conducted outside Japan in 2020. The seroprevalence in patients with cancer reported from Spain (n = 229) and Austria (n = 84) ranged from 3.6% to 27.9%,29,30 and that in HCWs ranged from 3.2% to 19.1% in Western countries.15,16,29,31,32 This difference is explained by the fact that the seroprevalence rate in Japan among the general population as of June 2020 was 0.1% (positive on both Roche Elecsys and Abbott Architect assays; the prevalence of positivity for either test was 0.4%), which is also lower than that reported in Western countries (1.0%-6.9%).33,34 Nevertheless, our study revealed the differences in baseline SARS-CoV-2 antibody levels in patients with cancer and HCWs without COVID-19 infection and how the cancer treatment affects the baseline SARS-CoV-2 antibody levels.
Conclusions
In this cross-sectional study of Japanese patients with cancer and HCWs, the seroprevalence of SARS-CoV-2 antibodies between the 2 groups was not different, but results suggest that comorbid cancer and treatment with systemic therapy, including chemotherapy and ICIs, may be associated with serum SARS-CoV-2 antibody levels. Further studies are needed to determine the influence of comorbid cancer and cancer treatment on the immune response to SARS-CoV-2.
eMethods 1. SARS-CoV-2 antibody test
eMethods 2. Information collected by the questionnaire
eTable 1. Multivariable regression analysis of the association between patients with cancer and SARS-CoV-2 antibody levels
eTable 2. Characteristics of seropositive patients with cancer
eTable 3. Multivariable regression analysis of the association between chemotherapy and SARS-CoV-2 antibody levels in patients with cancer
eTable 4. Multivariable regression analysis of the association between immune checkpoint inhibitors and SARS-CoV-2 antibody levels in patients with cancer
eFigure 1. ROC curves and cutoff values of SARS-CoV-2 antibodies
eFigure 2. SARS-CoV-2 antibody levels in patients with cancer and HCWs in the analysis population
eFigure 3. Association between clinical factors and SARS-CoV-2 seropositivity rates in patients with cancer
eFigure 4. SARS-CoV-2 antibody levels in patients who did and did not receive anti-cancer treatment
eFigure 5. Association between clinical factors and SARS-CoV-2 antibody levels in patients with cancer
References
- 1.WHO . Coronavirus Disease (COVID-19) Dashboard 2021. Accessed February 1, 2021. https://covid19.who.int
- 2.Patel A, Jernigan DB; 2019-nCoV CDC Response Team . Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(5):140-146. doi: 10.15585/mmwr.mm6905e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks JJ, Dinnes J, Takwoingi Y, et al. ; Cochrane COVID-19 Diagnostic Test Accuracy Group . Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6(6):CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337. doi: 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berghoff AS, Gansterer M, Bathke AC, et al. SARS-CoV-2 testing in patients with cancer treated at a tertiary care hospital during the COVID-19 pandemic. J Clin Oncol. 2020;38(30):3547-3554. doi: 10.1200/JCO.20.01442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108-1110. doi: 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertuzzi AF, Marrari A, Gennaro N, et al. Low incidence of SARS-CoV-2 in patients with solid tumours on active treatment: an observational study at a tertiary cancer centre in Lombardy, Italy. Cancers (Basel). 2020;12(9):2352. doi: 10.3390/cancers12092352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogado J, Obispo B, Pangua C, et al. COVID-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020;22(12):2364-2368. doi: 10.1007/s12094-020-02381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fillmore NR, La J, Szalat RE, et al. Prevalence and outcome of COVID-19 infection in cancer patients: a national Veterans Affairs study. J Natl Cancer Inst. 2020;djaa159. doi: 10.1093/jnci/djaa159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220-227. doi: 10.1001/jamaoncol.2020.6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088-1089. doi: 10.1016/j.annonc.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta V, Goel S, Kabarriti R, et al. case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935-941. doi: 10.1158/2159-8290.CD-20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anelli F, Leoni G, Monaco R, et al. Italian doctors call for protecting healthcare workers and boosting community surveillance during covid-19 outbreak. BMJ. 2020;368:m1254. doi: 10.1136/bmj.m1254 [DOI] [PubMed] [Google Scholar]
- 15.Moscola J, Sembajwe G, Jarrett M, et al. ; Northwell Health COVID-19 Research Consortium . Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324(9):893-895. doi: 10.1001/jama.2020.14765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11(1):3500. doi: 10.1038/s41467-020-17318-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 18.Noda K, Matsuda K, Yagishita S, et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci Rep. 2021;11(1):5198. doi: 10.1038/s41598-021-84387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park R, Lee SA, Kim SY, de Melo AC, Kasi A. Association of active oncologic treatment and risk of death in cancer patients with COVID-19: a systematic review and meta-analysis of patient data. Acta Oncol. 2021;60(1):13-19. doi: 10.1080/0284186X.2020.1837946 [DOI] [PubMed] [Google Scholar]
- 20.Solodky ML, Galvez C, Russias B, et al. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020;31(8):1087-1088. doi: 10.1016/j.annonc.2020.04.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumley SF, O’Donnell D, Stoesser NE, et al. ; Oxford University Hospitals Staff Testing Group . Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533-540. doi: 10.1056/NEJMoa2034545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016;18(1):10. doi: 10.1186/s13058-015-0669-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loulergue P, Alexandre J, Iurisci I, et al. Low immunogenicity of seasonal trivalent influenza vaccine among patients receiving docetaxel for a solid tumour: results of a prospective pilot study. Br J Cancer. 2011;104(11):1670-1674. doi: 10.1038/bjc.2011.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousseau B, Loulergue P, Mir O, et al. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: the VACANCE study. Ann Oncol. 2012;23(2):450-457. doi: 10.1093/annonc/mdr141 [DOI] [PubMed] [Google Scholar]
- 25.Läubli H, Balmelli C, Kaufmann L, et al. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J Immunother Cancer. 2018;6(1):40. doi: 10.1186/s40425-018-0353-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Channappanavar R, Twardy BS, Suvas S. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One. 2012;7(7):e39757. doi: 10.1371/journal.pone.0039757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuereder T, Berghoff AS, Heller G, et al. SARS-CoV-2 seroprevalence in oncology healthcare professionals and patients with cancer at a tertiary care centre during the COVID-19 pandemic. ESMO Open. 2020;5(5):e000889. doi: 10.1136/esmoopen-2020-000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabezón-Gutiérrez L, Custodio-Cabello S, Palka-Kotlowska M, Oliveros-Acebes E, García-Navarro MJ, Khosravi-Shahi P. Seroprevalence of SARS-CoV-2-specific antibodies in cancer outpatients in Madrid (Spain): A single center, prospective, cohort study and a review of available data. Cancer Treat Rev. 2020;90:102102. doi: 10.1016/j.ctrv.2020.102102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 Antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. doi: 10.1001/jama.2020.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudberg AS, Havervall S, Månberg A, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(1):5064. doi: 10.1038/s41467-020-18848-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. ; ENE-COVID Study Group . Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. doi: 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;180(12):1576-1586. doi: 10.1001/jamainternmed.2020.4130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. SARS-CoV-2 antibody test
eMethods 2. Information collected by the questionnaire
eTable 1. Multivariable regression analysis of the association between patients with cancer and SARS-CoV-2 antibody levels
eTable 2. Characteristics of seropositive patients with cancer
eTable 3. Multivariable regression analysis of the association between chemotherapy and SARS-CoV-2 antibody levels in patients with cancer
eTable 4. Multivariable regression analysis of the association between immune checkpoint inhibitors and SARS-CoV-2 antibody levels in patients with cancer
eFigure 1. ROC curves and cutoff values of SARS-CoV-2 antibodies
eFigure 2. SARS-CoV-2 antibody levels in patients with cancer and HCWs in the analysis population
eFigure 3. Association between clinical factors and SARS-CoV-2 seropositivity rates in patients with cancer
eFigure 4. SARS-CoV-2 antibody levels in patients who did and did not receive anti-cancer treatment
eFigure 5. Association between clinical factors and SARS-CoV-2 antibody levels in patients with cancer