Abstract
Background:
The concentration-response relationship between mortality and long-term exposure to fine particulate matter (PM2.5) has not been fully elucidated, especially at high levels of PM2.5 concentrations.
Objective:
We aimed to evaluate chronic effects of ambient PM2.5 exposure on deaths among Chinese adults in high-exposure settings.
Methods:
Participants of the Prediction for Atherosclerotic cardiovascular disease Risk in China (China-PAR) project were included from four prospective cohorts among Chinese adults aged ≥18 years old. The overall follow-up rate of the four cohorts was 93.4% until the recent follow-up survey that ended in 2015. The average of satellite-based PM2.5 concentrations during 2000–2015 at 1-km spatial resolution was assigned to each participant according to individual residence addresses. Based on the pooled analysis of individual data from the four cohorts, a Cox proportional hazards model was used to estimate the hazard ratio (HR) and corresponding 95% confidence intervals (95% CIs) for the association of PM2.5 exposure with mortality after multivariate adjustment.
Results:
A total of 116,821 participants were eligible in the final analysis. During a mean of 7.7 years of follow-up, 6,395 non-accidental deaths and 2,507 cardio-metabolic deaths occurred. The mean of PM2.5 concentration was 64.9 μg/m3 ranging from 31.2 μg/m3 to 97.0 μg/m3. For each 10 μg/m3 increment in PM2.5, the HR was 1.11 (95% CI: 1.08–1.14) for non-accidental mortality and 1.22 (95% CI: 1.16–1.27) for cardio-metabolic mortality. In addition, a weak exponential curve for the concentration-response association between mortality and PM2.5 was observed among Chinese adults.
Conclusions:
Our study provided important evidence of the long-term effects of PM2.5 exposure on deaths among Chinese adults. The findings expand our knowledge on concentration-response relationship in high-exposure environments, which is essential to address the urgent challenge of reducing the disease burden attributable to PM2.5 exposure in rapidly industrializing countries such as China.
Keywords: Satellite-based PM2.5, Long-term exposure, Cohort study, Mortality, Concentration-response relationship
1. Introduction
Ambient pollution of fine particulate matter (airborne particles ≤2.5 μm in aerodynamic diameter; PM2.5) has been a well-recognized challenge for both air quality improvement and public health promotion. As one of the leading risk factors for global disease burden, 2.9 million deaths worldwide were attributable to outdoor PM2.5 pollution in 2017 (GBD, 2018). In China, ambient PM2.5 pollution contributed to 26.3 million disability-adjusted life years (DALYs) and ranked the fourth in top risk factors of deaths in 2017 (Institute for Health Metrics and Evaluation, 2019).
Evidence on the association of short-term exposure to PM2.5 with daily mortality was reinforced by a recent study in 652 cities across the globe (Liu et al., 2019). Long-term exposure to ambient PM2.5 were also linked to increased risk of mortality in North America and Europe, where PM2.5 concentrations were relatively low (< 35 μg/m3) (Miller et al., 2007; Beelen et al., 2014; Pope et al., 2015; Badaloni et al., 2017; Di et al., 2017; Pinault et al., 2017; Lepeule et al., 2012; Cakmak et al., 2018; Cesaroni et al., 2013). However, the chronic effects of PM2.5 using population-based cohort data in high-exposure settings have not been investigated comprehensively. To our knowledge, increased mortality risk associated with long-term exposure to PM2.5 exposure in mainland China were reported by two cohort studies, one conducted among Chinese men and the other among adults aged ≥65 years (Yin et al., 2017; Li et al., 2018). The design of these studies limited the generalizability of their PM2.5-mortality risk estimates to the general public in China. Thus, assessing the chronic effects of high concentrations of PM2.5 among representative Chinese adults with different characteristics is still warranted. Furthermore, it is important to investigate whether the effect strength of PM2.5-mortality association could be modified by any subject characteristics, which is helpful for early prevention of disease based on differential susceptibility to air pollution.
In addition, previous findings for long-term exposure-response curves on all-cause mortality were inconsistent, especially over higher PM2.5 concentrations. For example, the Integrated Exposure-Response functions (IERs) fit a non-linear curve for the PM2.5-mortality association (Burnett et al., 2014); while the Global Exposure Mortality Model (GEMM) recently reported a near-linear shape using different study assumptions and cohort data (Burnett et al., 2018). Additional cohort studies are needed to examine the shape of exposure-mortality relationship, especially at high concentrations, which forms the basis of assessment in disease burden attributable to PM2.5 by the Global Burden of Disease (GBD) project and the World Health Organization (GBD, 2015; World Health Organization, 2019). Combining the broad coverage of satellite-based ambient PM2.5 concentrations with long-term follow-up data from the Prediction for Atherosclerotic cardiovascular disease Risk in China (China-PAR) project, we have quantified the chronic effects of PM2.5 exposure on incidence of hypertension and diabetes (Huang et al., 2019; Liang et al., 2019). In this study, using 1-km resolution satellite-based PM2.5 exposure estimates, we aimed to investigate both the magnitude and the shape of mortality risk associated with long-term exposure to ambient PM2.5 in Chinese adults, in order to support policy making and health promotion in high-exposure environments.
2. Materials and methods
2.1. Study population
Participants were selected from the prospective population-based China-PAR project, which involves four sub-cohorts including China MUCA (1992–1994) study (China Multi-Center Collaborative Study of Cardiovascular Epidemiology) (Zhao et al., 2003); China MUCA (1998) study (The Collaborative Study Group, 2001); InterASIA (International Collaborative Study of Cardiovascular Disease in Asia) (He et al., 2004); and CIMIC (Community Intervention of Metabolic Syndrome in China & Chinese Family Health Study) (Yang et al., 2016). The study design of the China-PAR project has been described in details elsewhere (Yang et al., 2016). Briefly, China MUCA (1992–1994) was established from 1992 to 1994, and used a cluster random sampling method to select participants aged 35 to 59 years from 14 clusters in China. China MUCA (1998) was initiated among 15 clusters in 1998 with similar design of China MUCA (1992–1994). The InterASIA study was set up at 2000–2001, and selected a nationally representative sample aged 35 to 74 years in China using a 4-stage stratified sampling method based on geographic region (northern versus southern China, divided by the Yangtze River) and urbanicity (urban versus rural). The CIMIC study was a community-based cohort conducted in 4 survey sites of central and eastern China, recruiting participants aged ≥ 18 years during 2007–2008. Together, the four sub-cohorts covered 15 provinces of China (Fig. S1), and each cohort had completed the follow-up survey at least once by the end of 2015.
A total of 127,840 Chinese adults (≥18 years) enrolled initially, of which 8,452 (6.6%) were lost to follow up. Because exposure data started in 2000, the follow-up information after that year was used. The subjects with prevalent cardiovascular disease or cancer before 2000 (n = 2,086) and additional death cases prior to 2000 (n = 382) were excluded. We further excluded 99 subjects without detailed address information. Finally, the remaining 116,821 participants were eligible for our analysis.
These studies have all been approved by the Institutional Review Board at the Fuwai Hospital in Beijing. Written informed consent was obtained from each participant before data collection.
2.2. Health data collection
The baseline and follow-up surveys for all sub-cohorts used comparable protocols and similar questionnaires to obtain information on demographics, residential address, geographic region, lifestyles (e.g., smoking, alcohol drinking, and working-related physical activity), and personal medical history. Smoking status was categorized as smoker or nonsmoker by asking the participant whether he or she had smoked more than 400 cigarettes or at least one cigarette per day for one year. Alcohol drinking was defined as alcohol consumption at least once per week during the last year. Physical examinations were conducted, and body mass index (BMI) was calculated as weight divided by squared height (kg/m2). Hypertension was defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg and/or using antihypertensive medicine in the past two weeks. In addition, blood samples were drawn from participants after fasting for at least ten hours to measure serum glucose and lipids levels in local qualified laboratories or Fuwai Hospital.
During the follow-up surveys, an integrated method of active follow-up was applied including performing face-to-face interviews and obtaining hospital records and death certificates. The hospital records were collected for all of death cases and participants with several chronic diseases occurred during the follow-up periods (e.g., myocardial infarction and stroke which were pre-defined in the survey protocol). We recorded physician diagnosis, date of diagnosis, symptoms, lab-testing results and other necessary information from collected hospital records. For death cases, local investigators also recorded cause of death, death location and date based on official death certificates besides hospital records provided by next of kin. We also double checked the vital status of participants based on other official health information sources such as local disease registries. An end-point assessment committee at Fuwai Hospital in Beijing reviewed medical history information and death certificates to adjudicate the final cause of death. All committee members were blinded to participants’ baseline risk factors, and discrepancies for disease diagnosis or the cause of death were solved by further discussions among committee members. Deaths were coded based on the International Classification of Diseases, 10th Revision. In this study, the following causes of death were considered: non-accidental causes (A00-R99) as well as cardio-metabolic diseases (I00-I99 and E10-E14).
2.3. Exposure assessment
Satellite-based monthly mean PM2.5 concentrations at 1-km spatial resolution from 2000 to 2015 in China were estimated using a machine learning algorithm (Xiao et al., 2018). Briefly, the Aerosol Optical Depth (AOD) product retrieved by the Multi-Angle Implementation Atmospheric Correction (MAIAC) algorithm from the Moderate Resolution Imaging Spectroradiometer (MODIS) instruments of U.S. National Aeronautics and Space Administration was used as a major predictor to estimate high-resolution PM2.5 concentration. Since the satellite data of MODIS were available after 2000 (Xiong et al., 2009; Levy et al., 2013), PM2.5 data after that were estimated. A multiple imputation model was fitted to fill missing in MAIAC AOD and then random forest and extreme gradient boosting models were adopted to predict PM2.5 concentration, incorporating meteorological factors, land use type, and road information. Based on ground-level measurements from national monitoring network after 2013, the prediction accuracy at the monthly level showed a R2 of 0.93 under 10-fold cross-validation. For the period before the availability of nationwide ground networks before 2013, the R2 was estimated to be 0.67 for the monthly prediction and 0.80 for the annual prediction, respectively, when comparing with available observations of Hong Kong, Taiwan, and the US Embassy.
Detailed residential addresses for participants were collected at each interview of the baseline and follow-up surveys. The investigators recorded the residential information of participant by asking which building/street/city she or he was living during the periods of surveys. Thus, the annual mean of PM2.5 exposure levels and exposure duration at each residential address were able to be estimated for every participant in each cohort. We assigned average PM2.5 estimates over the study period (from 2000 to 2015) to each participant based on the geocoded residential address. Considering the residential moving history, time-weighted average PM2.5 concentration was calculated for each participant with the weights defined as the period spent at each of his/her residence.
2.4. Statistical analysis
The baseline characteristics of the included participants were presented as mean ± standard derivation (SD) for continuous variables or as percentages for categorical variables. Person-years of follow-up were calculated from January 1, 2000 until the date of death or that of last follow-up. The stratified Cox proportional hazards model was employed to assess effects of long-term exposure to PM2.5 on mortality with strata defined as sub-cohort and geographic region. The hazard ratio (HR) and 95% confidence interval (95% CI) of mortality per 10 μg/m3 PM2.5 increase was reported when PM2.5 was considered as a continuous variable. Covariates in the multivariate adjusted analysis included age, sex, education level, urbanicity (urban/rural), BMI, total cholesterol, hypertension, diabetes, smoking, drinking, and work-related physical activity at baseline. Furthermore, sensitivity analysis was conducted to assess the robustness of results. First, we examined the associations of mortality with PM2.5 exposure after excluding participants who died within the first year after baseline. Second, considering temporal trends of PM2.5, a Cox regression analysis was re-run with time-varying exposures on a one-year time scale, using the same covariate variables as the main analysis.
We also assessed the potential non-linear effects of PM2.5 exposure on mortality by fitting penalized splines with 2 degrees of freedom for PM2.5. The reference to fit the concentration-response (C-R) curve was 31.2 μg/m3, which was the lowest annual average PM2.5 that our participants were exposed to.
In addition, subgroup analyses were performed by demographics, smoking, drinking, BMI categories, and personal medical history (i.e., hypertension and diabetes). We fitted a separate Cox regression model for each subgroup to obtain subgroup-specific estimates of HR. Student’s t-test was implemented for assessing statistically significant differences in the estimated HR between categories within each subgroup (e.g., male versus female), based on the point estimate and standard error (Di et al., 2017).
The statistical analyses were performed using SAS (version 9.4; SAS Institute Inc, Cary, NC) and R software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). All tests were two-sided, and a P-value < 0.05 was considered to be statistically significant.
3. Results
3.1. Summary statistics
There were 116,821 participants eligible in this analysis, and the numbers of participants included and excluded at each stage in the study are shown in a flow chart (Fig. S2). The cohort-specific follow-up information was shown in Table S1. During a mean (SD) of 7.7(3.3) years of follow-up, a total of 6,395 non-accidental deaths and 2,507 cardio-metabolic deaths occurred. Descriptive analysis of the total and cohort-specific participants at baseline is presented in Table 1. Overall, the mean (SD) age of participants was 51.6 (11.7) years old, and 59% were women. In addition, 31.8% and 5.5% of the participants were with hypertension and diabetes at baseline, respectively.
Table 1.
Baseline characteristics of the total and cohort-specific study participants.a
| Characteristics | Total | ChinaMUCA (1992-1994) | ChinaMUCA (1998) | InterASIA | CIMIC |
|---|---|---|---|---|---|
| Participants (n) | 116,821 | 13,629 | 10,289 | 13,396 | 79,507 |
| Age, y | 51.6 ± 11.7 | 52.7 ± 7.2 | 47.8 ± 7.1 | 49.1 ± 10.4 | 52.3 ± 12.8 |
| Women, % | 59.0 | 53.9 | 52.1 | 52.0 | 62.0 |
| Urban, % | 12.3 | 31.8 | 32.8 | 49.3 | 0.0 |
| Education (≥high school level), % | 14.2 | 14.3 | 27.7 | 31.9 | 9.6 |
| Working PA (≥medium level), % | 55.7 | 50.9 | 41.7 | 39.3 | 61.1 |
| Smoking, % | 27.5 | 39.9 | 37.3 | 37.1 | 22.5 |
| Alcohol drinkers, % | 19.6 | 26.7 | 26.5 | 24.2 | 16.6 |
| BMI | |||||
| <25 kg/m2 | 67.8 | 78.7 | 72.8 | 66.8 | 65.4 |
| 25–29 kg/m2 | 27.4 | 18.9 | 23.7 | 28.7 | 29.2 |
| ≥30 kg/m2 | 4.8 | 2.4 | 3.5 | 4.5 | 5.4 |
| SBP, mmHg | 127.5 ± 21.1 | 122.3 ± 18.7 | 122.8 ± 19.0 | 124.0 ± 20.3 | 129.6 ± 21.6 |
| DBP, mmHg | 78.8 ± 11.7 | 78.6 ± 11.4 | 78.1 ± 11.6 | 78.6 ± 11.1 | 79.0 ± 11.8 |
| Total Cholesterol, mg/dL | 175.2 ± 35.5 | 176.9 ± 37.6 | 184.9 ± 37.1 | 187.9 ± 38.1 | 171.5 ± 33.6 |
| Hypertension, % | 31.8 | 21.5 | 23.1 | 26.6 | 35.6 |
| Diabetes, % | 5.5 | 3.0 | 3.5 | 6.0 | 6.0 |
| PM2.5 concentration, μg/m3 | 64.9 ± 14.2 | 57.9 ± 16.0 | 56.4 ± 15.2 | 58.9 ± 13.9 | 68.2 ± 12.5 |
Abbreviations: Working PA, working-related physical activity; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Baseline characteristics are presented as mean±standard deviation for continuous variables or percentage for categorical variables.
3.2. Long-term exposure to PM2.5 and mortality
The mean (SD) of the 16-year PM2.5 exposure was 64.9 (14.2) μg/m3 ranging from 31.2 μg/m3 to 97.0 μg/m3. The annual PM2.5 levels across different regions had diverse ranges but kept similar temporal trends during 2000–2015 (Fig S3). Table 2 showed the HRs (95% CIs) for the association of mortality with a 10 μg/m3 increase in long-term PM2.5 exposure according to different models. In the full model (i.e., Adjusted Model 4), the risk of non-accidental mortality was significantly elevated with a HR of 1.11 (95% CI, 1.08–1.14) per 10 μg/m3 increase of PM2.5 concentration after adjusting for age, sex, education level, urbanicity (urban/rural), BMI, total cholesterol, hypertension, diabetes, smoking, drinking, and work-related physical activity at baseline. We also observed the association of PM2.5 exposure with death of cardio-metabolic disease, showing a HR of 1.22 (95% CI, 1.16–1.27) after adjustment for the same covariates.
Table 2.
Hazard ratio (95% CI) of non-accidental and cardio-metabolic mortality associated with a 10 μg/m3 increase in PM2.5.
| Hazard Ratio (95% CI) | ||
|---|---|---|
| Non-accidental Deaths | Cardio-metabolic Deaths | |
| No. of Deaths | 6,395 | 2,507 |
| Crude Model | 1.09 (1.06–1.12) | 1.25 (1.20–1.31) |
| Adjusted Model 1a | 1.10 (1.07–1.13) | 1.27 (1.22–1.32) |
| Adjusted Model 2b | 1.12 (1.09–1.15) | 1.31 (1.25–1.36) |
| Adjusted Model 3C | 1.12 (1.09–1.16) | 1.25 (1.19–1.30) |
| Adjusted Model 4d | 1.11 (1.08–1.14) | 1.22 (1.16–1.27) |
Model 1: adjusted for age and sex.
Model 2: Model 1 + adjusted for education and urbanicity (urban vs. rural).
Model 3: Model 2 + adjusted for BMI, hypertension, diabetes, and total cholesterol.
Model 4: Model 3 + adjusted for smoking, drinking, and working-related physical activity at baseline.
In the sensitivity analyses conducted to assess the robustness of our results, the effect estimates of PM2.5 exposure on mortality did not change substantially after excluding participants who died within the first year after baseline (Table S2). When the 16-year average PM2.5 exposure was replaced by the time-varying exposure at a one-year scale, the effects of PM2.5 on deaths were observed with HRs of 1.12 (95% CI, 1.09–1.15) for non-accidental mortality and 1.25 (95% CI, 1.19–1.30) for cardio-metabolic mortality (Table S3), which were similar with those in the main analysis.
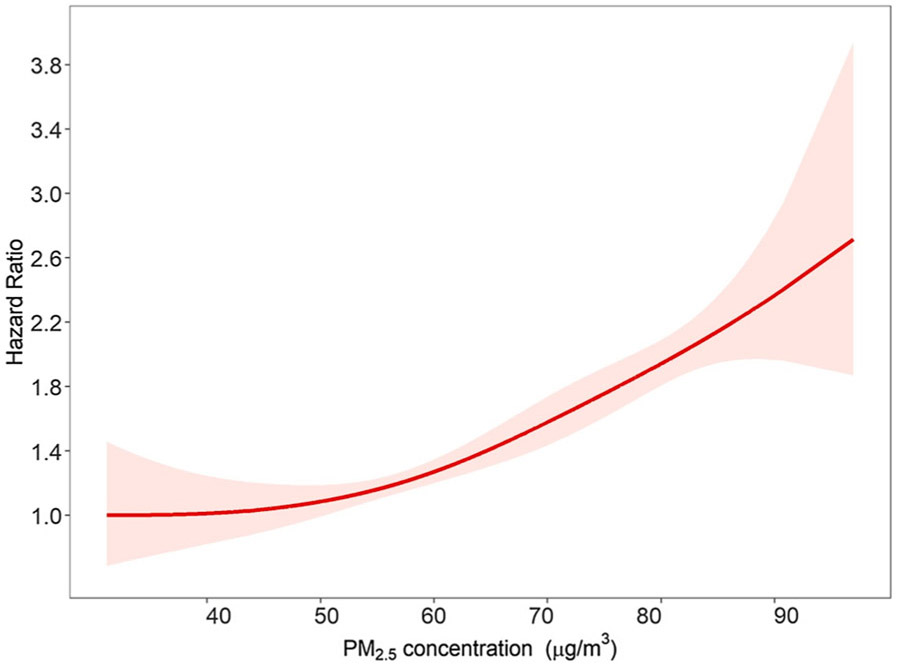
In addition, after fitting the penalized spline model with multiple degrees of freedom, the two degrees of freedom (df = 2) was used to avoid potential over-fitting, based on Bayesian Information Criterion for both non-accidental death and cardio-metabolic death (Table S4). The results with df = 2 showed that a weak exponential C-R curve between long-term PM2.5 exposure and mortality was observed, which revealed that the risk of total death increased substantially with the elevation of PM2.5 at high concentrations (Fig. 1). The C-R relationship for cardio-metabolic mortality was also fitted with visually steeper slope (Fig. 2).
Fig. 1.
Concentration-response relationship between non-accidental mortality and PM2.5 exposure. The curve results were from Model 4, and the reference concentration of PM2.5 was 31.2 μg/m3. The red line represents the point estimate and the shading indicates 95% CIs.
Fig. 2.
Concentration-response relationship between cardio-metabolic mortality and PM2.5 exposure. The curve results were from Model 4, and the reference concentration of PM2.5 was 31.2 μg/m3. The red line represents the point estimate and the shading indicates 95% CIs.
3.3. Subgroup analysis and effect modification
In subgroup analysis, the effect of a 10 μg/m3 increase in PM2.5 on non-accidental mortality was not modified by sex, age, smoking status, and other characteristics except hypertension. The non-accidental death risk associated with PM2.5 exposure was significantly higher among elevated BP groups, including participants in stage 1 hypertension with HR of 1.12 (95% CI, 1.04–1.20) and those in stage 2 hypertension with HR of 1.21 (95% CI, 1.15–1.27), respectively (Table 3). For the outcome of cardiometabolic death, difference in HR was only observed between the stage 2 hypertension group and the normal BP group (P = 0.04), and no differences were tested by other subgroups (Table S5).
Table 3.
Subgroup analysis and hazard ratio (95% CI) of non-accidental mortality associated with a 10 μg/m3 increase in PM2.5.
| No. of Death | Person-year | HR (95% CI)a | Pdifference | |
|---|---|---|---|---|
| Sex | ||||
| Male | 3,732 | 378,663 | 1.10 (1.06–1.14) | 0.87 |
| Female | 2,663 | 523,963 | 1.11 (1.06–1.16) | |
| Age, yrs | ||||
| <60 | 2,597 | 706,924 | 1.05 (1.00–1.10) | 0.28 |
| ≥60 | 3,797 | 195,639 | 1.09 (1.05–1.13) | |
| Urbanicity | ||||
| Rural | 5,486 | 736,094 | 1.12 (1.09–1.16) | 0.97 |
| Urban | 909 | 166,531 | 1.12 (0.93–1.34) | |
| BMI, kg/m2 | ||||
| <25 | 4,705 | 622,081 | 1.10 (1.06–1.13) | 0.51 |
| ≥25 | 1,682 | 280,063 | 1.12 (1.06–1.19) | |
| Smoking | ||||
| Nonsmoker | 3,728 | 632,577 | 1.12 (1.08–1.16) | 0.16 |
| Smoker | 2,639 | 266,374 | 1.07 (1.02–1.12) | |
| Drinking | ||||
| Excess | 581 | 71,242 | 1.11 (1.01–1.22) | 0.962 |
| Moderate | 378 | 63,864 | 1.20 (1.07–1.36) | 0.275 |
| None | 4,835 | 712,641 | 1.11 (1.07–1.14) | |
| BP subgroups | ||||
| Stage2 HTN | 1,912 | 142,928 | 1.21 (1.15–1.27) | <0.001 |
| Stage1 HTN | 1,098 | 119,115 | 1.12 (1.04–1.20) | 0.045 |
| Prehypertension | 1,817 | 297,716 | 1.11 (1.04–1.17) | 0.055 |
| Normal | 1,566 | 342,562 | 1.00 (0.93–1.08) | |
| Diabetes | ||||
| Yes | 643 | 41,972 | 1.12 (1.03–1.23) | 0.76 |
| No | 5,295 | 805,092 | 1.11 (1.07–1.14) | |
Abbreviations: BMI, body mass index; HTN, hypertension; HR, hazard ratio.
The HRs (95% CIs) were the results after adjustment of age, sex, education, urbanicity (urban vs. rural), BMI, hypertension, diabetes, total cholesterol, smoking, drinking, and working-related physical activity at baseline.
4. Discussion
This prospective study provided evidence that long-term exposure to higher PM2.5 concentrations increased mortality risk in Chinese adults aged ≥ 18 years. The risk of non-accidental mortality elevated 11% per 10 μg/m3 increase in PM2.5 concentration. Effect modification of hypertension for the adverse role of PM2.5 exposure was observed, which suggested that hypertensive patients may be more sensitive to PM2.5 exposure. In addition, our estimated C-R curve showed a weak exponential curve of mortality attributable to PM2.5 exposure within the concentration range of 31–97 μg/m3 in China.
The effect estimates for the association of PM2.5 exposure with non-accidental cause mortality in North American and European studies were similar to the current results (Di et al., 2017; Lepeule et al., 2012; Cakmak et al., 2018; Cesaroni et al., 2013); but their annual average concentration of PM2.5 were usually lower than 35 μg/m3. As a rapidly industrializing and high energy consumption country, the annual average PM2.5 in China approached 43 μg/m3 in 2017 (Bulletin, 2017); which was much higher than the World Health Organization Ambient Air-quality Guideline of 10 μg/m3. Direct evidence from Chinese population-based cohort data is urgently needed to identify the association between the long-term exposure of PM2.5 and mortality. In China, two previous studies reported the effect of PM2.5 exposure on overall deaths (Yin et al., 2017; Li et al., 2018). By including over 13,000 adults aged 65 years or older with a mean duration of 3.7 years, the Chinese Longitudinal Healthy Longevity Survey reported a HR of 1.08 (95% CI, 1.06–1.09) for mortality per 10 μg/m3 increase in PM2.5. The Chinese Male Cohort observed risk of non-accidental cause mortality elevated for a 10 μg/m3 increase with a HR of 1.09 (95% CI, 1.08–1.09) (Yin et al., 2017); but this cohort was originally designed to investigate the effects of smoking and enrolled only male participants (Niu et al., 1998). Compared to the aforementioned studies, the current cohort data from China-PAR project included study participants with a large sample size of 116,821 adults among both male and female aged ≥ 18 years, and the maximum follow-up period has reached 16 years with the average follow-up duration of 7.7 years. These important features of our study design allowed us to obtain robust estimates for the deleterious effect of long-term exposure to PM2.5, and provide the opportunity in subgroup analysis to compare potential differences in the effect strength of PM2.5 on mortality.
In subgroup analysis, associations of PM2.5 pollution with mortality were similar between non-smokers and ever-smokers, which was in accordance with the European Study of Cohorts for Air Pollution Effects and the two Chinese cohort studies (Beelen et al., 2014; Yin et al., 2017; Li et al., 2018). And we did not find any significant difference of HRs in categories stratified by alcohol drinking or other characteristics of participants except blood pressure. The subgroup analyses in Table 3 showed a clear trend for increased risks of non-accidental mortality with PM2.5 exposure across elevated BP groups. Multiple mechanistic pathways with complex interdependencies might be considered in the role of effect modification with blood pressure such as systemic inflammation, accelerated atherosclerosis, and altered cardiac autonomic function (Brook et al., 2010); but we should interpret these results with caution since we did not observe the effect modification for cardio-metabolic mortality which should be more sensitive by hypertension.
In addition, since meteorological factors such as ambient temperature could be an important confounder for the association of PM2.5 exposure with mortality, we conducted a sensitivity analysis by additional adjustment for temperature which was accessed from the European Center for Medium-Range Weather Forecasts (ECMWF) atmospheric reanalysis dataset of the global climate (fifth generation, ERA5) at a spatial resolution of 30 × 30 km. Compared with the main results, the association estimation after adjusting for temperature slightly decreased with HR of 1.08 (95% CI, 1.05–1.12) for non-accidental mortality and HR of 1.19 (95% CI, 1.14–1.25). The spatial resolution of temperature data did not well match the resolution PM2.5 dataset (1 × 1 km), so the role of temperature in association of long-term exposure to PM2.5 with mortality needs to be further investigated in the future.
To further test the robustness of results on the relationship between long-term PM2.5 exposure and mortality, we conducted several additional sensitivity analyses besides results shown in Tables S2 and S3. First, considering the potential heterogeneities of cohorts, we used Cox model without the strata of cohort and added the “cohort” as a covariate into the regression model. The HRs were 1.08 (95% CI, 1.04–1.10) for non-accidental mortality and 1.18 (95% CI, 1.13–1.23) for cardio-metabolic mortality per 10 μg/m3 PM2.5 increase (Table S6), which were similar to the main results. In addition, an alternative analysis was conducted to investigate associations of the exposure period before 2013 with non-accidental mortality and cardio-metabolic mortality (Table S7) since assessment of exposure lack of nation-wide ground monitoring data. The similar results to the main analyses suggested that the association analyses for mortality based on exposure assessment with and without monitoring data were comparable. Last, an additional sensitivity analysis adjusted for the average years of education at county-level as one of neighborhood socio-economic status (SES) indicators. There were no substantial changes after adjustment for the neighborhood education years (Table S8).
However, some important confounders such as household solid fuel combustion had not been accessed in the study. The pattern of solid fuel use has changed dramatically especially in rural China during the past decades. For example, the China Kadoorie Biobank (CKB) indicated that the proportion of self-reported solid fuel use decreased by two-thirds during 1968–2014 (Chan et al., 2017). The China Health and Nutrition Survey (CHNS) also showed proportion of rural households that use solid fuel fell substantially from 1991 to 2011 among 9 provinces in China (Liao et al., 2016). Because information of household solid fuel use was not collected during the baseline and follow-up periods (i.e., 2000–2015), we used the covariate of urbanicity as a crude approximation of exposure difference between urban and rural regions to conduct a subgroup analysis, and there were no significant differences for the effect estimations stratified by urbanicity. We need collect information of household solid fuel use in the future among our study participants, which will help us to obtain a more accurate estimation in PM2.5-mortality relationship after adjustment for change pattern of household PM2.5 pollution.
Another important feature of our study was that the PM2.5 exposure levels of participants covered much of high-exposure concentration range worldwide. In 2015, the GBD data indicated global population-weighted PM2.5 concentration has increased to 44.2 μg/m3 along with the highest estimated exposure for Qatar (107.3 μg/m3) (Cohen et al., 2017). In 2017, the PM2.5 pollution has been the fifth-ranked risk factor for global deaths, and has contributed to a loss of 142.5 million DALYs, which represented 5.7% of total DALYs worldwide (Institute for Health Metrics and Evaluation, 2019). In addition, disease burdens attributable to long-term exposure to PM2.5 varied substantially among regions. It reported that the highest PM2.5-attributable mortality in 2015 was in southern Asia, and trends for ambient air pollution contributing to burden diseases have substantially increased in low-income and middle-income countries from 1990 to 2015 (Cohen et al., 2017). In the view of decreasing ambient air pollution and its attributable burden of disease via policy action for the entire population at national level, especially in countries with high-pollution exposure and huge populations such as India and China, it urgently needs to obtain evidence on the associations of long-term exposure to PM2.5 on mortality and to link research evidence to the public policy development. With the annual average PM2.5 exposure of 31.2–97.0 μg/m3 among the study participants, our study greatly extended the range of exposures observed in cohorts conducted in Europe and North America. Moreover, the maximum level of PM2.5 exposure in our study was higher than that reported in the Chinese Male Cohort (84 μg/m3) (Yin et al., 2017). This allowed us to provide new evidence on the shape of outdoor PM2.5-mortality association within a broader exposure range, which will help to accurately assess the national and global disease burden attributable to long-term PM2.5 exposure.
In the past, due to lack of direct findings at higher PM2.5 concentrations, the GBD study had developed an IER model that incorporated HR data from indoor PM2.5 sources such as smoking and household use of solid fuel (Burnett et al., 2014). The IER model indicated a non-linear PM2.5-mortality relationship, and the effects on mortality tended to level off at higher PM2.5 concentrations. The Chinese Longitudinal Healthy Longevity Survey conducted among Chinese elder adults also supported the IER concept and had a non-linear pattern of all-cause mortality (Li et al., 2018). Nevertheless, the most recent GEMM model was constructed using data from 41 cohorts from 16 countries, including the Chinese Male Cohort (Yin et al., 2017); and displayed near-linear associations for non-accidental mortality (Burnett et al., 2018). An investigation recently reported that the estimation of cardiovascular disease burden attributable to PM2.5 pollution in Europe was much higher based on the GEMM function, compared with former GBD assessments (Lelieveld et al., 2019). Our risk functions fitted using independent data corroborated the near-linear shape of PM2.5-mortality relationship for the PM2.5 range of 50–85 μg/m3, although the gradient of HR slope in our analysis did not seem as steep as the GEMM one. Based on the results from the GEMM (Burnett et al., 2018), Chinese Male Cohort (Yin et al., 2017), and this China-PAR project, it suggested that the early IER model developed by the GBD study may underestimate the mortality risk over the PM2.5 concentration of 50 μg/m3. In addition, it seemed no statistically significant excess risk of mortality for the C-R function when PM2.5 concentration was below the 50 μg/m3. We fitted the C-R function using the lowest exposure level of 31.2 μg/m3, which was much higher than the reference levels reported from IER (Burnett et al., 2014), GEMM (Burnett et al., 2018), and the other two Chinese cohorts (Yin et al., 2017; Li et al., 2018); and the proportions of participants with exposure to 31–50 μg/m3 were 13.4% in the study. It was inferred that one explanation for the non-significant HR below the 50 μg/m3 might be the limited exposure gradient (31–50 μg/m3) among the relatively small number of participants, compared with those under the PM2.5 exposure of 50–97 μg/m3. Similarly, it was noted that the C-R curve for non-accidental mortality also showed a nearly flat line below PM2.5 of 40 μg/m3 in Chinese Male Cohort, and the authors explained that partially due to the negative association below 20 μg/m3 (Yin et al., 2017). In the future, we need more prospective cohort studies to investigate the mortality and PM2.5 concentrations especially within the medium range (such as 31–50 μg/m3) in China in order to obtain more accurate estimations in both the shape and the magnitude of PM2.5-mortality association spanning the global distribution of PM2.5 exposure.
The current analysis has several strengths. To our knowledge, this study is the first cohort study in mainland China to investigate the PM2.5-mortality relationship among both male and female adults aged ≥ 18 years. The findings indicated that the risks of non-accidental and cardiometabolic mortality increased 11% and 22%, respectively, per increment of 10 μg/m3. The significant trend for the exposure-mortality association was also observed across the quartiles of long-term exposure to PM2.5 among the study participants (Table S9). In addition, the China-PAR project had a high follow-up rate (93.4%), and there were no substantial differences in baseline characteristics between the participants lost to follow-up and those included in the study (Table S10), which suggested that potential selection bias due to loss to follow-up might be small. The individual covariate data were collected using unified protocols and comparable questionnaires through face-to-face interviews, which enhanced quality assurance of the surveys. Finally, using high-resolution (1-km) PM2.5 estimates derived from MAIAC AOD products and machine-learning approaches helped us capture the fine-scale PM2.5 variability, and allowed us to assign the historical exposure to each study participant.
Despite its strengths above, several limitations should be noted in our study. First, indoor use of solid fuel was not measured in our survey, which may generate a large amount of pollutants including PM2.5, and increased risk of all-cause mortality (Yu et al., 2018). The limitation in lack of adjustment for household solid fuel use may overestimate the effect strength for PM2.5-mortality relationship, especially in rural areas. The study was not able to account for the temporal changes of household solid fuel use, either. Under background of energy use pattern dramatically changed during the past decades in rural China (World Bank, 2013), historical and contemporary data of household solid fuel use among the study participants should be collected in the future, in order to obtain a more accurate estimation in PM2.5-mortality relationship. Second, the emerging of health effects associated with short-term exposure was not examined in the study. One research has investigated the mortality associated with both short- and long-term exposure of PM2.5 at 1 km spatial resolution in Beijing, China (Liang et al., 2018). However, the prospective study covered 15 provinces in China where daily satellite-based PM2.5 concentrations cannot be estimated accurately before 2013 mainly due to lack of nation-wide ground monitoring data. Further analyses would be performed with the mutual adjustment of long- and short-term exposure of PM2.5 if reliable daily estimates in the historical period were available in the future. Third, we were not able to adjust potential confounding for multiple pollutants, because high-resolution data of multiple gaseous pollutants, such as sulfur dioxide, nitrogen dioxide, and ozone, are not available currently. Fourth, the exposure assessment using the satellite-based PM2.5 estimates was not accurate enough in studies on ambient air pollution and health, although it was commonly used in previous studies (Di et al., 2017; Yin et al., 2017; Li et al., 2018). It is warranted to integrate data of ambient-origin PM2.5, indoor air pollution, and time-location patterns of participants to obtain more reliable personal exposure. Fifth, China experienced transitions in lifestyle during the past two decades. There has been a shift in diet from traditional to western pattern, and prevalence of smoking remains high in China (Li et al., 2016). The current analysis did not account for residual confounding in temporal changes of lifestyle factors such as amounts of smoking and dietary sodium intake due to data accessibility. Last, the major emission sources and chemical compositions of PM2.5 may have geographical differences, but we did not collect related information in the population-based study during the past years. The overall relationship of exposure-mortality in the study should be interpreted with caution, given different toxicities of adsorbed chemical compounds of PM2.5 (Brook et al., 2010). More independent investigations need to confirm the relationship between mortality and long-term exposure to PM2.5 with chemical compositions in China.
5. Conclusions
In summary, based on PM2.5 concentrations covering much of the global high-exposure range, this study provided direct evidence for non-accidental mortality associated with long-term exposure to PM2.5 in Chinese adults. The new findings on the concentration-response association will contribute in the national and global estimates of disease burden, and in the assessment on impacts of policy scenarios on projected air-quality improvements in public health.
Supplementary Material
Acknowledgements
The authors acknowledge the staffs and participants of the China-PAR project for their important participation and contribution. The study was supported by the National Key Research and Development Program of China (2017YFC0211703, 2016YFC0206503, and 2018YFE0115300), and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2017-I2M-1-004). The work of YL is partially supported by the MAIA science team at the JPL, California Institute of Technology, led by D. Diner (Emory subcontract No. 1588347).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.105589.
References
- Badaloni C, Cesaroni G, Cerza F, Davoli M, Brunekreef B, Forastiere F, 2017. Effects of long-term exposure to particulate matter and metal components on mortality in the Rome longitudinal study. Environ. Int 109, 146–154. [DOI] [PubMed] [Google Scholar]
- Beelen R, Raaschou-Nielsen o., Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. , 2014. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 383 (9919), 785–795. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. , 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121 (21), 2331–2378. [DOI] [PubMed] [Google Scholar]
- Bulletin 2017 on the State of China's Ecological Environment, http://www.mee.gov.cn/xxgk2018/?ClassInfoId=115. Data access 06/29/2019.
- Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA 3rd, et al. , 2018. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. U S A 115 (38), 9592–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett RT, Pope CA 3rd, Ezzati M, Olives C, Lim SS, Mehta S, et al. , 2014. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect 122 (4), 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak S, Hebbern C, Pinault L, Lavigne E, Vanos J, Crouse DL, et al. , 2018. Associations between long-term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environ. Int 111, 200–211. [DOI] [PubMed] [Google Scholar]
- Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, et al. , 2013. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ. Health Perspect 121 (3), 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Lam KBH, Kurmi OP, Guo Y, Bennett D, Bian Z, et al. , 2017. Trans-generational changes and rural-urban inequality in household fuel use and cookstove ventilation in China: A multi-region study of 0.5 million adults. Int. J. Hyg. Environ. Health 220 (8), 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. , 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389 (10082), 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. , 2017. Air pollution and mortality in the medicare population. N. Engl. J. Med 376 (26), 2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Risk Factors Collaborators, 2016. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053), 1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Risk Factor Collaborators, 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 392(10159), 1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Neal B, Gu D, Suriyawongpaisal P, Xin X, Reynolds R, et al. , 2004. International collaborative study of cardiovascular disease in Asia: design, rationale, and preliminary results. Ethn. Dis 14 (2), 260–268. [PubMed] [Google Scholar]
- Huang K, Yang X, Liang F, Liu F, Li J, Xiao Q, et al. , 2019. Long-term exposure to fine particulate matter and hypertension incidence in China. Hypertension 73 (6), 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation, https://vizhub.healthdata.org/gbd-compare. Data access 02/20/2019.
- Lelieveld J, Klingmuller K, Pozzer A, Poschl U, Fnais M, Daiber A, et al. , 2019. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J 40 (20), 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepeule J, Laden F, Dockery D, Schwartz J, 2012. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ. Health Perspect 120 (7), 965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RC, Mattoo S, Munchak LA, Remer LA, Sayer AM, Patadia F, et al. , 2013. The Collection 6 MODIS aerosol products over land and ocean. Atmos. Meas. Tech 6 (11), 2989–3034. [Google Scholar]
- Li Y, Wang DD, Ley SH, Howard AG, He Y, Lu Y, et al. , 2016. Potential Impact of Time Trend of Life-Style Factors on Cardiovascular Disease Burden in China. J Am Coll Cardiol 68 (8), 818–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang Y, Wang J, Xu D, Yin Z, Chen H, et al. , 2018. All-cause mortality risk associated with long-term exposure to ambient PM2·5 in China: a cohort study. Lancet Public Health. 3 (10), e470–e477. [DOI] [PubMed] [Google Scholar]
- Liang F, Xiao Q, Gu D, Xu M, Tian L, Guo Q, et al. , 2018. Satellite-based short- and long-term exposure to PM2.5 and adult mortality in urban Beijing, China. Environ. Pollut 242 (Pt A), 492–499. [DOI] [PubMed] [Google Scholar]
- Liang F, Yang X, Liu F, Li J, Xiao Q, Chen J, et al. , 2019. Long-term exposure to ambient fine particulate matter and incidence of diabetes in China: A cohort study. Environ. Int 126, 568–575. [DOI] [PubMed] [Google Scholar]
- Liao H, Tang X, Wei Y-M, 2016. Solid fuel use in rural China and its health effects. Renew. Sustain. Energy Rev 60, 900–908. [Google Scholar]
- Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al. , 2019. Ambient particulate air pollution and daily mortality in 652 cities. N. Engl. J. Med 381 (8), 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. , 2007. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med 356 (5), 447–458. [DOI] [PubMed] [Google Scholar]
- Niu SR, Yang GH, Chen ZM, Wang JL, Wang GH, He XZ, et al. , 1998. Emerging tobacco hazards in China: 2. Early mortality results from a prospective study. BMJ 317 (7170), 1423–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault LL, Weichenthal S, Crouse DL, Brauer M, Erickson A, Donkelaar AV, et al. , 2017. Associations between fine particulate matter and mortality in the 2001 Canadian Census Health and Environment Cohort. Environ. Res 159, 406–415. [DOI] [PubMed] [Google Scholar]
- Pope CA 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, et al. , 2015. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ. Res 116 (1), 108–115. [DOI] [PubMed] [Google Scholar]
- The Collaborative Study Group on Trends of Cardiovascular Diseases in China and Preventive Strategy, 2001. Current status of major cardiovascular risk factors in Chinese populations and their trends in the past two decades. Chin J. Cardiol 29(2), 74–79. [Google Scholar]
- World Bank, 2013. China: Accelerating Household Access to Clean Cooking and Heating. East Asia and Pacific Clean Stove Initiative Series. Washington, DC: World Bank. [Google Scholar]
- World Health Organization, 2019. Ambient air pollution: A global assessment of exposure and burden of disease. Available at https://www.who.int/phe/publications/air-pollution-global-assessment/en/. Accessed May 14, 2019.
- Xiao Q, Chang HH, Geng G., An, Liu Y, 2018. Ensemble machine-learning model to predict historical PM2.5 concentrations in China from satellite data. Environ. Sci. Technol 52 (22), 13260–13269. [DOI] [PubMed] [Google Scholar]
- Xiong X, Chiang K, Sun J, Barnes WL, Guenther B, Salomonson VV, 2009. NASA EOS terra and aqua MODIS on-orbit performance. Adv. Space Res 43 (3), 413–422. [Google Scholar]
- Yang X, Li J, Hu D, Chen J, Li Y, Huang J, et al. , 2016. Predicting the ten-year risks of atherosclerotic cardiovascular disease in Chinese population: The China-PAR project. Circulation 134, 1430–1440. [DOI] [PubMed] [Google Scholar]
- Yin P, Brauer M, Cohen A, Burnett RT, Liu J, Liu Y, et al. , 2017. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ. Health Perspect 125 (11), 117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Qiu G, Chan KH, Lam KH, Kurmi OP, Bennett DA, et al. , 2018. Association of solid fuel use with risk of cardiovascular and all-cause mortality in rural China. JAMA 319 (13), 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wu Y, Zhou B, Li Y, Yang J, 2003. Mean level of blood pressure and rate of hypertension among people with different levels of body mass index and waist circumference. Zhonghua Liu Xing Bing Xue Za Zhi. 24 (6), 471–475. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.