INTRODUCTION
Increasing rates of opioid use disorder have resulted in an epidemic of infectious complications of injection drug use (IDU). This includes outbreaks of human immunodeficiency virus (HIV)1 and hepatitis C virus (HCV),2 as well as increasing hospitalizations from skin and soft tissue infections (SSTIs), osteomyelitis, septic arthritis, bacteremia, central nervous system infections, and endocarditis.3-7 Despite increasing incidence of IDU-associated infectious diseases, the best approach to management is unclear, varies widely, and remains understudied.8-10 There is a critical need to identify the best ways to care for patients with IDU-associated infections.
To answer questions about the treatment of patients with IDU-associated infections, clinicians are tasked with applying the best available evidence, yet this research has often excluded people who inject drugs (PWID). Over the past few years, the literature has provided some answers to many of the fundamental questions in infectious diseases (IDs). With increased interest in evidence-based medicine, funding for pragmatic clinical trials, and democratization of the medical literature through social media, many long-held dogmas have been reversed based on robust clinical data. Examples include studies showing noninferiority of oral versus intravenous (IV) antibiotics for certain severe infections,11,12 shorter versus longer courses of antibiotics,13 and bactericidal versus bacteriostatic antibiotics.14 Care must be taken when applying the ID literature to PWID. In this article, the authors will first describe important differences between PWID and the general population represented in clinical trials. Next, they propose an approach to using the literature to inform the management of IDU-associated infections. The authors then apply these principles to important evidence-based practices and provide a framework for designing effective treatment plans for PWID with severe infections.
DIFFERENCES BETWEEN PEOPLE WHO INJECT DRUGS AND PATIENTS REPRESENTED IN CLINICAL TRIALS
Recognizing differences between PWID and patients typically represented in clinical trials is important to appropriately contextualize these data for patients with IDU-associated infections. Table 1 describes unique attributes of PWID and implications of how these differences from the general population may affect infection-related outcomes. Table 2 presents common drug-drug interactions relevant to PWID presenting with IDU-associated infections.
Table 1.
How people who Inject drugs differ from the general population represented In clinical trials for management of severe infections
| Characteristics of People Who Inject Drugs | Implications for Injection Drug Use- Associated Infections |
|---|---|
| Younger age and fewer comorbidities • Median age of IDU-endocarditis patients almost half that of non-IDU-associated endocarditis (33 vs 63 y)79 |
• More physiologic reserve to survive severe infections than older multimorbid patients80,81 • Less likely to experience life-threatening adverse events from antimicrobials82,83 • May be able to tolerate longer courses of riskier antimicrobials (such as trimethoprim-sulfamethoxazole) |
| More mental health disorders • 29% with depression, 22% have attempted suicide, and symptoms of post-traumatic stress disorder are common84 • Higher prevalence of substance-induced mood disorders, personality disorders, and anxiety disorders85,86 |
• Barriers to adhere to medical treatment plans • Drug interactions between psychoactive medications, illicit drugs, and antimicrobials |
| More chronic viral infections • Among PWID, global HIV prevalence is 18%, and in the United States it is 7%87,88 • More than 50% of PWID are antibodypositive for HCV and 9% have chronic hepatitis B virus infection88 |
• Immunodeficiency of advanced HIV increases the chances of both opportunistic and typical infections, as does chronic liver disease from HCV or HBV • Drug interactions between antiretroviral therapy (ART) and antimicrobials often used for the treatment of severe infections |
| Stigmatization by health care system • Many report experiences of dehumanization and discrimination89 • Experiences of trauma during prolonged hospitalization28 |
• Associated with delay in presenting for health care, self-treatment attempts, and seeking informal therapies from nonmedical personnel90,91 • Untreated withdrawal and undertreated pain fuel behaviors like leaving the hospital AMA (or early discharge) and in-hospital illicit drug use92,93 • Stigmatization of drug use may lead PWID to present with more advanced disease, creates barriers to completing care plans, and often results in early discharge without antimicrobials or follow-up30 |
| More social barriers to care • 60% report past-year homelessness94 • 74% uninsured, and 19% did not seek care from a medical provider within the last year95 |
• Difficulty adhering to medical treatment plans while homeless • Lack of access to follow-up medical care and difficulty paying for medications |
Table 2.
Common drug interactions between antimicrobials and medications for opioid use disorder
| Antibiotic Class | Concern | Antibiotic | Medication for Opioid Use Disorder | ||
|---|---|---|---|---|---|
| Methadone | Buprenorphine | XR-Naltrexone | |||
| Fluoroquinolones | QTc prolongation – risk for adverse CV events increased with structural heart disease, electrolyte disorders, and likely by use of multiple QTc prolonging agents (eg,; SSRI)96-100 | Moxifloxacin – highest QTc effect and strongest association with CV events among FQs101 Levofloxacin – lower QTc effect and association with CV events compared with moxifloxacin101 Ciprofloxacin – lower QTc effect and association with CV events compared with moxifloxacin and levofloxacin101 Delafloxacin – industrysponsored studies suggest no QTc effect; limited postmarketing data108,109 |
Associated with QTc prolongation, but weak association with CV events Most cases reported to the FDA had other risk factors for QTc prolongation102-104 |
In vitro blockage of cardiac potassium voltage-gated channels, but no QTc prolongation in the absence of concomitant CYP inhibitors105-107 | Not associated with QTc prolongation |
| Oxazolidinones | Serotonin toxicity – risk for serotonin toxicity increased by use of >1 serotonergic agent (eg, SSRI, MDMA, cocaine) | Linezolid – MAOI Most linezolid-associated serotonin toxicity cases reported were also on an SSRI110 One case control study showed similar risk for serotonin toxicity when linezolid is used with or without an SSRI111 Tedizolid – preclinical studies suggest less MAOI compared with linezolid and lower risk for serotonin toxicity Patients on SSRI excluded from clinical trials116 Limited postmarketing data |
Weak serotonin reuptake inhibitor One case report of serotonin toxicity associated with methadone and linezolid coadministration112 Package insert recommends careful observation with slow incremental methadone doses if used together with an MAOI or ≤14 d of stopping a MAOI113 |
No serotonin reuptake inhibitor activity predicted in vitro114 One published report of serotonin toxicity in patient taking tricyclic antidepressants Prescribing information does not recommend use together with an MAOI or ≤14 d of stopping a MAOI115 |
Not associated with serotonin toxicity |
| Rifamycins | CYP induction leading to increased metabolism of CYP substrates Induction can occur within hours of first dose Induction reversal can take up to 2 weeks after discontinuation | Rifampin – potent CYP3A inhibitor Rifabutin – less potent CYP3A inhibitor compared with rifampin Limited clinical data for bacterial infections compared with rifampin120 |
Methadone is a CYP substrate and rifampin-induced withdrawal is well described.117 Rifabutin does not appear to precipitate methadone withdrawal. Rifamycin/methadone combination not contraindicated, but careful monitoring needed118 | Buprenorphine is a CYP substrate, and rifampin coadministration can induce withdrawal Rifabutin decreases buprenorphine levels, but does not appear to precipitate withdrawal Buprenorphine decreases rifabutin levels, but clinical significance unclear119 Rifamycin/buprenorphine combination not contraindicated, but careful monitoring needed Consider rifabutin therapeutic drug monitoring |
Not metabolized by CYPs No known interaction with rifamycins |
Abbreviations: CV, cardiovascular; FQ, fluoroquinolone; MAOI, monoamine oxidase inhibitor; MDMA, 3,4-methylenedioxymethamphetamine (ecstasy); SSRI, selective serotonin reuptake inhibitor.
APPLYING EVIDENCE-BASED PRACTICES TO INJECTION DRUG USE-ASSOCIATED INFECTIONS
In order to apply the ID literature to PWID, the authors suggest answering 3 questions about each evidence-based practice considered:
What is the evidence for this practice in the general population?
What is the evidence for this practice specifically among PWID?
What are the risks, benefits, and implications for applying this practice to PWID?
The focus of this article is on management questions in the treatment of severe IDU-associated infections requiring hospitalization (Table 3). The purpose of this article is not to provide a comprehensive guide to the management of all IDU-associated infections, but rather to build a framework for applying the best available evidence to a vulnerable population in a thoughtful and informed manner.
Table 3.
Benefits and risks of implementing selected evidence-based practices for injection drug use-associated infections
| Benefits | Risks | |
|---|---|---|
| OPAT vs extended hospitalization | • Completing treatment in more acceptable environment • Increased autonomy, ability to reintegrate, and enter recovery • Avoid traumatic aspects of extended hospitalization |
• Nonadherence with potential for worsening infection • PICC-associated complications • Lack of close monitoring for toxicity and worsening infection • Exposure to drug use triggers |
| Oral vs intravenous antibiotics | • Helps avoid prolonged hospitalization • Increased autonomy, ability to reintegrate, and enter recovery • Lack of need for PICC • Allows most diverse discharge options |
• Nonadherence with potential for worsening infection • Less clinical experience and data for PWID • Lack of close monitoring for toxicity and worsening infection • Exposure to drug use triggers • Requires close outpatient follow-up that may not be feasible • More drug-drug interactions |
| Shorter vs longer course antibiotics | • Less risk for antibiotic adverse events • Potential for shorter hospitalization and need for IV antibiotics |
• Requires close outpatient follow up for response to treatment • Little clinical data specific to PWID |
| Long-acting IV vs standard oral or IV antibiotics | • IV bioavailability without the need to remain hospitalized • No need for PICC • Given long half-life, may be more tolerant of nonadherence |
• High cost • Logistical difficulties of finding infusion chair • Only for gram-positive organisms |
| Surgery vs medical management | • Decreased complications/tissue destruction by infection (eg, shortened duration of sepsis, fewer debilitating emboli) • Potential for shorter course antibiotics, and shorter hospitalization |
• Increased risk of reinfection, prosthetic device infection, especially in setting of ongoing drug use • Finite lifespan of many prosthetic devices |
OUTPATIENT PARENTERAL ANTIMICROBIAL THERAPY FOR INJECTION DRUG USE-ASSOCIATED INFECTIONS
Evidence for Outpatient Parenteral Antimicrobial Therapy in the General Population
Outpatient parenteral antimicrobial therapy (OPAT) allows patients to receive IV antimicrobials outside of the acute care hospital setting. Patients receiving OPAT usually require placement of a peripherally inserted central catheter (PICC) to facilitate antimicrobial infusions, either at home, in a skilled nursing facility (SNF), nursing home, or other institution. OPAT has been deemed safe and effective for a variety of severe infections, including endocarditis.15,16 The 2018 Infectious Diseases Society of America OPAT guidelines enumerate the benefits of OPAT, which include decreased hospital lengths of stay, reduced health care costs, fewer hospital acquired infections, and increased patient satisfaction, when compared with completing hospital-based antimicrobial therapy (HBAT).15 Yet, although there are definite benefits to OPAT overall, the harms have been less well-defined.17 For one of the most common OPAT indications, Staphylococcus aureus bacteremia, Townsend and colleagues18 documented an adverse event rate of 33% and 90-day readmission rate of 64% among patients receiving OPAT; however, there was no comparison to a group receiving HBAT. OPAT has become standard of care for most infections requiring an extended period of IV antimicrobials, and it is supported by strong evidence.
Evidence for Outpatient Parenteral Antimicrobial Therapy Among People Who Inject Drugs
Guidelines do not explicitly make a recommendation for or against providing OPAT to PWID, but recommend evaluation on a case-by-case basis.15 However, many individual OPAT programs, home infusion companies, and hospitals have guidelines prohibiting OPAT for patients with a history of substance use disorders (SUDs) and PWID in particular.9,10,19 Suzuki and colleagues20 performed a review of published OPAT cohorts including PWID. Successful completion of OPAT ranged from 72% to 100% among studies reporting this outcome. Among studies that directly compared PWID versus OPAT among other patients, differences in treatment failure, readmission, mortality, and reinfection rates were negligible. PICC complications ranged from 3% to 9% across the cohorts, although some programs utilized special tamper-proof devices and frequent nurse oversight that might not be standard of care or available in most OPAT programs. Among studies comparing PICC complications between PWID and other patients, there were no significant differences. Patients were discharged to a mix of home, SNF, medical respite programs, and substance use rehabilitation programs. Home OPAT had no worse outcomes than those completing OPAT in an SNF, and patients have far better experiences with home OPAT versus SNF.21,22
There is reason to believe that treatment of the underlying SUD, especially in the case of OUD, has major implications for the success of OPAT among PWID. In a pilot randomized controlled trial for patients with OUD requiring IV antibiotics, all received treatment with buprenorphine, and those randomized to home OPAT had equal success to those receiving HBAT.23,24 In 1 health system, an IV antibiotic risk score was implemented to assess addiction disease activity to guide OPAT decisions.25 Using this score, low-risk patients were eligible to complete antibiotic outside the hospital, which reduced mean hospital stay by 20 days.
Applying Outpatient Parenteral Antimicrobial Therapy Data to People Who Inject Drugs
One of the key determinants in considering the implementation of OPAT for PWID is the expected efficacy of the alternatives to OPAT. In this section, the authors presume that the IDU-associated infection in question requires at least daily doses of IV antibiotics. In such cases, the alternative to OPAT is prolonged hospitalization for HBAT. Prolonged hospitalizations for PWID can be traumatic and an antitherapeutic experience.26 These hospital stays are marked by untreated withdrawal, undertreated pain, stigmatization by health care providers, and subjection to restrictions on mobility off the ward.27-29 Early discharge (against medical advice or AMA) is common among patients receiving HBAT, often without any antibiotics or medical follow-up, and is associated with increased mortality.30-32 For patients who remain in the hospital, prolonged hospitalization can be a reachable moment and opportunity to initiate evidence-based therapies for SUDs.27,33 However, addiction as the underlying cause of disease often goes unacknowledged and untreated.30,34-37 OPAT is an effective intervention overall; success is possible among PWID, and the alternative to OPAT can be harmful and costly to patients. Effectiveness of OPAT for PWID depends on the individual patient’s SUD, access to addiction treatment (including medication for opioid use disorder [MOUD], when indicated), and SNF and home infusion company acceptance of PWID receiving OPAT/MOUD.38
ORAL ANTIBIOTICS FOR INJECTION DRUG USE-ASSOCIATED INFECTIONS
Evidence for Oral Antibiotics for Severe Infections in the General Population
Dogma has long dictated that severe bacterial infections should be treated with IV antibiotics. The preference for IV over oral antibiotics has been especially pervasive for osteomyelitis and endocarditis, yet recent studies have shown noninferiority of oral versus IV antibiotics for common severe infections among PWID. Many of these studies focus on the use of antibiotics with high oral bioavailability or combination therapy including at least 2 agents with differing mechanisms of action. Schrenzel and colleagues39 compared a fluoroquinolone/rifamycin combination versus flucloxacillin or vancomycin for severe staphylococcal infections, excluding left-sided endocarditis, and showed noninferiority of the oral regimen. There are multiple retrospective studies showing noninferiority of early switch to oral antibiotics—primarily linezolid—for uncomplicated SAB and other severe S aureus infections; however, they are prone to selection bias and should be confirmed by prospective studies.40-42
Two recent randomized controlled trials (RCTs) for the treatment of endocarditis and osteomyelitis have led to wider adoption of oral antibiotics for severe infections. The POET (Partial Oral Treatment of Endocarditis) trial compared an early switch to oral antibiotics for patients with left-sided endocarditis caused primarily by methicillin-sensitive S aureus (MSSA), Streptococcus species, and Enterococcus species.11 Patients with minimal valve complications were randomized to switch to oral combination therapy after at least 10 days of IV therapy or to continue on IV. The composite outcome rates were 12% in the IV arm and 9% in the oral arm, consistent with noninferiority. Long-term follow-up continued to show noninferiority of oral therapy.43 The OVIVA (Oral Versus Intravenous Antibiotics) study was a pragmatic clinical trial comparing oral versus IV therapy for bone and joint infections performed in the United Kingdom. Patients were randomized to oral or IV therapy after less than 7 days of IV lead-in. Treatment failure at 1 year was noninferior between the 2 arms (15% IV vs 13% oral), with more catheter-related complications in the IV group.
Evidence for Oral Antibiotics for Severe Infections Among People Who Inject Drugs
Studies of oral antibiotics for severe infections among PWID date back to the late 1980s but have not been rigorously studied in the modern era. The first attempt at using oral therapy for right-sided S aureus endocarditis was documented in 1989 when a cohort of 14 patients were treated with ciprofloxacin and rifampin for 4 weeks.44 An RCT of oral versus IV therapy for right-sided endocarditis among PWID published in 1996 showed noninferiority of oral therapy; few patients, however, had MRSA, and all remained inpatient despite receiving oral therapy.45 Of the high-quality RCTs noted previously, few included any PWID (POET included 5 PWID) or otherwise did not report on the number of patients with SUDs (OVIVA). In an observational study of oral versus IV therapy for MRSA bacteremia, 20% (N = 99) were PWID, of which 22 received oral antibiotics.41 Subgroup analysis of these 99 patients was not reported, but of the 5 total failures in the oral group, 2 were PWID. In sum, there is minimal contemporary data comparing outcomes of oral versus IV therapy among PWID for severe IDU-associated infections.
Applying Oral Antibiotic Data to People Who Inject Drugs
The potential benefits of oral therapy for PWID include shorter hospitalizations, more freedom, and lack of PICC-related complications. Although there is robust evidence to support the use of oral antibiotics for bone/joint infections and endocarditis, there are a few important limitations in applying these data to PWID. The use of long-term IV antibiotics often comes with weekly clinical follow-up and monitoring that might be lacking in the real-world application of oral antibiotics to PWID. In POET, patients receiving combination oral antibiotics were seen up to 3 times weekly with close follow-up of response to therapy. The health care contact that comes along with HBAT and OPAT (eg, home health nurse visits) might lead to greater adherence to IV than oral therapy. Data supporting oral antibiotics for severe infections should be applied to PWID with caution and are not a license to discharge patients with pills and minimal follow-up plans.46 Another consideration is that oral antibiotic regimens may have more potential drug interactions relevant to PWID including the common use of rifamycins in many well-studied oral regimens (see Table 2). OVIVA and POET included few patients with MRSA infection, which is common among PWID in the United States.47 Similarly, many studies used fluoroquinolone combination therapy, to which there is increasing resistance among S aureus isolates worldwide.48 The use of oral antibiotics for severe infections among PWID is promising and can be successfully implemented, but should include shared decision making with patients, with consideration of their social situation and addiction treatment options, rather than being a 1-size-fits-all approach.
Shorter-Course Antibiotics for Injection Drug Use-Associated Infections
Increasing evidence supports the idea that traditional lengths of antibiotic therapy can be shortened substantially without compromising outcomes and with fewer antibioticrelated adverse events.13,49,50 Most of these data have been accrued for pneumonia, urinary tract infections (UTIs), cellulitis, gram-negative bacteremia, and intraabdominal infections, with few rigorous clinical trials evaluating short-course therapy for infections typical among PWID, such as osteomyelitis and endocarditis. A systematic review and meta-analysis of treatment length of osteomyelitis—more than half were pediatric patients—showed noninferiority of shorter course overall, with an odds ratio of 1.50 (95% confidence interval [CI] 0.97-2.34) for treatment failure; however, subgroup analyses indicated some important differences.51 Patients with S aureus infections and those with vertebral osteomyelitis had more treatment failure when given less than 4 to 6 weeks of antibiotics. There was also no subgroup analysis of adult-only trials, which severely limits adaptation to PWID. An RCT of 2 versus 4 weeks of antibiotics for primarily small-joint septic arthritis showed noninferiority of a short course, as did a comparison of 6 versus 12 weeks for pyogenic vertebral osteomyelitis.52,53 In contrast, a prospective observational study of treatment for hematogenous vertebral osteomyelitis showed decreasing relapse with increasing length of treatment, especially among patients with MRSA infection and undrained abscesses.54
Evidence for Shorter Course Antibiotics for Severe Infections Among People Who Inject Drugs
Studies of short- versus longer-course antibiotics have almost systematically not included PWID. The only 2 studies specific to PWID evaluate a shorter antibiotic course of combination therapy including an aminoglycoside for right-sided S aureus endocarditis without a longer-course comparator.55,56 Chambers and colleagues55 performed a prospective study and administered 2 weeks of nafcillin (N = 50) or vancomycin (N = 3) both with tobramycin to 53 PWID. They found 94% and 33% (N = 1) cure rates with nafcillin and vancomycin, respectively. Ribera and colleagues56 performed an RCT among PWID to compare cloxacillin with versus without gentamicin for right-sided MSSA endocarditis. Cure rates were similar between the 2 groups (86%–89%, P>.2), indicating high success with short-course cloxacillin monotherapy. Of studies in the general population, only the study by Gjika and colleagues (2 vs 4 weeks for native joint septic arthritis) made mention of inclusion of PWID (N = 9 out of 154).
Applying Shorter-Course Antibiotic Data to People Who Inject Drugs
In comparison to data on oral versus IV antibiotics, the shorter- versus longer-course literature is more readily adaptable to PWID. The main caveat is that most of the data on management of osteomyelitis and septic arthritis were predicated on appropriate source control procedures. PWID with more complicated infections or multifocal infections with incomplete surgical management would call into question the applicability of shorter-course approaches. Apart from native joint septic arthritis with surgical drainage, the bone/joint literature dictates 4 to 6 weeks of antibiotics for most infections, and this seems appropriate to apply to PWID. Based on the prospective study by Park and colleagues,54 it would be reasonable to extend treatment of hematogenous vertebral osteomyelitis to longer than 6 weeks, especially in the setting of MRSA or undrained abscesses, which might be more common scenarios among PWID. As with the oral versus IV discussion, appropriate treatment of any infection requires follow-up and monitoring for response to treatment. Whether shorter or longer courses of antibiotics are used, access to postacute care ID services is crucial and ideally could be colocalized with management of the patient’s SUD.57
LONG-ACTING INTRAVENOUS ANTIBIOTICS FOR INJECTION DRUG USE-ASSOCIATED INFECTIONS
Evidence for Long-Acting Intravenous Antibiotics in the General Population
Dalbavancin and oritavancin are long half-life lipoglycopeptide antibiotics dosed once weekly intravenously for gram-positive infections, obviating the need for daily infusions or PICCs. Both are approved by the US Food and Drug Administration (FDA) for the treatment of SSTIs, but have been increasingly used off label for treatment of other infections. Two phase 2 RCTs have evaluated the efficacy of dalbavancin for non-SSTIs. Raad and colleagues58 compared dalbavancin for 2 weekly doses versus 14 days of vancomycin for central line-associated blood stream infections. Dalbavancin was statistically superior to vancomycin (success rate of 87% vs 50%, P<.05), but numbers were small (N = 67); no power calculation was presented, and the vancomycin arm included 11% MSSA infections, for which vancomycin is substandard therapy. The other RCT evaluated the efficacy of 2 higher-dose weekly doses of dalbavancin for osteomyelitis, performed in Ukraine. This study showed high success rates for dalbavancin (97%) for gram-positive osteomyelitis, although methodological flaws preclude strong conclusions about efficacy versus the standard of care arm, which did not represent usual practice (vancomycin was used for MSSA, and levofloxacin IV monotherapy was used for MSSA).
Real-world applications of dalbavancin and oritavancin for non-SSTI indications describe over 200 patients treated for osteomyelitis, endocarditis, bacteremia, and prosthetic joint infections with high success rates overall, but without comparison groups and limited reporting on adverse effects.59-64 It is unclear from these studies how many of these infections could have been treated using oral antibiotics.
Evidence for Long-Acting Intravenous Antibiotics Among People Who Inject Drugs
Since long acting lipoglycopeptides became available, there has been interest in applying these lineless antibiotics to PWID. A few retrospective cohorts have evaluated the efficacy of dalbavancin among vulnerable populations, primarily PWID. Among 32 PWID with severe S aureus infections treated by dalbavancin, 56% had a clinical response, 13% with clinical failure, but 31% were lost to follow up with unknown outcome.65 Bork and colleagues66 reported the outcome of 28 patients receiving dalbavancin for non-SSTI in Baltimore, Maryland, of whom 16 (57%) were PWID. The cohort was comprised of primarily orthopedic infections and endocarditis, with a reported cure rate of 71%, but no information on the subgroup comprised of PWID.28 Another group in Colorado included 11 PWID with non-SSTIs, but outcomes in this subgroup were not clearly described.67
Applying Long-Acting Intravenous Antibiotic Data to People Who Inject Drugs
The use of long-acting (LA)-IV antibiotics among PWID have the potential to address a few important problems in the management of IDU-associated infections. Adherence to daily oral antibiotics can be difficult for PWID with ongoing drug use and unstable social circumstances. The use of LA-IV for these infections is particularly encouraging given the high osteomyelitis success rate with only 2 doses of dalbavancin.68 Concerns regarding access to follow-up and ability to adhere to treatment plans are not significantly mitigated by the use of LA-IVs. In the cohort of PWID treated with dalbavancin for S aureus infections, only 53% completed the planned course of therapy.65 Although studies have documented cost savings by allowing earlier hospital discharge with LA-IV antibiotics, the use of oral antibiotics is likely to be even more cost-effective, further weakening the rationale for LA-IV therapy.59,67
SURGICAL PROCEDURES FOR THE TREATMENT OF INJECTION DRUG USE-ASSOCIATED INFECTIONS
Evidence for Surgical Interventions for Severe Infections in the General Population
With the exception of 1 RCT, data for indications and timing of valve surgery for infectious endocarditis are limited to observational data and subject to survivor and selection bias.69,70 A propensity score-matched meta-analysis of early valve surgery (≤20 days) versus conventional therapy (surgery >20 days) or no surgery found early valve surgery was associated with decreased all-cause mortality compared with conventional therapy (odds ratio 0.41 [95% CI 0.31–0.54]).69 The only randomized control trial evaluating timing of valve surgery for infectious endocarditis included patients with native left-sided infectious endocarditis who had large vegetations (>10 mm) and no urgent indication for surgery.70 Early surgery reduced in-hospital mortality and embolic events, but not 6-month all-cause mortality. Only 8 patients in this trial had S aureus endocarditis, and none were reported to be PWID.
Evidence for Surgical Interventions for Severe Infections Among People Who Inject Drugs
The data on surgical outcomes for infections among PWID are primarily retrospective studies of valve surgery for IDU endocarditis. Most found that postoperative mortality among those with IDU-endocarditis and non-IDU-endocarditis was similar in the short-term.71-73 The outcomes following the acute postoperative period among PWID appear to be worse, with 1 institution reporting a tenfold increase in mortality in the 3- to 6-month period following surgery.72 Long-term mortality among PWID with endocarditis is high. In a cohort with a mean age of 36 years, the 10-year survival was just 44%.7 Most deaths following valve surgery are related to ongoing IDU, and the need for reoperation was associated with increased mortality.74 Importantly, addiction treatment referral is strongly correlated with survival among PWID with infectious endocarditis (hazard ratio 0.29).32
Applying Surgical Intervention Data to People Who Inject Drugs
When considering surgical interventions for PWID with severe infections, it is important to consider that even in the best circumstances, addiction is a relapsing disease, and ongoing episodes of drug use are expected.75 In some cases, prosthetic material can be feasibly avoided, such as in tricuspid valve endocarditis. In this case, survival following tricuspid valve repair and replacement was similar, but repair was associated with lower risk for recurrent infection and need for reoperation.76 Additionally, PWID tend to be younger, and many prosthetic devices have a finite lifespan. Thus, prosthetic material should be avoided whenever feasible, but the desire to avoid surgery should never supersede the most effective course of action to cure a severe infection. It may be true that PWID have higher medium-term mortality after endocarditis valve surgery compared with those who do not use injection drugs; however, the important unanswered question is how those with IDU-associated endocarditis would have done without any surgery. There are conflicting retrospective data on whether valve surgery is a predictor of survival in IDU-associated endocarditis.7,32
Application of surgical literature to PWID is also limited by the scant data on the effect of MOUD and other addiction treatments on infection-related outcomes. Most retrospective studies of IDU-associated endocarditis surgery do not report information on SUD diagnoses or utilization of MOUD. Outcomes following implementation of MOUD for patients with IDU-associated infections are being researched actively. Early reports from a cohort of IDU-associated endocarditis patients in Massachusetts indicate reduced mortality among those who took at least 3 months of MOUD following diagnosis.77 Even without clear data on infection outcomes, MOUD should be routinely offered to patients with OUD based on strong evidence of decreasing overall mortality, retention in addiction treatment, and improved quality of life.78
SUMMARY
Successful management of IDU-associated infectious diseases requires a deliberate and earnest assessment of the literature and application to each unique patient. Clinical data supporting noninferiority of less-invasive, expensive, and dangerous approaches to infectious diseases should not be used as a license to deliver lower quality care to PWID. Instead, these data should be scrutinized to evaluate applicability to PWID, considering their unique challenges, before being carefully applied in practice. Fig. 1 provides a framework for considerations and treatment decisions for patients hospitalized with IDU-associated infections. Infectious disease, SUD, and environmental domains each play a vital role in the development of an individualized successful treatment plan. Most importantly, any treatment plan must be in line with a patient’s values and preferences. Future research should focus on increasing the inclusion of PWID into pragmatic clinical trials, improving assessment of SUD diagnoses and utilization of MOUD, and working on comparing interventions between PWID rather than comparing primarily with other patients.
Fig. 1.
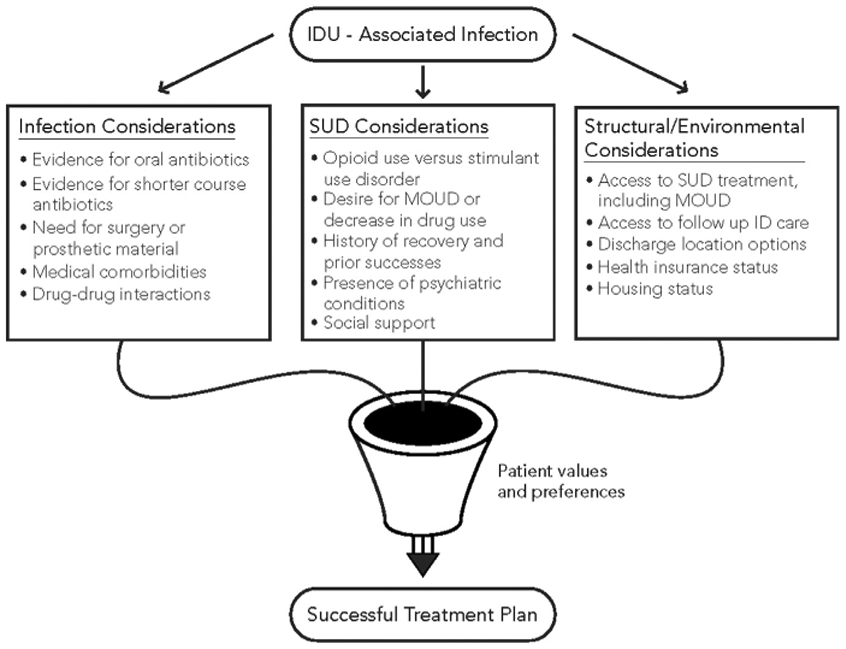
Factors to consider when developing an evidence-based treatment plan for IDU-associated infections. When confronted with an IDU-associated infection, clinicians must balance the best available ID literature (infection considerations) with a patient’s SUD (SUD considerations). Both must be realistic and feasible given the local environment and social circumstances of the patient (structural/environmental considerations). Finally, any successful plan must be filtered through each individual’s values and preferences.
KEY POINTS.
Application of the existing infectious disease (ID) literature to people who inject drugs (PWID) must consider their unique medical, psychological, and social challenges.
Outpatient parenteral antimicrobial therapy can be successful among select PWID with injection drug use-associated (IDU) infections, especially when the alternative is prolonged hospitalization for intravenous antibiotics.
Data supporting the use of oral antibiotics for severe bacterial infections should be applied with caution to PWID, and close ID follow-up and consideration of barriers to adherence to oral antibiotics are required.
Literature on surgical management of IDU-associated endocarditis suggests worse long-term outcomes compared with other causes of endocarditis, but there are no prospective data comparing medical versus surgical approaches for IDU-associated endocarditis and little information on the effect of addiction treatment.
ACKNOWLEDGMENTS
The authors would like to thank Zabrina Quidiello for graphic design assistance with the figure.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Cranston K, Alpren C, John B, et al. Notes from the field: HIV diagnoses among persons who inject drugs - Northeastern Massachusetts, 2015-2018. MMWR Morb Mortal Wkly Rep 2019;68(10):253–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep 2015; 64(17):453–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schranz AJ, Fleischauer A, Chu VH, et al. Trends in drug use-associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med 2018;170(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccarone D, Unick GJ, Cohen JK, et al. Nationwide increase in hospitalizations for heroin-related soft tissue infections: associations with structural market conditions. Drug Alcohol Depend 2016;163:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy NL, Baggs J, See I, et al. Bacterial infections associated with substance use disorders, large cohort of United States Hospitals, 2012-2017. Clin Infect Dis 2020. 10.1093/cid/ciaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straw S, Baig MW, Gillott R, et al. Long-term outcomes are poor in intravenous drug users following infective endocarditis, even after surgery. Clin Infect Dis 2019. 10.1093/cid/ciz869. [DOI] [PubMed] [Google Scholar]
- 8.Serota DP, Vettese T. New answers for old questions in the treatment of severe infections from injection drug use. J Hosp Med 2019;14:E1–7. [DOI] [PubMed] [Google Scholar]
- 9.Rapoport AB, Fischer LS, Santibanez S, et al. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018;5(7):ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanucchi L, Leedy N, Li J, et al. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med 2016;11(8):581–2. [DOI] [PubMed] [Google Scholar]
- 11.Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019;380(5):415–24. [DOI] [PubMed] [Google Scholar]
- 12.Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019;380(5):425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spellberg B Shorter is better. 2019. Available at: https://www.bradspellberg.com/shorter-is-better. Accessed January 4, 2020. [Google Scholar]
- 14.Wald-Dickler N, Holtom P, Spellberg B. Busting the myth of "static vs cidal": a systemic literature review. Clin Infect Dis 2018;66(9):1470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 2019;68(1):e1–35. [DOI] [PubMed] [Google Scholar]
- 16.Perica SJ, Llopis J, Gonzalez-Ramallo V, et al. Outpatient parenteral antibiotic treatment for infective endocarditis: a prospective cohort study from the GAMES Cohort. Clin Infect Dis 2019;69(10):1690–700. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell ED, Czoski Murray C, Meads D, et al. Clinical and cost-effectiveness, safety and acceptability of community intravenous antibiotic service models: CIVAS systematic review. BMJ Open 2017;7(4):e013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend J, Keller S, Tibuakuu M, et al. Outpatient parenteral therapy for complicated Staphylococcus aureus infections: a snapshot of processes and outcomes in the real world. Open Forum Infect Dis 2018;5(11):ofy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaton RA, Barr DA. Outpatient parenteral antibiotic therapy: principles and practice. Eur J Intern Med 2013;24(7):617–23. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki J, Johnson J, Montgomery M, et al. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis 2018;5(9):ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Couto HT, Robbins GK, Ard KL, et al. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis 2018;5(5):ofy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansour O, Arbaje AI, Townsend JL. Patient experiences with outpatient parenteral antibiotic therapy: results of a patient survey comparing skilled nursing facilities and home infusion. Open Forum Infect Dis 2019;6(12):ofz471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanucchi LC, Walsh SL, Thornton AC, et al. Integrated outpatient treatment of opioid use disorder and injection-related infections: a description of a new care model. Prev Med 2019;128:105760. [DOI] [PubMed] [Google Scholar]
- 24.Fanucchi LC, Walsh SL, Thornton AC, et al. Outpatient parenteral antimicrobial therapy plus buprenorphine for opioid use disorder and severe injection-related infections. Clin Infect Dis 2019;70(6):1226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton EF, Mathews RE, Lane PS, et al. A 9-point risk assessment for patients who inject drugs requiring intravenous antibiotics may allow health systems to focus inpatient resources on those at greatest risk of ongoing drug use. Clin Infect Dis 2018;68(6):1041–3. [DOI] [PubMed] [Google Scholar]
- 26.Fanucchi LC. Do persons with opioid use disorder and injection-related infections really need prolonged hospitalizations to complete intravenous antibiotic therapy? San Francisco (CS): ID Week; 2018. [Google Scholar]
- 27.Velez CM, Nicolaidis C, Korthuis PT, et al. "It’s been an experience, a life learning experience": a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med 2017;32(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bearnot B, Mitton JA, Hayden M, et al. Experiences of care among individuals with opioid use disorder-associated endocarditis and their healthcare providers: results from a qualitative study. J Subst Abuse Treat 2019;102:16–22. [DOI] [PubMed] [Google Scholar]
- 29.Alfandre D, Geppert C. Ethical considerations in the care of hospitalized patients with opioid-use and injection drug-use disorders. J Hosp Med 2019; 14(2):123–5. [DOI] [PubMed] [Google Scholar]
- 30.Serota DP, Niehaus ED, Schechter MC, et al. Disparity in quality of infectious disease vs addiction care among patients with injection drug use-associated staphylococcus aureus bacteremia. Open Forum Infect Dis 2019;6(7):ofz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health 2015;105(12):e53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodger L, Glockler-Lauf SD, Shojaei E, et al. Clinical characteristics and factors associated with mortality in first-episode infective endocarditis among persons who inject drugs. JAMA Netw Open 2018;1(7):e185220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trowbridge P, Weinstein ZM, Kerensky T, et al. Addiction consultation services - Linking hospitalized patients to outpatient addiction treatment. J Subst Abuse Treat 2017;79:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serota DP, Kraft CS, Weimer MB. Treating the symptom but not the underlying disease in infective endocarditis: a teachable moment. JAMA Intern Med 2017; 177(7): 1026–7. [DOI] [PubMed] [Google Scholar]
- 35.Jicha C, Saxon D, Lofwall MR, et al. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med 2019;13(1):69–74. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal ES, Karchmer AW, Theisen-Toupal J, et al. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016;129(5):481–5. [DOI] [PubMed] [Google Scholar]
- 37.Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug-related infections are missed: a population-based cohort study of inpatient and emergency department settings. Clin Infect Dis 2019;68(7): 1166–75. [DOI] [PubMed] [Google Scholar]
- 38.Wakeman SE, Rich JD. Barriers to post-acute care for patients on opioid agonist therapy; an example of systematic stigmatization of addiction. J Gen Intern Med 2017;32(1):17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrenzel J, Harbarth S, Schockmel G, et al. A randomized clinical trial to compare fleroxacin-rifampicin with flucloxacillin or vancomycin for the treatment of staphylococcal infection. Clin Infect Dis 2004;39(9):1285–92. [DOI] [PubMed] [Google Scholar]
- 40.Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis 2018;69(3):381–7. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen SCJ, Lagnf AM, Bhatia S, et al. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: one step closer. J Antimicrob Chemother 2019;74(2):489–98. [DOI] [PubMed] [Google Scholar]
- 42.Eliakim-Raz N, Hellerman M, Yahav D, et al. Trimethoprim/sulfamethoxazole versus vancomycin in the treatment of healthcare/ventilator-associated MRSA pneumonia: a case-control study. J Antimicrob Chemother 2017;72(9):2687. [DOI] [PubMed] [Google Scholar]
- 43.Bundgaard H, Ihlemann N, Gill SU, et al. Long-term outcomes of partial oral treatment of endocarditis. N Engl J Med 2019;380(14):1373–4. [DOI] [PubMed] [Google Scholar]
- 44.Dworkin RJ, Lee BL, Sande MA, et al. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet 1989;2(8671):1071–3. [DOI] [PubMed] [Google Scholar]
- 45.Heldman AW, Hartert TV, Ray SC, et al. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am J Med 1996;101(1):68–76. [DOI] [PubMed] [Google Scholar]
- 46.Seaton RA, Ritchie ND, Robb F, et al. From ‘OPAT’ to ‘COpAT’: implications of the OVIVA study for ambulatory management of bone and joint infection. J Antimicrob Chemother 2019;74(8):2119–21. [DOI] [PubMed] [Google Scholar]
- 47.Jackson KA, Bohm MK, Brooks JT, et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs - six sites, 2005-2016. MMWR Morb Mortal Wkly Rep 2018;67(22):625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diekema DJ, Pfaller MA, Shortridge D, et al. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 2019;6(Suppl 1):S47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spellberg B The new antibiotic mantra-"shorter is better". JAMA Intern Med 2016;176(9):1254–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wald-Dickler N, Spellberg B. Short-course antibiotic therapy-replacing Constantine units with "shorter is better". Clin Infect Dis 2019;69(9):1476–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang CY, Hsieh RW, Yen HT, et al. Short- versus long-course antibiotics in osteomyelitis: A systematic review and meta-analysis. Int J Antimicrob Agents 2019; 53(3):246–60. [DOI] [PubMed] [Google Scholar]
- 52.Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015;385(9971):875–82. [DOI] [PubMed] [Google Scholar]
- 53.Gjika E, Beaulieu JY, Vakalopoulos K, et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomised, non-inferiority trial. Ann Rheum Dis 2019;78(8):1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park KH, Cho OH, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis 2016;62(10):1262–9. [DOI] [PubMed] [Google Scholar]
- 55.Chambers HF, Miller RT, Newman MD. Right-sided Staphylococcus aureus endocarditis in intravenous drug abusers: two-week combination therapy. Ann Intern Med 1988;109(8):619–24. [DOI] [PubMed] [Google Scholar]
- 56.Ribera E, Gomez-Jimenez J, Cortes E, et al. Effectiveness of cloxacillin with and without gentamicin in short-term therapy for right-sided Staphylococcus aureus endocarditis. A randomized, controlled trial. Ann Intern Med 1996;125(12): 969–74. [DOI] [PubMed] [Google Scholar]
- 57.Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2019; 70(5): 968–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005;40(3):374–80. [DOI] [PubMed] [Google Scholar]
- 59.Bouza E, Valerio M, Soriano A, et al. Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int J Antimicrob Agents 2018; 51(4):571–7. [DOI] [PubMed] [Google Scholar]
- 60.Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the General Hospital of Vienna. Clin Infect Dis 2018;67(5):795–8. [DOI] [PubMed] [Google Scholar]
- 61.Chastain DB, Davis A. Treatment of chronic osteomyelitis with multidose oritavancin: a case series and literature review. Int J Antimicrob Agents 2019;53(4):429–34. [DOI] [PubMed] [Google Scholar]
- 62.Morata L, Cobo J, Fernandez-Sampedro M, et al. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother 2019;63(5):e02280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life Dalbavancin use in gram-positive infections. Int J Infect Dis 2019;81:210–4. [DOI] [PubMed] [Google Scholar]
- 64.Almangour TA, Perry GK, Terriff CM, et al. Dalbavancin for the management of gram-positive osteomyelitis: Effectiveness and potential utility. Diagn Microbiol Infect Dis 2019;93(3):213–8. [DOI] [PubMed] [Google Scholar]
- 65.Bryson-Cahn C, Beieler AM, Chan JD, et al. Dalbavancin as secondary therapy for serious staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis 2019;6(2):ofz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther 2019;8(2):171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrisette T, Miller MA, Montague BT, et al. Long-acting lipoglycopeptides: "line-less antibiotics" for serious infections in persons who use drugs. Open Forum Infect Dis 2019;6(7):ofz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis 2019;6(1):ofy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anantha Narayanan M, Mahfood Haddad T, Kalil AC, et al. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart 2016;102(12):950–7. [DOI] [PubMed] [Google Scholar]
- 70.Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012;366(26):2466–73. [DOI] [PubMed] [Google Scholar]
- 71.Hall R, Shaughnessy M, Boll G, et al. Drug-use and post-operative mortality following valve surgery for infective endocarditis: a systematic review and meta-analysis. Clin Infect Dis 2019;69(7):1120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shrestha NK, Jue J, Hussain ST, et al. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015;100(3): 875–82. [DOI] [PubMed] [Google Scholar]
- 73.Wurcel AG, Boll G, Burke D, et al. Impact of substance use disorder on midterm mortality after valve surgery for endocarditis. Ann Thorac Surg 2020;109(5):1426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguemeni Tiako MJ, Mori M, Bin Mahmood SU, et al. Recidivism is the leading cause of death among intravenous drug users who underwent cardiac surgery for infective endocarditis. Semin Thorac Cardiovasc Surg 2019;31(1):40–5. [DOI] [PubMed] [Google Scholar]
- 75.Schuckit MA. Treatment of opioid-use disorders. N Engl J Med 2016;375(4): 357–68. [DOI] [PubMed] [Google Scholar]
- 76.Yanagawa B, Elbatarny M, Verma S, et al. Surgical management of tricuspid valve infective endocarditis: a systematic review and meta-analysis. Ann Thorac Surg 2018;106(3):708–14. [DOI] [PubMed] [Google Scholar]
- 77.Kimmel SD, Walley AY, Berson D, et al. Medications for opioid use disorder following injection drug associated endocarditis. Orlando (FL): American Society of Addiction Medicine Conference; 2019. [Google Scholar]
- 78.Mancher M, Leshner AI. National Academies of Sciences Engineering and Medicine (U.S.). Committee on Medication-Assisted Treatment for Opioid Use Disorder. Medications for opioid use disorder save lives. In: Consensus study report of the national academies of sciences, engineering, medicine. Washington, DC: National Academies Press; 2019. Available at: https://www.ncbi.nlm.nih.gov/books/NBK538936/. [PubMed] [Google Scholar]
- 79.Leahey PA, LaSalvia MT, Rosenthal ES, et al. High morbidity and mortality among patients with sentinel admission for injection drug use-related infective endocarditis. Open Forum Infect Dis 2019;6(4):ofz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nasa P, Juneja D, Singh O. Severe sepsis and septic shock in the elderly: an overview. World J Crit Care Med 2012;1(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clifford KM, Dy-Boarman EA, Haase KK, et al. Challenges with diagnosing and managing sepsis in older adults. Expert Rev Anti Infect Ther 2016;14(2):231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antoniou T, Hollands S, Macdonald EM, et al. Trimethoprim-sulfamethoxazole and risk of sudden death among patients taking spironolactone. CMAJ 2015; 187(4):E138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ 2014;349:g6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colledge S, Larney S, Peacock A, et al. Depression, post-traumatic stress disorder, suicidality and self-harm among people who inject drugs: a systematic review and meta-analysis. Drug Alcohol Depend 2019;207:107793. [DOI] [PubMed] [Google Scholar]
- 85.Mackesy-Amiti ME, Donenberg GR, Ouellet LJ. Prevalence of psychiatric disorders among young injection drug users. Drug Alcohol Depend 2012; 124(1–2):70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mackesy-Amiti ME, Donenberg GR, Ouellet LJ. Prescription opioid misuse and mental health among young injection drug users. Am J Drug Alcohol Abuse 2015;41(1):100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burnett JC, Broz D, Spiller MW, et al. HIV infection and hiv-associated behaviors among persons who inject drugs - 20 cities, United States, 2015. MMWR Morb Mortal Wkly Rep 2018;67(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017;5(12):e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend 2019; 198:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilbert AR, Hellman JL, Wilkes MS, et al. Self-care habits among people who inject drugs with skin and soft tissue infections: a qualitative analysis. Harm Reduct J 2019;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monteiro J, Phillips KT, Herman DS, et al. Self-treatment of skin infections by people who inject drugs. Drug Alcohol Depend 2019;206:107695. [DOI] [PubMed] [Google Scholar]
- 92.Summers PJ, Hellman JL, MacLean MR, et al. Negative experiences of pain and withdrawal create barriers to abscess care for people who inject heroin. A mixed methods analysis. Drug Alcohol Depend 2018;190:200–8. [DOI] [PubMed] [Google Scholar]
- 93.Starrels JL, Barg FK, Metlay JP. Patterns and determinants of inappropriate antibiotic use in injection drug users. J Gen Intern Med 2009;24(2):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Linton SL, Cooper HL, Kelley ME, et al. Cross-sectional association between ZIP code-level gentrification and homelessness among a large community-based sample of people who inject drugs in 19 US cities. BMJ Open 2017;7(6): e013823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors among Persons Who Inject Drugs—National HIV Behavioral Surveillance: Injection Drug Use, 20 U.S. Cities, 2015. HIV Surveillance Special Report 18. Revised edition. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2018. Accessed January 21, 2020. [Google Scholar]
- 96.Meid AD, Bighelli I, Machler S, et al. Combinations of QTc-prolonging drugs: towards disentangling pharmacokinetic and pharmacodynamic effects in their potentially additive nature. Ther Adv Psychopharmacol 2017;7(12):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meid AD, von Medem A, Heider D, et al. Investigating the additive interaction of QT-prolonging drugs in older people using claims data. Drug Saf 2017;40(2):133–44. [DOI] [PubMed] [Google Scholar]
- 98.Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013;6(4):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vandael E, Vandenberk B, Vandenberghe J, et al. Development of a risk score for QTc-prolongation: the RISQ-PATH study. Int J Clin Pharm 2017;39(2):424–32. [DOI] [PubMed] [Google Scholar]
- 100.Vandael E, Vandenberk B, Willems R, et al. Risk management of hospitalized psychiatric patients taking multiple qtc-prolonging drugs. J Clin Psychopharmacol 2017;37(5):540–5. [DOI] [PubMed] [Google Scholar]
- 101.Gorelik E, Masarwa R, Perlman A, et al. Fluoroquinolones and cardiovascular risk: a systematic review, meta-analysis and network meta-analysis. Drug Saf 2019;42(4):529–38. [DOI] [PubMed] [Google Scholar]
- 102.Bart G, Wyman Z, Wang Q, et al. Methadone and the QTc interval: paucity of clinically significant factors in a retrospective cohort. J Addict Med 2017;11(6):489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ehret GB, Voide C, Gex-Fabry M, et al. Drug-induced long QT syndrome in injection drug users receiving methadone: high frequency in hospitalized patients and risk factors. Arch Intern Med 2006;166(12):1280–7. [DOI] [PubMed] [Google Scholar]
- 104.Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf 2005;14(11):747–53. [DOI] [PubMed] [Google Scholar]
- 105.Baker JR, Best AM, Pade PA, et al. Effect of buprenorphine and antiretroviral agents on the QT interval in opioid-dependent patients. Ann Pharmacother 2006;40(3):392–6. [DOI] [PubMed] [Google Scholar]
- 106.Katchman AN, McGroary KA, Kilborn MJ, et al. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exp Ther 2002;303(2):688–94. [DOI] [PubMed] [Google Scholar]
- 107.Schmith VD, Curd L, Lohmer LRL, et al. Evaluation of the effects of a monthly buprenorphine depot subcutaneous injection on QT interval during treatment for opioid use disorder. Clin Pharmacol Ther 2019;106(3):576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Litwin JS, Benedict MS, Thorn MD, et al. A thorough QT study to evaluate the effects of therapeutic and supratherapeutic doses of delafloxacin on cardiac repolarization. Antimicrob Agents Chemother 2015;59(6):3469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mogle BT, Steele JM, Thomas SJ, et al. Clinical review of delafloxacin: a novel anionic fluoroquinolone. J Antimicrob Chemother 2018;73(6):1439–51. [DOI] [PubMed] [Google Scholar]
- 110.Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006;42(11):1578–83. [DOI] [PubMed] [Google Scholar]
- 111.Karkow DC, Kauer JF, Ernst EJ. Incidence of serotonin syndrome with combined use of linezolid and serotonin reuptake inhibitors compared with linezolid monotherapy. J Clin Psychopharmacol 2017;37(5):518–23. [DOI] [PubMed] [Google Scholar]
- 112.Mastroianni A, Ravaglia G. Serotonin syndrome due to co-administration of linezolid and methadone. Infez Med 2017;25(3):263–6. [PubMed] [Google Scholar]
- 113.Roxane Laboratories I Dolophine hydrochloride cii. Available at: https://www.fda.gov/media/76020/download. Accessed January 21, 2020.
- 114.Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth 2005;95(4):434–41. [DOI] [PubMed] [Google Scholar]
- 115.Indivior. Suboxone® (buprenorphine and naloxone) sublingual film, for sublingual or buccal use CIII. 2019. Available at: https://www.suboxone.com/pdfs/prescribing-information.pdf. Accessed January 21, 2020. [Google Scholar]
- 116.Burdette SD, Trotman R. Tedizolid: the first once-daily oxazolidinone class antibiotic. Clin Infect Dis 2015;61(8):1315–21. [DOI] [PubMed] [Google Scholar]
- 117.Kreek MJ, Garfield JW, Gutjahr CL, et al. Rifampin-induced methadone withdrawal. N Engl J Med 1976;294(20):1104–6. [DOI] [PubMed] [Google Scholar]
- 118.Brown LS, Sawyer RC, Li R, et al. Lack of a pharmacologic interaction between rifabutin and methadone in HIV-infected former injecting drug users. Drug Alcohol Depend 1996;43(1–2):71–7. [DOI] [PubMed] [Google Scholar]
- 119.McCance-Katz EF, Moody DE, Prathikanti S, et al. Rifampin, but not rifabutin, may produce opiate withdrawal in buprenorphine-maintained patients. Drug Alcohol Depend 2011;118(2–3):326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crabol Y, Catherinot E, Veziris N, et al. Rifabutin: where do we stand in 2016? J Antimicrob Chemother 2016;71(7):1759–71. [DOI] [PubMed] [Google Scholar]


