Abstract
A wide number of neurological manifestations have been described in association with coronavirus disease 19 (COVID-19). We describe an unusual case of a young man who developed severe rhombencephalitis after COVID-19. He demonstrated clinical and radiological improvement with high dose corticosteroids, plasma exchange and intravenous immune globulin. Our findings, along with previously reported cases that we review here, support an autoimmune para- or post-infectious mechanism and highlight a possible role for immunotherapy in patients with rhombencephalitis after COVID-19.
Keywords: Brainstem encephalitis, Rhombencephalitis, COVID-19, SARS-CoV-2
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel strain of coronavirus identified in December 2019. Coronavirus disease 19 (COVID-19), the disease caused by SARS-CoV-2, is associated with neurological complications including headaches, seizures, stroke and encephalitis.(Ellul et al., 2020) The neurological sequelae are attributed to various mechanisms such as direct viral infection,(Baig et al., 2020) post- or para-infectious immune-mediated processes.(Zubair et al., 2020; Reichard et al., 2020) We describe a patient with brainstem and cerebellar inflammation consistent with rhomboencephalitis(Cleaver et al., 2020) following COVID-19, and a literature review of SARS-CoV-2-related rhomboencephalitis.(Llorente Ayuso and Torres Rubio, 2020; Wong et al., 2020; Khoo et al., 2020)
2. Case report
A 21-year-old man with type 1 diabetes mellitus developed mild fevers, cough, myalgias and had a positive nasopharyngeal PCR for SARS-CoV-2. He was managed conservatively at home, and he had a follow-up negative SARS-CoV-2 PCR 14 days later. Two weeks after, he developed progressive gait instability and dysarthria and within a week he was bedridden.
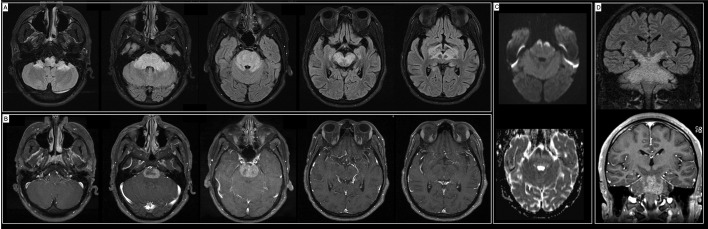
He then was evaluated in a community hospital. A repeat nasopharyngeal SARS-CoV-2 PCR was negative. Brain magnetic resonance imaging (MRI) showed extensive brainstem and cerebellum T2/fluid attenuated inversion recovery (FLAIR) hyperintense signal (Fig. 1 , A) with heterogeneous contrast enhancement (Fig. 1, B) and patchy areas of restricted diffusion (Fig. 1, C). Cerebrospinal fluid (CSF) revealed no pleocytosis, normal glucose and elevated protein. He had negative anti-GM1 IgG/IgM and anti-GQ1B antibodies. He received 1 g methylprednisolone intravenously for 5 days, intravenous immune globulin (IVIG) 2 g/kg over 5 days, followed by 5 cycles of plasmapheresis. He had no significant improvement and was discharged home.
Fig. 1.
(A) Axial MRI FLAIR sequence demonstrating extensive FLAIR signal abnormality involving the cerebral peduncles, midbrain, pons, and bilateral brachium pontis associated with (B) marked contrast enhancement of the pons, (C) restricted diffusion in the anterior portion of the pons. (D) Coronal views demonstrating the extensive signal abnormality in the braistem.
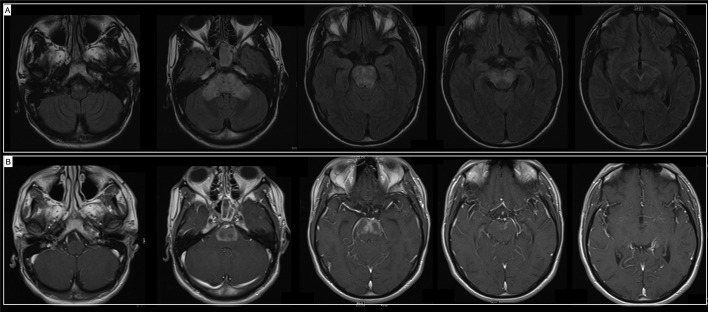
Ten days after hospital discharge, he presented to our institution after two generalized tonic-clonic seizures. His vital signs were normal, neurological examination was notable for limitation of leftward gaze, severe dysarthria and profound truncal and limb ataxia. He had slightly brisk reflexes and flexor plantar response bilaterally. Nasopharyngeal SARS-CoV-2 PCR was negative and serum SARS-CoV-2 IgG was positive. Systemic inflammatory markers were slightly elevated, with thrombocytosis 465 10E(Zubair et al., 2020)/mcL (150–400 10E(Zubair et al., 2020)/mcL) and erythrocyte sedimentation rate of 34 mm/h (normal 1–15 mm/h). Repeat brain MRI showed mild improvement of the signal abnormalities and decreased contrast enhancement (Fig. 2 A, B). CSF analysis revealed zero white blood cells, elevated protein count (122 mg/dL, normal 15–45 mg/dL), normal glucose, zero oligoclonal bands and normal IgG index. An extensive infectious workup was negative, including negative HIV, CSF bacterial and fungal cultures, PCR for HSV, VZV, EBV, CMV, and metagenomic next-generation sequencing (UCSF Clinical Laboratories - San Francisco, CA). Extensive antibody screening including serum and CSF autoimmune encephalopathy panels (Mayo Clinic Laboratories – Rochester, MN), serum anti-aquaporin 4 IgG and myelin oligodendrocyte glycoprotein IgG were negative. He had a positive glutamic acid decarboxylase-65 (GAD-65) in the serum (2.00 nm/L, normal ≤ 0.02 nm/L) and normal in CSF. Other autoimmune testing revealed slightly elevated anti-double-stranded DNA antibody titer (8.0 IU/mL, normal 0–4 IU/mL), with negative antinuclear antibodies, antineutrophil cytoplasmic antibodies, and extractable nuclear antigen.
Fig. 2.
(A) Improvement of the signal abnormality in the midbrain, demonstration of FLAIR signal hyperintensity in the bilateral superior cerebellar peduncles and bilateral pons. (B) Demonstrated enhancement in the bilateral pons, similar compared to prior MRI.
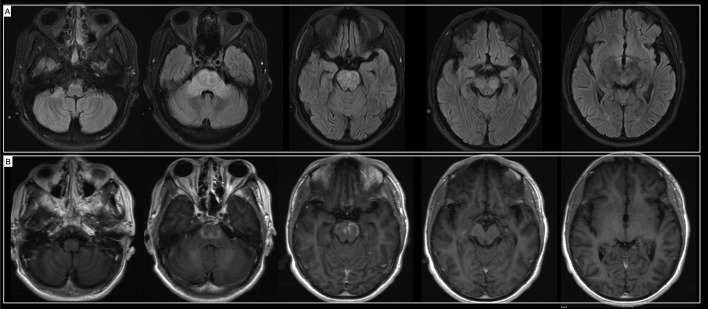
He was diagnosed with post-infectious autoimmune rhombencephalitis. With his severe symptoms and partial radiological improvement, he was treated again with 5 sessions of plasmapheresis followed by IVIG 2 g/kg. Levetiracetam was started for seizures. Clinically, he demonstrated some improvement with normalization of his extraocular movements and improvement of the dysarthria and ataxia. Repeat imaging prior to discharge showed significant improvement (Fig. 3 , A, B). He was discharged to an acute rehabilitation facility.
Fig. 3.
(A) Significantly improved FLAIR signal abnormalities, particularly of the cerebral peduncles and midbrain, pons, and medulla. (B) Enhancement of the bilateral pons significantly improved from prior MRI.
3. Methods
We conducted a systematic literature search in electronic databases PubMed and Scopus to identify studies reporting on “COVID-19” or “SARS-CoV- 2” with “brainstem encephalitis”, “rhombencephalitis”, “postinfectious brainstem encephalitis” and “Bickerstaff encephalitis” published between January 1, 2020 and February 28, 2021. We selected case studies that reported the association between COVID-19 and brainstem encephalitis. The search identified 29 articles. Based on the title and abstract, 3 papers were selected and are summarized in Table 1 .
Table 1.
Summary of cases of rhombencephalitis
| Patient | 1 (Wong et al., 2020) | 2 (Llorente Ayuso and Torres Rubio, 2020) | 3 (Khoo et al., 2020) | Our patient |
|---|---|---|---|---|
| Age/sex | 40-year-old man | 72-year-old woman | 65-year-old woman | 21-year-old man |
| Presenting symptoms | Fever, malaise, exertional dyspnea | Delirium and fever, bilateral interstitial pneumonia with hospital course complicated by myocardial infarction and shock. Discharged home after 22 days of hospitalization. | Fever, cough and myalgias | Fever, cough and myalgias |
| Days of respiratory symptoms before neurological presentation | 13 days | 30 days | 7 days | 21 days |
| Covid diagnosis | Positive nasopharyngeal PCR SARS-CoV-2 on initial presentation | Positive nasopharyngeal PCR SARS-CoV-2 on initial presentation, negative at the time of CNS symptoms | Positive nasopharyngeal PCR SARS-CoV-2 on initial presentation with neurological symptoms. CSF PCR for SARS-CoV-2 was negative. | Positive nasopharyngeal PCR SARS-CoV-2 on initial presentation, negative at the time of neurological symptoms. SARS-CoV-2 IgG antibodies: positive in serum, negative in CSF. |
| Neurologic symptoms | Unsteady gait, diplopia, oscillopsia, limb ataxia, altered sensation in right arm, hiccups | Dizziness, oscillopsia, and unsteadiness | Involuntary movements, diplopia, visual hallucinations and cognitive decline | Severe slurred speech, inability to walk and abnormal eye movements |
| Neurological exam | Mild bilateral facial weakness, tongue weakness, upbeat nystagmus on all directions of gaze and limb ataxia. | Bradypsychia, downbeat nystagmus and impairment of smooth pursuit. The left plantar response was extensor. Severe truncal ataxia and stimulus induced myoclonus. | Widespread stimulus-sensitive myoclonus, hyperekplexia, ocular flutter and convergence spasm, and ocular-facial synkinesis. | Severe dysarthria, truncal and limb ataxia, and abnormalities of horizontal extraocular movements |
| CSF findings | Normal | WBC of 0/mm3, glucose 70 mg/dL, protein 41 mg/dL, sterile cultures, IgG index of 0.5, and absence of CSF oligoclonal bands | WBC of 0.001 × 109/L (normal <0.005 × 109/L), normal protein and glucose with negative CSF oligoclonal bands. | WBC 0/mm3, protein 122 mg/dL, normal 15–45 mg/dL) with normal glucose. |
| Other workup | CRP marginally increased (50 mg/L), negative HIV | Negative HIV, extensive negative infectious workup in the CSF. Extensive autoimmune encephalitis antibody screening was negative. Positive anti-GD1a IgG antibodies in the serum. | CRP elevated (21 mg/L,), extensive autoimmune encephalitis antibody screening was negative. No occult malignancy on CT chest, abdomen and pelvis and FDG-PET | Extensive infectious and autoimmune encephalitis antibody screening was negative. No occult malignancy evidences on CT chest, abdomen and pelvis |
| MRI | Increased T2/FLAIR signal abnormality in the inferior cerebellar peduncle extending to the upper cervical cord. | T2/FLAIR hyperintense lesions in the caudal vermis and right flocculus, with contrast enhancement in the floor of the fourth ventricle. | Normal | Extensive brainstem and cerebellar increased T2/FLAIR signal, restricted diffusion and contrast enhancement |
| Treatment | Symptomatic - Gabapentin | IV methylprednisolone 1 g daily x 5 days followed by 1 mg/kg/day of PO prednisone. | IV methylprednisolone 1 g daily x 3 days, followed by oral prednisolone 1 mg/kg, with a plan to wean over a period of 4–6 months. | IV methylprednisolone 1 g daily x 5 days, IVIG 2 g/kg followed by 5 sessions of PLEX at outside institution without clinical improvement. On arrival to our institution, underwent 5 sessions of PLEX followed by IVIG 2 g/kg. |
| Outcome | Improvement in hiccups and nystagmus, oscillopsia and ataxia persisted at the time of discharge. | Significant improvement within days, with resolution of bradypsychia, nystagmus, myoclonus and improvement of ataxia. Imaging on day 24 with significant improvement. 2-month follow-up with mild unsteadiness. | On discharge from hospital (10 days after steroids), slow improvement was noticed. Cognition returned to her previous baseline, able to mobilize with support, and some improvement of myoclonus. | Normalization of his extraocular movements and improvement of the dysarthria and ataxia. Significant radiological improvement. |
| Diagnosis/presumed mechanism | Brainstem encephalitis and myelitis/ not discussed by the authors. | BBE/Post- or para-infectious | Postinfectious immune-mediated encephalitis | Postinfectious immune-mediated encephalitis |
Abbreviations: BBE Bickerstaff brainstem encephalitis; FLAIR: fluid attenuated inversion recovery, CRP: C-reactive protein, mg = milligram, kg = kilogram, g = gram, PLEX = plasmapheresis.
4. Case reviews
4.1. Case 1
Wong et al.(Wong et al., 2020) describe a 40-year-old man who presented with unsteady gait, diplopia, oscillopsia, limb ataxia, altered sensation in right arm, and hiccups 14 days after the onset of respiratory symptoms. Nasopharyngeal PCR SARS-CoV-2 was positive. Neurological examination revealed bilateral facial weakness, tongue weakness, upbeat nystagmus and limb ataxia. Imaging showed increased signal in the inferior cerebellar peduncle extending to the upper cervical spinal cord and normal CSF analysis. He was diagnosed with brainstem encephalitis and treated symptomatically with gabapentin with improvement of hiccups and nystagmus. Ataxia persisted upon discharge.
4.2. Case 2
Llorente Ayuso et al.(Llorente Ayuso and Torres Rubio, 2020) describe a 72-year-old woman who presented with dizziness, oscillopsia, and unsteadiness 8 days after a prolonged hospitalization for SARS-CoV-2 pneumonia complicated by myocardial infarction and cardiogenic shock. Neurological examination revealed inattention downbeat nystagmus and impairment of smooth pursuit, Babinski on the left, severe truncal ataxia and stimulus-induced myoclonus. CSF was normal, with normal IgG index and no oligoclonal bands. MRI revealed T2/FLAIR hyperintense lesions in the cerebellum and contrast enhancement in the floor of the fourth ventricle. Serum GD1a IgG antibodies were detected. She was treated with high-dose steroids and oral steroid taper, with significant clinical and radiological improvement. She was diagnosed with Bickerstaff's brainstem encephalitis, with a triad of ophthalmoplegia, ataxia, and depressed level of consciousness, typically a post-infectious syndrome.(Shahrizaila and Yuki, 2013)
4.3. Case 3
Khoo et al(Khoo et al., 2020) describe a 65-year-old woman who developed involuntary movements, diplopia and cognitive decline 7 days after a prodrome of fever, cough and myalgia. She was diagnosed with COVID-19 after a positive nasopharyngeal PCR SARS-CoV-2 and chest X-ray demonstrating bilateral pulmonary infiltrates. Brain MRI and CSF analysis were normal. CSF SARS-CoV-2 PCR was negative. Extensive antibody screening was negative. She was diagnosed with post-infectious immune-mediated encephalitis and started on high-dose steroids, with slow progressive improvement of her neurological symptoms.
5. Discussion
Neurological manifestations of COVID-19 are relatively common, described in 36%–84% of cases.(Mao et al., 2020; Helms et al., 2020) Neurological symptoms can be the presenting manifestation of SARS-CoV-2 infection or can develop later in the disease course.(Mao et al., 2020; Romero-Sánchez et al., 2020) COVID-19 is associated with the development of autoimmune neurological diseases, such as Guillain-Barré syndrome,(Toscano et al., 2020; Padroni et al., 2020) Miller Fisher syndrome,(Gutiérrez-Ortiz et al., 2020) acute necrotizing encephalitis,(Poyiadji et al., 2020) and acute disseminated encephalomyelitis.(Parsons et al., 2020) In 43 patients with neurological disorders associated with COVID-19, 12 developed a central nervous system (CNS) inflammatory syndrome thought to be associated with para-or post-infectious mechanisms. Of these patients, 2 developed encephalitis, 9 acute disseminated encephalomyelitis and 1 myelitis.(Paterson et al., 2020)
SARS-CoV-2 is known to access the CNS.(Baig et al., 2020) Different mechanisms of CNS invasion have been proposed including the trans-synaptic spread of the virus, direct viral entry along the olfactory bulb, invasion of the vascular endothelium via the angiotensin converting enzyme 2 receptor, to which SARS-CoV-2 binds to gain entry into the cells, and finally migration of infected leukocytes through the blood-brain barrier.(Ellul et al., 2020; Zubair et al., 2020) Our patient had a negative CSF SARS-CoV-2 IgG, and the patient reported by Khoo et al(Khoo et al., 2020) had a negative CSF SARS-CoV-2 PCR, which argues against direct viral injury. Furthermore, the brainstem lesions in our patient predominantly affect the white matter and spare the grey matter (Fig. 1, A), making an infectious process less likely.
COVID-19 is associated with a severe systemic inflammatory response,(Lan et al., 2020; Moore and June, 2020; Mehta et al., 2020) which can lead to a para- or post-infectious immune-mediated CNS damage.(Zubair et al., 2020; Romero-Sánchez et al., 2020; Guilmot et al., 2021) The presence of antiganglioside antibodies in patients with immune mediated syndromes supports the potential for an autoimmune reaction triggered by SARS-CoV-2 leading to neurological sequelae.(Gutiérrez-Ortiz et al., 2020; Guilmot et al., 2021) A high prevalence SSA/Ro and antinuclear antibodies has been detected in COVID-19 patients, further supporting the possibility of autoimmune phenomena in convalescent patients.(Zhou et al., 2020) Our patient had low titers of dsDNA, likely a false positive seen in other autoimmune conditions or following prior IVIG administration.(Attar and Koshak, 2010) His serum GAD-65 was minimally elevated, which is consistent with his type 1 diabetes mellitus(Mattozzi et al., 2020) and is likely predisposing him to other autoimmune disorders. The timing of the neurological symptom onset, the course and clinicoradiological improvement seen in this case series, suggest a para- or post-infectious immune-mediated mechanism. Other studies have shown inflammatory changes and elevated cytokines in CSF analysis,(Benameur et al., 2020; Picod et al., 2020; Muccioli et al., 2020) which further support this theory.
For patients with neurological sequelae from COVID-19, rehabilitation (both appropriate assessments and programs) will also be critical in their recovery, especially in patients who were admitted to the intensive care unit.(Curci et al., 2021; de Sire et al., 2021; Tuzun et al., 2021) Many COVID-19 patients experience fatigue(de Sire et al., 2021; Carfì et al., 2020) including in the post-acute/chronic phase, and intensive rehabilitation has been shown to improve outcomes.(Curci et al., 2021; Ferraro et al., 2021)
6. Conclusion
Our case adds to the literature describing post-infectious immune mediated neurological sequalae in patients with COVID-19. Even though we cannot rule out the possibility of direct viral CNS penetration by SARS-CoV-2, the timing, imaging and clinical features, along with improvement after immunotherapy, are most supportive of a para- or post-infectious disease mechanism in COVID-19 associated rhombencephalitis. Although it is unclear whether treatment with steroids, IVIG or plasmapheresis contributed to the improvement or if it merely followed the natural course of a monophasic illness, these cases highlight a possible role for these therapies in patients where a post- COVID-19 autoimmune rhombencephalitis is suspected after careful exclusion of infectious mimics, such as listeria, herpes simplex encephalitis, and tuberculosis.(Cleaver et al., 2020)
Ethical statement
We present a de-identified case report and review of the literature. Per Emory University Institutional/IRB rules, a study involving fewer than 5 patients is not reviewed as “research” and as such, does not require a study approval and is considered exempt. We avoid the use of any patient identifiable information anywhere in the text or imaging sections of the manuscript that would necessitate a consent requirement or an ethics committee approval.
Funding
None.
Conflict of interest statement
GG receives part-time salary support as a consultant for the Centers for Disease Control in reviewing acute flaccid myelitis cases for disease surveillance.
Acknowledgements
None.
References
- Attar S.M., Koshak E.A. Medical conditions associated with a positive anti-double-stranded deoxyribonucleic acid. Saudi Med. J. 2010;31(7):781–787. Jul. [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the cns: tissue distribution, host-virus inateraction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. Apr 1. [DOI] [PubMed] [Google Scholar]
- Benameur K., Agarwal A., Auld S.C., et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 2020;26(9):2016–2021. doi: 10.3201/eid2609.202122. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J., James R., Rice C.M. Rhomboencephalitis. Pract. Neurol. 2020 doi: 10.1136/practneurol-2020-002680. Dec 8. [DOI] [PubMed] [Google Scholar]
- Curci C., Negrini F., Ferrillo M., et al. Functional outcome after inpatient rehabilitation in post-intensive care unit COVID-19 patients: findings and clinical implications from a real-practice retrospective study. Eur. J. Phys. Rehabil. Med. 2021 doi: 10.23736/s1973-9087.20.06660-5. Jan 4. [DOI] [PubMed] [Google Scholar]
- de Sire A., Giray E., Ozyemisci Taskiran O. Chelsea physical assessment tool for evaluating functioning in post-intensive care unit COVID-19 patients. J. Med. Virol. 2021;5:2620–2622. doi: 10.1002/jmv.26867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/s1474-4422(20)30221-0. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro F., Calafiore D., Dambruoso F., Guidarini S., de Sire A. COVID-19 related fatigue: which role for rehabilitation in post-COVID-19 patients? A case series. J. Med. Virol. 2021;93(4):1896–1899. doi: 10.1002/jmv.26717. Apr. [DOI] [PubMed] [Google Scholar]
- Guilmot A., Maldonado Slootjes S., Sellimi A., et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021;268(3):751–757. doi: 10.1007/s00415-020-10108-x. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/wnl.0000000000009619. Aug 4. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;23:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo A., McLoughlin B., Cheema S., et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J. Neurol. Neurosurg. Psychiatry. 2020;9:1013–1014. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- Lan S.H., Lai C.C., Huang H.T., Chang S.P., Lu L.C., Hsueh P.R. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2020;56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente Ayuso L., Torres Rubio P. Beijinho do Rosário RF, Giganto arroyo ML, sierra-Hidalgo F. Bickerstaff encephalitis after COVID-19. J. Neurol. 2020:1–3. doi: 10.1007/s00415-020-10201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattozzi S., Sabater L., Escudero D., et al. Hashimoto encephalopathy in the 21st century. Neurology. 2020;94(2):e217–e224. doi: 10.1212/WNL.0000000000008785. Jan 14. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/s0140-6736(20)30628-0. Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. May 1. [DOI] [PubMed] [Google Scholar]
- Muccioli L., Pensato U., Cani I., Guarino M., Cortelli P., Bisulli F. COVID-19-associated encephalopathy and cytokine-mediated Neuroinflammation. Ann. Neurol. 2020;88(4):860–861. doi: 10.1002/ana.25855. Oct. [DOI] [PubMed] [Google Scholar]
- Padroni M., Mastrangelo V., Asioli G.M., et al. Guillain-Barré syndrome following COVID-19: new infection, old complication? J. Neurol. 2020;267(7):1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020;10:2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picod A., Dinkelacker V., Savatovsky J., Trouiller P., Guéguen A., Engrand N. SARS-CoV-2-associated encephalitis: arguments for a post-infectious mechanism. Crit. Care. 2020;24(1):658. doi: 10.1186/s13054-020-03389-1. Nov 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–e120. doi: 10.1148/radiol.2020201187. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/wnl.0000000000009937. Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrizaila N., Yuki N. Bickerstaff brainstem encephalitis and fisher syndrome: anti-GQ1b antibody syndrome. J. Neurol. Neurosurg. Psychiatry. 2013;84(5):576–583. doi: 10.1136/jnnp-2012-302824. May. [DOI] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., et al. Guillain-Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;26:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzun S., Keles A., Okutan D., Yildiran T., Palamar D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur. J. Phys. Rehabil. Med. 2021 doi: 10.23736/s1973-9087.20.06563-6. Jan 4. [DOI] [PubMed] [Google Scholar]
- Wong P.F., Craik S., Newman P., et al. Lessons of the month 1: A case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin. Med. 2020;20(3):293–294. doi: 10.7861/clinmed.2020-0182. May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Han T., Chen J., et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin. Transl. Sci. 2020;13(6):1077–1086. doi: 10.1111/cts.12805. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]