Abstract
Purpose
Nattokinase (NK), an active ingredient extracted from traditional food Natto, has been studied for prevention and treatment of cardiovascular diseases due to various vasoprotective effects, including fibrinolytic, antihypertensive, anti-atherosclerotic, antiplatelet, and anti-inflammatory activities. Here, we reported an antineovascular effect of NK against experimental retinal neovascularization.
Methods
The inhibitory effect of NK against retinal neovascularization was evaluated using an oxygen-induced retinopathy murine model. Expressions of Nrf2/HO-1 signaling and glial activation in the NK-treated retinae were measured. We also investigated cell proliferation and migration of human umbilical vein endothelial cells (HUVECs) after NK administration.
Results
NK treatment significantly attenuated retinal neovascularization in the OIR retinae. Consistently, NK suppressed VEGF-induced cell proliferation and migration in a concentration-dependent manner in cultured vascular endothelial cells. NK ameliorated ischemic retinopathy partially via activating Nrf2/HO-1. In addition, NK orchestrated reactive gliosis and promoted microglial activation toward a reparative phenotype in ischemic retina. Treatment of NK exhibited no cell toxicity or anti-angiogenic effects in the normal retina.
Conclusions
Our results revealed the anti-angiogenic effect of NK against retinal neovascularization via modulating Nrf2/HO-1, glial activation and neuroinflammation, suggesting a promising alternative treatment strategy for retinal neovascularization.
Keywords: Nattokinase, retinal neovascularization, oxygen-induced retinopathy, Nrf2, glial activation
Ischemic retinopathy is the leading cause of irreversible visual impairment and blindness among neonates (retinopathy of prematurity), working age adults (diabetic retinopathy), and the elderly (retinal vein occlusion).1 The common hallmark of these diseases is the presence of pathological neovascularization. In past decades, targeting vascular endothelial growth factor (VEGF) has been widely adopted and proven to be effective for macular edema secondary to diabetic retinopathy and retinal vein occlusion.2 Despite being achieved in great success, VEGF-based strategies could have drawbacks, including drug resistance and macular ischemia/atrophy.3 Compensatory neovascularization by expression of VEGF-independent factors after anti-VEGF therapy has been demonstrated.4 In addition, reactive gliosis and neuroinflammation have been proposed as the novel mechanisms for retinal neovascularization.5 Herein, exploration of new anti-angiogenic agents is warranted, including natural products with anti-angiogenic properties.
Nattokinase (NK), an alkaline protease composed of 275 amino acid residues, is the most active constituent in Natto, a traditional food made from soybean fermentation by Bacillus subtilis.6 NK has been developed for the prevention and health care of cardiovascular diseases because of its potent fibrinolytic and antithrombotic effects, as well as the profile of oral availability and safety.7 From the experimental and clinical studies, NK has recently been shown to exert multiple biological functions, including anti-atherosclerotic, antihypertensive, antiplatelet, lipid-lowering, anti-inflammatory, and neuroprotective activities.8–12 Based on the multiple preventive pharmacologic properties of NK, we hypothesized that it could possess vasoprotective effects by attenuating retinal neovascularization.
Recent studies have demonstrated the beneficial effects of NK in oxidative stress injury and LPS-induced inflammation,13 which plays a critical role in the regulation of retinal neovascularization.14 Nuclear factor-erythroid 2-related factor-2 (Nrf2), a cytoprotective transcriptional factor involved in the regulation of stress-induced antioxidant and anti-inflammatory responses, is a target molecule in the treatment of retinal pathological angiogenesis and hyperpermeability.15 Exogenous activation of Nrf2 could promote reparative angiogenesis, suggesting a potential therapeutic strategy for retinal neovascularization.16 In addition, the inflammatory mediators, such as TNF-α and IL-1β, initiated by oxidative stress could further trigger reactive oxygen species production, leading to a vicious feedback loop.17
In the present study, we aimed to assess the inhibitory effect of NK against retinal neovascularization using an oxygen-induced retinopathy (OIR) model and cultured vascular cells. The degree of retinal neovascularization as well as the activation of Nrf2/HO-1 pathway and glia-mediated neuroinflammation were evaluated upon the treatment of NK.
Methods
Oxygen-Induced Retinopathy Murine Model
C57BL/6J mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Animals were kept in a specific pathogen-free facility. All the experiments were conducted in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research and were approved by the local Animal Ethic Committee of Joint Shantou International Eye Center of Shantou University and The Chinese University of Hong Kong. The OIR model was established according to a previously described method.18 Briefly, mouse pups and their nursing mother were exposed to 75% oxygen using an Oxy Cycler system (BioSpherix, Inc., Parish, NY, USA) from postnatal day 7 (P7) to P12 and then returned to room air. Intravitreal injection of NK (AbMole BioScience, Houston, TX, USA; 1 µM dissolved in 10% [v/v] DMSO in PBS) or control vehicle (the same solvent) was performed at P12 and P14 in the right eye using a 5-µL Hamilton syringe with a 33-gauge needle (n = 6 in each group). For Nrf2 inhibition, Brusatol (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 100 nM was injected intravitreally together with NK at P12 and P14. For treatment with intraperitoneal injection, NK (5 µM) or control vehicle was injected intraperitoneally once a day from P12 to P16. At P17, mice were euthanized and the eyeballs were enucleated for further investigations. Mice under 6 g at P17 were excluded from further analysis.
Immunofluorescence Assay
For retinal cryosections, eyes were enucleated and embedded in optimal cutting temperature compound overnight. Eight-µm serial cryosections were prepared. The slices were probed with primary antibodies (1:100) at 4°C overnight followed by incubation with fluorescence-labeled secondary antibodies (1:1,000 for1 hour). For retinal wholemounts, eyes were fixed in 4% paraformaldehyde for 30 minutes and the retinae were carefully removed. After incubated with primary (1:100, 4°C, overnight) and respective secondary antibodies (1:1000 for 1 hour), and counterstained with 4′6-diamidino-2-phenylindole (DAPI, 1:1000 for 5 minutes), the retinae were washed extensively and flat-mounted on the slides. The primary antibodies include Isolectin (Invitrogen, Waltham, MA, USA), anti-Nrf2 antibody (Abcam, Cambridge, United Kingdom), anti-HO-1 antibody (Abcam), anti-Iba1 antibody (Wako Chemicals, Osaka, Japan), anti-GFAP antibody (Abcam), anti-CD86 antibody (Abcam), and anti-CD206 antibody (Santa Cruz Biotechnology, Dallas, TX, USA). The sections and flat mounts were observed using a confocal microscope (Carl Zeiss LSM700-ZEN 2009, Germany) or an automated upright fluorescence camera (Leica DM4000B, Solms, Germany). Areas of neovascularization were assessed using Image-Pro Plus 6.0 (Media Cybernetic, Rockville, MD, USA).
Retro-Orbital Injection of Fluorescein Isothiocyanate-Dextran
The OIR mouse pups at P17 were anesthetized with 10% chloral hydrate. The peri-orbital area of the right eye was gently exposed by two fingers. Fluorescein isothiocyanate (FITC)-dextran (50 mg/mL in 0.05 mL, molecular weight = 2000; Sigma-Aldrich) was then injected into the mouse's orbital venous sinus using a 27-gauge needle with a 1 mL syringe.19 After 5 minutes, the eyeballs were enucleated. The whole-mount retinae were carefully removed for imaging. There were six eyes in each group. The vascular leakage of retinal microvessels was calculated as the percentage of leakage area to the total area.
Western Blotting Assay
Retinal and cellular protein was harvested and homogenized in lysis buffer containing protease and phosphatase inhibitor mini tablets (Thermo Fisher Scientific, Waltham, MA, USA). The protein concentration was determined with bicinchoninic acid protein assay. Equal amounts of protein were loaded and Western blotting was performed as previously described. The gray intensity of proteins was measured using Image J software (National Institutes of Health, 9000 Rockville Pike, Bethesda, MD, USA). Primary antibodies include Anti-Nrf2 antibody (Abcam), anti-HO-1 antibody (Abcam), anti-VEGF antibody (Abcam), anti-bFGF antibody (Abcam), anti-IL-17 antibody (Abcam), anti-IL-6 antibody (Abcam), anti-TNF-α antibody (Abcam), and anti-MCP-1 antibody (Abcam). Nuclear expression of Nrf2 was detected using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher, No. 78833).
Hematoxylin-Eosin Staining
The eyeballs were fixed with 4% formalin overnight, embedded in paraffin and cut into 3-µm vertical slices. Sections were washed and stained with hematoxylin buffer for 10 minutess at room temperature. The sections were rinsed with deionized water and dipped in 1% Eosin solution for 15 seconds. After being rehydrated in alcohol gradient, the slices were washed and mounted. Histological analyses of retinal tissues were imaged under a light microscope (Leica DM4000B, Solms, Germany) for calculating neovascular cell nuclei beyond the inner limiting membrane.
Tunel Assay
The eyeballs were embedded in optimal cutting temperature compound overnight and sectioned. TUNEL staining (In Situ Cell Death Detection Kit, Fluorescein; Roche, IN, USA) was performed according to the manufacturer's instructions. The sections were photographed using a confocal microscope. For quantitative analysis, TUNEL+ cell nuclei were counted in six microscopic fields from six sections in each group.
Human Umbilical Vein Endothelial Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA; cat. no. CRL-1730) and cultured in tissue culture flasks (Corning, Lowell, MA, USA) coated with 0.5 µg/cm2 human fibronectin (HFN; Millipore, Billerica, MA, USA) in complete medium (CM) consisting of Endothelial Basal Medium (EBM-2MV, Lonza, Basel, Switzerland) supplemented with Single Quot Bullet Kits (Lonza) and 10% fetal bovine serum (FBS, PAA Laboratories, Pasching, Austria). Cells used for experiments were with five passages. EBM-2MV supplemented with hydrocortisone and ascorbic acid, but without serum or growth factors, was used to starve the cells.
Tube Formation Assay
Tube formation assays were carried out as described previously.20 HUVECs were starved for 24 hours in BM, harvested by trypsin detachment, seeded at a density of 5000 cells/well in 24-well plates precoated with Matrigel, and were treated with NK (1 or 2 µM) and/or Brusatol (0.1 µM) for 2 hours before being incubated in medium supplemented with 10 ng/mL VEGF. The images were taken at fourfold magnification using an inverted light microscope. The extent of tube formation was quantified by counting the number of meshes using ImageJ software.
Scratch Wound Assay
The scratch wound assay was performed as described previously.21 HUVECs were seeded at a density of 1 × 105 cells per well in 6-well culture plates and incubated at 37°C in 5% CO2 for 24 hours to create confluent monolayers. The monolayers were scratched using a sterile pipette tip and treated with NK (1 or 2 µM) and/or Brusatol (0.1 µM). After 2 hours, VEGF was added into the medium at a final concentration of 10 ng/mL. Before and after the 18-hour NK treatment, the cells were imaged, and cell migration was determined by the number of cells migrating into the scratched area.
Cell Transfection
Nrf2-siRNA constructs were diluted in buffer (Lipofectamine transfection reagent; Invitrogen Inc., Carlsbad, CA, USA) and transfected in culture medium of HUVECs at a working concentration of 100 nM for 24 hours before NK treatment. Phosphate buffer saline (PBS) and scrambled siRNA were used as control. The transfection efficacy was evaluated by western blot and found to be satisfactory (data not shown). The sequences for Nrf2-siRNA were 5ʹ-CCAACCAGUUGACAGUGAACUCAUU-3ʹ.
Statistical Analysis
All experiments were repeated three times. Data are presented as mean ± standard deviation (SD). Two-tailed Student's t-test was used to compare the differences between the two groups, and 1-way analysis of variance (ANOVA) was carried out to compare multiple groups with post hoc analysis. P < 0.05 was considered statistically significant.
Results
NK Reduced Retinal Neovascularization in the OIR Model
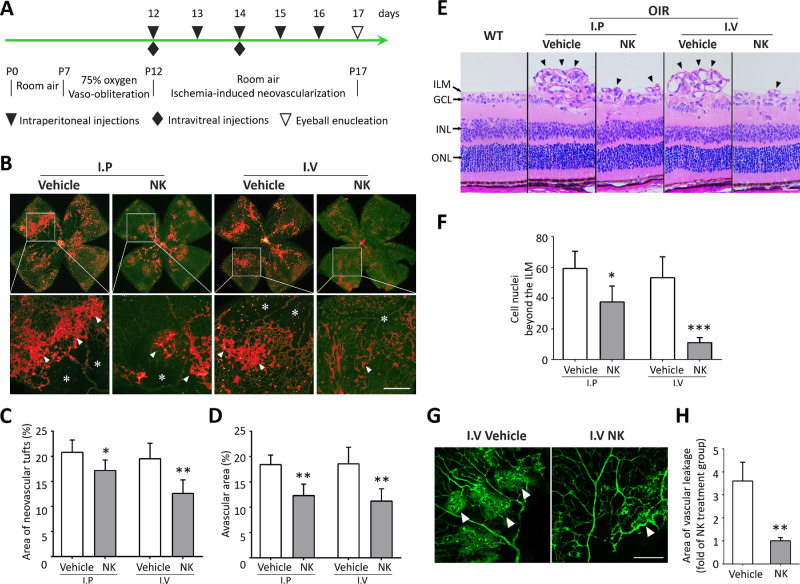
The anti-angiogenic effect of NK was evaluated using the murine model of OIR (Fig. 1A). NK was administered by either intravitreal injection or intraperitoneal injection. Isolectin staining on retinal whole-mounts on P17 was performed, which showed reduced neovascular tufts after NK treatment, particularly in the intravitreal injection group (Figs. 1B, 1C). Decreased avascular area was also observed, indicating a potential role of NK in promoting reparative angiogenesis. We also investigated retinal neovascularization by quantifying the number of neovascular nuclei on retinal sections. Consistently, the NK-treated retina presented with significantly less neovascular cells, in which intravitreal injection achieved better outcomes (Figs. 1D, 1E). Based on these results, intravitreal injection was used for NK administration in subsequent studies. We next investigated whether NK could improve vascular hyperpermeability, a distinctive feature of pathological angiogenesis. As shown, obvious leakage of FITC-dextran was observed in the OIR retinae, which could be largely attenuated after the treatment of NK (Figs. 1G, 1H). These data provide substantial evidence for the inhibitory effect of NK against retinal neovascularization.
Figure 1.
Nattokinase (NK) attenuated retinal neovascularization and vascular leakage in the oxygen-induced retinopathy (OIR) model. (A) The protocol showing the establishment of OIR model and treatment of NK in mice. (B) Representative images of Isolectin staining on retinal whole mounts with magnification in the region framed by white square. Neovascularization tufts (arrowheads) and avascular area (asterisks) were indicated in the magnified images. Scale bar: 50 µm. (C, D) Neovascularization and avascular area were quantified. There were six retinae in each group. (E) Representative images of H&E staining showing neovascular cells beyond the inner limiting membrane (arrowheads) in the retina. ILM, inner limiting membrane; GCL, ganglion cell layer; INL, inner nuclear layer; ONL: outer nuclear layer. Scale bar: 50 µm. (F) Neovascular cells beyond the ILM were analyzed. There were six retinae and six microscope fields per eye in each group. (G, H) Assessment of vascular leakage using retro-orbital injection of FITC-dextran. There were six retinae and six microscope fields per eye in each group were analyzed. Scale bar: 100 µm. I.P., intraperitoneal injection; I.V., intravitreal injection. Data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
NK Suppressed Neovascularization Via Activating Nrf2/HO-1 Pathway
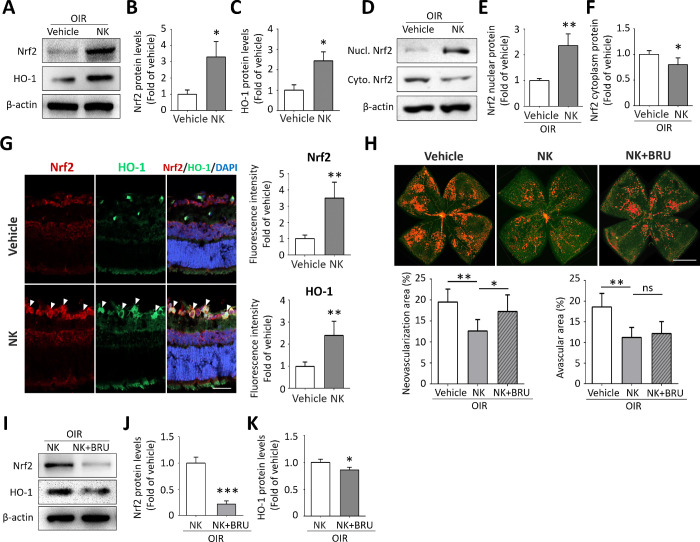
To explore the underlying molecular mechanism, we identified a significant increase in the expression of Nrf2 and its downstream target hemeoxygenase1-1 (HO-1) with NK treatment (Figs. 2A–C). Nrf2 activation was further clarified with increased Nrf2 nuclear translocation (Figs. 2D–F). Consistently, co-staining of Nrf2 and HO-1 confirmed their upregulation in the NK-treated retinae. Of note, Nrf2 and HO-1 were found mainly in the inner layer, where retinal ganglion cells and vascular endothelial cells locate (Fig. 2G). We next investigated whether NK worked through Nrf2/HO-1 signaling by combining NK with intravitreal injection of Brusatol, a specific inhibitor of Nrf2. As a result, Nrf2 inhibition largely abolished the anti-angiogenic effect of NK. Notably, the neovascularization tufts were found mainly in the central and mid-peripheral area of the retina, compared with that located in the mid-peripheral and peripheral area in the vehicle-treated retina. However, the inhibition of Nrf2 did not affect the avascular area in the NK-treated retina (Fig. 2H). Blockade of Nrf2 also significantly downregulated HO-1 expression in the NK-treated retina, further suggesting the involvement of Nrf2/HO-1 pathway in NK treatment (Figs. 2I–K). These data showed that NK inhibited retinal neovascularization, at least partially, through the activation of the Nrf2/HO-1 pathway.
Figure 2.
Nrf2/HO-1 signaling was responsible for the anti-neovascular effect of Nattokinase (NK). (A–C) NK treatment reduced protein expression of Nrf2 and HO-1 in the OIR retinae at P17 (n = 3). (D–F) NK-induced nuclear translocation of Nrf2 was observed by Western blot using the NE-PERTM Nuclear and Cytoplasmic Extraction Reagents. (G) Double immunofluorescent staining showing increased intensity of Nrf2 and HO-1 in the inner retinal layer. Scale bar: 50 µm. (H) Representative images of Isolectin staining on retinal whole mounts in the OIR retinae. Note that Nrf2 blockade by BRU (Brusatol) diminished the anti-neovascularization effect of NK in the OIR retina. Scale bar: 1 mm. Neovascularization and avascular area in retina treated with NK and BRU were quantified. There were six retinae in each group. (I–K) Co-administration of BRU reduced levels of Nrf2 and HO-1 in the NK-treated retinae (n = 3). Data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
NK Regulated Reactive Gliosis and Increased Reparative Microglia
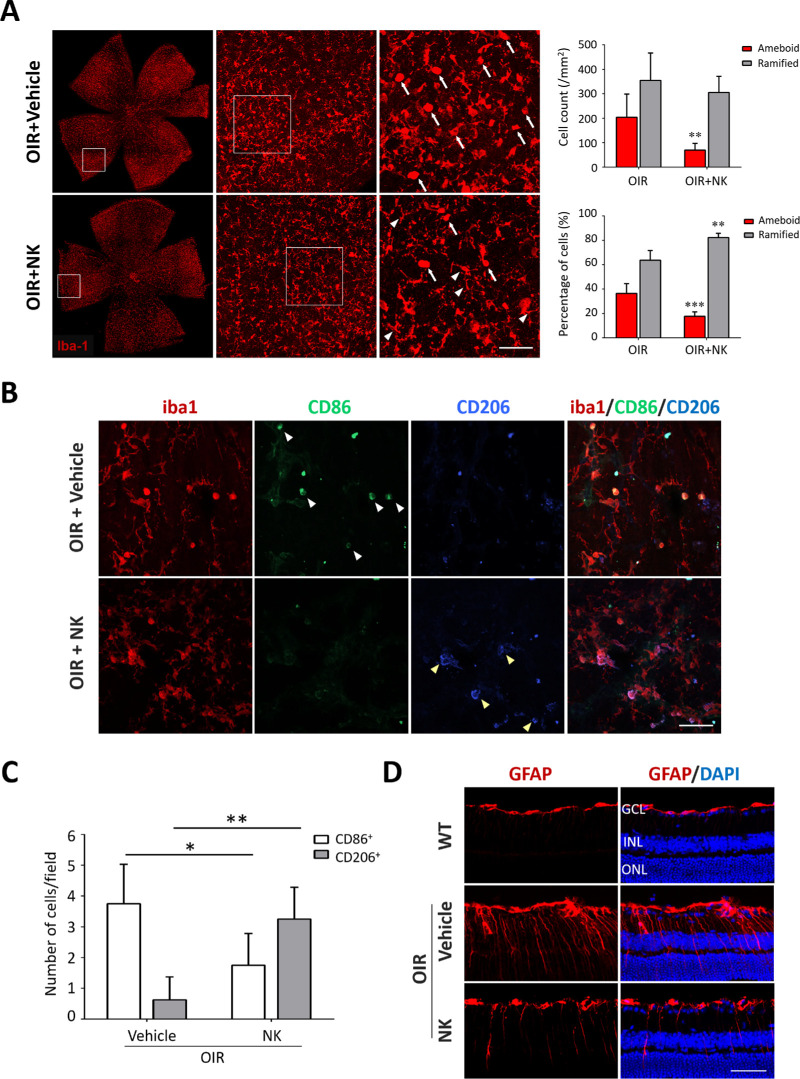
Glial activation is the hallmark of retinal neuroinflammation. To investigate whether NK could regulate glial activation and neuroinflammation in the OIR retinae, we carried out immunofluorescence staining on Iba1 and GFAP, the markers for microglia and astrocytes/Müller cells, respectively. Of note, the activated microglia in the OIR presented with enlarged somas and short lamellipodia, as compared with the resting ones with small cell bodies and long processes. Treatment of NK decreased the number of ameboid microglia (Fig. 3A). Because microglia exhibited a shift from an anti-inflammatory and neuroprotective phenotype to a pro-inflammatory and detrimental state in the central nervous system (CNS) diseases,22 we investigated the alteration of these microglia subsets using specific markers and found that NK treatment remarkably decreased pro-inflammatory while increased reparative microglia (Figs. 3B, 3C). In addition, enhanced GFAP immunoreactivity indicates reactive gliosis in astrocytes and Müller cells. In the OIR retinae, GFAP labeling demonstrated axial extent through the retina and much intensity in the astrocytes along the inner layer. NK markedly reduced GFAP immunoreactivity in astrocytes and Müller cell processes (Fig. 3D).
Figure 3.
Nattokinase (NK) orchestrated reactive gliosis and skewed M2 polarization of microglia. (A) Iba1+ microglia presented an activated “ameboid” phenotype in the OIR retina, featured with enlarged somas and short lamellipodia, compared with that presented small cell bodies and long processes in the normal retina. NK treatment reduced the number of ameboid microglia. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar: 50 µm. (B) Immunostaining of GFAP was used to mark astrocytes and Müller cells in the retina. In normal retina, GFAP was restricted to astrocytes in the GCL. In the OIR, intensive GFAP labeling on Müller cell showing axial extent throughout the retina was shown. Increased GFAP immunoreactivity in astrocytes around the blood vessels in the inner layer was noted. Scale bar: 50 µm. (C) Representative images of triple immunostaining on retinal whole mounts. Treatment of NK increased the expression of CD86 (white arrowheads), a marker for M1 microglia, whereas decreased the expression of CD206 (yellow arrowheads), a marker for M2 microglia. Scale bar: 50 µm. (D) The number of M1 microglia (iba1+CD86+) and M2 microglia (iba1+CD206+) was analyzed. There were three retinae and six microscope fields per eye in each group. Data are shown as mean ± SD. *P < 0.05, **P < 0.01.
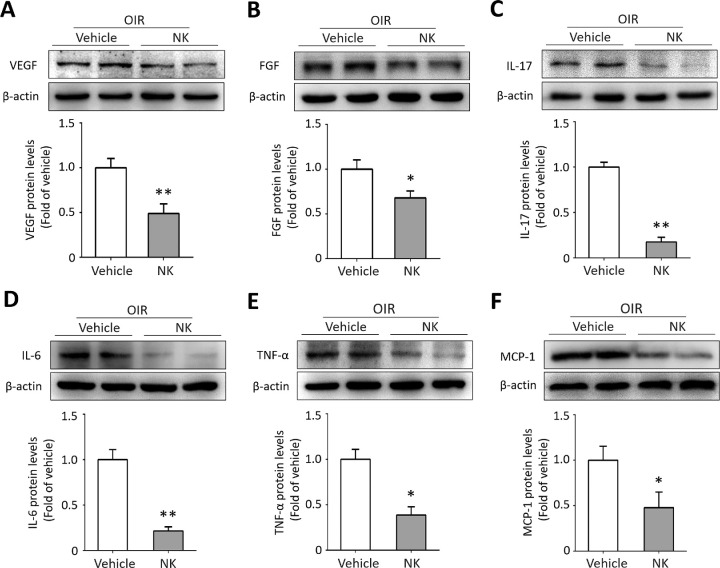
The ischemia insult elicits a robust amount of pro-angiogenic and inflammatory cytokines in the retina. We investigated the effect of NK on production of several cytokines. As expected, VEGF and bFGF, the two major pro-angiogenic factors, and the pro-inflammatory factors including IL-17, IL-6, and TNF-α cytokines, and MCP-1 chemokine, were downregulated with NK administration (Fig. 4). Collectively, these data indicated the role of NK in modulating reactive gliosis and neuroinflammation in the OIR retinae.
Figure 4.
Nattokinase (NK) reduced levels of proangiogenic factors and proinflammatory cytokines in the OIR. The relative intensity of Western blot assay is defined as the fold change of protein levels with β-actin. NK treatment significantly reduced expressions of VEGF (A), FGF (B), IL-17 (C), IL-6 (D), TNF-α (E), and MCP-1 (F) in the OIR retina. Six retinae from six mice in each group were used for Western blot (2 retinae per sample and 3 independent samples). One sample from each group was loaded for two consecutive lanes. Data are shown as mean ± SD. *P < 0.05, **P < 0.01.
NK Inhibited VEGF-Induced HUVEC Proliferation and Migration Via Nrf2 Activation
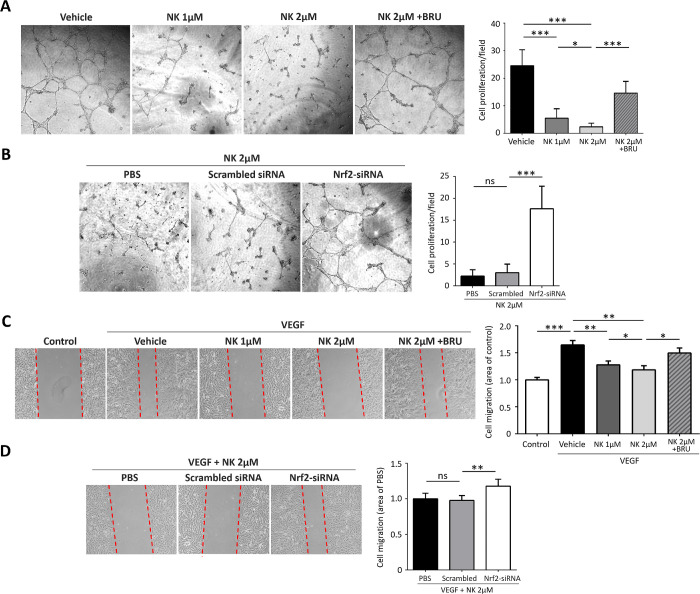
The in vitro effect of NK was explored by evaluating VEGF-induced proliferation and migration in HUVECs. Incubation of HUVECs with NK induced a significant decrease in cell proliferation and tube formation. However, co-treatment with NK and the Nrf2 inhibitor Brusatol largely abolished this effect, indicating that NK inhibited cell proliferation in part by activating Nrf2 (Fig. 5A). We next silenced Nrf2 expression using siRNA together with NK treatment to further confirm the involvement of Nrf2. As expected, Nrf2 deficiency promote tube formation in the NK-treated HUVECs (Fig. 5B). Consistently, pretreatment with NK inhibited VEGF-induced cell migration in HUVECs in a concentration-dependent manner, whereas Nrf2 inhibition or silencing partially reversed this process (Figs. 5C, 5D).
Figure 5.
Nattokinase (NK) inhibited proliferation and migration of human umbilical vein endothelial cells (HUVECs) in vitro. (A) Representative images and statistical analysis of VEGF-induced tube formation in HUVECs. Note that NK treatment significantly inhibited tube formation and that blockade of Nrf2 with BRU abolished this effect. (B) Nrf2 silencing using siRNA largely abolished the effect of NK against HUVEC proliferation. (C) Representative images and quantitative data of wounded monolayer of HUVECs with and without treatments. NK attenuated VEGF-induced HUVEC migration which was partially abolished by BRU. (D) Nrf2 silencing using siRNA partially promoted migration in NK-treated HUVECs. BRU, the specific Nrf2 inhibitor Brusotal. Data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
NK Treatment had No Visible Retinal Toxicity or Anti-Angiogenic Effects in Normal Vessels
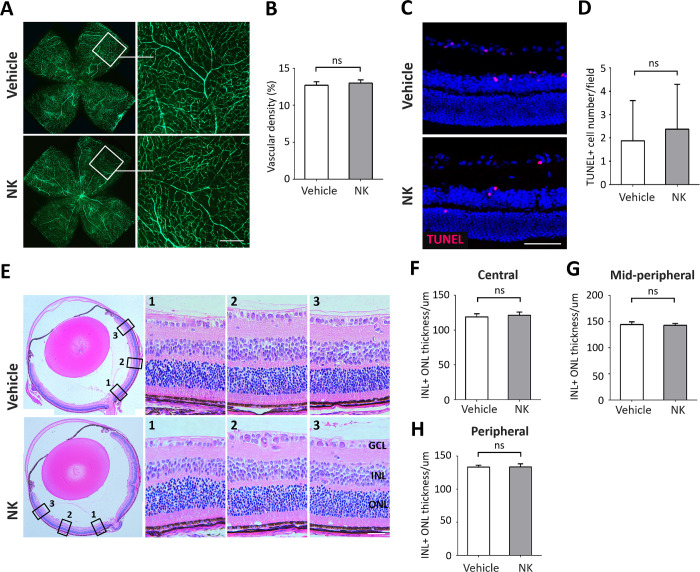
We administered NK intravitreally in normal mice and assessed the toxicity and anti-angiogenic effect of NK in the normal retina. Treatment with NK did not affect the density and morphology of retinal microvasculature as compared with vehicle-treated control (Figs. 6A, 6B). Injection of NK did not induce cell death (Figs. 6C, 6D) and had no impact on the structure and thickness in each layer of retina (Figs. 6E–H). These results confirmed the safety of NK administration in the eye.
Figure 6.
Nattokinase (NK) had no toxicity or antiangiogenic effect on the normal retina. Intravitreal injection of NK was performed at P12 and P14 in normal mice. Retina were removed for investigation at P17. (A) Assessment of retinal vessels using retro-orbital injection of FITC-dextran. No significant changes in vascular morphology with NK treatment were observed. Scale bar: 100 µm. (B) Statistical analysis on vascular density were shown. There were six retina and six microscope fields per eye in each group. (C) Images of retinal sections with TUNEL assay were shown. Scale bar: 50 µm. (D) Quantitative data showing TUNEL+ apoptotic cells in the retina. There were six retinae and six microscope fields per eye in each group. (E) A panoramic view of the whole retinal sections with H&E staining, with magnification in the central (location 1), mid-peripheral (location 2), and peripheral (location 3) regions framed by black square. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scare bar: 50 µm. (F, G) The total thicknesses in different regions of retina were measured and no significant changes were found. There were six retinae and six microscope fields per retina in each group were used for analysis. Data are shown as mean ± SD. ns, no significance.
Discussion
Since its discovery in 1987,23 NK, the most active ingredient of Natto, has been proved to exert multiple protective effects on cardiovascular health. Due to potent fibrinolytic and antithrombotic activities as well as antihypertensive, anti-atherosclerotic, lipid-lowering, and anti-apoptotic actions, NK is considered a promising alternative therapy in prevention and treatment of cardiovascular diseases.7 In addition, Chang et al. found less apoptosis and improved viability of vascular endothelia in the laser irradiation damage model, suggesting a vasoprotective effect of NK.24 In this study, we revealed the anti-angiogenic effect of NK against retinal neovascularization, indicating an antineovascular effect of this natural product.
The stress-response transcription factor Nrf2 and its downstream target HO-1 are well known for their antioxidative and cytoprotective properties. In ischemic retinopathy, Nrf2 was found highly expressed in the early period and then depleted continually with progression of the ischemia insults,16 indicating the breakdown of anti-ischemic defense system. Activation of Nrf2/HO-1 axis attenuated ischemia reperfusion injury and improved neurological function.25,26 In addition, Nrf2 was found to regulate ischemic revascularization and neurovascular repair, suggesting a potential therapeutic strategy for ischemic CNS diseases.16,27 Recently, the role of Nrf2 in retinal neovascularization has been revealed using RS9, a specific Nrf2 activator.15 These results also suggest a strong correlation between Nrf2 and ischemic retinopathy.
In this study, treatment of NK activated the Nrf2/HO-1 pathway in the OIR retina, whereas Nrf2 inhibition blocked the anti-angiogenic effect of NK, implicating that NK attenuated retinal neovascularization at least partially through the Nrf2/HO-1 pathway. In addition, we noted that Nrf2 and HO-1 mainly located in the inner retinal layer around the neovascular tufts, which was consistent with previous study that Nrf2 was expressed in vascular endothelial cells and retinal ganglion cells.16 The anti-angiogenic effect of NK via Nrf2 was further confirmed by our in vitro experiment that inhibition of Nrf2 largely abolished the inhibitory effect of NK on the migration, proliferation, and tube formation of HUVECs.
The anti-inflammatory and anti-apoptotic effects of NK on neurological diseases have also been investigated. Several studies on animal model of Alzheimer's disease found improved learning and memory capability with suppression of proinflammatory IL-6 and increase of anti-apoptotic Bcl-2 activity.11,28 Besides, treatment with NK could reduce the production of TNF-α and rescue neurons from apoptosis with peripheral nerve injury.29 The concept of inflammation-associated angiogenesis has recently been proposed and is believed to play a critical role in ischemic retinopathy.30 In this study, we investigated the anti-inflammatory effect of NK in the OIR retinae and found consistent results that NK decreased the levels of the critical proinflammatory cytokines and chemokines, of which IL-17 has been shown to promote ischemic retinopathy.31 Of note, NK mitigated neuroinflammation probably through modulating glial activation. Microglia activation toward a proinflammatory phenotype is considered the hallmark of retinal and CNS neuroinflammation.32 NK switched proinflammatory microglia into an anti-inflammatory and reparative state, implicating a potential immunoregulation function of this natural agent.
Hyperpermeability is a distinctive feature of pathological angiogenesis, which is directly responsible for retinal edema and visual loss.33 In this study, treatment of NK was able to suppress vascular leakage in the OIR. The role of Nrf2 signaling in regulating CNS vasopermeability has been proposed.34 In the retina, Nrf2 supplementation increased the expression levels of tight junction proteins and pericytes15 via defending reactive oxygen species or inflammation,35 indicating the capability of Nrf2 to protect the blood-retinal barrier. Based on these studies, we postulated that the antihyperpermeability activity of NK is, at least in part, due to the enhancement of Nrf2 expression.
NK was also found to exert the fibrinolytic and anti-apoptotic effects in a dose-dependent manner.36,37 Consistently, our in vitro results showed NK inhibited HUVEC proliferation and migration in a concentration-dependent manner. However, increasing dosage does not lead to more significant antineovascular effect in mouse models (data not shown). In addition, treatment of NK via intravitreal injection has stronger anti-angiogenic effect than intraperitoneal injection. The superiority of intravitreal NK delivery could be attributed to a higher local concentration in the retina tissue. In addition, it is noted that intravitreal injection of NK can induce posterior vitreous detachment.38 In diabetic retinopathy, a complete posterior vitreous detachment can reduce proliferation and neovascularization since it removes the “scaffolding” for new capillary growth and improves retinal ischemia by allowing the exchange of oxygen-rich aqueous fluid.39 These findings provide additional evidence for the superiority of NK administration via intravitreal injection than intraperitoneal injection. Finally, because NK can be administered orally, it is interesting to investigate the anti-angiogenic effect of NK through oral consumption.
One limitation of this study is that we deactivated Nrf2 signaling by using a specific inhibitor Brusatol. Although Brusatol is a very highly efficient suppressor of Nrf2 that functions by preventing Nrf2 from activation and migration into the nucleus,40 it can hardly bring a complete Nrf2 deficiency, as compared with using gene knock-out animals. Besides, there might be other potent signaling pathways involved in the NK-mediated effect against retinal neovascularization, although we detected levels of several pro-angiogenic and pro-inflammatory molecules including the HIF-1α, E-twenty six-1 (ETS-1), STAT3, AKT, and ERK1/2 signaling, which were found without significant alteration by NK treatment (Supplementary Fig. S1). Finally, the OIR model, although extensively used for assessment of experimental neovascularization, is not a precise correlate of human neovascular eye diseases. For instance, there is resolution of neovascularization in the late phase of OIR, which is seldom seen in human retinopathy of prematurity (ROP) diseases.41 In addition, studies have found glial activation and upregulation of proinflammatory cytokines, indicating the involvement of neuroinflammation in the OIR.42 Therefore, whether NK works for human neovascular diseases needs further investigation.
In summary, this study revealed the anti-angiogenic effect of NK against retinal neovascularization partially through the activation of Nrf2/HO-1 pathway and the regulation of glia-mediated neuroinflammation, suggesting its potential value to be developed as a new pharmaceutical product for the treatment of retinal neovascularization.
Supplementary Material
Acknowledgments
Supported by the Foundation for Distinguished Young Talents in Higher Education of Guangdong, China to Zijing Huang (No. 2018KQNCX076) and the Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program, China.
Disclosure: Z. Huang, None; T.K. Ng, None; W. Chen, None; X. Sun, None; D. Huang, None; D. Zheng, None; J. Yi, None; Y. Xu, None; X. Zhuang, None; S. Chen, None
References
- 1. Al-Shabrawey M, Elsherbiny M, Nussbaum J, Othman A, Megyerdi S, Tawfik A.. Targeting neovascularization in ischemic retinopathy: recent advances. Expert Rev Ophthalmol. 2013; 8: 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wells JA, Glassman AR, Ayala AR, et al.. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung EJ, Roh MI, Kwon OW, Koh HJ.. Effects of macular ischemia on the outcome of intravitreal bevacizumab therapy for diabetic macular edema. Retina. 2008; 28: 957–963. [DOI] [PubMed] [Google Scholar]
- 4. Bergers G, Hanahan D.. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenberg GA, Bjerke M, Wallin A.. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke. 2014; 45: 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Urano T, Ihara H, Umemura K, et al.. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis Cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem. 2001; 276: 24690–24696. [DOI] [PubMed] [Google Scholar]
- 7. Chen H, McGowan EM, Ren N, et al.. Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases. Biomark Insights. 2018; 13: 1177271918785130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren NN, Chen HJ, Li Y, McGowan GW, Lin YG.. A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia. Zhonghua Yi Xue Za Zhi. 2017; 97: 2038–2042. [DOI] [PubMed] [Google Scholar]
- 9. Fujita M, Ohnishi K, Takaoka S, Ogasawara K, Fukuyama R, Nakamuta H.. Antihypertensive effects of continuous oral administration of nattokinase and its fragments in spontaneously hypertensive rats. Biol Pharm Bull. 2011; 34: 1696–1701. [DOI] [PubMed] [Google Scholar]
- 10. Jang JY, Kim TS, Cai J, et al.. Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab Anim Res. 2013; 29: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fadl NN, Ahmed HH, Booles HF, Sayed AH.. Serrapeptase and nattokinase intervention for relieving Alzheimer's disease pathophysiology in rat model. Hum Exp Toxicol. 2013; 32: 721–735. [DOI] [PubMed] [Google Scholar]
- 12. Hsu RL, Lee KT, Wang JH, Lee LY, Chen RP.. Amyloid-degrading ability of nattokinase from Bacillus subtilis natto. J Agric Food Chem. 2009; 57: 503–508. [DOI] [PubMed] [Google Scholar]
- 13. Wu H, Wang Y, Zhang Y, et al.. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020; 32: 101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong A, Xie B, Shen J, et al.. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009; 219: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura S, Noguchi T, Inoue Y, et al.. Nrf2 activator RS9 suppresses pathological ocular angiogenesis and hyperpermeability. Invest Ophthalmol Vis Sci. 2019; 60: 1943–1952. [DOI] [PubMed] [Google Scholar]
- 16. Wei Y, Gong J, Xu Z, et al.. Nrf2 in ischemic neurons promotes retinal vascular regeneration through regulation of semaphorin 6A. Proc Natl Acad Sci USA. 2015; 112: E6927–E6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischer R, Maier O.. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. 2015; 2015: 610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Connor KM, Krah NM, Dennison RJ, et al.. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009; 4: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S, Li T, Luo Y, et al.. Retro-orbital injection of FITC-dextran is an effective and economical method for observing mouse retinal vessels. Mol Vis. 2011; 17: 3566–3573. [PMC free article] [PubMed] [Google Scholar]
- 20. Mohr T, Desser L.. Plant proteolytic enzyme papain abrogates angiogenic activation of human umbilical vein endothelial cells (HUVEC) in vitro. BMC Complement Altern Med. 2013; 13: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang C, Fu L, Fu J, et al.. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res. 2009; 15: 4017–4027. [DOI] [PubMed] [Google Scholar]
- 22. Tang Y, Le W.. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016; 53: 1181–1194. [DOI] [PubMed] [Google Scholar]
- 23. Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H.. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987; 43: 1110–1111. [DOI] [PubMed] [Google Scholar]
- 24. Chang CH, Chen KT, Lee TH, et al.. Effects of natto extract on endothelial injury in a rat model. Acta Med Okayama. 2010; 64: 399–406. [DOI] [PubMed] [Google Scholar]
- 25. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J.. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016; 73: 3221–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He M, Pan H, Chang RC, So KF, Brecha NC, Pu M.. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014; 9: e84800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei Y, Gong J, Xu Z, Duh EJ.. Nrf2 promotes reparative angiogenesis through regulation of NADPH oxidase-2 in oxygen-induced retinopathy. Free Radic Biol Med. 2016; 99: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhatt PC, Pathak S, Kumar V, Panda BP.. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer's disease by fermented soybean nanonutraceutical. Inflammopharmacology. 2018; 26: 105–118. [DOI] [PubMed] [Google Scholar]
- 29. Pan HC, Cheng FC, Chen CJ, et al.. Dietary supplement with fermented soybeans, natto, improved the neurobehavioral deficits after sciatic nerve injury in rats. Neurol Res. 2009; 31: 441–452. [DOI] [PubMed] [Google Scholar]
- 30. Wang T, Tsirukis DI, Sun Y.. Targeting neuroinflammation in neovascular retinal diseases. Front Pharmacol. 2020; 11: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Yang Z, Lai P, et al.. Bcl-6-directed follicular helper T cells promote vascular inflammatory injury in diabetic retinopathy. Theranostics. 2020; 10: 4250–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jha MK, Lee WH, Suk K.. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem Pharmacol. 2016; 103: 1–16. [DOI] [PubMed] [Google Scholar]
- 33. Inoue Y, Shimazawa M, Nakamura S, et al.. Both autocrine signaling and paracrine signaling of HB-EGF enhance ocular neovascularization. Arterioscler Thromb Vasc Biol. 2018; 38: 174–185. [DOI] [PubMed] [Google Scholar]
- 34. Zhao J, Moore AN, Redell JB, Dash PK.. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007; 27: 10240–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reyes JL, Molina-Jijón E, Rodríguez-Muñoz R, Bautista-García P, Debray-García Y, Namorado Mdel C. Tight junction proteins and oxidative stress in heavy metals-induced nephrotoxicity. Biomed Res Int. 2013; 2013: 730789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pais E, Alexy T, Holsworth RE Jr., Meiselman HJ. Effects of nattokinase, a pro-fibrinolytic enzyme, on red blood cell aggregation and whole blood viscosity. Clin Hemorheol Microcirc. 2006; 35: 139–142. [PubMed] [Google Scholar]
- 37. Ji H, Yu L, Liu K, et al.. Mechanisms of Nattokinase in protection of cerebral ischemia. Eur J Pharmacol. 2014; 745: 144–151. [DOI] [PubMed] [Google Scholar]
- 38. Takano A, Hirata A, Ogasawara K, et al.. Posterior vitreous detachment induced by nattokinase (subtilisin NAT): a novel enzyme for pharmacologic vitreolysis. Invest Ophthalmol Vis Sci. 2006; 47: 2075–2079. [DOI] [PubMed] [Google Scholar]
- 39. Akiba J, Arzabe CW, Trempe CL.. Posterior vitreous detachment and neovascularization in diabetic retinopathy. Ophthalmology. 1990; 97: 889–891. [DOI] [PubMed] [Google Scholar]
- 40. Cai SJ, Liu Y, Han S, Yang C.. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 2019; 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vähätupa M, Jääskeläinen N, Cerrada-Gimenez M, et al.. Oxygen-induced retinopathy model for ischemic retinal diseases in rodents [published online ahead of print September 16, 2020]. J Vis Exp, 10.3791/61482. [DOI] [PubMed] [Google Scholar]
- 42. Fischer F, Martin G, Agostini HT.. Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. J Neuroinflammation. 2011; 8: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.