Abstract
Purpose
To investigate the antifungal and anti-inflammatory effects of baicalein on Aspergillus fumigatus (A. fumigatus) keratitis and the underlying mechanisms.
Methods
The noncytotoxic antifungal concentration of baicalein was determined using CCK8, cell scratch assay, minimum inhibitory concentration, biofilm formation, scanning electron microscopy, propidium iodide uptake test and adherence assay in vitro and Draize test in vivo. In fungal keratitis (FK) mouse models, clinical score and plate count were used to evaluate FK severity, and myeloperoxidase assay and immunofluorescence staining were performed to examine neutrophil infiltration and activity. Real-time PCR, ELISA, and Western blot were performed to explore the anti-inflammatory activity of baicalein and the underlying mechanisms in vivo and in vitro.
Results
Baicalein at 0.25 mM (noncytotoxic) significantly inhibited A. fumigatus growth, biofilm formation, and adhesion in vitro. In A. fumigatus keratitis mice, baicalein mitigated FK severity, reduced fungal load, and inhibited neutrophil infiltration and activity. Baicalein not only suppressed mRNA and protein levels of proinflammatory factors IL-1β, IL-6, and TNF-α, but also inhibited the expression of thymic stromal lymphopoietin (TSLP) and TSLP receptor (TSLPR) in vivo and in vitro. In HCECs, mRNA and protein levels of IL-1β, IL-6, and TNF-α were significantly lower in the TSLP siRNA–treated group, while higher in the rTSLP-treated group than in the corresponding control. Baicalein treatment significantly inhibited rTSLP induced the expression of IL-1β, IL-6, and TNF-α.
Conclusions
Baicalein plays a protective role in mouse A. fumigatus keratitis by inhibiting fungal growth, biofilm formation, and adhesion, and suppressing inflammatory response via downregulation of the TSLP/TSLPR pathway.
Keywords: Baicalein, keratitis. Aspergillus fumigatus, TSLP; TSLPR
Fungal keratitis (FK) is a blinding corneal disease caused by fungal pathogens especially Fusarium and Aspergillus,1 which is becoming prevalent in developing countries associated with several risk factors such as trauma, long-term use of contact lenses, and corticosteroid abuse.2,3 However, current therapies like polyenes (natamycin, amphotericin) and azoles (voriconazole) have significant limitations,4 suggesting the need to explore new effective drugs.
Baicalein (5,6,7-trihydroxyflavone, BE) is a natural flavonoid, originally extracted from Scutellaria baicalensis, a Chinese herbal medicine that has been used for diarrhea, lung infections, and chronic liver disease for thousands of years.5–8 Recently, baicalein has been reported to have antifungal,9 antivirus,10 anti-inflammatory11 and anticancer12 effects in diverse disease models including hematological malignancies,13 neurodegenerative diseases,14 and inflammatory disorders.15 For example, baicalein has antifungal activity against Candida albicans and Candida krusei in vitro,16 and a combination of baicalein and amphotericin B could accelerate Candida albicans apoptosis.17 A recent preclinical study reported that baicalein inhibits SARS-CoV-2–induced lung injury by suppressing virus replication and inflammatory cell infiltration.10 In addition, baicalein exerts an anti-inflammatory effect on activated macrophages by inhibiting nitric oxide and cytokines such as interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and growth factors through modulating the endoplasmic reticulum stress CHOP/STAT pathway, suggesting it might be useful for inflammation related diseases.18 Moreover, baicalein inhibits non-small-cell lung cancer invasion and metastasis in the inflammatory microenvironment.19 However, whether baicalein has antifungal effect in vivo and how baicalein plays a role in FK have not been studied yet.
Although baicalein has been studied in various inflammation-related pathways,20 how it interacts with inflammatory mediators is still largely unknown. A recent study reported that baicalein is a novel thymic stromal lymphopoietin (TSLP) inhibitor through binding to the N-terminal helix in TSLP to break the interaction between TSLP and TSLPR, inhibiting the TSLP/TSLPR signaling pathway.21 TSLP is an epithelial cell-derived cytokine that acts as one of the central regulators of type 2 immunity and inflammatory diseases.22 In response to various stimuli including microbial infection, mechanical injury, and endogenous cytokines, TSLP expression and release in epithelial cells are increased,23 which in turn triggers the production of proinflammatory cytokines such as IL-4, IL-5, IL-9, IL-13, and TNF-α of the innate and adaptive immunity, driving various pathological inflammation.24–26 Notably, previous studies have shown that TSLP is involved in FK progression. Qingshan et al. reported that TSLP was highly expressed and secreted in Aspergillus fumigatus (A. fumigatus) infected human corneal epithelial cells (HCECs), facilitating FK development via inducing caspase-1-dependent pyroptosis.27 In addition, TSLP-activated dendritic cells could induce T helper type 2 (Th2) inflammation in mice with A. fumigatus keratitis.28 Inhibition of TSLP could mitigate the severity of FK, and downregulate neutrophil infiltration and the expression of proinflammatory factors IL-6 and IL-8, controlling excessive inflammation caused by corneal injury,29 which suggests TSLP could be a potential therapeutic target for FK treatment.
According to the existing evidence, we hypothesized that baicalein may exhibit antifungal and anti-inflammatory effects on FK, which could be associated with the TSLP/TSLPR pathway. This study investigated the role of baicalein in FK progression and its potential interactions with TSLP/TSLPR in A. fumigatus keratitis mouse models and HCECs.
Materials and Methods
Baicalein Solution Preparation
Baicalein powder (10 mg) purchased from MedChem Express (Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO, Solarbio, Beijing, China) at 125 mM, and further diluted using required solvents, including Dulbecco's Modified Eagle Medium (DMEM) for cell culture experiments, Sabourand Medium for in vitro fungal culture assays and phosphate-buffered saline (PBS) for animal experiments to obtain the desired concentrations of baicalein solution.
Cell Viability (Cell Counting Kit-8, CCK-8)
HCECs (obtained from the Ocular Surface Laboratory Zhongshan Ophthalmic Center, China) were inoculated in 96-well plates and grown for one to two days to 80% confluence (37°C, 5% CO2). Then HCECs were mixed with baicalein (0, 0.0625, 0.125, 0.25, 0.5 mM) or DMSO (0.2%) and incubated for 24 hours, followed by adding 10 µL of CCK-8 (Solarbio, Beijing, China) to each well and incubated for two hours. The absorbance at 450 nm was read using a microplate reader.
Cell Scratch Test
HCECs were seeded (3 × 105/mL) in 6-well plates with three parallel lines labeled by black marker on the bottom of each well. Then, three lines perpendicular to them were scratched using a 200 µL pipette tip in each well. HCECs per well were incubated with baicalein (0, 0.0625, 0.125, 0.25, and 0.5 mM) or DMSO (0.2%) for 24 hours. Cell migration was evaluated by measuring the width of the cell scratch at the same location at 0 and 24 hours under optical microscopy (Nikon, Tokyo, Japan, 100×). Experiments were repeated at least three times under the same conditions.30
Ocular Toxicology: The Draize Eye Test
Ocular toxicology study (Draize Eye Test) was performed to investigate the potential adverse effects of baicalein in normal mouse eyes. Baicalein (0.25, 0.4, and 0.5 mM) in dose of 5 µL was dropped into the conjunctival cul-de-sac of one eye in four mice. The contralateral eye treated with DMSO (0.2%) served as a control. Corneal fluorescein staining (CFS) was examined using slit-lamp microscopy under a cobalt blue light at one, three, and five days after corneal instillation in mice. CFS scores were evaluated according to the Organization for Economic and Cooperative Development (OECD) grading scale for ocular irritation. The scores involve weighting and summing six components of directly observable changes of the eye's anterior segment, including the density and area of corneal opacification, severity of iritis, conjunctival redness, edema, and discharge.31–36
Baicalein Minimum Inhibitory Concentration (MIC) on A. fumigatus
A. fumigatus conidia were prepared as described previously.37 Briefly, the conidia (China General Microbiological Culture Collection Center, Beijing, China) were rinsed using PBS to adjust the concentration to 5 × 107/mL and dilute to 5 × 105/mL for MIC. Baicalein was diluted in different concentrations (0.0625, 0.075, 0.125, 0.15, 0.25, and 0.5 mM) with Sabouraud medium containing spores and distributed to 96-well plates, and incubated at 37°C for 48 hours. The absorbance at 570 nm was measured and analyzed. Then, the supernatant in 96-well plates were discarded, and 50 µL of Calcofluor White Stain (Sigma-Aldrich) was added and incubated at room temperature (RT) for 30 minutes. The staining pictures were photographed using the fluorescence microscope (Nikon, Tokyo, Japan, 100×).
Propidium Iodide (PI) Staining
The conidia suspension of A. fumigatus (2 × 106 CFU/mL) was poured on a 6-well plate and incubated at 37°C for 24 hours. Then, the hyphae were washed, centrifuged (12,000 rpm, 10 minutes), and transferred to a new 6-well plate, and then incubated with 0.25 mM baicalein or 0.2% DMSO at 37°C for 24 hours. After harvesting and washing with sterile PBS, the fungal hyphae were incubated with PI (Sigma-Aldrich) solution for 15 minutes at RT in the dark. Images were photographed with fluorescence microscope (Nikon, Tokyo, Japan, 200×).38
Scanning Electron Microscopy (SEM)
A. fumigatus conidia (2 × 106 CFU/mL) was cultured in a 6-well plate (1 mL per well) at 37°C for 24 hours to form hyphae. Hyphae were washed, centrifuged (12,000 rpm, 10 minutes), and transferred to a new 6-well plate, and then incubated with 0.25 mM baicalein or 0.2% DMSO at 37°C for 24 hours. Then, hyphae were collected in EP tubes and fixed with 2.5% glutaraldehyde at 4°C for two hours. The samples were washed with PBS, then mixed with 1% (v/v) osmium tetroxide in PBS at 4°C for one hour. Subsequently, samples were gently dehydrated in graded ethanol, critical-point dried in CO2, coated with gold, and observed under SEM (JSM-840, JOEL Company, Japan) at 2000× and 5000× magnification (bar = 20 or 10 µm).9,38
Biofilm Formation and Inhibition Test
Quantitative determination of biofilm forming capacity was tested by a colorimetric microtiter plate assay.39 Briefly, the conidia of A. fumigatus were adjusted to a concentration of 5 × 105 CFU/mL. The suspension containing 90 µL Sabouraud medium with serial dilutions of baicalein (0.0625, 0.075, 0.125, 0.15, 0.25 and 0.5 mM) and 10 µL conidia were added to each well of a microtiter plate (in triplicate). Medium was added as the negative control for each plate. Subsequent to an incubation period of 48 hours at 37°C in aerobic conditions without shaking, wells were gently decanted and rinsed three times with 200 µL of sterile PBS (pH 7.2). After air drying, biofilms were fixed with 100 µL of 99% glutaraldehyde per well for 20 minutes, dried, and stained with 100 µL per well of 0.1% crystal violet (CV; Sigma-Aldrich) for 15 minutes. Unbound dye was rinsed with PBS. After air drying, dye bound to biofilm formed on the chamber was released with 100 µL of 33% acetic acid per well for 30 minutes at RT, without shaking. OD (Optical Density) of each well was measured at 620 nm using a microtiter plate reader.
Adhesion of A. fumigatus to HCECs
The chambered slides (four/slide) were inoculated with 1 mL complete medium containing HCECs and incubated overnight. Cell chambers were washed twice with sterile PBS, then 1 mL fresh media was added without antibiotics. Conidia suspension (2 × 105/mL) was combined with baicalein (0.25 mM) or DMSO immediately before application to the chambered slides. Each slide was incubated for three hours at 37°C under aerobic conditions. Then slides were washed three times with sterile PBS. After air drying, each slide was stained through hematoxylin and eosin (HE). The number of spores which adhered to the surface of HCECs were counted, averaged, and expressed as the number of adherent spores/cell. All specimens were observed and photographed by microscopy (Nikon, Tokyo, Japan, 400×).40
Cell Culture and A. fumigatus Stimulation
HCECs seeded in 12-well plates or 6-well plates were incubated at 37°C for 48 hours. Then, the cells were first stimulated with inactivated A. fumigatus hyphae (5 × 106 CFU/mL) for one hour, and treated with 0.25 mM baicalein or DMSO. After four hours of A. fumigatus stimulation, cells were collected for real-time PCR (RT-PCR) to detect mRNA levels of TSLP, TSLPR, IL-1β, IL-6 and TNF-α. After 24 hours of A. fumigatus stimulation, HCECs protein were collected for Western blot to detect TSLP protein level, and the supernatant were collected for enzyme-linked immunosorbent assay (ELISA) to detect protein levels of IL-1β, IL-6 and TNF-α.
TSLP RNA Interference (TSLP siRNA) and Recombinant TSLP (rTSLP) Treatment
TSLP siRNA was used to block TSP expression. HCECs suspensions of 1 × 105/mL seeded into 12- or 6-well plates were treated with TSLP siRNA (50 nM/mL, Ribobio, Guangzhou, China) or scramble siRNA (50 nM/mL, Ribobio, Guangzhou, China) for 48 hours. Cells were harvested for RT-PCR to evaluate transfection efficiency. HCECs were treated with human rTSLP (100 ng/mL, Peprotech, USA) or IgG (100 ng/mL, Bioss, China) for 12 hours. After transfection, cells were treated with A. fumigatus for one hour, then followed by 0.25 mM baicalein treatment. Then, cells were harvested after three hours, and cell protein and supernatant were collected 23 hours later for the future use.41
FK Murine Models
All treatments were performed on C57BL/6 mice (female, eight weeks, Jinan Pengyue Laboratory) according to the ARVO Statement regarding the Use of Animals in Ophthalmology and Vision Research. The method of establishing the FK mouse model was based on Che et al.42 First, each mouse was abdominally anesthetized with 4 mL of 8% chloral hydrate. Each left eye was a blank control. The right corneal stroma was injected with 2.5 µL A. fumigatus conidia suspension (2.5 × 106 CFU/mL) with a micro syringe. In the baicalein treatment group, 5 µL baicalein (0.25 mM) was spotted on the right eye three times a day, and 5 µL DMSO (0.2%) was spotted on the right eye three times a day as control. Based on the observation under a slit lamp at one, three, and five days post infection (p.i.), the severity of keratitis was evaluated by clinical score referred to Wu et al.43 The total score of 0 to 5 is mild infection, 6 to 9 is moderate infection, and 10 to 12 is severe infection. The corneas at days one, three, and five p.i. were removed for RT-PCR, ELISA, and Western blot. The corneas infected at three days were removed for Myeloperoxidase (MPO). The corneas infected at five days were used for plate count. The mouse eyeballs infected at three days were removed for immunofluorescence (IF).
Plate Count
The mice cornea infected after five days were taken (n = 6/group/time), and evenly ground with a glass. Each cornea was mixed with 1 mL PBS, then evenly dispersed on a Sabouraud agar plate. The plates were incubated under aerobic conditions at 37°C. The formation of aseptic colonies on the plate medium was observed every day. Then, the number of A. fumigatus colonies on the plate medium of the baicalein-treated group and the DMSO-treated group was counted.44
Myeloperoxidase (MPO) Assay
The mice corneas at three days p.i. (n = 6/group/time) were harvested and homogenized, then followed with the protocol of MPO test kit (Nanjing Jiancheng Institute of Bioengineering, China). MPO was measured spectrophotometrically at 460 nm at 37°C. The slope of the line was related to the MPO unit/g cornea.
Real-Time PCR (RT-PCR)
Total RNA of mouse cornea and HCECs were extracted using RNAiso Plus reagent (Takara, Dalian, China). RNA samples were reverse transcribed using HiScript III RT SuperMixcjj (Vazyme, Nanjing, China) to produce cDNA template. RT-PCR was performed referring to previous experimental research.45 The primer pair sequences used for RT-PCR are shown in the Table.
Table.
Target Gene Primer Sequence
| Gene | Primer | Nucleotide Sequence | GenBank |
|---|---|---|---|
| β-Actin (mouse) | F | 5’-GATTACTGCTCTGGCTCCTAGC-3’ | NM_007393.3 |
| R | 5’-GACTCATCGTACTCCTGCTTGC-3’ | ||
| IL-1β (mouse) | F | 5’-CGCAGCAGCACATCAACAAGAGC-3’ | NM_008361.4 |
| R | 5’-TGTCCTCATCCTGGAAGGTCCACG-3’ | ||
| TNF-α (mouse) | F | 5’-ACCCTCACACTCAGATCATCTT-3’ | NM_013693.3 |
| R | 5’-GGTTGTCTTTGAGATCCATGC-3’ | ||
| IL-6 (mouse) | F | 5’-TGATGGATGCTACCAAACTGGA-3’ | NM_001314054.1 |
| R | 5’-TGTGACTCCAGCTTATCTCTTGG-3’ | ||
| TSLP (mouse) | F | 5′-ACGGATGGGGCTAACTTACAA-3′ | NM_021367.2 |
| R | 5′-AGTCCTCGATTTGCTCGAACT-3′ | ||
| TSLPR (mouse) | F | 5′-TCACGGGGTGATGTCACAGT-3′ | NM_001164735.1 |
| R | 5′-AGTGCCATAACGGAACTCCAG-3′ | ||
| GAPDH (human) | F | 5′TGGCACCCAGCACAATGAA-3′ | NM_001101.5 |
| R | 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ | ||
| IL-1β (human) | F | 5′-GCTGATGGCCCTAAACAGATGAA-3′ | NM_000576.3 |
| R | 5′- TCCATGGCCACAACAACTGAC -3′ | ||
| TNF - α (human) | F | 5 ′-TGCTTGTTCCTCAGCCTCTT -3′ | NM_000594.4 |
| R | 5′-CAGAGGGCTGATTAGAGA GAGGT-3′ | ||
| IL-6 (human) | F | 5′-AAGCCAGAGCTGTGCAGATGAGTA-3′ | NM_000600.5 |
| R | 5′- TGTCCTGCAGCCACTGGTTC-3′ | ||
| TSLP (human) | F | 5′-ACGGATGGGGCTAACTTACAA-3′ | NM_033035.5 |
| R | 5′-AGTCCTCGATTTGCTCGAACT-3′ |
F, forward; R, reverse.
Western Blot
Mice corneas (n = 6/group/time) or HCECs were lysed in 196 µL RIPA (Solarbio) containing 2 µL PMSF (Solarbio) and 2 µL phosphatase inhibitor (MCE) for tow hours, and centrifuged at 12000 rpm for five minutes. BCA kit (Solarbio) was used for protein concentration determination. Then, total proteins were separated on SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane (Solarbio), which was then blocked in blocking buffer (Solarbio) for two hours. Subsequently, membranes were incubated with primary antibodies against TSLP (1:1000; Thermo), TSLPR (1:1000; Thermo), and β-actin (1:5000; Abcam) at 4°C overnight, followed by incubation with goat anti-rabbit secondary antibodies at 37°C for one hour. Then, the blots were visualized using enhanced chemiluminescence (ECL) (Thermo Fisher Scientific).
Enzyme-Linked Immunosorbent Assay (ELISA)
The HCECs supernatant and mouse corneas (n = 6/group/time) were collected and centrifuged. The ELISA kit (R&D Systems) was used to detect IL-1β, IL-6, and TNF-α protein levels according to the instructions. The absorbance was measured at 450 nm with a microplate reader.
Immunofluorescence Staining (IFS)
The immunofluorescence step refers to the previous study.46 Briefly, the eyeballs of the euthanized mice (n = 6/group/time) were removed at three days p.i. and completely embedded in the optimal cutting temperature (O.C.T.) (Sakura Tissue-Tek, Torrance, CA, USA) and frozen with liquid nitrogen. The corneas were cut by microtome into 10 μm thick slices, and the slices were soaked in acetone for 30 minutes. After being blocked with goat serum (1:100) for 30 minutes, the sections were incubated with anti-NIMP-R14 antibody (1:200; Santa Cruz, CA, USA) or anti-mouse TSLP antibody (20 µg/mL, Thermo) at 4°C, overnight. Then, slices were stained with FITC-conjugated goat anti-rat (1:200; Abcam) or FITC-conjugated goat anti-rabbit (1:200; Abcam) secondary antibody at RT for one hour. Cell nuclei were stained with DAPI (1:100; Solarbio) for 10 minutes. The slices were observed under the microscope and captured at 400× magnification.
HCECs seeded in poly-L-lysine-coated slips were stimulated by A. fumigatus for one hour, and then were incubated with 0.25 mM baicalein for seven hours. Slips were rinsed three times in PBS, fixed by 4% paraformaldehyde at RT for 15 minutes and then blocked in goat serum (1:10) for 30 minutes at RT. Slips were incubated with primary antibody against human TSLP (20 µg/mL, Thermo) at 4°C overnight, followed by one hour incubation with secondary antibody FITC-conjugated goat anti-rabbit antibody (1:200; Abcam) at RT. After being rinsed three times in PBS, slips were stained with DAPI (1:100; Solarbio). Fluorescence images were obtained using optical microscopy at 200× magnification.
Statistical Analysis
The experimental data were expressed in the form of mean ± SEM and analyzed by GraphPad 7.0 software. The clinical score in different groups was determined by Mann-Whitney U test. MIC, plate count, RT-PCR, Western blot, ELISA, and IFS were analyzed by an unpaired, two-tailed Student's t-test (GraphPad Prism; GraphPad, San Diego, CA, USA). One-way ANOVA with post hoc analysis was used for CCK-8 test. P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001) was considered as significant (ns = no significance). All experiments were repeated at least three times to ensure accuracy.
Results
Toxicity Evaluation of Baicalein in Vitro and In Vivo
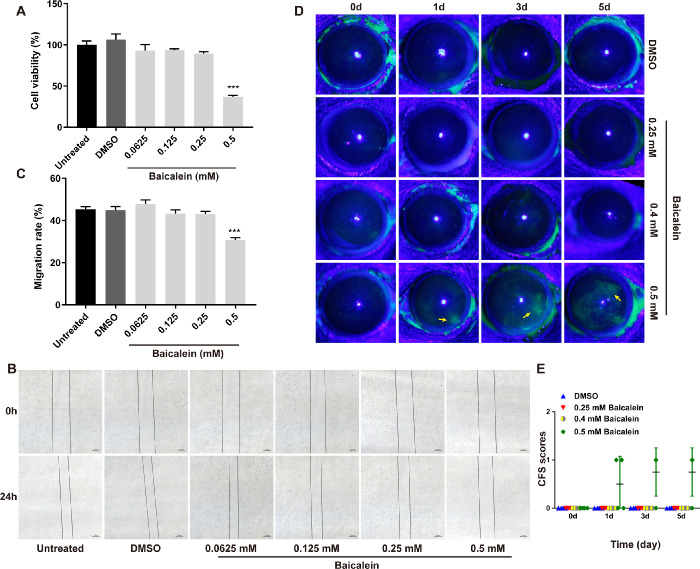
To determine a nontoxic baicalein concentration, HCECs and mouse eyes were treated with different concentrations of baicalein for 24 hours and five days, respectively. CCK-8 test revealed that the cell viability of HCECs was not affected by baicalein when its concentration was less than or equal to 0.25 mM (Fig. 1A), and cell scratch assay showed that 0.25 mM baicalein did not affect HCECs migration within 24 hours (Figs. 1B, C). To estimate the toxicity of baicalein in vivo, Draize eye test was used to assess the ocular lesions marked by sodium fluorescein staining. Mouse eyes exposed to DMSO or 0.25 and 0.4 mM baicalein for one, three, and five days showed no sodium fluorescein staining (Fig. 1D), and CFS scores were 0 (Fig. 1E). However, 0.5 mM baicalein treated mice displayed slight sodium fluorescein staining on the cornea at days one, three, and five (Fig. 1D) with higher CFS score (Fig. 1E), suggesting 0.5 mM baicalein could be toxic to mouse cornea, which is consistent with the adverse effects of 0.5 mM baicalein on HCECs (Figs. 1A–C). Thus, baicalein at 0.25 mM was considered as a nontoxic concentration and applied for the following in vitro and in vivo experiments.
Figure 1.
Toxicity evaluation of baicalein in vitro and in vivo. CCK-8 assay was performed on HCECs treated with DMSO or baicalein at 0.0625, 0.125, 0.25 and 0.5 mM for 24 hours (A). Scratch assay (B) and quantitative analysis (C) showed baicalein ≤ 0.25 mM hardly affected HCECs migration at 24 hours (bar = 100 µm). Representative mouse corneas of Draize eye test (D) and CFS scores (E) before and after treatment with baicalein at 0.25, 0.4, 0.5 mM or DMSO for one, three, and five days. Ocular lesions were marked by sodium fluorescein staining (yellow arrows). All data were mean ± SEM. CCK-8 test was analyzed using One-way ANOVA with post hoc analysis, and all other data were analyzed by an unpaired, two-tailed Student's t-test (***P < 0.001).
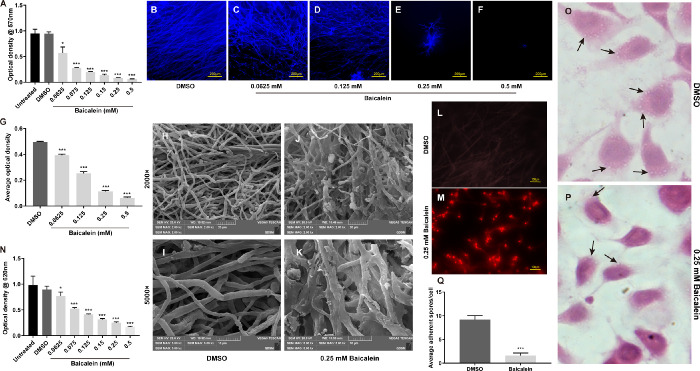
Baicalein Inhibits the Growth, Biofilm Formation, and Adhesion Ability of A. fumigatus
MIC showed that baicalein at 0.0625 mM started to inhibit the growth of A. fumigatus and prevented 90% A. fumigatus growth (MIC90) at 0.25 mM (Fig. 2A) at 48 hours. To detect how baicalein exerts its antifungal activity, mycelium growth, biofilm formation, and adhesion ability were tested at different concentrations of baicalein. Fluorescence staining showed that baicalein significantly inhibited hyphae growth in a concentration-dependent manner (Figs. 2B–G). Compared with the DMSO-treated tubular hyphae with smooth surfaces in control group (Figs. 2H, I), baicalein (0.25 mM) treated hyphae were shrunken and knotted, with evident efflux of a cotton-like substance (Figs. 2J, K). Additionally, the membrane integrity was examined by PI staining, in which nucleic acids exposed from damaged or compromised membranes were stained by red-fluorescent counterstain. An enhanced fluorescence intensity was observed in 0.25 mM baicalein-treated hyphae (Fig. 2M) in comparison to the DMSO-treated group (Fig. 2L), indicating baicalein may disrupt the membrane integrity of hyphae. Moreover, baicalein at 0.25mM significantly inhibited the biofilm formation of A. fumigatus at 48 hours (Fig. 2N). Compared with the DMSO group, HE staining showed a remarkable decreased number of conidia adhering to the surface of baicalein-treated (0.25 mM) HCECs, suggesting baicalein can inhibit the adhesion ability of A. fumigatus to HCECs (Figs. 2O–Q). These results demonstrated that baicalein may inhibit A. fumigatus growth through suppressing mycelium growth, biofilm formation, and adhesion abilities.
Figure 2.
Baicalein inhibited the growth, biofilm formation, and adhesion ability of A. fumigatus. For A. fumigatus, the minimum effective concentration (MEC) of baicalein was 0.0625 mM. MIC90 was 0.25 mM, and the minimum fungicidal concentration (MFC) was 0.5 mM in A. fumigatus at 48 hours (A). Fluorescence images of A. fumigatus hyphae (blue) treated with DMSO (B) or baicalein at 0.0625 (C), 0.125 (D), 0.25 (E) and 0.5 mM (F) for 48 hours (n = 6/group/isolate, bar = 200 µm) and quantitative analysis (G). SEM images (H–K) of A. fumigatus hyphae exposed to DMSO (H, bar = 20 µm; I, bar = 10 µm) or 0.25 mM baicalein (J, bar = 20 µm; K, bar = 10 µm) for 24 hours at 37°C. PI staining of A. fumigatus hyphae treated with DMSO (L) or 0.25 mM baicalein (M) for assessment of hyphae membrane integrity (bar = 100 µm). Biofilm formation activity of A. fumigatus was inhibited at 0.0625 to 0.5 mM baicalein for 48 hours in a concentration-dependent manner when compared with no baicalein treatment (N). HE staining images of HCECs treated with DMSO (O) or 0.25 mM baicalein (P) (magnification = × 40 µm) for three hours and quantitative analysis of spores on the cell surface (Q). All data are mean ± SEM and were analyzed by an unpaired, two-tailed Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
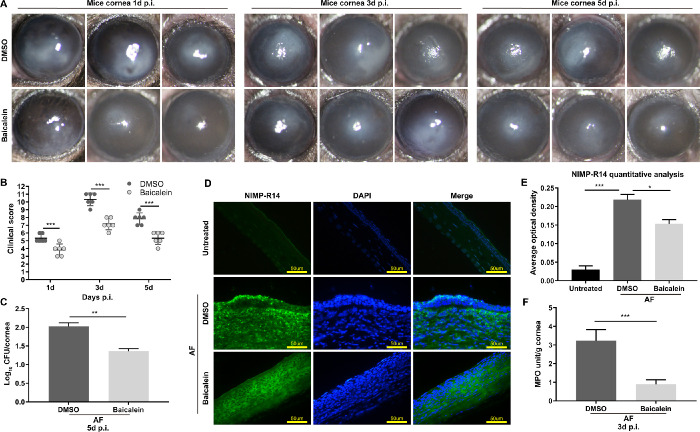
Baicalein Mitigates FK Severity, and Alleviates Fungal Load and Neutrophil Infiltration and Activity in Mice With A. fumigatus Keratitis
To evaluate the therapeutic effect of baicalein, 0.25 mM baicalein or DMSO were applied to an in vivo mouse model of A. fumigatus keratitis, and corneas were photographed under a slit lamp at one, three, and five days p.i. Compared with DMSO, baicalein treatment markedly reduced the ulcer area and corneal opacity, making the cornea more transparent (Fig. 3A). Consistently, clinical scores in baicalein-treated groups were much lower than DMSO-treated groups (Fig. 3B), which suggested baicalein treatment can improve the prognosis of mice with FK. In addition, plate count results showed that baicalein treatment significantly reduced the fungal load in A. fumigatus infected corneas in comparison to DMSO at five days p.i. (Fig. 3C). We then examined whether baicalein treatment could affect neutrophil infiltration and activity using immunofluorescence and MPO assay. Compared with DMSO, baicalein treatment not only reduced the number of infiltrated neutrophils in the infected corneal stroma (Figs. 3D, E), but also inhibited MPO activity level in corneas at three days p.i. (Fig. 3F). Thus, baicalein may mitigate the severity of FK in mice with A. fumigatus keratitis through decreasing fungal load and inhibiting neutrophil infiltration and activity.
Figure 3.
Effects of baicalein on A. fumigatus–infected mouse corneas. Photographs of mouse corneas in 0.25 mM baicalein-treated group and DMSO-treated group at one, three, and five days p.i. (A). Compared with the DMSO, baicalein treatment significantly reduced clinical scores at one, three, and five days p.i. (B). Quantitative analysis of fungal load in baicalein- or DMSO-treated mice cornea at five days p.i. (C). NIMP-R14 labeled neutrophils (green) in baicalein-treated group and DMSO-treated group at three days p.i. (bar = 50 µm) (D) and quantitative analysis (E). Green: NIMP-R14-FITC staining; blue: nuclear staining (DAPI); merge: neutrophil localization; magnification: 400×. MPO activity in baicalein-treated group was much lower than DMSO group at three days p.i. (F). All data are mean ± SEM, in which clinical scores were analyzed by Mann-Whitney U test, and other data were analyzed by an unpaired, two-tailed Student's t-test (n = 6/group/isolate, *P < 0.05, **P < 0.01, ***P < 0.001).
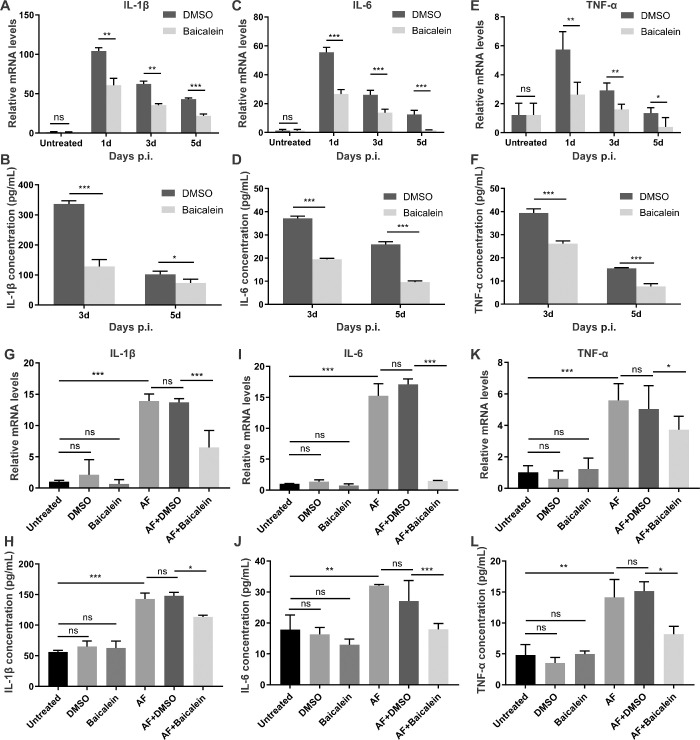
Baicalein Inhibits the Expression of Proinflammatory Cytokines Induced by A. fumigatus In Vivo and In Vitro
We then sought to detect whether baicalein could modulate the expression of proinflammatory mediators in A. fumigatus infected mouse corneas and HCECs. The mRNA and protein expressions of IL-1β, IL-6, and TNF-α were examined using RT-PCR and ELISA. In mice with FK, baicalein significantly reduced mRNA levels of IL-1β (Fig. 4A), IL-6 (Fig. 4C), and TNF-α (Fig. 4E) when compared with DMSO at one, three, and five days p.i. Consistently, treatment with baicalein versus DMSO showed decreased protein levels of IL-6 (Fig. 4D) and TNF-α (Fig. 4F) at three and five days p.i., but not for IL-1β (Fig. 4B),which was only significant at three days p.i. Similar results were obtained from A. fumigatus infected HCECs, in which baicalein remarkably inhibited mRNA levels of IL-1β (Fig. 4G), IL-6 (Fig. 4I), and TNF-α (Fig. 4K) at four hours after infection, and suppressed protein expressions of IL-1β (Fig. 4H), IL-6 (Fig. 4J), and TNF-α (Fig. 4L) within 24 hours following infection when compared with DMSO. Therefore, baicalein inhibited A. fumigatus–induced expression of proinflammatory cytokines including IL-1β, IL-6, and TNF-α in mouse corneas and HCECs, suggesting baicalein has anti-inflammatory activity in vivo and in vitro.
Figure 4.
Baicalein inhibits the expression of proinflammatory mediators induced by A. fumigatus in vivo and in vitro. In mouse model of keratitis, RT-PCR showed the mRNA levels of IL-1β (A), IL-6 (C), and TNF-α (E) were reduced significantly in baicalein (concentration = 0.25 mM) vs. DMSO-treated corneas at one, three, and five days p.i. ELISA analysis for protein levels of IL-1β (B), IL-6 (D), and TNF-α (F) at three and five days p.i. in baicalein- versus DMSO-treated mice (n = 6/group). In HCECs, RT-PCR showed the mRNA levels of IL-1β (G), IL-6 (I) and TNF-α (K) were reduced significantly in baicalein (concentration = 0.25 mM) vs. DMSO-treated cells at four hours after infection. ELISA analysis for protein levels of IL-1β (H), IL- 6 (J), and TNF-α (L) at 24 hours following infection in baicalein- versus DMSO-treated HCECs. All data are mean ± SEM and were analyzed using an unpaired, two-tailed Student's t-test (ns, no significance; *P < 0.05, **P < 0.01, ***P < 0.001).
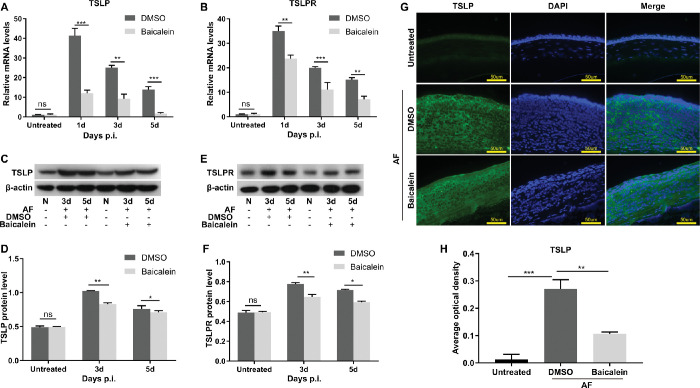
Baicalein Inhibits the Expression of Thymic Stromal Lymphopoietin (TSLP) and TSLP Receptor (TSLPR) In Vivo and In Vitro
As baicalein directly interacts with TSLP, we next explored whether TSLP is involved in the anti-inflammatory mechanism of baicalein in corneas of mice with A. fumigatus keratitis (Fig. 5) and HCECs (Fig. 6). In FK mouse models, baicalein significantly reduced A. fumigatus–induced elevated mRNA levels of TSLP (Fig. 5A) and TSLPR (Fig. 5B) at one, three, and five days p.i., and suppressed protein levels of TSLP (Figs. 5C, D) and TSLPR (Figs. 5E, F) at three and five days p.i. when compared with DMSO. Immunofluorescent assays revealed that TSLP was mainly located in corneal epithelium and stroma (Fig. 5G), and the secretion of TSLP was suppressed in baicalein-treated mice cornea versus The DMSO group at five days p.i. (Figs. 5G, H). These results suggested baicalein not only inhibited A. fumigatus–induced expression of TSLP and TSLPR in mouse corneas, but also alleviated TSLP secretion to corneal epithelium and stroma.
Figure 5.
Baicalein inhibited the expression levels of TSLP and TSLPR in mouse models with A. fumigatus keratitis. RT-PCR showed mRNA expressions of TSLP (A) and TSLPR (B) in A. fumigatus–infected mouse corneas treated with 0.25 mM baicalein or DMSO at one, three, and five days p.i. Western blotting results demonstrated protein expression levels of TSLP (C) and TSLPR (E) in baicalein (0.25 mM) or DMSO-treated FK mouse models at three and five days p.i. and quantitative analysis (D, F). Immunofluorescence images of TSLP (green) expression in corneal epithelium and stroma of FK mice treated with 0.25 mM baicalein or DMSO at three days p.i. Green: TSLP (FITC), blue: nucleus (DAPI), magnification 400× (bar = 50 µm) (G) and quantitative analysis (H) (n = 6/group/isolate). All data were mean ± SEM and were analyzed by an unpaired, two-tailed Student's t-test (ns, no significance; *P < 0.05, **P < 0.01, ***P < 0.001).
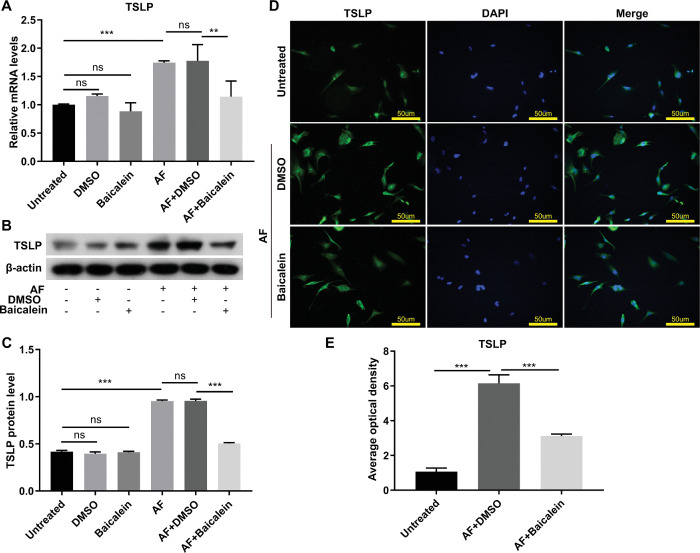
Figure 6.
Baicalein inhibited A. fumigatus–induced expression of TSLP in HCECs. RT-PCR demonstrated mRNA expression level of TSLP in HCECs treated with 0.25 mM baicalein or DMSO at four hours after A. fumigatus infection (A). Western blotting showed TSLP protein expression level in baicalein (0.25 mM) or DMSO-treated HCECs at 24 hours after infection (B) and quantitative analysis (C). Immunofluorescence images of TSLP (green) expression in A. fumigatus–infected HCECs with or without 0.25 mM baicalein treatment at eight hours after infection (D) and quantitative analysis (E). Green: TSLP (FITC), blue: nucleus (DAPI), magnification 200×. All data were mean ± SEM and were analyzed by an unpaired, two-tailed Student's t-test (ns, no significance; *P < 0.05, **P < 0.01, ***P < 0.001).
In addition, TSLP mRNA and protein expression levels were also tested in A. fumigatus infected HCECs (Fig. 6). Compared with DMSO, baicalein dramatically restrained the elevated TSLP mRNA (Fig. 6A) and protein levels (Figs. 6B, C) after infection, which is consistent with immunofluorescence staining that baicalein attenuated the overexpression of TSLP induced by A. fumigatus in HCECs (Figs. 6D, E). These data indicated that baicalein inhibited A. fumigatus–induced expression of TSLP in HCECs.
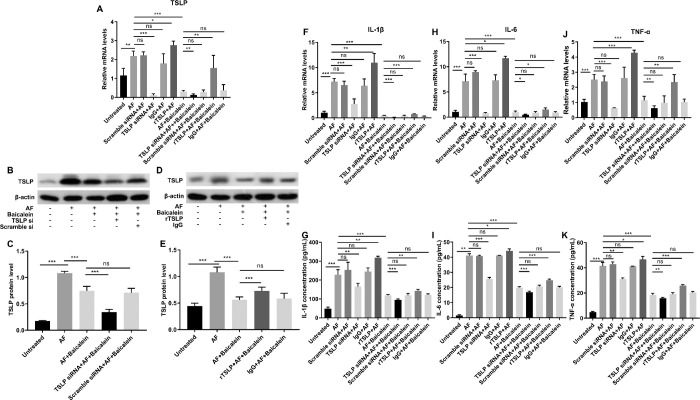
Baicalein Suppresses A. fumigatus–Induced Inflammatory Response by Inhibiting TSLP Signaling
HCECs were pretreated with TSLP siRNA or rTSLP followed by A. fumigatus stimulation, and then treated with baicalein or DMSO. RT-PCR and Western blot results showed, respectively, that TSLP mRNA and protein were markedly downregulated by TSLP siRNA (Figs. 7A–C) and were upregulated by rTSLP that partially counteracted the inhibitory effect of baicalein on the expression of TSLP (Figs. 7A, D, E). To further understand how TSLP is involved in FK-related inflammation, we examined the expression of proinflammatory mediators in response to TSLP knockdown and overexpression (Figs. 7F–K). The mRNA and protein levels of IL-1β (Figs. 7F, G), IL-6 (Figs. 7H, I), and TNF-α (Figs. 7J, K) were significantly lower in TSLP siRNA–treated group, while higher in rTSLP-treated group than in the corresponding control, indicating involvement of TSLP in A. fumigatus–induced inflammatory response. Baicalein treatment not only contributed to inhibit the expression of IL-1β (Figs. 7F, G), IL-6 (Figs. 7H, I), and TNF-α (Figs. 7J, K) in TSLP siRNA–treated groups, but also inhibited rTSLP-induced overexpression of these inflammatory mediators. These results suggested that baicalein suppresses A. fumigatus–induced inflammatory response by inhibiting TSLP signaling.
Figure 7.
Baicalein suppresses A. fumigatus–induced inflammatory response by inhibiting TSLP signaling. HCECs pretreated with TSLP siRNA or rTSLP were stimulated by A. fumigatus for one hour, then incubated with DMSO or 0.25 mM baicalein for three hours or twenty three hours. RT-PCR showed TSLP mRNA expression level in HCECs with or without 0.25 mM baicalein treatment (A). Western blot results of TSLP expression in TSLP siRNA (B) and rTSLP (D) treated HCECs and quantitative analysis (C, E). Quantification of mRNA levels of L-1β (F), IL-6 (H), and TNF-α (J) in TSLP siRNA, rTSLP, or non-pretreated HCECs with or without 0.25 mM baicalein treatment. ELISA demonstrated protein expression levels of L-1β (G), IL-6 (I), and TNF-α (K) in TSLP siRNA, rTSLP, or non-pretreated HCECs with or without 0.25 mM baicalein treatment (N, no treatment; AF, A. fumigatus stimulation). All data were mean ± SEM and were analyzed by an unpaired, two-tailed Student's t-test (ns, no significance; *P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
Baicalein is a plant-derived flavonoid with various pharmacological properties including antifungal, anti-inflammatory, and antitumor effects.15,47,48 However, it is still unclear whether baicalein has a therapeutic effect on FK. In this study, we demonstrated that baicalein improves A. fumigatus keratitis prognosis by inhibiting fungal load and suppressing inflammatory response via downregulation of the TSLP/TSLPR signaling pathway.
Baicalein has been reported to have potent antifungal activity against several fungal pathogens in vitro including Candida albicans (C. albicans), A. fumigatus, Trichophyton rubrum and Trichophyton mentagrophytes.9,49 For example, baicalein effectively inhibited the total growth of C. albicans via suppressing biofilm formation and metabolic activity,16 and restrained A. fumigatus growth by inducing deformation of the fungal surface structure and substance efflux in vitro.9 Consistently, we showed baicalein not only inhibited A. fumigatus mycelium growth and biofilm formation, but also disrupted hyphae structure and membrane integrity, and the MIC90 was 0.25 mM at 48 hours. Nevertheless, there is a lack of evidence to show the antifungal effect of baicalein in mammalian cells or animal models. In this study, we demonstrated that 0.25 mM baicalein inhibited adhesion ability of A. fumigatus to HCECs and reduced fungal load in A. fumigatus keratitis mouse models, which further confirmed the antifungal activity of baicalein in vivo. According to cell viability, migration ability, and Draize eye test results, baicalein ≤ 0.25 mM was nontoxic to corneal epithelial cells and mice corneas. Thus, baicalein at 0.25 mM was considered as the nontoxic effective antifungal concentration and applied to the following in vitro and in vivo experiments.
On the other hand, we revealed baicalein inhibited neutrophil infiltration and MPO activity in the infected corneal epithelium and stroma, indicating baicalein could attenuate A. fumigatus keratitis–induced inflammatory response. Neutrophil infiltration is known to be essential in innate response against fungal infection,50 promoting the release of various cytokines and chemokines to further attract immune cells to eliminate pathogens. However, the large number of proinflammatory factors and toxic substances released by neutrophils may result in continuous inflammation that causes cornea damage, even vision loss.51 Thus, limiting the excessive inflammatory response is of significance for FK treatment. In the present study, we showed that baicalein treatment markedly mitigated the severity of FK in mice by reducing the ulcer area and corneal opacity, making corneas more transparent, which suggested baicalein may improve the prognosis of FK not only through inhibiting fungal load, but also via suppressing inflammatory response. Baicalein has been demonstrated to possess an anti-inflammatory effect in diverse diseases via various mechanisms.52–54 For instance, baicalein inhibited LPS-enhanced neutrophil infiltration and MPO activity to protect rats from acute lung injury.53 Moreover, baicalein alleviated acute pancreatitis–caused pathological injury through dampening the NF-κB, MAPK, and STAT3 signaling pathways.55 Chen et al. reported that baicalein treatment improved histological and functional outcomes of traumatic brain injury by reducing the induction of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 in rats.56 Baicalein also inhibited vascular abnormality and neuron loss in diabetic retinas through ameliorating the expression of inflammatory factors including TNF-α, IL-1β, and IL-18.54 In this study, we showed baicalein suppressed the mRNA and protein expression of proinflammatory cytokines IL-1β, IL-6, and TNF-α in A. fumigatus–infected mouse corneas and HCECs, which further elucidated the anti-inflammatory activity of baicalein in FK.
Recently, baicalein was identified as a novel thymic stromal lymphopoietin (TSLP) inhibitor that blocks the interaction between TSLP and TSLPR, thus repressing the TSLP signaling pathway.21 TSLP is an IL-7–like proallergic cytokine that plays a central role in dendritic cell (DC) activation, inducing Th2 immune responses and initiating allergic and inflammatory diseases.57,58 Hiroki et al . showed that TSLP stimulated both DCs and innate lymphoid cells, facilitating the development of Th2 cell-mediated airway inflammation.25 TSLP also promotes the expression of proallergic cytokines IL-4 and TNF-α, while downregulating IL-10 and interferon-gamma, mediating pathological inflammation.26 Recent studies suggest TSLP may serve as a new target for FK treatment. TSLP knockdown reduced DC aggregation, maturation, and migration, and repressed Th2-attracting chemokines and cytokines to attenuate the inflammatory response in A. fumigatus–infected corneas, and alleviating the severity of FK.28 In contrast, pretreatment of exogenous TSLP with A. fumigatus resulted in severe keratitis with increased neutrophil infiltration and higher levels of Toll-like receptor (TLR)-2, TLR-4, IL-6, and IL-8.29 TLRs are located on the cell surface, recognizing pathogen-associated molecular patterns (PAMPs) to initiate antimicrobial immune responses.59 Our previous studies have shown A. fumigatus infection induced an elevated expression of TLRs,37, 60 and TLR-dependent induction of TSLP has been confirmed in HCECs in response to microbial components,29,58 which provides evidence for the TLRs mediated cross-talk between FK and TSLP.
Park et al. reported that the inhibition of TSLP signaling by baicalein could reduce eosinophilic airway inflammation, alleviating the severity of allergic reaction.21 However, the effects of baicalein on TSLP/TSLPR signaling in FK has not been investigated yet. Our study demonstrated that baicalein not only inhibited A. fumigatus–induced overexpression of TSLP and TSLPR in mouse corneas, but also alleviated TSLP secretion to corneal epithelium and stroma. Similar results were obtained in corneal epithelial cells, in which baicalein inhibited the upregulated expression of TSLP in A. fumigatus–infected HCECs. These results indicate baicalein may exert its anti-inflammatory activity through inhibiting the TSLP/TSLPR signaling pathway. To further understand the regulatory role of baicalein on the TSLP/TSLPR pathway, HCECs were pretreated with TSLP siRNA or rTSLP followed by A. fumigatus infection. The rTSLP-induced upregulated TSLP partially counteracted the inhibitory effect of baicalein on the expression of TSLP. Additionally, the mRNA and protein levels of proinflammatory factors IL-1β, IL-6, and TNF-α were significantly lower in the TSLP siRNA–treated group, while higher in the rTSLP-treated group than in the corresponding control, indicating the involvement of TSLP in A. fumigatus–induced inflammatory response. Notably, baicalein treatment significantly inhibited rTSLP-induced overexpression of IL-1β, IL-6, and TNF-α. These results suggested that baicalein suppresses A. fumigatus–induced inflammatory response by inhibiting TSLP/TSLPR signaling.
In summary, our study showed that baicalein plays a protective role in mouse A. fumigatus keratitis by inhibiting fungal growth, biofilm formation, and adhesion, and suppressing inflammatory response via downregulation of TSLP/TSLPR signaling pathway. Therefore, baicalein is expected to be a promising therapeutic drug or combined with other antifungal drugs in FK treatment.
Acknowledgments
Supported by the National Natural Science Foundation of China (No. 81470609; No. 81870632; No. 81500695) and the Natural Science Foundation of Shandong Province (No. ZR2019BH004).
Disclosure: Y. Zhu, None; X. Peng, None; Y. Zhang, None; J. Lin, None; G. Zhao, None
References
- 1. Austin A, Lietman T, Rose-Nussbaumer J.. Update on the management of infectious keratitis. Ophthalmology. 2017; 124(11): 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niu L, Liu X, Ma Z, et al.. Fungal keratitis: pathogenesis, diagnosis and prevention. Microb Pathog. 2020; 138: 103802. [DOI] [PubMed] [Google Scholar]
- 3. Kuo MT, Chang HC, Cheng CK, et al.. A highly sensitive method for molecular diagnosis of fungal keratitis: a dot hybridization assay. Ophthalmology. 2012; 119(12): 2434–2442. [DOI] [PubMed] [Google Scholar]
- 4. Lakhani P, Patil A, Majumdar S.. Challenges in the Polyene- and Azole-Based Pharmacotherapy of Ocular Fungal Infections. J Ocul Pharmacol Ther. 2019; 35(1): 6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou HC, Wang H, Shi K, et al.. Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules. 2018; 24(1): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao Q, Cui MY, Levsh O, et al.. Two CYP82D enzymes function as flavone hydroxylases in the biosynthesis of root-specific 4'-deoxyflavones in Scutellaria baicalensis. Mol plant. 2018; 11(1): 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou CC, Pan SL, Teng CM, et al.. Pharmacological evaluation of several major ingredients of Chinese herbal medicines in human hepatoma Hep3B cells. Eur J Pharm Sci. 2003; 19(5): 403–412. [DOI] [PubMed] [Google Scholar]
- 8. Shen J, Li P, Liu S, et al.. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J Ethnopharmacol . 2021; 265: 113198. [DOI] [PubMed] [Google Scholar]
- 9. Da X, Nishiyama Y, Tie D, et al.. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci Rep. 2019; 9(1): 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song J, Zhang L, Xu Y, et al.. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem Pharmacol. 2020; 183: 114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinda B, Dinda S, DasSharma S, et al.. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017; 131: 68–80. [DOI] [PubMed] [Google Scholar]
- 12. Bie B, Sun J, Guo Y, et al.. Baicalein: a review of its anti-cancer effects and mechanisms in hepatocellular carcinoma. Biomed Pharmacother. 2017; 93: 1285–1291. [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Gao Y, Wu J, et al.. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014; 354(1): 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sowndhararajan K, Deepa P, Kim M, et al.. Baicalein as a potent neuroprotective agent: a review. Biomed Pharmacother.2017; 95: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 15. Meresman GF, Götte M, Laschke MW.. Plants as source of new therapies for endometriosis: a review of preclinical and clinical studies. Hum Reprod Update. 2021; 27(2): 367–392. [DOI] [PubMed] [Google Scholar]
- 16. Cao Y, Dai B, Wang Y, et al.. In vitro activity of baicalein against Candida albicans biofilms. J Prosthodont. 2008; 32(1): 73–77. [DOI] [PubMed] [Google Scholar]
- 17. Fu Z, Lu H, Zhu Z, et al.. Combination of baicalein and amphotericin B accelerates Candida albicans apoptosis. Biol Pharm Bull. 2011; 34(2): 214–218. [DOI] [PubMed] [Google Scholar]
- 18. Kim YJ, Kim HJ, Lee JY, et al.. Anti-inflammatory effect of baicalein on polyinosinic⁻polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses. 2018; 10(5): 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X, Ruan Q, Zhai Y, et al.. Baicalein inhibits non-small-cell lung cancer invasion and metastasis by reducing ezrin tension in inflammation microenvironment. Cancer Sci. 2020; 00: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan GW, Zhang Y, Jiang X, et al.. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation. 2013; 36(6): 1584–1591. [DOI] [PubMed] [Google Scholar]
- 21. Park BB, Choi JW, Park D, et al.. Structure-activity relationships of baicalein and its analogs as novel TSLP inhibitors. Sci Rep. 2019; 9(1): 8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Corren J, Ziegler SF.. TSLP: from allergy to cancer. Nat Immunol. 2019; 20(12): 1603–1609. [DOI] [PubMed] [Google Scholar]
- 23. Roan F, Obata-Ninomiya K, Ziegler SF.. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019; 129(4): 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vannella KM, Ramalingam TR, Borthwick LA, et al.. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci Transl Med. 2016; 8(337): 337ra65. [DOI] [PubMed] [Google Scholar]
- 25. Kabata H, Flamar AL, Mahlakõiv T, et al.. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol. 2020; 13(4): 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soumelis V, Reche PA, Kanzler H, et al.. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002; 3(7): 673–80. [DOI] [PubMed] [Google Scholar]
- 27. Ji Q, Wang L, Liu J, et al.. Aspergillus fumigatus-stimulated human corneal epithelial cells induce pyroptosis of THP-1 macrophages by secreting TSLP. Inflammation. 2021; 44(2): 682–692. [DOI] [PubMed] [Google Scholar]
- 28. Sun L, Chen C, Wu J, et al.. TSLP-activated dendritic cells induce T helper type 2 inflammation in Aspergillus fumigatus keratitis. Exp Eye Res. 2018; 171: 120–130. [DOI] [PubMed] [Google Scholar]
- 29. Dai C, Wu J, Chen C, et al.. Interactions of thymic stromal lymphopoietin with TLR2 and TLR4 regulate anti-fungal innate immunity in Aspergillus fumigatus-induced corneal infection. Exp Eye Res. 2019; 182: 19–29. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Y, Lin J, Peng X, et al.. The role of netrin-1 in the mouse cornea during Aspergillus fumigatus infection. Int Immunopharmacol. 2019; 71: 372–381. [DOI] [PubMed] [Google Scholar]
- 31. Wilhelmus K. The Draize eye test. Surv Ophthalmol. 2001; 45(6): 493–515. [DOI] [PubMed] [Google Scholar]
- 32. Secchi A, Deligianni V.. Ocular toxicology: the Draize eye test. Curr Opin Allergy Clin Immunol. 2006; 6(5): 367–72. [DOI] [PubMed] [Google Scholar]
- 33. Nagai N, Sakurai S, Seiriki R, et al.. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology. 2014; 319;53–62. [DOI] [PubMed] [Google Scholar]
- 34. Barroso J, Pfannenbecker U, Adriaens E, et al.. Cosmetics Europe compilation of historical serious eye damage/eye irritation in vivo data analysed by drivers of classification to support the selection of chemicals for development and evaluation of alternative methods/strategies: the Draize eye test Reference Database (DRD). Arch Toxicol. 2017; 91(2): 521–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bengani L, Kobashi H, Ross AE, et al.. Steroid-eluting contact lenses for corneal and intraocular inflammation. Acta Biomater. 2020; 16: 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson S, Ahearne M, Hopkinson A.. An overview of current techniques for ocular toxicity testing. Toxicology. 2015; 327: 32–46. [DOI] [PubMed] [Google Scholar]
- 37. Zhan L, Peng X, Lin J, et al.. Honokiol reduces fungal load, toll-like receptor-2, and inflammatory cytokines in Aspergillus fumigatus keratitis. Invest Ophthalmol Vis Sci. 2020; 61(4): 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khani S, Seyedjavadi SS, Hosseini HM, et al.. Effects of the antifungal peptide Skh-AMP1 derived from Satureja khuzistanica on cell membrane permeability, ROS production, and cell morphology of conidia and hyphae of Aspergillus fumigatus. Peptides.2020; 123;170195. [DOI] [PubMed] [Google Scholar]
- 39. Mowat E, Butcher J, Lang S, et al.. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. Future Microbiol. 2007; 56: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 40. Peng X, Ekanayaka SA, McClellan SA, et al.. Characterization of three ocular clinical isolates of P. aeruginosa: viability, biofilm formation, adherence, infectivity, and effects of glycyrrhizin. Pathogens. 2017; 6(4): 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yuan K, Zhao G, Che C, et al.. Dectin-1 is essential for IL-1β production through JNK activation and apoptosis in Aspergillus fumigatus keratitis. Int Immunopharmacol. 2017; 52: 168–175. [DOI] [PubMed] [Google Scholar]
- 42. Che C, Li C, Lin J, et al.. Wnt5a contributes to dectin-1 and LOX-1 induced host inflammatory response signature in Aspergillus fumigatus keratitis. Cell Signal. 2018; 52: 103–111. [DOI] [PubMed] [Google Scholar]
- 43. Wu TG, Wilhelmus KR, Mitchell BM.. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003; 44: 210–216. [DOI] [PubMed] [Google Scholar]
- 44. Fan Y, Li C, Peng X, et al.. Perillaldehyde ameliorates Aspergillus fumigatus keratitis by activating the Nrf2/HO-1 signaling pathway and inhibiting dectin-1-mediated inflammation. Invest Ophthalmol Vis Sci. 2020; 61(6): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu G, Zhao G, Lin J, al et. FCN-A mediates the inflammatory response and the macrophage polarization in Aspergillus fumigatus keratitis of mice by activating the MAPK signaling pathway. Int Immunopharmacol . 2020; 83: 106473. [DOI] [PubMed] [Google Scholar]
- 46. Gu L, Lin J, Wang Q, et al.. Dimethyl itaconate protects against fungal keratitis by activating the Nrf2/HO-1 signaling pathway. Immunol Cell Biol . 2020; 98(3): 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsimplouli C, Demetzos C, Hadzopoulou-Cladaras M, et al.. In vitro activity of dietary flavonol congeners against human cancer cell lines. Eur J Nutr. 2012; 51(2): 181–190. [DOI] [PubMed] [Google Scholar]
- 48. Liau PR, Wu MS, Lee CK.. Scutellaria baicalensis Inhibitory Effects of Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities. Molecules. 2019; 24(11): 2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kang K, Fong WP, Tsang PW.. Antifungal activity of baicalein against Candida krusei does not involve apoptosis. Mycopathologia. 2010; 170(6): 391–396. [DOI] [PubMed] [Google Scholar]
- 50. Bian Z, Guo Y, Ha B, et al.. Regulation of the inflammatory response: enhancing neutrophil infiltration under chronic inflammatory conditions. J Immunol. 2012; 188(2): 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Willcox M. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007; 84(4): 273–278. [DOI] [PubMed] [Google Scholar]
- 52. Li D, Shi G, Wang J, et al.. Baicalein ameliorates pristane-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res Ther. 2019; 21(1): 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsai CL, Lin YC, Wang HM, et al.. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J Ethnopharmacol. 2014; 153(1): 197–206. [DOI] [PubMed] [Google Scholar]
- 54. Yang LP, Sun HL, Wu LM, et al.. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009; 50(5): 2319–27. [DOI] [PubMed] [Google Scholar]
- 55. Pu WL, Bai RY, Zhou K, et al.. Baicalein attenuates pancreatic inflammatory injury through regulating MAPK, STAT 3 and NF-κB activation. Int Immunopharmacol. 2019; 72: 204–210. [DOI] [PubMed] [Google Scholar]
- 56. Chen SF, Hsu CW, Huang WH, et al.. Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br J Pharmacol. 2008; 155(8): 1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin J, Xu R, Hu L, et al.. Interleukin-32 induced thymic stromal lymphopoietin plays a critical role in the inflammatory response in human corneal epithelium. Cell Signal. 2018; 49: 39–45. [DOI] [PubMed] [Google Scholar]
- 58. Ma P, Bian F, Wang Z, et al.. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009; 50(6);2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ren X, Wang L, Wu X. A potential link between TSLP/TSLPR/STAT5 and TLR2/MyD88/NFκB-p65 in human corneal epithelial cells for Aspergillus fumigatus tolerance. Mol Immunol. 2016; 71: 98–106. [DOI] [PubMed] [Google Scholar]
- 60. Xu R, Lin J, Zhao GQ, et al.. Aspergillus fumigatus production of interleukin-1β related to mammalian target of rapamycin/toll-like receptor 4 signaling pathway during infection of the mouse cornea. Int J Ophthalmol. 2018; 11(5): 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]