Abstract
Health problems associated with essential trace metals can result from both inadequate (i.e., low intake) and excessive exposures (i.e., from environmental and/or occupational source). Thus, measuring the exposure level is a real challenge for epidemiologists. Among non-invasive biomarkers that intend to measure long-term exposure to essential trace metals, the toenail is probably the biological matrix with the greatest potential.
This systematic review collects the current evidence regarding the validity of toenail clippings as exposure biomarker for trace metals such as boron, cobalt, copper, iron, manganese, molybdenum, selenium, silicon, vanadium and zinc. Special attention was paid to the time-window of exposure reflected by the toenail, the intraindividual variability in exposure levels over time in this matrix, and the relationship of toenail with other biomarkers, personal characteristics and environmental sources.
Our search identified 139 papers, with selenium and zinc being the most studied elements. The variability among studies suggests that toenail levels may reflect different degrees of exposure and probably correspond to exposures occurred 3–12 months before sampling (i.e., for manganese/selenium). Few studies assessed the reproducibility of results over time and, for samples obtained 1–6 years apart, the correlation coefficient were between 0.26 and 0.66. Trace metal levels in toenails did not correlate well with those in the blood and urine and showed low-moderate correlation with those in the hair and fingernails.
Available data suggests that for some elements (Se, Mn, Zn) toenail concentrations reflect long-term external exposures in fairly reproducible levels, while for other metals, this association has not yet been assessed. Among dietary factors, only toenail selenium showed clear associations with the intake of supplements or specific foods. The toenail levels could also represent occupational exposure, for instance, Mn exposure in welders. The scarcity of information on other essential trace elements, together with the great heterogeneity among studies makes the validation of the usage of toenails as biomarkers of exposure to these elements difficult. Standardization of sample collection, quality control, analytical techniques and reporting procedures might facilitate further research focused on the clear understanding of the significance of essential levels in this promising matrix and would enhance its utility in epidemiological research.
Keywords: Toenail, Biomonitoring, Biomarker, Exposure, Essential trace essential metals, Systematic review
1. Introduction
Essential trace metals/metalloids either are needed to maintain physiologically important functions, or are part of organic structures with vital functions in humans (Mertz, 1981). However, in many cases, excessive levels of these elements also pose a serious health risk. The typical source of these elements is diet, but occupational (e.g., Mn in welders) and environmental sources (e.g., environmental release of Zn by metal production industries or from combustion of coal in power stations) may also contribute to high exposure levels. Therefore, measurement of exposure at the individual level is a critical aspect in the research of the role of these elements in human health.
According to the World Health Organization (WHO) (WHO, 1996), the list of essential trace metals/metalloids -which will be called as metals in this review paper, since most of the elements studied belong to this group-, includes chromium (III) (Cr) [now under discussion] (Nigra et al., 2016), copper (Cu), cobalt (Co), iron (Fe), molybdenum (Mo), selenium (Se), zinc (Zn), and [manganese (Mn), which was added in 2001 to this group (Institute of Medicine (US), 2001). Vanadium (V), silicon (Si) and boron (B), not strictly considered essential elements, are often studied with them due to their potential health benefits (WHO, 1996; US EPA O, 2014).
A common approach to measure the exposure level to these essential trace metals is the use of biomarkers due to several reasons: they reflect both known and inadvertent exposures; they are not affected by recall bias, and they integrate all sources (i.e., diet, air and water) and routes of contact (Santonen et al., 2015). Among these, toenails are non-invasive matrices that have become quite popular in large epidemiological studies for their logistic advantages, namely very easy collection and storage and less influence of external contamination than fingernails and hair (Karagas et al., 2000). In addition, the slow rate of growth of toenails has made these suitable candidates for evaluating longer term exposures than other biomarkers such as blood or urine, a key issue in chronic diseases research (Hopps, 1977; Sukumar, 2006). However, the validity of these assumptions is unclear and the usefulness of measurement of essential trace metals in toenails as a biomarker of exposure is still uncertain. In this review, we condensed available data on essential trace metal levels in toenails and summarized the evidence on their validity as biological matrices of exposure. We paid special attention to a) stability over time and time-window of exposure, two aspects critical in evaluating long-term chronic exposures; b) the relationship with other commonly used biomarkers, such as blood and urine and; c) the association with personal characteristics and several sources, in order to better understand the factors that module or determine the levels of essential trace metals in this substrate.
2. Material and methods
This review is reported in accordance with the PRISMA publication standards (Moher et al., 2009) and the protocol was registered in PROSPERO, the international prospective register of systematic reviews (registration number CRD42018085822) (University of York, 2018).
2.1. Identification of studies
We conducted a systematic literature search for peer-reviewed papers providing original data on essential trace metal concentrations in toenails. The flow diagram of the study selection process is shown in Fig. A1 (Appendix A). Two reviewers (EG and BP) independently screened abstracts, reviewed full-text articles, extracted data and performed quality assessments. Initially, we searched the databases PubMed/MEDLINE, Web of Science and Scopus from inception to December 31, 2017, using the following exact searches: #1 Nail OR toenail; #2 Exposure OR biomonitoring OR biomarker; #3 Metals OR trace elements OR beryllium OR vanadium OR chromium OR cobalt OR nickel OR copper OR zinc OR arsenic OR selenium OR cadmium OR platinum OR lead OR uranium OR mercury OR thallium OR aluminium OR molybdenum OR manganese OR iron OR silicon OR boron; #4: #1 AND #2 AND #3. This search retrieved 2632 references. Then, we manually reviewed the reference lists of all included studies and identified 52 additional articles. Studies that reported original quantitative data on toenail metal concentrations in humans in English or Spanish were considered eligible. When the type of nail (fingernail/toenail) was not specified, the corresponding author was contacted for clarification. After exclusion of duplicates, 276 articles met the general inclusion criteria. From these, for the purpose of this review, we narrowed our scope to focus on the papers (139) with information on essential trace metals (Co, Cu, Fe, Mn, Mo, V and Zn) or metalloids (B, Se, Si); we did not include Cr due to its disputed essentiality (Nigra et al., 2016) and the carcinogenic role of Cr (VI) (IARC, 2012).
2.2. Data collection
Data from included studies were extracted into a customized spreadsheet. According to a purpose-designed protocol, the following data were extracted from each study: a) basic information (first author, country, year of publication, research project, studied trace metals); b) design features (type of epidemiological study, main objective, sample size, population sample method, participant characteristics, informed consent request, ethical committee approval); c) sample and analytical information (toenail type (i.e., big toe, all toes), sample preparation, analytical method, quality control measures, limits of detection and availability of other biological specimens with trace metal data); d) metal concentrations, i.e., measures of central tendency (geometric or arithmetic mean, median (p50)) and dispersion (standard deviation (SD) or range), when available; and e) correlation with other biomarkers, with personal characteristics, with environmental data, or with previous toenail measures.
Most studies reported metal levels in micrograms per gram (μg/g), but many authors chose to provide other units of concentration, namely nanograms per gram (ng/g); micromoles per kilogram (μmol/kg); nanomoles per gram (nmol/g); milligrams per kilogram (mg/kg); parts per million (ppm); and parts per billion (ppb). This variability in the units used to express metal concentrations was not related to any specific element or technique. For this review, we converted all units into μg/g to allow for an easier comparison among studies.
3. Results
3.1. Study characteristics
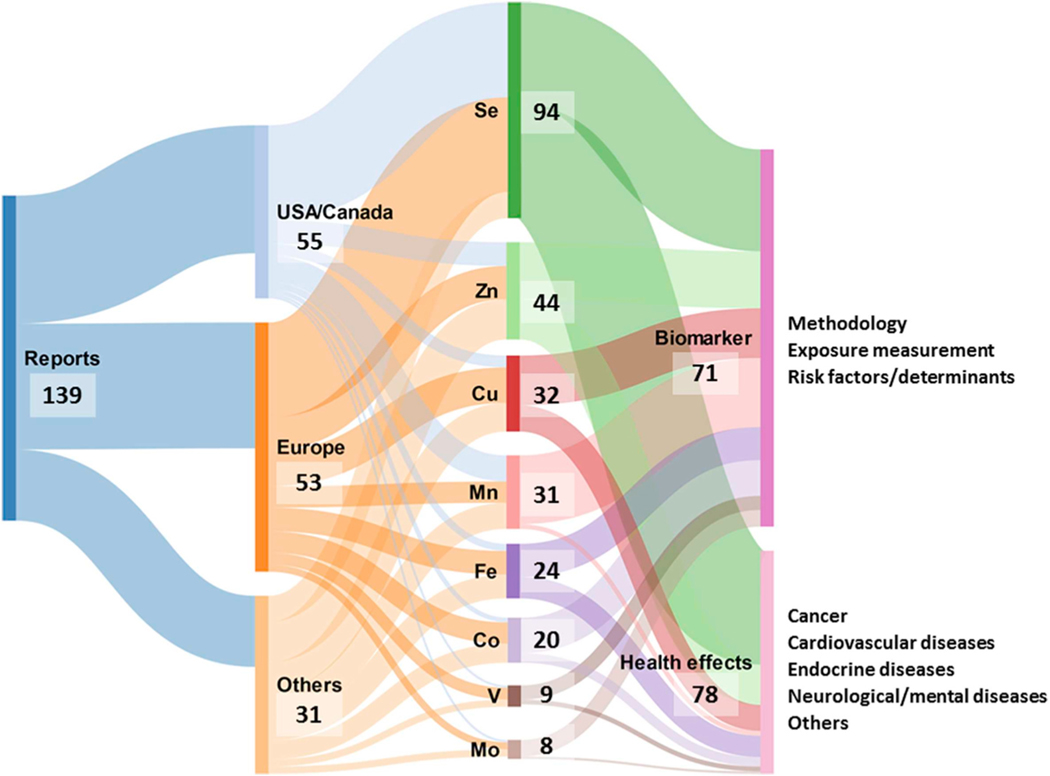
We identified a total of 139 manuscripts published between 1975 and 2017, which provided levels of essential metals in toenails, from 89 different research projects (number of papers per research project ranged from 1 to 9; 25 projects had 2 or more manuscripts). Almost 40% of the articles evaluated exposure either in the USA (49 papers, with data from 25 research projects) or in Canada (6 manuscripts, with data from 4 research projects) and another 39% of the papers included information from studies conducted in Europe (the Netherlands −13 manuscripts from 4 research projects-; Italy −7 papers from 4 projects-; and Poland −5 manuscripts from 5 research projects-were the most common locations). The remaining studies (21%) presented toenail essential metal levels from other countries, with a higher proportion of Asian countries in the recent years. One research project combined populations from more than one continent (EURAMIC study). The number of manuscripts has increased in the last decade (See Fig. A2, Appendix A), evidencing a growing interest in this biomarker. The characteristics of each paper, sorted by research study and year of publication, are detailed in Appendix A, Table A1. Search results are summarized in Fig. 1.
Fig. 1.
Graphical summary of the systematic review results. Sankey diagram. The first column represents the total number of articles included in the review. The second column shows the geographical distribution according to the place where the studies were conducted. The third column illustrates the number of articles that assessed each element in toenails (many articles deal with more than one element). The last column shows the main objective of the articles included in the review (some studies may share both objectives).
The majority of the research projects (n = 48) were focused on a single element, usually selenium. Overall, Se was the most studied essential trace element (n = 94 reports), followed by Zn (n = 44), Cu (n = 32), Mn (n = 31), Fe (n = 24), Co (n = 20), V (n = 9) and Mo (n = 8), while no data were found about toenail concentrations of B or Si. Regarding the aims of the articles, 71 used toenails as biomarkers of exposure to one or more essential metals or tried to identify their determinants, while 78 studied the association between toenail essential metal levels, mainly Se and Zn, and different health problems or biological characteristics (i.e., cholesterol levels). The most common outcome studied was cancer (Garland et al., 1995; Ghadirian et al., 2000), although other articles evaluated cardiovascular (Kardinaal et al., 1997), metabolic (Park and Seo, 2016), or hematologic effects (Lee et al., 2016), as well as neurologic (Meramat et al., 2017) or mental-health related problems (Colangelo et al., 2014) (Appendix A, Table A1).
The number of participants in each study was highly variable: 33 research projects included less than 100 individuals, 46 studies, between 100 and 1000 and 9 recruited more than 1000 participants (range: 12–9267). In one project (only 1 paper published), the sample size was not specified (Abdulrahman et al., 2012). Baseline characteristics of these subjects were heterogeneous across studies and depended on the aim of each investigation. Thus, each paper provided information describing some features of the specific population under study, such as age, sex, race, socioeconomic status, occupation, or dwelling place (See Appendix A, Table A1). However, in some cases, this description was limited to geographical area of recruitment, omitting basic data such as age or sex of the participants.
Regarding study design, most papers presented cross-sectional analyses (n = 59, which also included baseline levels in longitudinal projects), followed by case-control (30 reports) studies; prospective designs (case-cohort: 9 reports; nested case-controls: 13 reports; cohort: 25 reports) and randomized trials (3 reports). Convenience sampling was the typical strategy for recruitment and only 17 research projects reported participation rates, ranging from 41.8% (O’Rorke et al., 2012) to 98% 22; usually, in case-control studies participation was lower among controls.
Concerning ethical considerations, 47 articles declared having approval of an ethics committee and informed consent explicitly appeared as an inclusion criterion in 49 papers.
3.2. Analytical methodology: sample collection, nail preparation, analysis and quality control (Table A1)
Toenails clippings -which are the last end of the nail and constitute only a fraction of the total nail-were obtained using stainless-steel clippers or scissors. In regard to sample storage, studies that included information about this issue indicated that were kept in paper envelopes or in plastic bags/vials (polyethylene or non-specified), usually at room temperatures. The effects of long-term storage (6 years) were evaluated only for Se by St-Pierre et al., who concluded that the loss of humidity occurred with long-term storage led to increased Se concentrations (St-Pierre et al., 2006).
The influence of sampling date has not been commonly studied. Isolated reports have suggested that Zn levels might be higher in winter (Campos et al., 2008) and that there might be significant differences in metal toenail levels depending on the season of sampling collection, for Cu, but not for Zn (Wilhelm et al., 1991) or Se (Baskett et al., 1995).
Most of the projects (n = 40) did not identify the specific type of toenails analyzed. Among those that did, the majority (n = 36 studies) collected clippings from all toes; 8 projects used nails from big toes and, in another two studies, specimens from big toes and from the other toes were evaluated separately. Two projects provided partial information, reporting sampling from both feet (Ghadirian et al., 2000; Vinceti et al., 2005) or from right foot (Vinceti et al., 2012), or using nails from different feet for analysis of each metal, without specifying which toes were considered (Vinceti et al., 2005). Possible differences in metal levels among toes have only been explored for Se, comparing the big toe to the rest of toes (Baskett et al., 2001; Kok et al., 1989; Longnecker et al., 1993) or with a pool of all toes (van ‘t Veer et al., 1990), without finding significant variations. Also for this element, a high correlation between Se concentrations in the right and left toenails was observed in one study (r = 0.74) (Steven Morris et al., 1983).
In regard to the amount of bio-specimen, most papers (n = 105) did not provide any summary or descriptive data (i.e., mean or range) about the toenail mass used for the analyses. However, several authors (Kardinaal et al., 1997; Steven Morris et al., 1983; Mordukhovich et al., 2012; Platz et al., 2002; Sanders et al., 2014) remarked that the limit of detection (LOD) for trace metal determinations was dependent on toenail mass and at least 25 reports indicated that a minimum weight was required for the analyses, which was only specified in 15 papers (i.e., 10 mg in 12 of them). In some studies (Lee et al., 2016; Abdulrahman et al., 2012; Al-Saleh and Billedo, 2006; Goullé et al., 2009; Hartman et al., 2002; Michaud et al., 2002; Morris and Crane, 2013; Saat et al., 2013; Wongwit et al., 2004), researchers stated they analyzed aliquots of toenail within a mass range (mostly 10–50 mg). It should be noted that, according to some investigators, the weight of the clippings not only limits the possibility of measurement of trace metals, but also bias the levels obtained. Thus, Baskett et al. described an apparent systematic bias in metal concentrations (Se, in this case) that were inversely proportional to the mass of the sample (Baskett et al., 1995), which occurred in clipping samples weighing 23 mg or less. Additionally, Saint Pierre et al. (St-Pierre et al., 2006) found an inverse correlation between sample weight and Se concentration, while Colangelo et al. reported that toenail mass was positively associated with toenail Se levels (Colangelo et al., 2014). In some of the studies, this possible influence of nail weight in trace metal concentrations was considered and was corrected for. Thus, some authors adapted analytical procedures to fit small samples (Baskett et al., 1995); in other studies, toenail weight was considered as a potential confounding factor (Colangelo et al., 2014), or measured levels were normalized based on sample weight (Brockman et al., 2009). Also, regression models were fitted with log-transformed toenail element levels (the dependent variable) against the nail weight (the independent variable) (St-Pierre et al., 2006), or the residual of each observation was added to the predicted mean toenail value and the result was exponentiated (Garland et al., 1993, 1995, 1996).
In most studies, toenails were subjected to pretreatment after clipping and storage, before analysis, and the methods used were highly variable. Table A1 (Appendix A) presents a summary of this information, derived from each report included in the review. Nail polish in samples was generally removed with acetone and visible dirt was manually cleaned. Direct effects of nail polish (Ghadirian et al., 2000; van ‘t Veer et al., 1990; Krogh et al., 2003) or debris (van ‘t Veer et al., 1990) was assessed only for Se, and these factors did not seem to influence toenail Se concentrations. In order to reduce external contamination, toenails were cleaned by washing with detergent, deionized water, methanol, Triton solution, acetone, or sodium dodecylsulfate, assisted by a sonicator or an ultrasound bath. Subsequently, samples were dried in an oven, air-dried or freeze-dried.
Neutron activation analysis (NAA or instrumental NAA) was the most commonly used technique for element measurement (46 studies), followed by inductively coupled plasma spectrometry (ICP-MS, ICP-OES, ICP-AES) (29 studies) and atomic absorption spectrometry (AAS) (14 studies) (Appendix A, Table A1). In these two last cases, digestion of samples was performed in nitric acid, with or without chlorhydric acid, in perchloric acid, or in hydrogen peroxide, when needed; some reports specified a microwave digestion system or a sonicator. Certain research projects, like CARDIA, CLUE II, or ORDET, employed different techniques depending on the metals studied. Less common techniques used were thermal neutron flux (Graham et al., 1991) and acid digestion fluorometry (Hartman et al., 2002; Michaud et al., 2002; Alfthan et al., 1992; Männistö et al., 2000; Ovaskainen et al., 1993).
Sixty reports mentioned quality control procedures, which included the use of certified reference material (urine or hair) or homemade nail reference material, recovery analysis, procedural blanks, duplicate samples, or spike samples.
3.3. Essential trace metal concentrations in toenails across populations
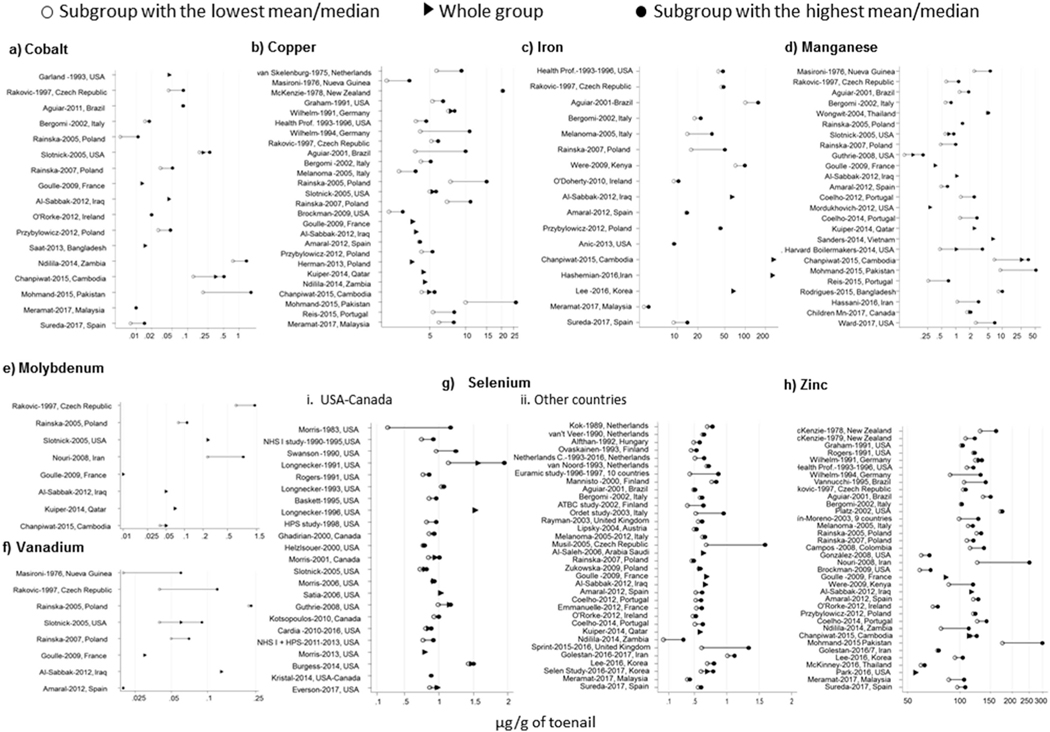
Tables B1–B8 (Appendix B) present the levels of each essential metal in each study and the subgroup of participants (mean (SD), median, range) extracted from the 139 studies, included in this review. Their main data are shown graphically in Fig. 2.
Fig. 2.
Essential metal levels [mean or median (μg/g dry weight] in human toenails (1975–2017).
Only 18 (9.5%) research projects, mostly those which used IPC-MS, provided information on LOD, and even fewer (n = 4), specified limits of quantification (LOQ). The LODs were calculated from procedural blank analyses, usually as three standard deviations from the mean blank reading (Al-Saleh and Billedo, 2006; Krogh et al., 2003; Herman et al., 2013; Przybylowicz et al., 2012; Ntihabose et al., 2017; Bouchard et al., 2017). Among the studies that reported LODs for specific metals, for Co, LODs ranged from 0.0003 (Goullé et al., 2009) to 0.01 μg/g (Chanpiwat et al., 2015); for Cu, from 0.009 (Przybylowicz et al., 2012) to 0.12 μg/g (Chanpiwat et al., 2015); for Fe, from 1.55 (Przybylowicz et al., 2012) to 2.93 μg/g (Chanpiwat et al., 2015); for Mn, from 0.001 (Goullé et al., 2009) to 0.33 μg/g (Chanpiwat et al., 2015); for Mo, from 0.0004 (Goullé et al., 2009) to 0.02 μg/g (Chanpiwat et al., 2015); for Se, from 0.0022 (Emmanuelle et al., 2012) to 0.02 μg/g (Goullé et al., 2009); and for Zn, from 0.01 (Goullé et al., 2009) to 0.279 μg/g (Przybylowicz et al., 2012). Only one study specified the LOD for V, which was 0.001 μg/g (Goullé et al., 2009). The percentage of samples with quantities under LOD, when available, depended on the element studied. It was low or inexistent for Zn and Se; in contrast, some studies reported a significant proportion of samples with quantities under the LOD for other metals, i.e., Cu, Fe, Mn and V (See Appendix B, Tables B1–B8).
Among the elements studied, Co, Mo, Se and V had the lowest levels, and most studies found mean values < 1 μg/g (Fig. 2a,e,f and g; Tables B1, B5, B.6, B.7). However, a couple of studies reported exposed populations with higher mean Co concentrations: metal workers in two towns of northern Italy (AMs: 18.90 and 53.79 μg/g (Sabbioni et al., 1994),); and young adults living near a mining area in Zambia (GM: 1.39 μg/g) (Ndilila et al., 2014). Morris et al. also reported exceptionally high Se levels (AM 8.27 μg/g) among people in USA who consumed misformulated supplements with excessive Se concentrations (Morris and Crane, 2013). In general, mean Se levels were higher in studies from USA-Canada, when compared with other countries.
Fe toenail concentrations (Table B3 and Fig. 2c) showed the highest variability across studies, with median levels ranging from 8.8 μg/g in a group of 60–80 year-old women from Spain (Sureda et al., 2017) to exceptionally high levels (median: 1434 μg/g) in subjects living in contaminated areas of Cambodia (Chanpiwat et al., 2015).
Mean Cu and Mn levels were usually below 10 μg/g (Tables B.2 and B.4, Fig. 2b–d, respectively), while mean Zn levels (Table B.8, Fig. 2h) ranged mostly from 50 to 200 μg/g. The highest Cu, Mn and Zn concentrations were found among subjects living in rural areas near a highly industrialized city in Pakistan (AM: 26.2 μg/g for Cu, 52.1 for Mn and 298 for Zn) (Mohmand et al., 2015). High concentrations of Mn were observed in individuals living in highly polluted areas of Cambodia (AM: 43.9 μg/g) (Chanpiwat et al., 2015) and among welders from a local boilermaker union in the USA (AM: 26.9–55.5 μg/g) (Laohaudomchok et al., 2011).
3.4. Toenail as a biomarker of integrated long-term exposure: time-window of exposure and intraindividual stability over time (Tables 1 and 2)
Table 1.
Reproducibility over time of essential metals in toenails and correlation with levels in other biological specimen.
| Toenail metal | Author | Year | N | Toenails reproducibility over time * | Hair | Urine | Whole blood | Serum | Plasma | Finger nail | Other toenails | Feces | Cord blood | Placenta | Saliva |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co | Garlande | 1993 | 127 | r = 0.35 (6-year) | |||||||||||
| Sabbioni | 1994 | 115 | r = 0.38 (ns) | r = 0.25 (ns) | |||||||||||
| Chanpiwate | 2015 | 180 | r = 0.20 | r = −0.08 (ns) | |||||||||||
| Cu | Wilhelm | 1991 | 461 | r = 0.18 | |||||||||||
| Garlande | 1993 | 127 | r = 0.26 (6-year) | ||||||||||||
| Herman | 2013 | 30 | r = 0.26 | ||||||||||||
| Kuiper | 2014 | 239 | (ns) | ||||||||||||
| Chanpiwate | 2015 | 180 | r = 0.10 (ns) | r = −0.08 (ns) | |||||||||||
| Fe | Garland | 1993 | 127 | r = 0.43 (6-year) | |||||||||||
| Chanpiwate | 2015 | 180 | r = 0.36 | r = −0.03 (ns) | |||||||||||
| Mn | Wongwitf | 2004 | 135 | r = 0.05 (ns) | (ns) | ||||||||||
| Kuiper | 2014 | 239 | (ns) | ||||||||||||
| Coelhoe | 2014 | 122 | r = 0.23 | ||||||||||||
| Chanpiwate | 2015 | 180 | r = 0.32 | r = −0.10 (ns) | |||||||||||
| Rodriguese | 2015 | 711 | r = 0.37–0.41 (6/7 months)b | r = 0.14 | Mother-infant r = 0.39 | r = 0.14 | |||||||||
| Hassanig | 2016 | 83 | r = 0.65 | r = 0.65 | |||||||||||
| Ntihabose | 2017 | 268 | r = 0.40 | r = 0.14 | |||||||||||
| Mo | Kuiper | 2014 | 239 | (ns) | |||||||||||
| Chanpiwate | 2015 | 180 | r = 0.08 (ns) | r = 0.00 (ns) | |||||||||||
| Se | Hunter | 1990b | 868 | r = 0.60 (5-year) | |||||||||||
| Morris | 1983 | 62 | r = −0.3 (ns) | r = 0.30 (ns) | Left-Right r = 0.74 | ||||||||||
| Kok | 1989 | 168 | Big-Small (nd) | ||||||||||||
| Van’t Veer | 1990 | 372 | Big-All (nd) | ||||||||||||
| Longnecker | 1991 | 142 | r = 0.60 | r = 0.91 | r = 0.89 | ||||||||||
| Alfthan | 1992 | 132 | r = 0.57 | ||||||||||||
| Garland | 1993 | 127 | r = 0.48 (6-year) | ||||||||||||
| Longnecker | 1993 | 12 | Big-Small (nd) | ||||||||||||
| Baskett | 2001 | 11 | Left-Right ** | ||||||||||||
| Krogh | 2003 | 80 | r = 0.57 (1-year)c | ||||||||||||
| Satiae | 2006 | 220 | r = 0.55 | ||||||||||||
| Al Salehd | 2006 | 691 | r = −0.16 | ||||||||||||
| Xun | 2010b | 69 | r = 0.56 (20-year) | ||||||||||||
| Vincetie | 2012 | 105 | r = 0.27 | ||||||||||||
| Coelhoe | 2014 | 122 | r = 0.33 | ||||||||||||
| Kuiperf | 2014 | 239 | (ns) | ||||||||||||
| Raymane | 2015 | 230 | r = 0.45 | ||||||||||||
| Punshonae | 2016 | 554 | h | ||||||||||||
| V | Rainska | 2007 | 33 | r = 0.61 | |||||||||||
| Zn | Mckenzie | 1979 | 110 | (ns) | (ns) | (ns) | |||||||||
| Wilhelma | 1991 | 461 | r = 0.03 (ns) | ||||||||||||
| Garland | 1993 | 127 | r = 0.58 (6-year) | ||||||||||||
| Chanpiwate | 2015 | 180 | r = −0.02 (ns) | r = 0.08 (ns) | |||||||||||
| Punshona | 2016 | 554 | (ns) |
N = number of participants (samples) in the study; r = correlation coefficient; (ns) = p > 0.05 except in Champiwat (2015) (p > 0.01) or author only stated that correlation was not statistically significant; (nd) = author stated that there were no differences.
= between parentheses is expressed the intervale of time between both measurements.
Log transformed.
Pregnant women: first trimester vs one month post-partum.
Not modified by age, menopausal status, smoking or acetone treatment.
No significant differences between mean levels in both specimens using t-test.
Spearman correlation.
Pearson correlation.
Partial correlation test.
=strong positive significant correlation with maternal toenails. Not significant with infant toenails.
Table 2.
Studies (Author year) reporting association of toenail essential trace metals to personal characteristics and non-dietary exposures.
▲ : Positive; ∅: No association; ▼: Negative; BMI: body max index; Socioecon: socioeconomic; exp: exposure; ♀: women; ♂: men.
The time-frame of exposure covered by toenails, investigated only for Se and Mn, was explored in studies that identified a specific moment in which the exposure occurred, which allowed to calculate the time elapsed until detection in toenail samples. Selenium was the most studied element and was evaluated in three research projects.
In the USA, 12 young men participated in a research conducted to evaluate the effect of one-year intake of Se-supplemented whole wheat bread on Se toenail levels. The participants were classified into three groups: those with high-dose (4.91 μmol Se/d), medium-dose (2.61 μmol Se/d) and low-dose (0.41 μmol Se/d), and the concentration of Se in toenail clippings was measured every 1–2 weeks for 2 years (Longnecker et al., 1993). Se peak levels were detected in the big toenail after 24–37 weeks vs. 12–24 weeks in other toes; this difference was attributed to the shorter nail bed in smaller nails. Selenium concentration in the big toenail was still high, even after one year of intake termination and remained over baseline for two years; this suggests that toenail clippings could provide a time-integrated measure of Se intake for over 26–52 weeks, serving as a good biomarker of Se intake for retrospective studies. Other studies included 10 middle-aged men and women provided with Se-76 supplementation, and the peak Se concentrations in toenails were observed at 16–32 (Baskett et al., 1995, 2001) and 16–50 (Baskett et al., 1998) weeks. Peak concentrations were also reported by Morris et al. at 34 weeks after ingestion of high Se misformulated products, while, in this study, baseline levels were recovered after 59 weeks (Morris and Crane, 2013). For Mn, the time-frame of exposure was evaluated in 3 studies conducted among occupationally exposed workers (welders). Exposure time reflected by toenail Mn concentrations was estimated between 7 and 9 (Grashow et al., 2014) and 7–12 months (Laohaudomchok et al., 2011; Ward et al., 2017) (Table 2).
Since one year is an insufficient period for the study of exposure for most chronic diseases, it is crucial to identify the extent to which individual point measurements of trace metals in toenails represent toenail concentration levels in the same person in the previous years.
This stability of essential trace metals over time was evaluated in 7 reports by estimating the within-person correlation among samples obtained at different moments (Table 1) (Baskett et al., 1995; Garland et al., 1993; Krogh et al., 2003; Hunter et al., 1990a; Rodrigues et al., 2015; Xun et al., 2010a). The usage of correlation coefficients to assess reproducibility is not ideal, but no studies used the intra-class correlation coefficient.
For most elements, this information was obtained from a single analysis carried out in 127 female nurses participating in the Nurse Health Study I (NHSI), which measured 5 trace metals (Co, Cu, Fe, Se and Zn) in 2 samples of toenails collected 6 years apart. Correlation values were around 0.5 for Zn, Se and Fe and less than 0.4 for Co and Cu (Garland et al., 1993).
In the case of Se and Mn, our search identified additional studies that evaluated the reproducibility of their measurements in different moments of time. For Se, the studies we identified reported a Pearson correlation coefficient of 0.57 among samples collected around 1 year apart from 80 women in the ORDET prospective study of whom 40 were pre-menopausal and 40 were postmenopausal (Krogh et al., 2003); a correlation coefficient of 0.60 using samples collected approximately 5 years apart, from the NHS I cohort (Hunter et al., 1990a); and a correlation coefficient of 0.56 using toenail Se samples collected 20 years apart from 64 participants in the CARDIA project (Xun et al., 2010a). Another exploratory research stated that Se levels were very stable in toenail samples from 2 men and 2 women, followed up for 15 months prior to Se supplementation (Baskett et al., 1995). For Mn, two studies have been conducted in this context: a study which reported correlation coefficients between 0.37 and 0.41 in pregnant women, with samples taken during the first trimester of pregnancy and one month after delivery (Rodrigues et al., 2015) and another exploratory analysis that evaluated clippings of the five toenails from the same individual, collected four times over a 9-month period, with a large variability in the Mn levels (Guthrie et al., 2008).
3.5. Correlation of trace elements in toenails with other biological matrices (Table 1)
To better understand toenail metal levels as biomarkers of exposure to essential trace elements, it is important to explore their relationship with other substrates commonly used in this field, especially blood and urine. A total of 20 studies with a variable number of participants (range: 30–868) evaluated the correlation of trace elements in toenails with other biological matrices collected concurrently (i.e., whole blood, serum, plasma, urine, hair, fingernails, feces, cord blood, placenta and saliva) (Table 1).
Hair:
Hair was the biological matrix more often compared with toenails (n = 10), probably due to its shared non-invasiveness and its similar keratin-based composition. For Mo, Se or Zn, no significant associations have been observed between levels in this substrate and in toenails (Wilhelm et al., 1991; Steven Morris et al., 1983; Chanpiwat et al., 2015; Kuiper et al., 2014; McKenzie, 1979). Other metals, such as Co and Cu, showed weak positive (< 0.30) or null correlation between both biomarkers (Wilhelm et al., 1991; Herman et al., 2013; Chanpiwat et al., 2015; Sabbioni et al., 1994). In contrast, the association was moderate (between 0.32 and 0.40) for Mn (Ntihabose et al., 2017; Chanpiwat et al., 2015) and Fe (Chanpiwat et al., 2015) and strong (r = 0.61) for V, (Rainska et al., 2007), although the interpretation of the latter result was limited by the small sample size of the study (n = 33).
Urine:
Our search identified two studies which revealed strong positive correlations between metal levels in toenails and urine for Mn (r = 0.65 (Hassani et al., 2016)) and Se (r = 0.60 (Longnecker et al., 1991)). In contrast, Co, Cu, Fe, Mo and Zn in toenails and in urine were not found to be correlated in other studies (Chanpiwat et al., 2015; Kuiper et al., 2014; McKenzie, 1979).
Blood:
Surprisingly, there were only data comparing these two matrices for Se, Mn, Co and Zn. In the case of Se, four studies showed positive and significant correlations with whole blood (r = 0.91 (Longnecker et al., 1991) and r = 0.45 (Rayman et al., 2015)); serum (r = 0.89 (Longnecker et al., 1991)); and plasma (r = 0.55 (Satia et al., 2006)). However, in the study by Al Saleh et al. a negative correlation between log-transformed Se concentrations in toenails and serum was reported (r = −0.16 (Al-Saleh et al., 2006)). Regarding Mn, Hassani et al. described a strong correlation between toenails and whole blood levels (r = 0.65 (Hassani et al., 2016)), but both biomarkers were not found to be related in a sample of welders studied by Wongwit et al. (2004); moreover, Rodrigues et al. reported a low correlation between infant toenails and cord blood concentrations (r = 0.14 (Rodrigues et al., 2015)). Finally, whole blood Co (Sabbioni et al., 1994) and serum Zn (McKenzie, 1979) levels were not significantly correlated with the corresponding toenail concentrations.
Fingernails:
Only three studies evaluated the correlation between toenails and fingernails, showing a low correlation for Mn (r = 0.23 (Coelho et al., 2014)) and a medium correlation for Se (r = 0.33 (Coelho et al., 2014) and r = 0.57 (Alfthan et al., 1992)). In these studies, as well as in the studies by Baskett et al. for Se (Baskett et al., 1995, 1998, 2001), fingernail concentrations were almost twice as high as those from toenails.
Other matrices:
For Mn levels in toenails, a weak correlation has been reported with the concentration in saliva (Ntihabose et al., 2017) and no correlation with levels in feces (Wongwit et al., 2004). A moderate correlation has been reported between infant and maternal toenail Mn levels (r = 0.39 (Rodrigues et al., 2015)). In the case of Se, a positive correlation was observed between maternal toenail and placenta Se concentrations (Punshon et al., 2016).
3.6. Correlation of essential metals with other trace metals in toenails (Table A2)
The correlation among essential trace elements in toenails or with other toxic metals was explored in 14 studies (Table A2, Appendix A). Most of the reported correlations were low-moderate (below 0.40) or non-significant, both for essential and non-essential elements. Among the essential metals group, the strongest correlations were observed between Fe and Co (r = 0.81) (Rainska et al., 2007) and between Mn and V (r = 0.40) (Masironi et al., 1976). Regarding the association between essential and toxic metals, Mn had the highest proportion of strong correlations (with aluminium (Al) (Guthrie et al., 2008), arsenic (As) (Mordukhovich et al., 2012; Sanders et al., 2014), barium (Ba) (Kuiper et al., 2014), cadmium (Cd) (Mordukhovich et al., 2012; Sanders et al., 2014), chromium (Cr) (Sanders et al., 2014), lead (Pb) (Mordukhovich et al., 2012) and uranium (U) (Kuiper et al., 2014)). Other strong correlations were reported for Co and tungsten (W) (Sabbioni et al., 1994), Cu and Cd (Wilhelm et al., 1991), Cu and Pb (Wilhelm et al., 1991; Kuiper et al., 2014), Se and As (negative correlation) (Burgess et al., 2014), V and Al (Masironi et al., 1976) and V and scandium (Sc) (Rainska et al., 2007).
3.7. Determinants of essential metals in toenails
Identification of the exposure sources or factors related to them is crucial in understanding the information provided by the measurement of essential metal levels in toenails. Most of the studies reported on this issue, although the huge variability in the variables studied, populations, designs and indicators made the calculation of quantitative summary estimators practically impossible. Notwithstanding, we have tried to summarize this information in order to give a general view of the published results. For this purpose, we have designed two tables that present, for each combination of metal and factor studied, the identification (i.e., author and date) of each report and the information about their possible relationship. Table 2 includes data for non-dietary factors and Table 3 includes the corresponding associations with intake of supplements, dietary estimates, foods and nutrients.
Table 3.
Studies (Author year) reporting association of toenail essential trace metals to supplements, foods or nutrients.
▲ : Positive; ∅: No association; ▼: Negative.
Ovaskainen 1993 excluded 2 due to Se>1.0mg/kg
Estimated by analysis of duplicate-plate foods
131 items grouped into 21 categories were assesssed in the food frequency questionnaire but only those shown in this table were reported
Non significant after adjusment
3.7.1. Personal and anthropometric characteristics (Table 2)
Age:
For toenail Se, most of the studies showed either no changes with age (Kardinaal et al., 1997; Colangelo et al., 2014; van ‘t Veer et al., 1990; Alfthan et al., 1992; Ovaskainen et al., 1993; Satia et al., 2006; Everson et al., 2017; Hashemian et al., 2017; Kristal et al., 2014; Lipsky et al., 2004; Morris, 2001; Morris et al., 2006; Swanson et al., 1990; van den Brandt et al., 1993; Xun et al., 2010b), or lower levels in elder individuals (Garland et al., 1995; Park and Seo, 2016; Hunter et al., 1990b; Jang et al., 2017; Park et al., 2011; Żukowska et al., 2009). Toenail concentrations of V were also inversely associated with age (Masironi et al., 1976; Rakovic et al., 1997; Slotnick et al., 2005) and a similar result was reported in the only study evaluating Co and Fe (Rakovic et al., 1997). The effect of age was inconsistent for Cu, Mo, Mn and Zn.
Sex:
Women generally had higher toenail Se concentrations than men (Lee et al., 2016; Baskett et al., 2001; Xun et al., 2010a; Rainska et al., 2007; Morris, 2001; Morris et al., 2006; Swanson et al., 1990; van den Brandt et al., 1993; Xun et al., 2010b; Żukowska et al., 2009). For Cu, Mo, Mn and V, the studies showed either higher levels in women (Coelho et al., 2014; Rakovic et al., 1997; Slotnick et al., 2005) or no variation by sex (Masironi et al., 1976; McKenzie et al., 1978; Reis et al., 2015). Fe levels were similar in both sexes (Sureda et al., 2017; Rakovic et al., 1997). For Zn, the available data showed very inconsistent results, with some reports finding no differences by sex (Park and Seo, 2016; Meramat et al., 2017; Przybylowicz et al., 2012; Sureda et al., 2017; Hashemian et al., 2017; Rakovic et al., 1997; McKenzie et al., 1978), while others showed higher concentrations either in women (Lee et al., 2016; Rainska et al., 2007; Bergomi et al., 2002) or in men (Campos et al., 2008; Gonzalez et al., 2008). Cobalt was the only metal for which levels were consistently higher in men (Sureda et al., 2017; Rakovic et al., 1997; Slotnick et al., 2005).
Differences by race/ethnic group have been evaluated for Mn and Se in a few papers. White people usually had higher toenail levels of Mn than other groups (Mordukhovich et al., 2012; Laohaudomchok et al., 2011); for Se, in some studies, levels were found to be higher in whites (Everson et al., 2017; Kristal et al., 2014), while other studies found lower concentrations in African-Americans (Colangelo et al., 2014; Kristal et al., 2014; Xun et al., 2010b, 2011). One study did not find any differences with respect to ethnicity (Satia et al., 2006).
The influence of body mass index (BMI) was studied only for Se, Zn and Fe. Neither Se (Kardinaal et al., 1997; Colangelo et al., 2014; Ovaskainen et al., 1993; Satia et al., 2006; Hashemian et al., 2017; Kristal et al., 2014; Lipsky et al., 2004; Morris et al., 2006; van den Brandt et al., 1993; Hunter et al., 1990b), nor Zn (Hashemian et al., 2017; Park et al., 2016; Vinceti et al., 2015) varied with this factor, while one study reported a positive link between Fe in female toenails and BMI (Garland et al., 1996).
Only a few studies evaluated the effect of reproductive factors, showing that parity may be positively associated with toenail Mn (Rodrigues et al., 2015) and Se concentrations (van Noord et al., 1993), while a later age at first birth may be associated to lower Fe levels (Garland et al., 1996). An older age at menarche was found to be associated with higher Se concentrations (Hunter et al., 1990a), but menopause did not modify levels of this element in toenails (Krogh et al., 2003).
3.7.2. Non-dietary lifestyle & social factors (Table 2)
Educational level was positively correlated with higher Se concentrations (Colangelo et al., 2014; Xun et al., 2010a, 2011; Rayman et al., 2015), while both higher education and monthly income were also associated with higher Mn content (Rodrigues et al., 2015). The relationship between educational level and toenail Zn levels was inconsistent (Park and Seo, 2016; Hashemian et al., 2017; Martin-Moreno et al., 2003).
Twenty out of twenty-four studies found an inverse relationship between tobacco consumption and toenail Se (Garland et al., 1995; Ghadirian et al., 2000; Kardinaal et al., 1997; Colangelo et al., 2014; Krogh et al., 2003; Emmanuelle et al., 2012; Hunter et al., 1990a; Xun et al., 2010a; Satia et al., 2006; Morris, 2001; Morris et al., 2006; Swanson et al., 1990; van den Brandt et al., 1993; Xun et al., 2010b; Hunter et al., 1990b; Park et al., 2011; Żukowska et al., 2009; Xun et al., 2011; van Noord et al., 1993; Virtanen et al., 1996), with a dose-response relationship reported in three of them (Kardinaal et al., 1997; van den Brandt et al., 1993; Hunter et al., 1990b). Smoking, however, seemed to be not related to toenail Mn (Wongwit et al., 2004; Grashow et al., 2014; Ward et al., 2017) or Zn concentrations (Park et al., 2016; Martin-Moreno et al., 2003), although results were not homogeneous (Campos et al., 2008; Coelho et al., 2014; Rakovic et al., 1997).
Sureda et al. reported higher levels of Co, Fe and Zn with physical activity only in elderly women (Sureda et al., 2017). However, this factor was not related to toenail Se (Sureda et al., 2017; Satia et al., 2006; Hashemian et al., 2017; Xun et al., 2010b).
3.7.3. Other environmental factors (Table 2)
With the aim of using toenail levels of trace metals as biomarkers of exposure, several authors evaluated the relationship between toenail trace metals levels and the diverse possible sources of environmental exposure.
Most of the metals showed variations according to dwelling place (Co (Chanpiwat et al., 2015), Cu (Chanpiwat et al., 2015; Ndilila et al., 2014; Mohmand et al., 2015); Fe 71,93,105; Mo (Nouri et al., 2008); Mn 35,55,76,93,107; Se (Colangelo et al., 2014; Steven Morris et al., 1983; Michaud et al., 2002; Emmanuelle et al., 2012; Ndilila et al., 2014; Hashemian et al., 2017; Morris et al., 2006; Xun et al., 2010b, 2011; Hunter et al., 1990b; Park et al., 2011; Yoshizawa et al., 1998) and Zn (Wilhelm et al., 1991; Ndilila et al., 2014; Mohmand et al., 2015; Were et al., 2009; Nouri et al., 2008). In this sense, living in high-polluted areas or near industries or mines was associated with higher levels of some essential trace elements such as Co (Chanpiwat et al., 2015), Mn (Sanders et al., 2014; Coelho et al., 2012, 2014) or Zn (Ndilila et al., 2014). In contrast, these exposures did not seem to be related to Cu, Fe, Se or V toenail concentrations (Rainska et al., 2007; Coelho et al., 2014; Reis et al., 2015).
Toenail Se was also related with the distribution of Se in forage crops (Hunter et al., 1990b) and soils (Morris et al., 2006). In 2012, after a period of Se soil fortification in Finland, an increase in toenail Se levels was observed (Michaud et al., 2002). Soil Cu concentrations were also positively associated with the toenail levels of this metal (Ndilila et al., 2014).
Some projects reported that trace metal concentrations in indoor dust could be important contributors to toenail levels of Co (Ndilila et al., 2014), Mn (Reis et al., 2015), Mo (Rakovic et al., 1997) and Zn (Ndilila et al., 2014; Raińska et al., 2005), while other studies reported consistent associations between Cu, Fe, Mo, Mn and Se in drinking water and in toenails (Ntihabose et al., 2017; Chanpiwat et al., 2015; Emmanuelle et al., 2012; Ndilila et al., 2014; Rodrigues et al., 2015; McKenzie et al., 1978; Reis et al., 2015). However, for Zn (Chanpiwat et al., 2015; McKenzie et al., 1978) and Co (Chanpiwat et al., 2015; Ndilila et al., 2014), results were inconsistent.
The role of occupation as a source of exposure has been assessed in several studies. In this case, the metal that received more attention was Mn, and the research was focused especially, but not only, on welders and boilermakers (Laohaudomchok et al., 2011; Grashow et al., 2014; Ward et al., 2017; Hassani et al., 2016; Coelho et al., 2012, 2014; Menezes et al., 2004a). Toenail Mn levels reflected exposure that occurred 7–12 months before clipping collection (Laohaudomchok et al., 2011; Grashow et al., 2014; Ward et al., 2017) and were correlated with the number of welding hours (Grashow et al., 2014) and with respirable airborne Mn concentrations (Ward et al., 2017). However, no differences were observed with respect to the type of personal protection equipment employed by workers (Wongwit et al., 2004). The consistent results obtained have led some authors to try to define threshold levels to identify the workers with excessive exposures (Ward et al., 2017). For the other metals, data were obtained from a few number of studies: one on workers of a phosphate fertilizer plant (Raińska et al., 2005), two on galvanizing companies (McKenzie, 1979; Menezes et al., 2004a, 2004b), another on tungsten-tin miners (Coelho et al., 2014) and another two on mixed (Sabbioni et al., 1994) or non-specified workers (Rakovic et al., 1997). Exposed workers showed higher levels of Cu (Raińska et al., 2005; Menezes et al., 2004b) and Fe (Rakovic et al., 1997), no association for Co (Rainska et al., 2007; Rakovic et al., 1997; Raińska et al., 2005; Menezes et al., 2004b), Se (Coelho et al., 2014), or V (Raińska et al., 2005; Menezes et al., 2004b) and contradictory results for Mo (Rakovic et al., 1997; Raińska et al., 2005) and Zn (McKenzie, 1979; Rakovic et al., 1997; Raińska et al., 2005).
3.7.4. Supplements, foods and nutrients (Table 3)
The role of toenails as biomarker of supplements intake has been explored for several essential elements, especially for selenium. The use of Se supplements significantly increased toenail Se levels (Garland et al., 1995; Baskett et al., 1995, 1998; Brockman et al., 2009; Ovaskainen et al., 1993; Guthrie et al., 2008; Satia et al., 2006; Kristal et al., 2014; Morris, 2001; Morris et al., 2006; Xun et al., 2010b; Hunter et al., 1990b; Park et al., 2011; Żukowska et al., 2009; Yoshizawa et al., 1998). Both organic and inorganic supplementation had a positive effect on toenail levels (Ovaskainen et al., 1993), with a dose-response effect (Hunter et al., 1990b). Supplement intake explained most of the variation and was the strongest predictor of toenail Se concentrations among supplements users (Ovaskainen et al., 1993; Satia et al., 2006). In contrast, toenail levels of Cu, Mn and Zn were unaffected by supplement use (Brockman et al., 2009; Guthrie et al., 2008; Gonzalez et al., 2008; Milunsky et al., 1992).
Regarding the dietary intake of essential metals, some authors compared the estimated exposure to Co, Mn, Se and Zn through diet with the corresponding levels in toenails. This intake was estimated by different methods, including food frequency questionnaires (FFQs), inventories with interviews and collection of diet composites. The most studied element was Se, with half of the reports showing a direct relationship of toenail levels with global Se intake (Longnecker et al., 1993; Morris and Crane, 2013; Ovaskainen et al., 1993; Longnecker et al., 1991; Swanson et al., 1990; van den Brandt et al., 1993; Longnecker et al., 1996) and the other half reporting no association (Satia et al., 2006; Hashemian et al., 2017; Morris et al., 2006; Hunter et al., 1990b; Vinceti et al., 2015; Kotsopoulos et al., 2010). For the other three metals, most of the reports did not show a good correlation between dietary intake and toenail levels. A number of studies also provided data on the relationship between toenail trace metals and specific foods or nutrients (Table 3).
The information obtained through this review suggests that there is a severe publication bias in this area, with many reports providing only the results for those nutrients with significant associations, without including the results corresponding to foods that do not appear to have a relationship with the studied metal in the report or in supplementary material. According to the available information, toenail Se levels seemed to be positively associated with beef, bread, vitamin C, zinc, folic acid, β-carotene, polyunsaturated fatty acids and dietary mercury intake (Ovaskainen et al., 1993; Xun et al., 2010a; Park et al., 2011), while rice and coffee (Park et al., 2011; Virtanen et al., 1996) were related to lower toenail Se concentrations.
The association of Se with eggs, dairy products, vitamin E and total energy intake was inconsistent among studies (Kardinaal et al., 1997; Ovaskainen et al., 1993; Xun et al., 2010a; Rayman et al., 2015; van den Brandt et al., 1993; Park et al., 2011; Yoshizawa et al., 1998). Regarding other essential elements (Zn, Mn, Cu), most studies found no association with dietary variables (Graham et al., 1991; Laohaudomchok et al., 2011; Ward et al., 2017; McKenzie et al., 1978; Milunsky et al., 1992); however, the intake of Zn, Mg, riboflavin, retinol, fiber and cholesterol was positively associated with toenail Zn levels in three studies (Gonzalez et al., 2008; Park et al., 2016; Bergomi et al., 2005), while the evidence for a correlation with animal proteins intake was inconsistent (Gonzalez et al., 2008; Bergomi et al., 2005).
Alcoholic beverages (Table 3) did not modify Zn toenail levels (Gonzalez et al., 2008; Martin-Moreno et al., 2003). For Se, the published data revealed no association (Garland et al., 1995; Kardinaal et al., 1997; Ovaskainen et al., 1993; Satia et al., 2006; van den Brandt et al., 1993; Hunter et al., 1990b) or a negative relationship with this risk factor (Colangelo et al., 2014; Xun et al., 2010a; Xun et al., 2010b; Park et al., 2011; Xun et al., 2011; van ‘t Veer et al., 1996). Positive associations were reported with Co, Cu, Mo and V (Garland et al., 1996; Rakovic et al., 1997).
4. Discussion
In this systematic review, we presented a comprehensive picture of the available evidence on the role of toenails as biological matrices of exposure to essential trace metals. In addition, we tabulated and homogenized published quantitative levels of essential trace metals measured in toenails in the literature, allowing for future comparisons among studies. Finally, we presented a synopsis of the evidence on factors and sources that may be associated with the concentrations of these elements in this matrix.
Toenails are gaining attention because of their indubitable logistical advantages for large epidemiological studies and because they are supposed to represent longer-term exposures compared with other biomarkers, which is an essential feature to study the involvement of certain agents in chronic diseases. Although toenails share many characteristics with fingernails, they have specific advantages that make them more desirable as a biomarker of exposure. Toenails’ rate of growth is slower; thus, compared with fingernails, toenails can reflect exposures that occurred further in the past. In addition, toenails are less frequently polished with nail varnish than fingernails and participants, especially females, may be less reticent to provide a sample. Some authors (Karagas et al., 2000) consider toenails to have a lower chance of exposure to external contamination, although in any case, both toenails and fingernails need to be pretreated and cleaned before being analyzed.
Another related sample is hair, as both biological matrices have a similar composition. However, in contrast to toenails, hair is frequently altered by cosmetic procedures such as dyeing, bleaching and permanent waving, which are known to modify hair content (Cuypers and Flanagan, 2018). The moment of exposure that is explored by hair samples is also difficult to standardize since it depends on the distance from scalp, and baldness may limit the study of elder men. In contrast, there is certified reference material for hair, which is used by many laboratories for nails.
The reason why toenails have been proposed as biological matrix to evaluate past exposures is that, after binding to keratin proteins present in nails, the concentrations of the elements deposited in them seem to remain stable in time, independent of changes in metabolic activities (Hopps, 1977; Sukumar, 2006). In this case, the researchers estimate that the levels of selected metals (i.e., Mn or Se) measured in toenail clippings reflect the exposure that occurred 6–12 months earlier, a time-window of exposure congruent with the estimated time required by toenails to grow (Yaemsiri et al., 2010). However, there is another critical issue: the use of toenails as a proxy for maintained exposures, implies that point measurements of the studied metals have to correlate well with ulterior determinations of the same element in the same individual. Our review shows that the stability of toenail levels of essential trace metals over time was examined in a few number of studies, which found that for some metals (i.e., Se and Zn), there was a good correlation among measurements even after years. However, for other elements (i.e., Fe, Mg, Mn, Co, Cu), intraindividual correlations coefficients were moderate or low, and obtained from a single study; in a third group (i.e., Mo and V), it has not been investigated. Thus, available information support the findings that for some essential metals, toenails may be a good biological matrix to measure long-term exposure, although data on this issue are scarce for other metals. Direct extrapolation of findings for one element to other elements might be risky; it implies that differences in the internal metabolism among trace elements do not play any role in their arrival and deposition in toenails. In fact, homeostasis of each essential trace element is tightly and specifically regulated because of their relevance in the organism, and this regulation may modify the relationship between exposure and the biomarker, making it more difficult to interpret.
It is also of interest to investigate the relationship of essential trace elements in toenails with their level in other commonly used biomarkers of exposure in the same individual. All but two studies showed non-significant correlations between levels in toenails and urine, suggesting either different exposure time-windows for both biospecimens or different metabolic pathways involved in the arrival of these elements to the kidney and toenail matrix. The correlation of whole blood, plasma and serum with toenails has only been assessed in a few studies, and the association was only consistent for Se. Not many reports have compared trace element concentrations in toenails with those in hair and fingernails. Surprisingly, correlations with hair or fingernail levels were also low to moderate; we expected them to be higher owing to the shared characteristics among these substrates (i.e., skin appendages with slow growth and similar composition) and their common use as long-term exposure biomarkers.
We were also interested in understanding whether toenails reflected external exposures and the sources that might be explored with them. It is noteworthy that for this substrate, there is great variability of concentrations among studies, suggesting that differences in external environmental exposures may have been reflected. In this case, because we evaluated essential elements for humans, we presumed that the diet was probably the main source of exposure in the general population. However, our review showed that mostly there was no evidence supporting a direct correlation between metal dietary intake estimates and corresponding levels in toenails; even for Se, the most frequently studied trace element, the results were quite conflicting, with half of the studies reporting no association between estimated global intake and toenail concentration. It should be noted that in many cases, dietary intake was estimated with FFQs, which are have known limitations as a tool to assess exposure to trace elements. FFQ-based individual micronutrient intake estimates have a high level of uncertainty; in addition to the possible inaccuracy of diet recording due to recall bias, such estimates are calculated with the help of food composition tables, which, in many cases, are standard and may not represent the real exposure in local settings.
In contrast, the relationship between the intake of supplements of Se and toenail concentration was very consistent, probably due to the high doses of the element in the supplements. For other elements (Zn, Mn, Cu), oral supplements were not related to toenail levels; however, there were no data for Fe, another element usually administered in supplements.
Regarding individual foods, it is difficult to review available evidence because of the obvious selective publication bias. A good example of this is the report of Park et al. on the determinants of toenail Se in NHS and NPFS participants (Park et al., 2011). The authors explain that they correlated toenail levels of this element with 21 food groups, but they only provide results for seven of them. Another limitation of assessing associations with many individual food items separately is that some associations will yield significant results just by chance (multiple comparisons).
Many exposures are related to the place of people’s residence and our data indicate that the dwelling place was a factor substantially affecting toenail metal concentrations. Indoor dust, which is closely related to environmental exposure, was also described as a non-negligible source of exposure for some metals (Co, Mn, Zn). Similarly, drinking water, which is related to the geographical area, represented an important source for some metals (Co, Cu, Fe, Mn, Se) in environments with high exposure levels. Finally, several studies showed that living near mining areas or in polluted regions significantly increased exposure to certain trace elements (Co, Mn, Zn). Based on these findings, it is of great importance that future studies consider the influence of geographical area, especially in multicentric epidemiological investigations.
Occupation is another important source of exposure to metals, which can occur through inhalation, ingestion or dermal absorption (Elder et al., 2015). However, its relevance and presence in the published literature seem less obvious in this subgroup of essential elements than for toxic metals such as Cd or As. However, workers are also exposed to these agents during specific industrial procedures and thus, occupation needs to be considered as a source of exposure to essential elements. The most studied metal in this field has been Mn and some authors have even tested the use of toenails to monitor exposure to Mn.
We also assessed the association of personal (age, sex, ethnicity, BMI) and socioeconomic and non-dietary lifestyle-related factors with metal levels. Age is one of the main factors that could modify toenail metal levels; for example, some studies suggested that age could change the rate of incorporation of elements such as Se into toenails, but also that differences between younger and elder people could reflect age-related variations in the total amount of the element in the body (Hunter et al., 1990b). Sex differences may also occur due to hormonal regulation and variations by race may be due to changes in enzyme activity. However, age, sex, ethnicity and BMI may also be associated to unidentified exposure patterns or confounders. In general, we did not identify any specific predictors of essential metals in toenails, with some exceptions (i.e., higher levels of Se in women, or inverse association with age for V). Of special interest is the inverse relationship between Se and tobacco use, which seems to be relatively consistent based on several reports and this relationship is consistent with the already described association between smoking and circulating selenium concentrations (Ellingsen et al., 2009).
In real world, mixed exposures are the rule and not the exception. The correlation among levels of different metals in toenails was explored only in a few studies. Some essential trace elements (i.e., Se) correlated negatively with toxic agents (i.e., Cd or As), suggesting that they are interrelated and share or interfere in the absorption or availability of toxic agents. Other essential metals (Co, Cu, Fe, Mn, Zn) correlated both with non-toxic and toxic metals, indicating common sources of exposure (i.e., diet or environment).
Another point of interest pertains to the quality and methodological details of reports included in this review and to the methodological issues they reflect. Our search found that relevant information such as inclusion/exclusion criteria was not always provided in the reports, making comparisons among studies difficult. Moreover, although quality control procedures have improved over time, there is no standardized protocol on toenail preparation, analyses and report, which would be essential owing to the challenges that this biomarker presents. Among them, we would highlight the absence of certified reference material for this substrate, a problem that has been addressed with diverse strategies in different studies. In addition, cleaning procedures should be homogeneously performed, as they are essential to avoid external contamination, especially for metals derived from nail polish, which may contain trace elements such as B, Fe or Zn (Favaro et al., 2005), or other elements such as hafnium, which may interfere with metal concentrations (St-Pierre et al., 2006). In addition, important information, such as limits of detection, was absent in many reports.
Regarding the analytic technique, neutron activation analysis (NAA) and inductively coupled mass spectrometry (ICP-MS) were the most frequently used techniques. In general, ICP-MS was the preferred technique for multi-elemental analysis, although NAA was also used to measure multiple metals. The validity of both techniques for detecting trace elements is well known; however, we did not find any study comparing their performance in toenails. As we previously mentioned, quantitative data on metal concentrations were most frequently reported using arithmetic means. However, the distribution of toenail metal concentration does not frequently follow a normal distribution and outliers are frequent. Consequently, geometric means or medians should be preferred when reporting results. Few studies reported the percentage of samples under LOD; probably, most samples had detectable levels of essential metals because of the low LOD that INAA and IPC-MS usually have, but if that were not the case, the lack of this information could impair the comparability among studies or affect the results. Another limitation was how LODs were determined, without giving a true indication of detectability, since the matrix effect is not being taken into account.
A relatively surprising finding was the general lack of information about sample mass, as it is known that the LOD and the metal concentrations in toenails are affected by sample size (Colangelo et al., 2014). Moreover, toenail type was frequently omitted, probably due to the assumption that metals are equally distributed in big and small toes. However, as their growth rate is different (Yaemsiri et al., 2010; Edwards and Schott, 1937), the time-windows represented by them is also different (Longnecker et al., 1991, 1993). Regarding which strategy would be better, we could state that using only big toes allows to explore a more defined and homogenous period of exposure; on the other hand, it is more probable to have a low amount of toenail mass available for the analyses. Notwithstanding, at least for Se, available research indicates that concentrations are similar among toes (Kok et al., 1989; Longnecker et al., 1993; van ‘t Veer et al., 1990). New investigations are needed to confirm if this statement applies to the other metals.
One of the main strengths of our review is its wide scope, which provides an overview of the existing information about the role of toenails as biological matrices of exposure to all essential metals. However, its major limitation is that the great heterogeneity of the studies, with respect to exposure or personal features of the subjects precluded any quantitative pooling of the results. Nevertheless, our tables intend to be useful instruments for those interested in investigating a specific metal, allowing anyone to easily locate all the studies that provide information in this context. Another limitation could be the exclusion of a number of studies that did not state the type of nail analyzed (i.e., fingernail or toenail), an issue that was not clarified by the corresponding authors after our request (see Fig. A1, flow diagram). In this case, we opted for being specific.
5. Conclusions
In summary, this review provided evidence on the role of toenails as biological matrices of exposure to essential metals. For some metals (Se, Mn, Zn), there is evidence that toenail metal concentrations can reflect long-term external exposures and the levels are reproducible, although for other elements, this information is unknown. There remain many uncertainties on the potential role of socioeconomic, lifestyle-related and other factors that affect the metal levels in toenails. Se levels can reflect exposure to certain foods or nutrients from diet or supplements and are modified by tobacco exposure; also Mn concentration can also indicate occupational exposure. Toenail levels of most of the essentials metals studied could also be affected by environmental factors such as pollution and the concentration of metals in soils or water, although reports are scarce for many trace elements. Standardization of sample collection, quality control, analytical techniques and reporting procedures might help boost research and better understand the implications of essential levels in this promising matrix, and would enhance the use of toenails in epidemiological research.
Supplementary Material
Acknowledgments
Funding sources
This work was supported by FIS grants PI12/00150, PI17CIII/00034 & PI18/00287 (Instituto de Salud Carlos III, State Secretary of R + D + I and European Union (ERDF/ESF, “Investing in your future”)).
Footnotes
Conflicts of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2019.108787.
References
- Abdulrahman FI, Akan JC, Chellube ZM, Waziri M, Abdulrahman FI, Akan JC, et al. , 2012. Levels of heavy metals in human hair and nail samples from maiduguri metropolis, Borno state, Nigeria. World Environ. 2 (4), 81–89. [Google Scholar]
- Al-Saleh I, Billedo G, 2006. Determination of selenium concentration in serum and toenail as an indicator of selenium status. Bull Environ Contam Toxicol. agosto de 77 (2), 155–163. [DOI] [PubMed] [Google Scholar]
- Alfthan G, Bogye G, Aro A, Feher J, 1992. The human selenium status in Hungary. J Trace Elem Electrolytes Health Dis. diciembre de 6 (4), 233–238. [PubMed] [Google Scholar]
- Baskett CK, Spate VL, Morris JS, Anderson HD, Mason MM, Reams CL, et al. , 1995. Investigation of the appearance of supplemental enriched Se-76 using the human nail as a dietary monitor. J Radioanal Nucl Chem 195 (1), 97–108 [Google Scholar]
- Radioanal J. Nucl. Chem. Articles, J. Radioanal. Nucl. Chem. Lett., J. Radioanal. Chem.: Int. J. dealing with all aspects of nucl. anal. methods. agosto de. [Google Scholar]
- Baskett CK, Morris JS, Spate VL, Mason MM, Cheng TP, Nichols TA, et al. , 1998. The effect of an enriched selenium-76 supplement on dietary monitors and glutathione peroxidase activity. J Radioanal Nucl Chem. 1 de octubre de 236 (1–2), 39–45. [Google Scholar]
- Baskett CK, Spate VL, Mason MM, Nichols TA, Williams A, Dubman I, et al. , 2001. Long-term selenium status in humans. J. Radioanal. Nucl. Chem. 1 de agosto de 249 (2), 429–435. [Google Scholar]
- Bergomi M, Vinceti M, Nacci G, Pietrini V, Brätter P, Alber D, et al. , 2002. Environmental exposure to trace elements and risk of amyotrophic lateral sclerosis: a population-based case-control study. Environ Res. junio de 89 (2), 116–123. [DOI] [PubMed] [Google Scholar]
- Bergomi M, Pellacani G, Vinceti M, Bassissi S, Malagoli C, Alber D, et al. , 2005. Trace elements and melanoma. J. Trace Elem. Med. Biol. 19 (1), 69–73. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Surette C, Cormier P, Foucher D, 2017. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology (Little Rock) 15 de julio de. [DOI] [PubMed] [Google Scholar]
- Brockman JD, Guthrie JM, Morris JS, Davis J, Madsen R, Robertson JD, 2009. Analysis of the toenail as a biomonitor of supranutritional intake of Zn, Cu, and Mg. J Radioanal Nucl Chem. 1 de febrero de 279 (2), 405–410. [Google Scholar]
- Burgess JL, Kurzius-Spencer M, Poplin GS, Littau SR, Kopplin MJ, Stürup S, et al. , 2014. Environmental arsenic exposure, selenium and sputum alpha-1 antitrypsin. J Expo Sci Environ Epidemiol. abril de 24 (2), 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FI, Koriyama C, Akiba S, Carrasquilla G, Serra M, Carrascal E, et al. , 2008. Toenail zinc level and gastric cancer risk in Cali, Colombia. J Cancer Res Clin Oncol. febrero de 134 (2), 169–178. [DOI] [PubMed] [Google Scholar]
- Chanpiwat P, Himeno S, Sthiannopkao S, 2015. Arsenic and other metals’ presence in biomarkers of Cambodians in arsenic contaminated areas. Int J Environ Res Public Health. noviembre de 12 (11), 14285–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho P, Costa S, Silva S, Walter A, Ranville J, Sousa ACA, et al. , 2012. Metal (loid) levels in biological matrices from human populations exposed to mining contamination–Panasqueira Mine (Portugal). J. Toxicol. Environ. Health Part A 75 (13–15), 893–908. [DOI] [PubMed] [Google Scholar]
- Coelho P, Costa S, Costa C, Silva S, Walter A, Ranville J, et al. , 2014. Biomonitoring of several toxic metal(loid)s in different biological matrices from environmentally and occupationally exposed populations from Panasqueira mine area. Portugal. Environ. Geochem. Health 36 (2), 255–269. [DOI] [PubMed] [Google Scholar]
- Colangelo LA, He K, Whooley MA, Daviglus ML, Morris S, Liu K, 2014. Selenium exposure and depressive symptoms: the coronary artery risk development in young adults trace element study. Neurotoxicology. marzo de 41, 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers E, Flanagan RJ, 2018. The interpretation of hair analysis for drugs and drug metabolites. Clin. Toxicol. 56 (2), 90–100. [DOI] [PubMed] [Google Scholar]
- Edwards Linden F., Schott Ralph G., 1937. The daily rate of growth of toenails. Ohio J. Sci. v37, 91–98. [Google Scholar]
- Elder A, Nordberg GF, Kleinman M, 2015. Routes of Exposure, Dose, and Toxicokinetics of Metals. En: Handbook on the Toxicology of Metals, fourth ed. Academic Press, San Diego, pp. 45–74. [Google Scholar]
- Ellingsen DG, Thomassen Y, Rustad P, Molander P, Aaseth J, 2009. The time-trend and the relation between smoking and circulating selenium concentrations in Norway. J. Trace Elem. Med. Biol. 23 (2), 107–115. [DOI] [PubMed] [Google Scholar]
- Emmanuelle B, Virginie M, Fabienne S, Isabelle I, Martine P-G, Bernard L, et al. , 2012. Selenium exposure in subjects living in areas with high selenium concentrated drinking water: results of a French integrated exposure assessment survey. Environ Int. abril de 40, 155–161. [DOI] [PubMed] [Google Scholar]
- Everson TM, Kappil M, Hao K, Jackson BP, Punshon T, Karagas MR, et al. , 2017. Maternal exposure to selenium and cadmium, fetal growth, and placental expression of steroidogenic and apoptotic genes. Environ Res. octubre de 158, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro PC, Bode P, De Nadai Fernandes EA, 2005. Trace elements in nail polish as a source of contamination of nail clippings when used in epidemiological studies. J Radioanal Nucl Chem. 1 de marzo de 264 (1), 61–65. [Google Scholar]
- Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, et al. , 1993. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. octubre de 2 (5), 493–497. [PubMed] [Google Scholar]
- Garland M, Morris JS, Stampfer MJ, Colditz GA, Spate VL, Baskett CK, et al. , 1995. Prospective study of toenail selenium levels and cancer among women. J Natl Cancer Inst. 5 de abril de 87 (7), 497–505. [DOI] [PubMed] [Google Scholar]
- Garland M, Morris JS, Colditz GA, Stampfer MJ, Spate VL, Baskett CK, et al. , 1996. Toenail trace element levels and breast cancer: a prospective study. Am. J. Epidemiol. 144 (7), 653–660. [DOI] [PubMed] [Google Scholar]
- Ghadirian P, Maisonneuve P, Perret C, Kennedy G, Boyle P, Krewski D, et al. , 2000. A case-control study of toenail selenium and cancer of the breast, colon, and prostate. Cancer Detect. Prev. 24 (4), 305–313. [PubMed] [Google Scholar]
- Gonzalez A, Peters U, Lampe JW, Satia JA, White E, 2008. Correlates of toenail zinc in a free-living U.S. population. Ann. Epidemiol. enero de 18 (1), 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullé JP, Saussereau E, Mahieu L, Bouige D, Groenwont S, Guerbet M, et al. , 2009. Application of inductively coupled plasma mass spectrometry multielement analysis in fingernail and toenail as a biomarker of metal exposure. J Anal Toxicol. marzo de 33 (2), 92–98. [DOI] [PubMed] [Google Scholar]
- Graham NM, Sorensen D, Odaka N, Brookmeyer R, Chan D, Willett WC, et al. , 1991. Relationship of serum copper and zinc levels to HIV-1 seropositivity and progression to AIDS. J. Acquir. Immune Defic. Syndr. 4 (10), 976–980. [PubMed] [Google Scholar]
- Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, Cavallari JM, 2014. Toenail metal concentration as a biomarker of occupational welding fume exposure. J. Occup. Environ. Hyg. 11 (6), 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie JM, Brockman JD, Morris JS, Robertson JD, 2008. The “One Source” cohort — evaluating the suitability of the human toenail as a manganese biomonitor. J Radioanal Nucl Chem. 1 de abril de 276 (1), 41–47. [Google Scholar]
- Hartman TJ, Taylor PR, Alfthan G, Fagerstrom R, Virtamo J, Mark SD, et al. , 2002. Toenail selenium concentration and lung cancer in male smokers (Finland). Cancer Causes Control. diciembre de 13 (10), 923–928. [DOI] [PubMed] [Google Scholar]
- Hashemian M, Murphy G, Etemadi A, Poustchi H, Brockman JD, Kamangar F, et al. , 2017. Toenail mineral concentration and risk of esophageal squamous cell carcinoma, results from the Golestan Cohort Study. Cancer Med. diciembre de 6 (12), 3052–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani H, Golbabaei F, Shirkhanloo H, Tehrani-Doust M, 2016. Relations of biomarkers of manganese exposure and neuropsychological effects among welders and ferroalloy smelters. Ind. Health 54 (1), 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M, Przybylowicz A, Florek E, Piekoszewski W, 2013. Method of determination of low copper concentration in human hair and nails. J Anal Chem. abril de 68 (4), 360–367. [Google Scholar]
- Hopps HC, 1977. The biologic bases for using hair and nail for analyses of trace elements. Sci Total Environ. enero de 7 (1), 71–89. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Morris JS, Stampfer MJ, Colditz GA, Speizer FE, Willett WC, 1990a. A prospective study of selenium status and breast cancer risk. JAMA. 5 de septiembre de 264 (9), 1128–1131. [PubMed] [Google Scholar]
- Hunter DJ, Morris JS, Chute CG, Kushner E, Colditz GA, Stampfer MJ, et al. , 1990b. Predictors of selenium concentration in human toenails. Am J Epidemiol. julio de 132 (1), 114–122. [DOI] [PubMed] [Google Scholar]
- IARC, 2012. Chromium VI compounds [Internet]. IARC Monographs on the evaluation of carcinogenic risks to humans. [citado 6 de noviembre de 2017]. Disponible en: http://monographs.iarc.fr/ENG/Monographs/vol100C/index.php. [Google Scholar]
- Institute of Medicine (US), 2001. Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc [Internet]. National Academies Press (US), Washington (DC) [citado 6 de noviembre de 2017]. Disponible en: http://www.ncbi.nlm.nih.gov/books/NBK222310/. [PubMed] [Google Scholar]
- Jang J, Morris JS, Park K, 2017. Toenail selenium levels and prevalence of dyslipidaemia among Korean adults. Br J Nutr. septiembre de 118 (6), 473–480. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Blum J, Klaue B, Weiss JE, Stannard V, et al. , 2000. Measurement of low levels of arsenic exposure: a comparison of water and toenail concentrations. Am J Epidemiol. 1 de julio de 152 (1), 84–90. [DOI] [PubMed] [Google Scholar]
- Kardinaal AF, Kok FJ, Kohlmeier L, Martin-Moreno JM, Ringstad J, Gómez-Aracena J, et al. , 1997. Association between toenail selenium and risk of acute myocardial infarction in European men. The EURAMIC study. European antioxidant myocardial infarction and breast cancer. Am J Epidemiol. 15 de febrero de 145 (4), 373–379. [DOI] [PubMed] [Google Scholar]
- Kok FJ, Hofman A, Witteman JC, de Bruijn AM, Kruyssen DH, de Bruin M, et al. , 1989. Decreased selenium levels in acute myocardial infarction. JAMA. 24 de febrero de 261 (8), 1161–1164. [PubMed] [Google Scholar]
- Kotsopoulos J, Chen Z, Vallis KA, Poll A, Ghadirian P, Kennedy G, et al. , 2010. Toenail selenium status and DNA repair capacity among female BRCA1 mutation carriers. Cancer Causes Control. mayo de 21 (5), 679–687. [DOI] [PubMed] [Google Scholar]
- Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, et al. , 2014. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. J Natl Cancer Inst. marzo de 106 (3), djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh V, Pala V, Vinceti M, Berrino F, Ganzi A, Micheli A, et al. , 2003. Toenail selenium as biomarker: reproducibility over a one-year period and factors influencing reproducibility. J. Trace Elem. Med. Biol. 17 (Suppl. 1), 31–36. [PubMed] [Google Scholar]
- Kuiper N, Rowell C, Nriagu J, Shomar B, 2014. What do the trace metal contents of urine and toenail samples from Qatar׳s farm workers bioindicate? Environ Res. mayo de 131, 86–94. [DOI] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, et al. , 2011. Toenail, blood, and urine as biomarkers of manganese exposure. J Occup Environ Med. mayo de 53 (5), 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee O, Moon JH, Kim SH, Chung YS, Sun GM, 2016. Toenail elemental analysis of Korean young adults by instrumental neutron activation analysis. J Radioanal Nucl Chem. marzo de 307 (3), 2571–2575. [Google Scholar]
- Lipsky K, Zigeuner R, Zischka M, Schips L, Pummer K, Rehak P, et al. , 2004. Selenium levels of patients with newly diagnosed prostate cancer compared with control group. Urology. mayo de 63 (5), 912–916. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Taylor PR, Levander OA, Howe M, Veillon C, McAdam PA, et al. , 1991. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am J Clin Nutr. mayo de 53 (5), 1288–1294. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Stampfer MJ, Morris JS, Spate V, Baskett C, Mason M, et al. , 1993. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. marzo de 57 (3), 408–413. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, et al. , 1996. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. julio de 7 (4), 384–390. [DOI] [PubMed] [Google Scholar]
- Männistö S, Alfthan G, Virtanen M, Kataja V, Uusitupa M, Pietinen P, 2000. Toenail selenium and breast cancer-a case-control study in Finland. Eur J Clin Nutr. febrero de 54 (2), 98–103. [DOI] [PubMed] [Google Scholar]
- Martin-Moreno JM, Gorgojo L, Riemersma RA, Gomez-Aracena J, Kark JD, Guillen J, et al. , 2003. Myocardial infarction risk in relation to zinc concentration in toenails. Br J Nutr. mayo de 89 (5), 673–678. [DOI] [PubMed] [Google Scholar]
- Masironi R, Koirtyohann SR, Pierce JO, Schamschula RG, 1976. Calcium content of river water, trace element concentrations in toenails, and blood pressure in village populations in New Guinea. Sci Total Environ. julio de 6 (1), 41–53. [DOI] [PubMed] [Google Scholar]
- McKenzie JM, 1979. Content of zinc in serum, urine, hair, and toenails of New Zealand adults. Am J Clin Nutr. marzo de 32 (3), 570–579. [DOI] [PubMed] [Google Scholar]
- McKenzie JM, Guthrie BE, Prior IA, 1978. Zinc anc copper status of Polynesian residents in the Tokelau Islands. Am J Clin Nutr. marzo de 31 (3), 422–428. [DOI] [PubMed] [Google Scholar]
- Menezes MÂBC, Sabino CVS, Franco MB, Maia ECP, Albinati CCB, 2004a. Assessment of workers’ contamination caused by air pollution exposure in industry using biomonitors. J. Atmos. Chem. 49 (1–3), 403–414. [Google Scholar]
- Menezes M, Maia ECP, Albinati CCB, Sabino CVS, Batista JR, 2004b. How suitable are scalp hair and toenail as biomonitors? J. Radioanal. Nucl. Chem. 259 (1), 81–86. [Google Scholar]
- Meramat A, Rajab NF, Shahar S, Sharif RA, 2017. DNA damage, copper and lead associates with cognitive function among older adults. J. Nutr. Health Aging 21 (5), 539–545. [DOI] [PubMed] [Google Scholar]
- Mertz W, 1981. The essential trace elements. Science. 18 de septiembre de 213 (4514), 1332–1338. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Hartman TJ, Taylor PR, Pietinen P, Alfthan G, Virtamo J, et al. , 2002. No Association between toenail selenium levels and bladder cancer risk. Cancer Epidemiol Biomarkers Prev. noviembre de 11 (11), 1505–1506. [PubMed] [Google Scholar]
- Milunsky A, Morris JS, Jick H, Rothman KJ, Ulcickas M, Jick SS, et al. , 1992. Maternal zinc and fetal neural tube defects. Teratology. octubre de 46 (4), 341–348. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group, T.P., 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine. 21 de julio de 6 (7), e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohmand J, Eqani SAMAS, Fasola M, Alamdar A, Mustafa I, Ali N, et al. , 2015. Human exposure to toxic metals via contaminated dust: bio-accumulation trends and their potential risk estimation. Chemosphere. agosto de 132, 142–151. [DOI] [PubMed] [Google Scholar]
- Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. , 2012. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect. enero de 120 (1), 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, 2001. Long-term selenium status in humans. Journal of Radioanalytical and Nuclear Chemistry. 1 de agosto de 249 (2), 429–435. [Google Scholar]
- Morris JS, Crane SB, 2013. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients. abril de 5 (4), 1024–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Spate VL, Ngwenyama RA, 2006. Determinants of selenium in the toenail biomonitor. J Radioanal Nucl Chem. 1 de agosto de 269 (2), 283–290. [Google Scholar]
- Ndilila W, Callan AC, McGregor LA, Kalin RM, Hinwood AL, 2014. Environmental and toenail metals concentrations in copper mining and non mining communities in Zambia. Int J Hyg Environ Health. enero de 217 (1), 62–69. [DOI] [PubMed] [Google Scholar]
- Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M, 2016. Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr Environ Health Rep 3 (4), 416–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri M, Chalian H, Bahman A, Mollahajian H, Ahmadi-Faghih M, Fakheri H, et al. , 2008. Nail molybdenum and zinc contents in populations with low and moderate incidence of esophageal cancer. Arch Iran Med. julio de 11 (4), 392–396. [PubMed] [Google Scholar]
- Ntihabose R, Surette C, Foucher D, Clarisse O, Bouchard MF, 2017. Assessment of saliva, hair and toenails as biomarkers of low level exposure to manganese from drinking water in children. Neurotoxicology (Little Rock) 1 de septiembre de. [DOI] [PubMed] [Google Scholar]
- Ovaskainen ML, Virtamo J, Alfthan G, Haukka J, Pietinen P, Taylor PR, et al. , 1993. Toenail selenium as an indicator of selenium intake among middle-aged men in an area with low soil selenium. Am J Clin Nutr. mayo de 57 (5), 662–665. [DOI] [PubMed] [Google Scholar]
- O’Rorke MA, Cantwell MM, Abnet CC, Brockman AJD, Murray LJ, 2012. FINBAR Study Group. Toenail trace element status and risk of Barrett’s oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. Int J Cancer. 15 de octubre de 131 (8), 1882–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Seo E, 2016. Association between toenail mercury and metabolic syndrome is modified by selenium. Nutrients. 12 de julio de 8 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Rimm E, Siscovick D, Spiegelman D, Morris JS, Mozaffarian D, 2011. Demographic and lifestyle factors and selenium levels in men and women in the U.S. Nutr. Res. Pract. agosto de 5 (4), 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Xun P, Li J, Morris SJ, Jacobs DR, Liu K, et al. , 2016. Longitudinal association between toenail zinc levels and the incidence of diabetes among American young adults: the CARDIA Trace Element Study. Sci. Rep. 6, 23155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Helzlsouer KJ, Hoffman SC, Morris JS, Baskett CK, Comstock GW, 2002. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. Prostate. 1 de septiembre de 52 (4), 288–296. [DOI] [PubMed] [Google Scholar]
- Przybylowicz A, Chesy P, Herman M, Parczewski A, Walas S, Piekoszewski W, 2012. Examination of distribution of trace elements in hair, fingernails and toenails as alternative biological materials. Application of chemometric methods. Cent Eur J Chem. octubre de 10 (5), 1590–1599. [Google Scholar]
- Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, Karagas MR, 2016. Placental metal concentrations in relation to maternal and infant toenails in a U.S. Cohort. Environ Sci Technol. 2 de febrero de 50 (3), 1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainska E, Biziuk M, Bode P, Dlugolecki P, Astel K, 2007. An evaluation of endemic exposure of citizens living in near a Gdansk phosphatic fertilizer plant. Pol. J. Environ. Stud. 16 (2), 243–250. [Google Scholar]
- Raińska E, Biziuk M, Sarbu C, Szczepaniak K, Frontasyeva MV, Culicov O, et al. , 2005. Assessment of phosphatic fertilizer production impact on occupational staff based on NAA of hair, nails, and inhald particles. J Environ Sci Health A Tox Hazard Subst Environ Eng 40 (12), 2137–2152. [DOI] [PubMed] [Google Scholar]
- Rakovic M, Foltýnová V, Pilecká N, Glagolicová A, Kucera J, 1997. Assessment of metals and metalloids in skin derivatives of volunteers from capital city of Prague, Czech Republic. Sb. Lek. 98 (2), 107–114. [PubMed] [Google Scholar]
- Rayman MP, Bath SC, Westaway J, Williams P, Mao J, Vanderlelie JJ, et al. , 2015. Selenium status in U.K. pregnant women and its relationship with hypertensive conditions of pregnancy. Br J Nutr. 28 de enero de 113 (2), 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis AP, Costa S, Santos I, Patinha C, Noack Y, Wragg J, et al. , 2015. Investigating relationships between biomarkers of exposure and environmental copper and manganese levels in house dusts from a Portuguese industrial city. Environ Geochem Health. agosto de 37 (4), 725–744. [DOI] [PubMed] [Google Scholar]
- Rodrigues EG, Kile M, Dobson C, Amarasiriwardena C, Quamruzzaman Q, Rahman M, et al. , 2015. Maternal-infant biomarkers of prenatal exposure to arsenic and manganese. J Expo Sci Environ Epidemiol. diciembre de 25 (6), 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saat NZM, Mohandas B, Ghazali AR, 2013. Study of heavy metals in hair and nails of among farmers in Kelantan, Malaysia. Res. J. Appl. Sci. 8 (3), 225–229. [Google Scholar]
- Sabbioni E, Minoia C, Pietra R, Mosconi G, Forni A, Scansetti G, 1994. Metal determinations in biological specimens of diseased and non-diseased hard metal workers. Sci Total Environ. 30 de junio de 150 (1–3), 41–54. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Miller SK, Nguyen V, Kotch JB, Fry RC, 2014. Toxic metal levels in children residing in a smelting craft village in Vietnam: a pilot biomonitoring study. BMC Public Health 14, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santonen T, Aitio A, Fowler BA, Nordberg M, 2015. Biological Monitoring and Biomarkers. En: Handbook on the Toxicology of Metals, fourth ed. Academic Press, San Diego, pp. 155–171. [Google Scholar]
- Satia JA, King IB, Morris JS, Stratton K, White E, 2006. Toenail and plasma levels as biomarkers of selenium exposure. Ann Epidemiol. enero de 16 (1), 53–58. [DOI] [PubMed] [Google Scholar]
- Slotnick MJ, Nriagu JO, Johnson MM, Linder AM, Savoie KL, Jamil HJ, et al. , 2005. Profiles of trace elements in toenails of Arab-Americans in the Detroit area, Michigan. Biol Trace Elem Res. noviembre de 107 (2), 113–126. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Kada R, Kennedy G, Zayed J, Ghadirian P, 2006. Variation of selenium concentration in toenails following long-term storage, washing and reactor irradiation. J Radioanal Nucl Chem. 1 de agosto de 269 (2), 347–349. [Google Scholar]
- Steven Morris J, Stampfer MJ, Willett W, 1983. Dietary selenium in humans toenails as an indicator. Biol Trace Elem Res. diciembre de 5 (6), 529–537. [DOI] [PubMed] [Google Scholar]
- Sukumar A, 2006. Human Nails as a Biomarker of Element Exposure. En: Reviews of Environmental Contamination and Toxicology. [Internet]. Springer, New York, NY, pp. 141–177. [citado 17 de noviembre de 2017]. 10.1007/0-387-30638-2_5 (Reviews of Environmental Contamination and Toxicology). Disponible en:. [DOI] [Google Scholar]
- Sureda A, Bibiloni MDM, Julibert A, Aparicio-Ugarriza R, Palacios-Le Blé G, Pons A, et al. , 2017. Trace element contents in toenails are related to regular physical activity in older adults. PLoS One 12 (10), e0185318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CA, Longnecker MP, Veillon C, Howe M, Levander OA, Taylor PR, et al. , 1990. Selenium intake, age, gender, and smoking in relation to indices of selenium status of adults residing in a seleniferous area. Am J Clin Nutr. noviembre de 52 (5), 858–862. [DOI] [PubMed] [Google Scholar]
- University of York Prospero. [Internet] [citado 6 de abril de 2018]. Disponible en: https://www.crd.york.ac.uk/prospero/.
- US EPA O, 2014. Issue Paper on the Human Health Effects of Metals. [Internet] US EPA; [citado 6 de noviembre de 2017]. Disponible en: https://www.epa.gov/osa/issue-paper-human-health-effects-metals. [Google Scholar]
- van den Brandt PA, Goldbohm RA, van’t Veer P, Bode P, Hermus RJ, Sturmans F, 1993. Predictors of toenail selenium levels in men and women. Cancer Epidemiol Biomarkers Prev. abril de 2 (2), 107–112. [PubMed] [Google Scholar]
- van Noord PA, Maas MJ, van der Tweel I, Collette C, 1993. Selenium and the risk of postmenopausal breast cancer in the DOM cohort. Breast Canc. Res. Treat. 25 (1), 11–19. [DOI] [PubMed] [Google Scholar]
- van t Veer P, van der Wielen RP, Kok FJ, Hermus RJ, Sturmans F, 1990. Selenium in diet, blood, and to enails in relation to breast cancer: a case-control study. Am J Epidemiol. junio de 131 (6), 987–994. [DOI] [PubMed] [Google Scholar]
- van t Veer P, Strain JJ, Fernandez-Crehuet J, Martin BC, Thamm M, Kardinaal AF, et al. , 1996. Tissue antioxidants and postmenopausal breast cancer: the European community multicentre study on antioxidants, myocardial infarction, and cancer of the breast (EURAMIC). Cancer epidemiol biomarkers prev. junio de 5 (6), 441–447. [PubMed] [Google Scholar]
- Vinceti M, Bassissi S, Malagoli C, Pellacani G, Alber D, Bergomi M, et al. , 2005. Environmental exposure to trace elements and risk of cutaneous melanoma. J Expo Anal Environ Epidemiol. septiembre de 15 (5), 458–462. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Crespi CM, Malagoli C, Bottecchi I, Ferrari A, Sieri S, et al. , 2012. A case-control study of the risk of cutaneous melanoma associated with three selenium exposure indicators. Tumori. junio de 98 (3), 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Grioni S, Alber D, Consonni D, Malagoli C, Agnoli C, et al. , 2015. Toenail selenium and risk of type 2 diabetes: the ORDET cohort study. J Trace Elem Med Biol. enero de 29, 145–150. [DOI] [PubMed] [Google Scholar]
- Virtanen SM, van’t Veer P, Kok F, Kardinaal AF, Aro A, 1996. Predictors of adipose tissue tocopherol and toenail selenium levels in nine countries: the EURAMIC study. European Multicentre Case-Control Study on Antioxidants, Myocardial Infarction, and Cancer of the Breast. Eur J Clin Nutr. septiembre de 50 (9), 599–606. [PubMed] [Google Scholar]
- Ward EJ, Edmondson DA, Nour MM, Snyder S, Rosenthal FS, Dydak U, 2017. Toenail Manganese: A Sensitive and Specific Biomarker of Exposure to Manganese in Career Welders. Ann Work Expo Health. 25 de noviembre de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Were FH, Njue WM, Murungi J, Wanjau R, 2009. Comparison of some essential and heavy metals in the toenails and fingernails of school-age children in Kenya. Bull Chem Soc Ethiop. abril de 23 (1), 117–122. [Google Scholar]
- WHO, 1996. Trace Elements in Human Nutrition and Health. [Google Scholar]
- Wilhelm M, Hafner D, Lombeck I, Ohnesorge FK, 1991. Monitoring of cadmium, copper, lead and zinc status in young children using toenails: comparison with scalp hair. Sci Total Environ. 15 de abril de 103 (2–3), 199–207. [DOI] [PubMed] [Google Scholar]
- Wongwit W, Kaewkungwal J, Chantachum Y, Visesmanee V, 2004. Comparison of biological specimens for manganese determination among highly exposed welders. Southeast Asian J Trop Med Public Health. septiembre de 35 (3), 764–769. [PubMed] [Google Scholar]
- Xun P, Liu K, Morris JS, Daviglus ML, Stevens J, Jacobs DR, et al. , 2010a. Associations of toenail selenium levels with inflammatory biomarkers of fibrinogen, high-sensitivity c-reactive protein, and interleukin-6: the CARDIA Trace Element Study. Am J Epidemiol. 1 de abril de 171 (7), 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun P, Liu K, Morris JS, Daviglus ML, He K, 2010b. Longitudinal association between toenail selenium levels and measures of subclinical atherosclerosis: the CARDIA trace element study. Atherosclerosis. junio de 210 (2), 662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun P, Bujnowski D, Liu K, Morris JS, Guo Z, He K, 2011. Distribution of toenail selenium levels in young adult caucasians and african Americans in the United States: the CARDIA trace element study. Environ Res. mayo de 111 (4), 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaemsiri S, Hou N, Slining MM, He K, 2010. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. abril de 24 (4), 420–423. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Willett WC, Morris SJ, Stampfer MJ, Spiegelman D, Rimm EB, et al. , 1998. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 19 de agosto de 90 (16), 1219–1224. [DOI] [PubMed] [Google Scholar]
- Żukowska J, Bode P, Biziuk M, 2009. Toenail selenium level among healthy residents of two Polish Districts. J Radioanal Nucl Chem. 1 de junio de 280 (3), 621–627. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.