Abstract
Despite the integration of salivary inflammatory cytokines into research across the biobehavioral, psychological, clinical, and health-related disciplines, there is little guidance regarding the biospecimen collection, handling, and storage practices that maximize the quality and validity of salivary cytokine data. Furthermore, associations between salivary cytokines and measures related to oral health are rarely assessed and accounted for in studies outside the oral health fields. To address these gaps, we examine the sensitivity of salivary interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor-α (TNF-α) to changes in saliva sample collection technique and cold chain management procedures. Using subsets of saliva samples collected from 150 healthy adults, we measure salivary IL-1β, IL-6, IL-8, TNF-α, and other oral health-related indices (i.e., blood contamination [transferrin], and salivary matrixmallotprotienase-8). In addition to examining changes in cytokine levels associated with sample collection technique and cold chain management procedures, we assess relations between cytokine concentrations and levels of other oral health-related measures. We found that IL-1β, IL-6, and IL-8 were more robust to changes in sample collection and cold chain management procedures than TNF-α, and all cytokines were positively associated with other oral health-related measures. Based on our findings, we recommend analyte-specific guidance for measuring and interpreting salivary cytokine concentrations.
Keywords: saliva, cytokine, inflammation, cold chain, best practices
Introduction
Over the past four decades, the assessment of biomeasures in oral fluids for research purposes has advanced with a great expansion in the range and type of measurable analytes. These developments have facilitated more complex, multisystem assessments of health and disease processes (e.g., see (Granger and Taylor, 2020)). In particular, the integration of salivary inflammatory cytokines into investigations of health and development has accelerated our understanding of the environmental sensitivity and cross-system complexities of inflammatory processes (e.g., (Riis et al., 2020)). Minimally-invasive, saliva-based assessments of inflammatory cytokines enable ecologically-valid evaluations of inflammatory processes among populations that are difficult to study using traditional, primarily blood-based, biospecimens. Measuring cytokines in saliva also allows us to study the dynamics of immune function over time as well as the multisystem physiologic response to environmental and psychosocial demands. Researchers across several disciplines have capitalized on these advantages integrating salivary inflammatory cytokines into studies of community health, stress and development, and mental, physical, and oral disease mechanisms (Chiang et al., 2012; Jaedicke et al., 2016; Moons et al., 2010; Riis et al., 2016; Slavish et al., 2015; Zefferino et al., 2006).
Despite the broad adoption of these measures by the research community, there is little information available regarding saliva sample collection and handling best practices that maximize salivary cytokine data validity and reliability. Prior investigations of these issues have been generally limited in scope, focusing on single analytes or protocols (e.g., (Minetto et al., 2007; Pramanik et al., 2012)). Also, while there is a wealth of research suggesting associations between oral inflammation and oral health (Belstrøm et al., 2017; Rhodus et al., 2005; St. John et al., 2004; Teles et al., 2009; Zhang et al., 2016), investigators outside the periodontal and oral health fields do not traditionally assess nor control for oral health in their studies of salivary cytokines. These inconsistencies and ambiguities in the collection and handling of biospecimens, and in the adjustment and interpretation of salivary cytokine data across studies, jeopardize data validity and hinder the synthesis of findings across investigations and disciplines.
Sample collection techniques
While salivary bioscientists have largely worked through the general recommended procedures for biospecimen collection protocols (Granger et al., 2012), there is little guidance available for investigators specifically interested in salivary cytokine determinations. There are two dominant methods for collecting whole saliva (i.e., passive drool and collection by absorbent material placed in the mouth). In theory, because the sample has not been changed in any way, whole saliva by passive drool is the method of choice. However, when participants are very young (< 3–4 years old), unable to follow instructions, or asleep/unconscious, saliva is more efficiently collected using absorbent swab materials. Previous studies have shown, however, that using absorbent materials to collect saliva can have deleterious effects on the measurement of some analytes, including salivary interleukin-6 (IL-6) (e.g., (Harmon et al., 2007; Minetto et al., 2007)). Yet, the impact of sample collection method on salivary cytokine concentrations has not been thoroughly examined in the published literature.
Sample handling procedures: Cold chain management
The microbial environment of the mouth is extremely diverse and can potentially affect oral fluid biomarker assessments (Maughan and Whiteson, 2020). The refrigeration of biospecimens is critical for protecting some analytes from degradation and restricting the activity of proteolytic enzymes and the growth of bacteria. For large-scale surveys and studies conducted in remote areas or with at-home sample collection, however, maintaining the cold chain through sample collection, shipping, and processing can be logistically complex and cost-prohibitive. The effects of various cold chain management procedures on salivary cytokine concentrations have not been reported and are important to delineate for logistical, practical, and scientific reasons.
Associations with other oral health-related markers
Salivary cytokines have long been investigated by oral health, periodontal, and dental researchers as correlates of oral diseases (e.g., gingivitis, periodontal disease, caries, salivary gland conditions (Belstrøm et al., 2017; Finoti et al., 2017; Sahibzada et al., 2017; St. John et al., 2004; Teles et al., 2009; Zhang et al., 2016)). Cytokine levels measured in oral fluids may reflect a mix of serum-derived cytokines infiltrating into the mouth via oral injuries and crevicular fluid, as well as cytokines expressed by the salivary glands, cells migrating from circulation into the oral mucosal immune compartment, or resident immune cells in the mouth (Brennan and Fox, 2010; Gröschl, 2009; Moutsopoulos and Konkel, 2018; Yue et al., 2013). Blood in oral fluid is more prevalent among individuals who suffer from poor oral health, which introduces the possibility that individuals with oral health problems have increased levels of both serum-derived and locally-produced cytokines. Prior studies have found significant relations between inflammatory salivary cytokine concentrations and smoking behaviors and tobacco smoke exposure, as well as oral health conditions and disease states (Javed et al., 2014; Rathnayake et al., 2013; Riis et al., 2015; Sharma et al., 2017; Zhang et al., 2016). Outside the fields of periodontology and oral health, however, the assessment and adjustment of salivary cytokine concentrations for oral health status and/or other markers of oral disease is not standard practice.
In this study, we address these gaps in our understanding of salivary cytokine measurement and interpretation by assessing changes in commonly examined proinflammatory salivary cytokine concentrations across conditions varying in sample collection, cold chain management, and storage procedures. We examine differences in measured analyte levels when passive drool saliva samples are subjected to swab filtration and exposed to cold chain procedures that deviate from current best practice protocols (i.e., immediate freezing and storage at −80°C with minimal thaws prior to assay). To evaluate and quantify relations between these cytokines and other oral health-related indices among healthy adults, we also examine their associations with measures of blood leakage and tissue repair and reconstruction in the mouth. Our goal is to establish the foundation for a set of best practices that maximize salivary cytokine data quality and facilitate the cross-study synthesis of findings.
Material and Methods
This investigation uses data from a study of 150 healthy adult participants (Mage= 24.17, age range 14.35 – 36.59 years, 49% female, 50% white, 73% non-Hispanic). This single-visit, laboratory-based study was conducted at the Johns Hopkins Institute for Clinical and Translational Research and aimed to assess the nature and distribution of analytes measured in serum and saliva. The study procedures, briefly summarized here, are the same as previously reported (e.g., (Riis et al., 2017)). Adult participants were recruited through community postings. Individuals reporting chronic or acute health conditions, medication use (except hormonal contraceptives), open wounds or sores in the mouth, or recent oral surgery were excluded from the study. Participants were asked to refrain from eating and drinking for at least one hour before the study visit. During the study visits, participants provided informed consent, completed demographic questionnaires, and provided whole, unstimulated saliva samples via passive drool.
The analyses presented in this paper use three subsamples drawn from the full sample of participants: 1) a subsample of 51 participants whose saliva samples were tested across saliva collection conditions (Mage= 24.51 years, age range 18.39 – 36.59 years, 53% male, 41% white, 77% non-Hispanic); 2) a subsample of 50 participants whose saliva samples were tested across a series of cold chain management conditions (Mage= 24.97 years, age range 14.35 – 34.04 years, 62% female, 60% white, 72% non-Hispanic); and 3) a subsample of 100 participants whose saliva samples were tested for several markers related to oral inflammation and oral health (Mage= 24.97 years, age range 14.35 – 34.04 years, 62% female, 60% white, 72% non-Hispanic). Subsamples were selected by laboratory staff based on saliva volume and without consideration of participant characteristics. All the participants included in the saliva collection conditions (n=51) were also included in the subsample of 100 participants whose saliva samples were tested for oral inflammatory and oral health-related markers.
Saliva Sample Handling
Upon collection, saliva samples were mixed by inversion and frozen to precipitate mucins. Samples were then thawed to room temperature and mixed by inversion, followed by vortexing for several seconds. Saliva was centrifuged at 3500 rpm (Sorvall ST40R) for 15 minutes, and the supernatant was transferred away from the resultant mucin and debris pellet into a 15 mL conical tube. Supernatant samples were mixed again by inversion and vortexing, after which the samples were divided into 500 μL aliquots in cryovials (Sarstedt cat# 72.694.106) and stored at −80˚C until assayed.
Sample Preparation Procedures
Aliquots from the passive drool saliva samples were systematically exposed to a series of conditions designed to test the stability of inflammatory cytokine concentrations across various sample collection and cold chain management procedures.
Sample collection conditions (passive drool vs. swab collection):
We measured cytokine concentrations in aliquots of the passive drool samples and compared them to levels measured from aliquots of the same saliva samples after filtration via collection swabs of various densities and characteristics. For each participant, 3.0 mL of whole saliva was thawed, vortexed to ensure homogeneity, and spun in a centrifuge at 3500 rpm for 15 minutes. Four 750 μL aliquots of each participant’s sample were then pipetted into four separate tubes (Sarstedt Salivette® without swab, cat# 51.1534.004), each containing one of three filters, and the fourth tube without a filter (i.e., the passive drool condition). The filters tested were: an ultra-light density filter (0.050 g/cc); a high-density filter (0.077 g/cc); and a medium-density filter (0.068 g/cc) with a proprietary 3% overlay designed to improve analyte recovery by preventing adherent molecules from bonding to the filter (Filtrona, cat# R-32073, R-32072, R-32074). The filtered samples were allowed to absorb for 5 minutes, then spun out through the Salivette basket assembly via centrifuge at 3500 rpm for 5 minutes. All samples were then refrozen at −20°C until testing.
Cold chain management conditions:
We examined changes in salivary cytokine concentrations associated with: 1) the amount of time saliva samples spent at room temperature (0 hours, 24 hours, 48 hours, and 72 hours); 2) the number of additional freeze/thaw (F/T) cycles the samples were exposed to (0, 2, and 4 cycles); and 3) short-term cold storage temperatures over a three month period (4°C, −20°C, and −80°C).
Hours at room temperature-
For each participant, a 3.0 mL aliquot of saliva was thawed, rehomogenized, and spun down in a centrifuge at 3500 rpm for 15 minutes. Four new aliquots of 750 μL each were then dispensed into 2.0 mL cryovial tubes. The zero (0) hour sample was immediately stored at −20°C, while the remaining samples were left on the bench at room temperature. At the same time on the following day (day 1), the 24 hour sample was moved to the −20°C freezer; the 48 hour sample was frozen at the same time on day 2, and on day 3 the final batch of samples (72 hours) was frozen as well. Samples were stored at −20°C until the day of testing when all samples were thawed and tested together.
Exposure to freeze/thaw cycles-
For each participant, a 2.0 mL aliquot was thawed and rehomogenized. Three new aliquots of 600 μL each were dispensed into 2.0 mL cryovial tubes, and all samples were immediately placed in a −20°C freezer. The next day (day 1), all 2- and 4-times F/T samples (2 F/T and 4 F/T) were removed from the freezer and placed on the bench at room temperature to thaw and sit for 4 hours, after which they were placed in the −20°C freezer. On day 2, this step was repeated for all 2 F/T and 4 F/T samples. On days 3 and 4, only the 4 F/T samples were removed from the freezer to thaw. Zero F/T samples (0 F/T) remained at −20°C for the duration of the sample preparation period and did not undergo any additional F/T cycles beyond the conventional sample handling procedures described above. All samples were then kept at −20°C until day of testing, at which point they were all thawed and tested together.
Short-term storage temperature-
For each participant, a 2.5 mL aliquot was thawed and rehomogenized. Three new aliquots of 750 μL each were then dispensed into 2.0 mL cryovial tubes. Each batch of samples was immediately placed into storage at their designated temperature of 4°C, −20°C, or −80°C. Samples remained in storage for three months. After the storage period was complete, all samples were thawed and tested together.
Salivary inflammatory cytokines and biomeasures related to oral health and immunity:
Aliquots used to assess associations between inflammatory cytokines and indices related to oral health and immunity were not exposed to any additional processing procedures beyond the conventional sample handling procedures described above.
Determination of Analyte Concentrations
This study focuses on four inflammatory cytokines commonly examined in the biobehavioral literature, namely salivary IL-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α). On the day of assay, all salivary aliquots were thawed to room temperature on the bench and spun at 3500 rpm for 15 minutes in a Sorvall ST40R centrifuge to pellet remaining mucins. Saliva was tested for IL-1β, IL-6, IL-8, and TNF-α using a V-PLEX Human Proinflammatory Panel II (4-Plex) electro-chemiluminescence sandwich immunoassay (MSD® cat# K15025C) following the manufacturer’s guidelines. Cytokine concentrations (pg/mL) were determined with MSD Discovery Workbench Software (v. 3.0.17) using curve fit models (4-PL with a weighting function option of 1/y2). Lower limits of detection (LLD) were as follows: IL-1β (0.04 pg/mL), IL-6 (0.06 pg/mL), IL-8 (0.04 pg/mL), and TNF-α (0.04 pg/mL). Intra- and inter-assay coefficients of variation (CVs) for the analyte data used to assess relations between salivary cytokines and indices related to oral health were ≤5% and <7.5%, respectively.
A subset of saliva samples was also tested for other analytes associated with oral health, including indices representing blood in saliva (transferrin; (Kivlighan et al., 2004)), and potential tissue degradation in the oral compartment (matrix metalloproteinase-8 [MMP8] (Taba et al., 2005)). Transferrin was measured using a salivary Blood Contamination Enzyme Immunoassay (Salimetrics cat# 1–1302) following manufacturer’s protocol. The sample volume for this test was 25 μL and the range of sensitivity was 0.08 to 6.6 mg/dL. The intra- and inter-assay CVs were 2.9% and 4.7%, respectively. MMP-8 was assessed using a commercially available kit following the manufacturer’s guidelines (DuoSet ELISA, R&D Systems, cat# DY908). Approximately 100 μL were used per sample, with saliva samples pre-diluted at 1:50. The test range of sensitivity was 62.5 to 4000 pg/mL, and intra- and inter-assay CVs were 3.7% and 5.2%, respectively.
Statistical Analyses
Statistical analyses examined within-saliva sample differences in measured cytokine levels associated with changes in saliva filtration and cold chain management procedures. We used non-parametric, dependent-samples Sign-Tests to assess changes in median cytokine concentrations across collection and management conditions. Spearman’s Rho correlations examined the degree of correspondence between cytokine concentrations exposed to the various experimental conditions as well as the associations between salivary cytokine concentrations and levels of salivary transferrin, and MMP-8. Non-parametric statistical analyses were employed due to the non-normal distributions of the analyte data. Tests of statistical significance were two-sided with an alpha of .05, and Bonferroni-corrected alpha levels (.05/the number of tests) were also examined for each cytokine in each condition. Analyses were conducted using the Basic Statistics and Data Analysis (BSDA) package (Arnholt and Evans, 2017) and Tools for Descriptive Statistics (DescTools) package (Signorell and et mult. al, 2020) in R (R Core Team, 2019).
Results
Sample Collection Technique
One individual was excluded from the saliva collection condition analyses due to unreliable assay results for all conditions and cytokines. Across all analytes and conditions, an additional 8 determinations also showed high intra-assay CVs, and 20 determinations were below the assays’ measurement ranges. These cases were excluded from analysis (analytic sample sizes range from 42 to 50 for all comparisons and correlations). The percent of censoring due to assay measurement thresholds was highest for TNF-α which had 6.5% censored values across all conditions and 0 to 14% censoring within condition.
Swab filtration and density was associated with differences in median concentrations of all cytokines (Figure 1). Compared to concentrations from passive drool aliquots (i.e., no filter condition), all median cytokine levels were lower when filtered through the ultra-light (0.05 g/cc) and high-density swabs (0.077 g/cc), although this difference was not statistically significant for IL-1β (median difference (MdnD), 95% confidence interval (CI) for MdnD (pg/mL)): passive drool vs. 0.05 g/cc: IL-6= 0.54, [0.27, 0.84]; IL-8= 54.07, [36.96, 91.45]; TNF-α= 0.31, [0.17, 0.68]; passive drool vs. 0.077 g/cc: IL-6= 0.77, [0.55, 2.35]; IL-8=23.75, [6.80, 48.08]; TNF-α= 0.58, [0.32, 0.83]; ps<0.01). The medium-density swab with the proprietary overlay (0.068 g/cc filter) significantly altered the median levels of IL-1β, IL-6, and IL-8 resulting in the highest measured concentrations for each analyte (MdnD, 95% CI for MdnD (pg/mL): 0.068 g/cc vs. passive drool: IL-1β= 5.17, [0.34, 9.50], IL-6= 0.39, [0.31, 0.54]; IL-8= 107.82, [70.73, 160.79]; 0.068 g/cc vs. 0.05 g/cc: IL-1β= 6.74, [5.19, 10.53], IL-6= 1.15, [0.76, 1.48]; IL-8= 178.99, [129.36, 253.56]; and 0.068 g/cc vs. 0.077 g/cc: IL-1β= 6.24, [1.86, 15.04], IL-6= 1.66, [1.02, 2.22]; IL-8= 157.89, [112.48, 188.70], ps<0.05). All differences were robust to corrections for multiple comparisons (Bonferroni-adjusted α=0.008) except those between IL-8 concentrations from the passive drool and 0.077 g/cc swab conditions and between IL-6 concentrations from the passive drool and 0.068 g/cc swab conditions.
Figure 1.
Swab filtration and density affected median levels of measured salivary cytokine concentrations.
*Denotes significant differences between passive drool concentrations (No Filter condition) and concentrations after filtration through a swab. The medium-density swab (0.68 g/cc) included a proprietary overlay designed to improve analyte recovery. The error bars are 95% bootstrap confidence intervals. Sample sizes differ based on missing values for each analyte per condition (n’s= 44–50).
Despite differences in median levels, there were strong positive correlations for IL-1β, IL-6, and IL-8 determinations across swab filtration and density conditions (Figure 2; ρs= 0.74–0.99, ps<0.001). Correlations of TNF-α concentrations across swab filtration and density conditions were weaker than those observed for the other cytokines (Figure 2; ρs= 0.47–0.60, ps<0.001). All associations remained significant when adjusted for multiple comparisons (Bonferroni-adjusted α=0.008).
Figure 2.
Salivary cytokine concentrations were significantly positively correlated across swab filtration and density conditions.
All correlations were statistically significant (ps<0.001). No Filter condition represents passive drool saliva collection. The medium-density swab (0.68 g/cc) included a proprietary overlay designed to improve analyte recovery. Sample sizes differ based on missing values for each analyte per condition (n’s= 42–50.)
Cold Chain Management
Hours at room temperature:
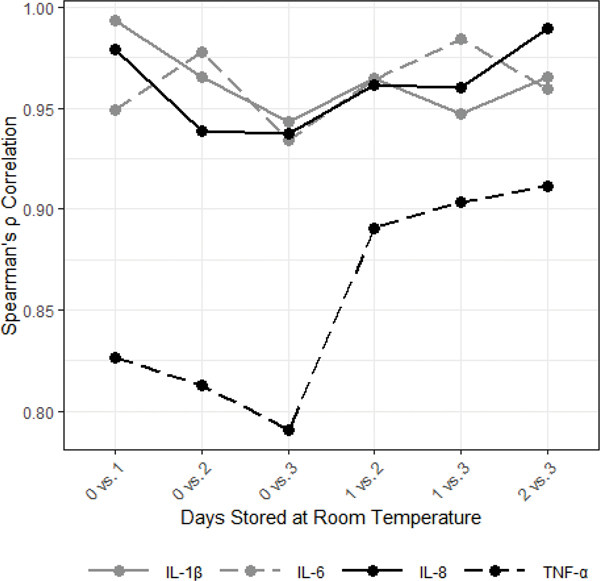
Storage at room temperature reduced median concentrations of all cytokines (Figure 3). For IL-1β, IL-6, and IL-8, significant losses in cytokine concentrations were observed after just one day of storage at room temperature, and each additional day at room temperature was associated with additional, statistically significant, reductions in concentrations (MdnD, 95% CI for MdnD (pg/mL)): IL-1β: 0 vs. 1 day= 8.27, [4.77, 11.58], 1 vs. 2 days= 16.33, [10.09, 24.07], 2 vs. 3 days= 19.50, [14.86, 26.76]; IL-6: 0 vs. 1 day= 0.30, [0.22, 0.42], 1 vs. 2 days= 0.29, [0.20, 0.35], 2 vs. 3 days= 0.16, [0.01, 0.24]; IL-8: 0 vs. 1 day= 16.44, [7.72, 28.22], 1 vs. 2 days= 10.18, [6.53, 14.06], 2 vs. 3 days= 11.62, [2.91, 21.02]; ps<0.05). For these cytokines, the changes in measured analyte concentrations associated with room temperature storage were relatively consistent across saliva samples; within-analyte correlations across conditions were all high indicating strong preservation of the ranking of individual sample determinations across the four conditions (Figure 4; ρs >0.93, ps<0.001). TNF-α concentrations exhibited a similar pattern when stored at room temperature (Figure 3), however, these changes were less consistent across samples resulting in lower cross-condition correlations (Figure 4; ρs= 0.79– 0.91, ps<0.001) and fewer significant changes in median levels across storage days (Figure 3). All results were robust to corrections for multiple comparisons (Bonferroni-corrected α=0.008) except the differences between IL-6 concentrations after 2 vs. 3 days and IL-8 concentrations after 1 vs. 2 days.
Figure 3.
Storage at room temperature decreased median salivary cytokine concentrations (N=50).
*Denotes significant differences between 0 days at room temperature and other conditions. The error bars are 95% bootstrap confidence intervals.
Figure 4.
Salivary cytokine concentrations were significantly positively correlated across room temperature storage conditions (N=50)
All correlations were statistically significant (ps<0.001).
Exposure to freeze/thaw cycles:
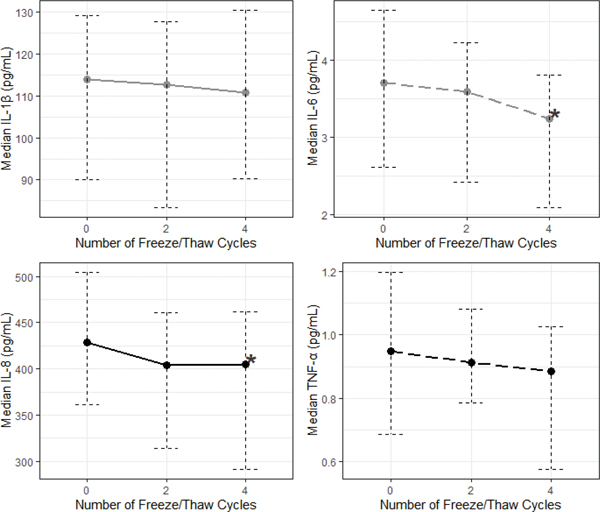
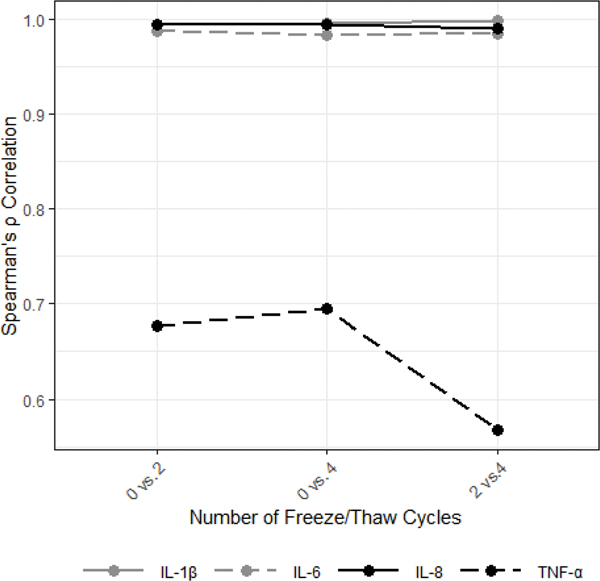
Exposing saliva samples to four additional F/T cycles resulted in significantly lower median levels of IL-6 and IL-8 (compared to 0 additional F/T cycles; Figure 5; MdnD, 95% CI for MdnD (pg/mL)): IL-6= 0.18, [0.10, 0.33], IL-8= 5.14, [1.49, 12.86]; ps<0.001). The relative ranking of individual samples based on their IL-1β, IL-6, and IL-8 determinations within the subsample was well-preserved across F/T conditions with high cross-condition correlations for each cytokine (Figure 6; ρs >0.98, ps<0.001). TNF-α showed less consistent changes in response to F/T cycle exposures, resulting in no significant median differences across conditions and low cross-condition correlations (Figure 6; ρs= 0.56–0.70, ps<0.001). All results were robust to Bonferroni corrections for multiple comparisons (Bonferroni-corrected α=0.017).
Figure 5.
Exposure to additional freeze/thaw cycles moderately decreased median concentrations of salivary IL-6 and IL-8 (N=50).
*Denotes significant differences between concentrations measured after 0 additional freeze/thaw cycles and those measured after 2 and 4 freeze/thaw cycles. The error bars are 95% bootstrap confidence intervals.
Figure 6.
Salivary cytokine concentrations were significantly positively correlated across freeze/thaw conditions (N=50).
All correlations were statistically significant (ps<0.001).
Short-term storage temperature:
One participant was excluded from the short-term storage temperature analyses for the −20°C condition due to unreliable determinations of all cytokines in this condition (analytic sample size for comparisons and correlations with the −20°C condition is 49).
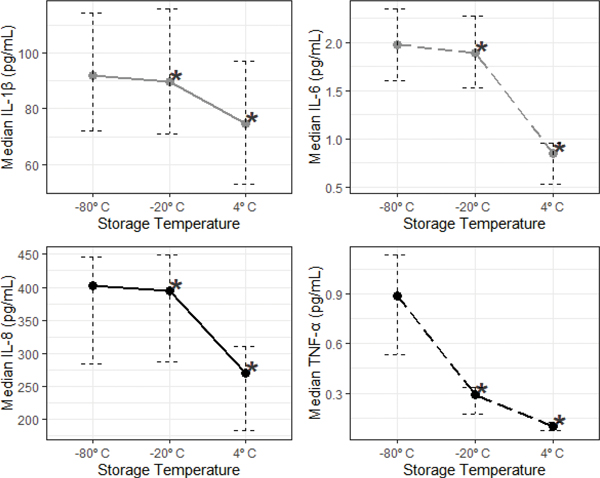
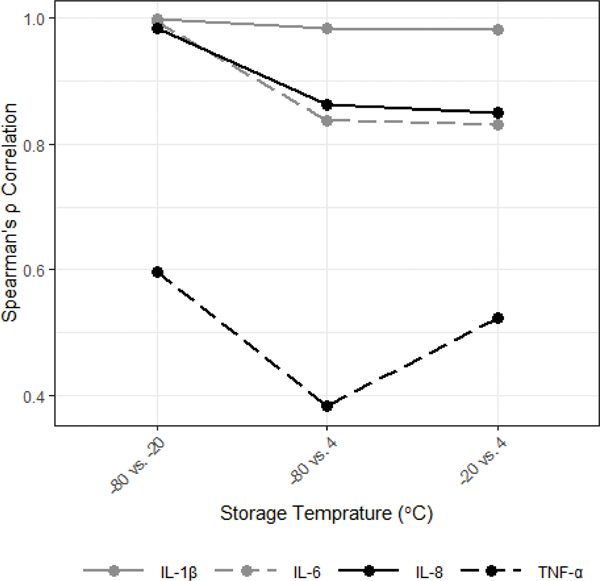
Median concentrations of all cytokines were significantly reduced when samples were stored at temperatures above −80°C for three months (Figure 7; MdnD, 95% CI for MdnD (pg/mL)): −80°C vs. −20°C: IL-1β= 3.72, [1.77, 5.35], IL-6= 0.07, [0.02, 0.13], IL-8= 20.51, [6.49, 30.78], TNF-α= 0.48, [0.30, 0.84]; −80°C vs. 4°C: IL-1β= 13.53, [8.17, 20.92], IL-6= 1.08, [0.86, 1.44], IL-8= 104.37, [96.91, 167.46], TNF-α= 0.79, [0.53, 1.15], ps<0.01). Similar reductions were seen comparing concentrations measured after storage at 4°C to storage at −20°C (Figure 7; MdnD, 95% CI for MdnD (pg/mL)): −20°C vs. 4°C: IL-1β= 10.02, [5.57, 15.89], IL-6= 1.04, [0.74, 1.34], IL-8= 109.19, [76.72, 146.58], TNF-α= 0.19, [0.14, 0.30]; ps<0.001). The impact of short-term storage temperature on the ranking of individual saliva samples based on their measured cytokine level was minimal for IL-1β, which showed high cross-condition correlations, and greatest for TNF-α, which showed the lowest cross-condition correlations (Figure 8; IL-1β: ρs>0.98; TNF-α: ρs=0.38–0.60; ps<0.01). All results remained significant when corrected for multiple comparisons (Bonferroni-corrected α=0.017).
Figure 7.
Storage above −80°C for three months decreased median salivary cytokine concentrations.
*Denotes significant differences between cytokine concentrations measured from samples stored at −80°C compared to those stored at −20°C and 4°C. The error bars are 95% bootstrap confidence intervals. Sample sizes differ based on missing values by condition (n’s=49–50).
Figure 8.
Salivary cytokine concentrations were significantly positively correlated across storage temperature conditions.
All correlations were statistically significant (ps<0.01). Sample sizes differ based on missing values by condition (n’s=49–50).
Associations between salivary inflammatory cytokines and measures related to oral health and immunity
Ten participants had concentrations of salivary MMP-8 that exceeded the assay’s measurement range, and an additional 6 MMP-8 determinations had high intra-assay CVs. These cases were excluded from analysis (analytic sample size for correlations with MMP-8 is 84). All salivary cytokine concentrations were significantly positively related to salivary transferrin and MMP-8 concentrations (Table 1).
Table 1.
Spearman’s Rho correlations showed salivary inflammatory cytokines were significantly positively associated with measures related to blood in saliva and potential tissue degradation in the oral compartment.
| Transferrin | MMP-8 | |
|---|---|---|
| IL-1β | .58 | .60 |
| IL-6 | .75 | .58 |
| IL-8 | .65 | .64 |
| TNF-α | .45 | .44 |
Note: All associations were statistically significant (ps<0.001; Bonferroni-corrected α=0.025). All analytes were measured in saliva. MMP-8= matrix metalloproteinase-8, IL= interleukin; TNF-α= tumor necrosis factor-α. For correlations with transferrin, n’s= 100; for correlations with MMP-8, n’s=84.
Discussion
In an effort to provide the field with a set of current best practice recommendations that support salivary cytokine data validity and reliability, we systematically assessed the impact of saliva sample filtration and cold chain procedures on measured concentrations of four of the most commonly-examined salivary inflammatory cytokines. These cytokines are often measured and investigated together using multiplexing assay technology which highlights the importance of understanding the effects of sample collection and handling protocols on each analyte alone, as well as differences in these effects across the four analytes. Overall, the findings suggest that alterations to current salivary bioscience best practice protocols (i.e., passive drool collection, immediate sample storage at −80°C, and minimal F/T cycles) result in significant changes in at least one of the four cytokine concentrations examined. Importantly, our results also show considerable differences in analyte sensitivity to these procedural changes.
Sample collection technique
The consistent, strong, positive correlations for IL-1β and IL-8 concentrations across swab filtration and density conditions suggest that these collection methods may have little effect on the overall ranking of individual determinations of these analytes within a study sample. Therefore, while swab filtration may affect the levels of IL-1β and IL-8 measured within a study, inferences regarding within- and between-person differences in cytokine concentrations may be minimally affected by sample collection technique (assuming one technique is used for all biospecimens). Similarly, our results suggest that using ultra-light and medium-density swabs to assess salivary IL-6 concentrations introduces minimal variability when considering within- and between-person effects in a single study. However, it is important to note that the medium density swab used in this investigation contained a proprietary overlay that may have affected measured analyte levels, so these results should not be generalized to all similar density swabs. In contrast to the interleukins, TNF-α was very sensitive to swab filtration and density conditions. Median concentrations for TNF-α dropped considerably when subjected to swab filtration with up to 14% of concentrations falling below the assay’s measurement threshold in the highest density swab condition. There were also more sporadic changes in TNF-α levels across swab conditions. Given TNF-α’s generally low concentrations in saliva, we recommend using passive drool to collect samples for TNF-α assessments. This will maximize the percent of detectable analyte concentrations and reduce censoring of TNF-α determinations due to the lower limits of the assay measurement range.
Overall, it is important to highlight that the effects of swab filtration and density on cytokine median concentrations and on the ranking of individual samples based on analyte concentrations were not consistent across cytokine nor was the pattern of findings consistent across filter densities. Studies should consider the effect of collection technique for each analyte of interest and standardize the collection approach across all participants. Our findings support the current recommendation for passive drool collection when planning to assess multiple analytes from a single biospecimen. If swab collection is used, these findings underscore the importance of standardizing collection protocols, reporting collection technique, and restricting the interpretation of cytokine concentrations to that technique. It is also important to note that, given the experimental protocols employed in this study, the swab effects we observed likely underestimate differences in analyte levels associated with swab vs. passive drool collection when implemented in a study protocol. When participants collect saliva by swab, additional factors, such as placement within the mouth and the stimulation of salivary flow due to chewing on the swab, may affect the constitution of the saliva collected and the resulting analyte levels. While these added sources of variability were not examined in our study (see (Minetto et al., 2007) for an evaluation of these issues with salivary IL-6), they are critical factors that should be considered when selecting and standardizing sample collection protocols.
Cold chain management
Our findings demonstrate the importance of cold chain management, minimizing F/T cycles, and storage in −80°C laboratory freezers for maximizing the validity of salivary cytokine measurements. For the interleukin cytokines, however, the findings suggest that deviations from these best practices will likely result in small changes in within- and between-subject effects for an individual study. The strong correlations for the interleukins across F/T cycles are particularly encouraging as F/T cycles may be difficult, or too costly, to avoid for larger studies and those examining multiple analytes per sample. The consistency of the rank ordering of saliva samples based on their analyte concentrations measured across room temperature conditions and three-month storage at −20°C vs. −80°C is also important to highlight for the interleukin cytokines. These findings suggest that field and home-based collection protocols that call for immediate sample storage in participants’ home refrigerators (which are typically set at 4°C with freezer settings just above −20°C) likely yield concentrations with a moderately high level of internal validity when examining differences within a specific analyte, even with imperfect compliance to freezing protocols.
High correlations across conditions, however, do not mean perfect preservation of the ranking of individual sample concentrations within the study subsample. For example, IL-6 concentrations measured after two F/T cycles were strongly correlated with the concentrations measured after no additional F/T cycles (ρ(48)=0.99, p<0.001). However, even at this very high correlation, there is variability in the effect of F/T cycles on individual cytokine determinations; 38% of our participants showed increases in IL-6 concentrations (median increase= 0.12 pg/mL) while 62% showed decreases in IL-6 concentrations (median decrease= 0.29 pg/mL) after two additional F/T cycles. This means that individual differences observed using biospecimens undergoing two additional F/T cycles will likely differ from those that would be observed if the samples were tested immediately upon arrival to the laboratory. For TNF-α, which consistently showed the lowest cross-condition correlation for all cold chain management procedures examined, this means that, for example, when comparing concentrations measured after two additional F/T cycles to those measured after no additional cycles, 46% of participants show increases (median increase= 0.16 pg/mL) and 54% show decreases (median decrease= 0.19 pg/mL) in TNF-α concentrations. This is reflected in a relatively low correlation across F/T conditions (ρ(48)= 0.68, p<0.001) and no significant changes in median TNF-α concentrations across F/T conditions due to non-systematic changes in TNF-α determinations across conditions.
TNF-α’s patterns of findings were consistently different and more erratic than those seen for the interleukins. Similar instability in TNF-α measurements have been reported in serum testing, particularly when examining changes in concentrations associated with storage at room temperature (Aziz et al., 2016; Skogstrand et al., 2008). In our study, TNF-α concentrations were lower than those of the other inflammatory cytokines, and this may have contributed to unreliable measurements across conditions. There may also be high levels of individual variability in the sensitivity of TNF-α to cold chain management procedures. Given the generally low levels of TNF-α, and the emerging nature of the investigation into salivary TNF-α stability, we recommend investigators follow the best practices for sample collection (passive drool), cold chain management, and storage (immediate freezing and storage at −80°Cwith minimal F/T cycles) when assessing TNF-α in saliva.
Our findings for the interleukins are also generally consistent with those from investigations of cold chain management procedures and cytokine levels measured in blood-based biospecimens. For example, prior work indicates that IL-1β, IL-6, and IL-8 concentrations are sensitive to cold chain procedures, and these effects may vary by sample handling and treatment protocols (Gong et al., 2019; Henno et al., 2017; Zhao et al., 2012). Also, similar to our results, IL-1β, IL-6, and IL-8 concentrations from pre-treated plasma samples have been shown to remain stable across several F/T cycles (three cycles for IL-1 β and six for IL-6 and IL-8) (Henno et al., 2017).
Associations between salivary inflammatory cytokines and measures related to oral health and immunity
In our sample of healthy young adults, we found strong positive associations between all four inflammatory cytokines and salivary markers related to blood leakage and potential tissue degradation in the mouth. The role of salivary cytokines in oral inflammation has been examined extensively in the periodontal and oral health literature (Belstrøm et al., 2017; Finoti et al., 2017; Sahibzada et al., 2017; St. John et al., 2004; Teles et al., 2009; Zhang et al., 2016) and should be seen as an opportunity, as well as a confound. There is an especially high prevalence of oral health problems in the United States. More than 40% of adults have periodontal disease, and oral health is worse among individuals earning lower incomes and racial/ethnic minorities (Centers for Disease Control and Prevention, 2016; Eke et al., 2018; U.S. Department of Health and Human Services, 2000). In many ways, the unequal distribution of oral health problems across the population mirrors patterns of disparities in systemic health problems, such as cardiometabolic and mood disorders (Centers for Disease Control and Prevention, 2016, 2013; U.S. Department of Health and Human Services, 2000), suggesting the possibility of common underlying mechanisms of disease etiologies. Oral inflammation, while a key component of oral disease, has also been associated with diabetes, depression, and cardiovascular disease (Borgnakke et al., 2013; Hashioka et al., 2018; Hsu et al., 2015; Lockhart et al., 2012; Mealey, 2006; Nascimento et al., 2019; Scannapieco et al., 2003). The study of salivary cytokines, therefore, opens up new opportunities to examine common biopsychosocial mechanisms underlying sociodemographic disparities in oral and systemic health.
Given our findings, and those in the periodontal and oral health literature, we advocate for increased cross-disciplinary collaborations to facilitate scientifically robust studies of salivary cytokines that consider the complexities of the oral environment and connections between oral, systemic, and emotional health. Additional research examining the sources of variation in salivary cytokine concentrations and how to best measure these analytes to reflect functioning in the compartment of interest is also needed. For example, we may be able to improve the correspondence between serum and salivary cytokine concentrations by adjusting measurements for indices of oral health. At a minimum, investigators aiming to use salivary cytokines as indicators of systemic, rather than oral, health, should be aware of, control for, and document the oral health status of their participants and note blood contamination in their saliva samples (Kivlighan et al., 2004).
Limitations
These recommendations for the collection, handling, and storage of saliva samples and the interpretation of cytokine concentrations are based on our data and current knowledge. It is therefore important to note gaps in our understanding, such as limited information about the measurement and meaning of salivary cytokines in the context of oral and/or systemic disease and how levels may be affected by developmental and aging processes. Our findings are limited by the generally healthy and relatively young sample examined in this study. Inclusionary criteria required that participants report no acute and/or chronic illnesses and no oral health concerns such as cuts or sores in their mouths. The effects of biospecimen collection technique and cold chain and storage procedures observed in this study would likely be different if examined in participants with physical and/or oral health concerns. Similarly, very young and elderly participants, as well as those exposed to specific antigens (e.g., a virus or bacteria), may have considerably different oral microbial environments. For these participants, we believe it is critical to adhere to sample collection, storage, and cold chain best practices to maximize data quality and validity (passive drool, immediate freezing and storage at −80°C). Relations between salivary cytokines and the other oral immune-related markers examined in this study may also vary by participant oral and physical health, as well as medication use. Additional investigations are needed to examine these relations in larger, more heterogeneous, study samples.
Our findings also highlight the need for additional research into valid and efficient methods for assessing and adjusting for oral health in biobehavioral investigations that use salivary biomeasures, including the utility and feasibility of biological, clinical, and/or self-report oral health measures. Further methodological studies are needed to assess the effects of long-term storage on analyte levels and the extent to which flow rate and sample volume affect measured analyte levels. Finally, additional studies are needed to further delineate diurnal variation in cytokine levels (Izawa et al., 2013; Nilsonne et al., 2016), as well as their sensitivity to participant and behavioral confounders (e.g., oral hygiene, eating/drinking behaviors).
Conclusions
The measurement of inflammatory cytokines in oral fluids has advanced scientific understanding of the biopsychosocial processes underlying health and illness. The promise of salivary cytokine measurement to continue to expand our knowledge of these processes, however, relies, in part, on the quality of our analyte data and on our ability to compare and synthesize findings across studies. Through the recommendations outlined in this paper, we aim to promote scientific inquiries into salivary cytokines that advance knowledge and contribute to a growing area of interdisciplinary investigations into inflammation and its associations with health and development. The analyte-specific recommendations suggested by our findings are important for investigators to consider when designing their research studies and procedures. Some analytes may be better suited for large-scale investigations with low levels of investigator controls (e.g., IL-1β) while others are best assessed when participant compliance with sample collection and storage procedures can be assured (e.g., TNF-α). Although highly specific to the research questions of interest, for investigators examining analytes with strong cross-condition correlations, the importance of changes in measured analyte levels across collection and management procedures is likely minimized if standard protocols are adopted across the study and the results are interpreted in the context of these protocols. Comprehensive assessments of salivary cytokines should also recognize the synergistic and pleiotropic mechanisms of inflammatory cytokines and choose a set of sample collection, handling, and storage procedures that maximizes data quality across multiple analytes, including oral immune-related indices that should be considered as potential covariates in salivary cytokine investigations.
Highlights.
Salivary IL-1β, IL-6, IL-8, and TNF-α were measured in healthy adults.
Cytokine changes were assessed across a series of saliva collection and handling procedures.
IL-1β, IL-6, and IL-8 were less sensitive to filtration and cold chain methods than TNF-α.
Cytokines were positively correlated with other indices related to oral health.
Best practices for salivary cytokine measurement and interpretation are provided.
Acknowledgement:
The data presented in this report were supported in part by NICHD (National Children’s Study, NCS, HHSN267200700048C) and NIH (Environmental Influences on Child Health Outcomes, ECHO, UH30D023332), PI DAG. We thank Mary Twomley, Jessica Bayer, Jessica Acevedo, Walter Worley, Kaitlin Smith, Hillary Piccerillo, Tatum Stauffer, and Andrew Huang for coordination of biospecimen collection and assay.
Footnotes
Disclosure statement: In the interest of full disclosure, DAG is founder and chief scientific and strategy advisor at Salimetrics LLC and Salivabio LLC and these relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California at Irvine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnholt A, Evans B, 2017. BSDA: Basic Statistics and Data Analysis. [Google Scholar]
- Aziz N, Detels R, Quint J, Li Q, Gjertson D, Butch A, 2016. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine 84, 17–24. 10.1016/j.cyto.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm D, Damgaard C, Könönen E, Gürsoy M, Gürsoy UK, 2017. Salivary cytokine levels in early gingival inflammation. J. Oral Microbiol. 9. 10.1080/20002297.2017.1364101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgnakke W, Ylostalo P, Taylor G, Genco R, 2013. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J. Periodontol. 84, S135–S152. 10.1902/jop.2013.1340013 [DOI] [PubMed] [Google Scholar]
- Brennan M, Fox P, 2010. Cytokine mRNA expression in the labial salivary glands of healthy volunteers. Oral Dis. 6, 222–226. 10.1111/j.1601-0825.2000.tb00117.x [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2016. Disparities in Oral Health [WWW Document]. URL https://www.cdc.gov/oralhealth/oral_health_disparities/index.htm
- Centers for Disease Control and Prevention, 2013. Centers for Disease Control and Prevention Health Disparities and Inequalities Report — United States, 2013. MMWR 62. [Google Scholar]
- Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE, 2012. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proc. Natl. Acad. Sci. U. S. A. 109, 1878–82. 10.1073/pnas.1120972109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ, 2018. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 149, 576–588.e6. 10.1016/j.adaj.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finoti LS, Nepomuceno R, Pigossi SC, Corbi SC, Secolin R, Scarel-Caminaga RM, 2017. Association between interleukin-8 levels and chronic periodontal disease. Med. (United States) 96. 10.1097/MD.0000000000006932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Liang S, Zeng L, Ni Y, Zhau S, Yuan X, 2019. Effects of blood sample handling procedures on measurable interleukin 6 in plasma and serum. J Clinc Lab Anal 33. 10.1002/jcla.22924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D, Taylor M. (Eds.), 2020. Salivary Bioscience: Foundations of Interdisciplinary Saliva Research and Applications. Springer International Publishing. [Google Scholar]
- Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, Out D, 2012. Focus on Methodology: Salivary bioscience and research on adolescence: An integrated perspective. J. Adolesc. 35, 1081–1095. 10.1016/j.adolescence.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Gröschl M, 2009. The physiological role of hormones in saliva. BioEssays 31, 843–852. 10.1002/bies.200900013 [DOI] [PubMed] [Google Scholar]
- Harmon A, Hibel L, Rumyantseva O, Granger D, 2007. Measuring salivary cortisol in studies of child development: watch out--what goes in may not come out of saliva collection devices. Dev Psychobiol 49, 495–500. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Inoue K, Hayashida M, Wake R, Oh-Nishi A, Miyaoka T, 2018. Implications of systemic inflammation and periodontitis for major depression. Front. Neurosci. 12, 1–7. 10.3389/fnins.2018.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henno L, Storjord E, Christiansen D, Bergseth G, Ludviksen J, Fure H, Barene S, Nielsen E, Mollnes T, Brekke O, 2017. Effect of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines – Consequences for defining reference values in healthy humans. Cytokine 97, 86–95. 10.1016/j.cyto.2017.05.014 [DOI] [PubMed] [Google Scholar]
- Hsu CC, Hsu YC, Chen HJ, Lin CC, Chang KH, Lee CY, Chong LW, Kao CH, 2015. Association of periodontitis and subsequent depression: A nationwide population-based study. Med. (United States) 94, 1–6. 10.1097/MD.0000000000002347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S, Miki K, Liu X, Ogawa N, 2013. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain. Behav. Immun. 27, 38–41. 10.1016/j.bbi.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Jaedicke K, Preshaw P, Taylor J, 2016. Salivary cytokines as biomarkers of periodontal diseases. Periodontol. 2000 70, 164–183. 10.1111/prd.12117 [DOI] [PubMed] [Google Scholar]
- Javed F, Ahmed H, Romanos G, 2014. Association between environmental tobacco smoke and periodontal disease: A systematic review. Environ. Res. 133, 117–122. [DOI] [PubMed] [Google Scholar]
- Kivlighan K, Granger D, Schwartz E, Nelson V, Curran M, Shirtcliff E, 2004. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva Katie. Horm. Behav. 46, 39–46. 10.1016/j.yhbeh.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Lockhart P, Bolger A, Papapanou P, Osinbowale O, Trevisan M, Levison M, Taubert K, Newburger J, Gornik H, Gewitz M, Wilson W, Smith S, Baddour L, 2012. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association?: A scientific statement from the American heart association. Circulation 125, 2520–2544. 10.1161/CIR.0b013e31825719f3 [DOI] [PubMed] [Google Scholar]
- Maughan H, Whiteson K, 2020. Saliva as a window into the human oral microbiome and metabolome, in: Taylor M, Granger D. (Eds.), Salivary Bioscience: Foundations of Interdisciplinary Saliva Research and Applications. Springer International Publishingr, pp. 139–155. [Google Scholar]
- Mealey B, 2006. Periodontal disease and diabetes: A two-way street. J. Am. Dent. Assoc. 137, 265–315. 10.14219/jada.archive.2006.0404 [DOI] [PubMed] [Google Scholar]
- Minetto MA, Gazzoni M, Lanfranco F, Baldi M, Saba L, Pedrola R, Komi PV, Rainoldi A, 2007. Influence of the sample collection method on salivary interleukin-6 levels in resting and post-exercise conditions. Eur. J. Appl. Physiol. 101, 249–256. 10.1007/s00421-007-0484-x [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, Taylor SE, 2010. Anger and fear responses to stress have different biological profiles. Brain. Behav. Immun. 24, 215–219. 10.1016/j.bbi.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel JE, 2018. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends Immunol. 39, 276–287. 10.1016/j.it.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento GG, Gastal MT, Leite FRM, Quevedo LA, Peres KG, Peres MA, Horta BL, Barros FC, Demarco FF, 2019. Is there an association between depression and periodontitis? A birth cohort study. J. Clin. Periodontol. 46, 31–39. 10.1111/jcpe.13039 [DOI] [PubMed] [Google Scholar]
- Nilsonne G, Lekander M, Åkerstedt T, Axelsson J, Ingre M, 2016. Diurnal variation of circulating interleukin-6 in humans: A meta-analysis. PLoS One 11, 1–17. 10.1371/journal.pone.0165799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik R, Thompson H, Kistler JO, Wade WG, Galloway J, Peakman T, Proctor GB, 2012. Effects of the UK Biobank collection protocol on potential biomarkers in saliva. Int. J. Epidemiol. 41, 1786–1797. 10.1093/ije/dys166 [DOI] [PubMed] [Google Scholar]
- R Core Team, 2019. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rathnayake N, Åkerman S, Klinge B, Lundegren N, Jansson H, Tryselius Y, Sorsa T, Gustafsson A, 2013. Salivary biomarkers of oral health - A cross-sectional study. J. Clin. Periodontol. 40, 140–147. 10.1111/jcpe.12038 [DOI] [PubMed] [Google Scholar]
- Rhodus N, Ho V, Miller C, Myers S, Ondrey F, 2005. NF-κB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 29, 42–45. [DOI] [PubMed] [Google Scholar]
- Riis J, Bryce C, Ha T, Hand T, Stebbins J, Matin M, Jaedicke K, Granger D, 2017. Adiponectin: Serum-saliva associations and relations with oral and systemic markers of inflammation. Peptides 91, 58–64. 10.1016/j.peptides.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Riis J, Byrne M, Hernandez L, Robles T, 2020. Salivary Bioscience, Immunity, and Inflammation, in: Granger D, Taylor M. (Eds.), Salivary Bioscience: Foundations of Interdisciplinary Saliva Research and Applications. Springer International Publishing, pp. 177–213. [Google Scholar]
- Riis J, Granger D, Dipietro J, Bandeen-Roche K, Johnson S, 2015. Salivary Cytokines as a Minimally-Invasive Measure of Immune Functioning in Young Children: Correlates of Individual Differences and Sensitivity to Laboratory Stress. Dev Psychobio 57, 153–167. 10.1002/cncr.27633.Percutaneous [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis J, Granger D, Minkovitz C, Bandeen-Roche K, DiPietro J, Johnson S, 2016. Maternal distress and child neuroendocrine and immune regulation. Soc. Sci. Med. 151, 206–214. 10.1016/j.socscimed.2015.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahibzada HA, Khurshid Z, Khan RS, Naseem M, Siddique KM, Mali M, Zafar MS, 2017. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 7, 21. 10.3390/diagnostics7020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco F, Bush R, Paju S, 2003. Associations Between Periodontal Disease and Risk for Atherosclerosis, Cardiovascular Disease, and Stroke. A Systematic Review. Ann. Periodontol. 8, 38–53. [DOI] [PubMed] [Google Scholar]
- Sharma V, Gupta N, Srivastava N, Rana V, Chandna P, Yadav S, Sharma A, 2017. Diagnostic potential of inflammatory biomarkers in early childhood caries - A case control study. Clin. Chim. Acta 471, 158–163. 10.1016/j.cca.2017.05.037 [DOI] [PubMed] [Google Scholar]
- Signorell A, et mult. al, 2020. DescTools: Tools for Descriptive Statistics. [Google Scholar]
- Skogstrand K, Ekelund C, Thorsen P, Vogel I, Jacobsson B, Norgaard-Pedersen B, Hougaard D, 2008. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods 336, 78–84. [DOI] [PubMed] [Google Scholar]
- Slavish D, Graham-Engeland J, Smyth J, Engeland C, 2015. Salivary Markers of Inflammation in Response to Acute Stress. 10.1111/j.1743-6109.2008.01122.x.Endothelial [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John M, Li Y, Zhou X, Denny P, Ho C-M, Montemagno C, Shi W, Qi F, Wu B, Sinha U, Jordan R, Wolinsky L, Park N-H, Liu H, Abemayor E, Wong D, 2004. Interleukin 6 and Interleukin 8 as Potential Biomarkers for Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. Arch Otolaryngol Head Neck Surg 130, 929–935. 10.1001/archotol.130.8.929 [DOI] [PubMed] [Google Scholar]
- Taba M, Kinney J, Kim AS, Giannobile WV, 2005. Diagnostic biomarkers for oral and periodontal diseases. Dent. Clin. North Am. 49, 551–571. 10.1016/j.cden.2005.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles R, Likhari V, Socransky SS, Haffajee AD, Teles RP; Likhari V; Socrensky SS; Haffajee AD, 2009. Salivary Cytokine Levels in Chronic Periodontitis and Periodontal Healthy Subjects. A cross-sectional Study. J Perodontal Res. 44, 411–417. 10.1111/j.1600-0765.2008.01119.x.Salivary [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2000. Oral Health in America: A Report of the Surgeon General, Oral Health in America: A Report of the Surgeon General. 10.1089/pop.2013.0038 [DOI]
- Yue Y, Yiu Q, Xu C, Loo W, Wang M, Wen G, Cheung M, Bai L, Dou Y, Chow L, Hao L, Tian Y, Li J, Yip A, Ng E, 2013. Comparative evaluation of cytokines in gingival crevicular fluid and saliva of patients with aggressive periodontitis. Int. J. Biol. Markers 28, 108–112. 10.5301/JBM.5000014 [DOI] [PubMed] [Google Scholar]
- Zefferino R, Facciorusso A, Lasalvia M, Narciso M, Nuzzaco A, Lucchini R, L’Abbate N, 2006. Salivary markers of work stress in an emergency team of urban police (1 degree step). G Ital Med Lav Erg. 28, 472–477. [PubMed] [Google Scholar]
- Zhang C, Cheng X, Li J, Zhang P, Yi P, Xu X, Zhou X, 2016. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 8, 133–137. 10.1038/ijos.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Qureshi F, Eastman P, Manning W, Alexander C, Robinson W, Hesterberg L, 2012. Pre-analytical effects of blood sampling and handling in quantitative immunoassays for rheumatoid arthritis. J. Immunol. Methods 378, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]