Abstract
The coronavirus disease 2019 (COVID-19) pandemic has brought unprecedented public health, and social and economic challenges. It remains unclear whether seasonal changes in ambient temperature will alter spreading trajectory of the COVID-19 epidemic. The probable mechanism on this is still lacking. This review summarizes the most recent research data on the effect of ambient temperature on the COVID-19 epidemic characteristic. The available data suggest that (i) mesophilic traits of viruses are different due to their molecular composition; (ii) increasing ambient temperature decreases the persistence of some viruses in aquatic media; (iii) a 1°C increase in the average monthly minimum ambient temperatures (AMMAT) was related to a 0.72% fewer mammalian individuals that would be infected by coronavirus; (iv) proportion of zoonotic viruses of mammals including humans is probably related to their body temperature difference; (v) seasonal divergence between the northern and southern hemispheres may be a significant driver in determining a waved trajectory in the next 2 years. Further research is needed to understand its effects and mechanisms of global temperature change so that effective strategies can be adopted to curb its natural effects. This paper mainly explores possible scientific hypothesis and evidences that local communities and authorities should consider to find optimal solutions that can limit the transmission of SARS-CoV-2 virus.
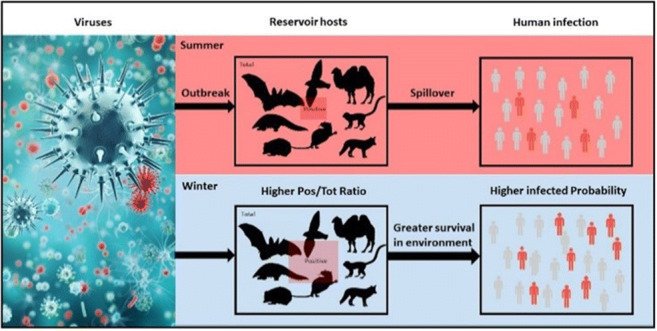
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-021-14625-8.
Keywords: COVID-19, Ambient temperature, Global seasonal change, Mammals , Trend prediction
Introduction
Since December 2019, a novel corona virus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has exhibited unprecedented human-to-human transmissibility, with more than 156 million confirmed cases of the coronavirus disease 2019 (COVID-19) and 3.26 million deaths in 220 countries and regions on May 8, 2021 (from the World Health Organization (WHO) COVID−19 Dashboard: https://covid19.who.int/). To date, there is no consensus on whether temperature can mitigate spreading of the virus, so efforts at control have focused on implementation of extensive public health interventions, such as social distancing, wearing of protective clothing, including face masks, and disinfection of surfaces and hands. To understand the effects of temperature and humidity on behavior of the SARS-CoV-2 virus, the first rapid response research fund was launched by the United States National Science Foundation for supporting decision-making on disease control.
From a perspective of environmental geosciences using a regression framework, results of previous studies on relationships between spatial and temporal distributions of SARS-CoV-2 and temperature have been summarized. Newly confirmed cases, in China and other countries worldwide, have been examined to show significantly relationships with ambient temperature (Wang et al. 2020a; Wang et al. 2020b). The optimal temperature for peak rate of transmission was centered at 8.72 °C, which indicates that cold environments facilitate survival and spread of droplet-mediated viral diseases and contributes to spreading the epidemic. Based on analysis of some countries where wide-spread infections have occurred, warm and humid weather in Singapore and Thailand might have been a factor in the more moderate trajectories of epidemics, which is compared to the relatively severe viral transmission in Japan, South Korea, Europe, and Iran. Based on results of a recent statistical study, the global pandemic was primarily between latitudes of 30° N ~ 50° N, a corridor with average temperatures of 5 ~ 11 °C. The number of cumulative cases in countries with an average temperature of more than 18 °C and an absolute humidity of more than 9 g/m3 from January to March 2020 is less than 6% of the total number of global confirmed cases (Bukhari and Jameel 2020). Due to limited and early-stage results on the current pandemic, the relative importance of climate drivers need to be further characterized (Baker et al. 2020).
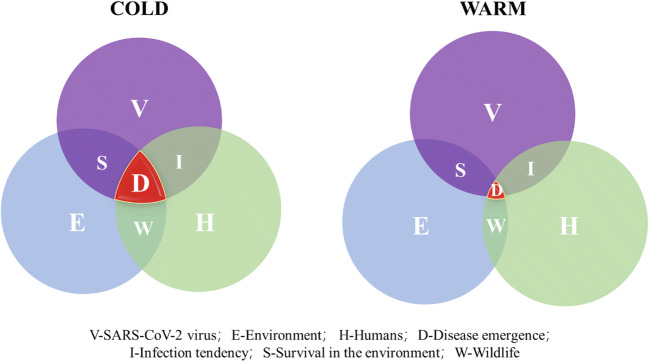
Epidemic disease emergence cannot occur without certain conditions being met, including the presence of the pathogen, humans, and environment media in which the virus can survive. A conceptual framework of viral outbreaks, transmission and occurrences of diseases that they cause, through interplay of these three essentials is provided (Fig. 1). There is a scientific hypothesis that the change of ambient temperature might affect infection tendency of different types of viruses, survival capacity in environmental media, and spreading among mammals as well as humans. The main objective of the present study is to review the limited temperature-dependent spatial and temporal distributions of the COVID-19 pandemic through existing data analyses. Viral mesophilic trait, survival in aquatic environment, body temperatures of hosts, and infected ratio of mammals were mainly discussed. Furthermore, based on global change of ambient temperature that differs from two hemispheres, we also predict the prevalent trend of COVID-19 epidemics in the next 2 years and demonstrate the seasonal epidemic characteristics of SARS-CoV-2 virus.
Fig. 1.
Ambient temperature can affect COVID-19 emergence through interactions among three essential elements of SARS-CoV-2 (purple), environmental media (blue), and uninfected humans (green). The red region represents emergence of an infective disease
Data analysis and discussion
Mesophilic traits of different viruses
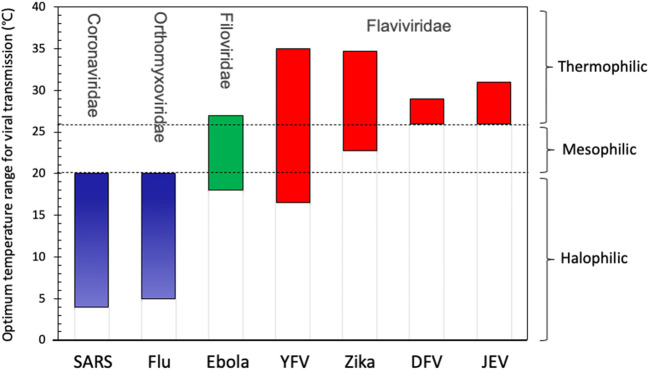
Several viruses that have caused outbreaks of infectious diseases such as Zika and Ebola show certain geographical distribution characteristics (Kraemer et al. 2019). Bibliometrics results show that optimal temperature ranges for survival and spreading can vary among seven major types of viruses that have caused public health emergency (Fig. 2). The optimal temperature range for transmission of coronavirus and influenza virus is usually below 20 °C, while some thermophilic viruses, including Zika, dengue fever virus (DFV), yellow fever virus (YFV), and Japanese encephalitis virus (JEV), tend to outbreak and spread in tropical areas (>25°C). For example, outbreak and spreading of Zika virus have been reported frequently in the past decades; however, the epidemic trajectory showed obvious geographical distribution characteristics. It was mainly located in tropical and subtropical areas, and did not cross into temperate zone (Caminade et al. 2017; Messina et al. 2016).
Fig. 2.
Mesophilic traits of viruses that have caused major public health emergencies in the last decades include severe acute respiratory syndrome (SARS) coronavirus, influenza (Flu), Ebola, yellow fever virus (YFV), Zika, dengue fever virus (DFV), and Japanese encephalitis virus (JEV)
In our opinion, the probable reason of temperature-dependent characteristics of viruses can be explained from principle of structural biology. Chemical bonding and non-bonding forces that maintain the protein structure are inversely proportional to temperature, which results in loss of structural integrity of the whole virus or partial function areas at high temperatures (Pain 1987). Some in silico techniques, such as molecular docking and molecular dynamic simulation, have been used to assess the stability of critical main proteases of the SARS-CoV-2, which indicated that temperature could interfere with virus replication (Bhardwaj et al. 2021; Sharma et al. 2021). Structural denaturation of proteins that make up viruses may trigger subsequently irreversible inactivation (Bhardwaj et al. 2020; Han and Kral 2020).
Survival capacity of viruses in aquatic media
In the present study, we compared durations of survival of various pathogens in aquatic environments under different ambient temperatures (4 °C, 15 °C, and 20-25 °C), results of which suggested that temperature was a significant factor influencing viability and survival of viruses (Table S1). Most viruses such as CoV-229E, Poliovirus, Feline CoV, and Adenoviruses can survive longer in cooler environments. Therefore, disinfection of drinking water can prevent transmission of SARS-CoV-2 through aquatic media. These findings demonstrate that higher temperature and humidity can reduce durations of survival of most viruses in aquatic media. Based on an extreme case of cluster transmission in a toilet, some researchers, however, have come to different conclusions. For nine patients who were infected, within a week, after entering the same public bathroom (Luo et al. 2020), the authors alerted the public to the fact that higher temperature and humidity have limited ability to suppress spread of the novel coronavirus, and the spread might not be weakened.
The SARS-CoV-2 virus as well as other viruses has been reported to be shed outside bodies of infected organisms, through aerosol, feces, and urine, which ultimately enter environmental media (Banik and Ulrich 2020; Chen et al. 2020). Persistence in air, water, soil, or on physical surfaces is a vital determinant of transmissibility. In general, viruses transmitted in droplets tend to persist longer at low-temperature and low-humidity environment (Chan et al. 2011), and on certain surfaces, including glass and steel (Wood et al. 2010). In the present study, we compared survival capacity of various viruses in aquatic environments under cold (4 °C) and warm (>15 °C) conditions (Fig. 3), which suggested that ambient temperature was a vital factor to influence the time to kill 99% of viruses (T99). Four corona viruses including 229E, Feline CoV, Porcine CoV, and Rat CoV can survive much longer in cold environments. These findings demonstrate that higher temperature and humidity can reduce durations of most of corona viruses in aquatic media. Median T99 of Poliovirus and Adenoviruses in cold condition extended at 2.9 and 1.44 times, respectively. Hepatitis A does not show any difference under warm and cold conditions. Despite of this, higher ambient temperature cannot completely prevent the spread of virus through environmental media. Luo C. et al. (2020) reported an extreme case of cluster transmission of SARS-CoV-2 in a public bathroom, where nine patients were confirmed to be infected through indirect contact. The authors alerted the public that higher temperature and humidity have limited ability to suppress spread of SARS-CoV-2 virus, and the spread might not be weakened.
Fig. 3.
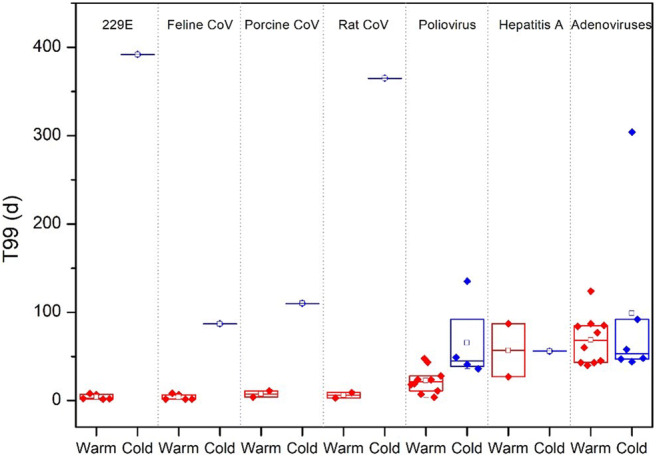
Durations of survival, based on 99% inactivation of virus (T99, days) at different ambient temperatures (4 °C in blue and >15 °C in red). The horizontal lines represent the 95th centiles, and the boxes represent the 25th and 75th centiles. Median are shown as the hollow box (data sources: Table S1)
Several direct testing on survival of SARS-CoV-2 in environmental media were reported in the past year. SARS-CoV-2 can survive for 7 days in outer layers of surgical masks. Absorbent materials like cotton are safer than unabsorbent materials for protection from virus infection. Risk of transmission via touching contaminated paper is low (Ren et al. 2020). The spread of the SARS-CoV-2 was reduced by increasing temperature from −13.2 to 19 °C and ambient concentration of ozone (Yao et al. 2020). More in-depth researches need to be performed as time goes on. Compared with other coronaviruses, SARS-CoV-2 may have greater survival capacity, which contributes to strong susceptibility. Therefore, it is very vital promoting effective environmental elimination in high-risk areas.
Infected ratio of mammals and ambient temperature
Mammals is an unneglected reservoir host to the SARS-CoV-2, and has opportunity in cross-species spillover to humans by close contact. To explore the relationships between ambient temperature and ratio of infection incidence in mammals, infected ratios of coronavirus (including α and β subtype) through polymerase chain reaction (PCR) testing were reviewed from existing literatures and database (Table S2). For mammals in five orders, including Chiroptera, Rodentia, Carnivora, Artiodactyla, and Erinaceomorpha, ratios between number of positive samples and number of total samples in mammals were computed. The average monthly minimum ambient temperatures (AMMAT) at the sampling regions were calculated at the same time. As shown in Fig. 4, the Pos/Tot ratios of mammals were inversely proportional to AMMAT, with a coefficient of determination (R2) of 0.6546 (F = 18.95, p = 0.001, RSS = 0.0166, RMSE = 0.0408). The internal validation had been performed to overcome overfitting (R2 − QCV2 = 0.2546 < 0.3, RMSECV = 0.053). Our finding indicates that a 1° increase in AMMAT was associated with 0.72% fewer mammalian individuals that were infected by the SARS-CoV-2.
Fig. 4.
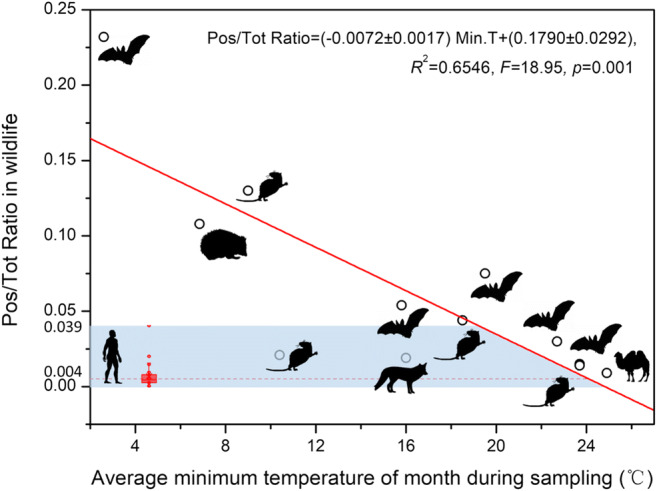
The relationship between average monthly minimum ambient temperatures (AMMAT) and ratio of infection incidence in mammals and human. Incidence of expression is expressed as the Pos/Tot ratio of mammals which was determined based on coronavirus detection by PCR. Red line is a linear fitting with minimum root mean square deviation (RMSE). Blue tape describes the range of cumulative infected ratio in top 31 countries worldwide. Animal silhouettes visually represent each mammalian order which were downloaded from PhyloPic (www.phylopic.org)
If we regard humans as a member of mammals, the ratio of cumulative infected cases by SARS-CoV-2 was investigated and global average infection ratio was quantified as 0.4% (0~3.9%), which was lower than that of mammals (Table S3). Based on the linear correlation model, the corresponding AMMAT was 24.9°C, at which SARS-CoV-2 virus can spread in most geographic areas worldwide.
These results also suggested that some mammals are more susceptible hosts than humans. There are some evidences that SARS-CoV-2 was detected in body of kitten, dog, tiger, and lion (Sabateeshan and Graham 2020; Sit et al. 2020). It was also found from ferret farms in Denmark, Spain, and the Netherlands, where cross-species transmission of SARS-CoV-2 has occurred from ferret to humans (Richard et al. 2020). Therefore, mutual infection between mammals and humans needs to be paid more attentions.
Proportion of zoonotic viruses in mammals and their body temperature
Several studies have shown that mammals may carry a variety of zoonotic viruses, which are prone to cross-species spillover to humans (Carlson et al. 2019; Mollentze and Streicker 2020). However, it is not clear whether the capacity to carry zoonotic viruses is related to body temperature of mammals. Therefore, we are trying to explore the normal body temperature of mammals and humans. Normal body temperatures of 62 mammals in 7 orders and 20 families were obtained from the existing literatures, including Chiroptera, Rodentia, Cetartiodactyla, Carnivora, Didelphimorphia, Primates, and Lagomorpha. A human body’s normal temperature, also referred to as euthermia or normothermia, is typically within the range of 36.5-37.5 °C, concerning race, circadian rhythm, age, and sex. The proportion of zoonotic viruses in 7 orders was predicted from a comprehensive analysis of mammalian host–virus relationships, which suggests that Chiroptera, Primate, and Rodentia harbor a significantly higher proportion of zoonotic viruses than other mammalian orders (Olival et al. 2017). As shown in Fig. 5, mammals in four orders (Primates, Chiroptera, Carnivora, and Rodentia), whose body temperatures were close to humans, had a higher proportion of zoonotic viruses and thus exhibited greater potential for viral spillover to humans. Body temperature of mammals, higher or lower than normal human body temperature, possesses fewer zoonotic virus. Although there is limited data on different species, it is a probable significant predictor of zoonotic potential to assess if a newly discovered mammalian virus could infect people.
Fig. 5.
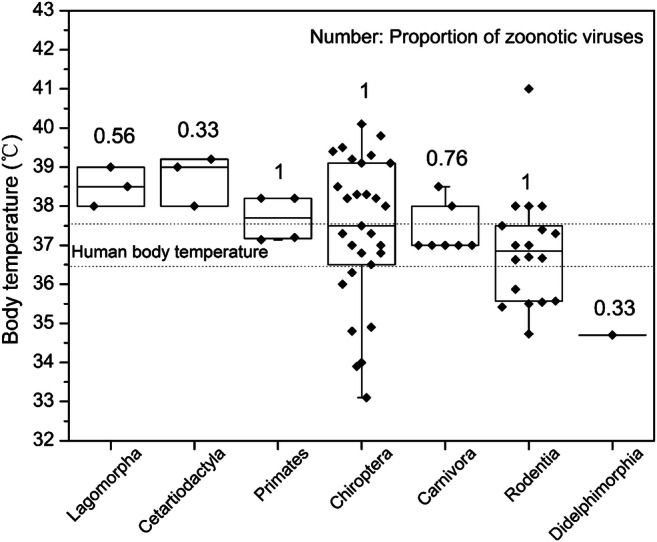
Proportion of zoonotic viruses and normal body temperatures of 62 mammals in 7 orders and 20 families (data sources: Table S4). Horizontal lines represent the 95th centiles, and the boxes represent the 25th and 75th centiles. The range of human body temperature is shown between two dotted lines. Proportion of zoonotic viruses is from an existing literature reported by K. J. Olival et al. (2017)
Global epidemic trajectory and seasonal change
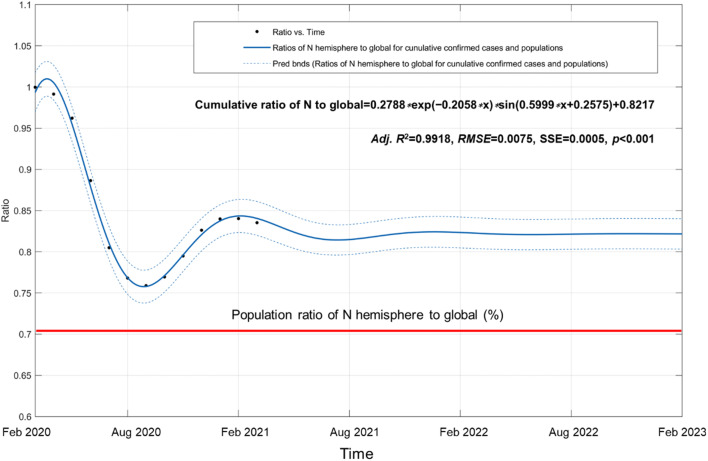
In the past year since the COVID-19 outbreak, SARS-CoV-2 virus has transmitted to more than 220 countries and regions worldwide. Variation of cumulative confirmed ratios between two hemispheres is a good indicator to indicate seasonal trends of the COVID-19 pandemic. Based on confirmed cases reported by the WHO (https://covid19.who.int/), cumulative ratios of the N hemisphere to those of global from Feb 29, 2020 to Feb 28, 2021 alternate in waves between two hemispheres (Fig. 6 and Table S5). A sinusoidal decay model was used to fit this trend, which suggested that under the current control measures, community communication will fluctuate with the seasons between two hemispheres. Then, the predicted cumulative ratios are going to stabilize at 82.17% in the next 2 years. Seasonal alternation between the northern and southern hemispheres is one of the important climatic drivers for the spread of SARS-CoV-2 virus (Figure S1). In winter time of the S hemisphere (up to Aug 2020), cumulative ratio of the N hemisphere to global dropped to a minimum value of 75.89% for the high speed at which the SARS-CoV-2 virus spreads. Preliminary epidemiological results have also demonstrated a significant increase in the number of confirmed cases in Brazil, Argentina, Chile, Peru, South Africa, and southern Australia during that period. A latest study in Brazil also reported that a decrease of 1°C was associated with an increase of 4.89% of total, daily, confirmed cases of COVID-19, which suggests greater severity in winter (Prata et al. 2020).
Fig. 6.
Global COVID-19 epidemic trajectory between two hemispheres during the period between Feb 2020 and Feb 2023. Dotted line shows 95% confidence intervals. Robustness of fitting model was evaluated by use of the adjusted coefficient of determination (adj R2), sum of squares due to error (SSE), root mean square deviation (RMSE), and F value using one-way analysis of variance (ANOVA) with the level of significance at α = 0.05. Means and variances were compared with Bonferroni and Tukey’s multiple tests. The red line represents the population ratio of the N hemisphere to global with 70.18%, published by the United Nations Population Division (UNPD) in 2019 (Table S4)
Conclusions
It is concluded that ambient temperature can play a role in influencing spatial and temporal distributions of viral transmission that has been usually overlooked in epidemiologic research. Three probable drivers to alter the pandemic trajectory are viral mesophilic traits, survival capacities in aquatic environmental media, and cross-species spillover from mammals to humans. Understanding the intrinsic connection and revealing the mechanism can help mitigate epidemic spread all over the world. It is the first reported study that infected ratio of mammals was inversely proportional to AMMAT at the sampling regions (R2 = 0.6546, F = 18.95, p = 0.001). A 1°C increase in the AMMAT was related to a 0.72% fewer mammalian individuals that would be infected by SARS-CoV-2 virus. Our findings also indicate that seasonal alternations between the northern and southern hemispheres could be a significant factor in determining a waved trajectory of the COVID-19 pandemic.
Therefore, international cooperation and joint research are of paramount importance. Some key scientific issues need to be further developed in three areas. These include (1) behavior, effect, and toxicological mechanism in the multi-process from natural environment to human, animal to environment to human, and human to human; (2) in addition to temperature, multi-factors should be focused, such as humidity, lighting, wind speed, extreme climate, susceptible people, natural and man-made environment types, and environmental pollution characteristics; (3) developing in silico techniques and statistical models to assess spreading spatial and temporal distributions in local, regional, and global scales.
Materials and methods
Data extraction
Literatures were obtained from the ISI Web of Knowledge Core Collection (1975-2020) and Chinese Science Citation Database (1989-2020) databases on July 12, 2020. To investigate the mesophilic traits of viruses, reference search strategy is virus [Topic] AND transmission [Topic] AND optimal temperature [Topic] and 86 publications were obtained. After checking abstract and full text, 15 papers on seven types of viruses satisfied the following selection criteria (Brady et al. 2014; Chan et al. 2011; Fiszon et al. 1989; Fouque and Reeder 2019; Hamlet et al. 2018; Kiseleva et al. 2010; Lambrechts et al. 2011; Li et al. 2014; Mordecai et al. 2013; Ng et al. 2014; Tesla et al. 2018; Tramonte and Christofferson 2019; Watanabe et al. 2013; Zhang et al. 2015; Zhang et al. 2004).
Body temperatures of mammals in different orders were obtained under existing literatures. The searching strategy is mammal [Topic] AND normal body temperature [Topic] OR normothermia [Topic]. After reviewing full text of 189 publications, 34 papers on normal body temperature of 62 mammals in 7 orders and 20 families were selected for further analysis (An 2005; Bevanger and Broseth 1998; Chen and White 2006; Deavers and Hudson 1981; Duan et al. 2019; Fleming 1980; Fons and Sicart 1976; Geiser 1984, 1986; Geiser 1988; Geiser and Baudinette 1987; Harlow 1981; Heldmaier and Steinlechner 1981; Hudson 1965; Hudson and Scott 1979; Jensen et al. 2009; Jing and Sun 1982; Kennedy and Macfarlane 1971; Kulzer 1965; Lee et al. 1985; Liu 1983; Liu et al. 2004; Macmillen 1965; Morrison and McNab 1962; Morton and Lee 1978; Noll 1979; Sheng 1982; Sun 2005; Thompson 1985; Tucker 1965; Wang 2003; Wang and Hudson 1970; Wei and Huang 1983; Yang et al. 2012). And then, keywords were set as coronavirus [Topic] AND mammal [Topic] AND PCR [Topic] OR polymerase chain reaction [Topic] to search both databases. Twelve papers reporting total number of samples and coronavirus positive number for mammalian individuals were screened (August et al. 2012; Berto et al. 2018; Castanheira et al. 2014; Ge et al. 2017; McIver et al. 2020; Nziza et al. 2020; Ommeh et al. 2018; Quan et al. 2010; Saldanha et al. 2019; Tsoleridis et al. 2016; Valitutto et al. 2020; Wacharapluesadee et al. 2015). Information on sampling time and location is summarized in Table S1. Average ambient minimum and maximum temperatures at the sampling regions were extracted from available data on World Meteorological Organization website (https://public.wmo.int/en). Global cumulative confirmed cases are derived from WHO Coronavirus Disease (COVID-19) Dashboard (https://covid19.who.int/). Population data are publicly available from open data of the World Bank (https://data.worldbank.org.cn/). Animal silhouettes visually represent each mammalian order which were downloaded from PhyloPic (www.phylopic.org).
Statistical analysis
Linear regression analysis was performed between the Pos/Tot ratio of different mammals and average monthly minimum ambient temperature (AMMAT). Robustness of fitting model was evaluated by use of the adjusted coefficient of determination (adj R2), sum of squares due to error (SSE), root mean square deviation (RMSE), and F value using one-way analysis of variance (ANOVA) with the level of significance at α = 0.05. Means and variances were compared with Bonferroni and Tukey’s multiple tests. To reduce the probability of overfitting the model to the training data, depending on presence/absence of one sample in the training set, models were internally validated by use of the cross-validated, leave-one-out technique (LOOCV) (Golbraikh et al. 2003; Tropsha et al. 2003). Following the LOOCV algorithm, each mammal was removed, one at a time, from the training set. The cross-validated correlation coefficient, QCV2, and cross-validated root-mean-square errors of prediction, RMSECV, were calculated from the sum of the squared differences between the observed and estimated toxicity. The recommended reference criteria stated that R2 should be greater than 0.6 and that the difference between R2 and QCV2 should be less than 0.3 (Eriksson et al. 2003). We used the QSAR toolbox in the SYBYL X1.1 program (Tripos, Inc., MO, USA) for leave-one-out calculations. Statistical analyses were completed with SPSS Statistics 17.0 (IBM Inc., NY, USA), G*Power 3.1.9.2 (program written by Franz Faul, Kiel University, Germany), and Origin Pro 8.0 (OriginLab Inc., MA, USA).
Supplementary Information
The supporting information (SI) provides details of supplementary data. The SI contains one figure and four tables with list of the details of survival time (days) for ninety-nine percent of viruses inactive (T99) in aquatic environment, coronavirus RNA-positive samples reported in the literature with their positive:total ratio (i.e., Pos/Tot ratio) and average monthly ambient minimum and maximum temperatures at the sampling regions, normal body temperature of 62 mammals in 7 orders and 20 families, and ratios of N hemisphere to global for cumulative confirmed cases and populations. (DOCX 439 kb)
Author contribution
Conceptualization: YSM; data curation: MCS, JM; formal analysis: BQZ, YQZ; methodology: YSM, FCW, FGZ; writing, original draft: YSM, FGZ; writing, reviewing and editing: KMYL, JPG, FCW.
Funding
The present study was supported by fund for building world-class universities (disciplines) of Renmin University of China “Interdisciplinary platform for ecological civilization”, and the Canada Research Chairs program of the Natural Sciences and Engineering Research Council of Canada and a visiting distinguished professorship of Baylor University.
Data availability
The datasets developed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yunsong Mu, Email: muyunsong@ruc.edu.cn.
Fangang Zeng, Email: zengfg@ruc.edu.cn.
References
- An D (2005) The evaluation of high environment temperature and thermoregulation of high yield dairy cow. Master Thesis, Nanjing Agricultural University, 61 pp
- August TA, Mathews F, Nunn MA. Alphacoronavirus detected in bats in the United Kingdom. Vector-Borne Zoonotic Dis. 2012;12:530–533. doi: 10.1089/vbz.2011.0829. [DOI] [PubMed] [Google Scholar]
- Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369:315–319. doi: 10.1126/science.abc2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik RK, Ulrich AK. Evidence of short-range aerosol transmission of SARS-CoV-2 and call for universal airborne precautions for anesthesiologists during the COVID-19 pandemic. Anesth Analg. 2020;131:e102–e104. doi: 10.1213/ANE.0000000000004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berto A, Anh PH, Carrique-Mas JJ, Simmonds P, Van Cuong N, Tue NT, Van Dung N, Woolhouse ME, Smith I, Marsh GA, Bryant JE, Thwaites GE, Baker S, Rabaa MA, consortium V (2018): Detection of potentially novel paramyxovirus and coronavirus viral RNA in bats and rats in the Mekong Delta region of southern Viet Nam. Zoonoses Public Hlth 65, 30-42 [DOI] [PMC free article] [PubMed]
- Bevanger K, Broseth H. Body temperature changes in wild-living badgers Meles meles through the winter. Wildl Biol. 1998;4:97–101. doi: 10.2981/wlb.1998.006. [DOI] [Google Scholar]
- Bhardwaj VK, Singh R, Sharma J, Rajendran V, Purohit R, Kumar S. Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors. J Biomol Struct Dyn. 2020;10:1–13. doi: 10.1080/07391102.2020.1766572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj VK, Singh R, Das P, Purohit R. Evaluation of acridinedione analogs as potential SARS-CoV-2 main protease inhibitors and their comparison with repurposed anti-viral drugs. Comput Biol Med. 2021;128:104117. doi: 10.1016/j.compbiomed.2020.104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Golding N, Pigott DM, Kraemer MUG, Messina JP, Reiner RC, Scott TW, Smith DL, Gething PW, Hay SI (2014) Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasite Vector 7 [DOI] [PMC free article] [PubMed]
- Bukhari Q, Jameel Y (2020) Will coronavirus pandemic diminish by summer?. Available at SSRN: https://ssrn.com/abstract=3556998
- Caminade C, Turner J, Metelmann S, Hesson JC, Blagrove MS, Solomon T, Morse AP, Baylis M. Global risk model for vector-borne transmission of Zika virus reveals the role of El Nino 2015. Proc Natl Acad Sci U S A. 2017;114:119–124. doi: 10.1073/pnas.1614303114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CJ, Zipfel CM, Garnier R, Bansal S. Global estimates of mammalian viral diversity accounting for host sharing. Nat Ecol Evol. 2019;3:1070–1075. doi: 10.1038/s41559-019-0910-6. [DOI] [PubMed] [Google Scholar]
- Castanheira P, Duarte A, Gil S, Cartaxeiro C, Malta M, Vieira S, Tavares L. Molecular and serological surveillance of canine enteric viruses in stray dogs from Vila do Maio, Cape Verde. BMC Vet Res. 2014;10:91. doi: 10.1186/1746-6148-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Peiris JS, Lam SY, Poon LL, Yuen KY, Seto WH. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Advances in Virology. 2011;2011:734690. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, White CE. Comparison of rectal, microchip transponder, and infrared thermometry techniques for obtaining body temperature in the laboratory rabbit (Oryctolagus cuniculus) J Am Assoc Lab Anim Sci. 2006;45:57–63. [PubMed] [Google Scholar]
- Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, Yang Y, Liu B, Wang W, Wei C, Yang J, Ye G, Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Deavers DR, Hudson JW. Temperature regulation in two rodents (Clethrionomys gapperi and Peromyscus leucopus) and a shrew (Blarina brevicauda) inhabiting the same environment. Physiol Zool. 1981;54:94–108. doi: 10.1086/physzool.54.1.30155808. [DOI] [Google Scholar]
- Duan S, Yang Y, Yang F, Li Y, Liu Q, Zhao Y, Ma S, He Z. Preliminary study on the infection of rhesus monkeys by human coxsackie B2 virus. Chin J Comp Med. 2019;29:52–60. [Google Scholar]
- Eriksson L, Jaworska J, Worth AP, Cronin MT, McDowell RM, Gramatica P. Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs. Environ Health Perspect. 2003;111:1361–1375. doi: 10.1289/ehp.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszon B, Hannoun C, Garciasastre A, Villar E, Cabezas JA. Comparison of biological and physical-properties of human and animal a(H1N1) Influenza-viruses. Res Virol. 1989;140:395–404. doi: 10.1016/S0923-2516(89)80118-9. [DOI] [PubMed] [Google Scholar]
- Fleming MR. Thermoregulation and torpor in the Sugar Glider, Petaurus Breviceps (Marsupialia: Petauridae) Aust J Zool. 1980;28:521–534. doi: 10.1071/ZO9800521. [DOI] [Google Scholar]
- Fons R, Sicart R (1976) Contribution a la connaissance du métabolisme énergétique chez deux crocidurinae: suncus etruscus (savi, 1822) et crocidura russula (hermann, 1780) (insectivora, soricidae). Mammalia 40 [PubMed]
- Fouque F, Reeder JC. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infect Dis Poverty. 2019;8:51. doi: 10.1186/s40249-019-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XY, Yang WH, Zhou JH, Li B, Zhang W, Shi ZL, Zhang YZ. Detection of alpha- and betacoronaviruses in rodents from Yunnan, China. Virol J. 2017;14:98. doi: 10.1186/s12985-017-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F. Correlates of torpor in the insectivorous marsupial Sminthopsis murina. Aust Mammal. 1984;7:185–191. doi: 10.1071/AM84020. [DOI] [Google Scholar]
- Geiser F. Thermoregulation and torpor in the Kultarr, Antechinomys laniger (Marsupialia: Dasyuridae) J Comp Physiol B. 1986;156:751–757. doi: 10.1007/BF00692755. [DOI] [Google Scholar]
- Geiser F. Daily torpor and thermoregulation in Antechinus (Marsupialia): influence of body mass, season, development, reproduction, and sex. Oecologia. 1988;77:395–399. doi: 10.1007/BF00378050. [DOI] [PubMed] [Google Scholar]
- Geiser F, Baudinette RV. Seasonality of torpor and thermoregulation in three Dasyurid marsupials. J Comp Physiol B. 1987;157:335–344. doi: 10.1007/BF00693360. [DOI] [Google Scholar]
- Golbraikh A, Shen M, Xiao Z, Xiao YD, Lee KH, Tropsha A. Rational selection of training and test sets for the development of validated QSAR models. J Comput Aided Mol Des. 2003;17:241–253. doi: 10.1023/A:1025386326946. [DOI] [PubMed] [Google Scholar]
- Hamlet A, Jean K, Perea W, Yactayo S, Biey J, Van Kerkhove M, Ferguson N, Garske T. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Negl Trop Dis. 2018;12:e0006284. doi: 10.1371/journal.pntd.0006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YX, Kral P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14:5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HJ. Torpor and other physiological adaptations of the badger (Taxidea-Taxus) to cold environments. Physiol Zool. 1981;54:267–275. doi: 10.1086/physzool.54.3.30159941. [DOI] [Google Scholar]
- Heldmaier G, Steinlechner S. Seasonal pattern and energetics of short daily torpor in the Djungarian hamster, Phodopus sungorus. Oecologia. 1981;48:265–270. doi: 10.1007/BF00347975. [DOI] [PubMed] [Google Scholar]
- Hudson JW. Temperature regulation and torpidity in the pygmy Mouse, Baiomys taylori. Physiol Zool. 1965;38:243–254. doi: 10.1086/physzool.38.3.30152836. [DOI] [Google Scholar]
- Hudson JW, Scott IM. Daily torpor in the laboratory mouse, Mus musculus Var. Albino. Physiol Zool. 1979;52:205–218. doi: 10.1086/physzool.52.2.30152564. [DOI] [Google Scholar]
- Jensen SA, Mundry R, Nunn CL, Boesch C, Leendertz FH. Energy metabolism and nocturnal hypothermia in two tropical passerine frugivores, Manacus vitellinus and Pipra mentalis. J Wildl Dis. 2009;45:542–546. doi: 10.7589/0090-3558-45.2.542. [DOI] [PubMed] [Google Scholar]
- Jing S, Sun R. Studies on development of thermoregulation in Mongolian gerbil, Meriones Uguiculatus. Acta Ecol Sin. 1982;2:189–199. [Google Scholar]
- Kennedy PM, Macfarlane WV. Oxygen consumption and water turnover of the fat-tailed marsupials Dasycercus cristicauda and Sminthopsis crassicaudata. Comp Biochem Phys A. 1971;40:723–732. doi: 10.1016/0300-9629(71)90257-X. [DOI] [PubMed] [Google Scholar]
- Kiseleva I, Larionova N, Kuznetsov V, Rudenko L. Phenotypic characteristics of novel swine-origin influenza A/California/07/2009 (H1N1) virus. Influenza Other Resp. 2010;4:1–5. doi: 10.1111/j.1750-2659.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer MUG, Cummings DAT, Funk S, Reiner RC, Faria NR, Pybus OG, Cauchemez S. Reconstruction and prediction of viral disease epidemics. Epidemiol Infect. 2019;147:1–7. doi: 10.1017/S0950268818002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulzer E. Temperaturregulation bei Fledermäusen (Chiroptera) aus verschiedenen Klimazonen. Zeitschrift für vergleichende Physiologie. 1965;50:1–34. doi: 10.1007/BF00388050. [DOI] [Google Scholar]
- Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. P Natl Acad Sci USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TF, Mora F, Myers RD. Effect of intracerebroventricular vasopressin on body temperature and endotoxin fever of macaque monkey. Am J Phys. 1985;248:674–678. doi: 10.1152/ajpregu.1985.248.6.R674. [DOI] [PubMed] [Google Scholar]
- Li XL, Gao XY, Ren ZP, Cao YX, Wang JF, Liang GD. A spatial and temporal analysis of Japanese encephalitis in Mainland China, 1963–1975: A Period without Japanese Encephalitis Vaccination. PLoS One. 2014;9:e99183. doi: 10.1371/journal.pone.0099183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y (1983) A preliminary study on canine distemper of the suspected Ailuropoda melanoleuca. Chinese J Wildlife:50–51
- Liu Y, Zhang Q, Wang Z (2004) A comparative study on the metabolic heat production capacity of three rodents, Abstracts of the 6th Congress and Symposium of the veterinary branch of the Chinese Zoological Society, Hunan
- Luo C, Yao L, Zhang L, Yao MC, Chen XF, Wang QL, Shen HB (2020) Possible transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a public bath center in Huai’an, Jiangsu province, China. JAMA Netw Open 3 [DOI] [PubMed]
- Macmillen RE. Aestivation in the cactus mouse, Peromyscus eremicus. Comp Biochem Physiol. 1965;16:227–248. doi: 10.1016/0010-406X(65)90062-9. [DOI] [PubMed] [Google Scholar]
- McIver DJ, Silithammavong S, Theppangna W, Gillis A, Lange CE. Coronavirus surveillance of wildlife in the Lao People’s Democratic Republic detects viral RNA in rodents. Arch Virol. 2020;165:1869–1875. doi: 10.1007/s00705-020-04683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JP, Kraemer MU, Brady OJ, Pigott DM, Shearer FM, Weiss DJ, Golding N, Ruktanonchai CW, Gething PW, Cohn E, Brownstein JS, Khan K, Tatem AJ, Jaenisch T, Murray CJ, Marinho F, Scott TW, Hay SI. Mapping global environmental suitability for Zika virus. eLife. 2016;5(2016-04-10):5. doi: 10.7554/eLife.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollentze N, Streicker DG. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc Natl Acad Sci U S A. 2020;117:9423–9430. doi: 10.1073/pnas.1919176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, Moor E, McNally A, Pawar S, Ryan SJ, Smith TC, Lafferty KD. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol Lett. 2013;16:22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- Morrison P, McNab BK. Daily torpor in a Brazilian murine opossum (Marmosa) Comp Biochem Physiol. 1962;6:57–68. doi: 10.1016/0010-406X(62)90043-9. [DOI] [Google Scholar]
- Morton SR, Lee AK. Thermoregulation and metabolism in Planigale maculata (Marsupialia: Dasyuridae) J Therm Biol. 1978;3:117–120. doi: 10.1016/0306-4565(78)90003-7. [DOI] [Google Scholar]
- Ng S, Basta NE, Cowling BJ. Association between temperature, humidity and ebolavirus disease outbreaks in Africa, 1976 to 2014. Eurosurveillance. 2014;19:16–26. doi: 10.2807/1560-7917.ES2014.19.35.20892. [DOI] [PubMed] [Google Scholar]
- Noll UG. Body-temperature, oxygen-consumption, noradrenaline response and cardiovascular adaptations in the flying fox, Rousettus-aegyptiacus. Comp Biochem Physiol A Physiol. 1979;63:79–88. doi: 10.1016/0300-9629(79)90631-5. [DOI] [Google Scholar]
- Nziza J, Goldstein T, Cranfield M, Webala P, Nsengimana O, Nyatanyi T, Mudakikwa A, Tremeau-Bravard A, Byarugaba D, Tumushime JC, Mwikarago IE, Gafarasi I, Mazet J, Gilardi K. Coronaviruses detected in bats in close contact with humans in Rwanda. Ecohealth. 2020;17:152–159. doi: 10.1007/s10393-019-01458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ommeh S, et al. Genetic evidence of Middle East respiratory syndrome coronavirus (MERS-Cov) and widespread seroprevalence among camels in Kenya. Virol Sin. 2018;33:484–492. doi: 10.1007/s12250-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain RH. Temperature and macromolecular structure and function. Symp Soc Exp Biol. 1987;41:21–33. [PubMed] [Google Scholar]
- Prata DN, Rodrigues W, Bermejo PH. Temperature significantly changes COVID-19 transmission in (sub) tropical cities of Brazil. Sci Total Environ. 2020;729:138862. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan P-L, Firth C, Street C, Henriquez JA, Petrosov A, Tashmukhamedova A, Hutchison SK, Egholm M, Osinubi MOV, Niezgoda M, Ogunkoya AB, Briese T, Rupprecht CE, Lipkin WI (2010) Identification of a severe acute respiratory syndrome coronavirus-like virus in a leaf-nosed bat in Nigeria. mBio:e00208–e00210 [DOI] [PMC free article] [PubMed]
- Ren SY, Wang WB, Hao YG, Zhang HR, Wang ZC, Chen YL, Gao RD. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. 2020;8:1391–1399. doi: 10.12998/wjcc.v8.i8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Kok A, de Meulder D, Bestebroer TM, Lamers MM, Okba NMA, Fentener van Vlissingen M, Rockx B, Haagmans BL, Koopmans MPG, Fouchier RAM, Herfst S. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun. 2020;11:3496. doi: 10.1038/s41467-020-17367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabateeshan M, Graham D (2020) Lions, tigers and kittens too: ACE2 and susceptibility to CoVID-19. Evol Med Public Health:109–113 [DOI] [PMC free article] [PubMed]
- Saldanha IF, Lawson B, Goharriz H, Fernandez JRR, John SK, Fooks AR, Cunningham AA, Johnson N, Horton DL. Extension of the known distribution of a novel clade C betacoronavirus in a wildlife host. Epidemiol Infect. 2019;147:e169. doi: 10.1017/S0950268819000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J, Bhardwaj VK, Singh R, Rajendran V, Purohit R, Kumar S. An in-silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein-15 of SARS-CoV-2. Food Chem. 2021;346:128933. doi: 10.1016/j.foodchem.2020.128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H (1982) Adaptation of mammals to high temperature environment. Biol Teaching:13–15
- Sit THC, Brackman CJ, Ip SM, Tam KWS, Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J 2005 Study on infectious model of cevet for SARS-CoV isolated from human. Master Thesis, Shenyang Agricultural University, 64 pp
- Tesla B, Demakovsky LR, Mordecai EA, Ryan SJ, Bonds MH, Ngonghala CN, Brindley MA, Murdock CC (2018) Temperature drives Zika virus transmission: evidence from empirical and mathematical models. P Roy Soc B-Biol Sci 285 [DOI] [PMC free article] [PubMed]
- Thompson SD. Subspecific differences in metabolism, thermoregulation, and torpor in the western harvest mouse Reithrodontomys megalotis. Physiol Zool. 1985;58:430–444. doi: 10.1086/physzool.58.4.30156018. [DOI] [Google Scholar]
- Tramonte AR, Christofferson RC (2019) Investigating the probability of establishment of Zika virus and detection through mosquito surveillance under different temperature conditions. PLoS One 14 [DOI] [PMC free article] [PubMed]
- Tropsha A, Gramatica P, Gombar VK. The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb Sci. 2003;22:69–77. doi: 10.1002/qsar.200390007. [DOI] [Google Scholar]
- Tsoleridis T, Onianwa O, Horncastle E, Dayman E, Zhu MR, Danjittrong T, Wachtl M, Behnke JM, Chapman S, Strong V, Dobbs P, Ball JK, Tarlinton RE, McClure CP. Discovery of novel Alphacoronaviruses in European rodents and shrews. Viruses-Basel. 2016;8:9. doi: 10.3390/v8030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker VA. Oxygen consumption, thermal conductance, and torpor in the California pocket mouse Perognathus californicus. J Cell Comp Physiol. 1965;65:393–403. doi: 10.1002/jcp.1030650313. [DOI] [PubMed] [Google Scholar]
- Valitutto MT, Aung O, Tun KYN, Vodzak ME, Zimmerman D, Yu JH, Win YT, Maw MT, Thein WZ, Win HH, Dhanota J, Ontiveros V, Smith B, Tremeau-Brevard A, Goldstein T, Johnson CK, Murray S, Mazet J. Detection of novel coronaviruses in bats in Myanmar. PLoS One. 2020;15:e0230802. doi: 10.1371/journal.pone.0230802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacharapluesadee S, Duengkae P, Rodpan A, Kaewpom T, Maneeorn P, Kanchanasaka B, Yingsakmongkon S, Sittidetboripat N, Chareesaen C, Khlangsap N, Pidthong A, Leadprathom K, Ghai S, Epstein JH, Daszak P, Olival KJ, Blair PJ, Callahan MV, Hemachudha T. Diversity of coronavirus in bats from Eastern Thailand. Virol J. 2015;12:7. doi: 10.1186/s12985-015-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Study on the relationship between body temperature of growing pigs and temperature-humidity index of pig housing. J Zhejiang Univ (Agric & Life Sci) 2003;029:675–678. [Google Scholar]
- Wang LC-H, Hudson JW. Some physiological aspects of temperature regulation in the normothermic and torpid hispid pocket mouse, Perognathus hispidus. Comp Biochem Physiol. 1970;32:275–293. doi: 10.1016/0010-406X(70)90941-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Tang K, Feng K, Lin X, Lv W, Chen K, Wang F (2020a) High temperature and high humidity reduce the transmission of COVID-19. Available at SSRN: https://ssrn.com/abstract=3551767
- Wang M, Jiang A, Gong L, Luo L, Guo W, Li C, Zheng J, Li C, Yang B, Zeng J, Chen Y, Zheng K, Li H (2020b) Temperature significant change COVID-19 transmission in 429 cities. Available at medRxiv: 10.1101/2020.02.22.20025791
- Watanabe T, et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Huang W. The comparative study of gas metabolism in three rodents. Acta Theriol Sin. 1983;3:73–84. [Google Scholar]
- Wood JP, Choi YW, Chappie DJ, Rogers JV, Kaye JZ. Environmental persistence of a highly pathogenic avian Influenza (H5N1) virus. Environ Sci Technol. 2010;44:7515–7520. doi: 10.1021/es1016153. [DOI] [PubMed] [Google Scholar]
- Yang F, Chen L, Zhao Y, Lu S, Yu W, Wang J, Li Y, He Z. The comparison of infrared ear thermometer and mercury thermometer in temperature measurement of macaque. Lab Anim Comp Med. 2012;32:351–352. [Google Scholar]
- Yao M, Zhang L, Ma J, Zhou L. On airborne transmission and control of SARS-Cov-2. Sci Total Environ. 2020;731:139178. doi: 10.1016/j.scitotenv.2020.139178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shou S, Zhang P, Chen T. Synoptic and climatic features during SARS spreading in China in 2003. Meteorological Monthly. 2004;30:46–49. [Google Scholar]
- Zhang Y, Feng C, Ma CN, Yang P, Tang S, Lau A, Sun WJ, Wang QY. The impact of temperature and humidity measures on influenza A (H7N9) outbreaks-evidence from China. Int J Infect Dis. 2015;30:122–124. doi: 10.1016/j.ijid.2014.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supporting information (SI) provides details of supplementary data. The SI contains one figure and four tables with list of the details of survival time (days) for ninety-nine percent of viruses inactive (T99) in aquatic environment, coronavirus RNA-positive samples reported in the literature with their positive:total ratio (i.e., Pos/Tot ratio) and average monthly ambient minimum and maximum temperatures at the sampling regions, normal body temperature of 62 mammals in 7 orders and 20 families, and ratios of N hemisphere to global for cumulative confirmed cases and populations. (DOCX 439 kb)
Data Availability Statement
The datasets developed during the current study are available from the corresponding author on reasonable request.