Abstract
It is important to know whether SARS-CoV-2 is spread through the air conditioning systems. Taking the central air conditioning system as an example, we analyze the mechanism and potential health risk of respiratory virus transmission in air-conditioned rooms and propose a method to study the risk of virus transmission in central air conditioning systems by investigating the data from medical experiments. The virus carrying capacity and the decay characteristics of indoor pathogen droplets are studied in this research. Additionally, the effects of air temperature and relative humidity on the virus survival in the air or on surfaces are investigated. The removal efficiency of infectious droplet nuclei by using an air conditioning filter was then determined. Thus, the transmission risk during the operation of the centralized air conditioning system is evaluated. The results show that the indoor temperature and humidity are controlled in the range of 20–25 °C and 40–70% by central air conditioning during the epidemic period, which not only benefits the health and comfort of residents, but also weakens the vitality of the virus. The larger the droplet size, the longer the viruses survive. Since the filter efficiency of the air conditioning filter increases with the increase in particle size, increasing the number of air changes of the circulating air volume can accelerate the removal of potential pathogen particles. Therefore, scientific operation of centralized air conditioning systems during the epidemic period has more advantages than disadvantages.
Graphical abstract
Keywords: Central air-conditioned room, Environmental regulation, Circulating air;, espiratory viruses, Virus survival test;,Transmission risk
Introduction
Since the end of 2019, the COVID-19 epidemic caused by SARS-COV-2 coronavirus has spread rapidly around the world, reaching more than 200 countries and regions with global implication ("WHO announces COVID-19 outbreak a pandemic (http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic). At present, epidemic prevention and control have become a major issue in China and even all over the world. In particular, whether SARS-CoV-2 spreads through the air conditioning system has drawn wide attention (Correia et al. 2020).
First, we need to understand how SARS-CoV-2 is transmitted. At present, it has been determined that the main transmission routes are through droplets and physical contact (Hoseinzadeh et al. 2020). It is not clear whether aerosols constitute one of the transmission routes, and it has also been the focus of debate among scholars. Recently, a team of 239 interdisciplinary scientists signed a document urging the WHO and public health authorities to recognize the airborne transmission of SARS-CoV-2 (Morawska and Milton 2020). Studies have shown that most people spend 90% of their lives indoors (Klepeis et al. 2020; Wang et al. 2015). In a crowded confined space, the droplets exhaled by SARS-CoV-2-infected patients through breathing, speaking, coughing, and other activities will form virus-containing aerosols (Morawska 2006; Zhang et al. 2020), and the virus may spread through these aerosols, a risk that should not be underestimated. For example, on February 3, 2020, a case of COVID-19 was reported in Inner Mongolia, China. The patient had repeatedly entered the room of a symptomatic patient but did not have direct contact with the patient. This case suggests that SARS-CoV-2 may spread through the air (Wang and Du 2020). By analyzing trends of epidemic spread in Wuhan, China, Italy, and New York, the results of a recent study suggest that air contributes to the spread of SARS-CoV-2 (Zhang et al. 2020).
Regarding the risk of indoor virus transmission, in 2010, Chen and Liao (Chen and Liao 2010) studied the transmission risk of different influenza viruses in an indoor environment based on the Wells–Riley mathematical model, and the results showed that the risk of indoor virus infection was negatively correlated with the air changes per hour (ACH) rate, where ACH is the ratio of the amount of clean air entering a room (m3/h) to the room (m3) per hour. In 2013, Cheng and Liao (Cheng and Liao 2013) used a multicontrol measure model for influenza to study the risk control effect of H1N1 in indoor transmission and revealed that the uncontrollable rate of influenza decreased with increase in the ACH rate. In 2020, in response to the outbreak of COVID-19, some scholars assessed the transmission risk of SARS-CoV-2 in the air. The team of Buonanno (Buonanno et al. 2020) emphasized the importance of ventilation in indoor environments in reducing the risk of infection.
Can the coronaviruses spread through the air conditioning systems? There are three major opinions on this issue. (1) There is evidence showing that the viruses can spread through air conditioning systems. Since the Philadelphia Legionnaires incident in 1976 (Farnham et al. 2014), scholars have repeatedly argued that the operation of air conditioning is beneficial to the spread of germs. They believe that the droplets produced by pathogen carriers through coughing, sneezing, and other activities cannot be effectively contained even by powerful air conditioning airflows. On the contrary, inappropriate airflows of the air conditioner will help the spread of droplets (https://www.buildup.eu/en/practices/publications/covid-19-guidance-rehva; Jayaweera et al. 2020; Lu et al. 2020; Pan et al. 2020a, b; Siddiqui and Ahmed Khan 2020). From January to February 2020, an outbreak of COVID-19 occurred in an air-conditioned restaurant in Guangzhou, China. Lu et al. (Lu et al. 2020) concluded that air-conditioned ventilation promoted the spread of SARS-CoV-2 in the restaurant after examining the potential transmission routes of the case. Correia et al. (Correia et al. 2020) also pointed out that improper use of air conditioning systems may lead to SARS-COV-2 transmission after reviewing the possible impact of HVAC on building virus transmission. (2) There is no evidence showing that the viruses can spread through air conditioning systems. The argument is that there is currently no evidence that the viruses can spread through air conditioning systems. COVID-19 is a global pandemic, with more than 27 million confirmed cases (“WHO, WHO Coronavirus Disease (COVID-19) Dashboard 2020. https://covid19.who.int/,”). Statistically speaking, the sample size is large enough, and many confirmed COVID-19 cases can be traced back to the origin. However, none of the medical records so far has clearly indicated that the cause is due to the centralized air conditioning system, so it is impossible to determine whether the air conditioning system spreads the viruses (“https://health.clevelandclinic.org/does-air-conditioning-spread-the-coronavirus/,”). (3) There is evidence that air conditioning systems work against the spread of viruses. This opinion is represented by the American Society of Heating, Refrigeration and Air-Conditioning Engineers (ASHRAE) (“https://www.ashrae.org/technical-resources/resources,” ; “https://www.cibse.org/coronavirus-covid-19/coronavirus-covid-19-and-hvac-systems,”). In a statement (“https://www.ashrae.org/technical-resources/resources,” ; “https://www.cibse.org/coronavirus-covid-19/coronavirus-covid-19-and-hvac-systems,”) on the relationship between COVID-19 and HVAC in buildings, it is clearly stated that ASHRAE opposes the recommendation not to use the HVAC system during the epidemic and maintains that keeping the air conditioning on during this period can help control the spread of the viruses. The press release issued by the German Association of Raumlufttechnische Geraete Herstellerverband e.V. (RLT) on March 26, 2020 (“https://rlt-geraete.de/,”) also expressed support for the viewpoint of epidemic prevention by air conditioning operation. However, due to the lack of academic supporting evidence, it is clearly argued that this is a minority view.
The situation in Chinese COVID-19 epidemic prevention and control continues to improve. Under the conditions of normalization of prevention and control, it is required to accelerate the restoration of production and life order and actively promote the resumption of work and production in an orderly manner. Commercial industries such as restaurants have opened their doors one after another. The northern hemisphere has entered early winter after the hot summer, and the southern hemisphere has entered an early summer. Therefore, will the operation of the centralized air conditioning system with return air increase the risk of virus transmission during the epidemic? This is a matter of public concern, government worry, and academic controversy, which is well worth studying. However, the three views are now the mainstream controversy, and the deadlock of opposition cannot be broken. The key reason is that the opinions are either based on the observation of epidemiological phenomena or the empirical inference of their respective disciplines, without the support of relevant medical virus experiments. In this paper, we take the air conditioning system with single return air as an example and systematically study the risk of SARS-CoV-2 spreading in a centralized air conditioning operation room from a unique perspective based on medical experiments, thus filling in the gap.
Analysis and methodology of the transmission mechanism
A medical evidence-based experiment is a method to first analyze the mechanism of the influence of respiratory virus on indoor person-to-person transmission, then to use the medical experimental evidence of the indoor environment for virus survival and infection among animals, and furthermore to study the risk of virus transmission of the main measures for the operation and control of centralized air conditioning systems and finally the optimal operation strategy of centralized air conditioning for prevention and control during the outbreak to be put forward. The medical experiments include particle size distribution of exhaled droplets, evaporation concentration process and viral load of infected persons, the influence of temperature and humidity on virus decay and animal infection, and the effect of a filter on the removal of potential pathogen particles.
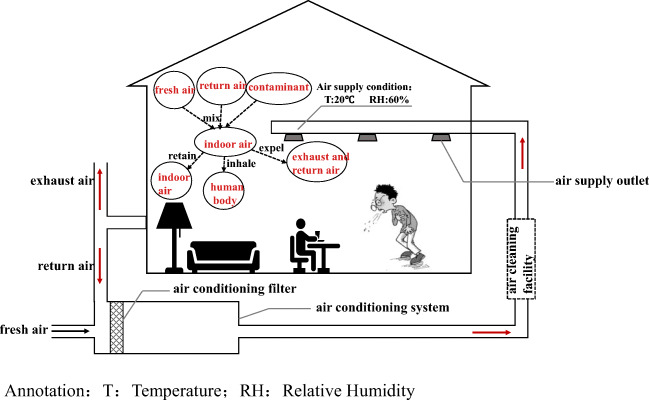
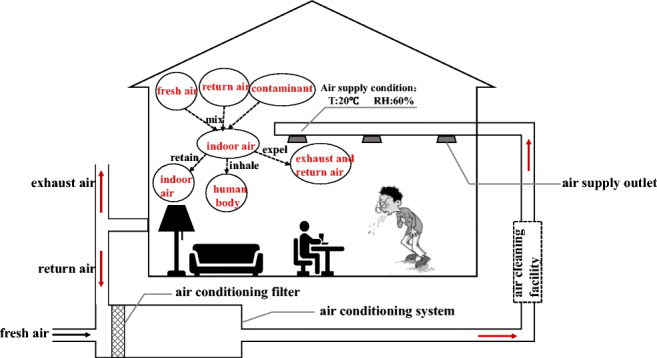
The COVID-19 is a respiratory infectious disease. Its transmission in the population includes three processes: occurrence, spread, and termination. The transmission process must have three basic links: the source of infection, the route of transmission, and the susceptible people (Lindsley et al. 2012; Zhao et al. 2010). Individual breathing activities and interpersonal social behavior are the main sources of transmission, and the factors affecting the transmission risk are very complex. In order to focus on the research topic, we only analyze the transmission mechanism of respiratory viruses in the indoor space of the primary return air centralized air conditioning system, as shown in Fig. 1. Through breathing, talking, coughing, sneezing, and other behaviors, the droplets or deposits on the surface of objects are released by the infected person into the ambient air. If the droplets or deposits are inhaled or touched by susceptible people again through close contact or close social activities, the infection spread might occur. When the viruses are inside of the host’s body, the temperature is appropriate, the water is sufficient, the protein nutrition composition is rich, the salt is moderate, the acid is balanced, and the environment is optimal. Once the droplets leave the host and enter the indoor environment, they must undergo the physical process of increasing the water evaporation and component concentration. The temperature, relative humidity, air speed, and surface characteristics of the environmental space have a direct impact on the droplets and droplet nuclei carrying pathogens in regard to particle size, distribution, and movement characteristics. Therefore, the new environment is also an important factor influencing the survival and spread of the viruses. The centralized air conditioning system that regulates indoor temperature and humidity, air quality, and airflow organization makes the mechanism of indoor virus transmission more complicated.
Fig. 1.
Schematic diagram of indoor SARS-CoV-2 transmission and air conditioning operation regulation. T temperature, RH relative humidity
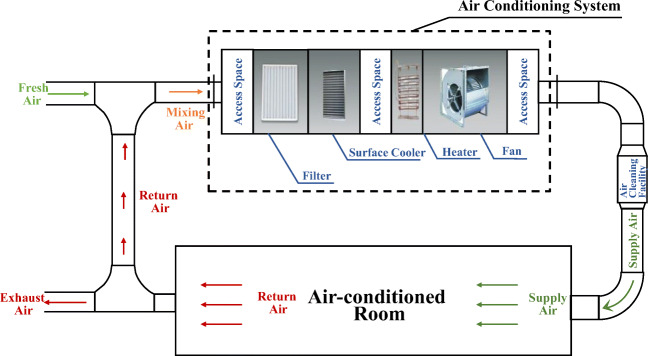
The main four functions of the conventional centralized air conditioning system are as follows. The first is to adjust the indoor temperature and relative humidity to an appropriate range for human comfort. The second is to reduce the concentration of pollutants through fresh air to ensure indoor hygiene requirements. The third is to partially remove indoor particulate pollutants through the primary and medium-efficiency filters installed in the air treatment box, thereby improving indoor air quality. For heavily polluted areas or special buildings such as hospitals, high-efficiency air purifiers are also installed in the systems to further improve indoor air quality. The fourth is to enhance the system’s heat and humidity load regulation capacity through partial return air and reduce the energy consumption of the air conditioning system. However, during the outbreak of the epidemic, some regulation functions of the conventional centralized air conditioning systems were questioned, which might increase the risk of epidemic transmission. For example, the return airflow processed by the air conditioning systems is returned to each room, which may cause the spread of potential pathogens. Then, after the filters and purifiers in the system capture the particles of potential pathogens, they may enter each room through the air supply again, and the pathogens collected on the filter layer may breed multiply. In addition, due to the various forms of airflow organization in the centralized air conditioning systems, small droplets and droplet nuclei can easily float in the air. The contact methods of infected and susceptible individuals in centralized air-conditioned rooms are uncertain. All of the above give people reason to doubt that air conditioning operation will increase the risk of transmission.
Due to the concerns about these potential risks, some professional institutions may suggest the following strategies when formulating guidelines for the operation and prevention of centralized air conditioning systems in public buildings during the epidemic (Pan et al. 2020a, b; “REHVA_COVID-19_guidance_document_ver2_20200403_ n.d.”). Firstly, turn on as little as possible or completely stop the use of centralized air conditioning systems. However, the downside is huge, because the amount of public buildings with air conditioning systems is massive, and the air conditioning system operation is the basic guarantee for restoring the social order of business. Stopping the operation of the air conditioning system may cause the indoor temperature and humidity too high or too low, and the indoor air quality will decrease, thus affecting the health of indoor personnel. Secondly, it is recommended to close the return air and operate all fresh air instead. However, since the design air volume of the fresh air system can only take a small part of the total load of the building, it is far from achieving indoor thermal comfort. The third is to transform and increase the fresh air systems, but this is a complex system project. For existing public buildings, the expansion of the pipeline system and equipment room is subject to large space constraints, and the practical conditions are limited. In particular, the increase in the fresh air volume has a significant impact on the air conditioning heating load, but the original matching host is difficult to bear, resulting in most of it impossible to implement. Fourth, it is suggested to try to open doors and windows for ventilation when the air conditioning heating system is running. However, since the amount of natural ventilation entering the room through doors and windows is greatly affected by the outdoor wind speed and is hard to control, the magnitude of fresh air changes (ACH) may be as high as 30~50/h (Becker et al. 2013) even when the doors and windows are 10% open. This approach may cause the air conditioning load to sharply increase. At this time, even if the system matched with the original design is running at full capacity, it is only a drop in the bucket. Therefore, this proposal not only wastes energy, but also sacrifices the comfort of indoor personnel.
In the field of built environment, the experts’ concern that the operation of centralized air conditioning systems may increase the risk of transmission is not groundless, and the prevention and control technology recommendations are also based on the common sense of environmental disciplines and are well founded. However, the transmission of the epidemic is a very complex interdisciplinary issue. It not only needs to conform to the common sense of indoor environmental control disciplines, but also needs to respect the basic laws of virus survival and spread. Only when these two are combined and the main contradiction of indoor transmission risk is recognized, can the prevention and control technology strategies be more accurate. The evidence-based medical experiment is not only the blind spot of the current research, but also the key to realize the breakthrough of knowledge.
Based on the above analysis, we propose a specific method of evidence-based medical experiments. First of all, droplets (droplet nuclei) are not only the pollution sources that diffuse in indoor environment with the air supply and return air of centralized air conditioning systems, but also the potential pathogen particles captured by the air filters and purifiers, the source of respiratory diseases such as the SARS-CoV-2 virus. Through the medical experiments, we can understand the viral load of the mouth, nose, and throat fluids of infected persons, the number and distribution of particle sizes of droplets released by various respiratory activities (breathing, speaking, coughing, and sneezing) of infected persons, and the difference of the potential virus carrying amount of different particle sizes. These form the basis for discriminating and analyzing the floating, settling, and moving trajectory of indoor pathogen particles and then allow studying the transmission risks of air conditioning system return air and filter operation.
Secondly, with the goal of keeping human health and comfort, the regulation of indoor temperature and relative humidity is the most important part of the operation of air conditioning systems. However, which kind of temperature and relative humidity is the most unfavorable for the survival of respiratory viruses and the most unfavorable for the spread of viruses is the question that must be answered through evidence-based medical experiments. Only by combining the advantages of human health and comfort with the disadvantages of virus survival and transmission, can a more scientific and precise control strategy be proposed for the operation air conditioning systems during the outbreak. The indoor temperature and humidity largely determine the physical fate of droplets exhaled from an infected person. Because under a certain temperature and humidity conditions, the water evaporation rate and the increase rate of the concentration of each component of the viral-containing liquid drops with different volumes or diameters in the air or on the surface of the object are different, which directly affects the speed of the decay process. While opening doors and windows or increasing the fresh air volume during the operation of the air conditioning system can reduce the concentration of indoor pathogens, it will significantly increase the air conditioning heating and cooling load and energy consumption. The coupling changes of indoor temperature and humidity will lead to changes in the law of virus survival and transmission, which is an important proposition that requires medical evidence to be answered.
Thirdly, the filter is also an important device in the air conditioning systems to remove particulate pollutants and improve air quality. During the epidemic, the one-time removal efficiency of potential pathogen particles and the potential risk of transmission also must be evidence based through medical experiments. The moisture of the droplets exhaled by an infected person evaporates quickly and becomes a droplet nucleus, which may float in the air for a long time and flow with the air conditioning systems, causing spread risk. More than 98% of the droplets are water, and the rest are protein nutrients and trace components. The ratio of the droplet core particle size to the initial particle size after evaporation is about 0.25~0.35 (Mao et al. 2020). Because the size of droplets varies widely and the volume of droplets of different particle sizes is proportional to the cube of the particle size, for example, the volume of a 10 μm particle is equivalent to more than 1000 times that of 1 μm, so the spread risk varies greatly. Therefore, for the transmission risk assessment of filters or air purifiers in air conditioning systems during the epidemic, attention should be paid not only to the filtration efficiency at the rated working condition (0.3), but also to the removal efficiency of particulate matter with a large particle size range, and the global analysis of the survival behavior of the viruses on the object surfaces (filter layers) should also be considered. These all need the support of medical experiment evidence.
Finally, based on the above methodology, the transmission risk of the operation of primary return air centralized air conditioning systems is discussed in depth.
Results
Transmission risk of indoor pathogens
The differences in virus concentration of different particle sizes
In the indoor environment, the source of the risk of virus transmission is that when there are virus carriers in the room, toxic droplets exhaled through coughing, sneezing, and other behaviors may be inhaled by susceptible people, thus causing the spread of the viruses (Olsen et al. 2003; Qu et al. 2020). We need to understand the viral load of the mouth, nose, and throat swabs of infected persons (or asymptomatic patients) through the medical measurements, as well as measure the particle size range and volume distribution of droplets released by respiratory activities into the room. It is the basis for the air conditioning and ventilation system to transmit the virus-containing droplets and nuclear particles and investigating the risk of virus disease transmission.
In medicine, the real-time RT-PCR virus nucleic acid test of the swab of the mouth, nose, and throat of the population or patient is generally used to judge the infection and its severity (Guarnieri and Balmes 2014). Since the outbreak of the COVID-19 epidemic, there have been many reports on the viral load data of patients. Zou et al. (Zou et al. 2020) and Cao et al. (Cao et al. 2020) tested 6 patients and 199 SARS-CoV-2 patients who participated in the treatment of throat swab viral load concentration in the early stage of the epidemic outbreak. The results showed that individual differences were significant in different patients, and viral load differences were significant on different dates after admission. Pan et al. (Pan et al. 2020a, b) found that viral load reached peak value five to six days after the onset of symptoms in the throat swabs of confirmed patients in Beijing. Tsang et al. (Tsang et al. 2020; Wang and Du 2020) tested saliva samples from 23 patients of different ages in two hospitals in Hong Kong and found that the viral load peaked in the first week after symptom onset and then decreased over time. Figure 2 presents a representative comparison of viral load changes in mouth, nose, and throat swabs from five medical reports involving 51 patients. It can be seen that the viral concentration of the COVID-19 patients (asymptomatic) varies greatly among different patients, and the same patient varies greatly at different time periods after infection, generally reaching the peak at four to six days after onset, with the maximum of 109 copies/mL. This suggests that if there are asymptomatic patients in a centrally air-conditioned indoor space, the virus concentration and transmission risk of the sprayed droplets or saliva droplets greatly differ.
Fig. 2.
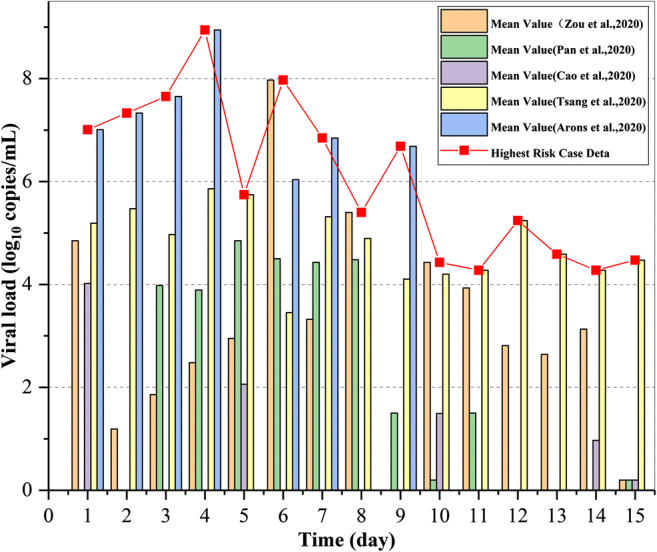
Trend of viral load in the body fluids of infected persons over time
Affected by the degree of infection, time, and other factors, although the virus concentration exhaled from different parts of the respiratory tract of an infected person is different, for a certain respiratory activity, the virus concentration in the exhaled droplets of various particle sizes can be considered to be the same. Therefore, when discussing the transmission risk of exhaled droplets, the number of viruses contained in droplets of different particle sizes is positively correlated with the total volume of droplets in the range of particle sizes, which can represent the initial level of virus concentration and transmission risk of such droplets.
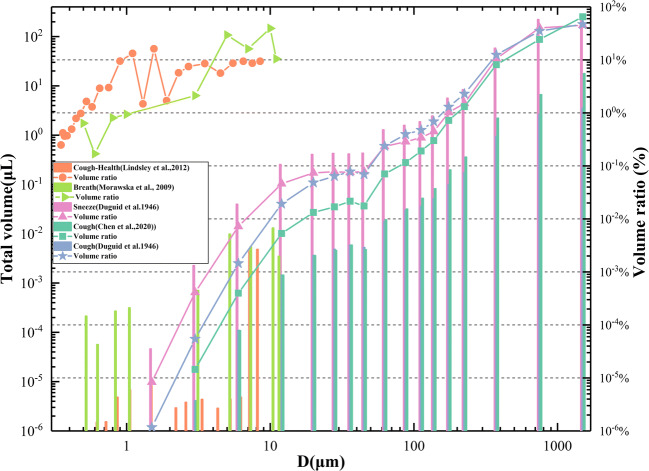
Figure 3 shows the analysis of total volume and volume ratio of the exhaled droplets in different particle size ranges based on five medical reports, such as breathing, sneezing, and coughing (Chen et al. 2020; Duguid 1946, 1954; Lindsley et al. 2012; Morawska et al. 2009). It can be seen that although the size distribution of droplets reported in different studies is different due to differences in instruments and methods (DuguidJP; “Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities 1994,” 1994; Loudon and Roberts 1967), the overall rules are similar. Infected persons exhale a large number of small-size particles but a small number of large-size droplets, with a large particle size range (1~2000 μm). Although the number of small particle size droplets is much higher than that of large particle size droplets (60~100 μm), because of their small single size, the cumulative volume of small particle size droplets is much smaller than that of large particle size droplets. Therefore, the risk of virus transmission of large droplets is much higher, with a difference of up to 3 to 4 orders of magnitude. It reminds us that the number of small droplets floating with the air in the air conditioning system may be much larger than that of larger droplets, but the total volume of the small droplets is much smaller than the total volume of the large droplets, so the small droplets are not the main contradiction of propagation.
Fig. 3.
Volume distribution and proportion of droplet corresponding to different particle sizes
Survival rate of spittle with different particle sizes
The droplets with a smaller particle size emitted by indoor infected persons float in the air, while those with a larger particle size settle on the surface. What are the effects of air conditioning on the survival and transmission risk of virus droplets with different particle sizes? Only through medical experiments, can this question be answered. In medicine, the virus culture solution which is similar to human saliva is usually atomized by a nozzle and then released into a small chamber or titrated to the surface of an object. After a certain period of time, samples are taken to detect changes in concentration to find the decay behavior.
Figure 4 shows the change of relative survival rate of respiratory viruses in the air with atomized droplet size according to 6 groups of experiments conducted by five scholars (Ge et al. 2014; Ijaz et al. 1985; Pyankov et al. 2012, 2018; Sattar et al. 1984). In order to eliminate the coupling effects of other factors, the selected experimental conditions are all air-conditioned environmental experimental conditions with a temperature of 18~25 °C and a relative humidity of 40~60% and are basically similar. The value C in C/C0 is the concentration value aerosolized for 1 hour after the virus atomization. The upper and lower limits of confidential intervals in the figure represent the maximum and minimum values of experimental data in the literature. It can be seen that the survival rate of the atomized droplet viruses in the air is positively correlated with the particle size; that is, the larger the atomized droplet size, the higher the survival rate and the greater the risk of transmission. The larger the particle size of the atomized droplets, the more viruses they can carry. Moreover, the longer the water takes to evaporate due to the heat and mass transfer between the droplets and the environment, the slower the survival environment of the virus deterioration, leading to a significant increase in the risk of transmission. The results suggest that although a small particle size droplet nucleus can float in the air for a longer period of time, the transmission risk is far inferior to the initial stage just after leaving the host.
Fig. 4.
The relative survival rate of respiratory viruses in the air varies with particle size
Figure 5 shows the relative survival rate of respiratory viruses obtained from 12 groups of experiments in 6 reports with the change of titration volume on different surfaces (Chin et al. 2020a, b; Doremalen et al. 2020; Doremalen et al. 2013; Greatorex et al. 2011; Sakaguchi et al. 2010; Warnes et al. 2015). In order to eliminate the coupling effects of other factors, the selected experimental conditions were all air-conditioned conditions with a temperature of 18~25 °C and a relative humidity of 40~60%, which are basically similar. C in C/C0 is the concentration value aerosolized for 1 hour after the virus atomization. The upper and lower limits of confidential intervals in the figure represent the maximum and minimum values of experimental data in the literature. It can be seen from this figure that the volume of the venomous droplet has a very obvious effect on the survival of the viruses on different surfaces: as the volume of the surface titrated liquid decreases, the survival rate of the virus on the surface drops rapidly. Specifically, the survival rate of the virus in a 1 μL droplet is 3 orders of magnitude lower than that in 500 μL droplet. These medical measured data strongly prove that the larger the volume of the surface droplets, the greater the risk of transmission, and therefore, they are the focus of epidemic prevention and control. The mechanism is that the size of the droplet volume directly affects the physical process of the viruses on the surface and then has a significant impact on virus decay. According to related literature research and testing, it takes only a few minutes for 1 μL droplets to reach equilibrium on the surface, while it may take several hours for 500 μL droplets to reach equilibrium, which creates better conditions for the survival of the viruses.
Fig. 5.
The relative survival rate of respiratory viruses on different surfaces with the titration volume
Temperature and humidity control method based on the medical experiments
Controlling the indoor temperature and relative humidity is the most important aspect of air conditioning systems. At present, we already know which temperature and relative humidity are most conducive to human health and comfort, and we have accumulated a wealth of knowledge in the field of building environment regulations. However, which temperature and humidity environment are the most unfavorable to the survival and transmission of respiratory viruses is a question that can only be answered through evidence-based medical experiments.
Medical experiments on the effect of temperature on virus survival
In terms of the influence of environmental temperature on virus viability, Casanova et al. (Casanova et al. 2010) inoculated 10 μL of TGEV and MHV containing 104~105 mpn into cell culture medium similar to human secretion at 4, 20, and 40 °C, respectively, and then placed them on a stainless steel surface carrier. After that, an appropriate sampling interval (1–24 hours or 7–28 days) was selected for each working condition, and then, the samples were taken out for physical examination of virus survival. Prussin et al. (Prussin et al. 2018) titrated 10 μL Phi6 virus solution with the concentration of 108~1010 pfu mL-1 into the polystyrene cell culture dish and cultured at different temperatures for two hours; then, the samples were taken out to analyze the survival of the viruses. They all found that higher temperature can reduce the relative survival rate of the viruses, and low temperature was more conducive to the survival of viruses, and this rule was also verified in the virus survival experiment of Chin et al. (Chin et al. 2020a). Chin et al. (Chin et al. 2020b) first incubated SARS-CoV-2 in virus medium for 14 days (the final concentration was 106.8 TCID50 mL-1), and then, they were placed in the environment of 4, 22, and 37 °C, respectively, and the stability of SARS-CoV-2 at different temperatures was measured.
Figure 6 presents the medical comparative experiment results of the virus survival rate under five different temperature working conditions with 50–70% relative humidity which is a relatively comfortable air-conditioned environment. It can be seen that when the relative humidity is at a certain condition and the temperature is low, the survival rate of the surface viruses is very high. With the increase in temperature, the relative survival rate decreases significantly, and when the temperature rises to above 35 °C, most of the viruses have lost their threat. This is mainly because the higher temperature leads to the faster evaporation rate of the surface droplets and the more rapid increase in various salt concentrations that is suitable for the survival of virus droplets and acid-base imbalance, causing the decline of the viruses. The effect of temperature on the virus survival rate in air is similar; that is, with the increase in temperature, the survival rate of viruses decreases significantly, but in the air, when the temperature reaches above 25 °C, the virus is basically inactivated (Colas de la Noue et al. 2014; Harper 1961).
Fig. 6.
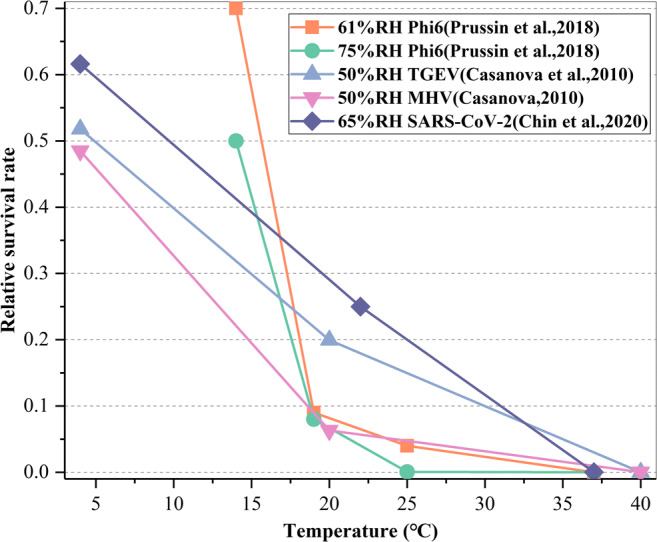
Ambient temperature affects the survival of viruses on surfaces
Medical experiments on the effect of relative humidity on virus survival
In terms of the influence of relative humidity on virus viability, Lin et al. (Lin and Marr 2020) carried out surface titration experiments on MS2 and Φ 6 phage viruses. They titrated 10 μL high-concentration virus suspension with concentration of 1010~1011 pfu mL-1 to a polystyrene cell culture plate, cultured for 1 hour under seven levels of relative humidity conditions in the range of 23~100%, and compared the ratio of virus concentration after exposure to that before exposure in the fixed droplet. The results showed that the relative survival rate of the MS2 virus was the lowest when the relative humidity was 55%, and the relative survival rate of Φ 6 virus was the lowest when the relative humidity was 75~85%. Prussin et al. (Prussin et al. 2018) and Casanova et al. (Casanova et al. 2010) also found similar behavior for virus survival in the range of 20–98% RH. Aimed at SARS-CoV-2, Smither et al. (Smither et al. 2020) and Biryukov et al. (Biryukov et al. 2020) respectively tested the survival of SARS-CoV-2 on tissue culture medium and different pore-free surfaces under different relative humidity conditions. The study also found that the survival rate of the viruses was low at medium relative humidity but higher at high relative humidity.
Figure 7 presents the results of medical comparative experiments on the effect of relative humidity on the relative survival rate of surface viruses obtained from five reports. In order to exclude the influence of other complex factors, the indoor temperature selected in the diagram is in the laboratory conditions with the temperature from 19 to 25 °C which is more comfortable for the human body. It can be seen that the influence of relative humidity on the relative virus survival rate is generally U-shaped, although the virus types are different. In other words, the relative virus survival rate is high when the relative humidity is low or extremely high. When the relative humidity is in the range of 50~70%, the relative survival rates of most viruses are low, which indicates that controlling the relative humidity at a medium level (50~70%) is beneficial to reducing the virus survival and inhibiting the virus transmission.
Fig. 7.
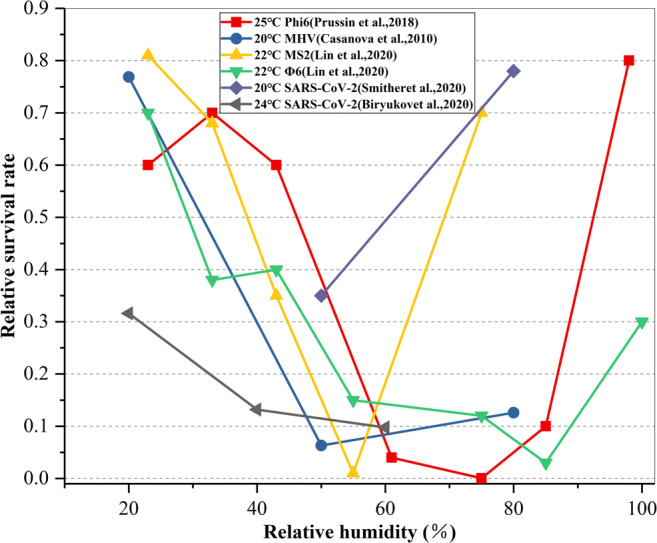
RH affects the survival of viruses on surfaces
People spend about 90% of their time indoors, and the respiratory disease infection is realized by the infected and susceptible individuals in the process of indoor social activities. However, due to the human ethics restriction, it is impossible to carry out human experiments on virus infection, so animal experiments are widely used in medicine. Generally, infected animals and susceptible animals are exposed in a closed space with comfortable temperature and humidity to simulate social behavior and observe the infection status. For example, the same cage experiments (Mubareka et al. 2009; Sia et al. 2020), adjacent cage experiments (Kim et al. 2020; S et al. 2009), short-distance exposure downstream (S et al. 2009), exposure to virus liquid smearing on the surface (Deng et al. 2020; Sia et al. 2020), and aerosol exposure of virus suspension (Kim et al. 2020; Sia et al. 2020), respectively, correspond to common close contact, close propagation, media transmission, and air transmission that are common in interpersonal social activities. Although in air-conditioned indoor spaces, the activity space for interpersonal social behavior is not as limited as in animal experiments, social distance and contact behavior are more random. Infected persons who enter the public buildings after investigation by various means are fewer than those who have participated in the experiment, and the virus concentration is generally not that high, but the evidence-based medical experiments can help readers understand the risk of virus transmission in the operation of the air conditioning system from another level. Some scholars have also carried out medical experiments on the influence of the temperature and humidity environment on the transmission of infection between animals, exposing a number of experimental animals that have been diagnosed with the viruses and a number of healthy animals in a limited box space with the same temperature and adjustable relative humidity. The changes in the viral load, infection rate, or mortality of the animals on each day after exposure were observed. It was found that the infection rate or mortality rate with the change of indoor air temperature and relative humidity has an influence similar to that of the virus survival rate (Herlocher et al. 2004; Marr et al. 2019).
Temperature and humidity control strategy based on medical experiment
The above medical experiments of the indoor temperature and humidity environment on virus survival and animal infection transmission suggest that during the outbreak of respiratory viruses, especially in the winter, raising the indoor air temperature through the air conditioning or heating systems can achieve thermal comfort for the human body, which is more conducive to inhibit virus activity and reduce the risk of infection between people. The suggestion to stop air conditioning or heating in winter will cause the indoor temperature to be low, which is not good for the health of indoor personnel, and may increase the risk of virus transmission. It is recommended that when the air conditioning or heating systems are running, opening windows for ventilation or increasing the amount of fresh air may cause the indoor temperature to be low or even result in the existing system having difficulty in bearing the heavy load. At this time, the indoor temperature is close to the outdoor, causing a great waste of energy, and may increase the risk of virus transmission. Since most air conditioning or heating systems, especially split air conditioners, do not humidify, the relative humidity in the room drops rapidly after heating, which may increase the risk of virus transmission. Therefore, an appropriate increase in relative humidity is a very important means. In summer, the operation of the air conditioning systems reduces the room temperature, although it may increase the virus activity and transmission risk to a certain extent, but due to its dehumidification effect, the indoor relative humidity can be brought to a moderate state, thereby generally inhibiting the virus activity. In particular, the pathogens released indoors by infected persons are in the form of droplets, so dehumidification can collect the potential pathogens in the air from the source, avoiding them from floating in the air, greatly reducing the harm to people indoors and reducing the spread risk.
Although based on the evidence-based medical experiments and combined with the basic principles of indoor environment regulation, the temperature and humidity regulation strategies of the air conditioning or heating systems during the outbreak of the epidemic have been established, due to the coupling complexity of the problem, there are still many issues worthy of more in-depth study.
One-time removal effect of the filter layers on particles containing pathogens
Whether in distributed or centralized environmental control equipment and systems, there are filters of various levels including primary efficiency, medium efficiency, and high efficiency. In some areas with more serious pollution, the owners of residential and public buildings will also install air purifiers to meet the requirements of higher indoor air quality. From the previous analysis, it is not difficult to find that the size of the droplets exhaled by indoor infected persons has a wide distribution range, and the droplet nuclei are formed after the water evaporates rapidly and floats in the air with the airflow generated by the air conditioner, which leads to the risk of transmission. Since the risk of virus transmission of droplet nuclei of different particle sizes is different, searching for the influence of filters (filter layers) on the removal of particles with different particle sizes based on related experiments is an important scientific basis for risk assessment of the spread of air conditioning system filters or air purifiers during the epidemic.
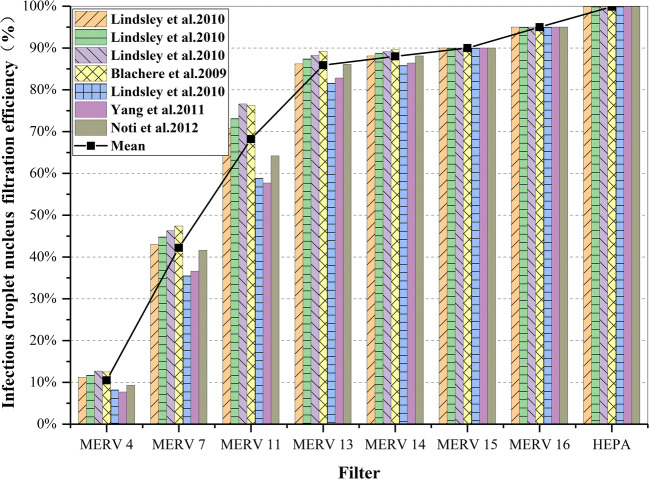
Some scholars (Emmerich et al. 2013; Miller-Leiden et al. 1996; Williamw et al. 1998) have found that particle filters commonly used in heating, ventilation and air conditioning (HVAC) systems can also be used to reduce the risk of airborne infectious diseases. For particles less than about 50 μm in diameter, the evaporation usually takes place within a few seconds (Chen and Zhao 2010). After rapid evaporation, the droplet core containing mixed solid particles (including any infectious particles) still exists, and its particle size is related to the initial droplet diameter, which is generally 26~35% of the initial diameter (Nardell 2001). Relevant experimental studies have been carried out (Blachere et al. 2009; Lindsley et al. 2010a, b; Noti et al. 2012; Yang et al. 2011), testing the one-time removal efficiency of different grades of filters for infectious droplet nuclei with particle sizes ranging from 0.3 to 10 μ. The results show that HVAC filters can effectively remove infectious droplet nuclei in the environment, and some high-efficiency filters can even reach more than 90%. Figure 8 summarizes the data of one-time filtration efficiency of HVAC filters of different grades on infectious droplet nuclei in the above studies. It can be seen from the figure that (1) the average filtration efficiency of infectious droplet nuclei ranges from 10.5% of MERV 4 filter to 99.9% of HEPA filter, and the filtration efficiency is significantly different. (2) The higher the filter level is, the better is the filtering effect. However, when the filter level reaches a certain level (MERV 13 and above), the filtering efficiency will be basically stable at above 90%. Therefore, regardless of the level of the air conditioner used indoors, it can achieve the effect of removing infectious droplets and reduce the risk of infection.
Fig. 8.
Comparative chart of infective-particle-size-weighted filtration efficiency for various HVAC filters
The filter efficiency of different filter layers varies with the particle size, as shown in Fig. 9 (Davies et al. 2013; Lee et al. 2008; Macintosh et al. 2008; Oberg and Brosseau 2008). From this figure, it can be further seen that different filter layers have different filtering effects on particles of different sizes in actual use. It can be found that for the same filter layer, the smaller the particle size, the lower the filtration efficiency. As the particle size increases, the filtration efficiency increases rapidly until a certain particle size where it becomes stable. Therefore, this figure can fully illustrate that the range of indoor pollutant particles removed by filters and air purifiers in air conditioning systems is mainly for larger particle sizes, and their filtering and purification effects are very significant, with an average efficiency of more than 80%.
Fig. 9.
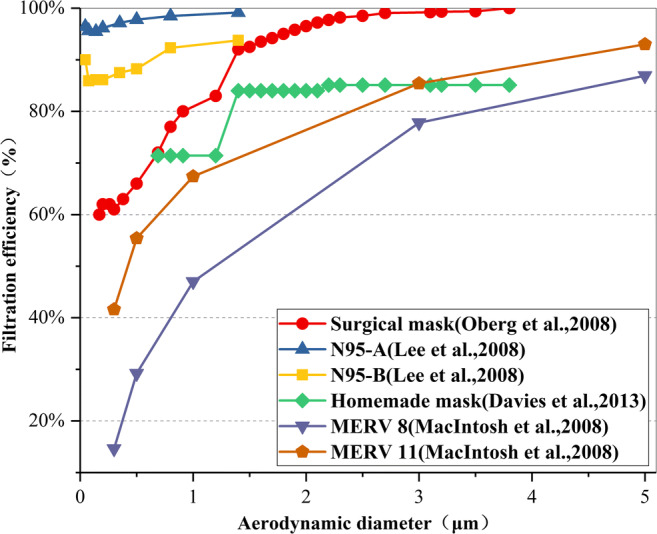
Variation rule of primary filtration efficiency of different filter layers with a particle diameter
Transmission risk of central air conditioning system operation
The centralized air conditioning systems with return air is representative. The principle of indoor temperature and humidity environment and air quality control is shown in Fig. 10. The heat and humidity loads generated by the envelope structure of each room and indoor personnel equipment enter the air conditioning unit through the return air. After passing through the primary (or middle) efficiency filter layer, the heat exchange is conducted with hot or cold water, and then, the humidification or dehumidification treatment is carried out. Finally, it is pressurized by the fan and sent to each room through the pipeline. In order to meet indoor hygiene requirements, a certain amount of outdoor fresh air must be introduced to dilute and reduce the concentration of indoor pollutants. The fresh air ratio is related to building functions, outdoor pollution, indoor load, etc. In civil buildings, the fresh air ratio can be as low as 5–10% or configured according to the minimum indoor air volume per person, and for hospital infectious wards, it can be as high as 100%. Primary (medium) efficiency filters are installed in air conditioning units (general civil buildings), as well as additional air purifiers for rooms in severely polluted areas, and even some special hospital buildings also install high-efficiency air purifiers to further improve indoor air quality. For a general air conditioning system, when the temperature of the cold and heat source is constant, the supply air temperature and relative humidity are basically determined, and the dynamic demand of the room air conditioning load is mainly adjusted by the supply air volume. When the amount of fresh air that meets the sanitary requirements of the room is constant, it is an inevitable choice to increase the number of return air cycles to enhance the heat and moisture load adjustment capacity of the air conditioning system. The corresponding number of cycle air changes (ACH) may be as high as ten times. The processed return airflow is sent to each room again, questioned by increasing the risk of virus transmission during the outbreak. Therefore, according to the aforementioned experimental evidence of the particle size distribution characteristics of the droplet nuclear particles exhaled by the indoor infected person and the filtration efficiency of the filter layer on different particles, combined with the operating characteristics of the centralized air conditioning system of ordinary public buildings, the propagation risks of different operating conditions corresponding to the typical filter purifier configuration are analyzed.
Fig. 10.
Airflow chart of the primary return air conditioning system
The air conditioning unit of the centralized air conditioning systems is generally equipped with primary and medium-efficiency filters, and there are also some better configured medium-efficiency filters. It is assumed that the comprehensive filtration efficiency of the full particle size range of the filter is 30, 50, and 70%. Due to the aggravation of environmental haze and PM2.5 pollution, more and more air purifiers are used independently in public buildings, and the call for designing air purification equipment in the system is gradually increasing. It is assumed that the comprehensive filtration efficiency of the full particle size range of the air purifier is 40, 75, and 95% (Cui et al. 2017), as shown in Table 1. In order to focus on the risk of transmission of droplets (droplet nuclei) of particles released into the room by the infected person during the epidemic period through the air conditioning system, we use the viral load of the infected person’s cough and the total volume of each exhaled droplet (Chen et al. 2020) to obtain the concentration C0 is the potential pathogenic particles discharged by the indoor infected person. It is supposed that the droplets are uniformly distributed in the indoor air and exist in the return airflow. After mixing with fresh air, they pass the filter or/and air purifier of the air conditioning unit and finally are sent to each room for continuous circulation. During the process, the natural settlement and reproduction of the droplets in the indoor and air conditioning system pipe network are not considered. Fresh air has a certain dilution effect on potential indoor pathogenic particulates, but the number of fresh air changes (usually 0.5~2 times) is much smaller than that of return air. Therefore, the impact of this is ignored when analyzing the risk of air conditioning system transmission.
Table 1.
The efficiency of different filter/purification equipment in the air conditioning system
| Filtration efficiency α1 | Purification efficiency α2 | |
|---|---|---|
| Minimum configuration | 30% | 40% |
| Medium configuration | 50% | 75% |
| Top configuration | 70% | 95% |
Since the transmission risk of centralized air conditioning mainly comes from the circulation of air, Fig. 11 shows the different grade filter and air purifier configurations of the air conditioning system and the corresponding indoor air circulation system’s removal effect on potential pathogens after one air change. In the figure, the relative concentration ratio C/C0 represents the relative size of indoor concentration of particulate matter of potential pathogen after one cycle. As it can be seen, (1) regardless of the level of air conditioning system configuration, it can effectively reduce the concentration of indoor pathogen particles, that is, effectively reducing the risk of indoor virus transmission. (2) For an ordinary centralized air conditioning system with only a filter, after the indoor air is circulated once, the relative concentration of pathogenic particles will decrease as the efficiency of the filter increases, thereby reducing the risk of transmission. (3) If an air purifier is installed on the basis of an ordinary air conditioning system, the effect of reducing the relative concentration of indoor pathogenic particles will be more obvious, and one cycle can reduce the risk of transmission by up to 98.5%.
Fig. 11.
Comparison of potential pathogen concentration changes in different configurations of air conditioning systems after one cycle of indoor air
In fact, in order to take away the waste heat and humidity in the room, the number of air changes of the circulating air of the central air conditioning system increases with the increase in load. Figure 12 is a diagram showing the relative concentration of pathogen particles corresponding to the change of the system running time under different air changes of circulating air when the air conditioning system is only equipped with a primary and medium-efficiency filter (that is, the medium configuration α1 = 50% and α2=0 in Table 1). It can be seen that (1) when the different air changes with the circulating air are 3 times/h, the relative concentration of pathogen particles can be reduced by about 90% after one hour. When the number of air changes of the circulating air reaches 7 times/h or more, the relative concentration of pathogen particles is only 0.1% in one hour. The risk of infection is very low. (2) Although the airflow organization of the centralized air conditioner may send the air from the room with the infected person to other rooms, after the circulating air is processed by the filtering device, most of the potential pathogen particles are captured and removed, which greatly reduces the risk of transmission. It is not difficult to predict that if an air purifier is independently purchased and put into operation in a centralized air conditioning system, it can achieve a better effect on removing potential indoor pathogen particle pollutants.
Fig. 12.
Changes in the relative concentration of pathogen particles with time under different cycles
The above analysis shows that the centralized air conditioning system operating normally during the epidemic has obvious effects of removing pathogenic particles and reducing the risk of transmission. First of all, the temperature and relative humidity are adjusted to the thermal comfort zone of the human body. Medical experiments have shown that it can accelerate the decline and death of viruses to a greater extent and is not conducive to the spread of the viruses between animals and persons. Secondly, the filter or air purifier in the air conditioning system has a better filtering and removal effect on the larger particle sizes of the pathogen particle carrier, and the medical experiments show that the larger the droplet nuclear virus load, the higher the virus load and the longer the survival time. This is the main contradiction of the respiratory virus transmission, so the risk of transmission after being captured and removed can be greatly reduced. The droplet nuclei remaining in the circulating air of the air conditioning system are mainly of small size. Although the number is large, the cumulative volume is small, and the decay is faster, the risk of transmission is lower. Of course, it does not mean that the droplet nuclei sent into each room in the circulating air are completely free from the risk of transmission. Small particles captured on the filter layer may be swept up by the airflow again, causing secondary diffusion. It is necessary to further understand the living environment and reproduction rules of the viruses from the medical perspective, especially the decay characteristics of the viruses in the external environment from the moment it leaves the host, and to find a more scientific answer by combining with the movement rules of droplet nucleus particles caused by air circulation in the centralized air conditioning system.
Conclusions and tips
In this paper, based on the existing medical experimental studies, the risk of the indoor SARS-COV-2 transmission is analyzed from three basic links of virus transmission. On the basis of the above, the risk is combined with the operation of the air conditioning systems. Taking the primary return air conditioning system as an example, the effect of air conditioning operation on the spread of SARS-CoV-2 in the indoor environment is comprehensively analyzed. The research results show the following:
Among three basic links of virus transmission, large particle size droplets exhaled by an infected person at the source of infection have the greatest risk in the process of indoor virus transmission, and the risk in the transport path comes next. For susceptible people, the risk is greater only if they have close contact with infected persons without adequate respiratory protection measures.
When the temperature is 20~25 °C and the relative humidity is 50~70%, the viability of the viruses in the air and on the surface is weak, and the inactivation is faster. Therefore, in an air-conditioned room, the air conditioning system can adjust the temperature and humidity to ensure human comfort and to create an indoor environment that is not conducive to the survival of SARS-CoV-2, thereby effectively inhibiting the spread of the viruses to a certain extent and reducing the risk of indoor virus transmission.
Because the virus content is proportional to the cube of the particle size and the efficiency of the filter or air purifier in the centralized air conditioner increases rapidly with the increase in the diameter of the pathogen-containing particles, an increase in the number of air changes of the circulating air accelerates the removal of potential pathogen particles. Therefore, the operation of the air conditioning system is helpful to solve the main contradiction. The return air circulation may also accelerate the mixing of the air in each room, but the small particle size, long floating time, and rapid decay of these droplet nuclei are secondary to the spread of the epidemic.
The operation of the air conditioning system can effectively reduce the concentration of potential pathogens in an air-conditioned room. The quantitative analysis shows that even under relatively poor cycle condition of 1 time/h, the residual rate of potential pathogens is only 40% after air conditioning operation for 1 hour, and the risk of infection is effectively reduced, while under the condition of good ventilation of 7 times/h and above, within 1 hour of air conditioning operation, the virus survival rate is only about 0.1% of the initial situation, and the infection risk is only one thousandth of the initial situation.
During the epidemic, people do not need to worry too much about whether SARS-CoV-2 will be transmitted through the air conditioning system. Scientific, reasonable, and safe use of an air conditioner can not only create a comfortable living environment, but also effectively reduce the risk of indoor infection.
Author contributions
Yonghong Jia was responsible for the data curation and original draft preparation. Yue Xiang, Shurui Guo, Lei Guo, and Zhu Cheng reviewed and edited the manuscript. Luyao Guo reviewed the manuscript. Yin Zhang and Li Zhang were responsible for the supervision. Enshen Long was involved in the conceptualization and methodology.
Funding
This work was supported by the National Natural Science Foundation of China (52078314) and the Sichuan Science and Technology Program (2019YFS0051 and 2020YFS0439).
Data availability
All data used during the study are available from the corresponding author on request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yonghong Jia, Email: Jiayonghong0301@163.com.
Yue Xiang, Email: xiangyue@cqust.edu.cn.
Shurui Guo, Email: Sherrygsr@outlook.com.
Lei Guo, Email: 370027526@qq.com.
Luyao Guo, Email: Gloriagly@outlook.com.
Zhu Cheng, Email: scchengzhu@126.com.
Yin Zhang, Email: cdzhangyin@163.com.
Li Zhang, Email: zhaolicabr@163.com.
Enshen Long, Email: longes2@163.com.
References
- Becker R, Haquin G, Kovler K (2013) Air change rates and radon accumulation in rooms with various levels of window and door closure. J Build Phys 38(3):234–261. 10.1177/1744259113506071
- Biryukov J, Boydston JA, Dunning RA, Yeager JJ, Wood S, Reese AL, Ferris A, Miller D, Weaver W, Zeitouni NE, Phillips A, Freeburger D, Hooper I, Ratnesar-Shumate S, Yolitz J, Krause M, Williams G, Dawson DG, Herzog A, Dabisch P, Wahl V, Hevey MC, Altamura LA (2020) Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere 5(4). 10.1128/mSphere.00441-20 [DOI] [PMC free article] [PubMed]
- Blachere FM, Lindsley WG, Pearce TA, Anderson SE, Fisher M, Khakoo R, Meade BJ, Lander O, Davis S, Thewlis RE, Celik I, Chen BT, Beezhold DH. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis. 2009;48(4):438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- Buonanno G, Stabile L, Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76(9):2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Liao CM. Probabilistic indoor transmission modeling for influenza (sub)type viruses. J Inf Secur. 2010;60(1):26–35. doi: 10.1016/j.jinf.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhao B. Some questions on dispersion of human exhaled droplets in ventilation room: answers from numerical investigation. Indoor Air. 2010;20(2):95–111. doi: 10.1111/j.1600-0668.2009.00626.x. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang N, Wei J, Yen H-L, Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020;176:106859. doi: 10.1016/j.buildenv.2020.106859. [DOI] [Google Scholar]
- Cheng Y-H, Liao C-M. Modeling control measure effects to reduce indoor transmission of pandemic H1N1 2009 virus. Build Environ. 2013;63:11–19. doi: 10.1016/j.buildenv.2013.01.014. [DOI] [Google Scholar]
- Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H-L, Chan MCW. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Lancet. 2020;1(1):E10. [Google Scholar]
- Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H-L, Chan MCW, Peiris M, Poon LLM. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microb. 2020;1(1):e10. doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas de la Noue A, Estienney M, Aho S, Perrier-Cornet JM, de Rougemont A, Pothier P, Gervais P, Belliot G. Absolute humidity influences the seasonal persistence and infectivity of human norovirus. Appl Environ Microbiol. 2014;80(23):7196–7205. doi: 10.1128/AEM.01871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia G, Rodrigues L, Gameiro da Silva M, Goncalves T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med Hypotheses. 2020;141:109781. doi: 10.1016/j.mehy.2020.109781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Yang X, Shen H, Li Q (2017) Experimental study on indoor air cleaner based on different types of air filter units. HV&AC
- Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7(4):413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, W., Bao, L., Gao, H., Xiang, Z., Qu, Y., Song, Z., . . . Qin, C. (2020). Rhesus macaques can be effectively infected with SARS-CoV-2 via ocular conjunctival route. bioRxiv doi:10.1101/2020.03.13.990036
- Doremalen V, Bushmaker T, Munster VJ (2013) Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eurosurveillance 18(38) [DOI] [PubMed]
- Doremalen N v, Morris DH, Holbrook MG, Gamble A, Williamson BN (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med:1564–1567 [DOI] [PMC free article] [PubMed]
- Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei[J] J Hyg (Lond) 1946;44(6):471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid JP. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinburgh Med J. 1954;52(11):385–401. [PMC free article] [PubMed] [Google Scholar]
- Emmerich SJ, Heinzerling D, Choi J-I, Persily AK. Multizone modeling of strategies to reduce the spread of airborne infectious agents in healthcare facilities. Build Environ. 2013;60:105–115. doi: 10.1016/j.buildenv.2012.11.013. [DOI] [Google Scholar]
- Farnham A, Alleyne L, Cimini D, Balter S. Legionnaires' disease incidence and risk factors, New York, New York, USA, 2002-2011. Emerg Infect Dis. 2014;20(11):1795–1802. doi: 10.3201/eid2011.131872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Kuehn TH, Abin M, Verma H, Bekele A, Mor SK, Goyal SM, Appert J, Raynor PC, Zuo Z. Airborne virus survivability during long-term sampling using a non-viable Andersen cascade impactor in an environmental chamber. Aerosol Sci Technol. 2014;48(12):1360–1368. doi: 10.1080/02786826.2014.984800. [DOI] [Google Scholar]
- Greatorex JS, Digard P, Curran MD, Moynihan R, Wensley H, Wreghitt T, et al. Survival of influenza A(H1N1) on materials found in households: implications for infection control. PLoS One. 2011;6(11):e27932. doi: 10.1371/journal.pone.0027932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi: 10.1016/s0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. (1994). [PubMed]
- Harper GJ. Airborne micro-organisms survival tests with four viruses. Epidemiol Infect. 1961;59(4):479–486. doi: 10.1017/S0022172400039176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlocher ML, Truscon R, Elias S, Yen H-L, Roberts NA, Ohmit SE, Monto AS. Influenza viruses resistant to the antiviral drug oseltamivir transmission studies in ferrets. J Infect Dis. 2004;190(9):1627–1630. doi: 10.1086/424572. [DOI] [PubMed] [Google Scholar]
- Hoseinzadeh E, Safoura J, Farzadkia M, Mohammadi F, Hossini H, Taghavi M. An updated mini-review on environmental route of the SARS-CoV-2 transmission. Ecotoxicol Environ Saf. 2020;202:111015. doi: 10.1016/j.ecoenv.2020.111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://health.clevelandclinic.org/does-air-conditioning-spread-the-coronavirus/.
- https://rlt-geraete.de/.
- https://www.ashrae.org/technical-resources/resources.
- https://www.buildup.eu/en/practices/publications/covid-19-guidance-rehva.
- https://www.cibse.org/coronavirus-covid-19/coronavirus-covid-19-and-hvac-systems.
- Ijaz MK, Brunner AH, Sattar SA. Survival characteristics of airborne human coronavirus 229E. J Gen Virol. 1985;66:2743–2274. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- Jayaweera M, Perera H, Gunawardana B, Manatunge J (2020) Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res 188:109819. 10.1016/j.envres.2020.109819 [DOI] [PMC free article] [PubMed]
- Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, Um J, Song MS, Jeong HW, Lai VD, Kim Y, Chin BS, Park JS, Chung KH, Foo SS, Poo H, Mo IP, Lee OJ, Webby RJ, Jung JU, Choi YK. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704–709. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepeis, N. E., Nelson, W. C., OTT, W. R., Robinson, J. P., S, A. M. T., Witzer, . . . Engelmann, W. H. (2020). The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. [DOI] [PubMed]
- Lee SA, Grinshpun SA, Reponen T. Respiratory performance offered by N95 respirators and surgical masks: human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann Occup Hyg. 2008;52(3):177–185. doi: 10.1093/annhyg/men005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Marr LC. Humidity-dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environ Sci Technol. 2020;54(2):1024–1032. doi: 10.1021/acs.est.9b04959. [DOI] [PubMed] [Google Scholar]
- Lindsley WG, Blachere FM, Davis KA, Pearce TA, Fisher MA, Khakoo R, Davis SM, Rogers ME, Thewlis RE, Posada JA, Redrow JB, Celik IB, Chen BT, Beezhold DH. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin Infect Dis. 2010;50(5):693–698. doi: 10.1086/650457. [DOI] [PubMed] [Google Scholar]
- Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, Palmer JE, Clark KE, Fisher MA, Khakoo R, Beezhold DH. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5(11):e15100. doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley WG, Pearce TA, Hudnall JB, Davis KA, Davis SM, Fisher MA, Khakoo R, Palmer JE, Clark KE, Celik I, Coffey CC, Blachere FM, Beezhold DH. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg. 2012;9(7):443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RG, Roberts RM. Droplet expulsion from the respiratory tract[J] Am Rev Respir Dis. 1967;95(3):435–442. doi: 10.1164/arrd.1967.95.3.435. [DOI] [PubMed] [Google Scholar]
- Lu J, Gu J, Li K, Xu C, Su W, Lai Z, Zhou D, Yu C, Xu B, Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh DL, Myatt TA, Ludwig JF, Baker BJ, Suh HH, Spengler JD. Whole house particle removal and clean air delivery rates for in-duct and portable ventilation systems. J Air Waste Manage Assoc. 2008;58(11):1474–1482. doi: 10.3155/1047-3289.58.11.1474. [DOI] [PubMed] [Google Scholar]
- Mao N, An CK, Guo LY, Wang M, Guo L, Guo SR, Long ES. Transmission risk of infectious droplets in physical spreading process at different times: a review. Build Environ. 2020;185:107307. doi: 10.1016/j.buildenv.2020.107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr LC, Tang JW, Van Mullekom J, Lakdawala SS. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface. 2019;16(150):20180298. doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Leiden S, Lobascio C, Nazaroff WW, Macher JM. Effectiveness of in-room air filtration and dilution ventilation for tuberculosis infection control. J Air Waste Manage Assoc. 1996;46(9):869–882. doi: 10.1080/10473289.1996.10467523. [DOI] [PubMed] [Google Scholar]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16:335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Morawska L, Milton DK (2020) It is time to address airborne transmission of COVID-19. Clin Infect Dis. 10.1093/cid/ciaa939 [DOI] [PMC free article] [PubMed]
- Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S et al (2009) Size distribution and sites of origin of droplets expelled during expiratory activities. J Aerosol Sci [DOI] [PMC free article] [PubMed]
- Mubareka S, Lowen AC, Steel J, Coates AL, Garcia-Sastre A, Palese P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J Infect Dis. 2009;199(6):858–865. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardell EA. Chapter 11: disinfecting air. McGraw-Hill, New York, NY: Indoor air quality handbook; 2001. [Google Scholar]
- Noti JD, Lindsley WG, Blachere FM, Cao G, Kashon ML, Thewlis RE, McMillen CM, King WP, Szalajda JV, Beezhold DH. Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin Infect Dis. 2012;54(11):1569–1577. doi: 10.1093/cid/cis237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg T, Brosseau LM. Surgical mask filter and fit performance. Am J Infect Control. 2008;36(4):276–282. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SJ, Chang HL, Cheung TY, Tang AF, Fisk TL, Ooi SP, et al. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349(25):2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- Pan, L., Yujing Zhang, Xu Yan, & Zhang, L. (2020a). Operation and management guidelines for air conditioning and ventilation systems in office and public places during COVID- 19 outbreak. Journal of Environmental Hygiene.
- Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411–412. doi: 10.1016/s1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin AJ, Schwake DO, Lin K, Gallagher DL, Buttling L (2018) Survival of the enveloped virus Phi6 in droplets as a function of relative humidity. Appl Environ Microbiol 84(12) [DOI] [PMC free article] [PubMed]
- Pyankov OV, Pyankova OG, Agranovski IE. Inactivation of airborne influenza virus in the ambient air. J Aerosol Sci. 2012;53:21–28. doi: 10.1016/j.jaerosci.2012.05.011. [DOI] [Google Scholar]
- Pyankov OV, Bodnev SA, Pyankova OG, Agranovski IE. Survival of aerosolized coronavirus in the ambient air. J Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G, Li X, Hu L, Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ Sci Technol. 2020;54:3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- REHVA_COVID-19_guidance_document_ver2_20200403_.
- S M, Lowen AC, Steel J. Transmission of influenza virus via aerosols and fomites in the guinea pig model[J] J Infect Dis. 2009;199(6):858–865. doi: 10.1086/597073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, H., Wada, K., Kajioka, J., Watanabe, M., Nakano, R., Hirose, T.a, Ohta H. Aizawa, Y. (2010). Maintenance of influenza virus infectivity on the surfaces of personal protective equipment and clothing used in healthcare settings. Environ Health Prev Med, 15(6), 344-349. doi:10.1007/s12199-010-0149-y [DOI] [PMC free article] [PubMed]
- Sattar SA, Ijaz MK, Johnson-Lussenburg CM, Springthorpe VS. Effect of relative humidity on the airborne survival of rotavirus SA11. Appl Environ Microbiol. 1984;47(4):879–881. doi: 10.1128/AEM.47.4.879-881.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, Kaewpreedee P, Perera RAPM, Poon LLM, Nicholls JM, Peiris M, Yen HL. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583(7818):834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R, Ahmed Khan N. Centralized air-conditioning and transmission of novel coronavirus. Pathog Glob Health. 2020;114(5):228–229. doi: 10.1080/20477724.2020.1765653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smither SJ, Eastaugh LS, Findlay JS, Lever MS (2020) Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg Microbes Infect 9(1):1415–1417. 10.1080/22221751.2020.1777906 [DOI] [PMC free article] [PubMed]
- Tsang KK-W, Os T-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/s1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Du G. COVID-19 may transmit through aerosol. Ir J Med Sci. 2020;189:1143–1144. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Cao S, Ma J, Huang N, Nie J, Wang Z, Duan X (2015) 5-time-activity factors related to air exposure. In: Duan X, Zhao X, Wang B, Chen Y, Cao S (eds) Highlights of the Chinese exposure factors handbook, vol 2015. Academic Press, pp 31–39
- Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface. Mater mBio. 2015;6:e01697–e01615. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO announces COVID-19 outbreak a pandemic (http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic).
- WHO, WHO coronavirus disease (COVID-19) dashboard 2020. https://covid19.who.int/.
- Williamw, Nazaro FF, Marknica S, Miller AL (1998) Framework for evaluating measures to control nosocomial tuberculosis transmission. Indoor Air
- Yang W, Elankumaran S, Marr LC. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J R Soc Interface. 2011;8(61):1176–1184. doi: 10.1098/rsif.2010.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ (2020) Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A 117(26):14857–14863. 10.1073/pnas.2009637117 [DOI] [PMC free article] [PubMed]
- Zhao S, Zhang A, Zhang X. Three basic steps in the epidemic of infectious diseases. 2010. [Google Scholar]
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used during the study are available from the corresponding author on request.